Abstract
This article describes the breast cancer statistics in Korea, including the incidence, type of surgical procedure, stage, and molecular subtype, using the Korean Breast Cancer Society (KBCS) and Korea Central Cancer Registry data. There were a total of 26,534 new breast cancer diagnoses in 2017 in Korea, of which 4,139 were carcinoma in situ cases and 22,395 were invasive cancer cases. The age standardized rate of breast cancer was 75.3 per 100,000 women in 2017 (63.0 of invasive carcinoma and 12.3 of carcinoma in situ), and it has been steadily increasing across all age groups. Breast cancer occurred most commonly in the 40–49 age group. Compared to 2016, breast conserving surgery (BCS) has increased, and 67.4% of patients were treated with BCS in 2017. The proportions of stage 0 and stage I have continued to increase, accounting for 60.7%. The most common subtype of breast cancer was hormone receptor (HR) positive and human epidermal growth factor receptor-2 (HER2) negative type comprising 65.9% of the cases, whereas HR negative and HER2 positive type was the rarest comprising 10.2% of the cases. The 5-year relative survival rate of breast cancer patients had increased by 14.0% from 79.2% in 1993–1995 to 93.2% in 2013–2017. It is essential to actively enter breast cancer data into the KBCS registry to improve our understanding.
Keywords: Breast neoplasms, Statistics, Epidemiology, Registries, Korea
INTRODUCTION
The International Agency for Research on Cancer reported a total of 18,078,957 cancer cases worldwide in 2018 including 2,088,849 breast cancer (11.6%) cases, making it the second most frequent type. Breast cancer was the most common cancer among women that accounted for 24.2% of the cases [1]. In Korea, there were 22,395 invasive breast cancer patients in 2017, making it the fifth most common cancer type (9.6%). In women, it was the most common cancer type and accounted for 20.3% of the cases [2]. A total of 9,555,027 people died of cancer worldwide in 2018, of whom 626,679 people died of breast cancer [1]. Among all cancer deaths, breast cancer ranked fifth (6.6%), while breast cancer was ranked first (15%) in women as the cause of death due to cancer [1]. In South Korea, a total of 79,153 people died of cancer in 2018, including 2,473 from breast cancer, and breast cancer ranked sixth (8.1%) among women as the cause of cancer death [2]. According to the data from the Korea Central Cancer Registry (KCCR), 26,534 cases of breast cancer occurred in Korea in 2017, including 22,395 and 4,139 cases of invasive cancer and carcinoma in situ, respectively.
The Korean Breast Cancer Society (KBCS) established the registration system in 1996. Since 2001, KBCS has administered the online registration system to collect and distribute the nationwide breast cancer information. This data includes not only physical parameters of breast cancer patients, such as sex, age, height, and weight, but also other valuable data for breast cancer research, such as molecular subtype, stage, and type of surgical procedure. This study analyzed the characteristics of breast cancer in Korea using the data registered in KBCS and KCCR in 2017 and investigated the breast cancer trends in Korea over the past 17 years.
DATA COLLECTION
Data sources
This study analyzed the breast cancer patients newly enrolled from January 1 to December 31, 2017. The analysis included the number, age, and incidence of breast cancer patients in Korea based on KCCR data, which includes both invasive cancer and carcinoma in situ (with International Classification of Disease, 10th version [ICD-10] code: C50 and D05) [3]. The KCCR began as a hospital-based nationwide cancer registry that was initiated by the Ministry of Health and Welfare in 1980. The KCCR annually publishes the incidence, mortality, and prevalence rates for the cancer-related data that has been collected since 1999.
This study, based on the data collected through the KBCS online breast cancer registry system (https://registry.kbcs.or.kr/ecrf/), analyzed the patient characteristics (including age, menopausal status, and treatment type) and disease characteristics (including tumor size, lymph node status, biological marker, and stage). The study was approved by the Institutional Review Board of Jeonbuk National University Hospital (approval No. 2020-03-031).
The Global Cancer Incidence, Mortality and Prevalence (GLOBOCAN) database contains information on 36 types of cancers from 185 countries organized by age and gender. The most recent data are for 2018. Moreover, this data is available online at the Global Cancer Observatory (http://gco.iarc.fr) [4].
Statistical analysis
A typical description of breast cancer incidence includes invasive cancer (ICD-10 code: C50) alone, while the inclusion of carcinoma in situ lesion is separately indicated.
Cancer incidence is defined as the number of newly occurred cases per 100,000 persons in a particular population for a year, and this is expressed as the crude rate (CR) and age-standardized rate (ASR). CR is defined as the number of newly occurred cancer patients in a particular population during an observation period and is typically expressed as the number of cancer diagnoses per 100,000 people [5]. The ASR is a weighted average of the age-specific rates, where the weights correspond to the proportions of corresponding age groups of a standard population [6]. The standard population in this study was adopted from Segi's world standard population [7].
The trend of the ASR is summarized as an annual percentage change (APC) using Joint regression [8]. In contrast, an average annual percent change (AAPC) is primarily used to identify the overall change over a set period of time, which analyzes the entire trend without considering a year-to-year change [9]. This study used a Joinpoint model 4.3.1 (National Cancer Institute, Bethesda, USA) to analyze the APC and AAPC.
The relative survival rate (RSR) compares the survival over a certain period of time between people with a specific disease and those without the disease. It is calculated by dividing the percentage of patients with the disease who are still alive at the end of the study period by the percentage of people in the general population of the same sex and age who are alive at the end of the same time period. The RSR shows whether the disease shortens the life-span. A p-value less than 0.05 was considered statistically significant. SPSS version 20.0 (IBM Corp., Armonk, USA) was used for statistical analysis.
BREAST CANCER STATISTICS IN KOREA IN 2017
Incidence
In 2017, a total of 26,534 patients were newly diagnosed with breast cancer. Of these, 22,395 (84.4%) and 4,139 (15.6%) patients were diagnosed with invasive cancer and carcinoma in situ, respectively. There were 104 (0.3%) cases in men, 9 of which were diagnosed as carcinoma in situ. The CR of breast cancer excluding carcinoma in situ was 86.9 per 100,000 women, while that including carcinoma in situ was 103.0 per 100,000 women. The ASR excluding carcinoma in situ was 63.0 per 100,000 women, while that including carcinoma in situ was 75.3 per 100,000 women.
In 2017, a total of 109,963 cases of cancer were newly reported in women, of which breast cancer was the most common. In terms of CR, invasive breast cancer ranked higher than thyroid cancer (78.5) and was the first among all cancers. Invasive breast cancer was ranked second only to thyroid cancer (68.9) in terms of ASR [2].
Age distribution
According to the data from KCCR and KBCS, the pattern of age distribution in breast cancer patients in 2017 was similar to that reported previously [10]. According to the KBCS registry data, the age of the youngest and the oldest patient diagnosed with breast cancer was 15 years 99 years, respectively. In the age distribution, the highest frequency of breast cancer was observed in the 40–49 years age group (4,174 cases, 35.9%), followed by the 50–59 years group (3,530 cases, 30.4%). According to the KCCR data, the 40–49 years group had the highest frequency of breast cancer with 8,867 cases (33.4%), and the 50–59 years group had the second highest frequency of breast cancer with 7,944 cases (29.9%) (Table 1).
Table 1. New cases of breast cancer diagnosed in 2017 in Korea by age.
| Age (yr) | KBCS registry data | KCCR data |
|---|---|---|
| < 20 | 5 (< 0.1) | 11 (< 0.1) |
| 20–29 | 117 (1.0) | 244 (0.9) |
| 30–39 | 1,157 (10.0) | 2,413 (9.1) |
| 40–49 | 4,174 (35.9) | 8,867 (33.4) |
| 50–59 | 3,530 (30.4) | 7,944 (29.9) |
| 60–69 | 1,803 (15.5) | 4,491 (16.9) |
| 70–79 | 693 (6.0) | 1,956 (7.4) |
| ≥ 80 | 133 (1.1) | 608 (2.3) |
| Total | 11,612 (100.0) | 26,534 (100.0) |
Values are presented as number (%).
KBCS = Korean Breast Cancer Society; KCCR = Korea Central Cancer Registry.
Surgical treatment and pathologic stage
According to the KBCS registry, out of 11,612 patients diagnosed with and registered as having breast cancer in 2017, 7,828 (67.4%) and 3,670 (31.6%) patients underwent BCS and mastectomy, respectively.
According to the stages classified by the American Joint Committee on Cancer and the International Union Against Cancer 7th edition [11], stage I was the most common with 4,995 patients (43.0%), followed by stage II with 3,424 patients (29.5%) and carcinoma in situ (stage 0) with 2,052 patients (17.7%) (Table 2). This included 274 and 271 patients who were diagnosed with stage 0 and stage I, respectively, in the postoperative pathologic stage after receiving neoadjuvant chemotherapy (NACT).
Table 2. Stage distribution of newly diagnosed breast cancer patients in 2017 in Korea (KBCS registry data).
| Stage* | No. of patients |
|---|---|
| 0 | 2,052 (17.7) |
| I | 4,995 (43.0) |
| II | 3,424 (29.5) |
| III | 898 (7.8) |
| IV | 100 (0.8) |
| Unknown | 143 (0.8) |
| Total | 11,612 (100.0) |
Values are presented as number (%).
KBCS = Korean Breast Cancer Society.
*Stage was based on the American Joint Committee on Cancer Cancer Staging Manual, seventh edition.
Molecular subtype pattern
The estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) status could be identified in a total of 9,674 patients out of the 11,612 enrolled in the KBCS. Hormone receptor (HR) positivity was defined as positivity in ER, PR, or both, and HER2 positivity was defined as 3+ expression or 2+ expression by immunohistochemistry with amplification by fluorescence in situ hybridization (FISH) or silver-enhanced in situ hybridization (SISH). Patients who were determined as 2+ of HER2 but did not undergo FISH or SISH tests (corresponding to 608 patients) were excluded from the analysis.
The results showed that the number of HR-positive and HER2-negative patients was 6,377 (65.9%), that of HR-positive and HER2-positive patients was 1,133 (11.7%), that of HR-negative and HER2-positive patients was 981 (10.2%), and that of HR-negative and HER2-negative patients was 1,183 (12.2%) (Table 3). When the distribution of molecular subtypes was analyzed by dividing the subjects into 3 age groups of < 40 years, 40–59 years, and ≥ 60 years, different patterns were observed for each age group. The proportion of HR positive and HER2 negative patients was the highest at 66.6% in the 40–59 years group and the lowest at 62.5% in the < 40 years group. The proportion of HR negative and HER2 negative patients was the highest at 17.8% in the < 40 year group and the lowest (11.8%) in the 40–59 years group (p < 0.001) (Supplementary Figure 1).
Table 3. Molecular subtype of newly diagnosed breast cancer in 2017 in Korea (KBCS registry data).
| Molecular subtype | No. of patients |
|---|---|
| HR (+), HER2 (−) | 6,377 (65.9) |
| HR (+), HER2 (+) | 1,133 (11.7) |
| HR (−), HER2 (+) | 981 (10.2) |
| HR (−), HER2 (−) | 1,183 (12.2) |
| Total | 9,674 (100.0) |
Values are presented as number (%).
KBCS = Korean Breast Cancer Society; HR = hormone receptor; HER2 = human epidermal growth factor receptor-2.
TREND OF BREAST CANCER IN KOREA
Incidence
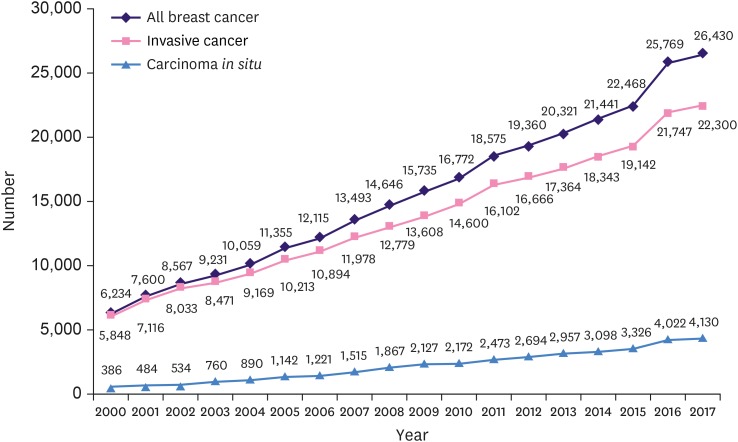
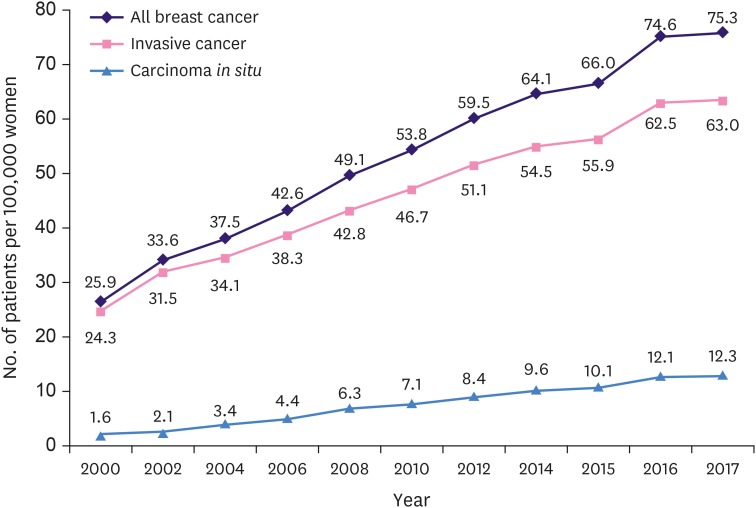
The ASR and the number of newly diagnosed female breast cancer patients is increasing in Korea every year. In 2017, 26,430 breast cancer patients, including carcinoma in situ, were diagnosed which was 4.2 times the number of patients (6,234) in 2000 (324% increase; R2 = 0.997, p < 0.001) (Figure 1). The breast cancer ASR in 2000 was overall 25.9 (24.3 for invasive carcinoma and 1.6 for carcinoma in situ), while the breast cancer ASR in 2017 was 75.3, 63.0, and 12.3 for overall, invasive carcinoma, and carcinoma in situ, respectively. The ASR for invasive carcinoma and carcinoma in situ increased by 2.6 and 7.7 times, respectively (Figure 2).
Figure 1. Number of newly diagnosed Korean female breast cancer patients.
Figure 2. Trends in age-standardized incidence rate of Korean female breast cancer.
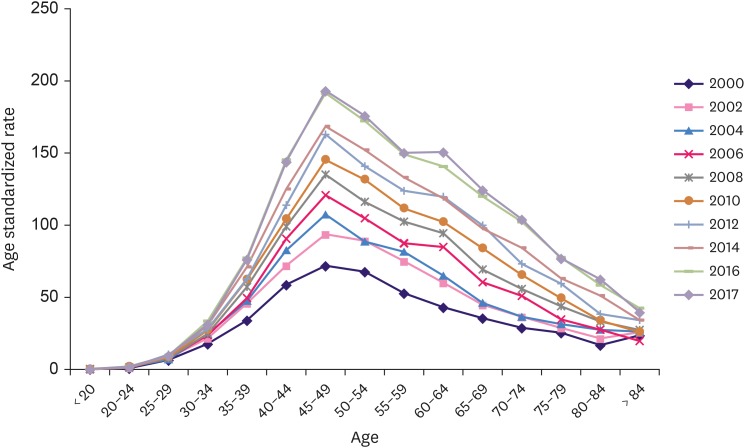
A steady increase in the change in invasive breast cancer ASR was observed across all age groups from 2000 to 2017. When the age groups were sub-divided by 5-year intervals, the ASR of the 70–74 years group 3.6 fold higher (from 28.9 to 104.1) than that in 2000s, and the increase was more significant in the ≥ 60 years group than in the < 60 years group (Figure 3).
Figure 3. Change of age-standardized incidence rate of invasive breast cancer.
The AAPC of the overall breast cancer ASR between 1999 and 2017 increased by 5.7%, and the AAPCs of invasive cancer and carcinoma in situ increased by 5.0% and 11.1%, respectively (Table 4).
Table 4. Trends in age-standardized incidence rates for breast cancer according to Joinpoint analysis (1999–2017).
| AAPC (1999–2017) | Trend 1 | Trend 2 | |||||
|---|---|---|---|---|---|---|---|
| Period | APC | 95% CI | Period | APC | 95% CI | ||
| All breast cancer | +5.7* | 1999–2007 | +7.4* | 6.2–8.6 | 2007–2017 | +4.9* | 4.3–5.5 |
| Invasive breast cancer | +5.0* | 1999–2002 | +10.0* | 3.8–16.5 | 2002–2017 | +4.7* | 4.4–5.1 |
| Carcinoma in situ | +11.1* | 1999–2008 | +18.0* | 15.7–20.3 | 2008–2017 | +7.9* | 6.8–8.9 |
AAPC = average annual percentage change (%); APC = annual percent change (%); CI = confidence interval.
*The APC or AAPC is a significantly different from zero (p < 0.05).
Median age and menopausal status at diagnosis
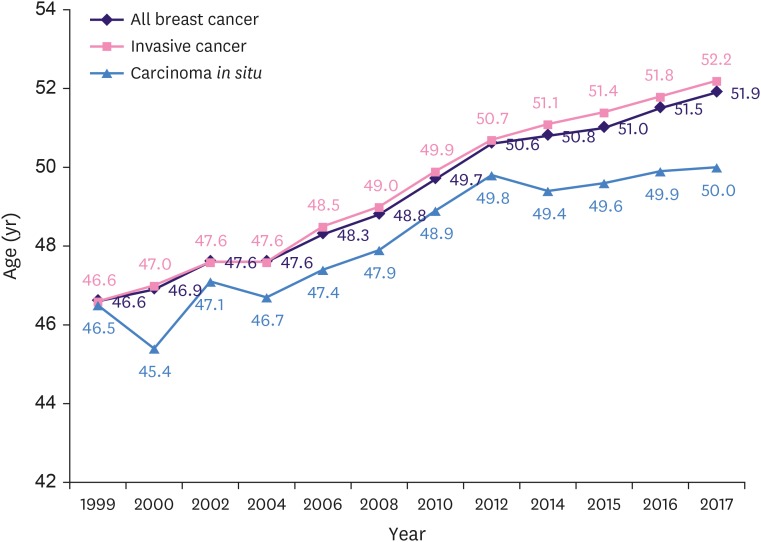
According to the KCCR data, since 1999 when the data enrollment began, the median age for all breast cancer patients, including invasive cancer and carcinoma in situ, has been continuously increasing. In 2017, the median age of all breast cancer patients, invasive cancer patients, and carcinoma in situ patients was 51.9, 52.2, and 50.0 years, respectively. The median age of all breast cancer patients had increased by 5.3 compared to 46.6 years in 1999, which was lower than that in the United States (Figure 4) [12]. Although the median age continues to increase every year, the age distribution graph for Korean breast cancer shows an inverted V shape (Supplementary Figure 2).
Figure 4. Trends in median age of breast cancer.
In 2002, 59.4% of the breast cancer patients were in the premenopausal state. However, since then, the number of postmenopausal breast cancer patients has gradually increased, and it was 53.5% in 2015 [10].
Biologic markers and stage distribution
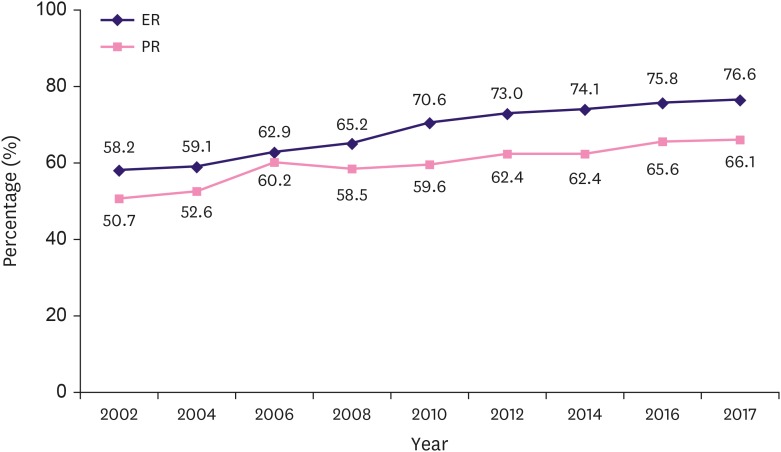
According to the KBCS data, the incidence of HR-positive breast cancer continued to increase from 2002 to 2017. Since 2002, the proportion of HR positive breast cancer has increased by 1% or more every year. This phenomenon was observed in both ER and PR, but it was particularly more noticeable in ER than in PR. The proportion of ER positive breast cancer patients increased from 58.2% in 2002 to 76.6% in 2017 (31.6% increase; R2 = 0.985, p < 0.001). The proportion of PR positive breast cancer patients increased from 50.7% in 2002 to 66.1% in 2017 (30.4% increase; R2 = 0.944, p < 0.001) (Figure 5).
Figure 5. Changes in the hormone receptor positive breast cancer.
ER = estrogen receptor; PR = progesterone receptor.
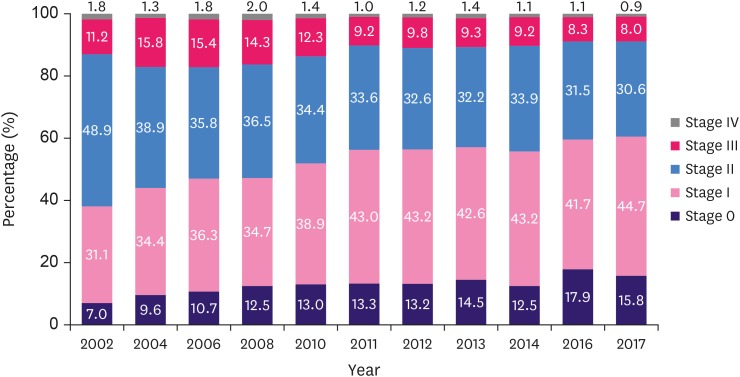
The proportion of stage 0 and stage I breast cancer patients is continuously increasing, and in 2017, it accounted for 60.5% of all cases, which was a 59.3% increase (R2 = 0.914, p < 0.001) from 38.1% in 2002. The analysis by stage shows that the increase in stage 0 was highest, from 7.0% in 2002 to 15.8% in 2017 (119.7% increase; R2 = 0.861, p < 0.001). Additionally, the proportion in stage I increased from 31.1% in 2002 to 44.7% in 2017 (43.7% increase; R2 = 0.968, p < 0.001). In contrast, the proportions in stages II and III decreased, and the decrease was significant in stage II (37.4% decrease; R2 = 0.889, p < 0.001) (Figure 6).
Figure 6. Changes in the stage distribution of breast cancer.
Surgical patterns
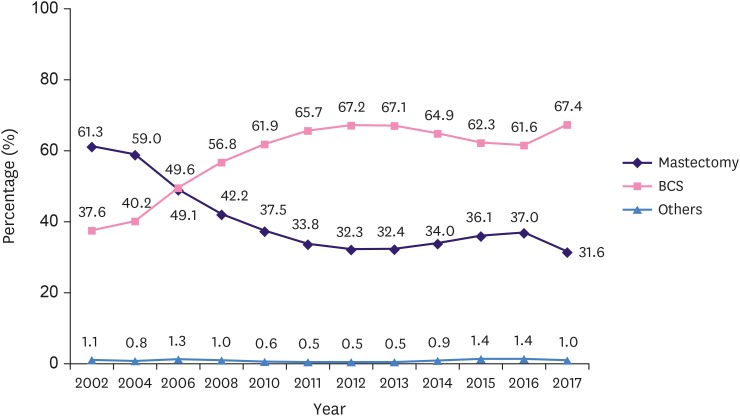
In 2002, the rate of BCS and mastectomy was 37.6% and 61.3%, respectively. Since then, the rate of BCS continued to increase, while that of mastectomy decreased. Thus, the rate of BCS in 2012 increased to 67.2% (126% increase; R2= 0.988, p < 0.001), while that of mastectomy decreased to 32.3% (47.3% decrease; R2 = 0.984, p < 0.001). Subsequently, however, the rate of BCS decreased slightly to 61.6% (9.1% decrease; R2 = 0.991, p < 0.001), while that of mastectomy increased slightly to 37.0% in 2016 (14.5% increase; R2 = 0.984, p < 0.001). In 2017, the rates of BCS and mastectomy were as 67.4%, and 31.6%, respectively; therefore, the rate of BCS increased since 2016 (Figure 7).
Figure 7. Changes in the surgical management of breast cancer.
BCS = breast conserving surgery.
Five-year and 10-year relative survival rates
The KCCR data analysis showed that the RSR of breast cancer is steadily increasing. The 5-year RSR of breast cancer patients diagnosed in 1993–1995 was 79.2%, and it increased steadily to 93.2% in 2013–2017. This is the highest rate among organ cancers that occur in women in Korea, including colon, stomach, lung, liver, cervix, uterine, pancreas, and gallbladder but not thyroid cancer. Moreover, the 10-year RSR of patients increased from 71.8% in 1993–1995 to 87.7% in 2008–2012 [2].
According to the local, regional, and distant cancer stages, based on the summary stage of the Surveillance, Epidemiology, and End Results (SEER) program, the 5-year RSR of breast cancer patients diagnosed in 2013–2017 was 98.7%, 92.2%, and 39.9%, respectively [2]. The 5-year RSR of breast cancer patients diagnosed in in 2007–2011 was 97.8%, 89.9%, and 34.5%, respectively, which suggested that survival rates had improved in the advanced stage as well as the early stage [2].
DISCUSSION
The ASR of most cancers occurring in women in Korea, except for breast and lung cancers showed a decreasing trend since 2007 [2]. However, the ASR of breast cancer has increased steadily since the cancer registry report in 1999, showing an average increase of 10.0% per year between 1999–2002 and 4.7% per year between 2002–2017. This is the largest increase among major cancers occurring in women [2]. As shown in Figure 3, the ASR of breast cancer increased across all age ranges and was particularly high in the ≥ 60 years group.
The causes of increase in breast cancer incidence are diverse and complex. There have been some reports that breast cancer incidence is increasing due to the expansion of screening mammography, which could be supported by the fact that the incidence of early stage breast cancer has increased [13,14]. The life-time screening rate in Korea has significantly increased from 55.9% in 2004 to 83.1% in 2013, and the APC in the screening rate with recommendation for the national breast cancer screening increased by 3.7%, from 33.2% in 2004 to 59.7% in 2013 [15]. Among the 11,162 breast cancer patients registered in the KBCS registry data in 2017, 5,347 (46.0%) patients were diagnosed as carcinoma in situ and small tumor size (≤ 1cm). It is highly probable that these patients would not be clinically detected without screening tests. The improvement in screening rate is considered as an important cause for breast cancer survival as well as increased incidence [16]. In addition, an important and well-known cause of increased incidence of breast cancer is the increased population exposure to risk factors of breast cancer such as reduced fertility and breast feeding, increased exposure to exogenous hormones, obesity and changes in diet and lifestyle [17].
One of the key characteristics of breast cancer in Korea is the higher incidence of young-age breast cancer than in Western countries. This trend is well-observed in other Asian countries. However, the incidence rate is the highest in the 50–54 years old age group in Japan, Singapore, and Taiwan, while the incidence rate is the highest in the 45–49 years old group in Korea [18]. In the United States, the age group with the highest incidence of breast cancer in 2017 was 60–69 years, and the median age was 62 years. This was largely different from those of Korea [19]. The analysis of racial incidence in the United States shows that American-Asians have a higher proportion of breast cancer incidence at a lower age than whites and blacks. In this regard, it is believed that racial factors in addition to social and cultural factors result in national differences [20]. The age group having the highest incidence of breast cancer in Korea from 2000 to 2017 is 40–50 years, while the peak point on the graph is gradually shifting to the right along the X-axis. Because social change took place in Korea within a relatively short time, there is a large difference between generations in terms of reproductive, diet, and lifestyle. If the young age population has a prolonged cumulative exposure to risk factors over time, the baseline risk of breast cancer will increase with age, as in the West, and the age distribution of breast cancer would be similar to that of the West. However, as shown in the racial analysis of the United States, there could be many other differences between Korea and the West [19].
Since 2011, postmenopausal and premenopausal state breast cancer patients accounted for more than 50% and 46–47% of cases, respectively, in Korea [10]. Thus, the importance of precision therapy strategy is emphasized more in premenopausal women than in postmenopausal women. In the KBCS registry data, the number of stage IV patients with distant organ metastasis was 100 (0.8%) in 2017, of whom 46 (46.0%) were premenopausal women. Currently, the endocrine therapeutic options are significantly limited for premenopausal women, as they are mostly focused on postmenopausal women. However, because the effect of endocrine therapy in premenopausal metastatic breast cancer women is equivalent to that of postmenopausal women, it is important in premenopausal metastatic breast cancer women as well [21]. In the recently published MONALEESA-7 trial, cyclin-dependent kinase 4/6 inhibitors have been shown to have clinically and statistically significant gains in overall survival of premenopausal women with metastatic cancer. Thus, Asian countries including Korea, where the incidence of young-age breast cancer is high, should focus on providing endocrine therapy for premenopausal metastatic breast cancer patients and respond promptly through changes in the national health insurance scheme [22].
A continued increase in HR-positive breast cancer rate is a common phenomenon observed in foreign countries [19]. Although no clear cause has been revealed, increased obesity may contribute to increase in breast cancer incidence [23]. HR-positive breast cancer is a favorable prognostic subtype, and it is thought to contribute to an increase in early-stage breast cancer and improvement in breast cancer survival rate [10]. Furthermore, an increase in HR-positive breast cancer and early stage breast cancer provides a variety of adjuvant treatment options. This increase would contribute to the improvement of quality of life for breast cancer patients [24].
The pathologic stage of breast cancer continues to show a favorable pattern over time. The proportion of stage 0 and stage I accounted for only 38.1% of all breast cancers cases in 2002, but it exceeded 50% from 2010 onwards and continued to increase to 60.5% in 2017. The most important cause of this change is the expansion of health checkup. An interesting fact about stage distribution by age group is that there was a difference in stage distribution between the groups of < 40 years, 40–59 years, and ≥ 60 years (p < 0.001) (Supplementary Figure 3). According to the Korean National Cancer Screening Survey, the breast cancer screening test rate is steadily increasing across all ages, including old age [25].
Additionally, a steady change was observed in the surgical procedure. The BCS, which was only 37.6% in 2002, increased to 67.2% in 2012. Although an increase in small-size breast cancer due to the expansion of health checkup could be the most important cause, the expansion of NACT would be one of the main causes [25]. However, it is well-known that the rate of BCS has not been increasing since 2012; rather, it has been decreasing, while the rate of mastectomy has been increasing. This phenomenon is e due to the expansion of breast magnetic resonance imaging, the development of breast reconstruction, the increase in genetic consultations, and the increased participation of patients in determining the surgical methods [26,27]. Unlike this recent trend, the KBCS registry data in 2017 showed an increasing tendency of the BCS, but it is difficult to determine whether such a result is a temporary phenomenon or the beginning of a new trend. The proportions of mastectomy and BCS vary according to country, region, and institution, as well as a patient's age, body mass index, and economic status, and the future pattern is uncertain [28]. In 2017, the proportion of mastectomy was the highest with 38%, 31.9%, and 31.6% in the group of < 40 years, 40–59 years, and ≥ 60 years, respectively. Further studies are necessary to determine the contribution of genetic consultation, breast density, patient preference, and rate of multicentric tumor to this trend. The rate of reconstruction after mastectomy was the highest with 39.1%, 33.7%, and 9.4% in the group of < 40 years, 40–59 years, and ≥ 60 years, respectively, as anticipated. To identify the change in the surgical procedure patterns, it will be necessary to analyze the data for the following several years.
Furthermore, a change was observed in the surgical patterns for axillary lymph node staging. Of 1,208 non-metastatic breast cancer patients with T1 or T2 who received BCS with N1, 440 patients (36.4%) underwent axillary lymph node dissection (ALND) after sentinel lymph node biopsy (SLNB), while 602 patients (49.8%) underwent SLNB only. The remaining patients underwent ALND without SLNB from the beginning. In 2015, 46.1% of 886 patients with the same conditions underwent ALND after SLNB, and only 36.0% underwent SLNB only. This is a meaningful statistic that is considered to reflect the era of American College of Surgeons Oncology Group (ACOSOG) Z0011 trial [26]. Of the 1,114 patients who received NACT, 873 patients (78.4%) underwent SLNB as axillary management. As shown in ACOSOG 1071 and SENTINA trials, SLNB after NACT has been adopted in practice at a high rate due to acceptable false negativity and a high success rate [27,28]. A recent study has reported that the application rate of the ACOSOG 1071 trial was 73% abroad, which is similar to the results of this study [29].
Despite many changes in stages and surgical patterns due to the expansion of medical checkups, the most significant and important change was in survival rate. The 5-year RSR of breast cancer diagnosed between 1993 and 1995 was 79.2%, but due to the continuous improvement, the 5-year RSR of breast cancer diagnosed between 2013 and 2017 was observed as 93.2%. The 5-year RSR of the local, regional, and distant stages of breast cancer diagnosed between 2007 and 2011 was 97.8%, 89.9%, and 34.5%, respectively, while the recent 5-year RSR of breast cancer diagnosed between 2013–2017 was 98.7%, 92.3%, 39.9%, respectively. Therefore, the survival gain was larger in distant and regional stages than in local stages. In the US SEER data, which can be compared with the data in the 1970s, the survival gain was the largest with 20% improvement in the regional stage and the smallest within 10% in the local stage [19]. The significant change in survival gains in regional and distant stages suggested that development in endocrine, chemotherapy, and target therapy have made significant contributions to improvement in breast cancer survival.
CONCLUSION
The incidence rate of breast cancer in Korea is steadily increasing across all ages, and the proportion of early breast cancer continues to increase. The proportion of young breast cancer patients is higher in Korea than in the West, and this has a heavy impact on society as well as individuals and families. Thus, this issue requires active national attention. Furthermore, because there is a difference in the molecular subtype and surgical treatment of breast cancer by age, tailored treatments should be actively considered. To increase the accuracy of Korean breast cancer statistics, increased active participation in the breast cancer registry and accurate data registration is required.
ACKNOWLEDGMENTS
We express our gratitude to all the members participating in the Korean Breast Cancer Society (KBCS) online registry.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Kim YS, Youn HJ.
- Data curation: Kang SY, Kim Z, Kim HY, Kim HJ, Park S, Bae SY, Yoon KH, Lee SB, Lee SK, Jung KW, Han J, Youn HJ.
- Formal analysis: Youn HJ.
- Investigation: Kim YS, Kim Z, Kim HY, Park S, Bae SY, Yoon KH, Lee SK, Jung KW.
- Methodology: Kang SY, Youn HJ.
- Writing - original draft: Kang SY, Youn HJ.
- Writing - review & editing: Kang SY, Youn HJ.
SUPPLEMENTARY MATERIALS
Molecular subtype distribution according to age group.
Trends in age distribution pattern of Korean female breast cancer.
Stage distribution according to age group.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Information Center. [Accessed January 22th, 2020]. https://www.cancer.go.kr.
- 3.World Health Organization. ICD-10 version: 2016. [Accessed January 23th, 2020]. https://icd.who.int/browse10/2016/en#/II.
- 4.International Agency for Research on Cancer. Cancer today. [Accessed January 22th, 2020]. http://gco.iarc.fr/today/home.
- 5.Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019;51:417–430. doi: 10.4143/crt.2019.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Cancer Institute. SEER cancer statistics review, 1975–2004. [Accessed January 22nd, 2020]. https://seer.cancer.gov/csr/1975_2004/
- 7.Segi M, Segi M. Cancer Mortality for Selected Sites in 24 Countries (1950–57) Sendai: Department of Public Health, Tohoku University School of Medicine; 1960. [Google Scholar]
- 8.National Cancer Institute. Previous version: SEER cancer statistics review, 1975–2013. [Accessed January 22th, 2020]. https://seer.cancer.gov/archive/csr/1975_2013/
- 9.Kim HJ, Luo J, Chen HS, Green D, Buckman D, Byrne J, et al. Improved confidence interval for average annual percent change in trend analysis. Stat Med. 2017;36:3059–3074. doi: 10.1002/sim.7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang SY, Kim YS, Kim Z, Kim HY, Lee SK, Jung KW, et al. Basic findings regarding breast cancer in Korea in 2015: data from a breast cancer registry. J Breast Cancer. 2018;21:1–10. doi: 10.4048/jbc.2018.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Joint Committee on Cancer. AJCC cancer staging manual. [Accessed January 22th, 2020]. https://cancerstaging.org/references-tools/deskreferences/Pages/default.aspx.
- 12.American Cancer Society. Breast Cancer Facts & Figures 2017–2018. Atlanta (GA): American Cancer Society; 2017. [Google Scholar]
- 13.Miller BA, Feuer EJ, Hankey BF. The increasing incidence of breast cancer since 1982: relevance of early detection. Cancer Causes Control. 1991;2:67–74. doi: 10.1007/BF00053123. [DOI] [PubMed] [Google Scholar]
- 14.Feuer EJ, Wun LM. How much of the recent rise in breast cancer incidence can be explained by increases in mammography utilization? A dynamic population model approach. Am J Epidemiol. 1992;136:1423–1436. doi: 10.1093/oxfordjournals.aje.a116463. [DOI] [PubMed] [Google Scholar]
- 15.Korean Statistical Information Service. [Accessed January 22th, 2020]. https://kosis.kr/eng/
- 16.Shulman LN, Willett W, Sievers A, Knaul FM. Breast cancer in developing countries: opportunities for improved survival. J Oncol. 2010;2010:595167. doi: 10.1155/2010/595167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkin DM, Fernández LM. Use of statistics to assess the global burden of breast cancer. Breast J. 2006;12(Suppl 1):S70–S80. doi: 10.1111/j.1075-122X.2006.00205.x. [DOI] [PubMed] [Google Scholar]
- 18.Lin CH, Yap YS, Lee KH, Im SA, Naito Y, Yeo W, et al. Contrasting epidemiology and clinicopathology of female breast cancer in Asians vs the US population. J Natl Cancer Inst. 2019;111:1298–1306. doi: 10.1093/jnci/djz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67:439–448. doi: 10.3322/caac.21412. [DOI] [PubMed] [Google Scholar]
- 20.Ko NY, Hong S, Winn RA, Calip GS. Association of insurance status and racial disparities with the detection of early-stage breast cancer. JAMA Oncol. 2020;6:385. doi: 10.1001/jamaoncol.2019.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park IH, Ro J, Lee KS, Kim EA, Kwon Y, Nam BH, et al. Phase II parallel group study showing comparable efficacy between premenopausal metastatic breast cancer patients treated with letrozole plus goserelin and postmenopausal patients treated with letrozole alone as first-line hormone therapy. J Clin Oncol. 2010;28:2705–2711. doi: 10.1200/JCO.2009.26.5884. [DOI] [PubMed] [Google Scholar]
- 22.Hurvitz SA, Im SA, Lu YS, Colleoni M, Franke FA, Bardia A, et al. Phase III MONALEESA-7 trial of premenopausal patients with HR+/HER2− advanced breast cancer (ABC) treated with endocrine therapy ± ribociclib: overall survival (OS) results. J Clin Oncol. 2019;37:LBA1008 [Google Scholar]
- 23.Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103:250–263. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suh M, Choi KS, Park B, Lee YY, Jun JK, Lee DH, et al. Trends in cancer screening rates among Korean men and women: results of the Korean National Cancer Screening Survey, 2004–2013. Cancer Res Treat. 2016;48:1–10. doi: 10.4143/crt.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318:918–926. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14:609–618. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 29.Palmer JA, Flippo-Morton T, Walsh KK, Gusic LH, Sarantou T, Robinson MM, et al. Application of ACOSOG Z1071: effect of results on patient care and surgical decision-making. Clin Breast Cancer. 2018;18:270–275. doi: 10.1016/j.clbc.2017.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Molecular subtype distribution according to age group.
Trends in age distribution pattern of Korean female breast cancer.
Stage distribution according to age group.