Abstract
Introduction
Childhood obesity and inactivity are associated with cardiovascular risk. Evidence is limited for exercise effects on arterial health in children.
Methods
One hundred seventy-five inactive children with overweight or obesity (8–11 years, ≥85th percentile BMI, 61% female, 87% Black, 73% with obesity) were randomized to an 8-month daily after-school aerobic exercise program (40 min/d, n=90) or a sedentary control condition (n=85). Carotid-femoral pulse wave velocity (PWV, primary outcome, arterial stiffness), fitness, adiposity, blood pressure (BP), glucose, insulin resistance, lipids, and C-reactive protein were measured at baseline and posttest (8 months). Adiposity, fitness, and BP were measured again at follow-up, 8–12 months later. Intent-to-treat analyses were conducted using mixed models.
Results
The study had 89% retention, with attendance of 59% in exercise and 64% in the control condition, and vigorous exercise participation (average heart rate 161±7 beats/min). Compared to controls, the exercise group had twice the improvement in fitness (VȮ2 peak, 2.7 (95% CI 1.8, 3.6) vs. 1.3 (0.4, 2.3) ml/kg/min) and adiposity (−1.8 (−2.4, −1.1) vs. −0.8 (−1.5, -0.1)%), each p=0.04, and a large improvement in HDL-cholesterol (0.13 (0.075, 0.186) vs. -0.028 (−0.083, 0.023) mmol/l, p<0.0001). There was no group×time effect on other outcomes at 8 months, or on any outcomes at follow-up. The change in PWV at 8 months correlated with changes in insulin and insulin resistance (both r=0.32), diastolic BP (r=0.24), BMI (r=0.22) and adiposity (r=0.18).
Conclusions
Eight months of aerobic exercise training improved fitness, adiposity, and HDL-cholesterol levels, but did not reduce arterial stiffness in children with excess weight. PWV improved as a function of insulin resistance, BP, BMI and adiposity. Weight loss may be required to improve arterial stiffness. Exercise benefits waned after discontinuing the program.
Trial Registration
This study is registered at www.clinicaltrials.gov NCT02383485.
Alarmingly, child obesity increased eightfold over the past 4 decades worldwide [1]. Cardiovascular disease has its origins in childhood. Children with obesity are likely to become adults with obesity who eventually develop cardiovascular disease [2]. Reductions in adult weight may not eliminate the risks that accrue from childhood obesity [3]. Little progress has been made in reducing obesity prevalence in U.S. children, particularly in minorities; thus, risk-reduction interventions are needed [4, 5, 6]. Elementary school offers an opportunity for population-wide intervention that could be more difficult before children enroll in school, or adolescents who have more choice in their activities.
Physical inactivity and low physical fitness are associated with arterial stiffness in young people [7, 8]. Pulse wave velocity (PWV) is a noninvasive measure of arterial stiffness, integrating several components of vascular health [9]. PWV assesses the speed of a pressure wave traveling distally through the arterial tree. Faster PWV indicates stiffer arteries, and independently predicts cardiovascular events and mortality in adults [10, 11, 12]. Carotid-femoral PWV measurement has been endorsed as the best measure of central arterial stiffness in children [9]. Peripheral measures of PWV are not as consistently linked with cardiovascular risk [13], and carotid-femoral PWV shows better tracking in young people than peripheral measures [14]. Black children have stiffer arteries than their peers [15, 16, 17]. PWV, an independent predictor of stroke, has been shown to be faster and to increase more quickly in young Blacks than Whites [16, 17]. There is limited evidence in children regarding the effect of lifestyle interventions, such as physical activity, on arterial stiffness [13].
The primary purpose of this prospective trial of an after-school exercise program was to assess changes in PWV after 8 months of the exercise vs. control intervention in children with overweight or obesity. Additionally the study tested the exercise effect on other cardiovascular risk markers, assessed effects 8–12 months after the intervention was completed, and tested associations between PWV and markers of cardiovascular risk. The hypothesis was that supervised after-school exercise would reduce PWV and adiposity, and improve fitness and cardiovascular risk.
METHODS
Participants
Children were recruited from schools for a parallel 2-arm trial investigating the effects of aerobic exercise on cognition and health [18]. This cardiovascular study is an a priori study ancillary to the cognitive study ( NCT02227095). The study was advertised via presentations and flyers distributed at 14 elementary schools in Augusta, Georgia in 2008–11. Prespecified inclusion criteria for the trial were: age 8–11 years, with excess weight (≥85th percentile BMI), inactive (no regular physical activity program ≥1 h/week), and no medical condition or medications that would affect study results or limit physical activity. There was no racial or sex bias in the selection of participants. Informed consent was obtained from a parent/guardian and assent from the child. The study was approved by the Medical College of Georgia (MCG) IRB. Photo and video consent were provided by study participants so depicted.
The parent study recruited, randomized (to after-school exercise or sedentary programs), intervened upon and tested four cohorts (25–50 children/school year, 2008–2012). After baseline testing was completed for each cohort, randomization (1:1, within school, race and sex) was performed by the study statistician using SAS®. The study coordinator enrolled children and informed families of the group assignment. Testing was conducted at the Georgia Prevention Institute and utilized the same instrumentation at baseline and posttest. Anthropometrics, BP, adiposity and treadmill test measures were repeated at follow-up (8–12 months after posttest, 2010–13).
Intervention
Both groups were offered an after-school program every school day for approximately 8 months (days offered, mean±SD=138±9). Participants were provided bus transportation after school to and from the Georgia Prevention Institute where they spent 1/2 h on homework and were offered snacks (e.g. fruit, crackers; no trans fat, low sugar) before the interventions were conducted. Both groups earned points redeemable for small weekly prizes for desired behaviors.
The exercise group engaged in instructor-led aerobic activities (e.g., tag, jump rope) for 40 min each day. Participants wore heart rate monitors every day (S610i; Polar Electro, Oy, Finland) with which they could monitor their own performance and from which data were collected daily. Points in the exercise group were earned for an average daily heart rate >140 beats/min, with more points for higher heart rates. The control group engaged in instructor-led sedentary activities (e.g., crafts, board games). Points in the control group were earned for participation and good behavior.
Measurements
The primary outcome, carotid-femoral PWV was measured with applanation tonometry (SphygmoCor, AtCor Medical, Sydney, Australia) in 142 children (72 exercise, 70 controls) at baseline, and 128 (66 exercise, 62 controls) at posttest who provided adequate quality data. (Funding for this measure began in the second year of the study.) PWV was automatically calculated from measurements of pulse transit time and the distance between the carotid and femoral arteries: PWV=distance (m)/transit time (s). Test-retest measurements of PWV were performed on 10 adults at 2 visits during a 7-day period to determine reproducibility. The one-way random effects model, single measure intraclass correlation coefficient was R=0.89.
A trained technician collected height (without shoes) and body weight (in light clothing) to calculate sex- and age-specific BMI percentiles and z-scores. Waist girth (cm) was then obtained at the midpoint between the lowest rib and the iliac crest. BP was measured 5 times at 1 min intervals after a 10-min rest using the Dinamap Pro 100 (Critikon Corporation, Tampa, Florida, USA); the last 3 measures were averaged. Adiposity (body fat, %) was measured by whole body dual-energy X-ray absorptiometry (QDR-4500W or Discovery W, Hologic Inc., Bedford, Massachusetts, USA).
Cardiovascular fitness (VȮ2 peak) was determined using a treadmill test (Modified Balke Protocol for Poorly Fit Children; SensorMedics 2000 treadmill, VMAX Spectra 29c (Yorba Linda, California, USA) or Cardiac Science TM65 treadmill, Parvo Medics TrueOne 2400 (Bothell, Washington, USA)) [19, 20]. The Progressive Aerobic Cardiovascular Endurance Run (PACER) 20m shuttle-run was done at the beginning and end of the interventions [21, 22].
Fasting blood samples were obtained at baseline (n=133) and posttest (n=104). (Some children refused or we had difficulty obtaining blood samples.) Assay personnel were blind to group assignment. Serum insulin was assayed using immunofluorescence technology on TOSOH AIA-II analyzer (TOSOH Corp., South San Francisco, California, USA). HOMA2-IR, an estimate of insulin resistance, was computed (https://www.dtu.ox.ac.uk/homacalculator/) [23]. Plasma triglyceride, total cholesterol, and high-density lipoprotein cholesterol (HDL) and serum glucose concentrations were measured using a SIRRUS analyzer (Stanbio Laboratory, Boerne, Texas, USA); low-density lipoprotein cholesterol (LDL) was calculated [24], and clinical cutoffs were applied [25]. Plasma C-reactive protein was measured using high-sensitivity ELISA (R&D System, Inc., Minneapolis, Minnesota, USA). Children with insulin values >350 pmol/L were excluded from analyses of insulin, glucose, and HOMA-2 IR due to likely noncompliance with fasting (n=10) [25].
To determine whether participants compensated for exercise by doing less physical activity during other times, physical activity outside the program was assessed at baseline and posttest (GT1M and GT3X, 30s epoch, vertical axis counts; ActiGraph, LLC, Fort Walton Beach, Florida, USA) [26]. Subjects were instructed to wear accelerometers during waking hours for 7 consecutive days during the school year, except while bathing and swimming. Valid wear time was defined as ≥4 days with ≥8 h/day of total wear time recorded (n=148 baseline, n=136 posttest). Non-wear time was defined as consecutive zeros equating to 20 min. Count thresholds determined time spent in moderate to vigorous physical activity (MVPA) [27]. To assess dietary compensation, a short form of the Youth/Adolescent Food Frequency Questionnaire estimated daily energy intake [28].
Statistical Analyses
Statistical analyses in 2013–19 used SAS (version 9.3 or higher, SAS Institute, Cary, North Carolina, USA) or SPSS (version 21 or higher, SPSS, Chicago, Illinois, USA) with two-sided α=0.05. Computer code is available on request (CLD, JLW). Group differences at baseline were determined by independent-samples t-tests if data were distributed normally, and Mann-Whitney U-tests otherwise. Chi-square tested differences in proportions. Insulin, insulin resistance, triglyceride and MVPA values were log-transformed to an approximate normal distribution. Pearson’s correlation analyses determined relationships between PWV and markers of cardiometabolic risk at baseline and changes over time. Partial correlations between PWV and cardiometabolic risk markers were calculated, adjusting for sex, race, and systolic BP. To replicate triglyceride/HDL ratio as a determinant of PWV, ANOVA compared PWV across three race-specific strata of triglyceride/HDL ratio (Whites: low triglyceride/HDL ratio <0.558 (1.28), high≥0.838 (1.92); Blacks: low<0.393 (0.90), high≥0.611 (1.40); conventional units in parentheses) [29].
Intention-to-treat analysis of each outcome used repeated-measures mixed models using all available data [30]. Planned sample size was 75/group based on prior studies with related data [31, 32]. Base models for each outcome included fixed effects of group, measurement time, and their interaction, controlling for cohort, sex, and race. Main effects, two- and three-factor interactions of sex×group×time and weight status (overweight/obesity)×group×time were examined for each outcome. If a three-factor interaction was not statistically significant, the model was reduced to examine the group×time interaction. Participant nested within group was a random effect. An unstructured covariance structure was assumed for baseline to posttest models. For three-timepoint models, an unstructured correlation structure between time points was found to provide the best fit with respect to Akaike’s and Bayesian Information Criteria. Final models contained at minimum cohort, sex, race, main effects, and the group×time interaction. Effect size was calculated (ηp2) for the two-timepoint models [33].
RESULTS
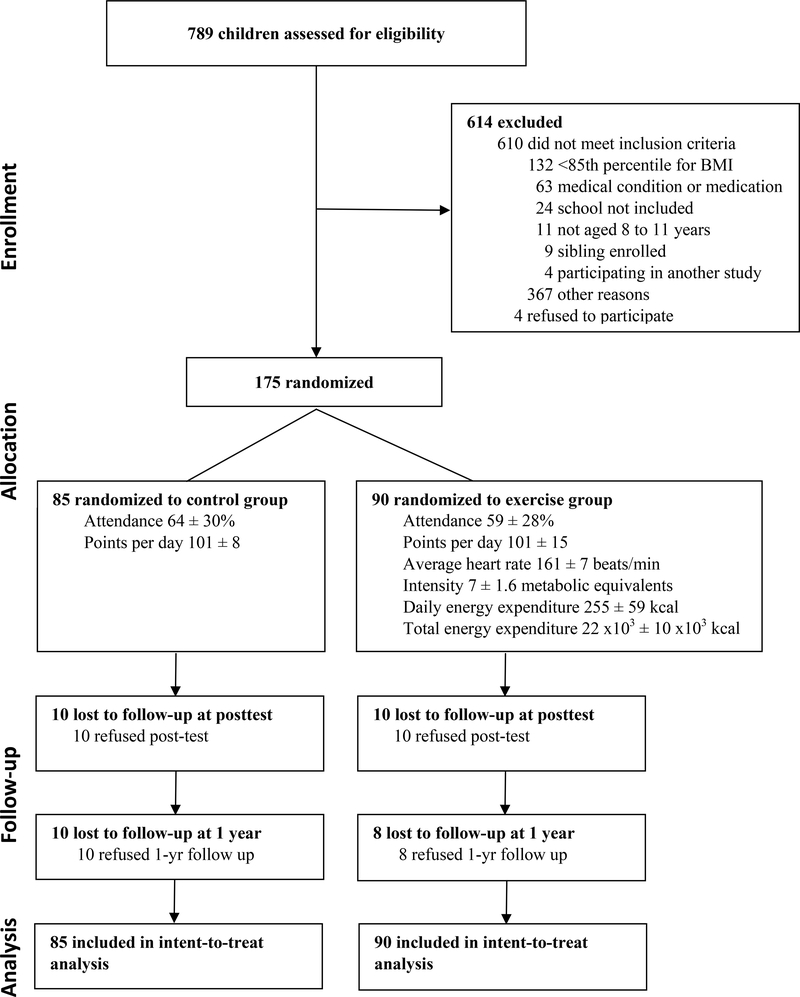
Participant flow is presented in Figure 1. Attendance was similar for the exercise (59±28%) and control groups (64±30%, p=0.26). Average heart rate during exercise was 161±7 beats/min during the intervention with exercise intensity of 6.8±1.6 METs. The mean daily energy expenditure during the exercise sessions was 1067±247 kJ/d (255±59 kcal/d), with a total 92∙103±42∙103 kJ (22∙103±10∙103 kcal) expended during the program. Sample games are shown in Figure S1. One hundred fifty-five subjects completed the study (89% retention).
Figure 1.
Participant flow diagram.
As shown in Table 1, baseline characteristics were similar between control (n=85) and exercise (n=90) groups. The children were predominantly Black (i.e., African-American) and had obesity (73%), a majority (59%) had prediabetes, mean glucose levels were at the upper limit of normoglycemia, and few were prehypertensive (3%) or hypertensive (5%). A third of participants had low HDL, and 14–16% had elevated cholesterol, LDL or triglycerides.
Table 1.
Baseline participant characteristics (mean [95% CI] or %)
| Characteristic | Total | Control | Exercise |
|---|---|---|---|
| N | 175 | 85 | 90 |
| Age, y | 9.7 [9.5, 9.8] | 9.7 [9.5, 9.9] | 9.6 [9.5, 9.8] |
| Female, % | 61 | 55 | 67 |
| Black, % | 87 | 85 | 90 |
| BMI, kg/m2 | 25.8 [25.0, 26.5] | 25.6 [24.4, 26.8] | 25.9 [25.0, 26.9] |
| z-score | 2.0 [1.9, 2.0] | 1.9 [1.8, 2.0] | 2.0 [1.9, 2.1] |
| Percentile | 96 [95, 97] | 96 [95, 97] | 97 [96, 97] |
| Obesity, % | 73 | 68 | 78 |
| Waist girth, cm | 76 [75, 78] | 76 [74, 79] | 77 [75, 79] |
| Body fat, % | 38 [36, 39] | 37 [35, 38] | 38 [37, 40] |
| Height, cm | 143 [141, 144] | 142 [141, 144] | 143 [141, 145] |
| VȮ2 peak, ml/kg/min | 29 [29, 30] | 30 [29, 31] | 29 [28, 30] |
| PACER laps | 11.9 [11.0, 12.8] | 12.5 [11.3, 13.8] | 11.3 [10.1, 12.5] |
| Moderate-vigorous physical activity, min/d | 28 [25, 30] | 29 [25, 32] | 26 [23, 30] |
| Accelerometer counts/d, thousands | 316 [298, 335] | 321 [295, 347] | 312 [285, 338] |
| Energy intake, kJ/d (kcal/d) | 10800 [9475, 12125] (2580 [2261, 2900]) | 11260 [9205, 13309] (2690 [2200, 3181]) | 10370 [8598, 12134] (2480 [2055, 2900]) |
| PWV, m/s | 5.1 [4.9, 5.2] | 5.1 [4.9, 5.2] | 5.1 [4.9, 5.3] |
| Systolic BP, mmHg | 104 [102, 105] | 102 [100, 104] | 105 [103, 107] |
| Diastolic BP, mmHg | 58 [57, 59] | 58 [56, 59] | 58 [56, 59] |
| Hypertension / pre-hypertension, % | 5 / 3 | 2 / 0 | 7 / 6 |
| Fasting glucose, mmol/l (mg/dl) | 5.6 [5.5, 5.7] (100 [99,102]) | 5.6 [5.4,5.7] (100 [98, 102]) | 5.6 [5.5, 5.7] (101 [99, 103]) |
| Prediabetes, % | 59 | 59 | 59 |
| Fasting insulin, pmol/l (μU/ml) | 150 [138, 163] (22 [20,23]) | 142 [124, 160] (20 [18, 23]) | 159 [142, 177] (23 [20, 26]) |
| HOMA2 insulin resistance | 2.8 [2.6, 3.0] | 2.6 [2.3, 3.0] | 3.0 [2.6, 3.3] |
| Total cholesterol, mmol/l (mg/dl) | 4.3 [4.2, 4.4] (166 [161, 172]) | 4.4 [4.2, 4.5] (168 [161, 176]) | 4.3 [4.0, 4.5] (164 [156, 172]) |
| High cholesterol, % | 14 | 15 | 12 |
| LDL, mmol/l (mg/dl) | 2.7 [2.6, 2.8] (103 [98, 108]) | 2.7 [2.5, 2.9] (103 [96, 110]) | 2.6 [2.4, 2.8] (102 [95, 110]) |
| High LDL, % | 15 | 18 | 13 |
| HDL, mmol/l (mg/dl) | 1.2 [1.2, 1.3] (47 [44, 50]) | 1.2 [1.1, 1.3] (47 [44, 50]) | 1.2 [1.1, 1.3] (47 [43, 51]) |
| Low HDL, % | 32 | 36 | 29 |
| Triglycerides, mmol/l (mg/dl) | 0.94 [0.86, 1.02] (83 [76, 90]) | 1.0 [0.9, 1.1] (89 [78, 101]) | 0.86 [0.77, 0.96] (76 [68, 85]) |
| High triglycerides, % | 16 | 19 | 12 |
| Triglyceride/HDL ratio (based on mmol/l) (based on mg/dl) | 0.91 [0.79, 1.03] (2.1 [1.8, 2.4]) | 0.98 [0.78, 1.18] (2.3 [1.8, 2.7]) | 0.83 [0.70, 0.96] (1.9 [1.6, 2.2]) |
| C-reactive protein, nmol/l (mg/l) | 21 [18, 24] (2.2 [1.8, 2.5]) | 20 [16, 24] (2.1 [1.7, 2.5]) | 21 [17, 26] (2.2 [1.7, 2.7]) |
HFZ, healthy fitness zone; NI-HR, Needs Improvement-Health Risk; NI-SR, Needs Improvement-Some Risk; PWV, carotid-femoral pulse wave velocity; BP, blood pressure; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol. Obesity is defined as BMI ≥95th percentile for age and sex. Conventional units are shown in parentheses.
Note: The only group difference was for high triglycerides (p=.03).
At baseline, girls had higher PWV (5.2±0.8 vs. 4.9±0.6 m/s, p=0.006), adiposity (39±6 vs. 35±8% body fat, p<0.001), insulin (166±69 vs. 127±65 pmol/L (24±10 vs. 18±9 μU/mL), p=0.002), insulin resistance (3.1±1.2 vs. 2.4±1.2, p=0.003), triglycerides (1.04±0.50 vs. 0.79±0.40 mmol/L (92±44 vs. 70±35 mg/dL), p=0.003), and triglyceride/HDL ratio (1.05±0.79 vs. 0.70±0.52 (2.4±1.8 vs. 1.6±1.2), p=0.01; conventional units in parentheses) than boys. Boys had higher fitness (31.6±5.6 vs. 28.1±4.8 mL/kg/min, p<0.001), MVPA (35±19 vs. 23±9 min/day, p<0.001) and HDL (1.3±0.44 vs. 1.2±0.34 mmol/L (51±17 vs. 45±13 mg/dL), p=0.02) than girls.
Baseline PWV (adjusted for sex, race, and where applicable, systolic BP) was associated with BMI, BMI z-score, waist girth, adiposity, systolic BP, and C-reactive protein (Table S1). Adjusting for covariates attenuated PWV associations with fitness variables and triglycerides. No associations were found between PWV and glucose, insulin, insulin resistance, lipid, or accelerometry measures (all p>0.10).
PWV was similar between the highest and lowest strata of triglyceride/HDL ratio (p=0.30), with no linear or quadratic trend detected (p=0.30, 0.49 respectively). Because there were only 8 children in the lowest stratum, with a mean triglyceride/HDL ratio similar to the middle stratum (2.09±1.05 vs. 2.09±0.57 (4.8±2.4, 4.8±1.3) respectively), the lower 2 strata were collapsed (n=34) and compared with the upper stratum (n=61, 2.2±0.04 (5.1±0.09)); a nearly significant result was obtained (p=0.07).
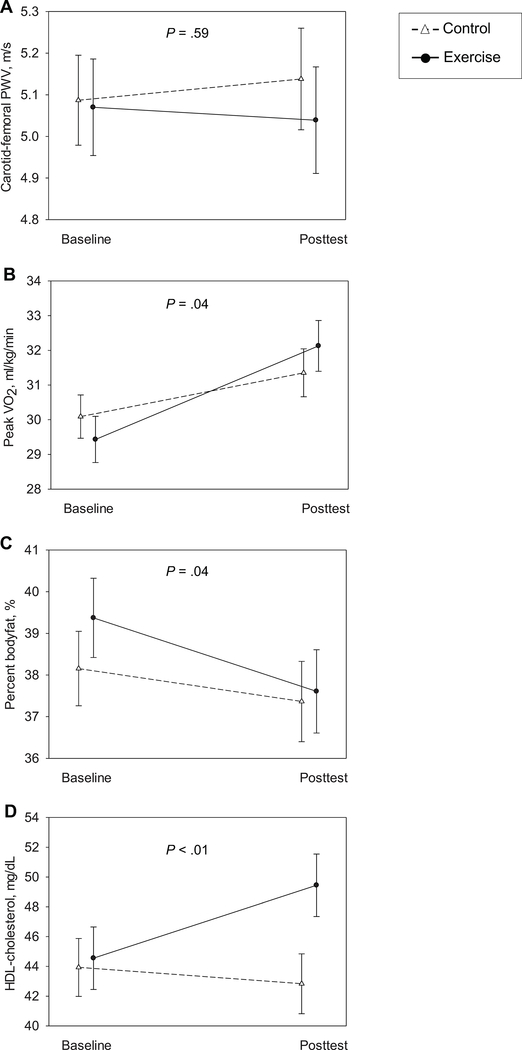
From baseline to posttest, there was no group×time effect on PWV. The exercise group had greater improvements than the control group in fitness (VȮ2 peak, 2.7 (1.8, 3.6) vs. 1.3 (0.4, 2.3) mL/kg/min, PACER, 2.6 (1.6, 3.7) vs. 0.9 (−0.2, 1.9) laps), adiposity (-1.8 (-2.4, -1.1) vs. -0.8 (-1.5, -0.1) %), and HDL (0.13 (0.08, 0.19) vs. -0.03 mmol/L (-0.08, 0.02), equivalent to (5.0 (2.9, 7.2) vs. -1.1 (-3.2, 0.9) mg/dL); all p≤0.04; Figure 2, Table 2). There was no group×time effect on BMI, BMI z-score, waist girth, height, BP, fasting glucose, fasting insulin, insulin resistance, total cholesterol, LDL, triglycerides, triglyceride/HDL ratio, or C-reactive protein. There was also no group×time effect on energy intake (kJ/day) or physical activity (MVPA and counts/day) outside the after-school programs, thus no evidence for caloric compensation. There were no significant three-way interactions.
Figure 2.
Group×time interactions for selected cardiovascular outcomes: carotid-femoral pulse wave velocity (PWV, A), VȮ2 peak (B), body fat (C), and high-density lipoprotein cholesterol (HDL, D).
Table 2.
Mean changes by group, group differences in change, and correlations of changes in PWV and with other variables
| Mean adjusted Δ by groupa | Group difference in Δ | Correlation of Δ in PWV with Δ in risk markers |
||||||
|---|---|---|---|---|---|---|---|---|
| Control | Exercise | Unadjusted | Adjustedb | |||||
| mean [95% CI] | mean [95% CI] | ηp2 | P | r | p | r | p | |
| Δ PWV, m/sec | 0.04 [−0.18, 0.26] | −0.02 [−0.23, 0.20] | .001 | .71 | - | - | - | - |
| Δ BMI, kg/m2 | 0.8 [0.5, 1.1] | 0.4 [0.1, 0.7] | .010 | .07 | .20 | .03 | .22 | .02 |
| Δ BMI z-score | −0.04 [−0.08, 0.01] | −0.10 [−0.14, −0.05] | .009 | .09 | .14 | .11 | .15 | .10 |
| Δ Waist girth, cm | 2.0 [1.0, 3.1] | 2.1 [1.1, 3.2] | .001 | .89 | .08 | .38 | .09 | .34 |
| Δ Percent body fat | −0.8 [−1.4, −0.1] | −1.8 [−2.4, −1.1] | .013 | .04 | .17 | .05 | .18 | .047 |
| Δ Height, cm | 4.6 [4.2, 4.9] | 4.8 [4.5, 5.1] | .004 | .23 | −.09 | .35 | −.09 | .34 |
| Δ VȮ2 peak, mL/kg/min | 1.3 [0.4, 2.3] | 2.7 [1.8, 3.6] | .012 | .04 | −.16 | .09 | −.13 | .16 |
| Δ PACER laps | 0.9 [−0.2, 1.9] | 2.6 [1.6, 3.7] | .017 | .02 | −.16 | .08 | −.17 | .07 |
| Δ Moderate-vigorous physical activity (min/day) | 4.0 [−0.4, 8.4] | 2.1 [−2.3, 6.5] | .001 | .54 | −.13 | .17 | −.14 | .16 |
| Δ Energy intake kJ/day (kcal/day) | −3318 [−5238, −1397] (−793 [−1252, −334]) | −3230 [−4858, −1180] (−722 [−1161, −282]) | .000 | .81 | −.15 | .11 | −.15 | .11 |
| Δ Systolic BP, mmHg | 0.3 [−1.3, 2.0] | 0.1 [−1.5, 1.7] | .000 | .87 | .03 | .78 | .11 | .24 |
| Δ Diastolic BP, mmHg | 0.0 [−1.1, 1.2] | 1.1 [−0.1, 2.2] | .005 | .21 | .21 | .02 | .24 | <.01 |
| Δ Fasting glucose, mmol/L (mg/dL) | 0.01 [−0.09, 0.11] (0.2 [−1.6, 1.9]) | −0.01 [−0.11, 0.09] (−0.1 [−1.9, 1.7]) | .000 | .82 | .18 | .19 | .21 | .094 |
| Δ Fasting insulin, pmol/L (μU/mL) | −4.2 [−26, 17] (−0.6 [−3.7, 2.5]) | 2.8 [−19, 25] (0.4 [−2.7, 3.6]) | .001 | .63 | .29 | .01 | .32 | .01 |
| Δ HOMA2 insulin resistance | −0.0 [−0.4, 0.3] | 0.1 [−0.3, 0.4] | .000 | .78 | .29 | .01 | .32 | .01 |
| Δ Total cholesterol, mmol/L (mg/dL) | −0.21 [−0.32, −0.09] (−8.0 [−12.4, −3.6]) | −0.07 [−0.27, 0.05] (−2.7 [−7.3, 1.9]) | .010 | .10 | .20 | .09 | .19 | .11 |
| Δ LDL, mmol/L (mg/dL) | −0.13 [−0.24, −0.03] (−5.1 [−9.2, −1.0]) | −0.16 [−0.27, −0.04] (−6.0 [−10.4, −1.7]) | .000 | .75 | .08 | .51 | .06 | .61 |
| Δ HDL, mmol/L (mg/dL) | −0.3 [−0.08, 0.02] (−1.1 [−3.2, 0.9]) | 0.13 [0.07, 0.19] (5.0 [2.9, 7.2]) | .061 | <.0001 | .04 | .74 | .07 | .57 |
| Δ Triglycerides, mmol/L (mg/dL) | −0.10 [−0.21, 0.01] (−9.1 [−19, 0.7]) | −0.08 [−0.19, 0.04] (−7.0 [−17, 3.2]) | .000 | .72 | .21 | .06 | .19 | .10 |
| Δ Triglyceride/HDL ratio | −0.26 [−0.62, 0.11] | −0.32 [−0.71, 0.06] | .000 | .83 | .17 | .15 | .13 | .25 |
| Δ C-reactive protein, nmol/L (mg/L) | 0.0 [−2.9, 3.8] (0.0 [−0.3, 0.4]) | −1.9 [−5.7, 1.0] (−0.2 [−0.6, 0.1]) | .004 | .35 | .08 | .50 | .08 | .51 |
PWV, carotid-femoral pulse wave velocity; Δ, change from baseline to posttest; ηp2, partial eta squared between-groups effect size; BP, blood pressure; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol.
Mean change (exercise – control group change from baseline to posttest) adjusted for cohort, race, and sex in mixed repeated-measures ANOVA. Conventional units are shown in parentheses.
Adjusted for sex, race, and where applicable, systolic BP.
Boldface highlights statistical significance (p<.05).
We examined relationships between the changes in PWV and markers of cardiometabolic risk during the 8 month intervention period (Table 2). As shown in Figure S2, there were associations between change in PWV and the changes in insulin, insulin resistance, diastolic BP, BMI, and adiposity, adjusted for covariates. There were no associations between change in PWV and changes in systolic BP, BMI z-score, waist girth, height, fitness, glucose, lipids, or C-reactive protein.
There were no group differences in change from baseline to follow-up (Table S2).
DISCUSSION
The current prospective, randomized trial in predominantly Black children with overweight or obesity shows that an 8-month supervised, vigorous (~7 METs) aerobic after-school exercise program did not affect arterial stiffness. The exercise treatment increased fitness levels, reduced adiposity, and increased HDL levels, with similar effects in boys and girls, and those with overweight or obesity. Reduced arterial stiffness was associated with improvements in insulin resistance, diastolic BP, BMI and adiposity in the whole sample. No lasting benefits of exercise on adiposity, fitness or BP were observed 8–12 months after completing the intervention.
Despite a substantial dose of aerobic training over an 8-month period in children at risk of cardiovascular disease, no exercise effect on PWV was observed. There was no reduction in insulin resistance, BMI or BP due to exercise, which might explain the null exercise effect on PWV, given associations among those changes. Null results of exercise on arterial stiffness have been reported in children and adolescents with obesity [34, 35, 36]. Horner et al. used carotid-femoral PWV, Lee et al. used brachial-ankle PWV, and Farpour-Lambert et al. used incremental elastic modulus [34, 35, 36]. The report of the exercise trial by Farpour-Lambert was equivocal [36]. The intent-to-treat analysis at 3 months showed null results for arterial stiffness. At 3 months, the control group was invited to join in the exercise program, and most of them did. At 6 months, arterial stiffness was reported to be improved in the exercise group, but this is unclear because it had risen in the exercise group during the first 3 months, and it rose in the control group once they were invited to participate in the exercise program. In contrast to the null results for PWV, a meta-analysis including 6 exercise trials in youth with obesity found a modest dose-response effect of exercise on carotid intima-medial thickness [37]. This suggests that other aspects of vascular health may be more related to fitness and more responsive to exercise. The current study was larger than previous studies assessing PWV, and offered a higher dose of exercise (5 days/week over 8 months) with intent-to-treat analyses on the gold standard measure of arterial stiffness in youth [13].
In this unusually long (8-month) exercise trial, participant growth may have masked exercise effects on PWV, insulin resistance, BP, and BMI [38, 39, 40, 41, 42]. For example, both the exercise and control groups gained nearly 5 cm in height, and showed improved fitness capacity. Distance from the carotid to femoral site is used to calculate PWV and may have changed over the course of the intervention. Some of the children may have undergone pubertal development during the study. In a prior study in a sample of similar age, 26% of the children had already begun thelarche or gonadarche at baseline [31]. Adults with more advanced arterial stiffness may be more responsive to exercise intervention. A study of young prehypertensive adults found reduced peripheral, but not carotid-femoral PWV with 2 months aerobic or strength training [43]. Meta-analyses of exercise studies in adults with prehypertension or hypertension found no effect on carotid-femoral PWV, while those with sicker CVD patients found consistent improvements in carotid-femoral PWV [44, 45].
In contrast with our prior, shorter intervention [31], we did not observe improved insulin resistance due to exercise in this study. However, the strongest associations of improvements in PWV in this predominantly Black study population enriched for prediabetes (59%) were with reductions in fasting insulin and insulin resistance (each r=0.32). This is salient because elevated BP, an obvious link between excess weight and stiffer arteries, is increasingly common in children with obesity [46] and when chronic, may lead to structural changes in artery walls. Despite the expected correlation of PWV with BP at baseline in this mostly normotensive sample, the association of BP reduction with improved PWV (r=0.24) was weaker than that of PWV and insulin or insulin resistance changes. A study in adolescents with type 1 diabetes showed that insulin resistance at baseline moderated the worsening of arterial stiffness over ~5 years, and like the present study, no association of PWV with insulin was observed at baseline [47]. Thus the current study aligns with previous results to emphasize intervening on insulin resistance as a modifiable protective factor to prevent arterial stiffness in youth. In contrast, a study focused on the effect of reducing insulin resistance on PWV (a weight-loss trial in otherwise healthy adults with overweight or obesity) found no link between reduced insulin levels and PWV, perhaps due to longer risk exposure [48].
This after-school exercise program reduced adiposity. The exercise group lost 1.8% body fat (5% of their baseline fat mass), twice as much as the control group. This effect is comparable to our prior 3-month exercise trial in children with excess weight [31, 49, 50]. Children can grow into their weight as they gain height if their weight gain is slowed [51]. Thus, weight loss is not always necessary in children with excess weight. Girls in particular need not go below 30% body fat to reach a healthy weight. A 10-year-old girl with 38% body fat who experiences a 1% reduction per year would attain a healthy body fat level by adulthood [52].
Reductions in adiposity and BMI were associated with reduced arterial stiffness, consistent with longitudinal studies linking PWV with weight change in young adults [16]. In the present study, PWV at baseline correlated with several measures of adiposity, consistent with other reports using carotid-femoral PWV (Table S1) [8, 13, 53, 54]. The studies that found lower PWV in children with obesity used peripheral measures (e.g., carotid-radial, carotid-dorsal pedis, brachial-ankle PWV) that are not as consistently linked with cardiovascular risk as carotid-femoral PWV. Children with obesity have higher carotid-femoral and lower carotid-radial PWV than their peers of normal weight, and adiposity correlated positively with carotid-femoral, and negatively with carotid-radial PWV measures [55, 56]. A study comparing adolescents who had normal weight (<85th percentile for BMI), had obesity, and had type 2 diabetes found a slightly lower carotid-radial PWV in those with obesity than those with normal weight, in contrast to a clearly graded increase in carotid-femoral PWV from adolescents with normal weight to those with obesity and those with diabetes [54]. Carotid-femoral PWV in adults predicts cardiovascular events and all-cause mortality [11], and is considered the gold standard measure of arterial stiffness in youth [13].
Increased total or regional adiposity could directly contribute to adverse vascular adaptations. For example, chronically increased cardiac output due to metabolic demands associated with excess fat might alter arterial properties via chronic differences in shear forces and wall stress. There is increasing recognition that perivascular adipose tissue exerts local control over vascular tone and in obesity, loses its normal vasodilatory effects [57]. Alternatively, chronic inflammation associated with obesity could lead to injury of the vascular wall [58]. Consistent with this idea, we observed an association between PWV and C-reactive protein at baseline. However, changes in PWV were unrelated to changes in systemic inflammation over time.
In contrast to our 3-month trial [31], a smaller exercise-induced benefit on fitness was observed in the current study (1.4 ml/kg/min greater than the control condition), with a 9% increase from baseline in the exercise group, more than double that in the control group. The exercise effects may have been partially obscured by the expected increase in aerobic fitness capacity with overall growth over the longer study period [59]. The current study sample had slightly lower adiposity and better aerobic fitness at baseline than our previous exercise trial [31]. Greater benefit from exercise is expected in populations at greater risk, who have more room to improve [60]. Improved aerobic fitness as a result of training could contribute to better arterial distensibility through mechanisms including favorable effects on weight or adiposity, BP, inflammation, and endothelial function [61]. In the current study, both baseline fitness measures correlated with PWV, but this did not survive adjustment, consistent with prior observations in children and adults [8, 34, 62]. There was no association between changes in PWV and aerobic fitness over the 8 months of the intervention, consistent with a report in adolescents with obesity [34]. Thus, the evidence does not support aerobic fitness as a direct determinant of arterial stiffness in children.
We observed a surprisingly large benefit of exercise on HDL (6 mg/dL greater than the control condition, a medium effect size) [49, 50]. The larger benefit on HDL might be attributable to the longer period of intervention, and therefore greater total energy expenditure, compared to our prior study [31]. Puberty cannot account for the benefit on HDL. Prepubertal boys and girls have HDL concentrations similar to those in women; as boys mature, their HDL levels drop [63]. Early pathological changes in the aortic walls of children with obesity represent a candidate mechanism to explain elevations in PWV. Although atherosclerotic plaques and calcification are rare in children, precursors to these plaques including fatty streaks and fibrous plaques develop in childhood [64]. Appearance of these features may be accelerated by obesity and its comorbidities. The association of PWV with triglycerides at baseline did not survive adjustment. We did not find relationships between any other serum lipids and PWV. We did not detect a statistically significant association between PWV and triglyceride/HDL ratio, a proposed independent determinant of arterial stiffness in youth with obesity [29]. In the current study, PWV was marginally faster in the highest level of triglyceride/HDL ratio (p=.07). This contrasts with a report in a large adolescent and young adult sample [29]. The children in this study were younger, predominantly Black, and excluded normal weight children, which restricted the range of lipid values. These results do not support lipid abnormalities as the cause of arterial stiffness in children with excess weight.
All participants in the current study received an intervention with healthy snacks, probably replacing what they would have eaten during this time. This minimal nutrition intervention may have favorably influenced outcomes in both groups. We found no evidence for compensatory changes in physical activity or diet outside of the after-school program. Other than the snacks provided during the interventions, there was no prescribed diet. Caloric restriction in combination with exercise may be expected to result in greater weight loss with similar or better effects on metabolic and cardiovascular parameters [65]. However, dietary restriction in children must be done carefully (e.g. focus on reducing intake of energy-dense, nutrient-poor foods, while encouraging consumption of nutrient-dense foods) to ensure adequate nutrition for growth. Studies of combined diet and exercise interventions cannot identify the specific effects of the exercise treatment, the focus of this study.
We observed a sex difference in PWV such that girls had stiffer arteries than boys, consistent with the literature in prepubertal children [66]. The girls in this sample were less fit and active, with worse adiposity, insulin resistance, HDL and triglyceride concentrations than boys. A combination of adverse lifestyle factors and earlier pubertal development in girls may account for these sex differences. The confounding of sex with these factors may have obscured their relationships with PWV. However, there were no apparent sex differences in response to the intervention, and sex was adjusted in analyses. Nonetheless, arterial stiffness in boys and girls changes differently with growth due to differences in arterial size and pubertal timing, which may have obscured exercise effects [13].
Limitations
While we did not detect a reduction in BMI or BMI z-score due to exercise, the observed reduction in adiposity is a meaningful measure of improved body composition. Imperfect attendance and incomplete follow-up are shared limitations of clinical studies. Fewer children provided fasting blood samples in this study than our prior, shorter intervention trial, limiting power for some outcomes [31]. We did not have enough White participants to test for race differences. Similar results may be expected in children with prediabetes and low HDL, however, this study may not generalize to children with prehypertension or hypertension.
Conclusions
A monitored 8-month exercise program did not reduce arterial stiffness, yet improved adiposity, fitness, and HDL in children with overweight or obesity. Reductions in insulin resistance, BP, BMI and adiposity were associated with improved arterial stiffness. Combined dietary and exercise intervention may be needed to reduce arterial stiffness in children. Unfortunately, no fatness, fitness or BP benefits of the exercise treatment were apparent within a year after the program ended. Widely accessible, sustained physical activity programs which children with excess weight enjoy are sorely needed to protect population health. Policies supporting promotion and clinician prescription of accessible health promotion programs could improve health trajectories and the societal burden of illness.
Supplementary Material
Acknowledgements
The authors acknowledge Amanda Gipson for assistance with accelerometry, and Barbara Gower at the University of Alabama at Birmingham for insulin, glucose, and lipid assays. This work was supported by NIH grants R01 HL087923 and P30 DK56336, and intramural support from MCG. The study was approved by the MCG IRB (Human Assurance Committee; 02-08-037, 09-04-287). Dr. Davis conceived the study, drafted the manuscript and oversaw data collection and analysis. Dr. Litwin assisted with drafting the manuscript. Dr. Pollock assisted with analyses and drafting the manuscript. Dr. Waller assisted with design of the study and conducted intent-to-treat statistical analyses. Dr. Zhu measured C-reactive protein and reviewed the manuscript. Drs. Dong and Kapuku assisted with design of the study, PWV data collection, and reviewed the manuscript. Dr. Bhagatwala assisted with quality control of the PWV data. Dr. Harris oversaw fitness testing and assisted with drafting the manuscript. Mr. Looney conducted the interventions and data collection, quality control of data, edited the video, and assisted with drafting the manuscript. Ms. Williams assisted with statistical analyses, quality control, and drafting the manuscript. Ms. Armento assisted with data analyses. Dr. Schmidt assisted with accelerometry analyses. Dr. Bassali consulted on design, provided medical oversight for the study, and assisted with drafting the manuscript. All authors reviewed the manuscript prior to submission. This study is registered at www.clinicaltrials.gov, NCT02383485. Contents of the article were presented at the 2013 American Heart Association meeting. No competing financial interests were reported by the authors of this paper.
Competing interests. This work was supported by NIH grants R01 HL087923 and P30 DK56336, and a Diabetes & Obesity Discovery Institute grant and Medical Scholars Program stipends from the Medical College of Georgia. Funders had no role in the design of the study, collection, analysis and interpretation of data, writing the report, or the decision to submit the report for publication. The findings and conclusions in this article are those of the authors and do not represent the official position of the NIH. This study is registered at www.clinicaltrials.gov, NCT02383485 and was approved by the MCG IRB (Human Assurance Committee).
Footnotes
No competing interests were reported by the authors of this paper.
REFERENCES
- 1.Risk Factor Collaboration NCD Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017; 390(10113): 2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park MH, Falconer C, Viner RM, Kinra S. The impact of childhood obesity on morbidity and mortality in adulthood: a systematic review. Obes Rev 2012; 13(11): 985–1000. [DOI] [PubMed] [Google Scholar]
- 3.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow- up of the Harvard Growth Study of 1922 to 1935. N Engl J Med 1992; 327(19): 1350–1355. [DOI] [PubMed] [Google Scholar]
- 4.Skelton JA, Cook SR, Auinger P, Klein JD, Barlow SE. Prevalence and trends of severe obesity among US children and adolescents. Acad Pediatr 2009; 9(5): 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA 2018; 319(16): 1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics 2018; 142(3): e20173459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards NM, Daniels SR, Claytor RP, Khoury PR, Dolan LM, Kimball TR et al. Physical activity is independently associated with multiple measures of arterial stiffness in adolescents and young adults. Metabolism 2012; 61(6): 869–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakuragi S, Abhayaratna K, Gravenmaker KJ, O’Reilly C, Srikusalanukul W, Budge MM et al. Influence of adiposity and physical activity on arterial stiffness in healthy children: the Lifestyle Of Our Kids study. Hypertension 2009; 53(4): 611–6. [DOI] [PubMed] [Google Scholar]
- 9.Urbina EM, Williams RV, Alpert BS, Collins RT, Daniels SR, Hayman L et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension 2009; 54(5): 919–50. [DOI] [PubMed] [Google Scholar]
- 10.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation 1999; 99(18): 2434–9. [DOI] [PubMed] [Google Scholar]
- 11.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010; 55(13): 1318–27. [DOI] [PubMed] [Google Scholar]
- 12.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 2006; 113(5): 657–63. [DOI] [PubMed] [Google Scholar]
- 13.Cote AT, Harris KC, Panagiotopoulos C, Sandor GG, Devlin AM. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol 2013; 62(15): 1309–19. [DOI] [PubMed] [Google Scholar]
- 14.Ye C, Pan Y, Xu X, Su S, Snieder H, Treiber F et al. Pulse wave velocity in elastic and muscular arteries: tracking stability and association with anthropometric and hemodynamic measurements. Hypertens Res 2016; 39(11): 786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lefferts WK, Augustine JA, Spartano NL, Atallah-Yunes NH, Heffernan KS, Gump BB. Racial differences in aortic stiffness in children. J Pediatr 2017; 180: 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wildman RP, Farhat GN, Patel AS, Mackey RH, Brockwell S, Thompson T et al. Weight change is associated with change in arterial stiffness among healthy young adults. Hypertension 2005; 45(2): 187–92. [DOI] [PubMed] [Google Scholar]
- 17.Thurston RC, Matthews KA. Racial and socioeconomic disparities in arterial stiffness and intima media thickness among adolescents. Soc Sci Med 2009; 68(5): 807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krafft CE, Schwarz NF, Chi L, Weinberger AL, Schaeffer DJ, Pierce JE et al. An eight month randomized controlled exercise trial alters brain activation during cognitive tasks in overweight children. Obesity 2014; 22: 232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowland TW. Aerobic Exercise Testing Protocols, Human Kinetics: Champaign, IL, 1993. [Google Scholar]
- 20.Rowland T Oxygen uptake and endurance fitness in children, revisited. Pediatr Exerc Sci 2013; 25(4): 508–514. [DOI] [PubMed] [Google Scholar]
- 21.Leger LA, Mercier D, Gadoury C, & Lambert J The multistage 20 metre shuttle run test for aerobic fitness. J Sports Sci 1988; 6(2): 93–101. [DOI] [PubMed] [Google Scholar]
- 22.The Cooper Institute. FITNESSGRAM/ACTIVITYGRAM Test Administration Manual, 4th ed. Human Kinetics: Champaign, IL, 2007. [Google Scholar]
- 23.George L, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Surrogate estimates of insulin sensitivity in obese youth along the spectrum of glucose tolerance from normal to prediabetes to diabetes. J Clin Endocrinol Metab 2011; 96(7): 2136–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18(6): 499–502. [PubMed] [Google Scholar]
- 25.Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011; 128(Suppl 5): S213–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ried-Larsen M, Brond JC, Brage S, Hansen BH, Grydeland M, Andersen LB et al. Mechanical and free living comparisons of four generations of the Actigraph activity monitor. Int J Behav Nutr Phys Act 2012; 9: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci 2008; 26(14): 1557–1565. [DOI] [PubMed] [Google Scholar]
- 28.Rockett HR, Berkey CS, Colditz GA. Comparison of a short food frequency questionnaire with the Youth/Adolescent Questionnaire in the Growing Up Today Study. Int J Pediatr Obes 2007; 2(1): 31–39. [DOI] [PubMed] [Google Scholar]
- 29.Urbina EM, Khoury PR, McCoy CE, Dolan LM, Daniels SR, Kimball TR. Triglyceride to HDL-C ratio and increased arterial stiffness in children, adolescents, and young adults. Pediatrics 2013; 131(4): e1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis CL, Pollock NK, Waller JL, Allison JD, Dennis BA, Bassali R et al. Exercise dose and diabetes risk in overweight and obese children: a randomized controlled trial. JAMA 2012; 308(11): 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbeau P, Johnson MH, Howe CA, Allison J, Davis CL, Gutin B et al. Ten months of exercise improves general and visceral adiposity, bone, and fitness in black girls. Obesity (Silver Spring) 2007; 15(8): 2077–2085. [DOI] [PubMed] [Google Scholar]
- 33.An ad hoc method for computing pseudo-effect size for mixed models. South Central SAS® Users Group Educational Forum; San Antonio, TX: South Central SAS® Users Group, 2016. [Google Scholar]
- 34.Horner K, Kuk JL, Barinas-Mitchell E, Drant S, DeGroff C, Lee S. Effect of aerobic versus resistance exercise on pulse wave velocity, intima media thickness and left ventricular mass in obese adolescents. Pediatr Exerc Sci 2015; 27(4): 494–502. [DOI] [PubMed] [Google Scholar]
- 35.Lee YH, Song YW, Kim HS, Lee SY, Jeong HS, Suh SH et al. The effects of an exercise program on anthropometric, metabolic, and cardiovascular parameters in obese children. Korean Circ J 2010; 40(4): 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farpour-Lambert NJ, Aggoun Y, Marchand LM, Martin XE, Herrmann FR, Beghetti M. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol 2009; 54(25): 2396–2406. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Hermoso A, Gonzalez-Ruiz K, Triana-Reina HR, Olloquequi J, Ramirez-Velez R. Effects of exercise on carotid arterial wall thickness in obese pediatric populations: a meta-analysis of randomized controlled trials. Child Obes 2017; 13(2): 138–145. [DOI] [PubMed] [Google Scholar]
- 38.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017; 140(3): e20171904. [DOI] [PubMed] [Google Scholar]
- 39.Dangardt F, Chen Y, Berggren K, Osika W, Friberg P. Increased rate of arterial stiffening with obesity in adolescents: a five-year follow-up study. PLoS One 2013; 8(2): e57454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hidvegi EV, Illyes M, Benczur B, Bocskei RM, Ratgeber L, Lenkey Z et al. Reference values of aortic pulse wave velocity in a large healthy population aged between 3 and 18 years. J Hypertens 2012; 30(12): 2314–2321. [DOI] [PubMed] [Google Scholar]
- 41.Ball GD, Huang TT, Gower BA, Cruz ML, Shaibi GQ, Weigensberg MJ et al. Longitudinal changes in insulin sensitivity, insulin secretion, and beta-cell function during puberty. J Pediatr 2006; 148(1): 16–22. [DOI] [PubMed] [Google Scholar]
- 42.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: Improvements to the 1977 National Center for Health Statistics version. Pediatrics 2002; 109(1): 45–60. [DOI] [PubMed] [Google Scholar]
- 43.Beck DT, Martin JS, Casey DP, Braith RW. Exercise training reduces peripheral arterial stiffness and myocardial oxygen demand in young prehypertensive subjects. Am J Hypertens 2013; 26(9): 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montero D, Roche E, Martinez-Rodriguez A. The impact of aerobic exercise training on arterial stiffness in pre- and hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol 2014; 173(3): 361–368. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Qi L, Xu L, Sun X, Liu W, Zhou S et al. Effects of exercise modalities on central hemodynamics, arterial stiffness and cardiac function in cardiovascular disease: Systematic review and meta-analysis of randomized controlled trials. PLoS One 2018; 13(7): e0200829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999–2008. Pediatrics 2012; 129(6):1035–1041. [DOI] [PubMed] [Google Scholar]
- 47.Shah AS, Black S, Wadwa RP, Schmiege SJ, Fino NF, Talton JW et al. Insulin sensitivity and arterial stiffness in youth with type 1 diabetes: the SEARCH CVD study. J Diabetes Complications 2015; 29(4): 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughes TM, Althouse AD, Niemczyk NA, Hawkins MS, Kuipers AL, Sutton-Tyrrell K. Effects of weight loss and insulin reduction on arterial stiffness in the SAVE trial. Cardiovasc Diabetol 2012; 11: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richardson JTE. Eta squared and partial eta squared as measures of effect size in educational research. Educ Res Review 2011; 6(2): 135–147. [Google Scholar]
- 50.Cohen J Statistical power analysis for the behavioral sciences, 2nd edn Erlbaum: Hillsdale, NJ, 1988. [Google Scholar]
- 51.Goldschmidt AB, Wilfley DE, Paluch RA, Roemmich JN, Epstein LH. Indicated prevention of adult obesity: how much weight change is necessary for normalization of weight status in children? JAMA Pediatrics 2013; 167(1): 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laurson KR, Eisenmann JC, Welk GJ. Development of youth percent body fat standards using receiver operating characteristic curves. Am J Prev Med 2011; 41(4 Suppl 2): S93–99. [DOI] [PubMed] [Google Scholar]
- 53.Koopman LP, McCrindle BW, Slorach C, Chahal N, Hui W, Sarkola T et al. Interaction between myocardial and vascular changes in obese children: a pilot study. J Am Soc Echocardiogr 2012; 25(4): 401–410 e1. [DOI] [PubMed] [Google Scholar]
- 54.Urbina EM, Kimball TR, Khoury PR, Daniels SR, Dolan LM. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J Hypertens 2010; 28(8): 1692–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pierce GL, Zhu H, Darracott K, Edet I, Bhagatwala J, Huang Y et al. Arterial stiffness and pulse-pressure amplification in overweight/obese African-American adolescents: relation with higher systolic and pulse pressure. Am J Hypertens 2013; 26(1): 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Charakida M, Jones A, Falaschetti E, Khan T, Finer N, Sattar N et al. Childhood obesity and vascular phenotypes: a population study. J Am Coll Cardiol 2012; 60(25): 2643–2650. [DOI] [PubMed] [Google Scholar]
- 57.Litwin SE. Good fat, bad fat: the increasingly complex interplay of adipose tissue and the cardiovascular system. J Am Coll Cardiol 2013; 62(2): 136–137. [DOI] [PubMed] [Google Scholar]
- 58.Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol 2011; 57(25): 2461–2473. [DOI] [PubMed] [Google Scholar]
- 59.Armstrong N Aerobic fitness and physical activity in children. Pediatr Exerc Sci 2013; 25(4): 548–560. [DOI] [PubMed] [Google Scholar]
- 60.Wilmore JH. Dose-response: variation with age, sex, and health status. Med Sci Sports Exerc 2001; 33(6 Suppl): S622–S634; discussion S640-S641. [DOI] [PubMed] [Google Scholar]
- 61.Okamoto T, Masuhara M, Ikuta K. Effect of low-intensity resistance training on arterial function. Eur J Appl Physiol 2011; 111(5): 743–748. [DOI] [PubMed] [Google Scholar]
- 62.Boreham CA, Ferreira I, Twisk JW, Gallagher AM, Savage MJ, Murray LJ. Cardiorespiratory fitness, physical activity, and arterial stiffness: the Northern Ireland Young Hearts Project. Hypertension 2004; 44(5): 721–726. [DOI] [PubMed] [Google Scholar]
- 63.Cresanta JL, Srinivasan SR, Webber LS, Berenson GS. Serum lipid and lipoprotein cholesterol grids for cardiovascular risk screening of children. Am J Dis Child 1984; 138(4): 379–387. [DOI] [PubMed] [Google Scholar]
- 64.Berenson GS, Wattigney WA, Tracy RE, Newman WP 3rd, Srinivasan SR, Webber LS et al. Atherosclerosis of the aorta and coronary arteries and cardiovascular risk factors in persons aged 6 to 30 years and studied at necropsy (The Bogalusa Heart Study). Am J Cardiol 1992; 70(9): 851–858. [DOI] [PubMed] [Google Scholar]
- 65.Okely AD, Collins CE, Morgan PJ, Jones RA, Warren JM, Cliff DP et al. Multi-site randomized controlled trial of a child-centered physical activity program, a parent-centered dietary-modification program, or both in overweight children: the HIKCUPS study. J Pediatr 2010; 157(3): 388–394, 394 e1. [DOI] [PubMed] [Google Scholar]
- 66.Rossi P, Frances Y, Kingwell BA, Ahimastos AA. Gender differences in artery wall biomechanical properties throughout life. J Hypertens 2011; 29(6): 1023–1033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.