Abstract
Microvascular diabetic complications are the most common causes of morbidity and mortality of patients with type 1 disease. Diabetic nephropathy is becoming the single most common cause of end stage renal failure, while diabetic retinopathy is the most common cause of blindness in working-age population. The main aim of the study was to evaluate the progression of late microvascular complications in type 1 diabetic patients treated by conventional or intensified insulin regimen over the period of 10 years. We selected a random sample of 32 patients, including 14 males and 18 females, aged 30,6 ± 11,8 years, with average duration of the disease of 4,8 ± 3,2 years. They did not show signs of overt diabetic nephropathy, while 5 patients had background retinopathy. All the patients had their fasting and postprandial gly-caemia, HbAlc, 24/hour proteinuria, blood pressure, height and weight measured and body mass index calculated (BMI). There was a trend towards increasing values of HbAlc (6.9 ±0.8 vs. 7.4 ± 1.0 %, p < 0.05), fasting glycaemia (6.8 ± 08 vs. 7.8 ± 1.2 mmol/l, p < 0.05), postprandial glycaemia (9.2 ± 1.5 vs. 11.3 ± 1.9 mmol/l, p <0.01), systolic and diastolic blood pressure values (120.0 ± 10.8 vs. 128.5 ± 16.8 mmHg, p<0.05; and 73.4 ± 8.1 vs. 79.8 ± 9.8 mmHg, p< 0.05) although no hypertensive patient was diagnosed. There were 11 persons (34.4%) with persistent proteinuria of 200 mg/24 hour or more and significant difference in overall proteinuria in 10 yrs period (121.3 ± 37.3 vs. 312.8 ± 109.9 mg/24 h, p< 0.001). Overall, 9 persons (28.1%) were diagnosed with simple, background retinopathy, but 6 of them (18.8%) had signs of proliferative form of the disease. The results indicate significant changes in progression of proteinuria in both groups although retinopathic progression was observed but was not significant in the intensively treated group.
Keywords: type 1 diabetes, insulin regimen, nephropathy, retinopathy
INTRODUCTION
Seriousness of metabolic disorder in diabetes mellitus is mostly exposed in the form of development of late microvascular and macrovascular complications. Diabetic nephropathy is becoming the most frequent cause of end-stage renal failure, and currently, in Europe and the USA, 25-35% of those requiring renal replacement therapy, dialysis or transplant, suffer from diabetes (1, 2, 3). It is also known that, in the developed countries, diabetic retinopathy is the most common cause of blindness in people aged 20 – 60 years (1). Prevention of onset and progression of these complications is still the most important task in comprehensive treatment of people suffering from diabetes. It requires early detection of incipient microvascular changes as well as their adequate and timely treatment (4, 5).
AIM
The main aim of this study is to evaluate the onset and progression of late microvascular complications in patients with type 1 disease who are treated either conventionally or by intensive insulin regimen, over the period of 10 years.
MATERIAL AND METHODS
We selected a random sample of 32 patients with type 1 diabetes mellitus, who were treated on outpatient or inpatient basis at the Department of Diabetes. Patients with nondiabetic renal diseases, those who manifest renal nephropathy or those with advanced form of diabetic retinopathy were excluded. Also, those with the signs of other autoimmune diseases and liver disease were excluded from the study sample. The study was designed on the principle “intention to treat” and baseline characteristics of subjects at the beginning of the study are presented in Table 1. All the patients had their fasting and postprandial blood glucose, glycosylated haemoglobin (HbA1c), 24-hour proteinuria, blood pressure, body height and weight measured and body mass index calculated. Blood glucose levels were determined in venous blood by glucoso-oxi-dase method. Glycosylated Hb was also measured from the venous sample, with micro column test by chromatography method, using reagents donated by the company Bio-Rad, Milan, Italy. Body weight, height and blood pressure were measured by validated instruments, Secca 770 scale, Secca 225 statometer, and calibrated sphygmo-manometer, Reister 600/306, Diplomat according to the CINDI protocol (6). 24 -hour proteinuria was measured by precipitation method with perchlorine acid followed by quantitative spectrophotometric reaction according to Gornall (Biuret reaction). All subjects had their eye fundus examined by direct and indirect ophtalmoscopy.
TABLE 1.
Characteristics of participants at baseline

STATISTICAL ANALYSIS
Data were expressed as mean values with standard deviation as a variability measure or proportions expressed as percentages. Differences between mean values in two periods were assessed using paired Student’s t-test and were considered significant at the level of p < 0.05. Differences between proportions were analyzed by X2 test.
RESULTS
In 10 years interval significant increase in glycosylated Hb values (HbA1c), fasting and postprandial blood glucose levels, systolic and diastolic blood pressure values, as well as body mass index values were noted (Table 2). However, the proportion of patients with higher levels of blood pressure (SBP > 135 mmHg, DKP >85mHg) was not significantly increased as well as the proportion of those who were overweight (BMI > 25 kg/m2) (Table 3). The total number of those who developed mild retinal changes was not significant but significant proportion of proliferative changes was noted in the total sample (Table 4). However, development of retinopathy was significantly more frequent in the group under conventional insulin regimen (p=0.019) (Table 6), while significant progression was not noted in the proportion of subjects under intensified insulin regimen (p=0.102) (Table 7). We observed significant increase in the mean values of urinary proteins (p<0.001), as well as larger proportion of subjects with values higher than 200 mg/ 24 h (p=0.001), as well as the percentage of those with values higher than 300 mg/24 h (p=0.015) (Table 5). An increase in the mean uroproteins was significantly higher in both groups, although the increment observed in the intensified insulin group was less pronounced (Table 6, Table 7).
TABLE 2.
Changes in metabolic control over 10 years period
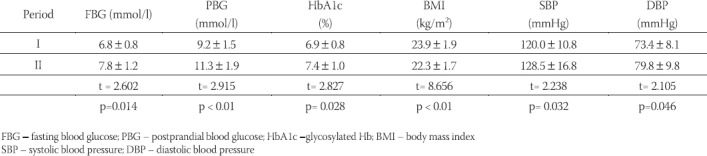
TABLE 3.
Changes in the level of metabolic control over 10 years period
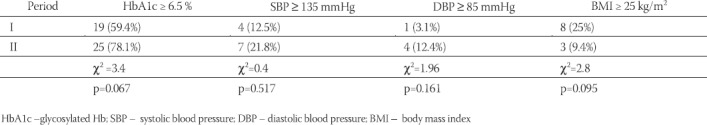
TABLE 4.
Changes in the grade of retinopathy in the two periods
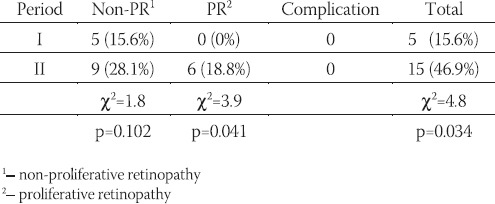
TABLE 5.
Levels of 24 h – proteinuria in the two periods (mg/24 h)
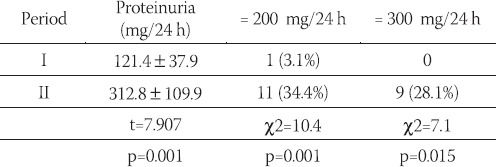
TABLE 6.
Progression of microvascular changes in conventionally treated patients

TABLE 7.
Progression of microvascular changes in intensively treated patients

DISCUSSION
Prevention of the development and early detection of the late microvascular complications is a priority in the treatment of people suffering from type 1 diabetes mellitus. Opinions on the mode of treatment have significantly changed since 1993, after the presentation of the results of the prospective, randomized Diabetes Control and Complication Trial (DCCT), which clearly showed that intensified treatment significantly delayed progression of the late microvascular changes (7). In our study relatively higher proportion of participants was under conventional than under intensified insulin treatment (Table 1). It is indicative that the former group had more significant increase of proteinuria (p<0.001), while the increase in the latter group was less expressed (p=0.021). Progression of retinopathy was also significant in the first group (p=0.019), which was not the case in the second, intensively treated group (p=0.102) although the trend of increase was clearly observed. It is well documented that incipient nephropathy is the marker of the development of progressive renal disease and that initial, timely treatment requires administration of ACE inhibitors (8). Decrease in progression of retinal changes was also noted in normotensive type 1 diabetic patients using lisinopril (9). Our results showed progression of microvascular changes over the period of 10 years which is consistent with the results of some larger previous studies (10). These results strongly indicate the need for more accurate identification of initial nephropatic changes in the phase of microalbuminuria so that timely implementation of adequate measures can be initiated. Also, promotion of intensified insulin regimen and preventive measures against increase in blood pressure values should attract more attention (11, 12).
CONCLUSION
We should invest more effort in trying to convince majority of type 1 diabetic patients to accept intensified insulin regimen in order to reduce the incidence and level of microvascular changes. Adequate diagnosis of incipient nephropathy and prevention of progression of renal disease require application of more sensitive method for timely detection of onset of microalbuminuric changes.
REFERENCES
- 1.Campbell IW, Lebovitz H. Diabetes mellitus. Oxford: Health press; 1996. p. 25. [Google Scholar]
- 2.Raine AEG. Evolution worldwide of the treatment of patients with advanced diabetic nephropathy by renal replacement therapy. In: Mogensen CE, editor. The Kidney and hypertension in Diabetes mellitus. Boston Kluwer Academic Publishers; 1994. pp. 449–458. [Google Scholar]
- 3.The 1993 USRDS Annual Data Report. Incidence and causes of treated ESRD. Am. J. Kid. Dis. 1993;22(Suppl.2):38–45. [Google Scholar]
- 4.Colucciello M. Diabetic retinopathy. Postgraduate medicine. 2004;116(1):57–64. doi: 10.3810/pgm.2004.07.1558. [DOI] [PubMed] [Google Scholar]
- 5.Molich ME. Management of early diabetic nephropathy. Am. J. Med. 1997;102(4):392–398. doi: 10.1016/s0002-9343(97)00118-6. [DOI] [PubMed] [Google Scholar]
- 6.Leparski E, Nussel E, editors. World Health Organization. Protocol and guidelines for monitoring and evaluation procedures. Berlin, Springer-Verlag: Countrywide integrated non-communicable diseases intervention programme (CINDI). Regional Office for Europe WHO; 1987. pp. 40–42. [Google Scholar]
- 7.The Diabetes Control and Complication Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 8.Lewis J, Hunsickler LG, Bain RP, Rohcle RD. The effect of angiotensin – converting enzyme inhibition on diabetic nephropa-thy. N. Engl. J. Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 9.The EUCLID, Study Group. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. Lancet. 1998;14(351):836–838. doi: 10.1016/s0140-6736(97)06209-0. [DOI] [PubMed] [Google Scholar]
- 10.Klein R, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14 year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophtalmology. 1998;105(10):1801–1815. doi: 10.1016/S0161-6420(98)91020-X. [DOI] [PubMed] [Google Scholar]
- 11.Aiello LM. Perspectives on diabetic retinopathy. Am. J. Ophtal-mol. 2003;136(1):122–135. doi: 10.1016/s0002-9394(03)00219-8. [DOI] [PubMed] [Google Scholar]
- 12.Donald SF, et al. Retinopathy in Diabetes. Diabetes Care. 2004;27:S84–S87. doi: 10.2337/diacare.27.2007.s84. [DOI] [PubMed] [Google Scholar]


