Abstract
Heat stress is one of the major abiotic stresses that impair plant growth and crop productivity. Plant growth-promoting endophytic bacteria (PGPEB) and humic acid (HA) are used as bio-stimulants and ecofriendly approaches to improve agriculture crop production and counteract the negative effects of heat stress. Current study aimed to analyze the effect of thermotolerant SA1 an isolate of Bacillus cereus and HA on tomato seedlings. The results showed that combine application of SA1+HA significantly improved the biomass and chlorophyll fluorescence of tomato plants under normal and heat stress conditions. Heat stress increased abscisic acid (ABA) and reduced salicylic acid (SA) content; however, combined application of SA1+HA markedly reduced ABA and increased SA. Antioxidant enzymes activities revealed that SA1 and HA treated plants exhibited increased levels of ascorbate peroxidase (APX), superoxide dismutase (SOD), and reduced glutathione (GSH). In addition, heat stress markedly reduced the amino acid contents; however, the amino acids were increased with co-application of SA1+HA. Similarly, inductively-coupled plasma mass-spectrometry results showed that plants treated with SA1+HA exhibited significantly higher iron (Fe+), phosphorus (P), and potassium (K+) uptake during heat stress. Heat stress increased the relative expression of SlWRKY33b and autophagy-related (SlATG5) genes, whereas co-application of SA1+HA augmented the heat stress response and reduced SlWRKY33b and SlATG5 expression. The heat stress-responsive transcription factor (SlHsfA1a) and high-affinity potassium transporter (SlHKT1) were upregulated in SA1+HA-treated plants. In conclusion, current findings suggest that co-application with SA1+HA can be used for the mitigation of heat stress damage in tomato plants and can be commercialized as a biofertilizer.
1. Introduction
Temperature plays a vital role in plant growth and development [1]. However, a rise in temperature beyond a threshold level causes irreversible damages to plant growth and development [1,2]. High temperatures affect all developmental stages of plants such as germination, vegetative growth, and gamete, seed and fruit development, resulting in crop yield reduction [3,4]. Lobell et al. [5] reported that high temperatures reduce grain yield per plant by 70% for each 1°C increase in temperature, resulting in 4–14% yield loss in rice. Similarly, You et al. [6] reported a 10% decrease in wheat yield with a 1°C increase in temperature. Furthermore, Intergovernmental Panel on Climate Change (IPCC) reported that India would likely suffer from a 10–40% loss in crop production by 2080–2100 due to heat stress [7].
Heat stress causes various physiological, biochemical, morphological and molecular changes that adversely affect plant growth, biomass, productivity and yield production either individually or in combination with other abiotic stresses [1,2,8]. Leaves and stem scorching, leaf abscission and senescence, root inhibition, shoot development and fruit damage are the physiological injuries observed with an increase in temperature [9,10]. Among the physiological processes, photosynthesis is more sensitive to heat stress and inhabit Photosystem II (PSII), which leads to a decrease in chlorophyll florescence, altering the photosynthetic pigment, foliar expansion and leaf senescence [11–13]. Biochemical changes that can occur in plants include the fluidity of membranes, organization of cellular structure, the structure of amino acids, an increase or decrease in the concentration of metabolites and osmolytes, a decrease in the synthesis of normal proteins, an increase in stress hormones like abscisic acid (ABA), a decrease in defense hormones like salicylic acid (SA), and the production of injurious reactive oxygen species (ROS) and antioxidants. Heat stress often leads to the accumulation of ROS such as superoxide radicals and hydrogen peroxide, which cause oxidative damage and disrupt metabolic homeostasis in plants [14–16]. However, plants activate antioxidant complex systems such as reduced glutathione (GSH), superoxide dismutase (SOD) and other macromolecules (proline, carbohydrates) that protect them from oxidative damages and scavenge excess ROS [14,17]. Molecular changes include the alteration of genes involved in the protection from heat stress. These genes are responsible for the expression of osmoprotectants, detoxifying enzymes and transporters and increases in the regulation of proteins called heat shock proteins (HSPs), heat stress transcription factors (HSFs), stress-induced proteins or stress proteins that are expressed and play key roles in conferring stress tolerance when plants are exposed to any stressors [18–20].
Heat stress tolerance is also achieved through genetic engineering, breeding programs, tissue culture, maturation and chemical fertilizer application, which are time consuming, costly and have adverse effect on the environment [20–23]. One alternative and ecofriendly approach for the improvement of agricultural crop production to ameliorate the negative effects of high temperature is the use of plant growth-promoting bacteria and chemicals such as humic substances. This biological technique is extremely popular and is broadly accepted all over the world [24–29]. Humic acid (HA) is a heterogeneous mixture of many compounds that enhance plant growth under normal and abiotic stress conditions [28–31]. The use of plant growth promoting bacteria is another approach for ameliorating the negative effects of abiotic stress (21–24). In the last couple of decades, a number of different researchers reported the use of plant growth-promoting bacteria (PGPB) for enhancing tolerance to heat stress in plants such as sorghum [26], chick pea [32], wheat [27,33], tomato [34], and potato [35]. Furthermore, PGPBs have the ability to produce phytohormones that help make plants tolerant against heat stresses by enhancing biofilm formation as well as reducing ABA level and HSP accumulation [24,26,27,32,33,36]. Previous reports indicated that significant changes in the root architecture in non-leguminous plants induced by humic substances may favor the fitness of bacteria-plant interactions, thus resulting in a significant increase in bacteria attachment and survival on plant surfaces as well as endophytic colonization [37–39]. Regarding the mutual interactions between plant roots and microorganisms, previous studies [39–41] reported that heat stress is a key factor in determining soil fertility as a candidate vehicle for PGPB and co-inoculation could be an excellent approach. Other studies [40,42,43] indicated a further benefit of the interaction between microorganisms and organic matter through biological substrate enrichment.
Tomato is one of the most popular and widely consumed vegetables grown worldwide. It is the second most popular vegetable after potato and is considered a good source of dietary minerals, vitamins, lycopenes and other essential nutrients [44,45]. Although tomato has the potential to be cultivated in every location throughout the world, high temperatures above its optimum temperature decrease growth, biomass and yield. According to Abdellatif et al. [46], high temperature affects all stages of tomato plants from the germination to reproductive phases and affects several physiological and biochemical processes dealing with final yield reduction. Canellas and Olivares [47] reported the combined application of PGPB and HA on several crops. Similarly, Busato et al. [48] applied a microbial suspension and a humic substance to plant substrates to promote seedling adaptation to stressful environments. Pishchik et al. [49] reported the impact of combined application of microbes and HA on tomato plants. However, least information are available on the combined effects of PGPB specifically endophytes and HA on tomato seedlings under heat stress conditions. Hence, in the present study, it was aimed to understand the combined effects of thermotolerant SA1 an isolate of Bacillus cereus and HA on tomato seedling’s growth and physiological changes at the biochemical, and molecular levels under heat stress.
2. Materials and methods
2.1. Isolation and screening of bacterial strains
Four plant samples (Artemisia princeps Pamp, Chenopodium ficifolium Smith, Oenothera biennis L. and Echinochloa crus-galli) were collected for the isolation of endophytic bacteria from the sand dunes at Pohang beach (latitude 36°7′56.2′′N, longitude 129° 23′55.1′′E) in the Republic of Korea. The method described by khan et al [50,51] was used for the isolation and screening of bacterial endophytes from the roots of the above mentioned plants.
Prior to bioassay assessment and molecular identification, pure cultures of the selected isolates were screened for the production of indole-3-acetic acid (IAA), siderophores and phosphate solubilization potential. Salkowski reagents were used for the initial confirmation of IAA production according to the protocol developed by Patten and Glick [52]. The detailed method described by Katznelson and Bose [53] and Khan et al [51] was assessed for phosphate solubilization potential. For siderophore production, the commonly used method introduced by Schwyn and Neilands [54] was followed. For bioassay assessment, bacterial isolates that revealed prominent plant growth-promoting traits in the initial screening were also screened on Waito-C (gibberellin [GA]-deficient) rice seedlings.
2.2. Molecular identification and screening for thermotolerant bacteria
Two different methods were used for the identification of thermotolerant bacteria. In the first method, multiple plant growth-promoting traits-producing bacteria were streaked on Luria-Bertani (LB) agar plates and kept at 30, 35, and 40°C for 6 days. The growth of the selected isolates was checked on a daily basis. In the second method, isolates were grown in LB broth media for 6 days consecutively. The culture was grown on a rotatory shaker at 35, 40, and 45°C. The growth of all the isolates was recorded using a spectrophotometer at 600 nm for 6 days. For molecular identification, rRNA was isolated using the detailed method presented by Sambrook and Russell [55]. Specific primers for 16S rDNA, 1492 Reverse (5′-CGG (T/C) TA CCT TGT TAC GAC TT-3′) and 27 Forward (5′-AGA GTT TGA TC (C/A) TGG CTC AG-3′) were used and amplified according to the protocol described by Khan et al. [50]. For the determination of nucleotide sequence homology for the selected isolates, the BLAST NCBI tool was used, while MEGA 6.1 was used for phylogenetic analysis as suggested by Tamura et al. [56].
2.3. In-vitro IAA, GA and organic acid quantification of isolate SA1
For the quantification of IAA, GA and organic acids, isolate SA1 was grown on LB media for 3 days and centrifuged at 5000Xg for 15 min. Following the method developed by Khan et al. [57], the culture broths of selected isolates were analyzed for IAA production. Similarly, for the extraction and quantification of the GA content, the method described by Khan et al [58] was followed and the data was calculated in nano-grams per milliliter. Organic acids were determined using Kang et al.’s method [59]. In brief, the culture broth of the bacterial isolate was centrifuged and the supernatant was filtered through 0.22-μm SmartPor Syringe Filter (P/N SPU0213-1). A total of 10 μL of the filtrate sample was injected into a high-performance liquid chromatography system (HPLC; Waters 600, Milford, MA, USA). For determination of the organic acids, the retention time and peak areas were compared with standards from Sigma-Aldrich, USA. All of the samples were analyzed in triplicate.
2.4. Plant-microbe growth and heat stress conditions
Vir Yegwang tomato seeds were purchased from Danong Co and surface sterilize with 70% EtoH followed by 2.5% NaOH and rinsed with deionized distilled water [60]. After surface sterilization, the seeds were germinated in an incubator (28°C). After 20 days of germination in the trays, uniform seedlings were selected for further processing. Plastic pots (440×270×195mm) were filled with three-time autoclaved field soil (M2 greenhouse at Kyungpook National University) and used for the growth of tomato. After 1 week of transplantation, 1L of freshly diluted bacterial culture(109 CFU/mL) was inoculated to each pot; this was repeated further two time after 5 days, similarly 500mg/L of humic acid was used [61,62], while autoclaved double distilled water were used for control tomato plants. At V3 stage plants were exposed to heat stress and plants sample were collected at 15 days. The experimental design was as follows: (a) Control (normal tomato), (b) Tomato with Isolate SA1, (c) Tomato with HA, (d) Tomato with SA1+HA, (e) heat stress (37°C), (f) heat stress with SA1, (g) heat stress with HA, (h) heat stress with SA1+HA in a growth chamber. Normal tomato plants were subjected to a 24-hour cycle at 28°C for 14 hours and 25°C for 10 hours with a relative humidity of 60 to 70% while the heat stress group was subjected to a 24-hour cycle at 37°C for 14 hours and 30°C for 10 hours with a relative humidity of 60 to 70%. Upon stress completion, growth attributes such as root/shoot length, biomass (fresh and dry weight) were recorded. The plants (shoot) were immediately harvested in liquid nitrogen and stored at -80 °C until further biochemical analyses. For chlorophyll estimation, a chlorophyll fluorometer (FIM 1500, ADC Bioscientific Ltd, UK) was used to measure chlorophyll fluorescence. The collected data were used for photosystem II (Fv/Fm) calculation as reported by Genty et al. [63].
2.5. Plant endogenous phytohormone quantification
The plant samples were subjected to endogenous phytohormone analysis and quantification under a controlled environment. Endogenous ABA was quantified according to the detailed method described by Asaf et al. [64] and Khan et al [65]. The pulverized shoot plant samples were treated with 30 mL of extraction solution containing 95% isopropanol, 5% glacial acetic acid, and 20 ng of [(±)– 3,5,5,7,7,7–d6]–ABA. The extracts were dried and methylated by adding diazomethane for GC-MS SIM (6890 N network GC system, and 5973 network mass-selective detector; Agilent Technologies, Palo Alto, CA, USA) analysis. On the other hand, SA was quantified following the method of Jan et al. [66]. Freeze-dried aerial parts were quantified using a HPLC system equipped with a fluorescence detector (excitation and emission at 3005 and 365 nm, respectively; Shimadzu RF-10AXL; Shimadzu, Kyoto, Japan) and fitted with a C18 reverse phase HPLC column (particle size 5 m, pore size 120 Å; HP Hypersil ODS; Waters Co., Milford, MA, USA) at a defined flow rate (1.0 ml/min).
2.6. Antioxidant analysis
LPO was analyzed according to the method of Bilal et al. [67]. Plant shoots were ground with liquid nitrogen, and 10 mM phosphate buffer (pH 7) was added. The reaction mixture was prepared by adding 0.2 ml of 8.1% sodium dodecyl sulfate, 1.5 ml of 20% acetic acid (pH 3.5), and 1.5 ml of 0.81% thiobarbituric acid to the supernatant, heating in boiling water for 60 min, and immediately cooling on ice to room temperature. Then, 5 ml of butanol:pyridine solution (15:1 v/v) was added. The upper layer was removed, and the resulting pink color was measured at 532 nm using a spectrophotometer. APX activity was measured following the method of Kim et al [68]. The plant sample was added to the reaction mixture (50 mM potassium phosphate pH 7, 0.5 mM ascorbate, 0.1 mM hydrogen peroxide, and 0.1 mM EDTA), and the decrease in absorbance was measured from 10 to 30 s at 290 nm using a spectrophotometer. The method of Khan et al. [69] was adapted for SOD activity assay. In brief, leaf samples (100 mg) were homogenized with 0.01 M phosphate buffer at pH 7.0 and centrifuged (17,000 × g for 15 min at 4°C). The supernatant was used as a crude enzyme extract and passed through a reaction mixture containing Tris-HCl buffer (2 ml) pH 8.2, double-distilled water (2 ml), and 2 mM pyrogallol (0.5 ml). The absorption of the assay mixture and blank (lacking pyrogallol or tissue homogenate) was measured at 470 nm using a spectrophotometer (Shimadzu, Kyoto, Japan) at 180 s intervals. The data are expressed as units/mg of protein. To determine the reduction in GSH concentration, each sample (500 mg) was treated with 2 ml of 10% trichloroacetic acid and centrifuged at 10,000 rpm for 15 min at 4°C. The resulting supernatant (1 ml) was combined with 0.5 ml of Ellman’s reagent and 3 ml of 15 mM sodium phosphate buffer (pH 7.4) and incubated for 5 min at 30°C. The absorbance was measured at 412 nm using a spectrophotometer [70,71]. All experiments were performed three times.
2.7. RNA extraction, cDNA synthesis, and qRT-PCR analysis
The protocol developed by Chan et al. [72] was adopted with some modifications. The total RNA was extracted from the crushed leaves using TRIzol™ reagent. The quality of RNA was examined by nanodrop. cDNA was synthesized using qPCRBIO cDNA Synthesis Kit from PCRBIOSYSTEMS. Quantitative real-time RT-PCR (qRT-PCR) was performed using qPCRBIO SYBR Green Kit from PCRBIOSYSTEM, using synthesized (1μl) cDNAs as templates and the gene-specific primers [73]. To normalize the level of relative expression of each gene, actin was used for each reaction and the expression level was calculated in control plants relatively with other treated plants. The reaction was performed in a 20μl volume containing 7μl ddH2O, 1μl primer, 10μl SYBR green and 1μl cDNA and the reaction was repeated trice (S1 Table). A total sample volume of 50 μl was subjected to the following conditions: initial denaturation at 94°C for 5 min, 40 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, extension at 72°C for 1 min, and final extension at 72°C for 5 min.
2.8. Amino acid quantification
For amino acid analysis, the detailed method developed by Kang et al. [60] was followed. Freeze-dried plant samples (100 mg) were hydrolyzed under vacuum in 6 N HCl in an ampulla tube for 24 h at 110°C followed by 80°C for 24 h. The solid residue was homogenized in 0.02 N HCl and filtered with a 0.45-m filter membrane. The amino acids were analyzed using an atomic amino acid analyzer (L-8900, Hitachi, Japan). The concentrations were measured by comparing with specific standards.
2.9. Statistical analysis
The experiments were performed in triplicate and the resulting products were used for further analysis. The differences among the mean values were compared with Duncan Multiple Range Test (DMRT) using the SAS (9.2, Cary, NC, USA) statistical software program. Graphical presentation was performed in Graph Pad Prism.
3. Results
3.1. Isolation, screening for indole-3-acetic acid, phosphate solubilization, siderophore production and bioassay assessment
From the roots of O. biennis, C. ficifolium, A. princeps and E. crus-galli, a total of 59 endophytic strains were isolated (S2 Table). These isolates were screened for different plant growth-promoting traits (PGP); i.e. IAA, GA, phosphate solubilization and siderophore production. Only 13 isolates showed multiple plant growth promoting (PGP) traits (S2 Table). These isolates were subsequently applied to Wito-C (gibberellin [GA]-deficient) rice and only eight isolates induced a significant increase in the growth attributes (root and shoot) compared to the other isolates and control plants.
3.2. Identification of thermotolerant bacteria
All the selected isolates were examined for their ability to grow at 25°C, 30°C, 35°C, 40°C and 45°C on both solid and liquid broth media. The results showed that the isolates grew at 40°C on solid as well as on broth medium. However, increasing the temperature to 45°C inhibited the growth of all isolates, and only SA1 showed tolerance to heat stress (S1 and S2 Figs). Therefore, isolate SA1 was selected for further investigation. For molecular identification and phylogenetic analysis of isolate SA1, 16S rRNA was amplified, sequenced and compared to the database of known 16S rRNA genes. Our results revealed that SA1 exhibited a high level of 16S sequence identity (99%) with B. cereus. The SA1 16S rRNA sequence was submitted to NCBI with GenBank accession no MH032605 (S3 Fig).
3.3. In-vitro IAA, GA and organic acid production of the bacterial isolates
The culture filtrate (CF) of isolate SA1 was quantified for phytohormones (IAA and GAs) using GC/MS and for organic acids using HPLC. The selected isolate SA1 produced significant amounts of IAA and GA (bioactive and non-bioactive) (Fig 1A and 1B). Organic acid analysis revealed that the CF of SA1 contained lactic acid, butyric acid, formic acid and succinic acid (Fig 1C).
Fig 1. Quantification of indole-3-acetic acid (IAA), gibberellins (GAs) and organic acids produced by SA1 an isolate of Bacillus cereus.
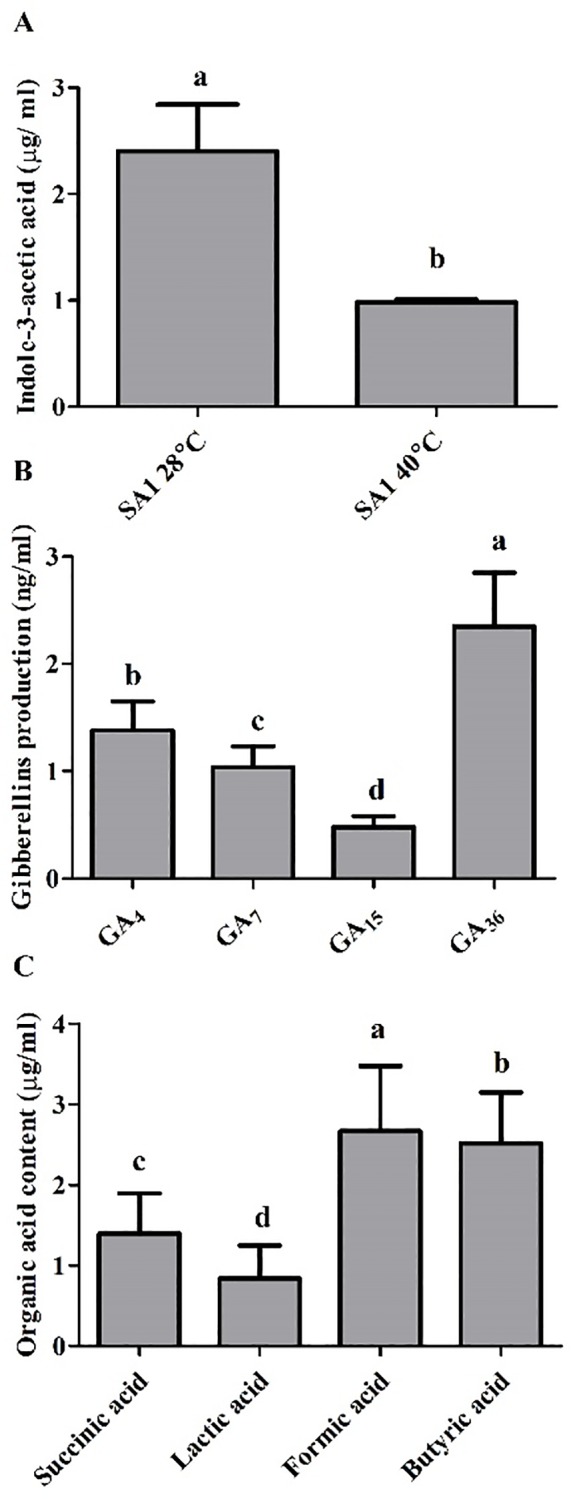
(A) IAA content was detected in culture broth (CB) of SA1, grown at 28°C and 40°C, through Gas chromatography–mass spectrometry (GC/MS-SIM) (B) GC/MS-SIM analysis and quantification of different GAs in CB of SA1 and its comparison with the internal standard. (C) Organic acid content analysis, detection and quantification through high-performance liquid chromatography system (HPLC) in relation to their respective standards. Each data is the mean of three replicates. Error bars represent standard errors. The bars presented with different letters are significantly different from each other as evaluated by Duncan’s Multiple Range Test (DMRT) analysis.
3.4. Ameliorative effects of isolate SA1 and HA against heat stress
Heat stress adversely affected the growth attributes of tomato; however, application of isolate SA1 and HA promoted the growth and biochemical parameters of tomato plants and significantly enhanced their thermotolerance. Under normal growth conditions, the application of HA, isolate SA1 and combined SA1+HA application significantly enhanced the shoot length by 22, 27 and 41% and the root length by 9, 6 and 19%, as well as the fresh weight (80–93%) and dry weight (61%-67%) when compared with normal control tomato plants (Table 1; Fig 2). Similar significant increases were observed in the root fresh and dry weight compared to the control plants (Table 1). However, when tomato plants were subjected to heat stress, combined application of SA1+HA significantly mitigated the adverse effects, with significantly higher growth attributes compared with sole SA and sole HA-inoculated plants and the control (stressed) plants (Fig 2). Heat stress significantly reduced the root and shoot lengths by 36 and 35% in control (heat stressed) plants, whereas combined application of HA+SA1 significantly enhanced the lengths up to 34 and 25% compared with sole SA1 and HA-treated plants. On exposure to heat stress, combined SA1+HA-treated plants displayed a significant restoration of fresh weight (1.5 fold) and dry weight (single fold) (Table 1). Similarly, chlorophyll fluorescence results showed a significant increase in SA1+HA-treated plants compared with control (stressed) plants (Table 1).
Table 1. Effect of SA1 an isolate of B. cereus, humic acid (HA) and SA1+HA on tomato plants under normal and heat stress.
| Control | SA1 | HA | HA+SA1 | |
|---|---|---|---|---|
| Normal Temperature | ||||
| SL (cm) | 32.66±1.7c | 40.33±1.3b | 41.83±3.7b | 46.5±5.3a |
| RL (cm) | 14.4.±1.5c | 15.7±1.5b | 15.3±1.6bc | 17.3±1.7a |
| SFW (g) | 23.96±1.7b | 43.26±2.3a | 44.66±3.3a | 46.33±2.3a |
| SDW (g) | 2.7±0.9b | 4.36±0.9a | 4.41±0.7a | 4.53±0.6a |
| RFW(g) | 16.80±1.1c | 21.19±1.9b | 22.26±2.1b | 30.08±1.6a |
| RDW(g) | 2.88±0.3c | 3.03±0.1bc | 3.54±0.4b | 4.23±0.3a |
| CF (Fv/Fm) | 0.81±0.02a | 0.81±0.03a | 0.82±0.04a | 0.84±0.04a |
| Heat stress | ||||
| SL (cm) | 21.16±1.7b | 26.66±2.5a | 26.33±1.7a | 28.5±3a |
| RL (cm) | 9.2±1.4c | 10.3±2.1b | 10.8±1.4b | 11.5±2.1a |
| SFW (g) | 12.3±1.1c | 22.33±1.8b | 21.13±1.6b | 29.06±1.7a |
| SDW (g) | 1.54±0.2c | 2.61±0.3b | 2.32±0.4ab | 2.96±0.2a |
| RFW(g) | 11.83±1.6b | 14.39±0.8ab | 12.47±1.3ab | 15.0±1.6a |
| RDW(g) | 1.1±0.2c | 1.5±0.2b | 1.7±0.1ab | 1.9±0.1a |
| CF (Fv/Fm) | 0.6±0.02c | 0.68±0.104b | 0.73±0.05b | 0.77±0.08a |
SL = Shoot length, SFW = shoot fresh weight, SDW = shoot dry weight, RL = root length, RFW = root fresh weight, RDW = root dry weight and CF = Chlorophyll fluorescence. Each data point is the mean of three replicates. Error bars represent standard errors. The bars presented with different letters are significantly different from each other as evaluated by Duncan’s Multiple Range Test (DMRT) analysis
Fig 2. Effects of selected SA1 an isolate of Bacillus cereus (SA1), humic acid (HA) and combined SA1+HA application on the growth of tomato plants under normal and heat stress.
3.5. Quantification of plant endogenous phytohormones
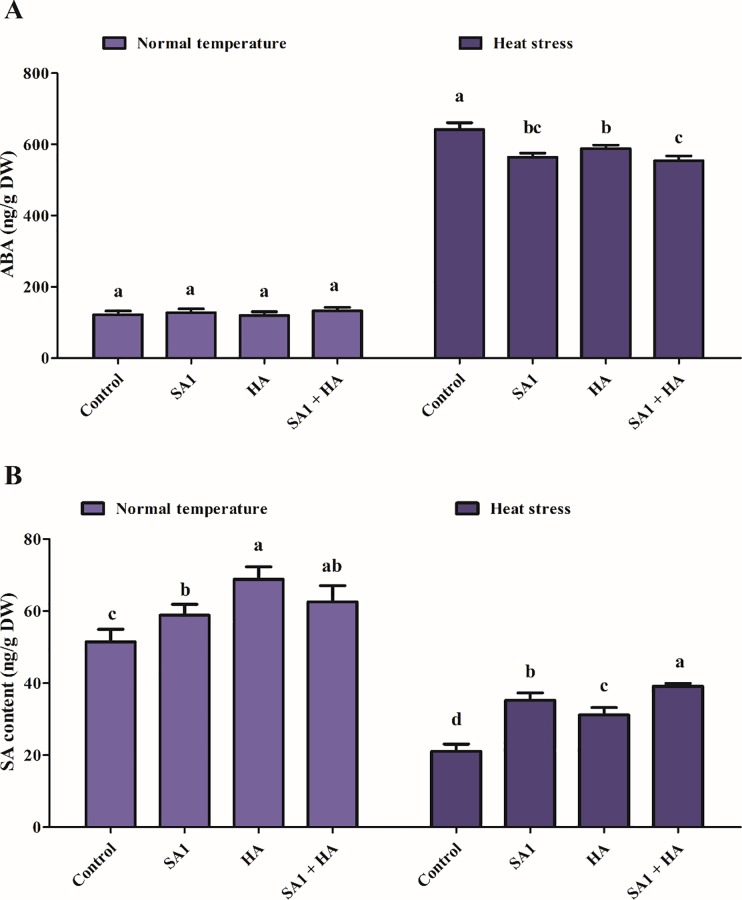
A significant increase in ABA content and a decrease in SA content were observed after exposure to heat stress. The endogenous ABA content showed a significant increase up to three-fold compared to control tomato plants (Fig 3A). In contrast, application with thermotolerant isolate SA1, HA and combined SA1+HA remarkably reduced the ABA content (1.5–2 fold). Similarly, both unstressed (normal temperature) and stressed (heat stressed) tomato plants were subjected to SA analysis. The results showed that in contrast to the endogenous ABA level, a decrease in SA content was observed in tomato plants exposed to heat stress. The endogenous SA content was significantly reduced from 48%; however, inoculation with SA1, HA and combined SA1+HA mitigated the heat stress and increased the SA content from 26.1 to 58.2% (Fig 3B).
Fig 3. (A) Endogenous abscisic acid (ABA) and (B) salicylic acid (SA) quantification in tomato plants inoculated with SA1 an isolate of Bacillus cereus (SA1), humic acid (HA) and combined SA1+HA application.
Each data point is the mean of at least three replicates. Error bars represent standard errors. The bars presented with different letters are significantly different from each other as evaluated by Duncan’s Multiple Range Test (DMRT).
3.6. Modulation of tomato antioxidant system under heat stress
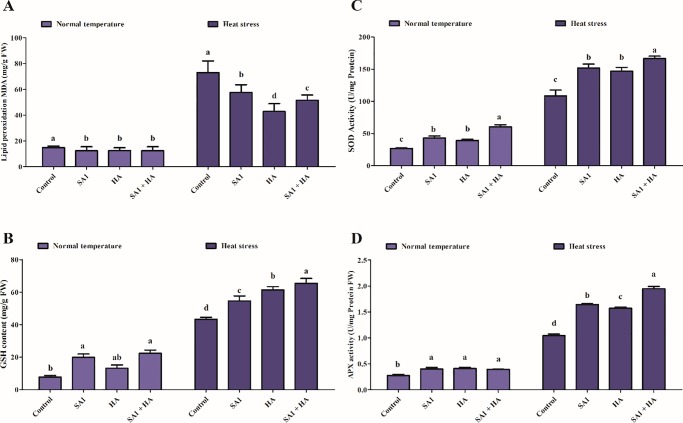
To assess the extent of lipid peroxidation in tomato due to heat stress-induced oxidative stress, the malondialdehyde (MDA) content was investigated (Fig 4). Heat stress activates MDA production, which in turn leads to the induction of lipid peroxidation. In the current study, not significant difference was observed in MDA content in the sole SA1, HA and SA1+HA treated tomato leaf tissues under normal temperatures (Fig 4A). However, when the tomato plants were exposed to heat stress, an increase in the MDA levels (385%) was observed compared to the combined application of SA1+HA (245%) and sole application of SA1 (285%) and HA (187%). The MDA content in HA-treated plants was significantly reduced under heat stress conditions relative to the MDA contents of the sole SA1-inoculated plants and combined SA1+HA-treated tomato plants. To further elucidate the oxidative stress mitigation, the GSH content in plants was examined. Under normal temperatures, SA1, HA and combined SA1+HA-treated plants showed significantly higher GSH content (90%-187%) compared with the control tomato plants (Fig 4B). Heat stress induced an increase in GSH content; however, a significantly higher amount of reduced GSH was generated in SA1 (607%), HA (695%) and combined SA1+HA-treated (740%) plants compared to the heat stressed control tomato plants (461%). In terms of SOD activity, the results indicated that sole application of SA1, HA and combined SA1+HA differentially regulated the SOD activities under normal and heat stress conditions. Under the normal temperature, no significant difference was observed in sole SA1 (469%), HA (450%) and combined SA1+HA-treated (523%) tomato plants. However, under heat stress, significant enhancement in the SOD activity of the combined SA1+HA-treated tomato plants was observed compared with the sole SA1 and HA-treated tomato plants and heat stress control (307%) plants (Fig 4C). Ascorbate peroxidase results showed a significant difference in tomato plants treated with sole or combined SA1+HA (41–49%) compared with control plants. However, under heat stress, significant enhancement in APX was observed in the combined SA1+HA-treated (608%) tomato plants compared with the sole SA1 (492%) and HA (470%) treated tomato plants and heat stressed control plants (283%) (Fig 4D).
Fig 4. Effect of SA1 an isolate of Bacillus cereus (SA1), humic acid (HA) and combined SA1+HA application on different antioxidants; (A) Lipid peroxidation (MDA), (B) Reduced glutathione (GSH), (C) Superoxide dismutase (SOD) and (D) Ascorbic peroxidase (APX) contents in tomato plants under normal and heat stress.
Each data point is the mean of three replicates. Error bars represent standard errors. The bars presented with different letters are significantly different from each other as evaluated by Duncan’s Multiple Range Test (DMRT) analysis.
3.7. Role of SA1 and HA in ion uptake during heat stress
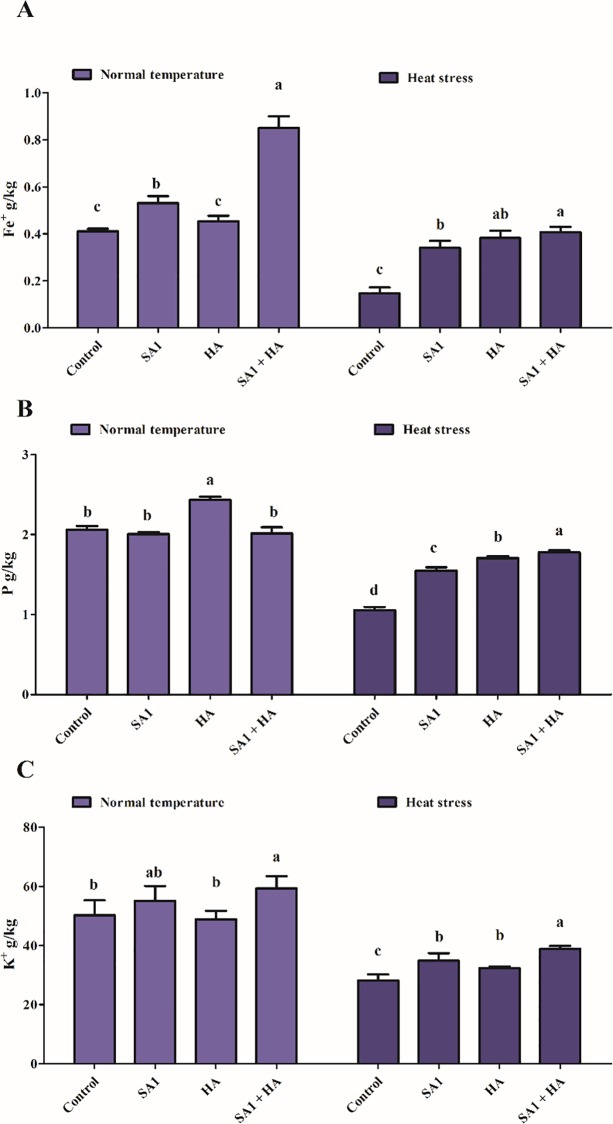
Inductively-coupled plasma mass spectrometry (ICP) results showed that under control conditions, SA1, HA and combined SA1+HA-treated plants had significantly higher amounts of iron (Fe+), phosphorus (P) and potassium (K) content compared to the control tomato plants under normal and heat stress conditions (Fig 5). Fe+ content revealed a significant difference in tomato plants treated with sole SA1 (28%), HA (6%) and combined SA1+HA (105%) under normal temperature. Heat stress significantly decreased the Fe+ content (63%); however, plants treated with SA1 (153%), HA (126%) and combined SA1+HA (158%) had a significantly higher content of Fe+ (Fig 5A). Furthermore, P and K content were significantly decreased in heat stressed control plants (49% and 43%). However, bacterial inoculation and HA mitigated heat stress and enhanced the content of P (45–67%) and K (11–34%) (Fig 5B and 5C).
Fig 5. Effect of SA1 an isolate of Bacillus cereus (SA1), humic acid (HA) and combined SA1+HA application on ion content; (A) iron (Fe+), (B) phosphorus (P), and (C) potassium (K+) content in tomato plants under normal and heat stress.
Each data point is the mean of three replicates. Error bars represent standard errors. The bars presented with different letters are significantly different from each other as evaluated by Duncan’s Multiple Range Test (DMRT) analysis.
3.8. Effect of isolate SA1 and HA on gene expression
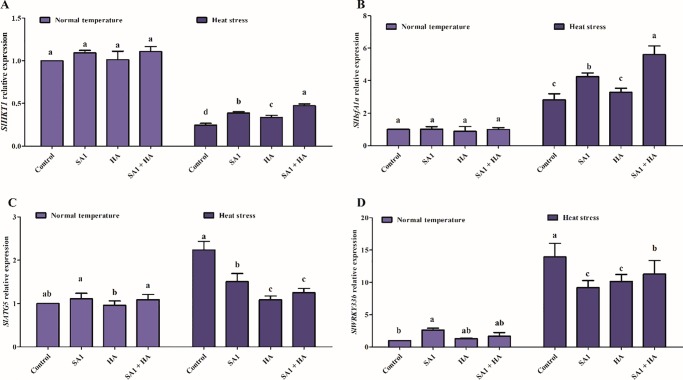
In response to heat stress, the transcriptional levels of SlHSFs, SlHSPs, high affinity potassium transporter (SlHAKT1), and SlWRKY33b were evaluated (Fig 6). SlHAKT1 gene expression was significantly upregulated in the combined HA and SA1-inoculated tomato plants (10%) compared to the control and sole HA (1.5%) and SA1-treated (9%) plants under normal conditions. Heat stress significantly reduced the expression level of SlHKT1 (5 fold) in control plants. However, SA1 inoculation and HA mitigated heat stress and enhanced SlHKT1 gene expression up to 2.5 and 3 fold (Fig 6A). SlHsfA1a is one of the master regulators required for induction of the heat stress response. SlHsfA1a expression results showed few differences in tomato plants under normal temperature, whereas the SlHsfA1a genes were upregulated (2.9-fold). However, combined application of SA1 and HA significantly enhanced the expression of SlHsfA1a (2.8 fold) compared to sole SA1 (1.4 fold) and HA (0.3 fold) application and control heat stressed plants (Fig 6B). Furthermore, heat stress-induced autophagy-related genes (ATGs) in the control plants were highly upregulated (1.2 fold) compared to the levels in the control plants. Co-application of SA1 and HA significantly reduced the expression of the SlATG5 gene (1.15 fold) (Fig 6C). The SlWRKY33b transcriptional factor plays a key role in plant stress responses. Our results showed that under normal growth conditions, there is a slight increase in the expression of SlWRKY33b genes in control and treated plants. When tomato plants were subjected to heat stress, the overexpression of SlWRKY33b genes was observed (13 fold) in control heat stressed plants. However, the application of SA1, HA and combined SA1 and HA significantly mitigated heat stress tolerance and the induced the downregulation of the SlWRKY33b genes (2.3–4 fold) (Fig 6D).
Fig 6. Relative expression of genes in tomato plants treated with SA1, humic acid (HA) and combined SA1+HA under normal and heat stress.
(A) SlHKT1, (B) SlHsfA1a, (C) SlWRKYY33b and (D) SlATG5. The values were calculated relative to those of actin gene expression and are the means of three replicates. Error bars represent standard errors. The bars presented with different letters are significantly different from each other as evaluated by DMRT analysis.
3.9. Amino acid quantification
Amino acid contents were determined to elucidate the regulation of physiological function with the inoculation of SA1, HA and combined application of SA1+HA in tomato plants under normal and heat stress conditions (Table 2). Under normal conditions, a slight increase was observed in aspartic acid, glutamic acid, alanine, phenylalanine, arginine and proline contents in SA1, HA and combined SA1+HA-treated plants compared with the levels in control plants. Under heat stress, the contents of aspartic acid (59%), glutamic acid (89%), arginine (113%) and proline (67%) were increased, while decreases were observed in alanine (33%) and phenylalanine (28%) contents. However, sole SA1, HA and combined SA1+HA application increased the content of aspartic acid (6, 12 and 10.57%), glutamic acid (18, 14 and 24%), arginine (31, 36 and 89%), alanine (4, 11 and 31%), phenylalanine (6, 28 and 49%) and proline (7, 13 and 3.24%) compared to the levels in un-inoculated tomato plants under heat stress (Table 2). Total amino acid results showed that heat stress markedly decreased the amino acid content up to 48% as compared to the levels in control plants. However, with the application of SA1, HA and SA1+HA, a significant increase in the total amino acid content was observed (85, 12 and 4.34%).
Table 2. Amino acids quantification in tomato plants inoculated with SA1 an isolate of B. cereus, humic acid (HA) and SA1+HA under normal and heat stress.
| Aspartic acid | Glutamic acid | Alanine | Phenylalanine | Arginine | Proline | |||
|---|---|---|---|---|---|---|---|---|
| Normal Temperature | ||||||||
| Control | 7.22±0.6b | 9.55±1.0b | 8.55±1.1c | 8.08±1.2c | 3.51±0.3b | 7.32±1.1c | ||
| SA1 | 10.34±1.2a | 10.46±2.0a | 9.98±1.0b | 9.02±0.9bc | 4.57±0.4a | 9.78±0.8ab | ||
| HA | 10.34±1.1a | 10.37±1.2a | 11.21±1.1a | 10.78±1.3b | 4.72±0.5a | 9.09±1.1b | ||
| HA+SA1 | 10.22±1.8a | 10.12±1.0a | 11.68±1.2a | 12.13±2.0a | 4.99±0.6a | 10.22±1.3a | ||
| Heat Stress | ||||||||
| Control | 11.54±1.9c | 18.11±2.1d | 5.73±0.8c | 5.77±0.5d | 7.49±1.0d | 12.26±1.3c | ||
| SA1 | 12.31±1.4b | 19.63±2.3c | 6.0±0.8b | 6.13±0.7c | 9.82±1.1c | 13.23±1.8b | ||
| HA | 12.98±1.1b | 20.67±1.9b | 6.37±0.9b | 7.40±1.0b | 10.21±1.3b | 13.92±1.7b | ||
| HA+SA1 | 13.89±1.8a | 22.48±3.2a | 7.51±1.1a | 8.65±1.3a | 14.20±1.5a | 15.10±1.2a | ||
Each data point is the mean of at least three replicates. Error bars represent standard errors. The bars presented with different letters are significantly different from each other as evaluated by Duncan’s Multiple Range Test (DMRT) analysis.
4. Discussion
Tomato is heat-sensitive and its growth, yield and quality are highly influenced by heat stress. High temperatures cause anatomical, physiological, morphological, and molecular changes in tomato plants, which affect plant growth and consequently affect plant yield. Presently, tomato is widely consumed raw or industrially processed and is considered a good source of nutritional properties and its different beneficial pigment constituents have paved the way for increased demand all over the world. Therefore, there is a need to boost tomato production under heat stress conditions. Consequently, substantial efforts have been extended to the use of physiochemical and ecofriendly biological approaches, which are currently being applied to alleviate plant heat stress. Recently, the co-administration of HA and the Bacillus genus as important plant bio-stimulants that improve the growth and productivity in different crops [41,49] while reducing the dependency on chemical fertilizers has begun to gain importance. Previous reports demonstrated that humic substances may favor bacteria-plant interactions and enhance the bacterial attachment and colonization [47,74]. Olivares et al. [40] and Canellas et al. [75] recently reported the beneficial effects of combined application of heat stress and PGPB on various crops like maize and tomato. These findings were consistent with the present results that tomato plants treated with isolate SA1+HA enhance root/shoot length, biomass and chlorophyll content under normal and heat stress condition (Table 1; Fig 2). Among the physiological activities of plants, photosynthesis is the most sensitive to heat stress because in the chloroplast, the stroma is severely affected by increased temperature [11–13,76–78]. Many plants species have evolved several mechanisms to guard the photosynthetic apparatus against heat stress damages by encoding for the production of HSPs that binds to thylakoid membranes and protect PSII and the electron transport chain [76,78–80]. However, under severe heat stress, these protective mechanisms may be inadequate to ensure plant viability. Our findings showed that an increase of chlorophyll florescence in treated tomato plants occurred under normal and heat stress conditions compared to un-inoculated tomato plants (Table 1). Similar results were also observed by other researchers [26,81], who reported that the use of thermotolerant agents increased total chlorophyll content in wheat and canola plants under heat stress. Enhanced chlorophyll content may also be the result of increased photosynthetic leaf area due to the application of microbes and HA compared to the control (stressed) plants [26,27]. This increase in chlorophyll content is probably due to enhanced moisture retention and the improvement of nutrient supply in the root zone. Canellas et al. [75] reported that co-application of PGPB and heat stress increased the net photosynthetic rate and nutrient uptake, which ultimately led to vigorous and healthy crop growth and productivity.
K, P and Fe+ are important elements required for plant growth and function regulation in several biochemical processes like protein synthesis, enzymatic activity and hormonal regulation [82–86]. Several physiological processes depend on Fe, K and P such as photosynthesis, stomatal regulation and abiotic stress tolerance. Under abiotic stress, K helps to maintain ion homeostasis, regulate osmatic balance and stomatal opening and enhance the antioxidant defense system in plants [87,88]. Baldotto et al. [89] revealed that pineapple plant growth was affected by Burkholderia strain inoculation and HAs increased the root and shoot growth, biomass and nutrient contents (nitrogen [N] 132%, P 131% and K 80%) compared to un-inoculated plants. These results are consistent with findings in the present study that tomato plants treated with SA1+HA exhibited enhanced P, K and Fe content under normal and heat stress condition (Fig 5). Previously, Schoebitz et al. [90] reported that combined administration of PGP microbes and HA increased N and K uptake and growth in blueberry plants compared to the control plants.
Phytohormones greatly respond to changing environmental conditions by regulating plant growth and are actively involved in the response to heat stress [91–93]. IAA is a key phytohormone that plays a key role in the growth, development and tolerance of plants to heat stress through the activation of antioxidant enzymes, regulation of gene expression, synthesis of osmoprotectants (proline), and enhanced accumulation of photosynthetic pigments [94]. It has been reported that IAA-producing bacteria increase the length and root surface of plants, helping the plants get better access to nutrients available in the soil [24,95]. Our bacterial isolate (SA1) was also capable of producing IAA and GAs (Fig 1A and 1B) and greatly mitigated the adverse effects of heat stress on tomato plants. Additionally, GAs play a prominent role in the alleviation of abiotic stress [96,97]. Stavang et al. [98] reported that GAs play a vital role in alleviating heat stress and enhanced growth in Arabdopasis and pea. Microbes that produced GAs are important for promoting plant growth and mitigating the adverse effects of heat stress [24,98,99]. Despite the existence of several forms of GA, biologically active GA is limited in different microorganisms [100,101]. The microbes used in our study produce bioactive GAs (Fig 1B). These biologically active GAs promote plant growth through reducing stress hormones like ABA [102–104]. When plants perceive stressful conditions, they regulate stress hormones like ABA through active chemical signals, which induce extreme sensitivity to stomatal conductance [104,105]. The prolific role of plant microbe interaction lies in mitigating the adverse effects of abiotic stress by reducing ABA content [24,25,106]. Similar results were observed in our study in which application of isolate SA1 and HA caused a decrease in ABA content and an increase in plant growth parameters (Fig 3A). Salicylic acid (SA) is another plant hormone that plays an important role in various physiological processes and biochemical reactions [107]. According to Zhang et al. [108] and Khan et al [107], in mutualistic plant-microbe interactions, SA induces systemic resistance in plants. Wang et al. [109] and Khan et al [107] suggested that SA can ameliorate abiotic stress by inducing ROS generation. The accumulation of SA has also been implicated in heat stress tolerance in various plants including Kentucky Bluegrass [23], grapevine [110], potato [111], bean and tomato [112], Arabidopsis [113] and grape plants [114]. Isolate SA1+HA application greatly enhanced the endogenous SA level in tomato plants under heat stress and normal conditions (Fig 3B). Our findings are in agreement with previous reports in which the ability of bacterial inoculum enhanced the endogenous levels of SA and contributed to the growth and development of plants under abiotic stress conditions [115,116].
When plants are subjected to environmental stress including heat stress, a variety of ROS are generated [117,118]. These ROS interfere with different organic molecules, resulting in the reduction of photosynthesis and subsequently reduced plant growth [117,119]. Under heat stress, plants are capable of counteracting ROS generation by the production of different antioxidant molecules (enzymatic and non-enzymatic) [117,118]. The key antioxidant enzymes that play an effective role in scavenging ROS are SOD, catalase (CAT), and peroxidase [120,121]. The activities of these enzymes usually increase under stress conditions [85,86,122–125]. Tomato plants treated with isolate SA1 and HA produced less ROS and showed an increase in antioxidant activities like SOD, MDA, GSH, and APX compared with un-inoculated plants under heat stress (Fig 4). Similar results were reported in previous studies [24,27,33,121,126], where PGPB enhanced the activity of different ROS scavenging enzymes under heat stress. Amino acids play a prominent role in the physiological and biochemical functions of plants such as the modulation of membrane permeability, osmolytes, ion uptake, enzymatic activity and tolerance to abiotic stress [127,128]. Amino acids also act as bio-stimulants for the growth attributes of plants and significantly mitigate injuries caused by abiotic stresses [121]. The co-application of SA1+HA exhibited a rescue effect and significantly enhanced the important endogenous amino acid content under heat stress compared with control heat stressed plants (Table 2). Several previous reports indicated that an increase in amino acid content increases tolerance to abiotic stress following inoculation with plant growth-promoting bacteria [120,129–133]. Heat stress reduced the accumulation of proline, but proline levels were enhanced in SA1+HA treated plants under control and heat stress conditions (Table 2). Similarly, proline accumulation is considered an adaptive mechanism under heat stress [134–137].
Plants contain several heat-stress-dependent genes such as HSFs and HSPs that enhance tolerance and protect the function of proteins under heat stress conditions. HSFs are essential for maintaining and restoring protein structure as well as stabilizing the condition of plants under heat stress [19,138,139]. Fragkostefanakis et al. [140] reported the upregulation of HSF level in bacteria-inoculated wheat seedlings during heat stress as well as a decrease in the HSP transcript levels. Similar SlHsfA1a transcriptional levels were observed in sole SA1, HA and combined SA1+HA application in tomato plants under heat stress (Fig 6). The induction of HSPs is usually accompanied by tolerance to heat and other stresses. Previous studies revealed that HSFs act as “molecular chaperones” and the over-expression of these genes and proteins is well established to enhance thermotolerance [18,20,141]. Similarly, SlWRKY33b, SlHAKT1 and SlATG5 proteins and play critical roles in tomato heat tolerance. HKT1 plays a crucial role and higher cellular K concentration during stress is critical for normal plant function [142]. We observed higher expression of the SlHKT1 gene in sole SA1, HA and combined SA1+HA-treated plants under normal and heat stress conditions. Ali et al. [143], Fairbairn [144] and Almeida [142] reported that higher expression of the SlHKT1 gene enhances stress tolerance and the accumulation of K content. Furthermore, based on previous reports, we evaluated the expression of SlWRKY33b and SlATG5 and found that both genes were highly expressed in plants grown under heat stress (37°C), indicating that both of these genes are highly correlated with heat stress. However, plants treated with bacteria and HA under the same degree of heat stress showed comparatively less regulation of both genes, suggesting that bacteria and HA also have key roles in heat tolerance. Our study was in agreement with that of Zhou [145], who reported that heat stress significantly induced the regulation of the SlATG gene and SlWRKY33, which physically interacts with the autophagy-related protein. Other reports demonstrated that silencing the SlWRKY33 gene in tomato reduces SlATG gene expression, which compromises tomato heat tolerance [146].
5. Conclusions
In conclusion, the present study demonstrated that isolate SA1 has the ability to produce biologically active metabolites such as GA, IAA and organic acids. Tomato plants treated with isolate SA1 and HA showed significant improvements in their growth attributes and chlorophyll fluorescence under normal and heat stress conditions. This improvement in plant growth was coupled with changes in endogenous phytohormones (ABA and SA), antioxidants (APX, SOD, GSH and LPO), and essential amino acids and the expression of genes such as SlWRKY33b, SlHKT1 and SlATG5. The stability and increased consistency of the tomato plant response to bacterial inoculation in the presence of HA indicated a promising co-friendly biological approach and physicochemical biotechnological tool to improve the growth and development of tomato under heat stress conditions.
Supporting information
(DOCX)
The isolates were preliminary sorted for single or multiple plant growth beneficial activities.
(DOCX)
Each data point is the mean of three replicates.
(DOCX)
Each data point is the mean of three replicates.
(DOCX)
(DOCX)
Abbreviations
- PGPEB
plant growth-promoting endophytic bacteria
- GA
gibberellin
- IAA
indole-3-acetic acid
- ABA
abscisic acid
- SA
salicylic acid
- APX
ascorbic acid peroxidase
- SOD
superoxide dismutase
- GSH
glutathione
- HSF
heat shock transcription factor
- SlHKT1
potassium channel HKT1
Data Availability
All data generated or analyzed during this study are included in this published article.
Funding Statement
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B04035601) to IJL.
References
- 1.Ihsan MZ, Daur I, Alghabari F, Alzamanan S, Rizwan S, et al. (2019) Heat stress and plant development: role of sulphur metabolites and management strategies. Acta Agriculturae Scandinavica, Section B—Soil & Plant Science 69: 332–342. [Google Scholar]
- 2.Hemmati H, Gupta D, Basu C (2015) Molecular Physiology of Heat Stress Responses in Plants In: Pandey GK, editor. Elucidation of Abiotic Stress Signaling in Plants: Functional Genomics Perspectives, Volume 2 New York, NY: Springer New York; pp. 109–142. [Google Scholar]
- 3.Firon N, Shaked R, Peet MM, Pharr DM, Zamski E, et al. (2006) Pollen grains of heat tolerant tomato cultivars retain higher carbohydrate concentration under heat stress conditions. Scientia Horticulturae 109: 212–217. [Google Scholar]
- 4.Sato S, Kamiyama M, Iwata T, Makita N, Furukawa H, et al. (2006) Moderate Increase of Mean Daily Temperature Adversely Affects Fruit Set of Lycopersicon esculentum by Disrupting Specific Physiological Processes in Male Reproductive Development. Annals of Botany 97: 731–738. 10.1093/aob/mcl037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lobell DB, Schlenker W, Costa-Roberts J (2011) Climate Trends and Global Crop Production Since 1980. Science 333: 616–620. 10.1126/science.1204531 [DOI] [PubMed] [Google Scholar]
- 6.You L, Rosegrant MW, Wood S, Sun D (2009) Impact of growing season temperature on wheat productivity in China. Agricultural and Forest Meteorology 149: 1009–1014. [Google Scholar]
- 7.Aggarwal PK (2008) Global climate change and Indian agriculture: impacts, adaptation and mitigation. Indian Journal of Agricultural Sciences 78: 911–919. [Google Scholar]
- 8.Pradhan GP, Prasad PVV, Fritz AK, Kirkham MB, Gill BS (2012) Effects of drought and high temperature stress on synthetic hexaploid wheat. Functional Plant Biology 39: 190–198. [DOI] [PubMed] [Google Scholar]
- 9.Vollenweider P, Gunthardt-Goerg MS (2005) Diagnosis of abiotic and biotic stress factors using the visible symptoms in foliage. Environ Pollut 137: 455–465. 10.1016/j.envpol.2005.01.032 [DOI] [PubMed] [Google Scholar]
- 10.Bita C, Gerats T (2013) Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Frontiers in Plant Science 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gururani MA, Mohanta TK, Bae H (2015) Current Understanding of the Interplay between Phytohormones and Photosynthesis under Environmental Stress. Int J Mol Sci 16: 19055–19085. 10.3390/ijms160819055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: An overview. Environmental and Experimental Botany 61: 199–223. [Google Scholar]
- 13.Camejo D, Rodríguez P, Angeles Morales M, Miguel Dell’Amico J, Torrecillas A, et al. (2005) High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. Journal of Plant Physiology 162: 281–289. 10.1016/j.jplph.2004.07.014 [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Thakur P, Kaushal N, Malik JA, Gaur P, et al. (2013) Effect of varying high temperatures during reproductive growth on reproductive function, oxidative stress and seed yield in chickpea genotypes differing in heat sensitivity. Archives of Agronomy and Soil Science 59: 823–843. [Google Scholar]
- 15.Kaushal N, Gupta K, Bhandhari K, Kumar S, Thakur P, et al. (2011) Proline induces heat tolerance in chickpea (Cicer arietinum L.) plants by protecting vital enzymes of carbon and antioxidative metabolism. Physiology and Molecular Biology of Plants 17: 203 10.1007/s12298-011-0078-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nahar K, Hasanuzzaman M, Alam MM, Fujita M (2015) Exogenous glutathione confers high temperature stress tolerance in mung bean (Vigna radiata L.) by modulating antioxidant defense and methylglyoxal detoxification system. Environmental and Experimental Botany 112: 44–54. [Google Scholar]
- 17.Driedonks N, Xu J, Peters JL, Park S, Rieu I (2015) Multi-Level Interactions Between Heat Shock Factors, Heat Shock Proteins, and the Redox System Regulate Acclimation to Heat. Frontiers in Plant Science 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yildiz M, Terzi H (2008) Small heat shock protein responses in leaf tissues of wheat cultivars with different heat susceptibility. Biologia 63: 521. [Google Scholar]
- 19.Xu Z-S, Li Z-Y, Chen Y, Chen M, Li L-C, et al. (2012) Heat shock protein 90 in plants: molecular mechanisms and roles in stress responses. International journal of molecular sciences 13: 15706–15723. 10.3390/ijms131215706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Queitsch C, Hong S-W, Vierling E, Lindquist S (2000) Heat Shock Protein 101 Plays a Crucial Role in Thermotolerance in Arabidopsis. The Plant Cell 12: 479–492. 10.1105/tpc.12.4.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jan N, Qazi HA, Ramzan S, John R (2018) Developing Stress-Tolerant Plants Through In Vitro Tissue Culture: Family Brassicaceae In: Gosal SS, Wani SH, editors. Biotechnologies of Crop Improvement, Volume 1: Cellular Approaches. Cham: Springer International Publishing; pp. 327–372. [Google Scholar]
- 22.Lamaoui M, Jemo M, Datla R, Bekkaoui F (2018) Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Frontiers in chemistry 6: 26–26. 10.3389/fchem.2018.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Y, Liu Y, Cao W, Huai M, Xu B, et al. (2005) Effects of Salicylic Acid on Heat Tolerance Associated with Antioxidant Metabolism in Kentucky Bluegrass. Crop Science 45: 988–995. [Google Scholar]
- 24.Park Y-G, Mun B-G, Kang S-M, Hussain A, Shahzad R, et al. (2017) Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLOS ONE 12: e0173203 10.1371/journal.pone.0173203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiwari S, Prasad V, Chauhan PS, Lata C (2017) Bacillus amyloliquefaciens Confers Tolerance to Various Abiotic Stresses and Modulates Plant Response to Phytohormones through Osmoprotection and Gene Expression Regulation in Rice. Frontiers in Plant Science 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali SZ, Sandhya V, Grover M, Kishore N, Rao LV, et al. (2009) Pseudomonas sp. strain AKM-P6 enhances tolerance of sorghum seedlings to elevated temperatures. Biology and Fertility of Soils 46: 45–55. [Google Scholar]
- 27.Ali SZ, Sandhya V, Grover M, Linga VR, Bandi V (2011) Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) under heat stress. Journal of Plant Interactions 6: 239–246. [Google Scholar]
- 28.Roomi S, Masi A, Conselvan GB, Trevisan S, Quaggiotti S, et al. (2018) Protein Profiling of Arabidopsis Roots Treated With Humic Substances: Insights Into the Metabolic and Interactome Networks. Frontiers in Plant Science 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah ZH, Rehman HM, Akhtar T, Alsamadany H, Hamooh BT, et al. (2018) Humic Substances: Determining Potential Molecular Regulatory Processes in Plants. Frontiers in plant science 9: 263–263. 10.3389/fpls.2018.00263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moraditochaee M (2012) Effects of Humic Acid Foliar Spraying and Nitrogen Fertilizer Management on Yield of Peanut (Arachis Hypogaea L.) in Iran. ARPN Journal of Agricultural and Biological Science 7: 289–293. [Google Scholar]
- 31.Khaleda L, Park HJ, Yun D-J, Jeon J-R, Kim MG, et al. (2017) Humic Acid Confers HIGH-AFFINITY K+ TRANSPORTER 1-Mediated Salinity Stress Tolerance in Arabidopsis. Molecules and cells 40: 966–975. 10.14348/molcells.2017.0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srivastava S, Yadav A, Seem K, Mishra S, Chaudhary V, et al. (2008) Effect of high temperature on Pseudomonas putida NBRI0987 biofilm formation and expression of stress sigma factor RpoS. Curr Microbiol 56: 453–457. 10.1007/s00284-008-9105-0 [DOI] [PubMed] [Google Scholar]
- 33.Abd El-Daim IA, Bejai S, Meijer J (2014) Improved heat stress tolerance of wheat seedlings by bacterial seed treatment. Plant and Soil 379: 337–350. [Google Scholar]
- 34.Issa A, Esmaeel Q, Sanchez L, Courteaux B, Guise J-F, et al. (2018) Impacts of Paraburkholderia phytofirmans Strain PsJN on Tomato (Lycopersicon esculentum L.) Under High Temperature. Frontiers in plant science 9: 1397–1397. 10.3389/fpls.2018.01397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bensalim S, Nowak J, Asiedu SK (1998) A plant growth promoting rhizobacterium and temperature effects on performance of 18 clones of potato. American Journal of Potato Research 75: 145–152. [Google Scholar]
- 36.Egamberdieva D, Wirth SJ, Alqarawi AA, Abd_Allah EF, Hashem A (2017) Phytohormones and Beneficial Microbes: Essential Components for Plants to Balance Stress and Fitness. Frontiers in Microbiology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carneiro JB Jr, Silveira SFd, Souza Filho GAd, Olivares FL, Giglioti ÉA (2004) Especificidade de anti-soro policlonal à Leifsonia xyli subsp. xyli. Fitopatologia Brasileira 29: 614–619. [Google Scholar]
- 38.da Silva SF, Olivares FL, Canellas LP (2017) The biostimulant manufactured using diazotrophic endophytic bacteria and humates is effective to increase sugarcane yield. Chemical and Biological Technologies in Agriculture 4: 24. [Google Scholar]
- 39.Olivares FL, Aguiar NO, Rosa RCC, Canellas LP (2015) Substrate biofortification in combination with foliar sprays of plant growth promoting bacteria and humic substances boosts production of organic tomatoes. Scientia Horticulturae 183: 100–108. [Google Scholar]
- 40.Olivares FL, Busato JG, de Paula AM, da Silva Lima L, Aguiar NO, et al. (2017) Plant growth promoting bacteria and humic substances: crop promotion and mechanisms of action. Chemical and Biological Technologies in Agriculture 4: 30. [Google Scholar]
- 41.Esringü A, Kaynar D, Turan M, Ercisli S (2016) Ameliorative Effect of Humic Acid and Plant Growth-Promoting Rhizobacteria (PGPR) on Hungarian Vetch Plants under Salinity Stress. Communications in Soil Science and Plant Analysis 47: 602–618. [Google Scholar]
- 42.Young C-C, Rekha PD, Lai W-A, Arun AB (2006) Encapsulation of plant growth-promoting bacteria in alginate beads enriched with humic acid. Biotechnology and Bioengineering 95: 76–83. 10.1002/bit.20957 [DOI] [PubMed] [Google Scholar]
- 43.Bacilio M, Moreno M, Bashan Y (2016) Mitigation of negative effects of progressive soil salinity gradients by application of humic acids and inoculation with Pseudomonas stutzeri in a salt-tolerant and a salt-susceptible pepper. Applied Soil Ecology 107: 394–404. [Google Scholar]
- 44.Beecher GR (1998) Nutrient content of tomatoes and tomato products. Proc Soc Exp Biol Med 218: 98–100. 10.3181/00379727-218-44282a [DOI] [PubMed] [Google Scholar]
- 45.Slavin JL, Lloyd B (2012) Health benefits of fruits and vegetables. Advances in nutrition (Bethesda, Md) 3: 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdellatif IMY, Abdel-ati YY, Abdel-Mageed YT, Hassan MAM (2017) Effect of Humic Acid on Growth and Productivity of Tomato Plants Under Heat Stress. Journal of Horticultural Research 25: 59–66. [Google Scholar]
- 47.Canellas LP, Olivares FL (2014) Physiological responses to humic substances as plant growth promoter. Chemical and Biological Technologies in Agriculture 1: 3. [Google Scholar]
- 48.Busato JG, Lima LS, Aguiar NO, Canellas LP, Olivares FL (2012) Changes in labile phosphorus forms during maturation of vermicompost enriched with phosphorus-solubilizing and diazotrophic bacteria. Bioresource Technology 110: 390–395. 10.1016/j.biortech.2012.01.126 [DOI] [PubMed] [Google Scholar]
- 49.Pishchik VN, Vorobyev NI, Ostankova YV, Semenov AV, Totolian Areg A, et al. (2018) Impact of Bacillus subtilis on Tomato Plants Growth and Some Biochemical Characteristics under Combined Application with Humic Fertilizer. International Journal of Plant & Soil Science 22: 1–12. [Google Scholar]
- 50.Khan AL, Waqas M, Kang SM, Al-Harrasi A, Hussain J, et al. (2014) Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J Microbiol 52: 689–695. 10.1007/s12275-014-4002-7 [DOI] [PubMed] [Google Scholar]
- 51.Khan MA, Asaf S, Khan AL, Ullah I, Ali S, et al. (2019) Alleviation of salt stress response in soybean plants with the endophytic bacterial isolate Curtobacterium sp. SAK1. Annals of Microbiology. [Google Scholar]
- 52.Patten CL, Glick BR (2002) Role of Pseudomonas putida Indoleacetic Acid in Development of the Host Plant Root System. Applied and Environmental Microbiology 68: 3795–3801. 10.1128/AEM.68.8.3795-3801.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katznelson H, Bose B (1959) Metabolic activity and phosphate-dissolving capability of bacterial isolates from wheat roots, rhizosphere, and non-rhizosphere soil. Can J Microbiol 5: 79–85. 10.1139/m59-010 [DOI] [PubMed] [Google Scholar]
- 54.Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160: 47–56. 10.1016/0003-2697(87)90612-9 [DOI] [PubMed] [Google Scholar]
- 55.Sambrook J, Russell D (2001) Molecular Cloning: A Laboratory Manual, 3rd edn: Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 56.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan AL, Halo BA, Elyassi A, Ali S, Al-Hosni K, et al. (2016) Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. 21: 58–64. [Google Scholar]
- 58.Khan MA, Asaf S, Khan AL, Jan R, Kang S-M, et al. (2019) Rhizobacteria AK1 remediates the toxic effects of salinity stress via regulation of endogenous phytohormones and gene expression in soybean. Biochemical Journal: BCJ 20190435. [DOI] [PubMed] [Google Scholar]
- 59.Kang SM, Khan AL, Hamayun M, Shinwari ZKS, Kim YH, et al. (2012) Acinetobacter calcoaceticus ameliorated plant growth and influenced gibberellins and functional biochemicals Pakistan journal of Botany 44: 365–372. [Google Scholar]
- 60.Kang S-M, Shahzad R, Bilal S, Khan AL, Park Y-G, et al. (2019) Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiology 19: 80 10.1186/s12866-019-1450-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Atiyeh RM, Lee S, Edwards CA, Arancon NQ, Metzger JD (2002) The influence of humic acids derived from earthworm-processed organic wastes on plant growth. Bioresource Technology 84: 7–14. 10.1016/s0960-8524(02)00017-2 [DOI] [PubMed] [Google Scholar]
- 62.Nikbakht A, Kafi M, Babalar M, Xia YP, Luo A, et al. (2008) Effect of Humic Acid on Plant Growth, Nutrient Uptake, and Postharvest Life of Gerbera. Journal of Plant Nutrition 31: 2155–2167. [Google Scholar]
- 63.Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta (BBA)—General Subjects 990: 87–92. [Google Scholar]
- 64.Asaf S, Khan MA, Khan AL, Waqas M, Shahzad R, et al. (2017) Bacterial endophytes from arid land plants regulate endogenous hormone content and promote growth in crop plants: an example of Sphingomonas sp. and Serratia marcescens. Journal of Plant Interactions 12: 31–38. [Google Scholar]
- 65.Khan MA, Khan AL, Imran QM, Asaf S, Lee S-U, et al. (2019) Exogenous application of nitric oxide donors regulates short-term flooding stress in soybean. PeerJ 7: e7741 10.7717/peerj.7741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jan R, Khan MA, Asaf S, Lee IJ, Kim KM (2019) Metal Resistant Endophytic Bacteria Reduces Cadmium, Nickel Toxicity, and Enhances Expression of Metal Stress Related Genes with Improved Growth of Oryza Sativa, via Regulating Its Antioxidant Machinery and Endogenous Hormones. Plants (Basel) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bilal S, Khan AL, Shahzad R, Asaf S, Kang S-M, et al. (2017) Endophytic Paecilomyces formosus LHL10 Augments Glycine max L. Adaptation to Ni-Contamination through Affecting Endogenous Phytohormones and Oxidative Stress. Frontiers in Plant Science 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim Y, Mun B-G, Khan AL, Waqas M, Kim H-H, et al. (2018) Regulation of reactive oxygen and nitrogen species by salicylic acid in rice plants under salinity stress conditions. PLOS ONE 13: e0192650 10.1371/journal.pone.0192650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khan MA, Ullah I, Waqas M, Hamayun M, Khan AL, et al. (2019) Halo-tolerant rhizospheric Arthrobacter woluwensis AK1 mitigates salt stress and induces physio-hormonal changes and expression of GmST1 and GmLAX3 in soybean. Symbiosis 77: 9–21. [Google Scholar]
- 70.Asaf S, Khan AL, Khan MA, Imran QM, Yun B-W, et al. (2017) Osmoprotective functions conferred to soybean plants via inoculation with Sphingomonas sp. LK11 and exogenous trehalose. Microbiological Research 205: 135–145. 10.1016/j.micres.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 71.Khan MA, Hamayun M, Iqbal A, Khan SA, Hussain A, et al. (2018) Gibberellin application ameliorates the adverse impact of short-term flooding on Glycine max L. Biochemical Journal: BCJ 20180534. [DOI] [PubMed] [Google Scholar]
- 72.Chan C-X, Teo S-S, Ho C-L, Othman RY, Phang S-M (2004) Optimisation of RNA extraction from Gracilaria changii (Gracilariales, Rhodophyta). Journal of Applied Phycology 16: 297–301. [Google Scholar]
- 73.Jan R, Khan MA, Asaf S, Lubna Lee I-J, et al. (2019) Metal Resistant Endophytic Bacteria Reduces Cadmium, Nickel Toxicity, and Enhances Expression of Metal Stress Related Genes with Improved Growth of Oryza Sativa, via Regulating Its Antioxidant Machinery and Endogenous Hormones. Plants 8: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Puglisi E, Pascazio S, Suciu N, Cattani I, Fait G, et al. (2013) Rhizosphere microbial diversity as influenced by humic substance amendments and chemical composition of rhizodeposits. Journal of Geochemical Exploration 129: 82–94. [Google Scholar]
- 75.Canellas LP, Balmori DM, Médici LO, Aguiar NO, Campostrini E, et al. (2013) A combination of humic substances and Herbaspirillum seropedicae inoculation enhances the growth of maize (Zea mays L.). Plant and Soil 366: 119–132. [Google Scholar]
- 76.Sharkey TD (2005) Effects of moderate heat stress on photosynthesis: importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant, Cell & Environment 28: 269–277. [Google Scholar]
- 77.Wang Q-L, Chen J-H, He N-Y, Guo F-Q (2018) Metabolic Reprogramming in Chloroplasts under Heat Stress in Plants. International journal of molecular sciences 19: 849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ortiz R, Sayre KD, Govaerts B, Gupta R, Subbarao GV, et al. (2008) Climate change: Can wheat beat the heat? Agriculture, Ecosystems & Environment 126: 46–58. [Google Scholar]
- 79.Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. International journal of molecular sciences 14: 9643–9684. 10.3390/ijms14059643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahammed GJ, Xu W, Liu A, Chen S (2018) COMT1 Silencing Aggravates Heat Stress-Induced Reduction in Photosynthesis by Decreasing Chlorophyll Content, Photosystem II Activity, and Electron Transport Efficiency in Tomato. Frontiers in Plant Science 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Glick BR, Liu C, Ghosh S, Dumbroff EB (1997) Early development of canola seedlings in the presence of the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2. Soil Biology and Biochemistry 29: 1233–1239. [Google Scholar]
- 82.Tariq A, Pan K, Olatunji OA, Graciano C, Li Z, et al. (2017) Phosphorous Application Improves Drought Tolerance of Phoebe zhennan. Frontiers in plant science 8: 1561–1561. 10.3389/fpls.2017.01561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cortina J, Vilagrosa A, Trubat R (2013) The role of nutrients for improving seedling quality in drylands. New Forests 44: 719–732. [Google Scholar]
- 84.Jin J, Lauricella D, Armstrong R, Sale P, Tang C (2014) Phosphorus application and elevated CO2 enhance drought tolerance in field pea grown in a phosphorus-deficient vertisol. Annals of Botany 116: 975–985. 10.1093/aob/mcu209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharma SS, Kaul S, Metwally A, Goyal KC, Finkemeier I, et al. (2004) Cadmium toxicity to barley (Hordeum vulgare) as affected by varying Fe nutritional status. Plant Science 166: 1287–1295. [Google Scholar]
- 86.Kumar P, Tewari RK, Sharma PN (2010) Sodium nitroprusside-mediated alleviation of iron deficiency and modulation of antioxidant responses in maize plants. AoB PLANTS 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Benbella M, Paulsen GM (1998) Efficacy of Treatments for Delaying Senescence of Wheat Leaves: II. Senescence and Grain Yield under Field Conditions. Agronomy journal 1998 v.90 no.3: pp. 332–338. [Google Scholar]
- 88.HongBo S, ZongSuo L, MingAn S (2005) Changes of anti-oxidative enzymes and MDA content under soil water deficits among 10 wheat (Triticum aestivum L.) genotypes at maturation stage. Colloids Surf B Biointerfaces 45: 7–13. 10.1016/j.colsurfb.2005.06.016 [DOI] [PubMed] [Google Scholar]
- 89.Baldotto LEB, Baldotto MA, Canellas LP, Bressan-Smith R, Olivares FL (2010) Growth promotion of pineapple ’vitória’ by humic acids and burkholderia spp. during acclimatization. Revista Brasileira de Ciência do Solo 34: 1593–1600. [Google Scholar]
- 90.Schoebitz M, López MD, Roldán A (2013) Bioencapsulation of microbial inoculants for better soil–plant fertilization. A review. Agronomy for Sustainable Development 33: 751–765. [Google Scholar]
- 91.Dobrá J, Černý M, Štorchová H, Dobrev P, Skalák J, et al. (2015) The impact of heat stress targeting on the hormonal and transcriptomic response in Arabidopsis. Plant Science 231: 52–61. 10.1016/j.plantsci.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 92.Peleg Z, Blumwald E (2011) Hormone balance and abiotic stress tolerance in crop plants. Current Opinion in Plant Biology 14: 290–295. 10.1016/j.pbi.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 93.Ahammed GJ, Li X, Zhou J, Zhou Y-H, Yu J-Q (2016) Role of Hormones in Plant Adaptation to Heat Stress In: Ahammed GJ, Yu J-Q, editors. Plant Hormones under Challenging Environmental Factors. Dordrecht: Springer Netherlands; pp. 1–21. [Google Scholar]
- 94.Siddiqui MH, Alamri SA, Al-Khaishany MYY, Al-Qutami MA, Ali HM, et al. (2017) Sodium nitroprusside and indole acetic acid improve the tolerance of tomato plants to heat stress by protecting against DNA damage. Journal of Plant Interactions 12: 177–186. [Google Scholar]
- 95.Yang J, Kloepper JW, Ryu C-M (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends in plant science 14: 1–4. 10.1016/j.tplants.2008.10.004 [DOI] [PubMed] [Google Scholar]
- 96.Vettakkorumakankav NN, Falk D, Saxena P, Fletcher RA (1999) A Crucial Role for Gibberellins in Stress Protection of Plants. Plant and Cell Physiology 40: 542–548. [Google Scholar]
- 97.Tuna AL, Kaya C, Dikilitas M, Higgs D (2008) The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environmental and Experimental Botany 62: 1–9. [Google Scholar]
- 98.Stavang JA, Lindgård B, Erntsen A, Lid SE, Moe R, et al. (2005) Thermoperiodic Stem Elongation Involves Transcriptional Regulation of Gibberellin Deactivation in Pea. Plant Physiology 138: 2344–2353. 10.1104/pp.105.063149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alonso-Ramírez A, Rodríguez D, Reyes D, Jiménez JA, Nicolás G, et al. (2009) Evidence for a Role of Gibberellins in Salicylic Acid-Modulated Early Plant Responses to Abiotic Stress in Arabidopsis Seeds. Plant Physiology 150: 1335–1344. 10.1104/pp.109.139352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee K-E, Adhikari A, Kang S-M, You Y-H, Joo G-J, et al. (2019) Isolation and Characterization of the High Silicate and Phosphate Solubilizing Novel Strain Enterobacter ludwigii GAK2 that Promotes Growth in Rice Plants. Agronomy 9: 144. [Google Scholar]
- 101.Bottini R, Cassan F, Piccoli P (2004) Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl Microbiol Biotechnol 65: 497–503. 10.1007/s00253-004-1696-1 [DOI] [PubMed] [Google Scholar]
- 102.Zhang HJ, Zhang N, Yang RC, Wang L, Sun QQ, et al. (2014) Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA 4 interaction in cucumber (C ucumis sativus L.). Journal of Pineal Research 57: 269–279. 10.1111/jpi.12167 [DOI] [PubMed] [Google Scholar]
- 103.Bashar KK, Tareq MZ, Amin MR, Honi U, Tahjib-Ul-Arif M, et al. (2019) Phytohormone-Mediated Stomatal Response, Escape and Quiescence Strategies in Plants under Flooding Stress. Agronomy 9: 43. [Google Scholar]
- 104.Verma V, Ravindran P, Kumar PP (2016) Plant hormone-mediated regulation of stress responses. BMC plant biology 16: 86–86. 10.1186/s12870-016-0771-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Ollas C, Dodd IC (2016) Physiological impacts of ABA–JA interactions under water-limitation. Plant molecular biology 91: 641–650. 10.1007/s11103-016-0503-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Curá JA, Franz DR, Filosofía JE, Balestrasse KB, Burgueño LE (2017) Inoculation with Azospirillum sp. and Herbaspirillum sp. Bacteria Increases the Tolerance of Maize to Drought Stress. Microorganisms 5: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khan N, Zandi P, Ali S, Mehmood A, Adnan Shahid M, et al. (2018) Impact of Salicylic Acid and PGPR on the Drought Tolerance and Phytoremediation Potential of Helianthus annus. Frontiers in Microbiology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang S, Moyne A-L, Reddy MS, Kloepper JW (2002) The role of salicylic acid in induced systemic resistance elicited by plant growth-promoting rhizobacteria against blue mold of tobacco. Biological Control 25: 288–296. [Google Scholar]
- 109.Wang Q-J, Sun H, Dong Q-L, Sun T-Y, Jin Z-X, et al. (2016) The enhancement of tolerance to salt and cold stresses by modifying the redox state and salicylic acid content via the cytosolic malate dehydrogenase gene in transgenic apple plants. Plant Biotechnology Journal 14: 1986–1997. 10.1111/pbi.12556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang LJ, Fan L, Loescher W, Duan W, Liu GJ, et al. (2010) Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol 10: 34 10.1186/1471-2229-10-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lopez-Delgado H, Dat JF, Foyer CH, Scott IM (1998) Induction of thermotolerance in potato microplants by acetylsalicylic acid and H2O2. Journal of Experimental Botany 49: 713–720. [Google Scholar]
- 112.Senaratna T, Touchell D, Bunn E, Dixon K (2000) Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regulation 30: 157–161. [Google Scholar]
- 113.Clarke SM, Mur LAJ, Wood JE, Scott IM (2004) Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. The Plant Journal 38: 432–447. 10.1111/j.1365-313X.2004.02054.x [DOI] [PubMed] [Google Scholar]
- 114.Wang L-J, Li S-H (2006) Salicylic acid-induced heat or cold tolerance in relation to Ca2+ homeostasis and antioxidant systems in young grape plants. Plant Science 170: 685–694. [Google Scholar]
- 115.Shahzad R, Khan AL, Bilal S, Waqas M, Kang S-M, et al. (2017) Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environmental and Experimental Botany 136: 68–77. [Google Scholar]
- 116.Shahzad R, Waqas M, Khan AL, Asaf S, Khan MA, et al. (2016) Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiology and Biochemistry 106: 236–243. 10.1016/j.plaphy.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 117.Arora A, Sairam RK, Srivastava GC (2002) Oxidative stress and antioxidative system in plants. Current Science 82: 1227–1238. [Google Scholar]
- 118.Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv 27: 84–93. 10.1016/j.biotechadv.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 119.LiXin Z, ShengXiu L, ZongSuo L (2009) Differential plant growth and osmotic effects of two maize (Zea mays L.) cultivars to exogenous glycinebetaine application under drought stress. Plant Growth Regulation 58: 297–305. [Google Scholar]
- 120.Khan N, Bano A, Babar MDA (2019) The stimulatory effects of plant growth promoting rhizobacteria and plant growth regulators on wheat physiology grown in sandy soil. Archives of Microbiology 201: 769–785. 10.1007/s00203-019-01644-w [DOI] [PubMed] [Google Scholar]
- 121.Khan N, Bano A, Rahman MA, Guo J, Kang Z, et al. (2019) Comparative Physiological and Metabolic Analysis Reveals a Complex Mechanism Involved in Drought Tolerance in Chickpea (Cicer arietinum L.) Induced by PGPR and PGRs. Scientific Reports 9: 2097 10.1038/s41598-019-38702-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environmental Chemistry Letters 8: 199–216. [Google Scholar]
- 123.Nazar R, Iqbal N, Masood A, Syeed S, Khan NA (2011) Understanding the significance of sulfur in improving salinity tolerance in plants. Environmental and Experimental Botany 70: 80–87. [Google Scholar]
- 124.Vaculík M, Landberg T, Greger M, Luxová M, Stoláriková M, et al. (2012) Silicon modifies root anatomy, and uptake and subcellular distribution of cadmium in young maize plants. Annals of Botany 110: 433–443. 10.1093/aob/mcs039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Singh S, Srivastava PK, Kumar D, Tripathi DK, Chauhan DK, et al. (2015) Morpho-anatomical and biochemical adapting strategies of maize (Zea mays L.) seedlings against lead and chromium stresses. Biocatalysis and Agricultural Biotechnology 4: 286–295. [Google Scholar]
- 126.Zhang L, Li S, Liang Z, Li S (2009) Effect of Foliar Nitrogen Application on Nitrogen Metabolism, Water Status, and Plant Growth in Two Maize Cultivars under Short-term Moderate Stress. Journal of Plant Nutrition 32: 1861–1881. [Google Scholar]
- 127.Hildebrandt Tatjana M, Nunes Nesi A, Araújo Wagner L, Braun H-P (2015) Amino Acid Catabolism in Plants. Molecular Plant 8: 1563–1579. 10.1016/j.molp.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 128.Hildebrandt TM (2018) Synthesis versus degradation: directions of amino acid metabolism during Arabidopsis abiotic stress response. Plant Mol Biol 98: 121–135. 10.1007/s11103-018-0767-0 [DOI] [PubMed] [Google Scholar]
- 129.Barbosa MAM, Lobato AKdS, Tan DKY, Viana GDM, Coelho KNN, et al. (2013) Bradyrhizobium improves nitrogen assimilation, osmotic adjustment and growth in contrasting cowpea cultivars under drought. Australian Journal of Crop Science 7: 1983–1989. [Google Scholar]
- 130.Vílchez JI, Niehaus K, Dowling DN, González-López J, Manzanera M (2018) Protection of Pepper Plants from Drought by Microbacterium sp. 3J1 by Modulation of the Plant’s Glutamine and α-ketoglutarate Content: A Comparative Metabolomics Approach. Frontiers in Microbiology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gagné-Bourque F, Bertrand A, Claessens A, Aliferis KA, Jabaji S (2016) Alleviation of Drought Stress and Metabolic Changes in Timothy (Phleum pratense L.) Colonized with Bacillus subtilis B26. Frontiers in Plant Science 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Santos AdA, Silveira JAGd, Guilherme EdA, Bonifacio A, Rodrigues AC, et al. (2018) Changes induced by co-inoculation in nitrogen–carbon metabolism in cowpea under salinity stress. Brazilian Journal of Microbiology 49: 685–694. 10.1016/j.bjm.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bhise KK, Bhagwat PK, Dandge PB (2017) Synergistic effect of Chryseobacterium gleum sp. SUK with ACC deaminase activity in alleviation of salt stress and plant growth promotion in Triticum aestivum L. 3 Biotech 7: 105 10.1007/s13205-017-0739-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.de Ronde JA, Laurie RN, Caetano T, Greyling MM, Kerepesi I (2004) Comparative study between transgenic and non-transgenic soybean lines proved transgenic lines to be more drought tolerant. Euphytica 138: 123–132. [Google Scholar]
- 135.Georgieva K, Fedina I, Maslenkova L, Peeva V (2003) Response of <emph type = "2">chlorina barley mutants to heat stress under low and high light. Functional Plant Biology 30: 515–524. [DOI] [PubMed] [Google Scholar]
- 136.Lv W-T, Lin B, Zhang M, Hua X-J (2011) Proline Accumulation Is Inhibitory to Arabidopsis Seedlings during Heat Stress. Plant Physiology 156: 1921–1933. 10.1104/pp.111.175810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yuan L, Tang L, Zhu S, Hou J, Chen G, et al. (2017) Influence of heat stress on leaf morphology and nitrogen—carbohydrate metabolisms in two wucai (Brassica campestris L.) genotypes. Acta Societatis Botanicorum Poloniae 86: 1–16. [Google Scholar]
- 138.Nakamoto H, Vigh L (2007) The small heat shock proteins and their clients. Cell Mol Life Sci 64: 294–306. 10.1007/s00018-006-6321-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Volkov RA, Panchuk II, Schöffl F (2003) Heat-stress-dependency and developmental modulation of gene expression: the potential of house-keeping genes as internal standards in mRNA expression profiling using real-time RT-PCR. Journal of Experimental Botany 54: 2343–2349. 10.1093/jxb/erg244 [DOI] [PubMed] [Google Scholar]
- 140.Fragkostefanakis S, Mesihovic A, Simm S, Paupière MJ, Hu Y, et al. (2016) HsfA2 Controls the Activity of Developmentally and Stress-Regulated Heat Stress Protection Mechanisms in Tomato Male Reproductive Tissues. Plant physiology 170: 2461–2477. 10.1104/pp.15.01913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hong S-W, Lee U, Vierling E (2003) Arabidopsis hot Mutants Define Multiple Functions Required for Acclimation to High Temperatures. Plant Physiology 132: 757–767. 10.1104/pp.102.017145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Almeida DM, Oliveira MM, Saibo NJM (2017) Regulation of Na+ and K+ homeostasis in plants: towards improved salt stress tolerance in crop plants. Genetics and molecular biology 40: 326–345. 10.1590/1678-4685-GMB-2016-0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ali A, Khan IU, Jan M, Khan HA, Hussain S, et al. (2018) The High-Affinity Potassium Transporter EpHKT1;2 From the Extremophile Eutrema parvula Mediates Salt Tolerance. Frontiers in Plant Science 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fairbairn DJ, Liu W, Schachtman DP, Gomez-Gallego S, Day SR, et al. (2000) Characterisation of two distinct HKT1-like potassium transporters from Eucalyptus camaldulensis. Plant Molecular Biology 43: 515–525. 10.1023/a:1006496402463 [DOI] [PubMed] [Google Scholar]
- 145.Zhou J, Wang J, Yu JQ, Chen Z (2014) Role and regulation of autophagy in heat stress responses of tomato plants. Front Plant Sci 5: 174 10.3389/fpls.2014.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang Y, Cai S, Yin L, Shi K, Xia X, et al. (2015) Tomato HsfA1a plays a critical role in plant drought tolerance by activating ATG genes and inducing autophagy. Autophagy 11: 2033–2047. 10.1080/15548627.2015.1098798 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
The isolates were preliminary sorted for single or multiple plant growth beneficial activities.
(DOCX)
Each data point is the mean of three replicates.
(DOCX)
Each data point is the mean of three replicates.
(DOCX)
(DOCX)
Data Availability Statement
All data generated or analyzed during this study are included in this published article.