Abstract
Purpose
This study aimed to determine the effect of an exercise intervention on subjective cancer-related fatigue (CRF) and pro-inflammatory cytokine levels in breast cancer survivors (BCS).
Methods
BCS with greater than moderate CRF (≥ 4) were recruited and randomly assigned to experimental or control groups. The experimental group participated in a 12-week exercise adherence program (Better Life after Cancer - Energy, Strength, and Support; BLESS). Interleukin (IL)-6 and tumor necrosis factor-α (TNF-α) levels were determined at 3 time points (M1: baseline, M2: post-intervention, and M4: 6 months after intervention). Subjective fatigue was measured using the Korean version of the revised Piper Fatigue Scale.
Results
In this analysis of participants with physiological fatigue measures available (19 experimental, 21 control), there were no statistically significant differences in IL-6 (F = 1.157, p = 0.341), TNF-α levels (F = 0.878, p = 0.436), and level of fatigue (F = 2.067, p = 0.118) between the 2 groups at baseline. Fatigue in the experimental group showed statistically significant improvement compared to the control only at M2 (p = 0.022). There was no significant relationship between subjective and physiological fatigue at the 3 measurement points.
Conclusion
The BLESS intervention improved CRF in BCS immediately at post-intervention, and this study presents clinical feasibility for the management of CRF in BCS in the early survivorship phase who are already experiencing fatigue.
Keywords: Breast neoplasms, Cytokines, Exercise, Fatigue
INTRODUCTION
Breast cancer (BC) is the most common cancer type among women worldwide, with 2.18 million newly-diagnosed cases in 2018, and is a major cause of cancer-related death [1]. However, early detection of BC and standard treatments such as surgery, chemotherapy, radiation therapy, and endocrine therapy have led to an increase in long-term breast cancer survivors (BCS). BCS experience various side effects not only during cancer diagnosis and treatment but also after completion of cancer treatment, of which fatigue is the most common. Cancer-related fatigue (CRF) is a distressing symptom experienced by a large number of BCS, which degrades quality of life (QoL), with 7% to 52% of BCS reporting severe fatigue [2]. Therefore, CRF is a clinical problem to be managed and improved over the long-term; however, effective and systematic intervention strategies are still required.
Physical activity in BCS is strongly recommended, as it has been reported in a systematic literature review to be associated with reduction in cancer mortality [3]. Exercise has also been shown to be a crucial intervention in significantly lowering the fatigue of BCS, particularly among various fatigue interventions such as drug therapy, training and counseling, and complementary therapies [4,5,6,7,8]. Exercise not only reduces fatigue but also improves negative emotions such as depression, anxiety, and distress, and leads to improved self-esteem and QoL [9,10,11]. However, although the short-term effectiveness of exercise has been verified to improve CRF in previous studies, there is a lack of related reports on exercise adherence from a longitudinal perspective [4,5,8,10].
Patients who experience CRF have an increase in the levels of pro-inflammatory cytokines (e.g., tumor necrosis factor-α [TNF-α], interleukin [IL]-1, and IL-6) [11,12]. As physical activity increases, systemic levels of IL-6 increase, while TNF-α levels decrease [12]. Strenuous physical activity rapidly increases IL-6 levels, and the response to moderate intensity repeated exercise also shows a long-term increase in IL-6 levels [13,14]. IL-6 and TNF-α were reported to be significantly associated with self-reported fatigue [15]. These pro-inflammatory cytokines have been used to test the effect of physical activity in the management of CRF, but a consensus has not been reached. Levels of IL-6 and TNF-α were reported to show no significant difference following an exercise intervention [16], and no consistent results were found in longitudinal studies of fatigue [17].
The National Institute of Health Symptom Science Model (NIH-SSM) focuses on improving patient outcomes, as well as QoL, by identifying complex symptoms with biological and clinical data and presenting directions for therapeutic and clinical interventions [18]. CRF in BCS is a complex phenomenon characterized by biobehavioral symptoms, and may be assessed by measuring pro-inflammatory cytokines; CRF can be reduced through an exercise intervention, leading to improvements in QoL. Therefore, this study aimed to identify the effects of a 12-week exercise intervention based on the NIH-SSM on CRF and pro-inflammatory cytokines in BCS.
METHODS
Study design
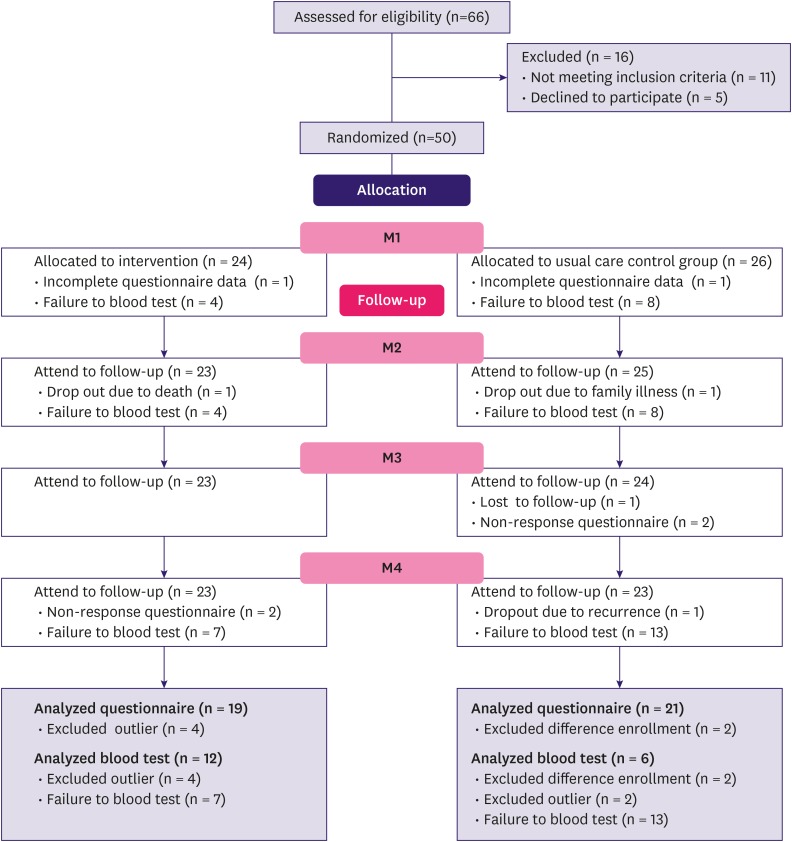
This study was based on data from the Better Life after Cancer - Energy, Strength, and Support (BLESS) study. BLESS is an exercise adherence intervention developed for BCS with greater than moderate fatigue, following a systematic theory-based approach [19]. Estimation of sample size was calculated according to the requirements for multivariate analysis of variance testing. We estimated 46 participants were needed, which was based on the effect size of 0.8 from a prior study [20,21]. Estimating a dropout rate of 20%, a total of 66 participants were assessed for eligibility and 50 BCS were randomly assigned to experimental (n = 24) and control (n = 26) groups (Figure 1). The mean participation rate was over 90%.
Figure 1. CONSORT diagram indicating number of participants who completed each measurement.
M1 = pre-intervention; M2 = post-intervention; M3 = after 1 month; M4 = after 6 months.
Participants
Participant data were obtained from the BLESS study [21], which was conducted at a tertiary hospital in Seoul, Korea, between 2017 and 2018. Ethics approval was given by the Severance Hospital Institutional Review Board (approval number 4-2017-0164). We recruited participants by posting recruitment announcements in the hospital cancer center's outpatient department and through social network systems targeting BCS. While details on inclusion and exclusion criteria are described elsewhere [21], participants were female BCS between 20–69 years who had completed surgery and chemotherapy and were within 5 years of diagnosis. Fatigue was screened on a 0–10 scale and those with greater than moderate fatigue (≥ 4) were eligible. Written informed consent was obtained from all participants before enrollment.
Intervention procedures
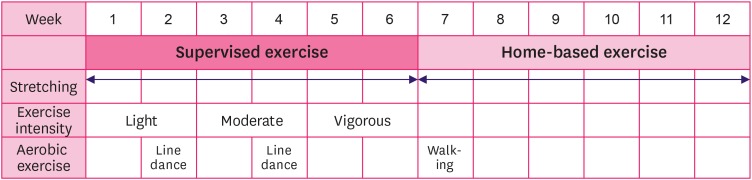
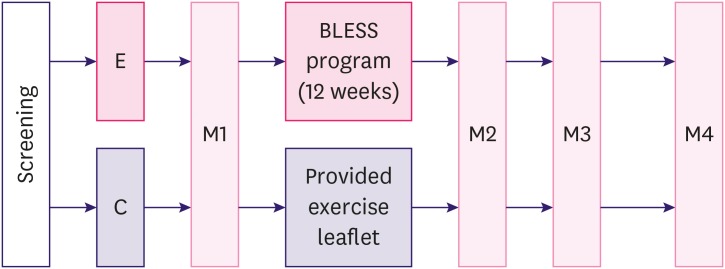
The BLESS exercise adherence program consisted of 8 exercise movements, specific to the needs of BCS, administered once a week for 12 weeks [19]. The experimental group participated in supervised exercise during the first 6 weeks, and then carried out the exercise program at home during the remaining 6 weeks. The BLESS program combined stretching and resistance exercise tailored to the limitations and needs of BCS with a gradual increase in intensity into light, moderate, and vigorous movements. Exercise video clips were provided to encourage adherence and promote accurate practice, and group exercise was done at every measurement point for the experimental group (Figure 2). The BLESS group also participated in small group sessions that aimed to activate social capital [21]. The control group was not prescribed a structured exercise program but received exercise-related handouts and offered the BLESS exercise at the end of the 12-week period. In order to minimize loss, during the follow up period after the end of the 12-week exercise program, we provided special activities e.g., candle making, calligraphy sessions, and other activities at follow up measurement points for both the experimental and the control groups. In order to test the effectiveness of the BLESS program, measurements were taken at 5 time points [21]: This manuscript focuses on the following time points: baseline (M1), post-intervention (M2; 12 weeks later), 1 month later (M3), and at 6 months (M4) after the intervention (Figure 3). Subjective fatigue was measured by self-report questionnaires at all time points, and physiological fatigue was measured at M1, M2, and M4.
Figure 2. BLESS exercise adherence intervention program for experimental group.
BLESS = Better Life after cancer – Energy, Strength, and Support.
Figure 3. Study design for the BLESS program.
BLESS = Better Life after cancer – Energy, Strength, and Support; E = experimental group; C = control group; M1 = baseline; M2 = post-intervention; M3 = after 1 month; M4 = after 6 months.
Measured parameters
Physiological fatigue
IL-6 and TNF-α levels were measured at M1, M2, and M4 using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (KOMA BIOTECH, Seoul, Korea). Briefly, 50 µL of plasma was loaded into wells coated with antibodies and incubated at room temperature for 4 hours on a microplate shaker. Wells were washed with the buffer provided and incubated with detection antibody at room temperature for 2 hours. Finally, plates were read with a spectrophotometric microplate reader at 450 nm. Cytokine concentrations were calculated from the standard curve and dilution factor.
Subjective fatigue
The revised Piper Fatigue Scale (R-PFS) [22] is a commonly used self-reported tool for cancer-related studies that can measure fatigue in a multidimensional manner. CRF was measured using the Korean version of R-PFS (R-PFS-K) [23]. This tool contains a total of 19 items with 4 subcategories: Behavioral/Severity (6 items), Affective Meaning (4 items), Sensory (4 items), and Cognitive/mood (5 items). The degree of fatigue currently experienced is assessed using a 0 to 10 scale; the higher the score, the more severe the fatigue. This tool previously showed high reliability (Cronbach's α = 0.97) [23]; in this study, Cronbach's α ranged from 0.92 to 0.94 across the subcategories.
Demographic and clinical factors
The examined demographic factors were age, marital status, income, employment status, religion, economic burden, education level, and whether they had children. Clinical factors related to BC included cancer stage, surgery type, time since diagnosis, chemotherapy, radiation therapy, endocrine therapy, target therapy, and triple-negative BC status.
Statistical analysis
The collected data were analyzed using SPSS for Windows version 23.0 (IBM Corp., Chicago, IL, USA). The homogeneity of the experimental and control groups were tested at baseline using t-test or chi-square test for each factor as appropriate. The CONSORT diagram presented in Figure 1 depicts the number of participants who completed each measurement. The effect of the BLESS program on the physiological and subjective fatigue measured at M1, M2, M3, and M4 was tested using a repeated-measures analysis of variance (ANOVA). Data could not be obtained in cases of missing questionnaire data at the particular time point or blood sampling not being performed due to refusal or insufficient blood volume. The physiological data of 6 participants who had much higher than average IL-6 and TNF-α levels due to health-related events, such as chemotherapy or radiation therapy in the last 3 months or undergoing periodontitis treatment, were identified as outliers and excluded from analysis, resulting in 40 participants. Differences in changes in the measured parameters were tested using a fixed effects model with a linear mixed model, which included all missing values in the analysis. M1 values were considered as baseline and comparisons were made between time points and groups; the interaction effect between time points and groups was also tested. Bonferroni correction was used to adjust for multiple comparisons. Time point differences were tested using repeated-measures ANOVA; 2 participants were excluded from this analysis due to enrolling at different time points. The level of statistical significance was set at p < 0.05.
RESULTS
Demographic and clinical factors
A total of 40 BCS with subjective fatigue data at all 4 time points were analyzed (19 in the experimental group, 21 in the control) and their demographic and clinical characteristics are shown in Table 1. There was no significant heterogeneity between the groups (p ≥ 0.05). The average age of participants was 49.0 ± 7.35 years. The majority of participants were diagnosed with either stage 1 or 2. More BCS had received breast conserving surgery than mastectomy. Most participants (85.8%–89.5%) had been diagnosed with BC within the last 2 years or less and had undergone not only chemotherapy but also radiation therapy. About half of the participants (45%) had undergone or were currently undergoing endocrine therapy (Table 1). The level of physical activity did not differ between the 2 groups at baseline [21].
Table 1. Demographics and clinical characteristics.
| Parameter | Exp. (n = 19) | Cont. (n = 21) | χ2 or t (p value) | |
|---|---|---|---|---|
| Mean ± SD (range) or No. (%) | Mean ± SD (range) or No. (%) | |||
| Age (years)* | 49.95 ± 8.12 (34–67) | 48.14 ± 6.67 (33–62) | ||
| ≤ 39 | 1 (5.3) | 2 (9.5) | 2.280 (0.623) | |
| 40–49 | 9 (47.4) | 10 (47.6) | ||
| 50–59 | 7 (36.8) | 9 (42.9) | ||
| ≥ 60 | 2 (10.5) | - | ||
| Marital status | 0.007 (0.935) | |||
| Married | 12 (63.2) | 13 (61.9) | ||
| Single/widowed/divorced | 7 (36.8) | 8 (38.1) | ||
| Income (KRW) | 0.973 (0.324) | |||
| < 3 million | 12 (63.2) | 10 (47.6) | ||
| ≥ 3 million | 7 (36.8) | 11 (52.4) | ||
| Employment status | 1.069 (0.301) | |||
| No | 13 (68.4) | 11 (52.4) | ||
| Yes | 6 (31.6) | 10 (47.6) | ||
| Religion | 0.043 (0.836) | |||
| No | 6 (31.6) | 6 (28.6) | ||
| Yes | 13 (38.4) | 15 (71.4) | ||
| Economic burden | 2.431 (0.119) | |||
| No | 8 (42.1) | 14 (66.3) | ||
| Yes | 11 (57.9) | 7 (33.4) | ||
| Education level | 2.824 (0.093) | |||
| ≤ High school | 14 (73.7) | 10 (47.6) | ||
| ≥ College | 5 (26.3) | 11 (52.4) | ||
| Children* | 0.302 (0.712) | |||
| No | 5 (26.3) | 4 (19.0) | ||
| Yes | 14 (73.7) | 17 (81.0) | ||
| Stage | 1.269 (0.530) | |||
| I | 4 (21.1) | 7 (33.4) | ||
| II | 10 (52.6) | 11 (52.4) | ||
| III | 5 (26.3) | 3 (14.2) | ||
| Surgery type | 0.935 (0.334) | |||
| Mastectomy | 3 (31.6) | 6 (28.6) | ||
| Breast conservation | 16 (68.4) | 15 (71.4) | ||
| Time since diagnosis (years)* | 0.974 (0.674) | |||
| < 1 | 6 (31.6) | 9 (42.9) | ||
| 1–2 | 11 (57.9) | 9 (42.9) | ||
| ≥ 2 | 2 (10.5) | 3 (14.2) | ||
| Chemotherapy* | 1.905 (0.488) | |||
| None | - | 2 (9.5) | ||
| Completed | 19 (100) | 19 (90.5) | ||
| Radiation therapy* | 1.778 (0.738) | |||
| None | - | 2 (9.5) | ||
| Completed | 18 (94.7) | 18 (85.7) | ||
| Currently | 1 (5.3) | 1 (4.8) | ||
| Endocrine therapy | 0.082 (0.775) | |||
| No | 10 (52.6) | 12 (57.2) | ||
| Yes | 9 (47.4) | 9 (42.8) | ||
| Target therapy* | 2.899 (0.217) | |||
| No | 11 (57.9) | 17 (81.0) | ||
| Yes | 7 (36.8) | 4 (19.0) | ||
| Unknown | 1 (5.3) | - | ||
| Triple-negative* | 1.655 (0.525) | |||
| No | 13 (68.4) | 12 (57.1) | ||
| Yes | 5 (26.3) | 5 (23.8) | ||
| Unknown | 1 (5.3) | 4 (19.1) | ||
Cont = control group; Exp = experimental group; SD = standard deviation; KRW = Korean Won.
*Fisher's exact test.
Differences in physiological fatigue
As presented in Table 2, although there were no differences in IL-6 (t = 0.861, p = 0.397) and TNF-α (t = 1.698, p = 0.111) levels between the 2 groups at M1, overall IL-6 levels showed an increasing trend over time (also noted in Supplementary Figure 1), which was statistically significant (F = 9.881, p = 0.002). The IL-6 levels showed statistically meaningful time effects, with M4 time point levels increasing to higher levels than at M1 and M2 time points (F = 9.492, p < 0.001). However, there was no significant group difference (F = 2.310, p = 0.148). There were no significant differences in TNF-α levels between groups (F = 1.039, p = 0.323) or between measurement time points (F = 1.475, p = 0.260). Regarding the effect of the BLESS program, there were no statistically significant differences in IL-6 (F = 1.157, p = 0.341) and TNF-α levels (F = 0.878, p = 0.436) between the experimental and control groups at M4. Further analysis of the changes in IL-6 and TNF-α levels in each group (Table 3) showed that IL-6 levels were elevated at M4 compared to M1 (p < 0.001) and M2 (p < 0.001) only in the experimental group, while TNF-α decreased at M4 compared to M1 (p = 0.042).
Table 2. Parameter estimates of IL-6 and TNF-α levels.
| Parameter | Time | Exp. (n = 12) | Cont. (n = 6) | Sources | F | p value | ||
|---|---|---|---|---|---|---|---|---|
| Mean ± SE | (95% CI) | Mean ± SE | (95% CI) | |||||
| IL-6 (pg/mL) | M1 | 14.11 ± 2.11 | (9.63–18.59) | 9.23 ± 2.99 | (2.90–15.57) | Group | 2.310 | 0.148 |
| M2 | 13.85 ± 2.16 | (9.26–18.43) | 9.18 ± 3.05 | (2.71–15.65) | Time | 9.881 | 0.002 | |
| M4 | 18.23 ± 2.07 | (13.83–22.62) | 11.40 ± 2.93 | (5.18–17.62) | Group × Time | 1.157 | 0.341 | |
| TNF-α (pg/mL) | M1 | 78.40 ± 15.13 | (46.33–110.47) | 50.35 ± 21.39 | (5.00–95.70) | Group | 1.039 | 0.323 |
| M2 | 74.18 ± 14.92 | (42.55–105.82) | 50.07 ± 21.10 | (5.33–94.81) | Time | 1.475 | 0.260 | |
| M4 | 60.73 ± 7.32 | (45.21–76.26) | 47.12 ± 10.36 | (25.16–69.07) | Group × Time | 0.878 | 0.436 | |
CI = confidence interval; Cont = control group; Exp = experimental group; IL-6= interleukin-6; M1 = baseline; M2 = post-intervention; M4 = after 6 months; SE = standard error; TNF-α = tumor necrosis factor-α.
Table 3. Pairwise comparisons estimate of IL-6 and TNF-α levels (n = 18).
| Group | Time points | Mean or mean difference | SE | p value | 95% CI | |
|---|---|---|---|---|---|---|
| Exp. + Cont. | M1 | IL-6 | 11.67 | 1.83 | NA | 15.55, 11.67 |
| M2 | IL-6 | 11.51 | 1.87 | NA | 15.48, 11.52 | |
| M4 | IL-6 | 14.81 | 1.80 | NA | 18.62, 14.81 | |
| M1 | TNF-α | 64.38 | 13.10 | NA | 92.15, 64.38 | |
| M2 | TNF-α | 62.13 | 12.92 | NA | 89.52, 62.13 | |
| M4 | TNF-α | 53.93 | 6.34 | NA | 67.37, 53.93 | |
| Exp. IL-6 | M1 | M2 | 0.26 | 0.27 | 0.349 | −0.31, 0.83 |
| M4 | −4.12 | 0.91 | < 0.001 | −6.05, −2.19 | ||
| M2 | M1 | −0.26 | 0.27 | 0.349 | −0.83, 0.31 | |
| M4 | −4.38 | 0.85 | < 0.001 | −6.18, −2.57 | ||
| M4 | M1 | 4.12 | 0.91 | < 0.001 | 2.19, 6.05 | |
| M2 | 4.38 | 0.85 | < 0.001 | 2.57, 6.18 | ||
| Cont. IL-6 | M1 | M2 | 0.05 | 0.38 | 0.897 | −0.75, 0.85 |
| M4 | −2.17 | 1.29 | 0.112 | −4.90, 0.56 | ||
| M2 | M1 | −0.05 | 0.38 | 0.897 | −0.85, 0.75 | |
| M4 | −2.22 | 1.20 | 0.084 | −4.77, 0.33 | ||
| M4 | M1 | 2.17 | 1.29 | 0.112 | −0.56, 4.90 | |
| M2 | 2.22 | 1.20 | 0.084 | −0.33, 4.77 | ||
| Exp. TNF-α | M1 | M2 | 4.22 | 2.02 | 0.054 | −0.07, 8.51 |
| M4 | 17.67 | 7.98 | 0.042 | 0.74, 34.59 | ||
| M2 | M1 | −4.22 | 2.02 | 0.054 | −8.51, 0.07 | |
| M4 | 13.45 | 7.72 | 0.101 | −2.91, 29.81 | ||
| M4 | M1 | −17.67 | 7.98 | 0.042 | −34.59, −0.74 | |
| M2 | −13.45 | 7.72 | 0.101 | −29.81, 2.91 | ||
| Cont. TNF-α | M1 | M2 | 0.28 | 2.86 | 0.922 | −5.78, 6.35 |
| M4 | 3.23 | 11.29 | 0.778 | −20.70, 27.17 | ||
| M2 | M1 | −0.28 | 2.86 | 0.922 | −6.35, 5.78 | |
| M4 | 2.95 | 10.92 | 0.790 | −20.19, 26.09 | ||
| M4 | M1 | −3.23 | 11.29 | 0.778 | −27.17, 20.70 | |
| M2 | −2.95 | 10.92 | 0.790 | −26.09, 20.19 | ||
CI = confidence interval; Cont = control group; Exp = experimental group; IL-6= interleukin-6; M1 = baseline; M2 = post-intervention; M4 = after 6 months; NA = not applicable; SE = standard error; TNF-α = tumor necrosis factor-α.
Differences in subjective fatigue
While a fatigue score of ≥ 4 out of 10 was an inclusion criterion, the mean total fatigue level of BCS at baseline was within moderate levels (5.35 experimental, 5.56 control) and did not differ between the groups (t = −0.374, p = 0.711). Subdomain fatigue scores at baseline did not differ either: behavioral/severity (t = −0.100, p = 0.921), affective meaning (t = −0.004, p = 0.997), sensory (t = −1.264, p = 0.214), and cognitive/mood (t = 0.014, p = 0.989). Regarding the effect of the BLESS program, the experimental group dropped to low (< 4) fatigue level immediately after the intervention (3.88 ± 0.32, p = 0.022) compared to the control group, which remained at moderate level (4.95 ± 0.31). The difference between the groups was statistically significant for fatigue level.
The total fatigue level showed statistically meaningful time effects, with levels decreasing across the time points (F = 9.492, p < 0.001). All of the following fatigue subdomain scores also showed time effects: behavioral/severity (F = 13.238, p < 0.001), affective meaning (F = 3.725, p < 0.05), sensory (F = 4.544, p < 0.01), and cognitive/mood (F = 5.949, p < 0.01) decreased over the time points. However, there were no significant differences in both total and subdomain fatigue scores when analyzed for group effects as well as for group-time effects (Table 4).
Table 4. Comparison of subjective fatigue levels.
| Parameter | Time | Exp. (n = 19) | Cont. (n = 21) | t | p value | Sources | F | p value |
|---|---|---|---|---|---|---|---|---|
| Mean ± SE (95% CI) | Mean ± SE (95% CI) | |||||||
| Total | M1 | 5.35 ± 0.41 (4.53–6.17) | 5.56 ± 0.39 (4.78–6.33) | −0.374 | 0.711 | Group | 1.784 | 0.189 |
| M2 | 3.88 ± 0.32 (3.22–4.53) | 4.95 ± 0.31 (4.32–5.57) | −2.393 | 0.022 | Time | 9.492 | < 0.001 | |
| M3 | 4.15 ± 0.41 (3.32–4.98) | 4.33 ± 0.41 (3.51–5.16) | −0.413 | 0.682 | Group × Time | 2.069 | 0.118 | |
| M4 | 3.68 ± 0.42 (2.87–4.53) | 4.59 ± 0.41 (3.77–5.40) | −1.415 | 0.166 | ||||
| Behavioral/severity | M1 | 5.60 ± 0.46 (4.74–6.37) | 5.66 ± 0.44 (4.68–6.24) | −0.100 | 0.921 | Group | 0.920 | 0.344 |
| M2 | 3.82 ± 0.43 (3.13–4.63) | 5.12 ± 0.41 (4.30–5.74) | −2.137 | 0.039 | Time | 13.238 | < 0.001 | |
| M3 | 4.04 ± 0.50 (2.98–4.70) | 4.24 ± 0.49 (3.46–4.87) | −0.492 | 0.626 | Group × Time | 2.513 | 0.069 | |
| M4 | 3.70 ± 0.52 (2.87–4.69) | 4.25 ± 0.50 (3.33–5.11) | −0.652 | 0.519 | ||||
| Affective meaning | M1 | 6.09 ± 0.57 (4.94–7.24) | 6.10 ± 0.55 (5.01–7.19) | −0.004 | 0.997 | Group | 0.627 | 0.433 |
| M2 | 5.04 ± 0.47 (4.10–5.98) | 5.86 ± 0.44 (4.96–6.75) | −1.245 | 0.221 | Time | 3.725 | 0.018 | |
| M3 | 5.09 ± 0.55 (3.99–6.19) | 5.17 ± 0.54 (4.08–6.26) | −0.139 | 0.890 | Group × Time | 1.290 | 0.290 | |
| M4 | 4.41 ± 0.55 (3.30–5.52) | 5.50 ± 0.53 (4.43–6.56) | −1.234 | 0.226 | ||||
| Sensory | M1 | 4.82 ± 0.53 (3.76–5.88) | 5.70 ± 0.50 (4.69–6.71) | −1.264 | 0.214 | Group | 3.557 | 0.067 |
| M2 | 3.47 ± 0.45 (2.57–4.37) | 4.87 ± 0.43 (4.01–5.73) | −2.153 | 0.038 | Time | 4.544 | 0.007 | |
| M3 | 3.84 ± 0.55 (2.73–4.95) | 4.36 ± 0.56 (3.23–5.48) | −0.676 | 0.504 | Group × Time | 0.440 | 0.726 | |
| M4 | 3.30 ± 0.53 (2.23–4.37) | 4.36 ± 0.51 (3.33–5.39) | −1.400 | 0.171 | ||||
| Cognitive/mood | M1 | 4.89 ± 0.44 (4.01–5.78) | 4.89 ± 0.42 (4.05–5.72) | 0.014 | 0.989 | Group | 0.903 | 0.348 |
| M2 | 3.34 ± 0.37 (2.58–4.09) | 4.08 ± 0.36 (3.36–4.79) | −1.383 | 0.175 | Time | 5.949 | 0.002 | |
| M3 | 3.78 ± 0.46 (2.84–4.72) | 3.79 ± 0.47 (2.84–4.74) | −0.092 | 0.927 | Group × Time | 1.082 | 0.366 | |
| M4 | 3.36 ± 0.44 (2.48–4.25) | 4.30 ± 0.42 (3.45–5.16) | −1.398 | 0.171 |
CI = confidence interval; Cont = control group; Exp = experimental group; M1 = baseline; M2 = post-intervention; M3 = after 1 month; M4 = after 6 months; SE = standard error.
Relationship between physiological and subjective fatigue
There was a significant strong positive correlation between IL-6 and TNF-α levels at M1, M2, and M4 (r = 0.658 to 0.992, p < 0.001). Subjective fatigue scores showed a significant moderate positive correlation at M1, M2, M3, and M4 (r = 0.444 to 0.694, p <0.001). However, there was no significant correlation between physiological and subjective fatigue at any of the time points (Table 5).
Table 5. Relationship between physiological and subjective fatigue.
| I (M1) | I (M2) | I (M4) | T (M1) | T (M2) | T (M4) | F (M1) | F (M2) | F (M3) | |
|---|---|---|---|---|---|---|---|---|---|
| I (M1) | - | ||||||||
| I (M2) | 0.819* | ||||||||
| I (M4) | 0.912* | 0.921* | |||||||
| T (M1) | 0.818* | 0.733* | 0.852* | ||||||
| T (M2) | 0.658* | 0.924* | 0.835* | 0.797* | |||||
| T (M4) | 0.952* | 0.954* | 0.866* | 0.988* | 0.992* | ||||
| F (M1) | −0.114 | 0.024 | 0.202 | 0.188 | 0.196 | 0.284 | |||
| F (M2) | −0.350 | −0.274 | −0.162 | −0.206 | −0.189 | −0.164 | 0.575* | ||
| F (M3) | −0.268 | −0.223 | 0.122 | −0.077 | −0.100 | −0.043 | 0.553* | 0.694* | |
| F (M4) | −0.062 | −0.021 | 0.215 | 0.257 | 0.242 | 0.295 | 0.498* | 0.566* | 0.444* |
F = Korean version of revised Piper Fatigue Scale; I = interleukin-6; M1 = baseline; M2 = post-intervention; M3 = after 1 month; M4 = after 6 months; T = tumor necrosis factor-α.
*p < 0.001.
DISCUSSION
This study examined the effects of the BLESS exercise adherence program on CRF and physiological parameters (IL-6 and TNF-α levels) among BCS. The findings from this study indicate that subjective perception of fatigue decrease over time in BCS, while physiological factors had distinct patterns. In contrast to previous studies that lacked a theoretical framework to explain the relationship between pro-inflammatory markers, self-reported fatigue scores, and effects of exercise [12,13,17,24], this study used the NIH-SSM framework [18] for the characterization of biobehavioral symptoms [25]. Using this model, we characterized CRF as being the result of a complex interaction between symptoms and pro-inflammatory cytokine levels according to the effects of the exercise program. Although the BLESS program did not lead to statistically significant improvement of physiological fatigue and there was a time effect of subjective fatigue decreasing overall, we found that subjective perception of fatigue decreased significantly in the experimental group at M2. As such, the theoretical basis for the usefulness of the NIH-SSM for CRF management in BCS was supported. The total fatigue mean score of BCS at baseline was at moderate level, ranging from 5.35 to 5.56 points, with a subsequent decrease in CRF over time. This baseline level is similar to the total fatigue mean score of 5.2 in a study using the R-PFS [22] with Spanish BCS within 2 years of BC diagnosis who had completed active treatment [26]. However, it is higher than the score of 3.83 in a study of Korean BCS 6 months to 2 years after completion of treatment [27], most likely because our study focused on recruiting BCS with greater than moderate CRF (≥ 4).
In several studies with BCS [4,5,6,7] exercise was a significant factor in improving fatigue, and a meta-analysis [8] showed that exercise interventions reduce CRF and brings short-term improvements in physical functioning of BCS. In this analysis, total fatigue level of the experimental group was statistically significantly lowered immediately after the BLESS program. This is in agreement with the results of a previous study of Korean breast cancer patients who completed chemotherapy [10]. Although this particular study reported that a 4-weeks exercise-based rehabilitation program reduced fatigue after 8 weeks, our study demonstrated that longer interventions that incorporate home-based exercise are feasible and effective for fatigue alleviation. The BLESS program was a feasible-program that provides accurate and safe exercise training designed by exercise professionals; starting with supervised exercise but transitioning to home-based exercise adherence.
The high participation rate of our study also suggests that BCS in the early survivorship stage who are experiencing fatigue may perhaps prefer exercise interventions that are longer in structure if they effectively engage participants. In the present study, the exercise participants' fatigue scores decreased over time, but moderate levels of CRF of 4 points or greater persisted in the control group. In the experimental group, both immediately after the exercise intervention and after 6 months, CRF was reduced to a mild level of 4 points or less, which has significance for clinical interpretation.
This study failed to find a significant relationship between physiological and subjective fatigue, which contrasts with Bower et al.'s [17] findings that IL-6 and TNF-α levels were associated with self-reported CRF over a period of 6 years after treatment (M = 4.3 years). Although it is possible our physiological measurement points may have not been optimal to sufficiently examine potential relationships between subjective and physiological fatigue, the mechanisms by which CRF occurs in cancer survivors are not yet fully explained. The prevailing consensus is that CRF is influenced by multidimensional factors such as physiological, psycho-emotional, and sociological factors [28]. The R-PFS tool used in this study is a self-reported fatigue assessment that is a multidimensional measure of the overall fatigue including behavioral/severity, affective meaning, sensory, and cognitive/mood aspects, whereas IL-6 and TNF-α measurement is an objective indicator that represents physiological mechanisms. Fatigue, like pain, is a subjective assessment of individual thresholds and strengths, so it should comprehensively cover mental, spiritual, and social fatigue as well as the physical fatigue experienced by patients. Another possible explanation is that our participants may have underestimated their fatigue level on self-report. In Korea, traditional Confucianism is deeply rooted, and many BCS struggle to continue to perform multiple family roles and bear the burden and stress of child rearing and living as a devoted mother and wife, which continues within the cancer trajectory. Even in the presence of extreme physical fatigue, it is possible that Korean BCS are less attuned to their level of fatigue due to the social constructs of perseverance and endurance. Therefore, the association between measures of subjective and physiological fatigue should be carefully interpreted in light of the socio-cultural context factors of BCS and explored further in future studies.
This study did not find significant group or group-time effects in IL-6 and TNF-α levels between the experimental and control groups. Our finding was consistent with reports of previous studies, which found no significant changes in IL-6, TNF-α, and other pro-inflammatory cytokine levels between groups after an exercise intervention [13,16,24]. In this study, however, there was a significant time effect of increasing IL-6 levels after 6 months compared to baseline, only in the experimental group, which supports the results of previous studies reporting that IL-6 levels show a long-term increase after moderate-intensity repeated exercise [12,13,14]. As elevation of pro-inflammatory cytokine levels is interpreted as an indicator of acute inflammation and can also reflect acute and chronic disease development and cancer prognosis [24,28], we should be careful in selecting pro-inflammatory cytokines for fatigue measurement.
This study presents some limitations. Although participant selection bias was minimized by the randomized clinical trial (RCT) design, a small sample size was used. We were also unable to prevent a high drop-out rate in blood testing in the longitudinal follow-up. This was especially notable in the control group, although they were provided with standard exercise information and special activities during the follow-up period. Therefore, the effectiveness of exercise adherence interventions should be studied further with larger sample size RCTs. Another limitation is that sampling was not conducted at the same time (e.g., morning or afternoon), but performed when the participants could come for sampling. Future studies should explore potential seasonal effects in pro-inflammatory cytokine levels by minimizing circadian changes through blood sampling at the same time of day, including all 4 seasons. In addition, although the BLESS program trained participants using light, moderate, and high-intensity exercises in addition to providing video clips, the home-based exercise was based on self-report, and exercise adherence according to exercise intensity could not be verified in detail.
Despite these limitations, this study was unique in that BCS already experiencing fatigue, a known barrier to practicing exercise, were recruited and successfully engaged over 12 weeks, with significant reduction in subjective fatigue at post-intervention. A strength of this study was that light, moderate, and vigorous intensity exercises tailored to BCS limitations and needs were developed and video clips were provided. However, a variety of creative multidisciplinary patient-centered exercise programs are in demand, so patients can adhere to exercise without time and space restrictions in a self-care context. Future studies could benefit from using augmented reality or virtual reality, after familiarization with the exercise program with an expert trainer. Also, this study employed a longitudinal design to evaluate the effects of exercise in CRF, reflecting both physiological and subjective fatigue, which had not been possible in previous cross-sectional studies.
Footnotes
Funding: This research was supported by the Basic Science Research Program through the National Research Foundation for Korea (NRF) funded by the Ministry of Education (grant number 2015R1D1A1A01059846).
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Kim SH, Song YK, Han J, Ko YH, Lee H, Kang MJ, Lee H, Kim S.
- Data curation: Kim SH, Song YK, Han J, Ko YH, Lee H, Kang MJ, Kim S.
- Formal analysis: Kim SH, Park H, Lee H, Kim S.
- Funding acquisition: Kim S.
- Investigation: Kim SH, Song YK, Han J, Ko YH, Lee H, Kang MJ, Kim S.
- Methodology: Kim SH, Park H, Lee H, Kim S.
- Project administration: Kim SH, Song YK, Han J, Ko YH, Lee H, Kang MJ, Kim S.
- Resources: Kim SH, Song YK, Han J, Ko YH, Lee H, Kang MJ, Park H, Lee H, Kim S.
- Software: Kim S.
- Supervision: Lee H, Kim S.
- Validation: Kim SH, Park H, Lee H, Kim S.
- Visualization: Kim SH, Kim S.
- Writing - original draft: Kim SH, Song YK, Han J, Ko YH, Lee H, Kang MJ, Park H, Kim S.
- Writing - review & editing: Kim SH, Song YK, Han J, Ko YH, Lee H, Kang MJ, Park H, Lee H, Kim S.
SUPPLEMENTARY MATERIAL
Pattern of physiological and subjective fatigue level according to time points. (A) Subjective fatigue over time in the experimental and control groups. (B) Interleukin-6 in the experimental and control groups across three measurement points. (C) Tumor necrosis factor-α in the experimental and control groups across three measurement points.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Abrahams HJ, Gielissen MF, Schmits IC, Verhagen CA, Rovers MM, Knoop H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: a meta-analysis involving 12 327 breast cancer survivors. Ann Oncol. 2016;27:965–974. doi: 10.1093/annonc/mdw099. [DOI] [PubMed] [Google Scholar]
- 3.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25:1293–1311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 4.Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961–968. doi: 10.1001/jamaoncol.2016.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilfiker R, Meichtry A, Eicher M, Nilsson Balfe L, Knols RH, Verra ML, et al. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med. 2018;52:651–658. doi: 10.1136/bjsports-2016-096422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20:123–133. doi: 10.1158/1055-9965.EPI-10-0988. [DOI] [PubMed] [Google Scholar]
- 7.Tian L, Lu HJ, Lin L, Hu Y. Effects of aerobic exercise on cancer-related fatigue: a meta-analysis of randomized controlled trials. Support Care Cancer. 2016;24:969–983. doi: 10.1007/s00520-015-2953-9. [DOI] [PubMed] [Google Scholar]
- 8.Juvet LK, Thune I, Elvsaas IK, Fors EA, Lundgren S, Bertheussen G, et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: a meta-analysis. Breast. 2017;33:166–177. doi: 10.1016/j.breast.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 9.McClellan R. Exercise programs for patients with cancer improve physical functioning and quality of life. J Physiother. 2013;59:57. doi: 10.1016/S1836-9553(13)70150-4. [DOI] [PubMed] [Google Scholar]
- 10.Do J, Cho Y, Jeon J. Effects of a 4-week multimodal rehabilitation program on quality of life, cardiopulmonary function, and fatigue in breast cancer patients. J Breast Cancer. 2015;18:87–96. doi: 10.4048/jbc.2015.18.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolak A, Kamińska M, Wysokińska E, Surdyka D, Kieszko D, Pakieła M, et al. The problem of fatigue in patients suffering from neoplastic disease. Contemp Oncol (Pozn) 2017;21:131–135. doi: 10.5114/wo.2017.68621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmer P, Baumann FT, Oberste M, Schmitt J, Joisten N, Hartig P, et al. Influence of personalized exercise recommendations during rehabilitation on the sustainability of objectively measured physical activity levels, fatigue, and fatigue-related biomarkers in patients with breast cancer. Integr Cancer Ther. 2018;17:306–311. doi: 10.1177/1534735417713301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogers LQ, Fogleman A, Trammell R, Hopkins-Price P, Vicari S, Rao K, et al. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integr Cancer Ther. 2013;12:323–335. doi: 10.1177/1534735412449687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieman DC, Dumke CL, Henson DA, McAnulty SR, Gross SJ, Lind RH. Muscle damage is linked to cytokine changes following a 160-km race. Brain Behav Immun. 2005;19:398–403. doi: 10.1016/j.bbi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Bower JE, Ganz PA, Irwin MR, Castellon S, Arevalo J, Cole SW. Cytokine genetic variations and fatigue among patients with breast cancer. J Clin Oncol. 2013;31:1656–1661. doi: 10.1200/JCO.2012.46.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swisher AK, Abraham J, Bonner D, Gilleland D, Hobbs G, Kurian S, et al. Exercise and dietary advice intervention for survivors of triple-negative breast cancer: effects on body fat, physical function, quality of life, and adipokine profile. Support Care Cancer. 2015;23:2995–3003. doi: 10.1007/s00520-015-2667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bower JE, Wiley J, Petersen L, Irwin MR, Cole SW, Ganz PA. Fatigue after breast cancer treatment: biobehavioral predictors of fatigue trajectories. Health Psychol. 2018;37:1025–1034. doi: 10.1037/hea0000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cashion AK, Gill J, Hawes R, Henderson WA, Saligan L. National Institutes of Health Symptom Science Model sheds light on patient symptoms. Nurs Outlook. 2016;64:499–506. doi: 10.1016/j.outlook.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S, Ko YH, Song Y, Kang MJ, Lee H, Kim SH, et al. Development of an exercise adherence program for breast cancer survivors with cancer-related fatigue-an intervention mapping approach. Support Care Cancer. 2019;27:4745–4752. doi: 10.1007/s00520-019-04785-2. [DOI] [PubMed] [Google Scholar]
- 20.Sandel SL, Judge JO, Landry N, Faria L, Ouellette R, Majczak M. Dance and movement program improves quality-of-life measures in breast cancer survivors. Cancer Nurs. 2005;28:301–309. doi: 10.1097/00002820-200507000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Kim S, Ko YH, Song Y, Kang MJ, Lee H, Kim SH, et al. Pre-post analysis of a social capital-based exercise adherence intervention for breast cancer survivors with moderate fatigue: a randomized controlled trial. Support Care Cancer. 2020 doi: 10.1007/s00520-020-05363-7. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 22.Piper BF, Dibble SL, Dodd MJ, Weiss MC, Slaughter RE, Paul SM. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25:677–684. [PubMed] [Google Scholar]
- 23.Lee EH. Construct validity of the revised Piper Fatigue Scale in Korean women with breast cancer. J Korean Acad Nurs. 1999;29:485–493. [Google Scholar]
- 24.Jones SB, Thomas GA, Hesselsweet SD, Alvarez-Reeves M, Yu H, Irwin ML. Effect of exercise on markers of inflammation in breast cancer survivors: the Yale exercise and survivorship study. Cancer Prev Res (Phila) 2013;6:109–118. doi: 10.1158/1940-6207.CAPR-12-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page GG, Corwin EJ, Dorsey SG, Redeker NS, McCloskey DJ, Austin JK, et al. Biomarkers as common data elements for symptom and self‐management science. J Nurs Scholarsh. 2018;50:276–286. doi: 10.1111/jnu.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galiano-Castillo N, Ariza-García A, Cantarero-Villanueva I, Fernández-Lao C, Díaz-Rodríguez L, Arroyo-Morales M. Depressed mood in breast cancer survivors: associations with physical activity, cancer-related fatigue, quality of life, and fitness level. Eur J Oncol Nurs. 2014;18:206–210. doi: 10.1016/j.ejon.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Seo MH, Lim KH. The effects of fatigue and distress on self-efficacy among breast cancer survivors. Korean J Adult Nurs. 2016;28:378–387. [Google Scholar]
- 28.Wang N, Yang Z, Miao J, Mi X, Liu S, Stern C, et al. Clinical management of cancer-related fatigue in hospitalized adult patients: a best practice implementation project. JBI Database Syst Rev Implement Reports. 2018;16:2038–2049. doi: 10.11124/JBISRIR-2017-003769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pattern of physiological and subjective fatigue level according to time points. (A) Subjective fatigue over time in the experimental and control groups. (B) Interleukin-6 in the experimental and control groups across three measurement points. (C) Tumor necrosis factor-α in the experimental and control groups across three measurement points.