Abstract
Type 2 diabetes mellitus (T2DM) is a serious public health problem accompanies with several complications. This study was conducted to evaluate the effects of chromium picolinate (CrPic) supplementation on the glycemic status and lipid profile in patients with T2DM. The patients with T2DM (n = 52) were randomly allocated into 2 groups. One group received 400 µg CrPic per day and the other group took placebo; the intervention duration was 8 weeks. Anthropometric indices and metabolic factors were measured at the beginning, and at end of the study. The patients were recommended not to change their normal diet, life style and medication. No significant changes were observed for weight, body mass index, and fasting blood glucose (FBG) in both groups; while intra-groups changes in homeostatic model assessment for insulin resistance (HOMA-IR) value was significant (p < 0.05). Results of analysis of covariance showed that there were significance differences between groups in total cholesterol, low density lipoprotein cholesterol and HOMA-IR at the end of the intervention adjusting for baseline levels (p = 0.035, 0.030 and < 0.001, respectively). In this study, oral supplementation with 400 µg CrPic for eight weeks did not alter FBG concentration as well as anthropometric parameters in individuals with T2DM. However, the modest beneficial effects of chromium supplementation on insulin resistance as indicated by HOMA-IR and lipid profile were found.
Keywords: Type 2 diabetes mellitus, Chromium, Blood glucose, Dyslipidemia
INTRODUCTION
Type 2 diabetes mellitus (T2DM) remains a serious public health problem, with high rates of morbidity and mortality in low- or middle-income countries [1]. The prevalence of T2DM is increasing at an alarming rate, so that the number of Iranian adults suffering from diabetes was more than four millions in 2011 and has been raised by 35% over the past years [2]. This chronic disease results from defects in insulin secretion and/or peripheral insulin resistance, that both deteriorate glycemic control [3].
The Diabetes Control and Complication Trial reported that tight blood glucose control noticeably slows the complications of diabetes; therefore many diabetic patients struggle to manage their blood glucose levels with existing treatments [4]. Furthermore, it is imperative to identify some preventive strategies to decrease the risk of diabetes and its complications.
Recently the dietary supplements including chromium were used to manage T2DM and improve glycemic control [5]. Chromium as an essential element is found in foods and dietary supplements that plays an important role in insulin function and glucose metabolism in mammalian [6]. There is evidence that in diabetic patients, subclinical chromium deficiency is associated with elevated blood glucose, insulin, and lipid levels that may adversely affect the management of diabetes [7] and its repletion after experimental dietary depletion led to improvement in glycemic status [8]. Chromium with the mechanism that expands insulin cell signaling through the low molecular weight chromium-binding substance increases the sensitivity of insulin receptors in the plasma membrane and improves glycemic control [9]. Some studies have shown that chromium could improve both glucose and insulin metabolism [3,10]. however, some studies reported no beneficial effect for chromium in T2DM patients [11,12,13]. Given that the content of the human diet is poor in terms of chromium and based on contradictory evidence regarding the effects of chromium, the present study was conducted to evaluate the effects of this supplement on fasting blood glucose (FBG), cholesterol, lipoproteins, insulin and Insulin resistance in T2DM patients.
MATERIALS AND METHODS
This study was a randomized, double-blind, placebo-controlled clinical trial which conducted on the patients who referred to the institute of Endocrine and Metabolism of Iran University of Medical Sciences. Approval was obtained from the ethical committee of Iran University of Medical Sciences (ethic code: IR.IUMS.REC 84.181) in accordance with Helsinki Declaration and a written informed consent was signed by all participants or their companions if they were illiterate. Patients were informed that supplements have no side effect and they are free to leave the study if they were unwilling to follow the intervention.
Subjects
We designed our study based on CONSORT statement for randomized clinical trials [14]. To calculate sample size, based on α = 0.05, effect size 80%, and a power of 90%, 25 persons have been estimated for each group [15]; however due to drop-out, 52 subjects with T2DM were recruited that 41 of them completed the study.
Inclusion criteria were patients over 40 years old with a documented history of T2DM for more than six months without any other chronic diseases, without the background of thyroid, liver, and any chronic diseases or under medical therapy for hyperlipidemia, not being pregnant or lactating, not consumption of any other supplements (antioxidants, minerals or vitamins, omega 3, and carnitine) or herbal medicines. The reluctance to follow up the study or occurrence of diabetes complications were considered as exclusion criteria. The patients were recommended not to change their routine diet and physical activity. Block randomization was done using Random Allocation Software to assign patients into 2 parallel groups with 4 participants in each block basis on age and sex. Following allocation, another person who was not involved in the study gave the supplements (indicated with code A and B) to patients.
Intervention
Subjects were randomly assigned to the chromium group (n = 22) who received two tablet of chromium picolinate (each one contained 200 µg chromium picolinate [CrPic]) (21st Century Health Care, Inc., Tempe, AZ, USA) per day and the control group (n = 19) who took placebo (starch). Patients were requested to consume one supplement/placebo with lunch and the other with dinner every day for eight weeks. Supplement and placebo were quite similar in size, shape and color. World Health Organization considered that supplementation with chromium should not exceed 250 µg/day [16], however across studies; doses of 400 or 1,000 µg/day appear to be safe and have had greater effects than lower doses [17]. Therefore we decided to investigate the effects of 400 µg/day of this supplement. Adherence of participants was assessed biweekly via telephone interview and they were asked to return the remained supplements at the end of intervention.
Anthropometric and dietary intakes assessments
Anthropometric indices were measured in light clothing and without shoes before and after the intervention. Body weight and height were measured in the fasting state using Seca scale (Seca, Hamburg, Germany) and a stadiometer attached to the scale, respectively. Body mass index (BMI) was calculated on the basis of weight (kg) divided by the square of height (m). Dietary intakes including energy, macronutrients (fiber, carbohydrate, protein, fat, cholesterol, saturated-, monounsaturated- and polyunsaturated fatty acids) and chromium were assessed by a 24-h recall questionnaire and were analyzed by Nutritionist IV software which modified for Iranian foods.
Sample collection and laboratory assessment
Approximately 5 mL of venous blood were obtained from all participants after a 12–14 hours overnight fasting. Blood specimens were poured into two tubes, one for provision serum (blood samples were centrifuged 10 minutes at 2500 r.p.m.) and the other one for measuring FBG (using enzymatic kits [Pars Azmoon Co., Tehran, Iran]). Serums were frozen at −80°C until analysis time.
Total cholesterol, high density lipoprotein cholesterol (HDL-C) and triglyceride levels in serum were measured by enzymatic kits (Pars Azmoon Co.). Serum chromium concentration was determined using flame atomic absorption spectrometry. Low density lipoprotein cholesterol (LDL-C) was calculated using the Friedwald equation because the triglyceride concentration of patients was less than 400 mg/dL. Insulin resistance calculated using the relevant equation homeostatic model assessment for insulin resistance (HOMA-IR) = FBG (mg/dL) × fasting insulin (µU/mL)/405 [18]. All assessments were done at baseline and at the end of intervention.
Statistical analysis
Data were analyzed using SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation and categorical variables as frequency and percentage. Normality distribution was evaluated by Kolmogorov-Smirnov test. Comparisons of variables between two groups at baseline were assessed through independent t-test and Mann-Whitney tests and at the end of intervention were evaluated using analysis of covariance (ANCOVA) after adjustment of dietary intakes and baseline amounts of each variable. Changes in biomarkers from the baseline to endpoint were compared within groups by paired sample/Wilcoxon tests and between groups by independent t-test. All significant tests were 2-tailed, and p-values under 0.05 were considered statistically significant.
RESULTS
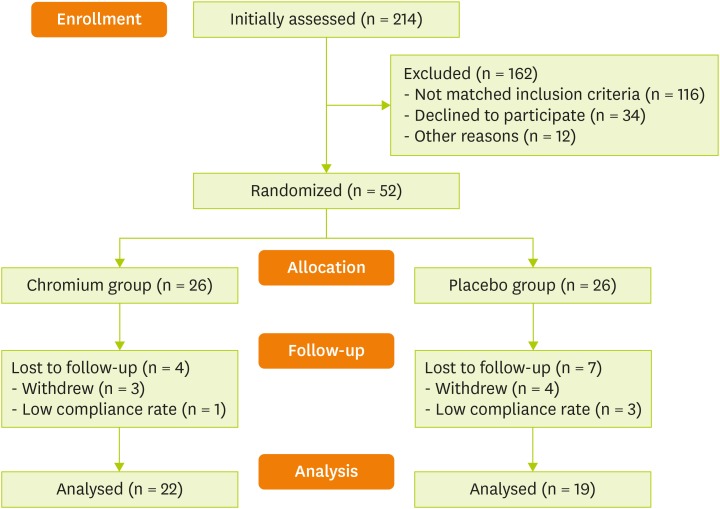
Out of 214 assessed patients at the beginning of the study, 52 of them were recruited in the present randomized clinical trial. As shown in Figure 1, 41 patients (22 in chromium group and 19 in placebo group) completed the study. No adverse effects or symptoms were reported by participants in both groups. There was no a significant difference between chromium and placebo groups in regard to age, sex, weight, height and BMI at the entry (Table 1).
Figure 1. CONSORT flowchart of study.
Table 1. Baseline characteristics of patients with type 2 diabetes mellitus.
| Variables | Chromium group (n = 22) | Placebo group (n = 19) | p value |
|---|---|---|---|
| Sex, female | 19 (86.3) | 15 (78.9) | NS |
| Age | 50.4 ± 6.0 | 51.4 ± 6.0 | NS |
| Weight | 77.3 ± 10.4 | 76.6 ± 8.8 | NS |
| Height | 164.2 ± 8.4 | 163.1 ± 5.1 | NS |
| BMI | 28.7 ± 5.0 | 28.8 ± 5.7 | NS |
Data are presented as number (percent) for sex and mean ± standard deviation for other variables. Data were analyzed using Fisher exact test for sex and Independent t-test for other variables.
NS, not significant; BMI, body mass index.
After intervention, BMI and its changes had no meaningful differences between two groups; also, within groups' changes were not statistically meaningful. Energy, macronutrients (carbohydrates, proteins, total fats (cholesterol, saturated-, monounsaturated- and polyunsaturated fatty acids)), fiber and chromium intakes of participants has been presented in Table 2. Comparison of dietary intakes indicated no significant differences between or within groups (p > 0.05).
Table 2. Dietary intakes of patients with type 2 diabetes mellitus.
| Variables | Chromium group (n = 22) | Placebo group (n = 19) | p value* | p value† | ||
|---|---|---|---|---|---|---|
| Baseline | Endpoint | Baseline | Endpoint | |||
| Energy (kcal) | 1,584.1 ± 368.1 | 1,528.4 ± 601.1 | 1,579.0 ± 633.0 | 1,536.4 ± 531.0 | NS | NS |
| Carbohydrate (g) | 230.9 ± 87.6 | 223.2 ± 131.3 | 235.9 ± 130.8 | 230.7 ± 103.3 | NS | NS |
| Protein (g) | 66.9 ± 27.8 | 61.3 ± 21.3 | 62.6 ± 23.0 | 62.7 ± 24.0 | NS | NS |
| Fat (g) | 46.1 ± 24.0 | 40.0 ± 20.0 | 43.0 ± 20.0 | 47.0 ± 25.0 | NS | NS |
| Cholesterol (mg) | 196.9 ± 12.0 | 220.3 ± 25.9 | 199.5 ± 21.4 | 169.8 ± 11.6 | NS | NS |
| SFA (g) | 13.0 ± 9.0 | 26.7 ± 7.0 | 10.8 ± 6.0 | 12.6 ± 9.0 | NS‡ | NS‡ |
| MUFA (g) | 6.1 ± 5.1 | 5.3 ± 3.8 | 5.1 ± 3.7 | 6.1 ± 5.0 | NS | NS |
| PUFA (g) | 2.5 ± 1.7 | 3.1 ± 3.3 | 3.3 ± 4.0 | 3.1 ± 2.0 | NS‡ | NS‡ |
| Chromium (µg) | 0.4 ± 0.4 | 0.4 ± 0.5 | 0.3 ± 0.2 | 0.3 ± 0.4 | NS | NS |
| Fiber (g) | 7.7 ± 4.9 | 6.5 ± 5.1 | 7.0 ± 4.9 | 8.0 ± 4.7 | NS | NS |
Data are presented as mean ± standard deviation.
NS, not significant; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
Between groups comparisons of *baseline and †endpoint using independent t-test except indicated with ‡which analyzed by Mann-Whitney test.
The levels of evaluated biomarkers as shown in Table 3 had non-significant differences between two groups at the baseline and the end of intervention. However comparison the changes of total cholesterol, LDL-C and HOMA-IR between two groups at the end of the intervention was significant (p = 0.035, 0.030 and < 0.001, respectively). Also, changes in levels of FBG, Insulin, triglyceride, HDL-C and chromium between groups were not significant (p > 0.05).
Table 3. Metabolic biomarkers of patients with type 2 diabetes mellitus before and after the intervention.
| Variables | Chromium group (n = 22) | Placebo group (n = 19) | p value† | p value‡ | p value§ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Endpoint | p value* | Changes | Baseline | Endpoint | p value* | Changes | ||||
| Cholesterol (mg/dL) | 210.1 ± 48.2 | 182.1 ± 37.4 | 0.002 | −28.0 ± 37.0 | 199.0 ± 48.2 | 194.6 ± 30.4 | NS | −4.4 ± 31.9 | NS | NS | 0.035 |
| Triglyceride (mg/dL) | 153.2 ± 51.3 | 138.4 ± 64.0 | NS | −14.8 ± 60.4 | 179.7 ± 146.5 | 142.1 ± 52.5 | NS | −37.6 ± 136.0 | NS | NS | NS |
| LDL-C (mg/dL) | 139.4 ± 46.3 | 109.6 ± 41.1 | 0.002 | −29.7 ± 40.4 | 188.7 ± 33.7 | 192.0 ± 30.1 | NS | 3.3 ± 34.6 | NS | NS | 0.030 |
| HDL-C (mg/dL) | 39.8 ± 9.6 | 42.0 ± 11.5 | NS | 2.1 ± 10.6 | 45.3 ± 9.0 | 44.0 ± 9.9 | NS | −1.3 ± 9.2 | NS | NS | NS |
| FBG (mg/dL) | 189.4 ± 71.3 | 158.1 ± 65.0 | NS | −31.3 ± 81.4 | 204.8 ± 68.5 | 178.2 ± 50.1 | NS | −26.6 ± 59.2 | NS | NS | NS |
| Insulin (µU/mL) | 9.9 ± 7.0 | 7.5 ± 3.3 | NS* | −2.5 ± 6.8 | 7.4 ± 3.4 | 7.0 ± 4.3 | NS | −0.4 ± 4.0 | NS* | NS | NS |
| HOMA-IR | 4.6 ± 1.2 | 2.9 ± 0.5 | 0.041 | −1.7 ± 0.3 | 3.7 ± 0.6 | 3.1 ± 0.5 | NS | −0.7 ± 0.3 | NS | NS | < 0.001 |
| Chromium (µg/L) | 2.3 ± 0.9 | 2.6 ± 0.9 | NS | 0.4 ± 1.4 | 2.2 ± 0.6 | 2.5 ± 0.6 | NS | 0.3 ± 0.9 | NS | NS | NS |
Data are presented as mean ± standard deviation.
NS, not significant; LDL-C, low-density lipoprotein; HDL-C, high-density lipoprotein; FBG, fasting blood glucose; HOMA-IR, homeostatic model assessment for insulin resistance.
*Within groups' analysis by paired t-test except indicated with which analyzed by Wilcoxon test. †Between groups' comparisons at baseline by independent t-test except indicated with which analyzed by Mann-Whitney test. ‡ANCOVA for between groups analysis after adjustment of dietary intakes and baseline levels of each variable, §independent t-test for between groups' changes analyses.
DISCUSSION
In the present study following supplementation of 400 µg/day CrPic for eight weeks in T2DM, total cholesterol, LDL-C and insulin resistance decreased significantly. However, no significant change was observed in the amounts of FBG, triglyceride and HDL-C, as well as weight and BMI.
Similar to our findings a recent study did not indicate any reduction in body weight and BMI in diabetic patients [19]. Briefly, the available evidence is not sufficient to establish the beneficial effects of chromium in patients with different BMI ranges with or without diabetes [20,21].
Although chromium supplementation was associated with decrease of FBG levels in diabetic patients [1], in the present study CrPic supplementation failed to affect the blood glucose levels while decreased HOMA-IR values. Considering chemistry and pharmacology of chromium, it was a safe nutrient for supplementation in T2DM [22]. Chromium is suggested as one of the supplements for controlling blood sugar which may be responsible for reducing myocellular lipids and enhance insulin sensitivity in subjects with T2DM [23]. Several hypothesis have explained the role of chromium in glucose/insulin metabolism. Chromium could play an important role in glucose transportation including impact on the main glucose transporter i.e. GLUT-4 [24]. Although the mechanisms of chromium action are unclear, it seems to stimulate the activity of insulin receptor kinases in the plasma membrane [25,26]. Furthermore, chromium may improve insulin receptor mRNA synthesis and increase the synthesis of insulin-like growth factor receptors [27].
While several researchers indicated a positive effect of chromium supplementation in type 2 diabetic patients [23,28]; some studies failed to show the beneficial effect of chromium supplementation in diabetes [11,12,29]. In term of glycemic control, our findings seem to contradict those found by Paiva et al. [3] which demonstrated a significant reduction in fasting glucose concentration. However, those patients received 600 µg CrPic/day which was a greater dose than used in our study.
Some studies have shown the benefits of supplementation with chromium for glycemic control of subjects with T2DM. Martin et al. [30] concluded that supplementation with 1,000 µg of CrPic for 6 months significantly improved insulin sensitivity and glycemic control, which the dose and the duration are greater than the present research. Komorowski and Juturu [31] found no benefits with regard to insulin sensitivity or glucose and lipid levels in 40 subjects with impaired glucose tolerance after 3 months of supplementation with 800 µg/day of CrPic. Inconclusive results on chromium supplementation and reduction of blood glucose and fasting insulin were also observed in a meta-analysis by Althuis et al. [32]. The reasons for the inconsistent findings may be attributed in part to the duration of the supplementation, the type of formulation, doses of supplements, and also the differences in disease condition, i.e., glucose intolerance status of the subjects [23,33].
In addition to its role in glucose metabolism, chromium supplementation has been shown to improve the lipid profile in particular the triglycerides concentration [6,34]. However, we did not observe any differences in the levels of triglyceride and HDL-C following CrPic treatment. These findings are supported by previous studies which evaluated the effect of chromium supplementation on lipid profile [9,35,36]. The inconsistency of CrPic impact on lipid profile may be explained in part by the fact that not all of the patients had the dyslipidemia.
In order to better monitoring the effects of crucial trace elements which related to T2DM, it is suggested that their levels in bio fluids should be measured. A lower levels of chromium has been reported in diabetic patients compared to healthy controls [37]. However due to the lack of an accurate diagnostic test, identifying individuals with deficiency is difficult [38]. To now there is no international acceptable value or range for the serum/plasma chromium concentration in the general population [39]. In this regard, studies reporting blood levels of chromium in large populations are sparse. However to investigate the effects of changes in chromium values, we measured the serum levels of chromium; the mean of chromium concentration in our population was 2.3 ± 0.9 and 2.2 ± 0.6 μg/L in chromium and placebo groups, respectively; that was higher than the previously reported values [40].
In spite of the finding of Anderson et al. [8] which reported that chromium repletion after experimental dietary depletion led to improved glycemic status, in the present study the increased serum concentration of chromium was not associated with improvement in glycemic status nor amelioration of triglyceride levels. We hypothesized that the lack of effects of chromium is due to the fact that the increase occurred in serum levels was not statistically meaningful, thus it was not sufficient to exert extra remarkable benefits. It is also possible that in patients with lower chromium concentrations the supplementation would result in beneficial effects [41]. Chromium concentration in serum has been elevated in both groups, however it was not significant. This increment is related to the consumption of chromium in the supplement group; also considering diet carbohydrate can reduce the chromium concentration [42] the elevation may be in part due to the insignificant reduction in carbohydrate intake in chromium and placebo groups.
The strengths of our study included the measurement of serum chromium levels; however there are a few limitations to be considered including the lack of information on the serum levels of other minerals supposed to affect T2DM such as magnesium as well as zinc. Thus their relationship and/or interaction with chromium and glucose homeostasis remained to be investigated. Also the sample size may not have been sufficient to detect differences between groups. Thus, further studies evaluating the effects of different doses and duration are necessary to better understand the effect of chromium supplementation on lipid metabolism in patients with T2DM.
CONCLUSION
In conclusion this study showed that supplementation of 400 µg CrPic resulted in no improvement in body weight and BMI. However, CrPic could attenuate LDL-C, total cholesterol, and HOMA-IR significantly; while no significant was occurred in FBG levels. It seems that CrPic supplementation might help T2DM patients to improve the disease-associated disturbance of lipid profile and insulin resistance.
ACKNOWLEDGEMENTS
We hereby acknowledge our sincere gratitude to all the staff of Endocrine and Metabolism Institute of Iran University of Medical Science for their support and cooperation. We also thank the Research vice-chancellor of Iran University of Medical Sciences due to financial supports.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
References
- 1.Huang H, Chen G, Dong Y, Zhu Y, Chen H. Chromium supplementation for adjuvant treatment of type 2 diabetes mellitus: results from a pooled analysis. Mol Nutr Food Res. 2018;62:1700438. doi: 10.1002/mnfr.201700438. [DOI] [PubMed] [Google Scholar]
- 2.Esteghamati A, Etemad K, Koohpayehzadeh J, Abbasi M, Meysamie A, Noshad S, Asgari F, Mousavizadeh M, Rafei A, Khajeh E, Neishaboury M, Sheikhbahaei S, Nakhjavani M. Trends in the prevalence of diabetes and impaired fasting glucose in association with obesity in Iran: 2005–2011. Diabetes Res Clin Pract. 2014;103:319–327. doi: 10.1016/j.diabres.2013.12.034. [DOI] [PubMed] [Google Scholar]
- 3.Paiva AN, Lima JG, Medeiros AC, Figueiredo HA, Andrade RL, Ururahy MA, Rezende AA, Brandão-Neto J, Almeida M. Beneficial effects of oral chromium picolinate supplementation on glycemic control in patients with type 2 diabetes: a randomized clinical study. J Trace Elem Med Biol. 2015;32:66–72. doi: 10.1016/j.jtemb.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Schaumberg DA, Glynn RJ, Jenkins AJ, Lyons TJ, Rifai N, Manson JE, Ridker PM, Nathan DM. Effect of intensive glycemic control on levels of markers of inflammation in type 1 diabetes mellitus in the diabetes control and complications trial. Circulation. 2005;111:2446–2453. doi: 10.1161/01.CIR.0000165064.31505.3B. [DOI] [PubMed] [Google Scholar]
- 5.Hua Y, Clark S, Ren J, Sreejayan N. Molecular mechanisms of chromium in alleviating insulin resistance. J Nutr Biochem. 2012;23:313–319. doi: 10.1016/j.jnutbio.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma S, Agrawal RP, Choudhary M, Jain S, Goyal S, Agarwal V. Beneficial effect of chromium supplementation on glucose, HbA1C and lipid variables in individuals with newly onset type-2 diabetes. J Trace Elem Med Biol. 2011;25:149–153. doi: 10.1016/j.jtemb.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Rajpathak S, Rimm EB, Li T, Morris JS, Stampfer MJ, Willett WC, Hu FB. Lower toenail chromium in men with diabetes and cardiovascular disease compared with healthy men. Diabetes Care. 2004;27:2211–2216. doi: 10.2337/diacare.27.9.2211. [DOI] [PubMed] [Google Scholar]
- 8.Anderson RA, Polansky MM, Bryden NA, Canary JJ. Supplemental-chromium effects on glucose, insulin, glucagon, and urinary chromium losses in subjects consuming controlled low-chromium diets. Am J Clin Nutr. 1991;54:909–916. doi: 10.1093/ajcn/54.5.909. [DOI] [PubMed] [Google Scholar]
- 9.Guimarães MM, Martins Silva Carvalho AC, Silva MS. Chromium nicotinate has no effect on insulin sensitivity, glycemic control, and lipid profile in subjects with type 2 diabetes. J Am Coll Nutr. 2013;32:243–250. doi: 10.1080/07315724.2013.816598. [DOI] [PubMed] [Google Scholar]
- 10.Cefalu WT, Hu FB. Role of chromium in human health and in diabetes. Diabetes Care. 2004;27:2741–2751. doi: 10.2337/diacare.27.11.2741. [DOI] [PubMed] [Google Scholar]
- 11.Kleefstra N, Houweling ST, Bakker SJ, Verhoeven S, Gans RO, Meyboom-de Jong B, Bilo HJ. Chromium treatment has no effect in patients with type 2 diabetes in a Western population: a randomized, double-blind, placebo-controlled trial. Diabetes Care. 2007;30:1092–1096. doi: 10.2337/dc06-2192. [DOI] [PubMed] [Google Scholar]
- 12.Kleefstra N, Houweling ST, Jansman FG, Groenier KH, Gans RO, Meyboom-de Jong B, Bakker SJ, Bilo HJ. Chromium treatment has no effect in patients with poorly controlled, insulin-treated type 2 diabetes in an obese Western population: a randomized, double-blind, placebo-controlled trial. Diabetes Care. 2006;29:521–525. doi: 10.2337/diacare.29.03.06.dc05-1453. [DOI] [PubMed] [Google Scholar]
- 13.San Mauro-Martin I, Ruiz-León AM, Camina-Martín MA, Garicano-Vilar E, Collado-Yurrita L, Mateo-Silleras B, Redondo Del Río MP. Chromium supplementation in patients with type 2 diabetes and high risk of type 2 diabetes: a meta-analysis of randomized controlled trials. Nutr Hosp. 2016;33:27. doi: 10.20960/nh.v33i1.27. [DOI] [PubMed] [Google Scholar]
- 14.Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P, CONSORT Group Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148:295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
- 15.Burmeister E, Aitken LM. Sample size: how many is enough? Aust Crit Care. 2012;25:271–274. doi: 10.1016/j.aucc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 16.EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) Scientific opinion on the safety of chromium picolinate as a source of chromium added for nutritional purposes to foodstuff for particular nutritional uses and to foods intended for the general population. EFSA J. 2010;8:1883. [Google Scholar]
- 17.Balk EM, Tatsioni A, Lichtenstein AH, Lau J, Pittas AG. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30:2154–2163. doi: 10.2337/dc06-0996. [DOI] [PubMed] [Google Scholar]
- 18.Katsuki A, Sumida Y, Gabazza EC, Murashima S, Furuta M, Araki-Sasaki R, Hori Y, Yano Y, Adachi Y. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care. 2001;24:362–365. doi: 10.2337/diacare.24.2.362. [DOI] [PubMed] [Google Scholar]
- 19.Guimarães MM, Carvalho AC, Silva MS. Effect of chromium supplementation on the glucose homeostasis and anthropometry of type 2 diabetic patients: double blind, randomized clinical trial: chromium, glucose homeostasis and anthropometry. J Trace Elem Med Biol. 2016;36:65–72. doi: 10.1016/j.jtemb.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Tian H, Guo X, Wang X, He Z, Sun R, Ge S, Zhang Z. Chromium picolinate supplementation for overweight or obese adults. Cochrane Database Syst Rev. 2013;(11):CD010063. doi: 10.1002/14651858.CD010063.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin RV, Phung OJ. Effect of chromium supplementation on glycated hemoglobin and fasting plasma glucose in patients with diabetes mellitus. Nutr J. 2015;14:14. doi: 10.1186/1475-2891-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan GJ, Wanko NS, Redman AR, Cook CB. Chromium as adjunctive treatment for type 2 diabetes. Ann Pharmacother. 2003;37:876–885. doi: 10.1345/aph.1C304. [DOI] [PubMed] [Google Scholar]
- 23.Cefalu WT, Rood J, Pinsonat P, Qin J, Sereda O, Levitan L, Anderson RA, Zhang XH, Martin JM, Martin CK, Wang ZQ, Newcomer B. Characterization of the metabolic and physiologic response to chromium supplementation in subjects with type 2 diabetes mellitus. Metabolism. 2010;59:755–762. doi: 10.1016/j.metabol.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broadhurst CL, Domenico P. Clinical studies on chromium picolinate supplementation in diabetes mellitus--a review. Diabetes Technol Ther. 2006;8:677–687. doi: 10.1089/dia.2006.8.677. [DOI] [PubMed] [Google Scholar]
- 25.Lewicki S, Zdanowski R, Krzyżowska M, Lewicka A, Dębski B, Niemcewicz M, Goniewicz M. The role of Chromium III in the organism and its possible use in diabetes and obesity treatment. Ann Agric Environ Med. 2014;21:331–335. doi: 10.5604/1232-1966.1108599. [DOI] [PubMed] [Google Scholar]
- 26.Vincent JB. Chromium: celebrating 50 years as an essential element? Dalton Trans. 2010;39:3787–3794. doi: 10.1039/b920480f. [DOI] [PubMed] [Google Scholar]
- 27.Wiernsperger N, Rapin J. Trace elements in glucometabolic disorders: an update. Diabetol Metab Syndr. 2010;2:70. doi: 10.1186/1758-5996-2-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X, Palanichamy K, Ontko AC, Rao MN, Fang CX, Ren J, Sreejayan N. A newly synthetic chromium complex--chromium(phenylalanine)3 improves insulin responsiveness and reduces whole body glucose tolerance. FEBS Lett. 2005;579:1458–1464. doi: 10.1016/j.febslet.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 29.Trumbo PR, Ellwood KC. Chromium picolinate intake and risk of type 2 diabetes: an evidence-based review by the United States Food and Drug Administration. Nutr Rev. 2006;64:357–363. doi: 10.1111/j.1753-4887.2006.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 30.Martin J, Wang ZQ, Zhang XH, Wachtel D, Volaufova J, Matthews DE, Cefalu WT. Chromium picolinate supplementation attenuates body weight gain and increases insulin sensitivity in subjects with type 2 diabetes. Diabetes Care. 2006;29:1826–1832. doi: 10.2337/dc06-0254. [DOI] [PubMed] [Google Scholar]
- 31.Komorowski J, Juturu V. Chromium supplementation does not improve glucose tolerance, insulin sensitivity, or lipid profile: a randomized, placebo-controlled, double-blind trial of supplementation in subjects with impaired glucose tolerance: response to Gunton et al. Diabetes Care. 2005;28:1841–1842. doi: 10.2337/diacare.28.7.1841-a. [DOI] [PubMed] [Google Scholar]
- 32.Althuis MD, Jordan NE, Ludington EA, Wittes JT. Glucose and insulin responses to dietary chromium supplements: a meta-analysis. Am J Clin Nutr. 2002;76:148–155. doi: 10.1093/ajcn/76.1.148. [DOI] [PubMed] [Google Scholar]
- 33.Anderson RA. Chromium and polyphenols from cinnamon improve insulin sensitivity. Proc Nutr Soc. 2008;67:48–53. doi: 10.1017/S0029665108006010. [DOI] [PubMed] [Google Scholar]
- 34.Bahijiri SM, Mira SA, Mufti AM, Ajabnoor MA. The effects of inorganic chromium and brewer's yeast supplementation on glucose tolerance, serum lipids and drug dosage in individuals with type 2 diabetes. Saudi Med J. 2000;21:831–837. [PubMed] [Google Scholar]
- 35.Iqbal N, Cardillo S, Volger S, Bloedon LT, Anderson RA, Boston R, Szapary PO. Chromium picolinate does not improve key features of metabolic syndrome in obese nondiabetic adults. Metab Syndr Relat Disord. 2009;7:143–150. doi: 10.1089/met.2008.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Król E, Krejpcio Z, Byks H, Bogdański P, Pupek-Musialik D. Effects of chromium brewer's yeast supplementation on body mass, blood carbohydrates, and lipids and minerals in type 2 diabetic patients. Biol Trace Elem Res. 2011;143:726–737. doi: 10.1007/s12011-010-8917-5. [DOI] [PubMed] [Google Scholar]
- 37.Badran M, Morsy R, Soliman H, Elnimr T. Assessment of trace elements levels in patients with type 2 diabetes using multivariate statistical analysis. J Trace Elem Med Biol. 2016;33:114–119. doi: 10.1016/j.jtemb.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Gibson RS. Principles of nutritional assessment. Oxford: Oxford University Press; 2005. [Google Scholar]
- 39.Chen S, Jin X, Shan Z, Li S, Yin J, Sun T, Luo C, Yang W, Yao P, Yu K, Zhang Y, Cheng Q, Cheng J, Bao W, Liu L. Inverse association of plasma chromium levels with newly diagnosed type 2 diabetes: A case-control study. Nutrients. 2017;9:294. doi: 10.3390/nu9030294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basaki M, Saeb M, Nazifi S, Shamsaei HA. Zinc, copper, iron, and chromium concentrations in young patients with type 2 diabetes mellitus. Biol Trace Elem Res. 2012;148:161–164. doi: 10.1007/s12011-012-9360-6. [DOI] [PubMed] [Google Scholar]
- 41.Bailey CH. Improved meta-analytic methods show no effect of chromium supplements on fasting glucose. Biol Trace Elem Res. 2014;157:1–8. doi: 10.1007/s12011-013-9863-9. [DOI] [PubMed] [Google Scholar]
- 42.Kozlovsky AS, Moser PB, Reiser S, Anderson RA. Effects of diets high in simple sugars on urinary chromium losses. Metabolism. 1986;35:515–518. doi: 10.1016/0026-0495(86)90007-7. [DOI] [PubMed] [Google Scholar]