Abstract
This study aims to investigate and compare the expressions of leptin and ghrelin in the gastrointestinal tracts of calves and cows. The mRNA expression of leptin in the rumen, abomasum, and jejunum of calves was significantly higher than that in cows. In both calves and cows, abomasum ghrelin mRNA expression was significantly higher than that in other gastrointestinal tracts. In calves, leptin protein expression in the abomasum was the highest. In addition, leptin protein expression in the abomasum and jejunum of calves was significantly higher than that in cows. Results indicated that leptin in the abomasum and jejunum plays an important role during the suckling period in a ruminant.
Keywords: cattle, ghrelin, intestine, leptin
Leptin is a 16-kD peptide hormone secreted by white adipose tissue [20]. Leptin plays an important role in controlling and managing fat mass and weight by acting on the hypothalamus to inhibit appetite and hypermetabolism [7]. Leptin is secreted in the white adipose tissue as well as in the placenta, skeletal muscle, and pancreas [15, 17, 19]. Leptin that is produced in the internal secretion cells of the stomach acts on the hypothalamus and subsequently causes short-term appetite inhibition [1, 16]. In addition, leptin produced in the chief cells (that secrete pepsinogen) is secreted in the gastric juice [4]. Leptin in the gastric juice decreases pepsin secretion and gastric acid production [3], increases the level of oligopeptide transporter PepT-1, and inhibits glucose transportation by SGLT-1 in the small intestine [5].
Ghrelin is a peptide isolated as a growth hormone secretagogue from the stomach of the rat [14]. Ghrelin plays various roles in the stimulation of growth hormone secretion, gastrointestinal motility, pancreatic endocrine secretion, glucose and lipid metabolism, and control of food intake [18]. The main source of circulating ghrelin is the endocrine secretion of the gastric mucosa. However, outside the stomach, ghrelin is produced in the bowels, pancreas, and hypothalamus [13]. In addition, ghrelin is expressed in the human salivary gland [6], suggesting that ghrelin in the saliva affects food intake and gastrointestinal tract function.
In ruminants, circulating leptin acts on the hypothalamus and affects energy metabolism [2]. In addition, ghrelin is primarily produced in the abomasum and plays a role that it is similar to that in monogastric animals [10]. However, the expression of leptin and ghrelin in the gastrointestinal tract of ruminants is unclear. Therefore, we aim to investigate the expression of leptin and ghrelin in the gastrointestinal tracts of calves and cows.
The study was approved by the Animal Care Committee of NARO Hokkaido Agricultural Research Center. This committee was established under the Laboratory Animal Control Guidelines, which is largely consistent with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health in the USA (NIH publication no. 86-23, revised in 1985). The subjects were four Holstein male calves weighing 48.1 ± 1.3 kg (mean ± SEM) aged 2 weeks and four Holstein cows weighing 664 ± 22 kg aged 5.2 ± 0.3 years, tested 20 weeks post-parturition. Cattle were fed according to the Japanese Feeding Standard designed to meet the requirements of total digestible nutrients and crude protein. Calves were fed whole milk twice a day. The gastrointestinal tracts (rumen, reticulum, omasum, abomasum, duodenum, jejunum, ileum, and colon) of cattle were immediately collected after slaughter. Collected tissue samples were then immediately frozen in liquid N2 and stored at −80°C until use.
Total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). RT-PCR, cloning, sequencing, and RT-PCR were performed as previously described [9]. The primer sequences for leptin (Accession, U43943) amplification were 5′-GGCTGTCCACAGGAGAAGAG-3′ (forward primer) and 5′-CATGATGCTCCCTGGATTCT-3′ (reverse primer). The primer sequences for ghrelin (Accession, BC148027) amplification were 5′-AGAAGCCATCAGGCAGACT-3′ (forward primer) and 5′-GTTGAACCGGATTTCCAGCT-3′ (reverse primer). The primer sequences for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Accession, BC102589) amplification were 5′-TCAACGGGAAGCTCACTGG-3′ (forward primer) and 5′-CCCCAGCATCGAAGGTAGA-3′ (reverse primer). The nucleotide sequences for these primers were designed based on the GenBank database. After an initial denaturation at 95°C for 1 min, 40 cycles of amplification were performed under the following conditions: 94°C for 15 sec, 53°C–58°C (leptin: 55°C, ghrelin: 58°C, GAPDH: 53°C) for 20 sec, and 72°C for 40 sec. The amplification products from mRNAs were predicted to be 317, 106, and 273 base pairs for leptin, ghrelin, and GAPDH, respectively. Post-PCR melting curves confirmed the specificity of the single-target amplification.
Western blotting was performed as previously described [9]. Membrane protein and cytoplasmic protein samples (25 µg) were electrophoresed on SDS polyacrylamide gels and then transferred to a nitrocellulose membrane by semi-dry transfer. The membranes were then blocked with 5% (w/v) ECL blocking agent (GE Healthcare, Chicago, IL, USA) in phosphate-buffered saline containing 0.1% Tween 20 (PBS-T) for 2 hr at room temperature and then probed for 2 hr with primary antibody diluted to 1:5,000 in PBS-T. Rabbit anti-leptin (Funakoshi Co., Ltd., Tokyo, Japan) was used as a primary antibody. After washing with PBS-T, the membranes were incubated for 30 min with secondary antibodies that were diluted to 1:10,000 in PBS. Mouse anti-rabbit IgG conjugated to horseradish peroxidase (Cosmo Bio Co., Ltd., Tokyo, Japan) was used as a secondary antibody. Immunodetection was performed using chemiluminescence kit (ECL-Prime; GE Healthcare). Negative control blots were only probed with the secondary antibody. As an internal standard, western blotting was performed using β-actin antibody (ab8229; Abcam, Cambridge, UK) and the relative abundance was normalized with respect to β-actin. Densitometric analysis for bands were performed using the analysis software CS Analyzer (ATTO Co., Ltd., Tokyo, Japan).
Results are expressed as mean ± SEM. Statistical significance between two groups was determined using Student’s t-test. Statistical significance in many groups was a one-way ANOVA followed by Bonferroni multiple range test. A P value of <0.05 was considered to be statistically significant.
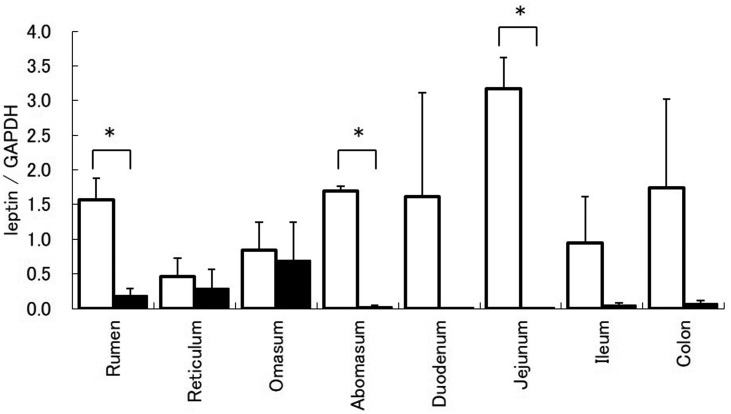
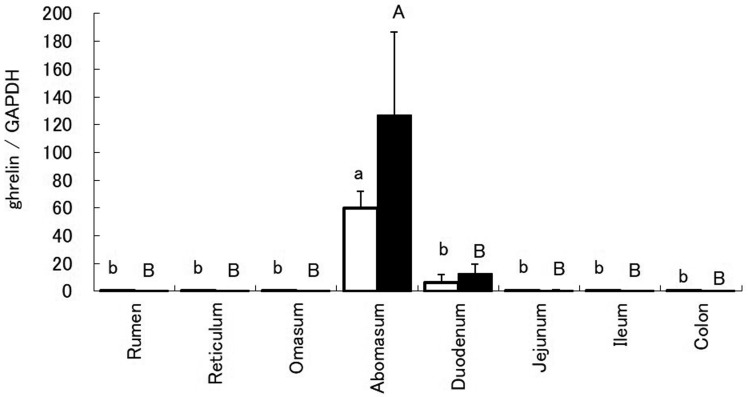
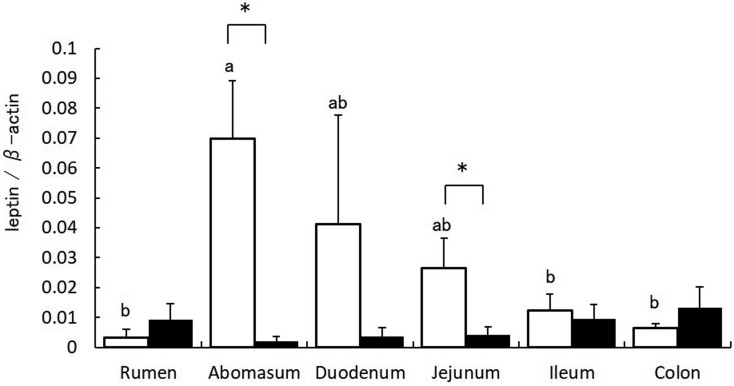
Leptin mRNA expression in the rumen, abomasum, and jejunum of calves was significantly higher than that in cows (Fig. 1). In both calves and cows, abomasum ghrelin mRNA expression was significantly higher than that in other gastrointestinal tracts (Fig. 2). Immunoblots revealed protein bands for leptin in all gastrointestinal tracts of both calves and cows. Densitometric analysis revealed that leptin expression in the abomasum of calves was the highest (Fig. 3). In addition, protein expression of leptin in the abomasum and jejunum of calves was significantly higher than that in cows.
Fig. 1.
Leptin mRNA expression in the gastrointestinal tract of calves (white bar) and cows (black bar). Leptin mRNA expression relative to that of glyceraldehyde-3-phosphate dehydrogenase mRNA is represented as mean ± SEM for each region of the gastrointestinal tract. * indicates P<0.05 (t-test).
Fig. 2.
Ghrelin mRNA expression in the gastrointestinal tract of calves (white bar) and cows (black bar). Ghrelin mRNA expression relative to that of glyceraldehyde-3-phosphate dehydrogenase mRNA is represented as mean ± SEM for each region of the gastrointestinal tract. Values without common letters (ab, AB) are significantly different (P<0.05, Bonferroni test).
Fig. 3.
Densitometric analysis of leptin protein in the gastrointestinal tract of calves (white bar) and cows (black bar). Leptin expression relative to that of β-actin is represented as mean ± SEM for each region of the gastrointestinal tract. Values without common letters (ab) are significantly different (P<0.05, Bonferroni test). * indicates P<0.05 (t-test).
In this study, leptin expression in the rumen, abomasum, duodenum, and jejunum of calves was high (Fig. 1). In addition, in calves, the highest leptin level was found in the abomasum (Fig. 3). Leptin, secreted by the stomach, acts on the hypothalamus and causes short-term appetite inhibition [16]. In addition, leptin is secreted by the stomach into the gastric juice that increases PepT-1 and inhibits glucose transportation by SGLT-1 in the small intestine [5]. Thus, it is suggested that leptin produced in the abomasum of suckling calves acts via the endocrine and exocrine systems. In addition, results demonstrated that leptin mRNA expression in the rumen of calves was high, but the protein expression was low. These combined observations suggest that leptin mRNA expression in the rumen is regulated post-transcriptionally. In contrast, the expression of leptin mRNA and protein significantly decreased in cows, and leptin protein expression was low in all gastrointestinal tracts. In ruminants, the rumen dramatically changes after weaning, which is important for digestion and absorption [8]. Therefore, it seems that because suckling calves have digestion and absorption mechanisms like monogastric animals, leptin expression in the gastrointestinal tract is similar to that in monogastric animals. However, these findings suggest that, due to changes in the digestion and absorption mechanisms after weaning, leptin secreted by the abomasum is not important.
In both calves and cows, jejunum ghrelin expression was significantly higher than that in all other gastrointestinal tracts (Fig. 2). The abomasum is the main ghrelin-producing anatomical structure in cows [10]. In addition, the results of the present study suggest that the abomasum is a primary ghrelin-producing anatomical structure in calves as well. Furthermore, the analysis of ghrelin mRNA expression revealed that cows during their lactation period showed higher expression than in calves. Lactating cows preferentially distribute nutrients between mammary glands that are necessary for milk production, and the growth hormone regulates this mechanism as well as lactation [11]. Ghrelin plays an important role in this nutrient allocation process through its impact on growth hormone secretion [12]. Therefore, we suggest that, in cows, ghrelin mRNA expression is high and ghrelin produced in the abomasum regulates lactation.
Acknowledgments
This study was partly supported by a Grants-in-Aid to Cooperative Research from Rakuno Gakuen University, 2013.
REFERENCES
- 1.Bado A., Levasseur S., Attoub S., Kermorgant S., Laigneau J. P., Bortoluzzi M. N., Moizo L., Lehy T., Guerre-Millo M., Le Marchand-Brustel Y., Lewin M. J.1998. The stomach is a source of leptin. Nature 394: 790–793. doi: 10.1038/29547 [DOI] [PubMed] [Google Scholar]
- 2.Bartha T., Sayed-Ahmed A., Rudas P.2005. Expression of leptin and its receptors in various tissues of ruminants. Domest. Anim. Endocrinol. 29: 193–202. doi: 10.1016/j.domaniend.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 3.Brzozowski T., Konturek P. C., Konturek S. J., Pajdo R., Duda A., Pierzchalski P., Bielański W., Hahn E. G.1999. Leptin in gastroprotection induced by cholecystokinin or by a meal. Role of vagal and sensory nerves and nitric oxide. Eur. J. Pharmacol. 374: 263–276. doi: 10.1016/S0014-2999(99)00314-3 [DOI] [PubMed] [Google Scholar]
- 4.Cammisotto P. G., Renaud C., Gingras D., Delvin E., Levy E., Bendayan M.2005. Endocrine and exocrine secretion of leptin by the gastric mucosa. J. Histochem. Cytochem. 53: 851–860. doi: 10.1369/jhc.5A6620.2005 [DOI] [PubMed] [Google Scholar]
- 5.Ducroc R., Guilmeau S., Akasbi K., Devaud H., Buyse M., Bado A.2005. Luminal leptin induces rapid inhibition of active intestinal absorption of glucose mediated by sodium-glucose cotransporter 1. Diabetes 54: 348–354. doi: 10.2337/diabetes.54.2.348 [DOI] [PubMed] [Google Scholar]
- 6.Gröschl M., Topf H. G., Bohlender J., Zenk J., Klussmann S., Dötsch J., Rascher W., Rauh M.2005. Identification of ghrelin in human saliva: production by the salivary glands and potential role in proliferation of oral keratinocytes. Clin. Chem. 51: 997–1006. doi: 10.1373/clinchem.2004.040667 [DOI] [PubMed] [Google Scholar]
- 7.Hâkansson M. L., Brown H., Ghilardi N., Skoda R. C., Meister B.1998. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J. Neurosci. 18: 559–572. doi: 10.1523/JNEUROSCI.18-01-00559.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi H., Yonezawa T., Kanetani T., Terada F., Katoh K., Obara Y.2005. Expression of mRNA for Na+/glucose transporter 1 (SGLT1) and fatty acid translocase (CD36) in the ruminant gastrointestinal tract before and after weaning. Anim. Sci. J. 76: 339–344. doi: 10.1111/j.1740-0929.2005.00273.x [DOI] [Google Scholar]
- 9.Hayashi H., Maruyama S., Fukuoka M., Kozakai T., Nakajima K., Onaga T., Kato S.2013. Fatty acid-binding protein expression in the gastrointestinal tract of calves and cows. Anim. Sci. J. 84: 35–41. doi: 10.1111/j.1740-0929.2012.01038.x [DOI] [PubMed] [Google Scholar]
- 10.Hayashida T., Murakami K., Mogi K., Nishihara M., Nakazato M., Mondal M. S., Horii Y., Kojima M., Kangawa K., Murakami N.2001. Ghrelin in domestic animals: distribution in stomach and its possible role. Domest. Anim. Endocrinol. 21: 17–24. doi: 10.1016/S0739-7240(01)00104-7 [DOI] [PubMed] [Google Scholar]
- 11.Itoh F., Komatsu T., Kushibiki S., Hodate K.2006. Effects of ghrelin injection on plasma concentrations of glucose, pancreatic hormones and cortisol in Holstein dairy cattle. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 143: 97–102. doi: 10.1016/j.cbpa.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa T., Fujioka H., Ishimura T., Takenaka A., Fujisawa M.2007. Ghrelin expression in human testis and serum testosterone level. J. Androl. 28: 320–324. doi: 10.2164/jandrol.106.000810 [DOI] [PubMed] [Google Scholar]
- 13.Kojima M., Kangawa K.2008. Structure and function of ghrelin. Results Probl. Cell Differ. 46: 89–115. doi: 10.1007/400_2007_049 [DOI] [PubMed] [Google Scholar]
- 14.Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K.1999. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656–660. doi: 10.1038/45230 [DOI] [PubMed] [Google Scholar]
- 15.Masuzaki H., Ogawa Y., Sagawa N., Hosoda K., Matsumoto T., Mise H., Nishimura H., Yoshimasa Y., Tanaka I., Mori T., Nakao K.1997. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat. Med. 3: 1029–1033. doi: 10.1038/nm0997-1029 [DOI] [PubMed] [Google Scholar]
- 16.Oliver P., Picó C., De Matteis R., Cinti S., Palou A.2002. Perinatal expression of leptin in rat stomach. Dev. Dyn. 223: 148–154. doi: 10.1002/dvdy.1233 [DOI] [PubMed] [Google Scholar]
- 17.Reddy S., Lau E. M., Ross J. M.2004. Immunohistochemical demonstration of leptin in pancreatic islets of non-obese diabetic and CD-1 mice: co-localization in glucagon cells and its attenuation at the onset of diabetes. J. Mol. Histol. 35: 511–519. doi: 10.1023/B:HIJO.0000045963.10002.4b [DOI] [PubMed] [Google Scholar]
- 18.Ueno H., Yamaguchi H., Kangawa K., Nakazato M.2005. Ghrelin: a gastric peptide that regulates food intake and energy homeostasis. Regul. Pept. 126: 11–19. doi: 10.1016/j.regpep.2004.08.007 [DOI] [PubMed] [Google Scholar]
- 19.Wang J., Liu R., Hawkins M., Barzilai N., Rossetti L.1998. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 393: 684–688. doi: 10.1038/31474 [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M.1994. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432. doi: 10.1038/372425a0 [DOI] [PubMed] [Google Scholar]