Abstract
Dapagliflozin is a selective sodium-glucose cotransporter 2 (SGLT2) inhibitor; it reduces glucose reabsorption via the kidney and increases the glucose excretion in urine. This inhibitor functions through a unique insulin-independent mechanism, and is therefore a potential new approach for the treatment of hyperglycemia in patients with diabetes. In this study, we evaluated the effectiveness of the SGLT2 inhibitor, dapagliflozin, by using a rat model of type 1 diabetes. Type 1 diabetes was induced by a single intraperitoneal injection of 60 mg/kg streptozotocin (STZ). The STZ-induced rats showed marked hyperglycemia and other metabolic abnormalities. We clarified the hypoglycemic effect of the combination treatment of dapagliflozin with a low dose of insulin compared with dapagliflozin alone and insulin alone in 3-week and 8-week studies. Our results showed that dapagliflozin in combination with a low dose of insulin significantly lowered hyperglycemia, hypercholesterolemia, and hypertriglyceridemia. Furthermore, the antioxidant status and body weight were improved. In contrast, treatment with dapagliflozin alone did not improve the blood glucose levels, lipid profile, antioxidant status, or body weight. These findings suggested that in type 1 diabetes, dapagliflozin was effective in combination with a low dose of insulin; however, the administration of dapagliflozin alone did not achieve a significant effect.
Keywords: dapagliflozin, insulin, type 1 diabetes
Diabetes mellitus (DM) is a group of metabolic diseases characterized by increased blood glucose levels (hyperglycemia). This abnormal elevation in blood glucose occurs as a result of defects in insulin secretion, insulin activity, or both [22]. Type 1 DM results from the autoimmune destruction of pancreatic β-cells, which leads to insulin deficiency, a defect that may cause hyperglycemia, polydipsia, polyuria, polyphagia, ketoacidosis, and other abnormalities [5, 8].
Experimentally, type 1 DM can be induced by the injection of streptozotocin (STZ), which exerts selective toxicity to pancreatic β-cells and therefore induces diabetes [35]. Rats injected with STZ show a marked decrease in pancreatic insulin content and hyperglycemia; thus, they are a suitable model for insulin-dependent diabetes (Type 1) [38].
Type 1 DM appears to be the most common form of canine diabetes [31], and insulin injections are commonly used as an effective treatment for long-term glycemic control, as in the case of humans [12]. However, insulin therapy significantly increases the risk of hypoglycemia, which may be life-threatening [6].
Recently, glucose reabsorption by the renal proximal tubule has been shown to play an essential role in glucose homeostasis [24]. Glucose reabsorption from the glomerular filtrate into the renal tubular epithelial cells is mediated by sodium glucose co-transporters (SGLT) [30]. These co-transporters comprise six isoforms that are specialized in the cotransport of sodium and glucose across different cell types [28, 41]; the two major isoforms are SGLT1 and SGLT2 [28]. Approximately 90% of the filtered glucose is reabsorbed through SGLT2, a low-affinity, high-capacity transporter located predominantly in the S1 segment of the renal proximal tubule, and the remainder is reabsorbed through SGLT1, a high-affinity, low-capacity transporter located in the S2 and S3 segments [16, 30, 40]. Therefore, SGLT2 plays an important role in renal glucose reabsorption [2]. This provides the basis for the clinical development of SGLT2 inhibitors, which inhibit glucose reabsorption, and results in the induction of urinary glucose excretion followed by reduced plasma glucose levels [15]. As SGLT2 inhibitors have an insulin-independent mechanism, this class of compounds may be of benefit as an adjunctive therapy in patients whose pancreatic function is diminished, or in patients who have insulin resistance. Therefore, treatment with SGLT2 inhibitors may be appropriate in all stages of type 2 DM [17]. Previous studies have shown that this class of drugs may also be useful in the treatment of type 1 DM [13, 42]. Dapagliflozin is a selective SGLT2 inhibitor. Our study examined whether the combination of dapagliflozin with a low dose of insulin could be effective in the treatment of diabetes in a rat model of type 1 diabetes. We evaluated the effect of treatment mainly through evaluation of hyperglycemia, hyperlipidemia, and antioxidant status.
MATERIALS AND METHODS
Materials
Streptozotocin was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Insulin (humulin N) was purchased from Eli Lilly & Co. (Tokyo, Japan). For administration, insulin was diluted with physiological saline and administered subcutaneously. Dapagliflozin was purchased from Cayman Chemical Co. (Ann Arbor, MI, USA). To prepare the stock solution, dapagliflozin was dissolved in ethanol, and then stored at −20°C. Prior to use, dapagliflozin was diluted in phosphate buffer saline and administered orally.
Animals
Forty 7-week-old male Sprague-Dawley rats were purchased from Sankyo Labo Service Corp., Inc. (Tokyo, Japan). The rats were subjected to a 1-week adaptation period prior to the start of the experiment. The rats were housed in stainless steel cages at a controlled temperature of 21 ± 1°C with a 12-hr light/dark cycle. The rats were provided with a standard commercial diet from Sankyo Labo Service Corporation, Inc. and given ad libitum access to water. All animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals, and as specified in the experimental protocol approved by the Ethics Committee of the Faculty of Agriculture, Tokyo University of Agriculture and Technology (approval number 28-37).
Induction of diabetes
Streptozotocin (STZ) was dissolved in 0.1 M citrate buffer (pH=4.5). The rats were pretreated with a single intraperitoneal injection of 60 mg/kg STZ to induce experimental diabetes. The manifestations of diabetes mellitus were confirmed through the measurement of blood glucose level at 72 hr following STZ injection.
Experimental design
After confirmation of the induction of diabetes in the STZ-injected rats, the animals were divided into groups and subjected to the following treatments for 3 and 8 weeks:
Group 1 (n=4), control animals; group 2 (n=4), diabetic untreated animals; group 3 (n=4), diabetic animals that received dapagliflozin at a dose of 0.1 mg/kg dose orally once per day [14]; group 4 (n=4), diabetic animals that received insulin treatment. Insulin doses were individually adjusted to maintain normoglycemic states; thus, insulin doses varied between 3 and 5 U/rat and were administered subcutaneously once per day. In a preliminary study, a dose of 2 U/rat of insulin alone did not sufficiently lower blood glucose. In group 5 (n=4), diabetic animals received a combination treatment of 0.1 mg/kg dapagliflozin orally once per day and insulin at a dose of 1.5–2.5 U/rat dose, which was administered subcutaneously once daily (half the insulin dose used in group 4).
Blood glucose levels and body weight were measured once per week. The animals in the 3- and the 8-week studies were sacrificed after 3 and 8 weeks of treatment, respectively. At the end of each study, the animals were anesthetized with isoflurane. The blood samples were collected from the abdominal vena cava and the tissues were removed after opening the abdomen. The blood samples were then centrifuged at 3,000 × g for 10 min at 4°C. The plasma was extracted and stored at −80°C prior to lipid profile analysis. The liver samples were stored at −80°C until analysis in the antioxidant assay. In the 8-week study only, tissues (pancreas, kidney and parts of the liver) from each animal were immediately fixed in 10% phosphate buffered formalin for histopathological examination.
Blood glucose measurement
The blood glucose levels were measured from a drop of blood obtained by tail vein puncture using Glutest Neo Alpha and a Glutest New Sensor purchased from Sanwa Kagaku Kenkyusho Co., Ltd. (Nagoya, Japan).
Biochemical analysis
Plasma triglyceride and total cholesterol levels were analyzed by using a Fuji Dri-Chem 7000 (Fujifilm Corp., Tokyo, Japan).
Liver antioxidants
Briefly, for the glutathione (GSH) assay, parts of the liver were homogenized in cold phosphate buffer, and centrifuged at 10,000 × g for 15 min at 4°C. The supernatants were deproteinated prior to use in the GSH assay kit (Cayman Chemical Co.). In the superoxide dismutase (SOD) assay, the liver tissue was homogenized in cold sucrose buffer (0.25 M sucrose, 10 mM Tris, 1 mM EDTA, pH 7.4), and centrifuged at 10,000 × g for 60 min at 4°C. The supernatants were subsequently used in the SOD assay kit (Dojindo Molecular Technologies, Japan).
Histopathology
Tissues from the liver, kidney, and pancreas were surgically removed from anesthetized animals, and immediately fixed in 10% neutral buffered formalin after 8-week experiments. The samples were embedded in paraffin blocks, and sliced into 5-µm-thick sections. The sections were stained with hematoxylin and eosin, and periodic acid-Schiff (PAS), and subsequently examined under a light microscope.
Statistical analysis
All data were presented as the mean ± standard deviation (SD). Analysis of variance (ANOVA) with Tukey’s post hoc multiple comparison test was used for the statistical analysis of the data. P values of less than 0.05 were considered significant. Statistical analysis was computed by using GraphPad Prism 7 software.
RESULTS
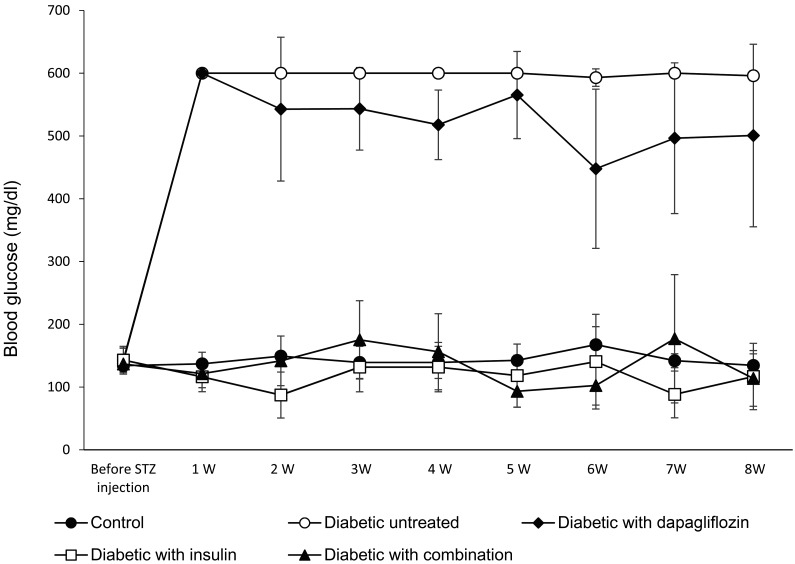
In a preliminary study in which 2 U/rat of insulin alone was administered to diabetic rats, one of the diabetic rats was able to normalize blood glucose (132 mg/dl) and seven rats were unable to lower their blood glucose level (569 ± 40.9 mg/dl). As shown in Fig. 1, the animals in the untreated diabetic group in the 8-week study developed severe hyperglycemia. Although the oral administration of dapagliflozin alone did not significantly decrease the blood glucose levels, the levels were significantly decreased in the high-dose insulin and the combination-treated groups. In the 3-week study (data shown in the Supplementary Fig. 1), the blood glucose levels were similar to the first 3 weeks of the 8-week study.
Fig. 1.
Weekly blood glucose measurements to evaluate the hypoglycemic effect of treatment with dapagliflozin (0.1 mg/kg) alone, insulin (3–5 U/rat) alone, or a combination of dapagliflozin (0.1 mg/kg) and insulin (1.5–2.5 U/rat) in the 8-week study. The values are expressed as the mean ± standard deviation.
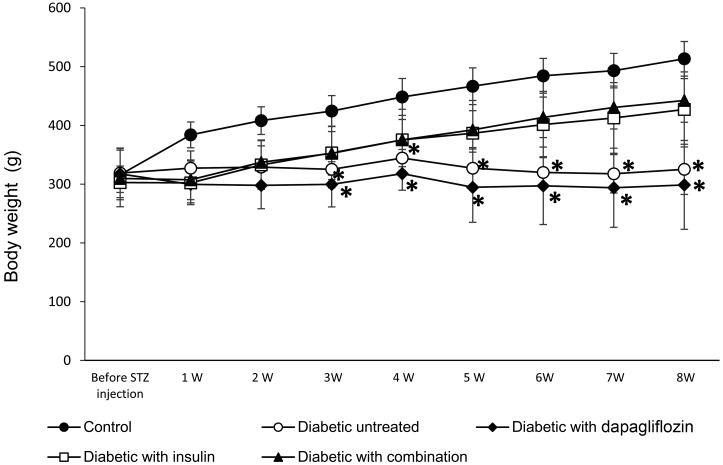
In the 8-week study, the body weight of animals in the untreated diabetic group and the dapagliflozin-treated group was not significantly increased compared with the normal growth observed in the control group (Fig. 2). In contrast, the body weight in groups treated with insulin alone and in combination with dapagliflozin was increased from the second week of treatment, in a comparable manner the control group. In the 3-week study (data shown in the Supplementary Fig. 2), the changes in body weight were similar to those in the first 3 weeks of the 8-week study.
Fig. 2.
Weekly body weight measurements to evaluate the hypoglycemic effect of treatment with dapagliflozin (0.1 mg/kg) alone, insulin (3–5 U/rat) alone, or a combination of dapagliflozin (0.1 mg/kg) and insulin (1.5–2.5 U/rat) for the 8-week study. The values are expressed as the mean ± standard deviation. *P<0.05 versus the control group.
In the 3-week study, the plasma total cholesterol levels were significantly increased in the untreated diabetic group compared with the control group. In the dapagliflozin-treated and the insulin-treated groups, the cholesterol levels decreased, but these changes were not significant. However, the combination treatment significantly decreased the plasma cholesterol. In the 8-week study, the total cholesterol levels were increased significantly in the untreated diabetic group. Although the oral administration of dapagliflozin alone did not significantly decrease the plasma cholesterol levels, it was significantly reduced in diabetic rats treated with insulin alone or in combination with dapagliflozin (Table 1).
Table 1. Plasma total cholesterol, plasma triglyceride, liver glutathione and liver superoxide dismutase activities at the end of the 3-week and 8-week studies.
| Control | Diabetic untreated | Diabetic with dapagliflozin | Diabetic with insulin | Diabetic with combination | ||
|---|---|---|---|---|---|---|
| Plasma total cholesterol (mg/dl) | 3-week | 67.0 ± 7.8 | 116.5 ± 34.3a) | 81.3 ± 16.7 | 81.5 ± 7.9 | 75.6 ± 4.0b) |
| 8-week | 60.8 ± 5.7 | 104.0 ± 13.5a) | 95.0 ± 25.3 | 62.0 ± 14.5b) | 58.0 ± 13.3b,c) | |
| Plasma triglyceride (mg/dl) | 3-week | 141.8 ± 32.5 | 643.3 ± 98.2a) | 569.7 ± 186.8a) | 278.5 ± 233.2b) | 151.3 ± 67.6b,c) |
| 8-week | 152.5 ± 31.8 | 453.0 ± 192.6 | 531.5 ± 196.4a) | 283.5 ± 162.5 | 92.5 ± 47.3b,c) | |
| Liver glutathione (nmol/mg protein) | 3-week | 15.8 ± 2.6 | 5.0 ± 1.4a) | 9.3 ± 2.1a) | 10.8 ± 1.9a,b) | 12.3 ± 1.9b) |
| 8-week | 24.3 ± 1.9 | 8.0 ± 2.2a) | 9.5 ± 5.9a) | 21.8 ± 4.6b,c) | 19.4 ± 4.8b,c) | |
| Liver superoxide dismutase activities (U/mg protein) | 3-week | 617.8 ± 130.9 | 386.3 ± 61.5a) | 337.3 ± 107.5a) | 326.8 ± 88.2a) | 370.3 ± 64.4b,c) |
| 8-week | 556.3 ± 85.3 | 389.5 ± 70.1 | 368.7 ± 69.1 | 642.8 ± 150.0b,c) | 706.3 ± 120.1b,c) |
The values are expressed as mean ± standard deviation. a) P<0.05 versus control group, b) P<0.05 versus diabetic untreated group, c) P<0.05 versus diabetic treated with dapagliflozin group.
In the 3-week study, the plasma triglyceride levels were significantly increased in the untreated diabetic group and the dapagliflozin-treated group compared with the control group. However, treatment with insulin alone and in combination with dapagliflozin significantly decreased triglyceride levels. In the 8-week study, the untreated diabetic group exhibited increases in plasma triglyceride levels, but these were not significant. The diabetic group treated with dapagliflozin showed significant increase in triglyceride levels. In the insulin-treated group, the triglyceride levels decreased, but these changes were not significant. In contrast, the triglyceride levels were significantly decreased in diabetic rats that received the combination treatment (Table 1).
In the 3-week study, the liver GSH levels significantly decreased in the untreated diabetic group and the dapagliflozin-treated group compared with the control group. The insulin-treated group also exhibited a significant decrease in GSH compared with the control group, but a significant increase compared with the diabetic untreated group. The GSH levels were significantly increased in the combination-treated group. In the 8-week study, GSH levels were significantly decreased in both the untreated diabetic group and the dapagliflozin-treated diabetic group; however, the insulin- and the combination-treated groups showed a significant increase (Table 1).
In the 3-week study, the SOD activities in the liver were significantly decreased in the untreated diabetic group and three of treated groups. In the 8-week study, the liver SOD activities were decreased in the untreated diabetic group and the dapagliflozin-treated diabetic group, but were not significantly different to the control group. However, the insulin-treated group and the combination-treated group showed a significant increase compared with the untreated diabetic group and the dapagliflozin-treated diabetic group (Table 1).
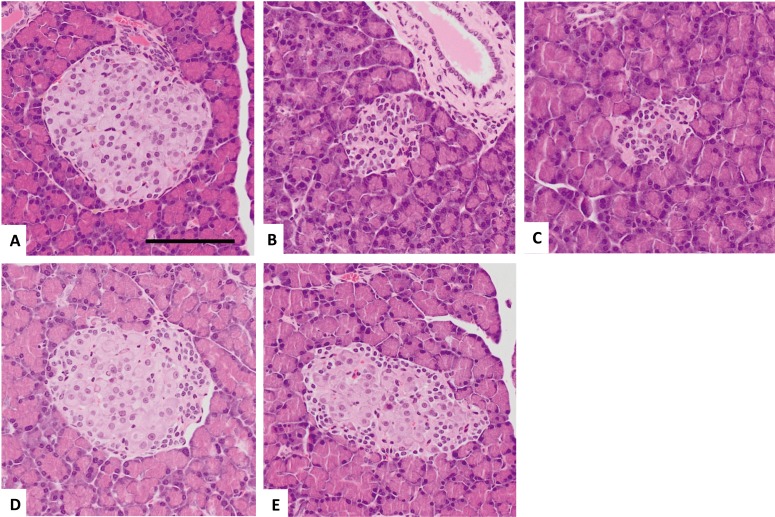
In the pancreas, the control animals showed several normal islets of Langerhans, which were normally scattered in the acinar glands. The cells in these islets of Langerhans had a homogenous, eosinophilic cytoplasm with round-to-oval nuclei containing fine chromatin. The diabetic animals had few islets of Langerhans, and these were small in size, and contained atrophic islet cells with a scant cytoplasm and condensed nucleus. These islets of Langerhans were similar in appearance to those in the dapagliflozin-treated group. The size of the islets of Langerhans in the insulin- and the combination-treated groups had nearly returned to normal; however, several cells in the periphery were still atrophic with a relatively scant cytoplasm and condensed nucleus. In the center of the islets, the cells appeared to have a normal eosinophilic cytoplasm with round or oval nuclei containing fine chromatin. There were no pathological changes in the acinar or ductal cells (Fig. 3).
Fig. 3.
Representative images of histopathological findings in the pancreas in the control group (A), diabetic untreated group (B), dapagliflozin (0.1 mg/kg)-treated group (C), high-dose insulin (3–5 U/rat) group (D), combined treatment of insulin (1.5–2.5 U/rat) and dapagliflozin (0.1 mg/kg) (E). (A) A normal islet of Langerhans. (B, C) An atrophic islet of Langerhans. (D, E) A normal-sized islet of Langerhans. Note the swollen cells in the center and small, densely packed cells in the periphery. H&E stain. Scale bar=100 µm.
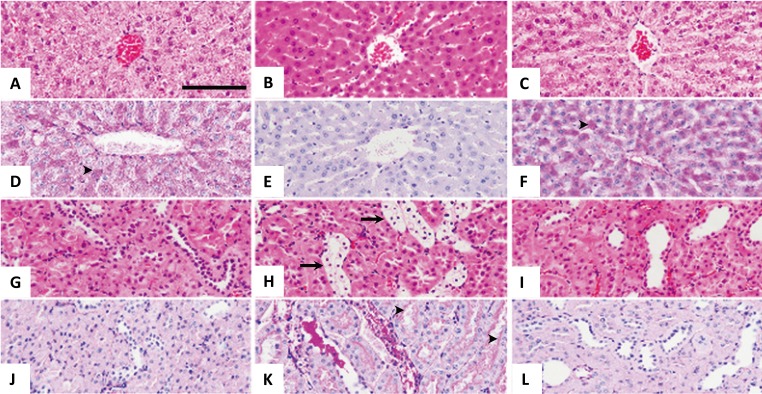
In the liver, the hepatocytes of control animals had normal lobular structures, which had a granular or vacuolated eosinophilic cytoplasm with round or oval nuclei containing fine chromatin. The diabetic animals showed a homogenous, eosinophilic cytoplasm with a relatively condensed nucleus. Similar changes in the hepatocyte appearance were observed in the dapagliflozin-treated group (not included in Fig. 4). After treatment with insulin or the combination of insulin and dapagliflozin, the hepatocytes became normal in appearance with a granular or vacuolated eosinophilic cytoplasm with a round to oval nucleus containing fine chromatin. There were glycogen deposits in the hepatocytes of the control animals (Fig. 4D) and in those of animals that were treated with a combination of insulin and dapagliflozin (Fig. 4F). Similar glycogen deposits in the hepatocyte appearance were observed in the group treated with insulin alone (not shown in Fig. 4). Clear deposits of glycogen were not detected in the hepatocytes of the diabetic animals (Fig. 4E). In addition, clear deposits of glycogen were not detected in the hepatocytes treated with dapagliflozin (not included in Fig. 4).
Fig. 4.
Representative images of histopathological findings in the liver (A–F), and kidney (G–L) in the control group (A, D, G, J), diabetic untreated group (B, E, H, K), and combined treatment group, of low-dose insulin (1.5–2.5 U/rat) and dapagliflozin (0.1 mg/kg) (C, F, I, L). (A) Normal hepatocytes with a granular or pale cytoplasm in the centrilobular region. (B) Hepatocytes with a homogenous eosinophilic cytoplasm in the centrilobular region. (C) Normal hepatocytes with a granular or pale cytoplasm in the centrilobular region. (D) Glycogen deposition in the hepatocytes, as indicated by the arrowheads. (E) No glycogen deposition in the hepatocytes. (F) Glycogen deposition in the hepatocytes, as indicated by the arrow heads. (G) Normal proximal and distal tubules. (H) Renal epithelial cells with a clear cytoplasm in the distal tubules, as indicated by the arrow. (I) Normal proximal and distal tubules. (J) No glycogen deposition in the epithelial cells of distal tubules. (K) Glycogen deposition in the epithelial cells of distal tubules, as indicated by the arrows. (L) No glycogen deposition in the epithelial cells of distal tubules. (A–C, G–I) H&E stain; (D–F, J–L) PAS reaction. Scale bar=100 µm.
In the kidney, control animals had normal nephrons that contained glomeruli and proximal and distal renal tubules. The cells of the proximal renal tubules had a round or oval nucleus and a homogenous, eosinophilic, cuboidal cytoplasm with prominent brush borders. In contrast, the cells of the distal renal tubules had a relatively thin, weakly eosinophilic cytoplasm with no prominent brush borders, but had a round or oval nucleus. The intraluminal spaces in the distal renal tubules were wider than those in the proximal renal tubules. In the diabetic animals, the cells in the distal renal tubules that were swollen had a clear cytoplasm, prominent cell boundaries, and a condensed nucleus (Fig. 4H). Similar morphologies were observed for cells in the renal tubules in the dapagliflozin-treated group (not included in Fig. 4). Glycogen deposits were observed in the swollen cells of diabetic animals (Fig. 4K). Similar glycogen deposits were observed in the dapagliflozin-treated group (not shown in Fig. 4). With the combination treatment, the proximal renal tubules became normal in appearance with a round or oval nucleus, and a homogenous, eosinophilic, cuboidal cytoplasm with prominent brush borders, similar to the control animals (Fig. 4L). Similar morphologies were observed for cells in the renal tubules in the insulin-treated group (not included in Fig. 4).
DISCUSSION
The marked increase in the incidence of DM requires new therapies to target this chronically devastating disease and its complications [17]. Hyperglycemia damages the blood vessels and nerves; in turn, this develops into major diabetic complications. However, intensive treatment for hyperglycemia is known to lower the risks of these complications in patients with diabetes [42]. Among the various anti-diabetic drugs, SGLT2 inhibitors have a unique mechanism of action. These drugs increase urinary glucose excretion, thereby lowering the blood glucose concentration [37]. Currently, SGLT2 inhibitors are the focus of interest for a possible therapy for DM. In our study, dapagliflozin was used in combination with a low dose of insulin for the treatment of type 1 DM. This combination may help to decrease the potential side effects associated with insulin treatment alone, such as hypoglycemia. We also compared the effect of this combination treatment with the effects of each drug alone.
Insulin is the most common and effective treatment for type 1 diabetes. The effect of insulin on glycemia occurs through cellular glucose uptake mediated by glucose transporter type 4 (GLUT4) [19], and the inhibition of glycogenolysis and gluconeogenesis [9]. Our results showed that treatment with 1.5–2.5 U of insulin in combination with dapagliflozin significantly decreased the blood glucose level, to a similar level as treatment with 3–5 U of insulin alone. These results were comparable with those in a previous study [23], that showed that, in STZ-treated rats, the combination of low insulin dose with empagliflozin decreased blood glucose levels to a level similar to that of monotherapy with a full dose of insulin, suggesting that empagliflozin has an insulin-sparing effect. Furthermore, treatment using empagliflozin alone decreased the blood glucose levels in the type 1 DM animal model. However, our results showed that treatment with dapagliflozin alone did not significantly decrease the levels of blood glucose (Fig. 1).
Body weight was significantly lower in untreated diabetic rats compared with the control rats. The lower body weight in type 1 DM indicates the catabolic state of poorly controlled glycemia. Under these conditions, the metabolic processes, lipolysis, and oxidative degradation of amino acids are increased; they degrade the greatest energy and tissue reserves in the animal, thereby decreasing the body weight [27]. The body weight was not improved by dapagliflozin treatment, which may be attributable to the continuous catabolic state. In the insulin- and the combination-treated groups, body weight was significantly improved and comparable with the control group, perhaps owing to an improvement in the catabolic state as an outcome of the proper glycemic control in these animals (Fig. 2).
Abnormalities in the lipid profile are one of the most common complications of DM [21]. Insulin not only plays an important role in glucose metabolism, but also in lipid metabolism. Therefore, insulin deficiency is closely correlated with abnormalities of lipid metabolism in type 1 diabetes [39]. In this study, insulin treatment improved the triglyceride levels in both studies; however, insulin only reduced hypercholesterolemia in the 8-week study. Our results agreed with another study [11] that stated that the effective insulin treatment improved the plasma lipid profile; however, the non-significant decrease in the triglyceride levels in the 8-week study could be attributable to the variations in the values within the insulin-treated group. The administration of insulin in combination with dapagliflozin reduced hypertriglyceridemia and hypercholesterolemia in both studies and achieved similar or possibly better results than the insulin treatment alone (Table 1). These results suggested that the administration of dapagliflozin in combination with a low dose of insulin could reduce hyperlipidemia, which is a better effect than treatment with a full dose of insulin alone. Hence, this treatment may reduce the risk of diabetic complications.
Chronic hyperglycemia produces reactive oxygen species (ROS) through various mechanisms, including the advanced glycation end-product formation, the polyol pathway, glucose auto-oxidation, and overproduction of mitochondrial superoxides [1]. These mechanisms negatively impact the ability of the antioxidant defense system to correct and balance the ROS overproduction [34]. The combination of the increased production of ROS and decreased capacity of the cellular antioxidant defense system results in diabetic oxidative stress [37]. Thus, the reduction of hyperglycemia may be a helpful measure to decrease oxidative stress [29].
Glutathione, a major endogenous antioxidant, ameliorates free radical-mediated damage. Studies have shown that the tissue GSH concentrations in STZ-induced diabetic rats were significantly lower than those in the control rats [10]. The decreased GSH levels in diabetic rats may be due to its increased consumption that is required to relieve oxidative stress [4]. This is consistent with our results that GSH levels were significantly decreased in the diabetic untreated group and the dapagliflozin-treated group. In addition, the depletion of hepatic GSH was corrected with insulin treatment; this finding was consistent with those of previous results [20]. In the combination-treated group, the GSH levels were similar to the insulin-treated group (Table 1).
Superoxide dismutase is an important defense enzyme that catalyzes the dismutation of superoxide radicals to produce hydrogen peroxide and molecular oxygen [25], subsequently reducing the toxic effects caused by their radicals [34]. It was reported that in uncontrolled diabetes, the activity of superoxide dismutase was decreased [7] as a result of SOD glycation [18]. In this study, the SOD activity was low in the diabetic untreated group and the three treated groups. The SOD activity was significantly improved after 8 weeks of treatment with insulin alone and insulin in combination with dapagliflozin (Table 1). Our results suggest that the combination of dapagliflozin with a low dose of insulin significantly improved the antioxidant status in diabetic rats, with an effect that was similar to a full dose of insulin alone. This improvement may be due to the antihyperglycemic effect of the treatment, which reduces the oxidative stress burden.
In the pancreas of the untreated diabetic rats and dapagliflozin-treated rats, the islets of Langerhans were noticeably smaller than those of the control rats. However, insulin treatment and the combined insulin and dapagliflozin treatment improved the size of the islets of Langerhans (Fig. 3D or 3E). Our results were consistent with those of a previous study, in which at 8 weeks after STZ injection, the islet size was significantly decreased compared with the control rats, and insulin treatment improved the islet size in diabetic rats [26]. However, there were swollen cells in the center and small, densely packed cells in the periphery of normal-sized islets in the rats treated with insulin or the combination of insulin and dapagliflozin (Fig. 3E). These findings implied that insulin secretion has not functionally improved in normal-sized islets over the 8-week experiment. However, blood glucose levels were controlled for up to 8 weeks by the same dose of insulin and dapagliflozin (Fig. 1) and hypoglycemia was not seen in any treated rats. Therefore, further studies are needed to confirm the effect of the combination treatment of insulin and dapagliflozin on the islets of Langerhans.
The liver of the untreated diabetic rats and dapagliflozin-treated rats showed glycogen depletion in the hepatocytes. These changes were confirmed by the PAS reaction, which is specific to glycogen. The decrease in hepatic glycogen content in diabetes is due to the lack of insulin in the diabetic state, which results in the inactivation of the glycogen synthase systems [36]. However, in the insulin- and combination-treated groups, this glycogen depletion was reversed, and was most likely caused by the re-activation of the glycogen synthase systems in a normal insulin-controlled state (Fig. 4).
The kidneys of the untreated diabetic rats and the dapagliflozin-treated rats showed glycogen deposits in the distal convoluted tubules. Our results are consistent with those of a previous study [33] that observed that prolonged hyperglycemia was the only driving force for glycogen accumulation in the renal tubules. As diabetes is prolonged, more glycogen accumulates and spreads into the renal tubules. However, it is not yet clear whether glycogen accumulation in the renal tubules is an inevitable change in the diabetic condition in humans, as an early phase in pathogenesis that will contribute to the end-stage diabetic nephropathy that is always associated with the end-stage nephropathy, or if it has a role in inducing a pathway that leads to the pathophysiological changes observed in diabetic nephropathy [3, 32]. These renal changes were reversed in the insulin- and combination-treated groups, as shown by the glycogen deposits in the liver tissues (Fig. 4).
Our results suggested that in type 1 DM, the combination treatment of dapagliflozin with a low dose of insulin was able to correct hyperglycemia, which, in turn, improved the lipid profile and antioxidant status. These effects may help to reduce the risk of diabetic complications and hypoglycemia. Furthermore, the effect of this combination treatment appears to be more potent than that of dapagliflozin treatment alone; however, further investigations are required. In conclusion, the combination of dapagliflozin with a low dose of insulin is a potential treatment for patients with type 1 DM.
Supplementary Material
REFERENCES
- 1.Baskol G., Baskol M., Kocer D.2007. Oxidative stress and antioxidant defenses in serum of patients with non-alcoholic steatohepatitis. Clin. Biochem. 40: 776–780. doi: 10.1016/j.clinbiochem.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 2.Blondel O., Bailbe D., Portha B.1990. Insulin resistance in rats with non-insulin-dependent diabetes induced by neonatal (5 days) streptozotocin: evidence for reversal following phlorizin treatment. Metabolism 39: 787–793. doi: 10.1016/0026-0495(90)90120-2 [DOI] [PubMed] [Google Scholar]
- 3.Caramori M. L., Mauer M.2003. Diabetes and nephropathy. Curr. Opin. Nephrol. Hypertens. 12: 273–282. doi: 10.1097/00041552-200305000-00008 [DOI] [PubMed] [Google Scholar]
- 4.Coskun O., Kanter M., Korkmaz A., Oter S.2005. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol. Res. 51: 117–123. doi: 10.1016/j.phrs.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 5.Cryer P. E.2004. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N. Engl. J. Med. 350: 2272–2279. doi: 10.1056/NEJMra031354 [DOI] [PubMed] [Google Scholar]
- 6.Cryer P. E.2009. Preventing hypoglycaemia: what is the appropriate glucose alert value? Diabetologia 52: 35–37. doi: 10.1007/s00125-008-1205-7 [DOI] [PubMed] [Google Scholar]
- 7.Culotta V. C.2000. Superoxide dismutase, oxidative stress, and cell metabolism. Curr. Top. Cell. Regul. 36: 117–132. doi: 10.1016/S0070-2137(01)80005-4 [DOI] [PubMed] [Google Scholar]
- 8.Daneman D.2006. Type 1 diabetes. Lancet 367: 847–858. doi: 10.1016/S0140-6736(06)68341-4 [DOI] [PubMed] [Google Scholar]
- 9.Edgerton D. S., Lautz M., Scott M., Everett C. A., Stettler K. M., Neal D. W., Chu C. A., Cherrington A. D.2006. Insulin’s direct effects on the liver dominate the control of hepatic glucose production. J. Clin. Invest. 116: 521–527. doi: 10.1172/JCI27073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ewis S. A., Abdel-Rahman M. S.1995. Effect of metformin on glutathione and magnesium in normal and streptozotocin-induced diabetic rats. J. Appl. Toxicol. 15: 387–390. doi: 10.1002/jat.2550150508 [DOI] [PubMed] [Google Scholar]
- 11.Feitosa A. C., Feitosa-Filho G. S., Freitas F. R., Wajchenberg B. L., Maranhão R. C.2013. Lipoprotein metabolism in patients with type 1 diabetes under intensive insulin treatment. Lipids Health Dis. 12: 15–22. doi: 10.1186/1476-511X-12-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman E. C., Nelson R. W.2004. Diabetes mellitus. pp. 486–538. In: Canine and Feline Endocrinology and Reproduction, 3rd. ed., W.B. Saunders, Philadelphia. [Google Scholar]
- 13.Fujimori Y., Katsuno K., Ojima K., Nakashima I., Nakano S., Ishikawa-Takemura Y., Kusama H., Isaji M.2009. Sergliflozin etabonate, a selective SGLT2 inhibitor, improves glycemic control in streptozotocin-induced diabetic rats and Zucker fatty rats. Eur. J. Pharmacol. 609: 148–154. doi: 10.1016/j.ejphar.2009.03.007 [DOI] [PubMed] [Google Scholar]
- 14.Han S., Hagan D. L., Taylor J. R., Xin L., Meng W., Biller S. A., Wetterau J. R., Washburn W. N., Whaley J. M.2008. Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes 57: 1723–1729. doi: 10.2337/db07-1472 [DOI] [PubMed] [Google Scholar]
- 15.Handlon A. L.2005. Sodium glucose co-transporter 2 (SGLT2) inhibitors as potential antidiabetic agents. Expert Opin. Ther. Pat. 15: 1531–1540. doi: 10.1517/13543776.15.11.1531 [DOI] [PubMed] [Google Scholar]
- 16.Hediger M. A., Coady M. J., Ikeda T. S., Wright E. M.1987. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. Nature 330: 379–381. doi: 10.1038/330379a0 [DOI] [PubMed] [Google Scholar]
- 17.Kapur A., O’Connor-Semmes R., Hussey E. K., Dobbins R. L., Tao W., Hompesch M., Smith G. A., Polli J. W., James C. D., Jr, Mikoshiba I., Nunez D. J.2013. First human dose-escalation study with remogliflozin etabonate, a selective inhibitor of the sodium-glucose transporter 2 (SGLT2), in healthy subjects and in subjects with type 2 diabetes mellitus. BMC Pharmacol. Toxicol. 14: 26–36. doi: 10.1186/2050-6511-14-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashiwagi A.2001. Complications of diabetes mellitus and oxidative stress. Japan Med. Assoc. J. 44: 521–528. [Google Scholar]
- 19.Kahn B. B.1996. Lilly lecture 1995. Glucose transport: pivotal step in insulin action. Diabetes 45: 1644–1654. doi: 10.2337/diab.45.11.1644 [DOI] [PubMed] [Google Scholar]
- 20.Kim S. K., Woodcroft K. J., Khodadadeh S. S., Novak R. F.2004. Insulin signaling regulates γ-glutamylcysteine ligase catalytic subunit expression in primary cultured rat hepatocytes. J. Pharmacol. Exp. Ther. 311: 99–108. doi: 10.1124/jpet.104.070375 [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni C. R., Joglekar M. M., Patil S. B., Arvindekar A. U.2012. Antihyperglycemic and antihyperlipidemic effect of Santalum album in streptozotocin induced diabetic rats. Pharm. Biol. 50: 360–365. doi: 10.3109/13880209.2011.604677 [DOI] [PubMed] [Google Scholar]
- 22.Loghmani E.2005. Diabetes mellitus: type 1 and type 2. pp. 167–182. In: Guidelines for Adolescent Nutrition Services (Stang, J. and Story, M. eds.), Center for Leadership, Education and Training in Maternal and Child Nutrition, Minneapolis. [Google Scholar]
- 23.Luippold G., Klein T., Mark M., Grempler R.2012. Empagliflozin, a novel potent and selective SGLT-2 inhibitor, improves glycaemic control alone and in combination with insulin in streptozotocin-induced diabetic rats, a model of type 1 diabetes mellitus. Diabetes Obes. Metab. 14: 601–607. doi: 10.1111/j.1463-1326.2012.01569.x [DOI] [PubMed] [Google Scholar]
- 24.Mather A., Pollock C.2011. Glucose handling by the kidney. Kidney Int. Suppl. 79120: S1–S6. doi: 10.1038/ki.2010.509 [DOI] [PubMed] [Google Scholar]
- 25.McCord J. M., Keele B. B., Jr, Fridovich I.1971. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc. Natl. Acad. Sci. USA 68: 1024–1027. doi: 10.1073/pnas.68.5.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novikova L., Smirnova I. V., Rawal S., Dotson A. L., Benedict S. H., Stehno-Bittel L.2013. Variations in rodent models of type 1 diabetes: islet morphology. J. Diabetes Res. 2013: 965832. doi: 10.1155/2013/965832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira G. O., Braga C. P., Fernandes A. A. H.2013. Improvement of biochemical parameters in type 1 diabetic rats after the roots aqueous extract of yacon [Smallanthus sonchifolius (Poepp.& Endl.)] treatment. Food Chem. Toxicol. 59: 256–260. doi: 10.1016/j.fct.2013.05.050 [DOI] [PubMed] [Google Scholar]
- 28.Pajor A. M., Wright E. M.1992. Cloning and functional expression of a mammalian Na+/nucleoside cotransporter. A member of the SGLT family. J. Biol. Chem. 267: 3557–3560. [PubMed] [Google Scholar]
- 29.Park K. S., Kim J. H., Kim M. S., Kim J. M., Kim S. K., Choi J. Y., Chung M. H., Han B., Kim S. Y., Lee H. K.2001. Effects of insulin and antioxidant on plasma 8-hydroxyguanine and tissue 8-hydroxydeoxyguanosine in streptozotocin-induced diabetic rats. Diabetes 50: 2837–2841. doi: 10.2337/diabetes.50.12.2837 [DOI] [PubMed] [Google Scholar]
- 30.Quamme G. A., Freeman H. J.1987. Evidence for a high-affinity sodium-dependent D-glucose transport system in the kidney. Am. J. Physiol. 253: F151–F157. [DOI] [PubMed] [Google Scholar]
- 31.Rand J. S., Fleeman L. M., Farrow H. A., Appleton D. J., Lederer R.2004. Canine and feline diabetes mellitus: nature or nurture? J. Nutr. 134Suppl: 2072S–2080S. doi: 10.1093/jn/134.8.2072S [DOI] [PubMed] [Google Scholar]
- 32.Raptis A. E., Viberti G.2001. Pathogenesis of diabetic nephropathy. Exp. Clin. Endocrinol. Diabetes 109Suppl 2: S424–S437. doi: 10.1055/s-2001-18600 [DOI] [PubMed] [Google Scholar]
- 33.Renno W. M., Abdeen S., Alkhalaf M., Asfar S.2008. Effect of green tea on kidney tubules of diabetic rats. Br. J. Nutr. 100: 652–659. doi: 10.1017/S0007114508911533 [DOI] [PubMed] [Google Scholar]
- 34.Saravanan G., Ponmurugan P.2011. Ameliorative potential of S-allyl cysteine on oxidative stress in STZ induced diabetic rats. Chem. Biol. Interact. 189: 100–106. doi: 10.1016/j.cbi.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 35.Schnedl W. J., Ferber S., Johnson J. H., Newgard C. B.1994. STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes 43: 1326–1333. doi: 10.2337/diab.43.11.1326 [DOI] [PubMed] [Google Scholar]
- 36.Shirwaikar A., Rajendran K., Dinesh Kumar C., Bodla R.2004. Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin-nicotinamide type 2 diabetic rats. J. Ethnopharmacol. 91: 171–175. doi: 10.1016/j.jep.2003.12.017 [DOI] [PubMed] [Google Scholar]
- 37.Son S. M., Whalin M. K., Harrison D. G., Taylor W. R., Griendling K. K.2004. Oxidative stress and diabetic vascular complications. Curr. Diab. Rep. 4: 247–252. doi: 10.1007/s11892-004-0075-8 [DOI] [PubMed] [Google Scholar]
- 38.Tahara A., Matsuyama-Yokono A., Nakano R., Someya Y., Shibasaki M.2008. Hypoglycaemic effects of antidiabetic drugs in streptozotocin-nicotinamide-induced mildly diabetic and streptozotocin-induced severely diabetic rats. Basic Clin. Pharmacol. Toxicol. 103: 560–568. doi: 10.1111/j.1742-7843.2008.00321.x [DOI] [PubMed] [Google Scholar]
- 39.Tahara A., Kurosaki E., Yokono M., Yamajuku D., Kihara R., Hayashizaki Y., Takasu T., Imamura M., Li Q., Tomiyama H., Kobayashi Y., Noda A., Sasamata M., Shibasaki M.2014. Effects of sodium-glucose cotransporter 2 selective inhibitor ipragliflozin on hyperglycaemia, oxidative stress, inflammation and liver injury in streptozotocin-induced type 1 diabetic rats. J. Pharm. Pharmacol. 66: 975–987. doi: 10.1111/jphp.12223 [DOI] [PubMed] [Google Scholar]
- 40.Turner R. J., Moran A.1982. Heterogeneity of sodium-dependent D-glucose transport sites along the proximal tubule: evidence from vesicle studies. Am. J. Physiol. 242: F406–F414. [DOI] [PubMed] [Google Scholar]
- 41.Wright E. M., Turk E.2004. The sodium/glucose cotransport family SLC5. Pflugers Arch. 447: 510–518. doi: 10.1007/s00424-003-1202-0 [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto K., Uchida S., Kitano K., Fukuhara N., Okumura-Kitajima L., Gunji E., Kozakai A., Tomoike H., Kojima N., Asami J., Toyoda H., Arai M., Takahashi T., Takahashi K.2011. TS-071 is a novel, potent and selective renal sodium-glucose cotransporter 2 (SGLT2) inhibitor with anti-hyperglycaemic activity. Br. J. Pharmacol. 164: 181–191. doi: 10.1111/j.1476-5381.2011.01340.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.