Abstract
Purpose
Endocrine therapy is a standard treatment for hormone receptor-positive breast cancer, which accounts for 60%–75% of all breast cancer. Hormone receptor positivity is a prognostic and predictive biomarker in breast cancer. Approximately 50%–80% of breast cancer is also positive for androgen receptor (AR), but the prognostic and predictive value of AR expression in breast cancer is controversial. Here, we investigated AR expression and its prognostic value in patients with surgically resected breast cancer in Korea.
Methods
We retrospectively reviewed the medical records of patients who had surgically resected breast cancer to collect AR expression data and other clinicopathological data. The optimal cut-off for AR positivity was determined using a receiver operating characteristic curve analysis.
Results
We reviewed 957 patients with surgically resected breast cancer from June 2012 to April 2013. The median follow-up was 62 months, and relapse events occurred in 101 (10.6%) patients. Unlike the cut-off value of 1% or 10% in previous reports, 35% was determined to be best for predicting relapse-free survival (RFS) in this study. At the cut-off value of 35%, 654 (68.4%) patients were AR-positive. AR expression was more prevalent in luminal A (87.6%) and luminal B (73.1%) types than in human epidermal growth factor receptor 2-positive (56.2%) or triple-negative (20.6%) types. AR expression of ≥ 35% was significantly related to longer RFS in a multivariate analysis (hazard ratio, 0.430; 95% confidence interval, 0.260–0.709; p = 0.001).
Conclusion
We propose a cut-off value of 35% to best predict RFS in patients with surgically resected breast cancer. AR expression was positive in 68.4% of patients, and AR positivity was found to be an independent prognostic factor for longer RFS.
Keywords: Breast; Carcinoma; Receptors, androgen; Recurrence; Survival
INTRODUCTION
Breast cancer is the most common cancer in women worldwide, with 2.08 million cases diagnosed annually [1]. It is also the second most common cancer in women in Korea, with 21,747 new cases diagnosed in 2016 [2]. Estrogen receptor (ER)- and/or progesterone receptor (PR)-positive breast cancer accounts for 60%–75% of all cases of breast cancer. ER and/or PR are prognostic factors for better survival and are also predictive markers for endocrine therapy in hormone receptor-positive breast cancer patients [3,4]. The survival of hormone receptor-positive breast cancer patients varies [5], and detailed classification using new prognostic biomarkers is required. Also, distinct subtypes of triple-negative breast cancer (TNBC) were identified using a gene expression analysis. The luminal AR subtype, which is characterized by androgen receptor (AR) signaling, is one of the subtypes with a distinct prognosis and therapeutic target [6]. Approximately 50%–80% of breast cancer cases are AR-positive, both in hormone receptor-positive and -negative breast cancers [7,8]. Various androgen agonists and antagonists are available and widely used in clinical practice. In this context, AR could be a good candidate as a novel prognostic marker in breast cancer.
AR expression is reported to be more common in ER-positive tumors (up to 90%). However, AR is also positive in 20%–40% of ER-negative tumors [9,10]. Additionally, AR expression is more prevalent in primary tumors (79.9%–82.9%) than in metastatic lesions (60.2%–73.5%), and its concordance rate of expression is 60.6%–66.7% between primary and metastatic cancer tissues [9]. The role of AR signaling in breast cancer differs in ER-positive and -negative tumors. AR serves as a potential tumor suppressor in breast cancer cells from ER-positive tumors. Cells with higher AR expression showed a greater anti-proliferative effect by cell cycle arrest and interfered with ER-mediated transactivation of tumor cells. Conversely, AR has a potential role as an oncogene in ER-negative breast cancer cells by dysregulating and enhancing human epidermal growth factor receptor 2 (HER2) expression [11]. The role of AR as a prognostic factor predicting survival in breast cancer has varied in previous reports. Disparities across study results are mainly due to the heterogeneous composition of the breast cancer subgroup as well as a lack of consensus regarding the AR cut-off point. From the literature review by Ricciardelli et al. [10], who collated 53 previous reports regarding AR and breast cancer outcomes, AR was found to be predictive of either overall survival (OS) or relapse-free survival (RFS), disease-free survival (DFS), and metastasis-free survival in 48% of the studies. For ER-positive tumors, AR was reported to be a better prognostic marker for breast cancer in 62% (5/8) of the studies (hazard ratio [HR] ranging from 0.22 to 0.65). However, the role of AR in ER-negative tumors as a prognostic marker was seen to be more controversial. The prognostic value of AR was reported to be significant in only 39% of the studies. Furthermore, the role of AR expression was found to be beneficial in some studies and harmful in others. There were several limitations to these previous studies. First, the cut-off for AR positivity was arbitrary. In most of the studies, the cut-off points were 1% or 10% and were not enough to predict breast cancer survival. Moreover, more than 90% of the studies were conducted in non-Asian regions. Most of the studies involving Asian patients studied the prognostic role of AR in TNBC patients, with inconclusive results.
In this study, we investigated AR expression in all surgically resected breast cancer patients during the study period so as to reflect the real proportion of AR positivity in Korean patients. Furthermore, we investigated the prognostic role of AR expression for RFS in early breast cancer and proposed the optimal cut-off point for AR positivity in these patients.
METHODS
Study population
Between June 2012 and June 2013, a total of 1,127 breast cancer patients who underwent surgery for breast cancer were screened. Patients without invasive cancer (n = 141, in situ carcinoma only) or who were initially stage IV cancer (n = 29) and received palliative resection were excluded from the analysis. Finally, 957 patients were enrolled in the study.
Detailed eligibility criteria were as follows: 1) pathologically confirmed invasive breast carcinoma; 2) stage I–III disease; 3) completely resected by surgery; 4) available pathological data (including AR status); and 5) available follow-up data.
The study protocol was reviewed and approved by the Institutional Review Board (IRB) at Seoul National University Hospital (IRB number 1910-134-1072). As the study was performed as a retrospective medical record review and caused less than minimal harm to the subjects, informed consent from each patients were waived. The recommendations of the Declaration of Helsinki for biomedical research involving human subjects were also followed.
Clinicopathological data collection and breast cancer subtypes
Clinical characteristics (age at diagnosis, date of diagnosis, date of surgery, neo-/adjuvant therapy, surgical stage, date of last visit, and date of relapse) and laboratory test results (follicle-stimulating hormone, luteinizing hormone, and estradiol levels at diagnosis for determining menopausal status) were obtained through a retrospective review of the electronic medical record system. We also reviewed immunohistochemistry (IHC) data, including ER, PR, HER2, Ki-67, and AR expression. IHC was performed as previously described [12]. Anti-AR antibody (anti-AR; Thermo Scientific, Carlsbad, USA) and IHC examination were performed according to our hospital's routine protocols [13]. In cases of HER2 IHC 2+, fluorescent in situ hybridization (FISH) was performed to determine HER2 positivity. Positivity thresholds for classification were ER ≥ 1%, PR ≥ 1%, HER2 = IHC 3+ (> 10% invasive tumor cells with intense and circumferential membrane staining), and/or FISH positivity (HER2:CEP17 ratio ≥ 2.0) [14]. The high Ki-67 threshold of ≥ 14% was based on work by Cheang et al. [15], in which 14% was found to best discriminate between luminal A and B tumors. Since a consensus on the optimal cut-off point for AR expression has not yet been established, we collected the data as an absolute value (percentage of positive cells).
Breast cancer was further classified into several groups according to molecular alterations and cellular composition. The subtypes are closely associated with different breast cancer outcomes. In this study, we classified breast cancer patients into 4 subgroups, luminal A, luminal B, HER2-enriched, and TNBC groups, according to the definitions adopted by the 2013 St. Gallen Consensus Panel [16]. The definitions of the subgroups are as follows: 1) luminal A: ER-positive, PR-positive, HER2-negative, and Ki-67-low. A PR cut-off of ≥ 20% was adopted from Prat et al. [17], which was found to be the best for distinguishing between luminal A and luminal B types; 2) luminal B: ER-positive and HER2-positive, independent of other parameters, or ER-positive, HER2-negative, and at least one of Ki-67-high, PR-negative, or PR-low (< 20%); 3) HER2-enriched: ER and PR absent, with HER2 overexpressed or amplified; 4) TNBC: ER-, PR-, and HER2-negative.
Statistical analysis
To determine the optimal cut-off value for AR expression, we performed a receiver operating characteristic (ROC) curve analysis. The optimal cut-off point for best predicting RFS was determined using Youden's index (J), which maximized sensitivity and specificity. RFS was determined as the interval between the date of surgery or initiation of neoadjuvant chemotherapy and the date when disease relapse or progression was first documented, or the date of death from any cause. Local, regional, and distant relapse were all included as disease relapse, while contralateral breast cancer was not regarded as a relapse. The Kaplan-Meier method and the Cox proportional hazard regression model were used for the survival analyses. Log-rank tests were used to compare RFS between different groups, according to hormone receptor status and HER2 status. The hazard function is the instantaneous failure rate at time, which is the probability of an event in the next small interval. Differences between AR expression levels according to the clinicopathological characteristics were examined using a one-way analysis of variance for the continuous variables (age and RFS) and χ2 tests for the remaining variables. All statistical tests were 2-sided, with the level of significance established at a p-value of < 0.05. Statistical analyses were performed using SPSS version 21.0 (IBM, Armonk, USA). The ROC analysis and ROC curve comparison were performed using MedCalc® version 19 (MedCalc Software Ltd., Ostend, Belgium).
RESULTS
Patient characteristics
A total of 957 patients with a median age of 50 years (range, 22–81 years) were analyzed in this study, with a median follow-up duration of 62.0 months. At the data cut-off in July 2018, 101 patients (10.6%) had experienced relapse events. Fourteen patients (13.9%) had a loco-regional relapse, and 87 (86.1%) experienced distant metastases. The median RFS was not reached at that time.
The baseline characteristics of the 957 patients are described in Table 1. Five hundred and eleven (53.4%) were pre-/peri-menopausal. Two-thirds of the patients (604, 63.1%) received breast-conserving surgery, and the histology was predominantly invasive ductal carcinoma (88.4%). Pathological stages I, II, and III accounted for 46.2%, 43.2%, and 10.2% patients, respectively. One hundred and fifty-one patients (15.8%) received neoadjuvant chemotherapy, and 508 (53.1%) received adjuvant chemotherapy. Regarding the breast cancer subtypes, luminal A, luminal B, HER2-positive, and TNBC types were 36.2%, 39.2%, 8.5%, and 16.2%, respectively.
Table 1. Patients' characteristics.
| Characteristic | Value (n = 957) | |
|---|---|---|
| Sex (male:female) | 5 (0.5):952 (99.5) | |
| Age, median (range) | 50 (22–81) | |
| Menopausal status (pre-menopause:post-menopause) | 511 (53.4):446 (46.6) | |
| Surgery (mastectomy:BCS) | 353 (36.9):604 (63.1) | |
| Histology (IDC/ILC/other) | 846 (88.4)/48 (5.0)/63 (6.6) | |
| Pathological stage | ||
| 0 | 4 (0.4)* | |
| IA/IB | 435 (45.5)/7 (0.7) | |
| IIA/IIB | 289 (30.2)/124 (13.0) | |
| IIIA/IIIB/IIIC | 66 (6.9)/1 (0.1)/31 (3.2) | |
| Neoadjuvant chemotherapy | 151 (15.8) | |
| Adjuvant treatment | ||
| Chemotherapy | 508 (53.1) | |
| Radiation therapy | 615 (64.3) | |
| Hormonal therapy | 720 (99.8% of hormone receptor positive patients) | |
| Trastuzumab | 136 (90.1% of HER2-positive patients) | |
| Breast cancer subtypes | ||
| Luminal A | 346 (36.2) | |
| Luminal B (HER2-negative/-positive) | 305 (31.9)/70 (7.3) | |
| HER2-positive | 81 (8.5) | |
| Triple-negative | 155 (16.2) | |
Values are presented as number (%).
BCS = breast-conserving surgery; IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma; HER2 = human epidermal growth factor receptor 2.
*Pathologic complete response (ypT0/Tis ypN0) after neoadjuvant chemotherapy.
AR expression in surgically resected patients
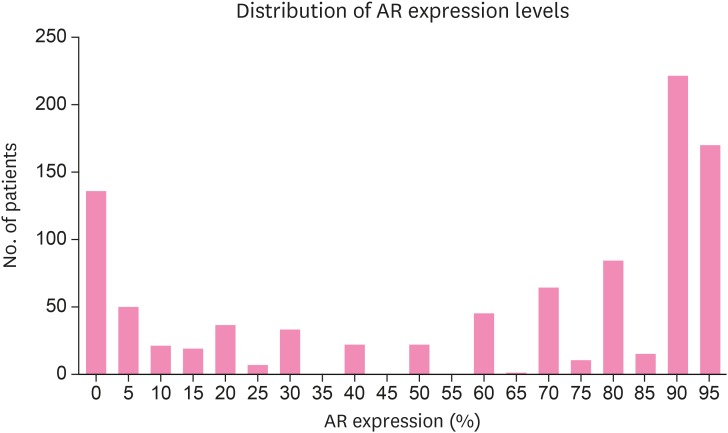
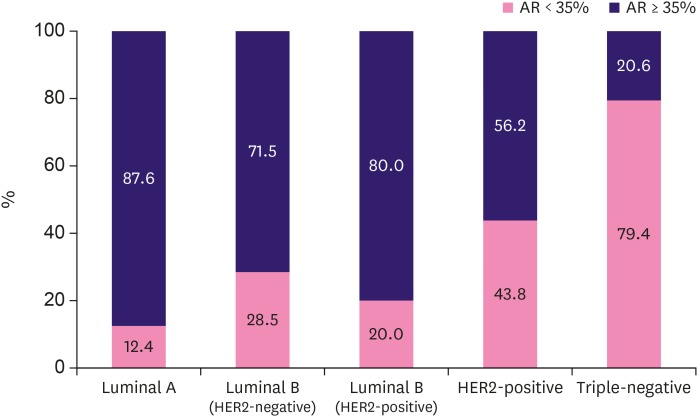
As a consensus on the best cut-off value for AR positivity has not been determined, we first investigated AR expression against the continuous variables. The median value of AR expression was 80% (range, 0%–95%; Figure 1). According to a literature review of previous studies covering AR expression in breast cancer patients, 29 out of 44 (75%) of the studies arbitrarily selected either 1% or 10% as the cut-off points for AR positivity [10]. From the Cox regression analysis of RFS according to AR expression as a continuous variable, the HR was 0.982 (95% confidence interval [CI], 0.976–0.988; p < 0.001). Therefore, we could assume that with an increase in 1% of AR expression, the risk of RFS would fall by 1.8% (HR, 0.982) with a p-value of less than 0.001. We further intended to determine the optimal cut-off point of AR expression in surgically resected breast cancer patients, which could predict the oncological outcomes, in particular the relapse. We performed a ROC analysis to identify the best cut-off point, and the area under the ROC curve (AUC) was 0.698 (95% CI, 0.667–0.727; p < 0.001) (Supplementary Figure 1). Youden's index was highest at the AR of 35% (J = 0.3221; 95% CI, 0.2146–0.3979) with a sensitivity of 60.4% and specificity of 71.8%. Meanwhile, with the cut-off criterion 1% and 10%, the sensitivity and specificity for relapse were 31.7%/89.0% and 47.6%/81.4%, respectively. ROC curves of each of the cut-off points were compared. The graph with the 35% cut-off point had a larger AUC (0.661; 95% CI, 0.630–0.691) than the other traditionally used cut-off points (AUC of cut-off 1% 0.603 with 95% CI, 0.572–0.635 and AUC of cut-off 10% 0.651, with 95% CI, 0.620–0.682) (Supplementary Figure 2). Thus, we selected 35% as the optimal cut-off point for AR expression and defined AR-positive as AR expression of ≥ 35% in tumor cells. The number of AR-positive patients was 830 (86.8%), 770 (80.6%), and 654 (68.4%) at the cut-off values of 1%, 10%, and 35%, respectively (Table 2). Consistent with previous reports, the number of AR-positive patients was much higher (577/721, 80.0%) among hormone receptor-positive patients compared with hormone receptor-negative patients (77/236, 32.6%). The absolute value of AR expression was higher (median, 85; range, 0–95; mean, 69.37 ± 30.751) in hormone receptor-positive patients than in hormone receptor-negative patients (median, 3.0; range, 0–95; mean, 28.21 ± 36.723). Also, AR positivity differed according to breast cancer subtype (Figure 2). AR-positivity was more common in luminal A (87.6%) and luminal B types (71.5% and 80.0% luminal B HER2-negative and -positive patients), while much less common in HER2-positive (56.2%) and TNBC types (20.6%).
Figure 1. AR expression distribution. Histogram showed AR expression levels and their distribution in surgically resected breast cancer patients. Median value of AR expression was 80% (range, 0–95).
AR = androgen receptor.
Table 2. Androgen receptor expression distribution and positive patients according to the various cut-off values.
| Cut-off values | 1% | 10% | 35% | |||
|---|---|---|---|---|---|---|
| < 1 | ≥ 1 | < 10 | ≥ 10 | < 35 | ≥ 35 | |
| No. (%) | 126 (13.2) | 830 (86.8) | 186 (19.4) | 770 (80.6) | 302 (31.6) | 654 (68.4) |
Figure 2. AR expression according to breast cancer subtype. AR positivity differed in breast cancer subtypes. AR positivity was more common in luminal subtypes than in HER2-positive or triple-negative breast cancer types.
AR = androgen receptor; HER2 = human epidermal growth factor receptor 2.
AR expression and clinical outcomes of patients
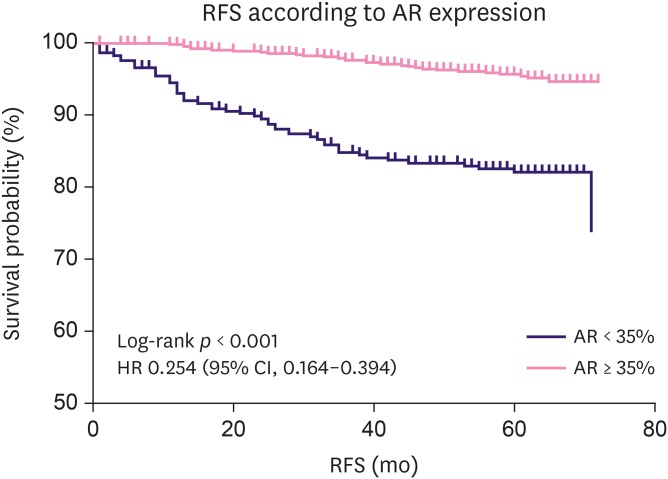
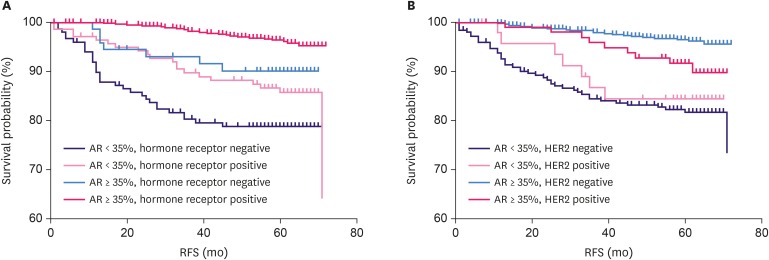
AR positivity (≥ 35%) was significantly related to a low proliferation index (Ki-67 < 14%, p < 0.001). Conversely, AR < 35% was more common at an advanced pathological stage, with 25%, 26.1%, 35.6%, and 39.8% in stage 0, I, II, and III, respectively. AR expression of ≥ 35% was significantly associated with longer RFS in the overall patient population using the Kaplan-Meier analysis (log-rank p < 0.001, Figure 3). AR positivity at the cut-off point of 1% and 10% were also associated with a longer RFS in the Kaplan-Meier analyses (both with log-rank p < 0.001, Supplementary Figure 3). However, as the cut-off point of 35% was found to have the highest Youden's index, we selected 35% as the cut-off value for further analysis. From the univariate analysis with Cox regression, AR positivity (≥ 35%) reduced relapse risk by 74.6% (HR, 0.254; 95% CI, 0.164–0.394). The relationship between AR expression and RFS according to the hormone receptor and HER2 status was also analyzed (Figure 4). AR positivity (≥ 35%) was significantly related to longer RFS in hormone receptor-positive (log-rank p < 0.001, Figure 4A) and HER2-negative (log-rank p < 0.001, Figure 4B) patients. Meanwhile, AR-positive patients showed a trend of longer survival, but this was not statistically significant in hormone receptor-negative or HER2-positive patients (log-rank p = 0.074 and p = 0.205, respectively).
Figure 3. AR expression and RFS in the overall patient population. AR positivity (expression level ≥ 35%) was significantly associated with longer RFS in the overall population of surgically resected breast cancer patients by the Kaplan-Meier method (log-rank p < 0.001). From the univariate analysis by Cox regression, the HR for RFS was 0.254 (95% CI, 0.164–0.394) in AR-positive patients.
AR = androgen receptor; RFS = relapse-free survival; HR = hazard ratio; CI = confidence interval.
Figure 4. AR expression and RFS according to hormone receptor and HER2 status. AR positivity was significantly associated with longer RFS in hormone receptor-positive breast cancer (n = 721) (A) and HER2-negative tumors (n = 806) (B) (both log-rank p < 0.001). In hormone receptor-negative (n = 236) and HER2-positive (n = 151) patients, AR positivity tended to show a longer RFS, but this was not statistically significant (log-rank p = 0.074 and p = 0.205, respectively).
AR = androgen receptor; RFS = relapse-free survival; HER2 = human epidermal growth factor receptor 2.
Univariate and multivariate analyses for RFS
We performed univariate and multivariate Cox proportional hazard regression analyses of multiple clinicopathological variables and RFS (Table 3). Age (< 40 vs. ≥ 40 years), ER and/or PR status (positive vs. negative), AR positivity (≥ 35% vs. < 35%), and pathological stage (stage II, III vs. 0, I) were significantly associated with RFS in both the univariate and multivariate analyses. AR ≥ 35% significantly reduced relapse risk by 57% (HR, 0.430; 95% CI, 0.260–0.709; p = 0.001) in the multivariate analysis. Therefore, we assumed that AR positivity was associated with longer RFS irrespective of other clinicopathological factors, especially hormone receptor positivity and pathological stage, which are well established prognostic markers.
Table 3. Univariate and multivariate analyses for relapse-free survival.
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||
| Age (< 40 yr) | 2.393 | 1.494–3.834 | < 0.001 | 2.796 | 1.510–5.177 | 0.001 | |
| Menopausal status (premenopausal) | 0.827 | 0.542–1.263 | 0.379 | 0.591 | 0.552–1.630 | 0.063 | |
| ER and/or PR (positive) | 0.309 | 0.202–0.417 | < 0.001 | 0.489 | 0.297–0.804 | 0.005 | |
| HER2 (positive) | 1.424 | 0.847–2.395 | 0.183 | 0.949 | 0.552–1.630 | 0.849 | |
| AR (≥ 35%) | 0.254 | 0.164–0.394 | < 0.001 | 0.430 | 0.260–0.709 | 0.001 | |
| P stage (II, III) | 6.225 | 3.305–11.723 | < 0.001 | 5.397 | 2.859–10.189 | < 0.001 | |
| Breast cancer subtypes | < 0.001 | - | - | - | |||
| Luminal A | 0.097 | 0.045–0.211 | < 0.001 | ||||
| Luminal B (HER2−) | 0.391 | 0.235–0.650 | < 0.001 | ||||
| Luminal B (HER2+) | 0.468 | 0.224–1.055 | 0.068 | ||||
| HER2-positive | 0.550 | 0.270–1.119 | 0.099 | ||||
| TNBC | 1.000 | - | - | ||||
HR = hazard ratio; CI = confidence interval; ER = estrogen receptor; PR = progesterone receptor; AR = androgen receptor; HER2 = human epidermal growth factor receptor 2; TNBC = triple-negative breast cancer.
DISCUSSION
Breast cancer is not a single disease, but rather refers to a variety of disease entities with various biological, pathological, and clinical features. Significant efforts have been made to precisely classify the breast cancer subgroups as this is considered crucial for determining treatment strategies and prognostic significance. With the advance of high-throughput gene expression analysis, breast cancer intrinsic subtypes have been widely studied and have found to be associated with distinct clinical outcomes. Five distinct subtypes of breast cancer were identified from gene expression profiles, luminal A, luminal B, HER2 over-expressed, basal, and normal-like tumors. Breast cancer outcomes such as RFS and OS of the subtypes were found to be significantly different [18,19,20,21,22]. As obtaining the gene expression profile from array data is not always feasible, subtype determination by clinicopathological factors using ER, PR, HER2, and Ki-67 immunohistostaining was introduced [15]. Further studies have shown that determining subtypes using clinicopathological criteria could result in a convenient and straightforward approximation of breast cancer intrinsic subtypes, and help to predict different prognoses among them [15,23,24]. However, the current clinicopathological subtype of breast cancer has limitations in that there are still a number of patients in the same subgroup that have different outcomes. Therefore, additional prognostic factors are required.
Approximately 55%–90% of breast cancers are positive for AR, and its expression level varies according to the breast cancer subtype [7,8]. AR expression is quite common in breast cancer, and AR expression levels in the tumor tissue can be determined simply with IHC. Therefore, AR could play a role in predicting breast cancer prognosis in addition to the current clinicopathological examinations.
The role of AR as a prognostic factor in breast cancer had been widely studied but is still inconclusive. Disparities across study results are mainly due to a lack of consensus regarding the AR cut-off point. Most of the studies arbitrarily used 1% or 10% as the cut-off point, which were not enough to predict breast cancer survival [10]. For example, most studies that adopted 10% as an AR cut-off point only performed a univariate analysis for DFS or OS and consequently could not conclude whether the role of AR expression is an independent prognostic factor for breast cancer survival [25,26]. Even studies that performed both univariate and multivariate analyses for DFS and/or OS with AR expression targeted only a limited subset of patients and provided conflicting results of the effect of AR expression on breast cancer survival [27,28,29]. Additionally, most of the studies selected those commonly used AR cut-off points, which had not demonstrated the whole distribution of AR expression previously. The other limitation of the previous reports was that most of the studies were performed in only a small subgroup of the patients, and only a few studies included an Asian population.
In this study, we retrospectively reviewed AR expression status in surgically resected breast cancer patients. AR was revealed to be a prognostic marker for RFS or OS in several studies, but the optimal cut-off value was not determined. We performed a ROC analysis and used Youden's index to determine a cut-off value of 35% to best predict RFS with adequate sensitivity and specificity. AR positivity (≥ 35%) was identified in 68.4% of all patients and was more common in hormone receptor-positive patients, 87.5% in luminal A type and 71.5%, and 80.0% in luminal B HER2-negative and -positive patients, respectively. In HER2-positive and TNBC patients, AR positivity was lower at 56.2% and 20.6%, respectively. High AR expression was related to favorable clinical features, lower pathological stage (0, I), and lower Ki-67 index. Using the Cox regression, AR positivity at the cut-off value of 35% was found to be an independent prognostic factor for RFS in surgically resected patients (HR, 0.430; 95% CI, 0.260–0.709; p = 0.001). We confirmed that a significant proportion of the surgically resected breast cancer patients were AR-positive. High AR expression was found to be an independent prognostic factor of RFS.
However, our study has several limitations. Due to the rather short follow-up time for early breast cancer patients, we could not confirm the prognostic effect of AR expression on OS. Further follow up studies are needed to confirm the prognostic role of AR and the adequacy of the cut-off value of 35% for OS. Another limitation is that we analyzed AR expression in curatively resected breast cancer patients. Thus, AR expression level in stage IV metastatic breast cancer was not reviewed, and the prognostic role of AR expression in advanced breast cancer was not examined in this study. Finally, we determined the cut-off of 35% to be the best prognostic value for RFS, but there was no data to confirm whether we could use the same value as a predictive marker to select patients for AR inhibitor treatment. To determine the predictive value of AR expression, further prospective clinical trials with AR inhibitors are necessary. Nevertheless, this is the first large-scale study to analyze AR expression in surgically resected breast cancer patients in all subtypes of patients, including both ER-positive and -negative patients in Asia, with Korean patients. Furthermore, unlike the previous studies that used arbitrary cut-off values of 1% or 10% for AR positivity, we used the ROC analysis method to determine a value that maximized the sensitivity and specificity to predict breast cancer relapse after surgery and proposed a cut-off value of 35%.
In conclusion, AR expression (≥ 35%) in breast cancer patients was observed in 68.4% of surgically resected patients. Elevated AR was found to be an independent predictor of longer RFS (HR, 0.430; p = 0.001), irrespective of other well-known clinical factors including age, pathological stage, and hormone receptor positivity. AR positivity can be easily tested using conventional IHC methods and is quite commonly observed in breast cancer. Therefore, testing AR with other clinicopathological factors might be helpful to further define the outcomes of surgically resected breast cancer patients.
ACKNOWLEDGMENTS
We would like to thank H. Nikki March, Ph.D., from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Yang Y, Min A, Noh DY, Im SA.
- Data curation: Yang Y, Woo GU, Im SA.
- Formal analysis: Yang Y, Min A, Im SA.
- Investigation: Yang Y, Im SA.
- Methodology: Yang Y.
- Project administration: Yang Y, Im SA.
- Resources: Lee KH, Ryu HS, Kim TY, Lee DW, Lee HB, Moon HG, Han W, Noh DY, Park IA, Im SA.
- Supervision: Im SA.
- Validation: Yang Y.
- Visualization: Yang Y.
- Writing - original draft: Yang Y, Im SA.
- Writing - review & editing: Yang Y, Min A, Ryu HS, Woo GU, Suh KJ, Lee DW, Im SA.
SUPPLEMENTARY MATERIALS
ROC curve analysis of androgen receptor expression and breast cancer relapse. ROC analysis in the overall population showed an AUC of 0.698 (95% CI, 0.667–0.727, p < 0.001).
Comparison of multiple ROC curve and AUC according to the variable AR cut-off points. Multiple ROC curves were plotted by the AR cut-off value at 1%, 10% and 35%, respectively. Their AUC values were 0.603 (95% CI, 0.872–0.635), 0.651 (95% CI, 0.620–0.682) and 0.661 (95% CI, 0.630–0.691), respectively.
AR expression and RFS according to the variable AR cut-off points. AR positivity was also associated with an increased RFS using 1% and 10% as cut-off points. (A) AR expression of ≥ 1% was associated with longer RFS (log rank p < 0.001) with HR of 0.173 (95% CI, 0.087–0.341). (B) AR expression of ≥ 10% was associated with longer RFS (log rank p < 0.001) with HR of 0.130 (95% CI, 0.073–0.230).
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019;51:417–430. doi: 10.4143/crt.2019.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher B, Redmond C, Fisher ER, Caplan R. Relative worth of estrogen or progesterone receptor and pathologic characteristics of differentiation as indicators of prognosis in node negative breast cancer patients: findings from National Surgical Adjuvant Breast and Bowel Project Protocol B-06. J Clin Oncol. 1988;6:1076–1087. doi: 10.1200/JCO.1988.6.7.1076. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett JM, Brookes CL, Robson T, van de Velde CJ, Billingham LJ, Campbell FM, et al. Estrogen receptor and progesterone receptor as predictive biomarkers of response to endocrine therapy: a prospectively powered pathology study in the Tamoxifen and Exemestane Adjuvant Multinational trial. J Clin Oncol. 2011;29:1531–1538. doi: 10.1200/JCO.2010.30.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inic Z, Zegarac M, Inic M, Markovic I, Kozomara Z, Djurisic I, et al. Difference between luminal A and luminal B subtypes according to Ki-67, tumor size, and progesterone receptor negativity providing prognostic information. Clin Med Insights Oncol. 2014;8:107–111. doi: 10.4137/CMO.S18006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakis S, Kotoula V, Eleftheraki AG, Batistatou A, Bobos M, Koletsa T, et al. The androgen receptor as a surrogate marker for molecular apocrine breast cancer subtyping. Breast. 2014;23:234–243. doi: 10.1016/j.breast.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol. 2010;23:205–212. doi: 10.1038/modpathol.2009.159. [DOI] [PubMed] [Google Scholar]
- 9.Bronte G, Bravaccini S, Ravaioli S, Puccetti M, Scarpi E, Andreis D, et al. Androgen receptor expression in breast cancer: what differences between primary tumor and metastases? Transl Oncol. 2018;11:950–956. doi: 10.1016/j.tranon.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricciardelli C, Bianco-Miotto T, Jindal S, Butler LM, Leung S, McNeil CM, et al. The magnitude of androgen receptor positivity in breast cancer is critical for reliable prediction of disease outcome. Clin Cancer Res. 2018;24:2328–2341. doi: 10.1158/1078-0432.CCR-17-1199. [DOI] [PubMed] [Google Scholar]
- 11.Hickey TE, Robinson JL, Carroll JS, Tilley WD. Minireview: the androgen receptor in breast tissues: growth inhibitor, tumor suppressor, oncogene? Mol Endocrinol. 2012;26:1252–1267. doi: 10.1210/me.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keam B, Im SA, Kim HJ, Oh DY, Kim JH, Lee SH, et al. Prognostic impact of clinicopathologic parameters in stage II/III breast cancer treated with neoadjuvant docetaxel and doxorubicin chemotherapy: paradoxical features of the triple negative breast cancer. BMC Cancer. 2007;7:203. doi: 10.1186/1471-2407-7-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Na HY, Choe JY, Shin SA, Choung HK, Oh S, Chung JH, et al. Proposal of a provisional classification of sebaceous carcinoma based on hormone receptor expression and HER2 status. Am J Surg Pathol. 2016;40:1622–1630. doi: 10.1097/PAS.0000000000000728. [DOI] [PubMed] [Google Scholar]
- 14.Wolff AC, Hammond ME, Allison KH, Harvey BE, Mangu PB, Bartlett JM, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Arch Pathol Lab Med. 2018;142:1364–1382. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 15.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prat A, Cheang MC, Martín M, Parker JS, Carrasco E, Caballero R, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol. 2013;31:203–209. doi: 10.1200/JCO.2012.43.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Z, Fan C, Oh DS, Marron JS, He X, Qaqish BF, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006;7:96. doi: 10.1186/1471-2164-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 21.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5:2929–2943. [PMC free article] [PubMed] [Google Scholar]
- 23.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallejos CS, Gómez HL, Cruz WR, Pinto JA, Dyer RR, Velarde R, et al. Breast cancer classification according to immunohistochemistry markers: subtypes and association with clinicopathologic variables in a peruvian hospital database. Clin Breast Cancer. 2010;10:294–300. doi: 10.3816/CBC.2010.n.038. [DOI] [PubMed] [Google Scholar]
- 25.Honma N, Horii R, Iwase T, Saji S, Younes M, Ito Y, et al. Clinical importance of androgen receptor in breast cancer patients treated with adjuvant tamoxifen monotherapy. Breast Cancer. 2012;20:323–330. doi: 10.1007/s12282-012-0337-2. [DOI] [PubMed] [Google Scholar]
- 26.Doberstein K, Milde-Langosch K, Bretz NP, Schirmer U, Harari A, Witzel I, et al. L1CAM is expressed in triple-negative breast cancers and is inversely correlated with androgen receptor. BMC Cancer. 2014;14:958–970. doi: 10.1186/1471-2407-14-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pistelli M, Caramanti M, Biscotti T, Santinelli A, Pagliacci A, De Lisa M, et al. Androgen receptor expression in early triple-negative breast cancer: clinical significance and prognostic associations. Cancers (Basel) 2014;6:1351–1362. doi: 10.3390/cancers6031351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ricciardi GR, Adamo B, Ieni A, Licata L, Cardia R, Ferraro G, et al. Androgen receptor (AR), E-cadherin, and Ki-67 as emerging targets and novel prognostic markers in triple-negative breast cancer (TNBC) patients. PLoS One. 2015;10:e0128368. doi: 10.1371/journal.pone.0128368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schippinger W, Regitnig P, Dandachi N, Wernecke KD, Bauernhofer T, Samonigg H, et al. Evaluation of the prognostic significance of androgen receptor expression in metastatic breast cancer. Virchows Arch. 2006;449:24–30. doi: 10.1007/s00428-006-0213-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ROC curve analysis of androgen receptor expression and breast cancer relapse. ROC analysis in the overall population showed an AUC of 0.698 (95% CI, 0.667–0.727, p < 0.001).
Comparison of multiple ROC curve and AUC according to the variable AR cut-off points. Multiple ROC curves were plotted by the AR cut-off value at 1%, 10% and 35%, respectively. Their AUC values were 0.603 (95% CI, 0.872–0.635), 0.651 (95% CI, 0.620–0.682) and 0.661 (95% CI, 0.630–0.691), respectively.
AR expression and RFS according to the variable AR cut-off points. AR positivity was also associated with an increased RFS using 1% and 10% as cut-off points. (A) AR expression of ≥ 1% was associated with longer RFS (log rank p < 0.001) with HR of 0.173 (95% CI, 0.087–0.341). (B) AR expression of ≥ 10% was associated with longer RFS (log rank p < 0.001) with HR of 0.130 (95% CI, 0.073–0.230).