Abstract
Hippocampal subfield segmentation on in vivo MRI is of great interest for cognition, aging and disease research. Extant subfield segmentation protocols have been based on neuroanatomical references, but these references often give limited information on anatomical variability. Moreover, there is generally a mismatch between the orientation of the histological sections and the often anisotropic coronal sections on in vivo MRI. To address these issues, we provide a detailed description of hippocampal anatomy using a post-mortem dataset containing 9 specimens of subjects with and without dementia, which underwent a 9.4 tesla MRI and histological processing. Post-mortem MRI matched the typical orientation of in vivo images and segmentations were generated in MRI space, based on the registered annotated histological sections. We focus on the following topics: the order of appearance of subfields, the location of subfields relative to macroanatomical features, the location of subfields in the uncus and tail and the composition of the dark bank, a hypointense layer visible in T2-weighted MRI. Our main findings are that: 1) there is a consistent order of appearance of subfields in the hippocampal head, 2) the composition of subfields is not consistent in the anterior uncus, but more consistent in the posterior uncus, 3) the dark band consists only of the CA-stratum lacunosum moleculare, not the strata moleculare of the dentate gyrus, 4) the subiculum/CA1 border is located at the middle of the width of the hippocampus in the body in coronal plane, but moves in a medial direction from anterior to posterior, and 5) the variable location and composition of subfields in the hippocampal tail can be brought back to a body-like appearance when reslicing the MRI scan following the curvature of the tail. Our findings and this publicly available dataset will hopefully improve anatomical accuracy of future hippocampal subfield segmentation protocols.
Keywords: Hippocampal subfields, segmentation, MRI, ex vivo, in vivo, histology
Introduction
The hippocampus plays a crucial role in declarative memory (Squire et al., 2004; Moscovitch et al., 2006), spatial navigation (Eichenbaum, 2017) and perception discrimination (Hodgetts et al., 2017) and is of great importance for research on development, aging and diseases, such as Alzheimer’s disease, temporal lobe epilepsy and depression (Small et al., 2011). Importantly hippocampal subfields, cornu ammonis (CA) 1, 2, 3, dentate gyrus (DG) and subiculum (SUB), are functionally and cytoarchitectonically distinct (Insausti and Amaral, 2012; Ding and Van Hoesen, 2015) and as such are also thought to be dissociably vulnerable to different pathologies (Braak and Braak, 1991; Small et al., 2011). Over the past years, MRI has been extensively used in order to visualize and measure the inner structure of the hippocampus in vivo. In particular, T2-weighted MR scans with high resolution in the coronal plane but relatively thick slices are being utilized (Yushkevich et al., 2015a; Wisse et al., 2017b). The high in-plane resolution allows for the assessment of several individual subfields and these subfields are indeed increasingly investigated in relation to cognition, lifespan changes and disease (Zeineh et al., 2003; Bakker et al., 2008; Burggren et al., 2008; Mueller et al., 2009; Yassa et al., 2010; Kerchner et al., 2010; Pluta et al., 2012; La Joie et al., 2013; Olsen et al., 2013; Wisse et al., 2015; de Flores et al., 2015; Kyle et al., 2015; Daugherty et al., 2016; Hodgetts et al., 2017).
The T2-weighted MRI scans with high in-plane resolution visualize a white matter band which accounts for a large portion of the border between the DG and adjacent CA and SUB. However, other borders between subfields cannot be readily visualized on this type of in vivo MRI scans, that is: the boundary between CA1/CA2, CA2/CA3 subfields and between the SUB and CA1 (Yushkevich et al., 2015a). In segmentation protocols, these borders are often approximated utilizing heuristic rules based on the available neuroanatomical literature (Duvernoy, 2005; Mai et al., 2008; Insausti and Amaral, 2012; Ding and Van Hoesen, 2015; Ding et al., 2016). Unfortunately, limited information is available with regard to anatomical variability of subfield boundaries. Often only one picture or specimen is displayed in the anatomical references, leaving ambiguity regarding variation around the subfield borders and overall shape of the hippocampus. It is therefore often unclear how representative the displayed case is and how much error is introduced basing one’s in vivo segmentation protocol on this particular reference.
Moreover, there is a mismatch between the orientation of histological sections in most studies or atlases and the orientation of the, often thick, slices of in vivo MRI. Whereas high-resolution T2-weighted MRI scans are typically acquired perpendicular to the long axis of the hippocampus, histological sectioning is usually not oriented along this axis in neuroanatomical literature (Duvernoy, 2005; Mai et al., 2008; Insausti and Amaral, 2012). This difference cannot be easily overcome because of the 2-dimensionality of both modalities and it is unclear how appropriate standard neuroanatomical references are for determining subfield borders on in vivo MRI with such a different angulation. One exception is the study by Ding and van Hoesen (2015) describing subfields in the hippocampal head, the anterior portion of the hippocampus, in 15 post-mortem specimens sliced perpendicular to the long axis of the hippocampus. While this is a valuable resource for researchers using in vivo MRI to study hippocampal subfields, information on other regions such as the hippocampal tail, the posterior portion of the hippocampus, is still lacking.
In this study, we utilized a dataset consisting of post-mortem MRI and accompanying serial histology sections of the hippocampus in nine intact hippocampal specimens from subjects with and without dementia (Adler et al., 2018). The advantage of the post-mortem MRI, acquired at 0.2×0.2×0.2 mm3 resolution, is that it can be sliced in any direction to match the common orientation of the T2-weighted in vivo MRI scans. This data was used to provide quantitative and qualitative descriptions of several hippocampal regions and subfield borders addressing the following key topics:
The order of appearance of hippocampal subfields along the long axis in the head.
The location of CA2 and CA3 relative to hippocampal head digitations.
A description of the uncus.
A characterization of the dark band.
The location of subfield borders relative to dark band length in the hippocampal body, the middle portion of the hippocampus.
The location of the SUB-CA1 boundary along the hippocampal body long axis.
The order of disappearance of hippocampal subfields along the long axis in the tail.
A detailed description of the tail.
The general aim of this paper is thus to take advantage of post-mortem data to inform in vivo segmentation by better characterizing the anatomy of the hippocampus and by investigating the utility of gross anatomical landmarks, which can also be observed on in vivo MRI, for segmentation purposes. We include both dementia and non-dementia cases to describe hippocampal subfield anatomy in both groups and investigate if dementia has an effect on subfield anatomy, as both groups are also of major interest in in vivo hippocampal subfield studies. The present manuscript is organized into a first section describing briefly the global methodology followed by 8 sections corresponding to each of the topics mentioned above, each including its own methods, findings, and discussion sections.
Methods
Dataset
Human brain specimens were obtained in accordance with the University of Pennsylvania Institutional Review Board guidelines. Preconsent during life and/or next-of-kin consent at death was given for all cases. Nine specimens from nine subjects for whom both ex vivo MRI and histological sectioning were available, were selected from the ex vivo hippocampus atlas dataset described in Adler et al. (2018) (note that we do not include the additional 22 specimens from this paper for whom only postmortem MRI is available but no histology data). Seven of the specimens were from the brain bank operated by the National Disease Research Interchange (NDRI) and two specimens were from autopsies performed at the University of Pennsylvania Center for Neurodegenerative Disease Research (CNDR). For specimens from NDRI, only clinical designation as dementia or control was available. For CNDR specimens, pathological diagnoses were available. See Table 1 for the demographics of the specimens. Specimens were fixed in 10% formalin solution for a minimum of 21 days and imaged on a Varian 9.4 T animal scanner at 0.2×0.2×0.2 mm3 (0.16×0.16×0.16 mm3 in one specimen) resolution. Hereafter, all nine specimens underwent serial histological processing. The histology protocol, the approach for matching histology to MRI, and the approach for mapping cytoarchitectural subfield boundaries from histology space to MRI space are described in Adler et al. (2018). In short, specimens were cut into 1 cm thick blocks (46 in total, for 9 specimens), which were imaged again with 0.2×0.2×0.2 mm3 resolution MRI. Blocks were embedded in paraffin and sectioned on a vibratome with 5 μm thickness and 200 μm spacing. Sections were stained using the Kluver-Barrera method (Kluver and Barrera, 1953), and digitally scanned at 0.5 μm × 0.5 μm resolution. For each block, the scanned sections were reconstructed in 3D and aligned to the block MRI using the interactive software HistoloZee (http://www.nitrc.org/projects/historecon). The resulting alignment was used to initialize the graph-theoretic automated histology reconstruction algorithm described in Adler et al. (2014), wherein each histology section undergoes linear and deformable registration to the neighboring sections and to the matched slice of the MRI scan. Note that not all MRI blocks had corresponding histology sections, as some blocks had poor staining and were thus unusable. Moreover, some sections were also torn or folded during the preparation.
Table 1.
Demographics and MRI acquisition parameters.
| Sample IDa | Age (years) | Sex | Group | Fixation (days) | Acquired MRI resolution (mm × mm × mm) |
|---|---|---|---|---|---|
| NDRI01-R | 89 | F | OD | 108 | 0.16×0.16×0.16b |
| NDRI02-R | 76 | M | NC | 256 | 0.2×0.2×0.2 |
| NDRI05-Lc | 61 | M | AD | 66 | 0.2×0.2×0.2 |
| NDRI08-R | 75 | M | NC | 523 | 0.2×0.2×0.2 |
| NDRI09-R | 78 | F | NC | 217 | 0.2×0.2×0.2 |
| NDRI12-L | 81 | F | NC | 527 | 0.2×0.2×0.2 |
| NDRI14-L | 90 | F | AD | 442 | 0.2×0.2×0.2 |
| CNDR02-Rd | 60 | M | AD | 82 | 0.2×0.2×0.2 |
| CNDR10-Le | 73 | M | OD | 22 | 0.2×0.2×0.2 |
AD=Alzheimer’s disease; NC=Non-dementia control; OD=Other dementia
ID number also includes hemisphere denotation: -L=left, -R=right hemisphere
All measurements were performend in 0.2×0.2×0.2 mm3.
Note that in Adler et al. (2018) mistakenly NDRI06-l instead NDRI05-L was mistakenly listed to have histology data available.
Pathological diagnosis: High Alzheimer’s Disease neuropathologic change
Pathological diagnosis: Progressive supranuclear palsy
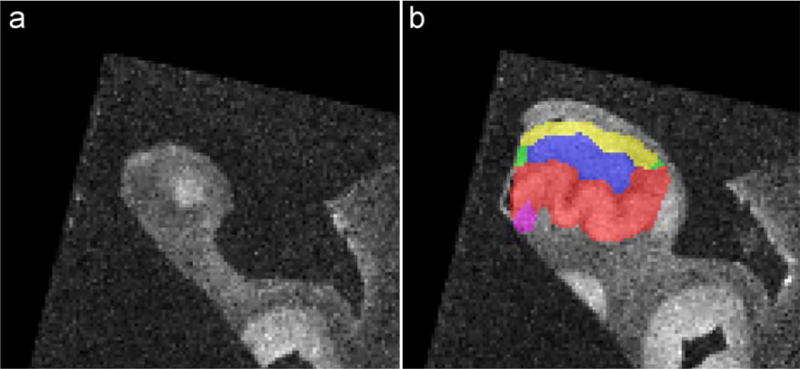
Boundaries between hippocampal subfields CA1, CA2, CA3, DG and SUB (and in some subjects the Hippocampal Amygdala Transition Area (HATA)) were annotated in each histology image by author JBP on the basis of cytoarchitectural features, following the anatomical protocol by Ding and van Hoesen (2015). Note that SUB in this study refers to the subicular complex, consisting of the prosubiculum, subiculum proper, presubiculum and parasubiculum (Ding, 2013). The annotations were reviewed by an expert neuroanatomist (author SLD) and modified based on his feedback. Annotations were mapped into the block-space MRI of each specimen and used to guide the manual segmentation of hippocampal subfields in MRI space. All segmentations were warped into the space of the whole MRI (Figure 1) and each MRI was rigidly aligned with the template from Adler et al. (2018), to approximate similar orientation for each MRI; that is approximately perpendicular to the long axis of the hippocampus. These segmentations in MRI space were used for all sections except the one regarding the characterization of the dark band. For this section, the segmentations in the space of the histology sections were used, which were also based on the annotations performed by author JBP. Seven regions were labeled: CA1, CA2, CA3, DG-SM, DG-G/H (DG granular cell layer/Hilus), SRLM and SUB. For certain questions, the hippocampus was also considered along its antero-posterior axis into the 3 subregions: head, body and tail. The uncal apex was used in order to define the head/body boundary (Poppenk et al., 2013). The body/tail boundary is classically defined using external landmarks such as the separation of the fornix (Malykhin et al., 2017) or the colliculi (Berron et al., 2017). However, these external landmarks were not available in our data. As a consequence, we use a working definition for the tail as the four most posterior slices with a 2 mm gap starting 1 mm anterior to the most posterior tip of the tail. Note that the dataset used in the present manuscript is publicly available (https://www.nitrc.org/projects/pennhippoatlas/).
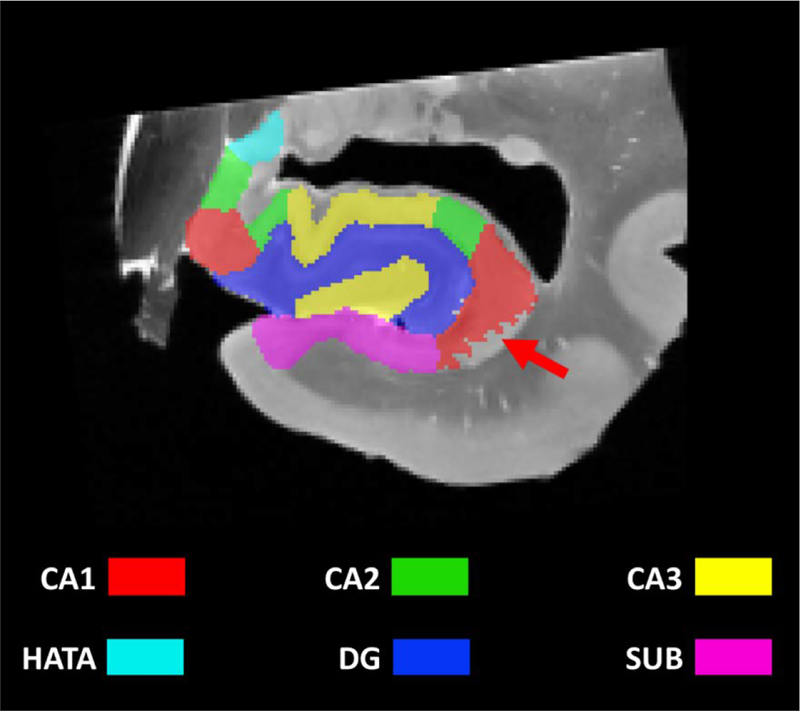
Figure 1.
Coronal image with subfield segmentation in the head of the hippocampus.
Because histological sectioning did not cover the full length of all specimens and because histological sectioning and MRI acquisition were not oriented exactly the same way, the available segmentation was partial for some MRI slices (red arrow).
HATA=Hippocampal Amygdala Transition Area; CA=cornu ammonis, DG=dentate gyrus, SUB=subiculum
Analyses
Each of the questions posed in this paper requires a slightly different approach and composition of the data described above, partly because histological sectioning did not cover the full length of all specimens. The approach for each question is therefore outlined in the results sections. Which specimens are used for each question is shown in Table 2. Measurements for almost all sections were performed in the coronal plane in ITKSNAP (Yushkevich et al., 2006 - http://www.itksnap.org), except the section on the subfield borders related to dark band length, which was performed in ImageJ (Schneider et al., 2012).
Table 2.
Overview of which specimens were used for each of the research questions.
| NDRI01-R | NDRI02-R | NDRI05- L | NDRI08-R | NDRI09-R | NDRI12- L | NDRI14- L | CNDR02-R | CNDR10- L | |
|---|---|---|---|---|---|---|---|---|---|
| Ordering of subfields – Head | x | x | x | x | |||||
| Relation anterior appearance CA1, CA2 and CA3 to digitations | x | x | x | x | x | x | x | x | |
| Characterization of the uncus | x | x | x | x | x | x | x | x | x |
| The dark band | x | x | x | x | x | x | x | x | x |
| Subfields borders relative to dark band length | x | x | x | x | x | x | x | x | x |
| SUB-CA1 boundary along the hippocampal body long axis | x | x | x | x | x | x | x | x | |
| Ordering of subfields - Tail | x | x | x | x | x | x | |||
| Tale of the tail | x | x | x | x | x | x | x | x |
CA=cornu ammonis; SUB=subiculum
Results
Order of appearance of hippocampal subfields along the long axis - Head
Aim:
To characterize the order in which different hippocampal subregions appear when slicing the hippocampal head along the hippocampal long axis. A particular emphasis was put on CA subfields due to the anatomical complexity of these structures in the hippocampal head. For instance, the extent of the typical X-shape of CA3 anterior to the separation of the uncus, as described in Wisse et al. (2017), was characterized.
Approach:
Because histological sectioning was not covering the entire length of the hippocampal head for all specimens, only 4 out of the 9 specimens could be used to address the aim of the present section. Note that this group is composed of 2 dementia and 2 non-dementia cases. The most posterior slice of the head was defined as the most posterior slice in which the uncal apex is visible (Poppenk et al., 2013). Distances were estimated as the number of slices separating two regions multiplied by the slice thickness (0.2 mm). Distances were also reported as a percentage of the hippocampal head length.
Results:
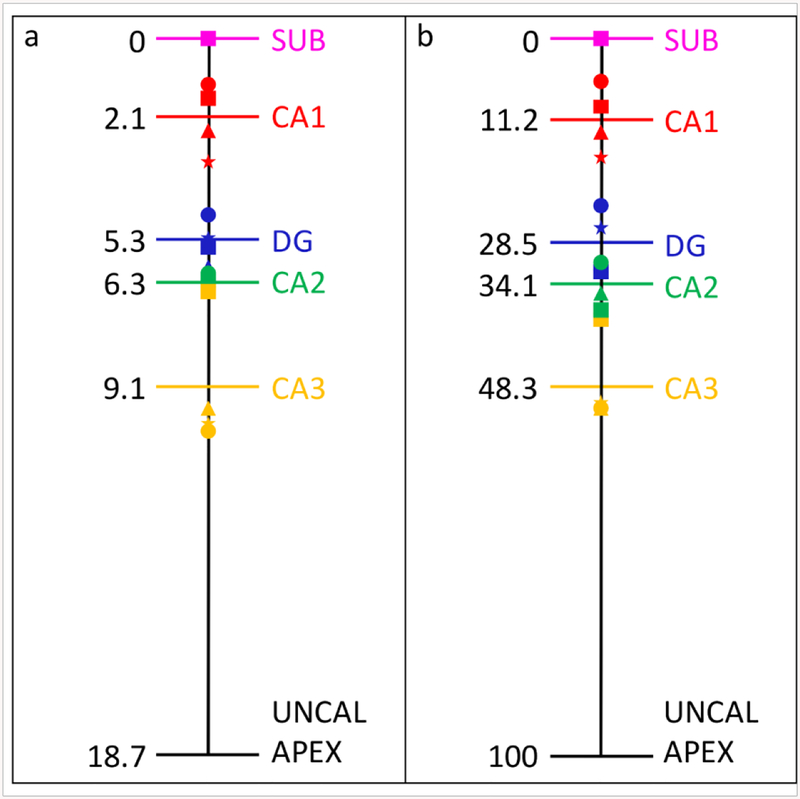
The average length of the hippocampal head was 18.7 (± 1.4) mm. The subfields’ order of appearance was consistent across the four specimens. Indeed, the SUB was always the first subfield to appear in the most anterior part of the hippocampus, followed by CA1 which appeared after an average distance of 2.1 (± 0.9) mm (11.2 ± 6.3 % of the head length). Posteriorly, the DG appeared after a distance of 3.2 (± 0.8) mm (17.3 ± 9 % of the head length). CA2 then became visible 1.0 (± 0.4) mm further (5.6 ± 3 % of the head length), followed by CA3 which appeared after a distance of 2.8 (± 1.7) mm (14.2 ± 8.9 % of the head length). Note that distances are given relative to the preceding subfield. See Figure 2 for the distances in millimeters and percentages. Because of the small sample size we were not able to make a comparison between the dementia and non-dementia cases.
Figure 2.
Order of appearance of subfields and distances between the subfields in millimeters (a) and percentages (b). Each shape labels one specific subject.
CA=cornu ammonis; DG=dentate gyrus; SUB=subiculum
Interestingly, CA1 appeared lateral to SUB (Figure 3 a) and progressed medially when moving posteriorly (Figure 3 b) in all cases. CA2 appeared in two different locations: 1) in the uncus (Figure 3 c–1) and 2) superior to the DG (Figure 3 c–2). The order of appearance of one of these regions relative to the other was not consistent across subjects: CA2 first appeared in the uncus in two specimens, while it appeared superior to the DG in the two other specimens. The average distance between the first appearance of CA2 in these different locations was 2.1 (± 1.7) mm (11 ± 8.7 % of the head length).
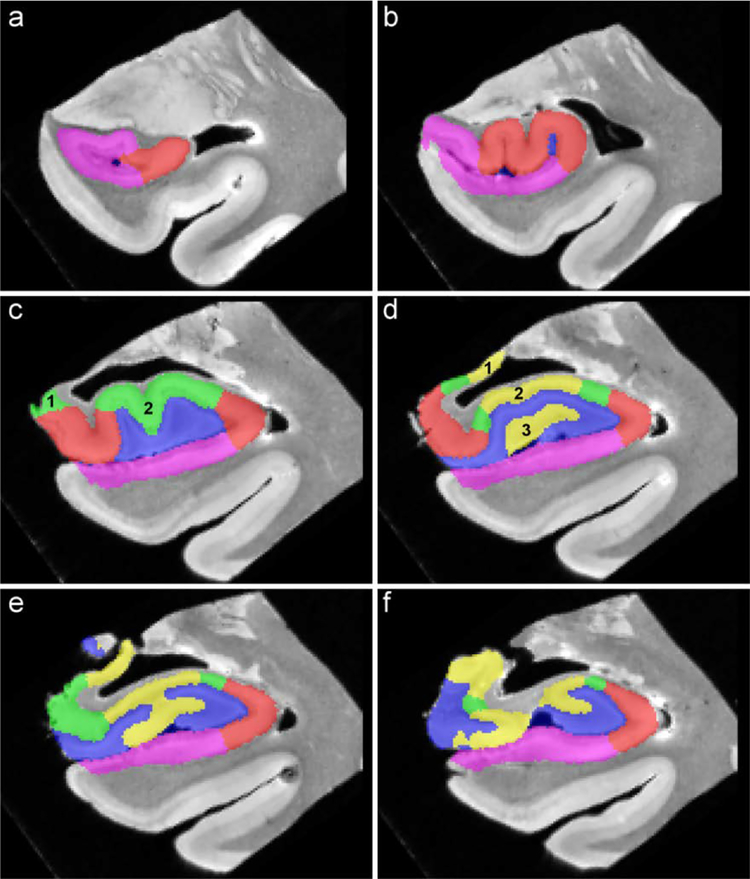
Figure 3.
Order of appearance of subfields showed in one specimen, from anterior to posterior.
CA1 appeared lateral to SUB (a) while CA2 appeared in 2 different locations (c) and CA3 appeared in 3 different locations (d). X-shaped CA3 was observed for several slices (e) the uncus separates from the hippocampus (f).
CA=cornu ammonis
CA3 could be observed in three different locations: 1) in the uncus (Figure 3 d–1), 2) superior to the DG (Figure 3 d–2) and 3) within the DG (Figure 3 d–3). Once again, no clear pattern arose from our observations: CA3 first appeared in the uncus in two subjects while it was first visible superior to the DG in the two remaining specimens. The average distance between the appearance of CA3 in the first and the second location was 0.8 (± 0.7) mm (4.5 ± 4.3 % of the head length), while the third region appeared on average 0.7 (± 1) mm (3.8 ± 5.3 % of the head length) after the appearance of the second region.
Several slices posterior to the appearance of CA3 within the DG, this region merged with the portion of CA3 located superior to the DG and formed an X-shaped CA3 region (Figure 3 e). In order to describe this particular feature of CA3, a larger group consisting of seven specimens was used. First, the X-shaped CA3 was observed in each of the seven hippocampi. The first slice in which the X-shaped CA3 appeared was 2.9 (± 0.9) mm (16 ± 6 % of the head length) posterior to the appearance of CA3 within the DG. The X-shape CA3 was then visible for 2.14 (± 0.8) mm (13.2 ± 5.1 % of the head length), until the uncus separates from the lateral portion of the hippocampus (Figure 3 f).
Discussion:
The aim of the present section was to better characterize the order of appearance of the different hippocampal subfields. Our analyses, performed on 4 specimens, revealed that the hippocampal subfields always emerged in the same order: SUB was the first region to become visible anteriorly, followed by CA1, DG, CA2 and CA3, respectively. Although some variability in distances between subfields could be observed, a similar order of appearance can be observed in the Ding and van Hoesen paper (2015).
Interestingly, CA1 appeared posteriorly to SUB after a mean distance of 1.9 mm. Given the fact that most of the in vivo high resolution images used to segment subfields use a 2 mm slice thickness, this observation seems to indicate that the very first segmented slice should only contain SUB. Note that the location of appearance of CA1 was consistent across specimens. Other studies also reported distances in order to better describe the order of appearance of the different subfields. For example, Insausti & Amaral (2012) provided a histological estimate of the locations, along the temporal lobe rostrocaudal axis, of the different components of the hippocampal formation. This paper reported a distance between SUB and DG of around 6 mm and between SUB and the uncal apex of 13 mm. While our results are close for the SUB to DG distance (5.3 mm), it is less similar for the distance between SUB and the uncal apex (18.7 mm). Another paper, based this time on in vivo scans acquired perpendicular to the long axis of the hippocampus, reported values closer to the ones in the current paper, with a SUB to uncal apex distance of 16.5 mm while the SUB to DG distance is 7.7 mm (note that while in vivo MRI generally does not provide definitive distances between subfields, the anterior tip of the SUB, i.e. the hippocampus, and DG and the posterior uncal apex can easily be identified on high resolution in vivo MRI). The differences between the histological reference (Insausti and Amaral, 2012), the in vivo MRI reference (Berron et al., 2017) and the current paper are likely mostly due to differences between modalities resulting from changes in brain tissue after death and during processing, as previously reported for same subject in vivo and ex vivo MRI (Wisse et al., 2017a) and for shrinkage during histological processing (Mouritzen Dam, 1979; Amunts et al., 2005). This warrants caution towards using absolute distances obtained from postmortem data for in vivo segmentation, and indicates that relative values, such as the percentages we have provided in Figure 2, may be more useful.
Another important observation is that CA2 and CA3 do not appear in a unique location but rather in different places: in the uncus or superior to the DG for CA2 while CA3 emerges in the uncus, superior to the DG and within the DG. However, no consistent pattern was found across specimens as, for example, CA2 first appeared in the uncus for 2 specimens while it appeared superior to the DG for the other 2 specimens. Unfortunately, these findings do not provide clear guidance for the segmentation of CA2 and CA3 in the hippocampal head and a larger sample is needed to further characterize the location of appearance of CA2 and CA3. Lastly, our observations showed that the portion of CA3 encompassed within the DG merged with the portion of CA3 located above the DG to form an X-shaped CA3 region (see also Wisse et al., 2017a). To our knowledge, this feature has never been incorporated in any segmentation protocol. This X-shaped CA3 was visible for an average length of 2.14 mm, which indicates, given the typical slice thickness of images used for subfield segmentation, that at least a 2 mm slice should be segmented with this X-shaped pattern for CA3. This is an important finding given that this particular slice makes up a considerable portion of CA3, a region that is of great interest in studies investigating, for example, pattern separation and pattern completion (Bonnici et al., 2012; Berron et al., 2016) or the effects of stress on the hippocampus (Sapolsky et al., 1990; Sousa et al., 1999). In addition to the present description of 4 specimens, the anatomy of each subfield in the hippocampal head can be visualized in the Supplementary Video showing the 3D template described in Adler et al. (2018).
Location of CA2 and CA3 relative to hippocampal head digitations
Aim:
In vivo segmentation often utilizes gross anatomical landmarks to develop heuristic rules for border placements and hippocampal head digitations could be useful landmarks. We aimed to characterize the location of the most anterior portion of CA2 and CA3 superior to DG in relation to the digitations.
Approach:
In eight subjects the anterior end of the CA2 and CA3 segmentation was available. The number of digitations was characterized on coronal slices as well as the location of the most anterior portion of CA2 and CA3 relative to the digitations. In contrast to the previous section, we did not examine the CA2 and CA3 in the uncal region or within the DG but only superior to DG. It was not possible to relate the appearance of CA1 to digitations as the digitations are not present in all cases this far anterior.
Results:
The results are displayed in Table 3. The majority of subjects had 3 digitations (62.5%) with a range of 2–4. The location of the most anterior CA2 was as follows: on the top of the most lateral digitation (42.8%; Figure 4 a), on the top of the second most lateral digitation (28.6%; Figure 4 b) or on the top of the first and second most lateral digitation (28.6%; Figure 4 c). The location of the most anterior CA3 was as follows: in the indentation between the two most lateral digitations (57.1%; Figure 4 d), on the top of the most lateral digitation (33.3%; Figure 4 e) or on the top of the second most lateral digitation (14.3%; Figure 4 f). When the most anterior CA3 was located at the top of one of the lateral digitations, it extended to the indentation between the two digitations within 1 mm. While there was a slight difference in number of digitations between non-dementia and dementia cases (3.33 vs 2.60), there were no clear differences between the groups on the location of appearance of CA2 or CA3 (data not shown).
Table 3.
Number of digitations and the location of the most anterior CA2 and CA3 relative to the digitations.
| N | Characterization | |
|---|---|---|
| Number of digitations (mean (range)) | 8 | 2.88 (2–4) |
| 2 digitations (n (%)) | 2 (25) | |
| 3 digitations (n (%)) | 5 (62.5) | |
| 4 digitations (n (%)) | 1 (12.5) | |
| Location of most anterior CA2 | 7 | |
| Top most lateral digitation (n (%)) | 3 (42.8) | |
| Top second most lateral digitation (n (%)) | 2 (28.6) | |
| Top of first and second most lateral digitation (n (%)) | 2 (28.6) | |
| Location of most anterior CA3 | ||
| Top most lateral dig (n (%)) | 6** | 2 (33.3) |
| Between two most lateral digitations (n (%)) | 7 | 4 (57.1) |
| Top second most lateral digitation (n (%)) | 7 | 1 (14.3) |
CA=cornu ammonis
Segmentation on the most lateral digitation was missing for one specimen.
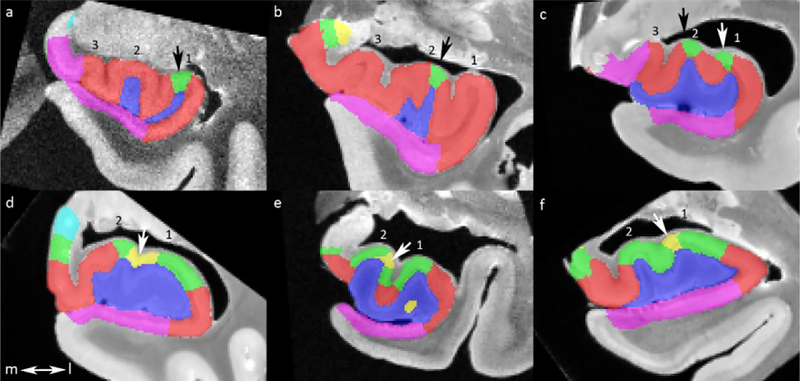
Figure 4.
Location of appearance of most anterior CA2 (a-c) and CA3 (d-f) relative to the digitations. All digitations are numbered from a lateral to medial direction. CA2 either appears on the top of the most lateral digitation (a), on the top of the second most lateral digitations (b) or both (c). CA3 appears either in the indentation between the two most lateral digitation (d), on the top of the second most lateral digitation (e) or on the top of the most lateral digitation (f). Note that slices from six different subjects were selected.
CA=cornu ammonis; l=lateral; m=medial
Discussion:
We did not observe an association between the digitations and the location in which CA2 or CA3 would appear. Given the considerable variability, it seems unlikely that digitations are a useful landmark, especially for CA2. Any possible segmentation rule utilizing the digitations would likely be incorrect for a considerable portion of subjects, especially since the sample size is small and the actual between subject-variability in the anterior appearance of CA2 might be larger than what was observed here. For CA3, a segmentation rule could be envisioned where it is located at the indentation between the two most lateral digitations. However, the extent of CA3 was still very variable between subjects, and replication of this finding in a larger dataset is needed.
Characterization of the uncus
Aim:
As the uncus is a relatively unknown hippocampal region, we aim to characterize the composition of subfields and the variability thereof in the uncus.
Approach:
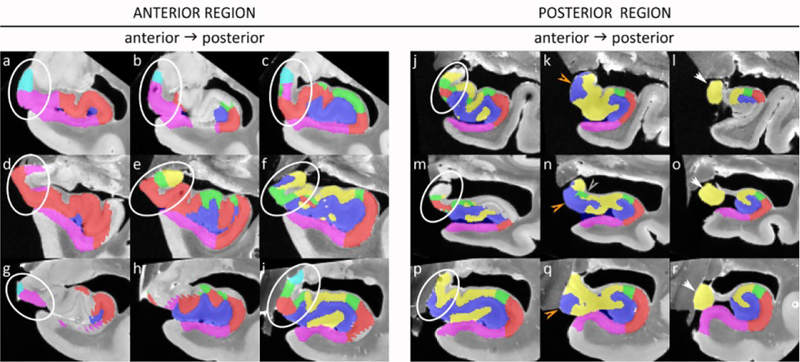
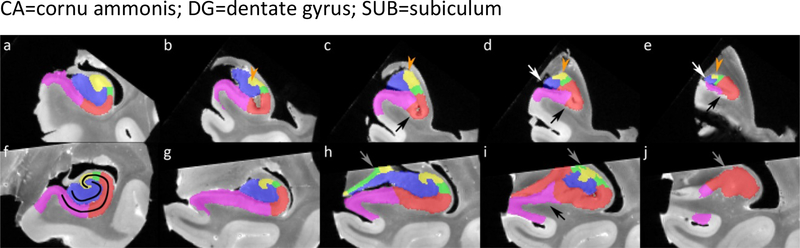
All nine subjects were used for the characterization of the uncus. As the uncus is a complex region we aimed to simplify it by dividing the region up in an anterior and a posterior region. Note that this division is only for practical reasons and not based on cytoarchitecture or function. The working definition for the anterior border is the most anterior slice of where the hippocampal sulcus reaches the cerebrospinal fluid in the ambiens cisterna. The posterior border is the most posterior slice of the uncal apex. The anterior uncus was visualized selecting the first slice 1 mm posterior to the appearance of the hippocampal sulcus, and two slices posterior to the first slice both with a 2 mm gap. The same approach was taken for the posterior uncus, with the most posterior slice 1 mm anterior to the tip of the uncus and two additional slices anterior to this slice with a 2 mm gap. Sections of three representative specimens are displayed in Figure 5.
Figure 5.
The anterior (a-i) and posterior (j-r) uncal region is shown for three specimens. In the anterior region a variety of subfields can be observed in different compositions, with two or more subfields present in all sections but not in a consistent manner. In the posterior region more consistency can be observed, with only CA3 being present in the most posterior selected slice, CA3 and DG present in the middle slice, although CA2 is also present in one specimen (n). However, in the most anterior section (j, m, p) more variety can be observed.
Note that because of slight differences in angulation of the histology sections, certain parts of the segmentation are missing (for example in b, g, h, l and m).
Results:
In the anterior region (Figure 5 a–i) great variability can be observed both in presence and in location of subfields. At least two or more subfields can be observed in all sections, but not in a consistent manner in each of the specimens or in an anterior-to-posterior direction. For the posterior region greater consistency can be observed (Figure 5 j–r), with only CA3 present in the most posterior slice and CA3 and DG present in the middle slice. Although it should be noted that the location of DG (inferiomedial or superiomedial) differed between subjects and that CA2 was also present in one specimen (n). The anterior slice of the posterior region, at 5 mm anterior to the tip of the uncus, showed less consistency in location or presence of subfields.
While it is possible that more consistency can be observed when selecting sections from specimens that more closely match in shape, for example, we did not observe this with more dense sampling (i.e with a 1 mm gap; data not shown). Moreover, the 2 mm gap between slices closely matches the slice thickness in the typical T2-weighted images.
The great variability in the presence and location of subfields in the uncus made it difficult to compare non-dementia and dementia cases.
Discussion:
While the uncus has been described in previous atlases, to the best of our knowledge, none of these atlases aimed to characterize the variability. We observed great variability in anterior and middle regions of the uncus in presence, location and composition of hippocampal subfields, but more consistency was observed in the most posterior slices.
When comparing Figure 5 with the existing literature (Mai et al., 2008; Insausti and Amaral, 2012; Ding and Van Hoesen, 2015; Ding et al., 2016), some consistencies can be observed. The HATA is generally present in more anterior regions together with the SUB, which is followed by CA1, then CA2 and subsequently DG and CA3 in more posterior regions. However, the exact location of the subfields and spacing between them in an anterior-to-posterior direction seems very variable. Only the posterior region seems to be more consistent with CA3 present as the only subfield in the most posterior uncal apex and the DG and CA3 being present in the section 2 mm anterior to this. Although CA2 can be present as well and the actual location of the DG can be variable.
Based on these observations, it is still unclear how the uncus can be segmented in in vivo scans. A larger dataset with more complete coverage of histology sections is needed to further characterize the uncal region. An interesting solution for segmentation of the uncus can be found in the protocol by Dalton et al. (2017), who grouped the different subfields into one ‘uncus’ label because they found this region differentiable in fMRI studies (Zeidman et al., 2015; Zeidman and Maguire, 2016).
Finally, we investigated the role of the curvature of the hippocampal head in the observed variability of the uncus in the coronal plane, similar as for the curvature of the tail (last section). However, reslicing the hippocampal head along the curvature in the medial direction did not reveal a more consistent morphometry in the hippocampal head (data not shown). It should be noted, though, that the curvature of the uncus is more complex than that of the tail which may have prevented us from finding more consistent morphometry.
The dark band
Aim:
The term “dark band” refers to a hypointense line appearing within the grey matter of the hippocampus on high resolution T2 images (Figure 6 a). This feature is very frequently used in segmentation protocols as the boundary between DG and SUB/CA1–3 (La Joie et al., 2010; Wisse et al., 2012; Berron et al., 2017; Dalton et al., 2017). However, it remains unclear what hippocampal layers the dark band represents. More precisely, it is not clear whether it reflects the stratum radiatum lacunosum moleculare (SRLM), the DG stratum moleculare (DG-SM) or both SRLM and DG-SM. As a consequence, an incorrect classification of the dark band in in vivo protocols could lead to inaccurate measures for DG and SUB/CA1–3. The aim of the present section was thus to determine the nature of the dark band.
Figure 6.
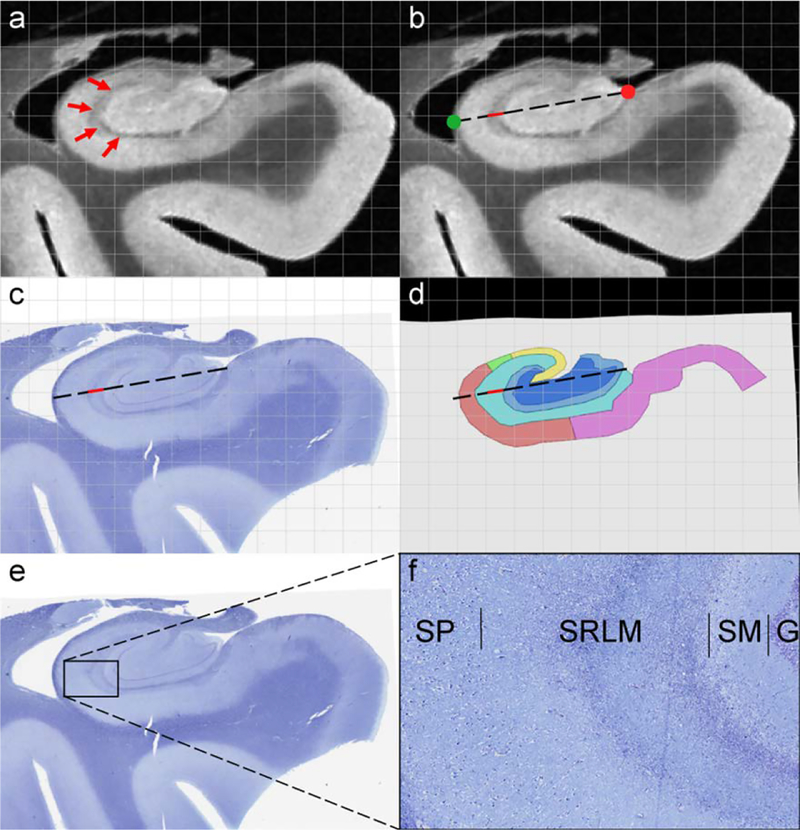
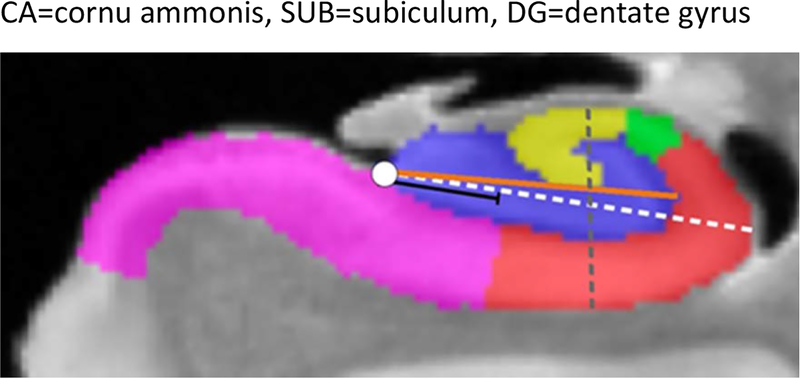
Characterization of the dark band. The dark band thickness was measured on MRI following the dashed line starting at the medial anchor point (red circle) to the most lateral point of the hippocampus (green circle) (b; dark band measurement is depicted in red). The corresponding histology section (c) and its segmentation (d) were registered to the MRI, where measurements along the same dashed line were performed. F) shows magnification of the slice showed in (e). 7 regions were labeled: CA1 (red), CA2 (green), CA3 (yellow), DG-SM (light blue), DG-G/H (dark blue), SRLM (cyan) and SUB (pink)
CA=cornu ammonis; DG-SM=dentate gyrus stratum moleculare; DG-SM=dentate gyrus granular cell layer/Hilus; SRLM=stratum radiatum lacunosum moleculare; SUB=subiculum; SP= stratum pyramidale; SM=stratum moleculare; G= granule cell layer
Approach:
Data from all 9 subjects were used for this section. Note that in this section we use the segmentations directly performed on the histology sections. The MRI image (Figure 6 b), histology image (Figure 6 c) and segmentation image (Figure 6 d) were opened in the same window using ITK-SNAP. Measurements were performed on the first body slice that contained a full segmentation. Moreover, the registration on the selected slices was carefully checked and found to be excellent, which is crucial for the current aim (see also the grid on Figure 6).
First, a line was drawn from the point where the DG meets the SUB (Figure 6 b – red circle) to the most lateral point of the hippocampus (Figure 6 b – green circle). The dark band thickness was measured in MRI scans following this line (Figure 6 b). As the MRI, histology and segmentation were aligned and opened in the same window, the line used to measure the dark band thickness in the MRI image was also automatically shown on the segmentation image so that the histological nature of the dark band could be evaluated (Figure 6 d). The thickness of CA-SRLM and DG-SM were also measured on the segmentation images following the same line. Lastly, the thickness of the dark band was correlated to the thickness of CA-SRLM and DG-SM using Spearman’s rank correlations.
Results:
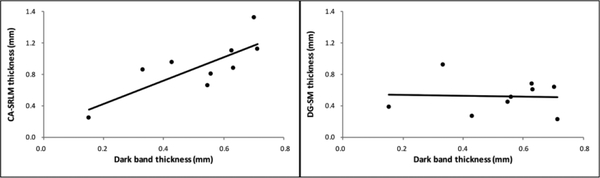
The average dark band thickness was 0.52 (± 0.18) mm (Non-dementia cases: 0.56 ± 0.16 mm; Dementia cases: 0.49 ± 0.22 mm). Interestingly, compared to the segmentation performed on histology, the dark band was exclusively located in CA-SRLM, similar as the example shown in Figure 6 c. Based on histology, the average CA-SRLM thickness was 0.91 (± 0.54) mm (Non-dementia cases: 1.02 ± 0.34 mm; Dementia cases: 0.82 ± 0.37 mm) and the average DG-SM was 0.52 (± 0.22) mm (Non-dementia cases: 0.67 ± 0.18 mm; Dementia cases: 0.40 ± 0.18 mm). The dark band thickness was significantly correlated with the thickness of CA-SRLM (rho=0.77; p= 0.02) but not with the thickness of DG-SM (rho= −0.03; p=0.93) (Figure 7).
Figure 7.
Correlation between the thickness of the dark band measured on MRI and the thickness of CA-SRLM and DG-SM measured on histology sections.
CA=cornu ammonis; DG-SM=dentate gyrus stratum moleculare; SRLM=stratum radiatum lacunosum moleculare
Discussion:
Our observations, based on a comparison of the dark band on MRI with the histology segmentations, indicate that the dark band mapped only onto the CA-SRLM. Note that the dark band was not corresponding to the full SRLM but more specifically to the stratum lacunosum moleculare. This finding was supported by the fact that the dark band thickness significantly correlated with the thickness of CA-SRLM but not with the thickness of DG-SM. A similar observation can be made in Figure 7 in Duvernoy (2005), further confirming our findings. This information is particularly relevant as segmentation protocols differ on this point. For example, the dark band is split between SUB/CA1 and DG by some authors (Wisse et al., 2012; Berron et al., 2017), while others include it in CA1 (La Joie et al., 2010) or in DG (Malykhin et al., 2010; Kulaga-Yoskovitz et al., 2015). How the dark band is handled, i.e. split between the subfields or included in the DG on in vivo MRI, may have affected study results, for example regarding subfield atrophy in AD. According to neuropathological studies, neurofibrillary tangle pathology affects CA1 more than DG (Milenkovic et al., 2014) and our recent study (Adler et al., 2018) shows that SRLM is severely affected in AD. Thus, the fact that DG is sometimes reported to be similarly or more affected than CA1 in in vivo MRI studies (Lim et al., 2013; Khan et al., 2015; Yushkevich et al., 2015b) might potentially be due to the erroneous inclusion of SRLM and thus artificially increase the observed effect of AD on the DG.
Finally, the observation that the widths of the dark band, CA-SRLM, DG-SM were all less than 1 mm thick, stress the need of high resolution in vivo images (< 1mm) to visualize and measure such thin layers. Note that these measures were overall thinner in dementia cases than in non-dementia cases. However, the sample size is too modest to perform any statistical analyses.
Subfield borders relative to the dark band in the body
Aim:
To determine the location of the borders of SUB/CA1, CA1/CA2 and CA2/CA3 relative to the length of the dark band in the hippocampal body, repeating the study by Steve et al. (2017).
Approach:
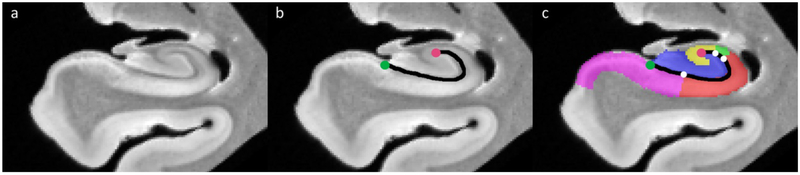
In all specimens, the most anterior body slice, posterior to the uncus and containing a segmentation with all relevant borders was selected (within a range of 2 mm). In addition, we selected the closest slice 5, 10 and 15 mm posterior to the uncus (within a range of 2 mm). The freehand line tool in ImageJ (Schneider et al., 2012) was used to measure the length of the dark band, drawing a line through the midpoint of the dark band starting at the most medial point of the DG (inferior anchor point) and ending at the most superior medial point in the curvature of CA3 (superior anchor point; Figure 8 b). Note that we chose to draw the line through the midpoint of the dark band, in contrast to Steve et al. (2017) who drew their line at the outer border of the SRLM on histology sections, as this boundary cannot be observed on MRI (see previous section). An advantage of using the midpoint is that the dark band is also visible on in vivo MRI, which would allow for in vivo application of this method in the future. Following this line, the distance was measured from the most medial point of the DG to each of the borders SUB/CA1, CA1/CA2, CA2/CA3 (Figure 8 c). Averages of the border location were calculated in absolute distances and % of SRLM length for the whole group and separately for the non-dementia controls and dementia patients.
Figure 8.
Measurement of dark band length and relative border locations of subfields in the hippocampal body.
The measurement of SRLM length started at the inferior anchor point (green circle) at the most medial point of the DG (b) going through midpoint of the SRLM to the superior anchor point (purple circle) at the CA3 inflexion point. Subsequently the distance from the inferior anchor point (green circle) to each of the border locations (white circles) was measured (c).
CA=cornu ammonis; DG=dentate gyrus; SRLM=stratum radiatum lacunosum moleculare
Results:
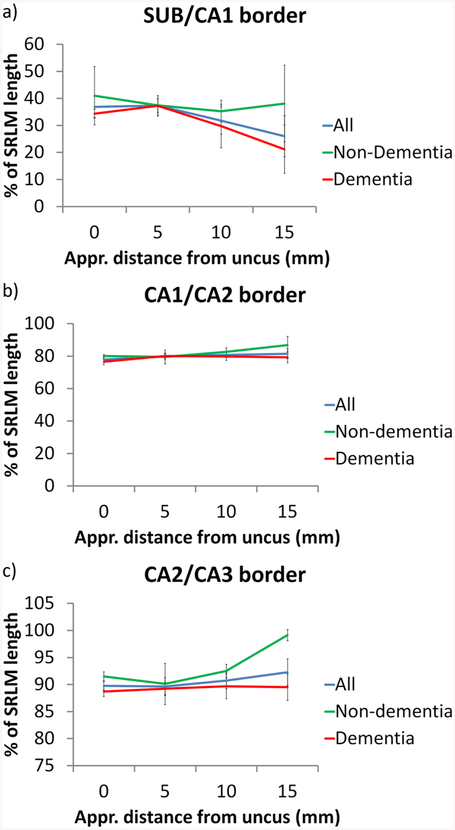
The results are displayed in Table 4 and Figure 9. The SRLM length is on average 1.13 cm, but decreases from anterior to posterior. The same holds for the absolute distance from the inferior anchor point (most medial point of the DG) to each of the borders. The SUB/CA1 border is on average located at 33.0% of the SRLM length from the inferior anchor point, the CA1/CA2 border at 80.0% and the CA2/CA3 border at 90.6%. As can be observed from the standard error, the location of the SUB/CA1 border is more variable than the other borders. From anterior to posterior, the SUB/CA1 border moves to a more inferiomedial location, with a difference of 10.9% from the first to fourth slice. As the SRLM length is appr. 1.13 cm, 10.9% translates to appr. 1.2 mm, which would mean 3 voxels of the typical 0.4×0.4 mm2 in vivo T2-weighted MRI scan. For the two other borders a similar tendency can be observed, although in a superiomedial direction towards the superior anchor point. However, the difference between the first and fourth slice is only about 3% which translates to 0.3 mm and is less than a voxel of the typical 0.4×0.4 mm2 in vivo T2-weighted MRI scan. Supplementary Figure S1 displays the most extreme examples of the change in border locations.
Table 4.
Total length of SRLM and location of the subfield borders relative to the length of SRLM in the group, and separately for non-dementia and dementia patients. The results from Steve et al. (2017) are shown for comparison.
| 1st body slice Mean (range) | 2nd body slice Mean (range) | 3rd body slice Mean (range) | 4th body slice Mean (range) | Mean of four slices (SE) | Steve et al. (2017) Mean (range) | |
|---|---|---|---|---|---|---|
| All | N=8 | N=9 | N=8 | N=7 | N=6 | |
| Distance posterior to uncus (mm) | 0.03 (−1.00; 1.60) | 5.00 (4.00; 6.00) | 10.30 (10.00; 11.20) | 15.06 (14.40; 16.00) | - | - |
| Total SRLM length (cm) | 1.27 (0.99; 1.62) | 1.19 (0.93; 1.61) | 1.07 (0.76; 1.39) | 0.98 (0.77; 1.19) | 1.13 (0.07) | 1.62 (1.39–1.96) |
| SUB/CA1 border (cm) | 0.47 (0.31; 0.75) | 0.44 (0.27; 0.63) | 0.35 (−0.01; 0.47) | 0.27 (−0.06; 0.60) | 0.38 (0.06) | 0.16 (−0.04; 0.46) |
| CA1/CA2 border (cm) | 1.00 (0.70; 1.31) | 0.95 (0.71; 1.33) | 0.87 (0.54; 1.18) | 0.81 (0.53; 1.05) | 0.91 (0.07) | 1.27 (1.04; 1.55) |
| CA2/CA3 border (cm) | 1.14 (0.87; 1.46) | 1.06 (0.82; 1.45) | 0.97 (0.65; 1.30) | 0.92 (0.63; 1.15) | 1.02 (0.08) | 1.57 (1.38; 1.87) |
| SUB/CA1 border (% of SRLM) | 36.9 (29.6; 62.7) | 37.3 (28.8; 50.5) | 31.8 (−1.6; 42.9) | 26.0 (−8.2; 52.3) | 33.0 (4.8) | 9.7 (−2.1; 26.2) |
| CA1/CA2 border (% of SRLM) | 77.9 (70.7; 81.2) | 79.9 (69.6; 89.7) | 80.8 (71.1; 86.7) | 81.4 (68.3; 92.3) | 80.0 (2.0) | 78.4 (69.8; 83.0) |
| CA2/CA3 border (% of SRLM) | 89.8 (87.1; 93.1) | 89.6 (80.8; 98.2) | 90.7 (85.4; 96.7) | 92.3 (82.1; 100.2) | 90.6 (1.7) | 97.5 (92.1; 100.7) |
| Non-dementia subjects | N=3 | N=4 | N=3 | N=2 | N=6 | |
| SUB/CA1 border (% of SRLM) | 41.0 (29.6; 62.7) | 37.4 (28.8; 45.7) | 35.3 (29.2; 42.9) | 38.1 (23.9; 52.3) | 38.0 (8.2) | 9.7 (−2.1; 26.2) |
| CA1/CA2 border (% of SRLM) | 80.1 (78.5; 81.2) | 79.5 (69.6; 89.7) | 82.6 (71.1; 86.7) | 86.8 (81.3; 92.3) | 82.3 (3.3) | 78.4 (69.8; 83.0) |
| CA2/CA3 border (% of SRLM) | 91.5 (90.3; 93.1) | 90.1 (87.4; 98.2) | 92.5 (90.6; 94.8) | 99.1 (98.1; 100.2) | 93.3 (1.7) | 97.5 (92.1; 100.7) |
| Dementia subjects | N=5 | N=5 | N=5 | N=5 | - | |
| SUB/CA1 border (% of SRLM) | 34.3 (30.4; 39.3) | 37.2 (29.3; 50.5) | 29.7 (−1.6; 42.6) | 21.2 (−8.2; 40.8) | 30.6 (5.6) | - |
| CA1/CA2 border (% of SRLM) | 76.6 (70.7; 80.7) | 80.1 (75.2; 83.1) | 79.8 (71.1; 84.7) | 79.3 (68.3; 86.6) | 79.0 (2.3) | - |
| CA2/CA3 border (% of SRLM) | 88.7 (87.1; 91.7) | 89.3 (86.2; 92.1) | 89.7 (85.4; 96.7) | 89.5 (82.1; 96.6) | 89.3 (1.7) | - |
CA=cornu ammonis; SRLM=stratum radiatum lacunosum molecular; SUB=subiculum
Figure 9.
Graphs of the SUB/CA1 (a), CA1/CA2 (b) and CA2/CA3 (c) borders relative to the length of SRLM over the length of the hippocampal body in the whole group and separately in the non-dementia and dementia subjects.
SRLM=stratum radiatum lacunosum moleculare, SUB=subiculum
With regard to the non-dementia and dementia subjects, some differences can be observed. The shift in the SUB/CA1 border in an inferiomedial direction from anterior to posterior seems to be mainly driven by the dementia subjects (13.1 vs 2.9%). However, given the small sample size, it is unclear how robust these findings are. The shift in the superiomedial direction towards the superior anchor point for the CA1/CA2 and CA2/CA3 borders seems to be driven by the non-dementia subjects, although it should be noted that the measurements 15 mm posterior to the uncus could only be performed in 2 non-dementia subjects. It is therefore unclear how robust these findings are.
When comparing our results from the non-dementia subjects to Steve et al. (2017; also only including non-dementia subjects), the location of the CA1/CA2 border (82.3% vs 78.4) and the CA2/CA3 border (93.3% vs 97.5) is fairly similar, however the SUB/CA1 border is located more laterally in our dataset (away from the inferior anchor point – 9.7 vs 38.0%).
Discussion:
Our measurements of the location of the CA1/CA2 and CA2/CA3 border relative to the SRLM length fairly closely matched the ones reported by Steve et al. (2017), but the SUB/CA1 border location was considerably different. For SUB/CA1 the border difference was 28.3%, which roughly translates to 3.2 mm and 8 0.4×0.4 mm2 voxels on a typical in vivo T2-weighted MRI scan. It is likely that this difference is largely due to potential differences in cytoarchitectonic border utilized in the segmentation protocols; for example, due to the inconsistent annotation of prosubiculum as a separate region (Insausti and Amaral, 2012; Ding and Van Hoesen, 2015). However, individual variability and slight differences in the line placement (midpoint of the dark band and outer border of the SRLM) could also have played a role in the observed differences. The age range in our non-dementia subjects was also smaller, although it is unclear how that could have affected the border definition. Given the inconsistencies between the studies for the SUB/CA1 border, it seems unwarranted to use this method for in vivo segmentation of this border. The CA1/CA2 and CA2/CA3 borders showed a similarity between the studies and SRLM could therefore be a useful marker for in vivo segmentation. However, the curvilinear tool to measure SRLM length is to the best of our knowledge only implemented in ImageJ and its use was difficult and time consuming. Potentially, an adaptation of this rule can be envisioned, where, for example, the CA1/CA2 border is approximately placed 20 % from the superior anchor point, that is about 2.3 mm, or 5 to 6 0.4×0.4 mm2 voxels. However, note that the results in the Steve et al. (2017) paper were slightly different and perhaps a weighted average of percentages in the two papers should be used to guide in vivo segmentation.
The potential difference between dementia and non-dementia subjects in the change in the inferiomedial direction of the SUB/CA1 border from anterior to posterior is notable, where the dementia subjects showed a large change in border location. A potential explanation is that the dementia patients show disproportionate DG atrophy in the posterior region, diminishing the distance from the inferior anchor point, which depends on the medial border of the DG, to the SUB/CA1 border. However, this seems unlikely as the dementia group is composed of patients with different types of dementia and the largest group consists of AD patients for whom disproportionate posterior DG atrophy has not been reported, to the best of our knowledge. Another possibility is that this difference is a spurious finding, which is very likely given the small sample size. Future studies in larger sample sizes are needed to further investigate this.
SUB-CA1 boundary along the hippocampal body long axis
Aim:
The border between hippocampal subfields CA1 and SUB has been a challenge in the past due to its variability between different segmentation protocols where the border has been placed at different locations along the medial-lateral axis (Yushkevich et al., 2015a). Here our aim was to investigate the location of the border between CA1 and SUB relative to the width of the hippocampus and the DG and whether the border between CA1 and SUB changes along the longitudinal axis of the hippocampal body. In addition, we examined whether there is evidence for differences between non-dementia controls and dementia subjects and whether the location of the SUB/CA1 border depends on the shape of the hippocampus in the coronal plane.
Approach:
We analyzed the location of the SUB/CA1 border relative to the width of the hippocampus (defined here as the CA+DG fields) along the long axis of the hippocampal body beginning at the uncal apex in 8 specimens. To that end, the overall width of the hippocampus was defined as the longest distance between a medial anchor point (see Figure 10, white dashed line) and the lateral border of the hippocampus. The position of the SUB/CA1 border was calculated relative to the overall width (black line). We followed this approach for one slice every 5mm (resulting in 25 slices) between the uncal apex and the last segmented hippocampal slice. It should be noted that the most posterior slices might be located in the hippocampal tail. However, this was difficult to assess as the commonly used external landmarks to determine the tail are not present in these excised specimens. Due to missing portions of the segmentations, we included also slices within a range of +/− 0.8mm in case the exact slice was not available. If there was no slice available within this range, this hippocampus was excluded for this slice position. This resulted in a maximum of 5 measurements within 8 subjects (mean 4.5, range 4–5)). In cases where the SUB/CA1 border was not clear cut but rather a diagonal line, we used the estimated midpoint. To ensure that the results are not dependent on the width of the CA fields per se, we did the same analysis again but focused on the SUB/CA1 boundary relative to the DG width only (defined as the longest distance between a medial anchor point and the most lateral border of the DG - see Figure 10, orange line). In order to characterize the overall hippocampal shape, we calculated a shape index by dividing hippocampal width by height. To that end, we measured the hippocampal width and height on the slice that was 10mm posterior to the uncal apex. Hippocampal height was measured from the most superior CA to the most inferior CA1 or SUB voxel (Figure 10, grey line). Lower shape index values are indicative of a rather flat hippocampus, higher numbers indicate a rounder hippocampal shape (see Figure 11).
Figure 10.
Approach to measure the relative position of the SUB/CA1 border. The white dashed line indicates the longest distance between the medial anchor point (white dot) and the most lateral border of the hippocampus. The black line indicates position of the SUB/CA1 border in percent with respect to the white dashed line. The orange line indicates the longest distance between the medial anchor point and the most lateral border of the DG. The grey line was used to measure hippocampal height from the most superior CA to the most inferior CA1 or SUB voxel.
CA=cornu ammonis, SUB=subiculum, DG=dentate gyrus
Figure 11.
Examples of one flat- and one round-shaped hippocampi. Hippocampi are presented with increasing shape index where higher numbers indicate more round shapes. A median split of the shape index was used to subdivide all hippocampi in two respective groups.
Results:
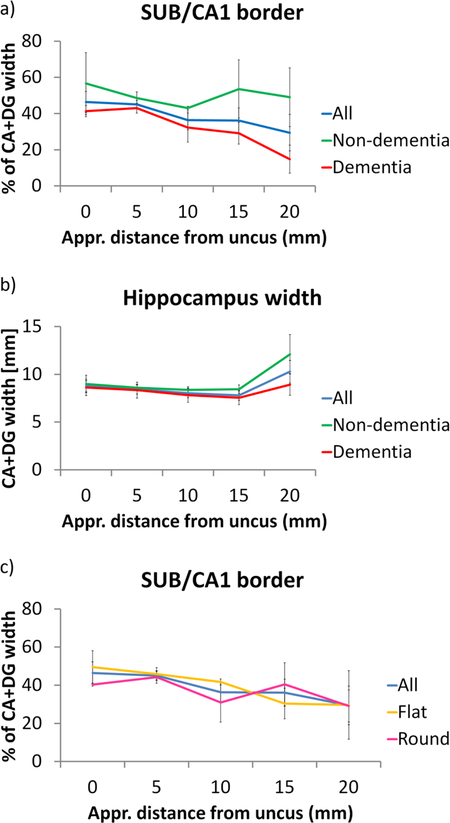
We found differences along the longitudinal axis of the hippocampal body (see Table 5). While the border of CA1 and SUB is at 46.5 % of the hippocampal width in anterior sections of the hippocampal body, it is better characterized by 29.4 % in more posterior sections (Figure 12 a). A similar result (from 60 % in anterior to 32 % in posterior sections) was found in the analysis where we defined the SUB/CA1 boundary relative to DG only (Supplementary Figure S2).
Table 5.
SUB/CA1 border positions relative to hippocampal width in the hippocampal body and anterior tail along the longitudinal hippocampal axis. Positions are indicated in percent relative to the overall hippocampal width (DG+CA1) or the DG width only (DG)
| Reference | Slice position* | 0mm Mean (SD) | 5mm Mean (SD) | 10mm Mean (SD) | 15mm Mean (SD) | 20mm Mean (SD) |
|---|---|---|---|---|---|---|
| CA+DG | All cases | 46.5 (14.2) | 45.1 (6.3) | 36.3 (14.8) | 36.1 (18.7) | 29.4 (26.8) |
| CA+DG | Non-dementia | 56.6 (24.21) | 48.6 (5.8) | 43 (1.6) | 53.6 (22.8) | 49 (28.1) |
| CA+DG | Dementia | 41.4 (6.2) | 43 (6.1) | 32.3 (18.1) | 29.2 (13.5) | 14.8 (15.4) |
| DG | All cases | 60.7 (17.7) | 55.4 (8.7) | 47.7 (15.6) | 41.8 (25.8) | 32.6 (30) |
| DG | Non-dementia | 77.3 (21.2) | 58.1 (2.9) | 49.6 (8.4) | 64.1 (29.3) | 55.3 (34.6) |
| DG | Dementia | 52.4 (9.7) | 53.7 (11) | 46.6 (19.6) | 32.9 (20.9) | 15.6 (10) |
CA=cornu ammonis; SUB=subiculum; DG=dentate gyrus; Mean (SD) in percent
Starting one slice posterior to the uncus
Figure 12.
SUB/CA1 border in the hippocampal body along the longitudinal axis and potential confounds. a. SUB/CA1 border relative to the CA+DG width in the whole group and separately in the non-dementia and dementia subjects. b. Overall hippocampus width in the whole group and separately in the non-dementia and dementia subjects. c. SUB/CA1 border relative to the CA+DG width in the whole group and separately in the flat- and round-hippocampi specimen.
CA=cornu ammonis; DG=dentate gyrus; SUB=subiculum
By comparing data from non-dementia controls and dementia subjects, we found that this trend was mainly driven by the patient group (note that this is a similar finding as in the previous section). While the boundary in controls is best described at around 50% for the entire length, it changes from 41.4 % in anterior to 14.8% in posterior slices in dementia subjects (see Table 5). A very similar group difference has been found in the analysis where we defined the SUB/CA1 boundary relative to DG only.
In order to examine whether this result is driven by subfield-specific atrophy, we compared the width of the hippocampus (including CA fields and DG) in different sections along the hippocampal long axis (Figure 12 b). The hippocampal width remained stable along most of the longitudinal slices with an increase in width in the last slice. Although this increase was more pronounced in non-dementia compared to the dementia subjects, the same trend was observed in both groups. We found a very similar result when only considering the DG width (data not shown).
Finally, given that the overall hippocampus shape is quite variable and can be characterized as rather flat and wide or rounder, we examined whether the overall shape has an influence on the measured SUB/CA1 border. To that end, we tested whether there was a relationship between the shape index and the position of the SUB/CA1 border by calculating a spearman correlation coefficient. However, no correlation was observed (p=0.75). In addition, we divided all hippocampi into two groups of flat or round shape based on a median split using the shape index. While we found that the SUB/CA1 border shifts more medial for both, round and flat, hippocampi, we did again not find a clear difference between groups which would suggest that hippocampal shape cannot explain our findings (Figure 12 c). Finally, we compared hippocampal shape between subjects with dementia and controls and found slightly lower shape indices in subjects with dementia (meancontrols = 0.82, SDcontrols = 0.11; meandementia= 0.66, SDdementia = 0.05) which indicates more flat-shaped hippocampi. However, as our shape index was not related to the location of the SUB/CA1 border, this can likely not explain the observed differences between dementia and non-dementia cases.
Discussion:
In this section, we found that the SUB/CA1 border shifts from lateral to medial when moving from anterior to posterior across all examined 8 hippocampi. This shift in the SUB/CA1 border can also be observed in the Duvernoy atlas (Duvernoy, 2005), however, this has not been quantified before. By splitting our sample according to dementia status, we found that this effect was driven by the patient population. Furthermore, we analyzed whether overall hippocampal width or shape can explain the observed effects. While we found slight differences in hippocampal width of the last slice between dementia subjects and controls, those could not account for the observed differences in the SUB/CA1 border. Likewise, we did not observe clear differences between round- and flat-shaped hippocampi that could account for the observed effects as the SUB/CA1 border shifts medially for both types in posterior slices.
Although we have to be very careful given that our results rest on a very small sample, our finding, when replicated, would have important implications. Our results can inform and optimize future segmentation protocols by accounting for a lateral to medial shift of the SUB/CA1 boundary in posterior body slices which would result in a more accurate characterization of the CA1 and SUB subfield. Again, it should be noted that this shift only occurs after 10 mm, which may already be considered tail on in vivo MRI. While there was an indication that this effect was pronounced in the dementia cases compared to the non-dementia cases (see also previous section where a similar effect for the SUB/CA1 was found in the dementia cases), the small sample size and the mixed dementia group precludes any strong conclusions. A larger sample is needed to confirm these findings before any definite conclusions can be drawn.
Ordering of hippocampal subfields along the long axis - Tail
Aim:
To characterize the order of disappearance of the different hippocampal subfields along the long axis.
Approach:
Because the number of available specimens with histological sectioning covering the hippocampal tail completely was too small, the approach employed in the section regarding the order of appearance of subfields could not be used here. Instead, the most posterior slice on which the hippocampus was visible was identified (Figure 13 a). Then, the slice 2 mm anteriorly was selected and subfields were described on this specific slice (Figure 13 b). In six specimens this slice contained sufficient coverage of the segmentation. This distance of 2mm was chosen to fit with the slice thickness of most high-resolution in vivo scans specifically acquired for subfield segmentation. We also repeated this analysis on slices 1mm anterior to the last slice on which the hippocampus was visible. Note that the segmentation was missing on this particular slice for one specimen (analysis performed in five specimens).
Figure 13.
Order of disappearance of subfields in the hippocampal tail in one specimen. The most posterior slice on which the hippocampus was visible was identified (a) and subfields were evaluated 2 mm anterior (b).
Results:
Every subfield (SUB, DG, CA1, CA2, CA3) was present 2 mm anterior to the most posterior slice in all specimens. Results were less consistent 1mm anterior to the most posterior slice, where all subfields were present for 2 specimens, CA1 and SUB were present for 2 specimens and CA1, 2 and 3 were present for 1 specimen. No clear pattern could be observed when comparing the non-dementia vs the dementia cases.
Discussion:
The aim of this section was to evaluate the order of disappearance of the different hippocampal subfields. Although we could not give as much detail as in the section regarding the order of appearance of subfields, our analyses showed that SUB, DG, CA1, CA2 and CA3 were visible 2 mm prior to the last hippocampal slice. As most images on which subfields are segmented are acquired with a slice thickness of 2 mm, this result suggests that the most posterior slice on which the hippocampus is visible should include all subfields. However, results were less consistent 1 mm prior to the last hippocampal slice, which might question the feasibility of segmenting subfields in the tail in images with slice thickness < 1 mm. More details regarding the hippocampal tail are given in the following section. This finding could have implications for labs that aim to include the tail in their segmentation protocol. One limitation to note is that it was difficult to establish the most posterior slice of the hippocampal tail for some specimens.
The tale of the tail
Aim:
The tail is not well characterized with regard to shape and the composition of the subfields. In this section, we aim to characterize the shape of tail, the composition of subfields and the variability thereof.
Approach:
Out of nine, only one specimen was excluded because the histology annotations only covered the anterior portion of the hippocampus. While there are some gaps in the segmentations, the remaining eight subjects were deemed to have sufficient coverage of the posterior part of the hippocampal to warrant inclusion. As noted in the method section, as a working definition, we selected the four most posterior slices with a 2 mm gap starting 1 mm anterior to the most posterior tip of the tail. Note that this is a different selection of slices than in the previous section. Moreover, to characterize the 3-dimensional shape of the tail, we used the segmentations generated by the post-mortem atlas (Adler et al., 2018) as these segmentations have full coverage of the tail.
Results:
Based on the coronal sections of the tail, the cases can be divided into two groups, although these types are likely part of the same continuum. See Figure 14 and Supplementary Figure S3. The first group is characterized by a body-like appearance until the last slice of the hippocampus; that is SUB, CA1, 2 and 3 appear in the same order and location as in the body, surrounding the DG (b-e). It should be noted that the appearance is not exactly the same as in the body, as the DG is often protruded medially (d,e) and the CA3 does not necessarily curve inwards creating a c-shaped DG, but rather is located superior of DG (d,e). Moreover, CA1 protrudes laterally (d, e). Finally more folds can be observed, especially in CA1 (see supplementary Figure S3).
Figure 14.
Tail slices of two specimens. The left column displays a body slice (a, f) of each of the specimens for comparison. In f the typical body-like shape is emphasized by two black lines showing two interlocking ‘C-s’. In the first row, a body-like shape can be observed in all tail sections; that is SUB, CA1, 2 and 3 appear in the same order and location as in the body, surrounding the DG (b-e). It should be noted that the appearance is not exactly the same as in the body, as the DG is often protruded medially (white arrows in d and e)) and the CA3 does not necessarily curve inwards creating a c-shaped DG, but rather is located superior of DG (orange arrowhead in d and e). Moreover, CA1 protrudes laterally and can be folded (black arrows in c-e). In the second specimen, the appearance of the tail cannot be brought back to a body-like appearance. In more anterior slices, it appears as if the CA region, in this case CA2 and CA3, wraps around the DG from the medial end and meets up with the more lateral CA3 region (grey arrows h-j). In more posterior regions, the SUB may be located more medially, no longer adjacent to the DG (black arrow in i). In the last few slices, only CA1 and SUB are present (j) in this particular case.
CA=cornu ammonis; DG=dentate gyrus; SUB=subiculum
Conversely, in the second group, the appearance of the tail cannot be brought back to a body-like appearance. In more anterior slices, it appears as if the CA regions wrap around the DG from the medial end and meet up with the more lateral CA3 region (h, i). In more posterior regions, SUB may be located more medially, no longer adjacent to the DG (i, j). In the last few slices, potentially only CA1 and SUB are present (j), although this is more variable when taking all cases into account (Supplementary Figure S3; see also previous section). In general, there is a lot of variability in the shape of the tail and in the location and presence of the subfields, even within the two groups (see Supplementary Figures S3). In both groups, dementia and non-dementia subjects were present, indicating that this appearance is not related to diagnosis.
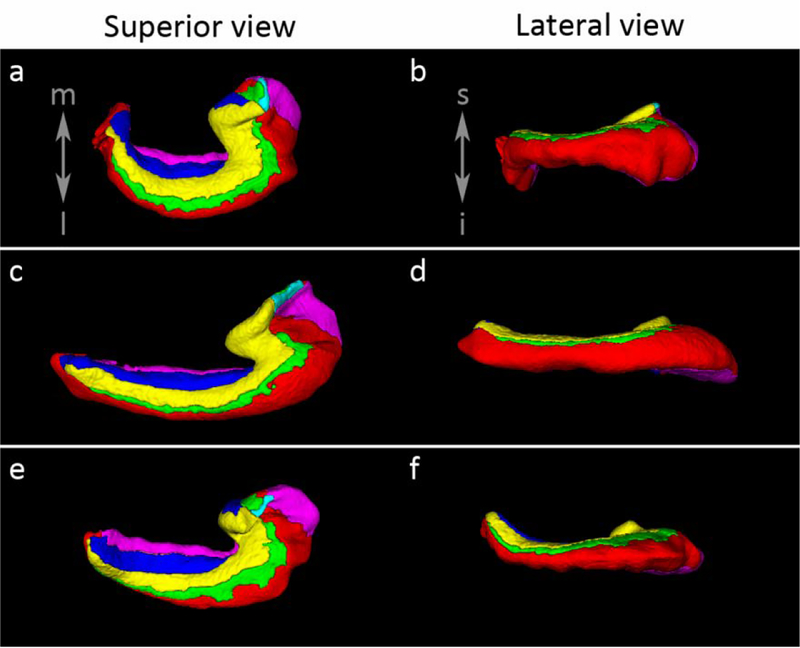
As noted, the groups are likely part of a continuum. As reported previously in Adler et al., (2018), the appearance of the tail in coronal sections is related to its curvature. In Figure 15 we show the curvature of the tail of several specimens in 3D renderings of the segmentations, indicating variability ranging from curvature in a medial direction to a curvature in a superior direction to an approximately straight hippocampus, observed from both views. Three specimens showed a strong angulation in the medial direction and two in a superior direction. Re-slicing the posterior hippocampus following the curvature of the tail either in the medial or in the superior direction as in Figure 15, the appearance of the tail in the coronal plane was more body-like (Figure 16). This was observed for the two subjects with a curvature in the superior direction and for the three subjects with a curvature in the medial direction (data not shown). Incidentally, the three specimens with a curvature in the medial direction make up group 2 identified by the coronal section indicating that indeed the different appearances of the hippocampal tail in the coronal plane are dependent on the curvature of the tail. In group 1, re-slicing the posterior hippocampus following the superior curvature of the tail, for those cases with a strong superior curve, also led to a more body-like appearance of the tail with less lateral protrusions of CA1 and less medial protrusions of the DG.
Figure 15.
Illustration of the curvature of the tail in three specimens. In the first specimen (upper row, a-b) the tail shows a strong curve in the medial direction, observed in the superior view, and a slight curve in the inferior direction, as shown in the lateral view. In the second specimen, the tail shows very little curving in either direction (c-d). Finally in the third specimen (e-f), very limited curving is observed in the medial direction, however, stronger curving is observed in the superior direction in the lateral view.
Figure 16.
Reslicing of the hippocampal tail. Examples of two specimens are shown, one with curvature of the hippocampus in superior direction (a-d) and one with curvature in the medial direction (e-h). In a the sagittal plane of the first specimen is shown with the white line indicating the location of the coronal cut in b, sliced perpendicular to the long axis of the hippocampus. Then the specimen is resliced at the point of the yellow dot perpendicular to the hippocampal tail at that point. In d the angulated coronal section is shown at the white line indicated in c. The hippocampus has a more body-like (see Figure 14 f) appearance in d, though rotated, than in b. In e the axial plane of the second specimen with the white line indicating the coronal cut in f, sliced perpendicular to the long axis of the hippocampus. Then the specimen is resliced at the point of the yellow dot perpendicular to the hippocampal tail at that point. In h the angulated coronal section is shown at the location of the white line in g. Again, it can be observed that the hippocampus is more body-like in h then in f.
Discussion:
The characterization of the hippocampal tail in coronal sections led to the identification of two subgroups, one group with a more body-like appearance and one with a different appearance. In fact, these types can also be found in anatomical atlases, with Harding et al., (1998) showing a more body-like shape throughout the length of the hippocampal tail, whereas the other type is shown, for example, in Duvernoy (2005), Insausti and Amaral (2012), Ding et al. (2016). Interestingly, when reslicing the hippocampal tail orthogonal to the curve of the tail, whether it curves medial or superior, we observed that the appearance of the hippocampus is more body-like in all cases. While we previously reported this for the shape of the hippocampus using raw MR images for a curvature in the medial direction (Adler et al., 2018), in the current study we also show this for the curving in the superior direction and we show that the subfield composition is body-like. This indicates that the variability observed in shape and subfield composition is likely driven partly by the angulation of the tail. This is potentially helpful for subfield segmentation on in vivo images with isotropic data, however, it is unfortunately less helpful for the typical anisotropic images with thick slices where reslicing of the tail is not possible. The current observations regarding the variability in shape of the tail and the composition of subfields, indicate that subfield segmentation in the tail on anisotropic in vivo data, with coronal sections acquired perpendicular to the long axis of the hippocampus, is very complicated. In this context, it may be safer to assign a single label to the tail instead of segmenting subregions. More post-mortem data is needed to further characterize this variability. However, hopefully the current description of the tail can be used to better interpret fMRI findings in the hippocampal tail. Potentially, future studies with high-resolution isotropic data might be able to better resolve the tail in three dimensions and to accurately segment the tail along its curvature, for example using elegant methods like the one described in DeKraker et al. (2018).
General discussion
In this study we examined a post-mortem dataset containing nine specimens from non-dementia and dementia cases with a post-mortem MRI and histology sections, used for subfield delineation. We utilized this dataset to provide quantitative and qualitative descriptions of hippocampal subfields with the aim to provide information for in vivo segmentation and reference material to improve interpretation of findings from fMRI studies. Our main findings, although preliminary, are the following: 1) there is a consistent order of appearance of subfields in the hippocampal head, 2) the location of the anterior CA2 and CA3 and the composition and location of subfields in the anterior uncus is less consistent, 3) the characterization of the posterior uncus shows a more consistent pattern than the anterior portion with only CA3 present in the most posterior slice and CA3 and DG present in more anterior slices, 4) the dark band, as visible on MRI, was shown to consist only of part of the CA-SRLM, not DG-SM, and should therefore be considered as part of the CA (or SUB) regions, 5) the CA1/SUB border is located at the middle of the width of the hippocampus, however, this border moves in a inferiomedial direction approximately 10 mm posterior to the tip of the uncus, and 6) the characterization of the location and composition of subfields in the hippocampal tail shows great variability, which can be brought back to a body-like shape when reslicing the MRI scan following the curvature of the tail. While this latter finding is unlikely to benefit current anisotropic T2-MRI scans with 2 mm thick slices, it might be useful for currently available high resolution isotropic data or be an incentive for the acquisition of isotropic data in the future.
To the best of our knowledge, this is the first study to characterize variability in important regions such as the tail and the uncus. This work also leads to novel findings, for example, regarding what subfield layers the dark band consists of. Moreover, the unique aspect of the current dataset is the availability of histology and isotropic 0.2×0.2×0.2 mm3 post-mortem MRI, which enabled histology-based segmentations in the space of the post-mortem MRI and the ability to reslice the MRI in any desired plan. This allowed us to match the slice orientation of more in vivo T2-MRI scans, that is perpendicular to the long axis of the hippocampus. Moreover, it allowed us investigate the hippocampal tail in more detail with the ability to reslice images following the curvature of the tail.
While nine specimens is a substantial number for the current post-mortem dataset, the ability to draw conclusions regarding variability, particularly in a mixed group of dementia and non-dementia cases, is limited. Especially, since there were gaps between the segmentations as the histology sections did not fully cover the specimens. We therefore warrant caution regarding our findings and recommend to interpret them in the larger context of neuroanatomy references, such as Duvernoy (2005), Insausti and Amaral (2012) and Ding et al. (2016). An additional factor that could have affected our findings is the fact that segmentation was performed on every third 0.2 mm slice in the head and tail and every fifth 0.2 mm slice in the body, before interpolation. However, we believe that this should not have affected any conclusions regarding in vivo segmentations, as the typical 2 mm slice thickness is much larger than the number of slices over which we interpolated the histology-based segmentations. Moreover, while all samples were rigidly aligned along the long axis, the coronal slices can only approximate an orientation perpendicular to the long axis of the hippocampus, given the curvature of the hippocampus in different planes. While this could have affected some of our results, many of our findings were robust across specimens and in line with anatomical references. Additionally, the same issue holds for anisotropic in vivo MRI scans. Any potential inconsistent findings due to the angulation of the post-mortem MRI will therefore also be problematic on in vivo MRI. Moreover, a previous study directly comparing in vivo and ex vivo MRI of the hippocampal formation in the same subjects reported that in vivo tissue appeared slightly smaller than the ex vivo tissue (Wisse et al., 2017a), potentially due to tissue changes during or after death (Shen et al., 1993; Maxeiner and Behnke, 2008), to brain extraction, for example by a relief of intracranial pressure after autopsy, or to handling of the tissue. This potential small difference in size between the in vivo and ex vivo domain should be taken into account when translating the information for in vivo segmentation, which mainly may have affected sections where we have reported exact values, for example of thickness or distance. Here relative values may be more useful (e.g. distances reported as a percentage of the hippocampal head length in the section “Order of appearance of hippocampal subfields along the long axis - Head“). Another point of attention is that both in the dementia patients, and in older individuals in general, several pathologies may be present in the hippocampus which may affect hippocampal subfields differentially, e.g. neurofibrillary tangle pathology is thought to affect CA1 first (Braak et al., 2006; Lace et al., 2009). However, most of our dementia patients were likely in more advanced stages of the disease where the hippocampus is more globally affected. It is still possible though that comorbid pathology or pathologies in the non-demented subjects may have affected certain hippocampal subfields differentially. However, it should be noted that many in vivo studies aim to study hippocampal subfields in aging and dementia. We therefore included non-dementia and dementia cases explicitly to provide information on hippocampal anatomy in these groups. Finally, our histology annotations were based on cytoarchitecture, but exact boundaries and divisions of regions within the hippocampus varies across protocols and laboratories (Wisse et al., 2017b; Olsen et al., 2019). This may further limit the generalizability of our findings. Our study therefore preferably needs to be replicated in a larger sample with annotations from different neuroanatomists, preferably also including other patient groups (e.g. patients with lesions) and several datasets with both hemispheres to assess interhemispheric variability. Lastly, it is important to keep in mind that the anatomical description provided in the present manuscript arise from observations of post-mortem scans with a resolution of 0.2×0.2×0.2mm3. Thus, these results may not directly translate to high resolution images acquired in vivo, mainly because of their anisotropic nature. Indeed, in order to achieve a sufficient in plane resolution to visualize subfield boundaries (< 0.5×0.5mm2), researchers often acquire images with thick slices (1–2mm). This trade-off between in plane resolution and slice thickness is essentially due to time limit for subject comfort. As a consequence, partial volume effects occurring because of an important slice thickness can be an issue for in vivo segmentation. The use of additional images, such as T1 images with standard resolution (i.e. 1×1×1mm3 resolution) might help guiding the segmentation. Hopefully, this limitation regarding in vivo image resolution should be solved in the future with new technical advances in MRI equipment.
In the meantime, our findings, although preliminary, will hopefully advance in vivo segmentation of hippocampal subfields and potentially also the harmonization effort that aims to develop a single harmonized protocol for hippocampal subfields (Yushkevich et al., 2015a; Wisse et al., 2017b; Olsen et al., 2019, https://www.hippocampalsubfields.com). As this dataset is publicly available, we invite other researchers to explore these data and further investigate hippocampal anatomy.
Supplementary Material
Acknowledgments:
We gratefully acknowledge the tissue donors and their families. We also thank the staff at the NDRI brain bank and at CNDR for performing the autopsies and making tissue available for this project. This work was supported by the National Institute of Health (Grants R01 AG056014, P30 AG010124 and R01 AG055005). We acknowledge the donors of Alzheimer’s Disease Research, a program of the BrightFocus Foundation (to L.E.M.W.) and of Fondation Philippe Chatrier (to R.D.F.) for support of this research.
Footnotes
Conflict of interest statement:
D.A.W. received consultation fees from Eli Lilly, Janssen, and Merck. D.A.W. receives grant support from Avid Radiopharmaceuticals/Eli Lilly, Biogen, Functional Neuromodulation, and Merck. J.B.T. has received research support from Eli Lilly. J.Q.T. may accrue revenue in the future on patents submitted by the University of Pennsylvania wherein he is co-inventor, and he received revenue from the sale of Avid to Eli Lilly as co-inventor on imaging-related patents submitted by the University of Pennsylvania.
Data accessibility statement:
The data that support the findings of this study are openly available in release_1.0.0 at https://www.nitrc.org/projects/pennhippoatlas/, (Adler et al., 2018)
References
- Adler DH, Pluta J, Kadivar S, Craige C, Gee JC, Avants BB, Yushkevich P a. 2014. Histology-derived volumetric annotation of the human hippocampal subfields in postmortem MRI. Neuroimage 84:505–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler DH, Wisse LEM, Ittyerah R, Pluta JB, Ding S-L, Xie L, Wang J, Kadivar S, Robinson JL, Schuck T, Trojanowski JQ, Grossman M, Detre JA, Elliott MA, Toledo JB, Liu W, Pickup S, Miller MI, Das SR, Wolk DA, Yushkevich PA. 2018. Characterizing the human hippocampus in aging and Alzheimer’s disease using a computational atlas derived from ex vivo MRI and histology. Proc Natl Acad Sci:201801093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. 2005. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 210:343–52. [DOI] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CEL. 2008. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science 319:1640–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berron D, Schutze H, Maass A, Cardenas-Blanco A, Kuijf HJ, Kumaran D, Duzel E. 2016. Strong Evidence for Pattern Separation in Human Dentate Gyrus. J Neurosci 36:7569–7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berron D, Vieweg P, Hochkeppler A, Pluta JB, Ding S-L, Maass A, Luther A, Xie L, Das SR, Wolk DA, Wolbers T, Yushkevich PA, Düzel E, Wisse LEM. 2017. A protocol for manual segmentation of medial temporal lobe subregions in 7 Tesla MRI. NeuroImage Clin 15:466–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnici HM, Chadwick MJ, Kumaran D, Hassabis D, Weiskopf N, Maguire EA. 2012. Multi-voxel pattern analysis in human hippocampal subfields. Front Hum Neurosci 6:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Tredici K. 2006. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. 1991. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–59. [DOI] [PubMed] [Google Scholar]
- Burggren a C, Zeineh MM, Ekstrom a D, Braskie MN, Thompson PM, Small GW, Bookheimer SY. 2008. Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. Neuroimage 41:1177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton MA, Zeidman P, Barry DN, Williams E, Maguire EA. 2017. Segmenting subregions of the human hippocampus on structural magnetic resonance image scans: An illustrated tutorial. Brain Neurosci Adv 1:239821281770144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty AM, Bender AR, Raz N, Ofen N. 2016. Age differences in hippocampal subfield volumes from childhood to late adulthood. Hippocampus 26:220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKraker J, Ferko KM, Lau JC, Köhler S, Khan AR. 2018. Unfolding the hippocampus: An intrinsic coordinate system for subfield segmentations and quantitative mapping. Neuroimage 167:408–418. [DOI] [PubMed] [Google Scholar]
- Ding S-L. 2013. Comparative anatomy of the prosubiculum, subiculum, presubiculum, postsubiculum, and parasubiculum in human, monkey, and rodent. J Comp Neurol 521:4145–62. [DOI] [PubMed] [Google Scholar]
- Ding S-L, Van Hoesen GW. 2015. Organization and detailed parcellation of human hippocampal head and body regions based on a combined analysis of Cyto- and chemoarchitecture. J Comp Neurol 523:2233–2253. [DOI] [PubMed] [Google Scholar]
- Ding S-L, Royall JJ, Sunkin SM, Ng L, Facer BAC, Lesnar P, Guillozet-Bongaarts A, McMurray B, Szafer A, Dolbeare TA, Stevens A, Tirrell L, Benner T, Caldejon S, Dalley RA, Dee N, Lau C, Nyhus J, Reding M, Riley ZL, Sandman D, Shen E, van der Kouwe A, Varjabedian A, Write M, Zollei L, Dang C, Knowles JA, Koch C, Phillips JW, Sestan N, Wohnoutka P, Zielke HR, Hohmann JG, Jones AR, Bernard A, Hawrylycz MJ, Hof PR, Fischl B, Lein ES. 2016. Comprehensive cellular-resolution atlas of the adult human brain. J Comp Neurol 524:3127–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. 2005. The Human Hippocampus, Functional Anatomy, Vascularization and Serial Sections with MRI. Third edit Berlin, Germany: Springer-Verlag. [Google Scholar]
- Eichenbaum H. 2017. The role of the hippocampus in navigation is memory. J Neurophysiol 117:1785–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Flores R, La Joie R, Landeau B, Perrotin A, Mézenge F, de La Sayette V, Eustache F, Desgranges B, Chételat G. 2015. Effects of age and Alzheimer’s disease on hippocampal subfields: comparison between manual and FreeSurfer volumetry. Hum Brain Mapp 36:463–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding a J, Halliday GM, Kril JJ. 1998. Variation in hippocampal neuron number with age and brain volume. Cereb Cortex 8:710–8. [DOI] [PubMed] [Google Scholar]
- Hodgetts CJ, Voets NL, Thomas AG, Clare S, Lawrence AD, Graham KS. 2017. Ultra-High-Field fMRI Reveals a Role for the Subiculum in Scene Perceptual Discrimination. J Neurosci 37:3150–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Amaral DG. 2012. Hippocampal Formation. Third Edit Elsevier. [Google Scholar]
- La Joie R, Fouquet M, Mézenge F, Landeau B, Villain N, Mevel K, Pélerin A, Eustache F, Desgranges B, Chételat G. 2010. Differential effect of age on hippocampal subfields assessed using a new high-resolution 3T MR sequence. Neuroimage 53:506–14. [DOI] [PubMed] [Google Scholar]
- La Joie R, Perrotin A, de La Sayette V, Egret S, Doeuvre L, Belliard S, Eustache F, Desgranges B, Chételat G. 2013. Hippocampal subfield volumetry in mild cognitive impairment, Alzheimer’s disease and semantic dementia. NeuroImage Clin 3:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Hess CP, Hammond-Rosenbluth KE, Xu D, Rabinovici GD, Kelley DAC, Vigneron DB, Nelson SJ, Miller BL. 2010. Hippocampal CA1 apical neuropil atrophy in mild Alzheimer disease visualized with 7-T MRI. Neurology 75:1381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W, Westman E, Jones N, Wahlund L-O, Mecocci P, Vellas B, Tsolaki M, Kłoszewska I, Soininen H, Spenger C, Lovestone S, Muehlboeck J-S, Simmons A, AddNeuroMed consortium and for the Alzheimer’s Disease Neuroimaging Initiative. 2015. Automated Hippocampal Subfield Measures as Predictors of Conversion from Mild Cognitive Impairment to Alzheimer’s Disease in Two Independent Cohorts. Brain Topogr 28:746–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluver H, Barrera E. 1953. A method for the combined staining of cells and fibers in the nervous system. J Neuropathol Exp Neurol 12:400–3. [DOI] [PubMed] [Google Scholar]
- Kulaga-Yoskovitz J, Bernhardt BC, Hong S-J, Mansi T, Liang KE, van der Kouwe AJW, Smallwood J, Bernasconi A, Bernasconi N. 2015. Multi-contrast submillimetric 3 Tesla hippocampal subfield segmentation protocol and dataset. Sci Data 2:150059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle CT, Stokes JD, Lieberman JS, Hassan AS, Ekstrom AD. 2015. Successful retrieval of competing spatial environments in humans involves hippocampal pattern separation mechanisms. Elife 4:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lace G, Savva GM, Forster G, de Silva R, Brayne C, Matthews FE, Barclay JJ, Dakin L, Ince PG, Wharton SB. 2009. Hippocampal tau pathology is related to neuroanatomical connections: an ageing population-based study. Brain 132:1324–34. [DOI] [PubMed] [Google Scholar]
- Lim HK, Hong SC, Jung WS, Ahn KJ, Won WY, Hahn C, Kim IS, Lee CU. 2013. Automated segmentation of hippocampal subfields in drug-naïve patients with Alzheimer disease. AJNR Am J Neuroradiol 34:747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Majtanik M, Paxinos G. 2008. Atlas of the human brain. New York, USA: Academic Press, Elsevier, New York, USA. [Google Scholar]
- Malykhin NV, Huang Y, Hrybouski S, Olsen F. 2017. Differential vulnerability of hippocampal subfields and antero-posterior hippocampal subregions in healthy cognitive aging. Neurobiol Aging. [DOI] [PubMed] [Google Scholar]
- Malykhin NV, Lebel RM, Coupland NJ, Wilman a H, Carter R. 2010. In vivo quantification of hippocampal subfields using 4.7 T fast spin echo imaging. Neuroimage 49:1224–30. [DOI] [PubMed] [Google Scholar]
- Milenkovic I, Petrov T, Kovacs GG. 2014. Patterns of Hippocampal Tau Pathology Differentiate Neurodegenerative Dementias. Dement Geriatr Cogn Disord 38:375–388. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. 2006. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr Opin Neurobiol 16:179–190. [DOI] [PubMed] [Google Scholar]
- Mouritzen Dam A. 1979. Shrinkage of the brain during histological procedures with fixation in formaldehyde solutions of different concentrations. J Hirnforsch 20:115–9. [PubMed] [Google Scholar]
- Mueller SG, Laxer KD, Barakos J, Cheong I, Garcia P, Weiner MW. 2009. Subfield atrophy pattern in temporal lobe epilepsy with and without mesial sclerosis detected by high-resolution MRI at 4 Tesla: Preliminary results. Epilepsia 50:1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RK, Carr VA, Daugherty AM, La Joie R, Amaral RSC, Amunts K, Augustinack JC, Bakker A, Bender AR, Berron D, Boccardi M, Bocchetta M, Burggren AC, Chakravarty MM, Chételat G, de Flores R, DeKraker J, Ding S-L, Geerlings MI, Huang Y, Insausti R, Johnson EG, Kanel P, Kedo O, Kennedy KM, Keresztes A, Lee JK, Lindenberger U, Mueller SG, Mulligan EM, Ofen N, Palombo DJ, Pasquini L, Pluta J, Raz N, Rodrigue KM, Schlichting ML, Lee Shing Y, Stark CEL, Steve TA, Suthana NA, Wang L, Werkle-Bergner M, Yushkevich PA, Yu Q, Wisse LEM. 2019. Progress update from the hippocampal subfields group. Alzheimer’s Dement Diagnosis, Assess Dis Monit 11:439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RK, Palombo DJ, Rabin JS, Levine B, Ryan JD, Rosenbaum RS. 2013. Volumetric analysis of medial temporal lobe subregions in developmental amnesia using high-resolution magnetic resonance imaging. Hippocampus 23:855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta J, Yushkevich P, Das S, Wolk D. 2012. In vivo analysis of hippocampal subfield atrophy in mild cognitive impairment via semi-automatic segmentation of T2-weighted MRI. J Alzheimer’s Dis 31:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. 2013. Long-axis specialization of the human hippocampus. Trends Cogn Sci 17:230–40. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE. 1990. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci 10:2897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small S a, Schobel S a, Buxton RB, Witter MP, Barnes C a. 2011. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 12:585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Paula-Barbosa MM, Almeida OF. 1999. Ligand and subfield specificity of corticoid-induced neuronal loss in the rat hippocampal formation. Neuroscience 89:1079–87. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. 2004. the Medial Temporal Lobe. Annu Rev Neurosci 27:279–306. [DOI] [PubMed] [Google Scholar]
- Steve TA, Yasuda CL, Coras R, Lail M, Blumcke I, Livy DJ, Malykhin N, Gross DW. 2017. Development of a histologically validated segmentation protocol for the hippocampal body. Neuroimage 157:219–232. [DOI] [PubMed] [Google Scholar]
- Wisse LEM, Adler DH, Ittyerah R, Pluta JB, Robinson JL, Schuck T, Trojanowski JQ, Grossman M, Detre JA, Elliott MA, Toledo JB, Liu W, Pickup S, Das SR, Wolk DA, Yushkevich PA. 2017a. Comparison of In Vivo and Ex Vivo MRI of the Human Hippocampal Formation in the Same Subjects. Cereb Cortex 27:5185–5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse LEM, Biessels GJ, Stegenga BT, Kooistra M, van der Veen PH, Zwanenburg JJM, van der Graaf Y, Geerlings MI. 2015. Major depressive episodes over the course of 7 years and hippocampal subfield volumes at 7 tesla MRI: The PREDICT-MR study. J Affect Disord 175:1–7. [DOI] [PubMed] [Google Scholar]
- Wisse LEM, Daugherty AM, Olsen RK, Berron D, Carr VA, Stark CEL, Amaral RSC, Amunts K, Augustinack JC, Bender AR, Bernstein JD, Boccardi M, Bocchetta M, Burggren A, Chakravarty MM, Chupin M, Ekstrom A, de Flores R, Insausti R, Kanel P, Kedo O, Kennedy KM, Kerchner GA, LaRocque KF, Liu X, Maass A, Malykhin N, Mueller SG, Ofen N, Palombo DJ, Parekh MB, Pluta JB, Pruessner JC, Raz N, Rodrigue KM, Schoemaker D, Shafer AT, Steve TA, Suthana N, Wang L, Winterburn JL, Yassa MA, Yushkevich PA, la Joie R. 2017b. A harmonized segmentation protocol for hippocampal and parahippocampal subregions: Why do we need one and what are the key goals? Hippocampus 27:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse LEM, Gerritsen L, Zwanenburg JJM, Kuijf HJ, Luijten PR, Biessels GJ, Geerlings MI. 2012. Subfields of the hippocampal formation at 7 T MRI: in vivo volumetric assessment. Neuroimage 61:1043–9. [DOI] [PubMed] [Google Scholar]
- Yassa M a, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CEL. 2010. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage 51:1242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Amaral RSC, Augustinack JC, Bender AR, Bernstein JD, Boccardi M, Bocchetta M, Burggren AC, Carr VA, Chakravarty MM, Chételat G, Daugherty AM, Davachi L, Ding S-L, Ekstrom A, Geerlings MI, Hassan A, Huang Y, Iglesias JE, La Joie R, Kerchner GA, LaRocque KF, Libby LA, Malykhin N, Mueller SG, Olsen RK, Palombo DJ, Parekh MB, Pluta JB, Preston AR, Pruessner JC, Ranganath C, Raz N, Schlichting ML, Schoemaker D, Singh S, Stark CEL, Suthana N, Tompary A, Turowski MM, Van Leemput K, Wagner AD, Wang L, Winterburn JL, Wisse LEM, Yassa MA, Zeineh MM. 2015a. Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: Towards a harmonized segmentation protocol. Neuroimage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. 2006. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 31:1116–1128. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Pluta JB, Wang H, Xie L, Ding S-L, Gertje EC, Mancuso L, Kliot D, Das SR, Wolk D a. 2015b. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum Brain Mapp 36:258–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidman P, Lutti A, Maguire EA. 2015. Investigating the functions of subregions within anterior hippocampus. Cortex 73:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidman P, Maguire EA. 2016. Anterior hippocampus: The anatomy of perception, imagination and episodic memory. Nat Rev Neurosci 17:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. 2003. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science 299:577–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.