Abstract
PURPOSE:
Allogeneic stem cell transplant (alloSCT) is considered in diffuse large B cell lymphoma (DLBCL) patients with chemorefractory disease or who have relapsed following autologous stem cell transplant (autoSCT). Here we present the first report of alloSCT using R-BEAM conditioning regimen in DLBCL patients.
PATIENTS AND METHODS:
We retrospectively compared long-term alloSCT outcomes of DLBCL who received either R-BEAM (n=47) or reduced intensity conditioning (RIC) regimens (n=23).
RESULTS:
Seventy patients (median age, 53 years) with DLBCL received alloSCT between January 2005 and December 2017. Median number of pretransplant therapies was 3, and 17 patients (24%) received prior autoSCT. All received rituximab as a front line or salvage therapy prior to alloSCT. The donor was unrelated in 42 patients (60%) and peripheral blood stem cells were commonly used (96%). The 6-month cumulative incidence of grade III-IV aGVHD was 36.2% and 8.7% for R-BEAM and RIC, respectively (p=0.03). Median follow-up of surviving patients after R-BEAM and RIC was 3.1 and 5.5 years, respectively. Three-year overall survival (OS) after R-BEAM and RIC was 34.4% and 43.4%, respectively (p=0.48). At 3-years, R-BEAM was associated with similar relapse rate (25.5% vs 26.1%, p=0.96), non-relapse mortality (NRM) (39.7% vs 39.1%, p=0.98), and relapse-free survival (RFS) (34.8% vs 34.7%, p=0.75) compared with RIC. In multivariable analysis, lower Karnofsky performance score was associated with lower OS (HR 0.96, p=0.05), whereas chemorefractory disease was associated with higher relapse risk (HR 8.8, p=0.04). No difference in OS, relapse, NRM or RFS was noticed between R-BEAM and RIC.
CONCLUSION:
R-BEAM regimen seems feasible, and results in equivalent rates of long-term OS, relapse, NRM and RFS compared to RIC. However, significantly higher rate of severe acute GVHD was noticed.
Keywords: Diffuse large B cell lymphoma, R-BEAM, Transformed Diffuse large B cell lymphoma, Reduced intensity conditioning regimen, Allogeneic stem cell transplantation
Introduction
Approximately 60–70% of patients with diffuse large B-cell lymphoma (DLBCL) achieve cure with rituximab and anthracycline containing chemoimmunotherapy (R-CHOP) 1. Cure rates differ according to the cell of origin, being lower among patients with non-germinal center (non-GCB) subtype of DLBCL 2. The role of autologous stem cell transplantation (autoSCT) in relapsed chemosensitive DLBCL was established by the PARMA study 3. This study included relapsed DLBCL patients without bone marrow and CNS involvement. Patients with response to salvage chemotherapy were randomized to autoSCT or four courses of conventional salvage chemotherapy, and demonstrated improvement in 5-year event-free and overall survival in favor of autoSCT. Patients with primary refractory disease, however, do not benefit from autoSCT. In addition, the CORAL study reported that 75 of 255 patients (29%) who underwent consolidative autoSCT for chemosensitive DLBCL experience disease progression with shortened overall survival (OS) of 9.9 months 4. Patients whose disease progressed less than 1 year from autoSCT had a significantly shorter OS compared with those who progressed beyond 1 year (8.2 vs. 26.7 months, P = 0.01) 5.
Allogeneic hematopoietic stem cell transplant (alloSCT) can provide long-term disease control mediated by graft-versus-lymphoma (GVL) effect and tumor-free grafts, ultimately reducing disease recurrence 6. AlloSCT using myeloablative conditioning regimens (MAC) significantly reduces relapse risk, but at the expense of higher non-relapse mortality (NRM) due to GVHD and/or infection 7. Few single center retrospective studies have similarly shown lower NRM with reduced intensity conditioning (RIC) and non-myeloablative (NMA) regimens, but with somewhat higher relapse rates, and improved long-term OS 8–11. The majority of these studies utilized myeloablative regimens of cyclophosphamide (CY)/total body irradiation (TBI), busulfan (bu)/CY, bu/fludarabine(flu), and bu/melphalan, or the non-myeloablative regimens of flu/melphalan, flu/TBI, and flu/CY. At our institution, we predominantly use R-BEAM (rituximab, carmustine, etoposide, cytarabine, melphalan) as a conditioning regimen for DLBCL undergoing both autoSCT and alloSCT, if there was no significant exposure to chemotherapeutic agents used in R-BEAM. The CIBMTR conducted a survey among 56 participants to define RIC regimens and < 500 cGy of TBI as a single fraction or 800 cGy in fractionated doses, busulfan dose <9 mg/kg, melphalan dose <140 mg/m2, or thiotepa dose < 10 mg/kg were considered RIC regimens. However, only 32% of the participants agreed that BEAM regimen should be considered as a RIC regimen 12. Based on this finding, we consider BEAM as MAC regimen. In addition, pre-transplant rituximab exposure was associated with lower rate of acute GVHD, NRM and improvement in progression-free and overall survival 13. Therefore, we added rituximab with BEAM regimen. This is the first report extensively evaluating the efficacy of RBEAM conditioning regimen in DLBCL patients undergoing alloSCT and comparing the outcomes to RIC regimens.
Methods
Patient population
We conducted a retrospective study of consecutive adult DLBCL patients who underwent related or unrelated alloSCT for primary induction failure or relapsed DLBCL at Karmanos Cancer Institute (KCI). Patients with prior autoSCT, bone marrow, treated CNS involvement and transformed DLBCL from indolent non-Hodgkin’s lymphoma were included. The Wayne State University Institutional Review Board approved this study. This research work is carried out in accordance with the code of ethics of the Declaration of Helsinki for experiments involving humans.
The cell of origin of DLBCL, germinal center (GCB) and non-germinal center (non-GCB), was decided as per algorithm by Hans et al by immunohistochemistry (IHC) for CD10, MUM1 and BCL6 14. Those with either CD10-positive or CD10-negative/BCL6-positive/MUM1-negative were considered to have the GCB subtype, while those with CD10-negative/BCL6-negative or CD10-negative/BCL6-positive/MUM1-positive results were considered to have the non-GCB subtype. Fluorescence in situ hybridization (FISH) for c-MYC, BCL2 and BCL6 was reviewed when available. Lymphomas with a c-MYC translocation by FISH were designated as MYC-positive. Double hit lymphoma (DHL) was defined as concurrent rearrangements of MYC and BCL2 and/or BCL6. Demographic and transplant details for all patients were collected. Pretransplant comorbidity index was calculated using HSCT-CI formula 15. Disease status prior to alloSCT was as per Lugano classification 16. Acute and chronic GVHD classification and grading was as per physician discretion using standard criteria 17,18. Patients were followed until the last follow up or death.
Conditioning regimen and GVHD prophylaxis
R-BEAM employed rituximab 375mg/m2 (day −8), carmustine 300mg/m2 (day −7), etoposide (VP16) and cytarabine each given at 100mg/m2 twice daily (days −6 to −3), and melphalan 140mg/m2 (day −2). RIC regimens included (1) busulfan 130mg/m2 (day −6 & −5)/Flu 30mg/m2 (day −6 to −2)/TBI 200cGy (day 0), (2) Flu 30mg/m2 (day −6 to −2)/melphalan 140mg/m2 (day −2)/TBI 200cGy (day 0). Busulfan was dosed pharmacokinetically to achieve AUC of 5000 ng x h/ml.
GVHD prophylaxis consisted of tacrolimus, mycophenolate and/or thymoglobulin. Tacrolimus was administered intravenously (0.03 mg/kg/day) starting on day −3 and tapered starting around day +56 in the absence of active GVHD with a goal of tapering off completely by day +180. IV mycophenolate (MMF) was initiated at 15 mg/kg twice daily from day −3 and stopped at day +30. We use rabbit thymoglobulin in combination with tacrolimus and MMF in unrelated donor transplants 2009 onwards and thymoglobulin was administered at a total dose of 4.5 mg/kg in divided doses (day −3: 0.5 mg/kg; day −2: 1.5 mg/kg; and day −1: 2.5 mg/kg).
Statistics
Definitions and study endpoints
The objectives were to compare OS, relapse rate, NRM, RFS, and GVHD-free relapse-free survival (GRFS) between R-BEAM and RIC group.
Statistical methods
Baseline patient characteristics were summarized using count and percentage for categorical variables and median and range for continuous variables. Chi-square or Fisher’s exact tests were used for categorical variables and Kruskal-Wallis tests were used for continuous variables to compare between two groups (R-BEAM vs. other RIC regimens). The length of hospital stay was calculated as the time from the date of admission prior to transplantation to the date of discharge post transplantation. OS was defined as the time from the date of transplantation to death from any cause. Patients who were alive were considered censored at the date of last observation. Relapse-free survival (RFS) was defined as the time from the date of transplantation to the date of relapse or death from any cause. Patients who were alive without relapse were considered censored at the date of last observation. GVHD-free/relapse-free survival (GRFS) was defined as the time from the date of transplantation to the date of grade III-IV acute GVHD (aGVHD), extensive chronic GVHD (cGVHD), disease relapse, or death from any cause, whichever occurs first. Kaplan-Meier estimates were used to summarize the distributions of GRFS, RFS and OS. The cumulative incidences of aGVHD and cGVHD were calculated with relapse or death without GVHD as competing risks. When calculating the cumulative incidence of grade II-IV aGVHD, grade III-IV aGVHD and extensive cGVHD, Grade I GVHD and limited cGVHD were ignored, respectively. The cumulative incidences of relapse and NRM were calculated with death without relapse for relapse and death with relapse for NRM, respectively, as competing risks. Univariable and multivariable Cox proportional hazards regression models were fit to assess associations between four prior chosen predictors (admit KPS, disease status at transplant, donor type, and group) and survival benefit (RFS and OS). For relapse and NRM, the proportional sub distribution hazards regression model in competing risks was used for univariable and multivariable analyses with the four predetermined covariates. The proportional hazard assumption was checked, and no violation was found. The follow-up time was calculated using the reverse Kaplan-Meier estimate.
Results
Patient characteristics
From January 2005 through December 2017, 70 patients with DLBCL underwent alloSCT. Median age was 53 years (range, 25–68). Sixty-four patients (91%) had primary DLBCL, and six (9%) had transformed DLBCL (Table 1). All patients received rituximab-containing front line or salvage chemoimmunotherapy before the alloSCT. Immunohistochemistry (IHC) studies indicating the type of DLBCL were available in 38 patients: 29 (41%) had GCB subtype, while nine (13%) had non-GCB subtype. CD5 expression by IHC was available in 38 patients: 10 (14%) had positive and 28 (39%) had negative expression. C-MYC, BCL2 and BCL6 positivity by FISH was observed in eight (11%), nine (13%) and two (3%) patients, respectively. Double expressor, double hit and triple hit lymphoma was identified in seven, four and one patients, respectively.
Table 1.
Baseline patient characteristics
| R-BEAM (N = 47) |
RIC regimensa (N = 23) |
All (N = 70) |
p | |
|---|---|---|---|---|
| Age at transplant (year) - median (range) | 52 (25,68) | 56 (28,67) | 53.5 (25,68) | 0.255 |
| Sex - no. (%) | 0.169 | |||
| Male | 32 (68) | 11 (48) | 43 (61) | |
| Female | 15 (32) | 12 (52) | 27 (39) | |
| Race - no. (%) | 0.273 | |||
| Caucasian | 40 (85) | 22 (96) | 62 (89) | |
| AA | 2 (4) | 1 (4) | 3 (4) | |
| Othersb | 5 (11) | 0 (0) | 5 (7) | |
| Subgroup - no. (%) | >0.99 | |||
| Primary DLBCL | 43 (91) | 21 (91) | 64 (91) | |
| Transformed DLBCL | 4 (9) | 2 (9) | 6 (9) | |
| Disease stage at diagnosis - no. (%) | 0.636 | |||
| 1 | 2 (4) | 1 (4) | 3 (4) | |
| 2 | 6 (13) | 4 (17) | 10 (14) | |
| 3 | 11 (23) | 8 (35) | 19 (27) | |
| 4 | 28 (60) | 10 (43) | 38 (54) | |
| LDH at diagnosis - median (range)^ | 391 (175,3861) | 381 (214,790) | 386 (175,3861) | 0.468 |
| Number of therapy prior to AlloHSCT - median (range) | 2 (1,6) | 3 (1,5) | 3 (1,6) | 0.008 |
| Time to AlloHSCT from diagnosis, year - median (95% CI) | 1.32 (0.99,2.48) | 2.18 (1.61,4.65) | 1.60 (1.28,2.48) | 0.695 |
| Prior transplant - no. (%) | <0.001 | |||
| Yes | 2 (4) | 15 (65) | 17 (24) | |
| Duration from prior autologous to allogenic transplant (month) – median (range) | 176.56 (174.85,178.26) | 27.08 (5.54,107.97) | 28.42 (5.54,178.26) | 0.015 |
| Disease status at transplant - no (%) | 0.212 | |||
| Complete remission | 13 (28) | 8 (35) | 21 (30) | |
| Partial remission | 3 (6) | 2 (9) | 5 (7) | |
| Relapse | 14 (30) | 10 (43) | 24 (34) | |
| Refractory | 17 (36) | 3 (13) | 20 (29) | |
| Cell of origin - no. (%)& | >0.99 | |||
| GCB | 21 (45) | 8 (35) | 29 (41) | |
| NonGCB | 7 (15) | 2 (9) | 9 (13) | |
| Stage at transplant - no. (%)* | 0.946 | |||
| 1 | 1 (2) | 0 (0) | 1 (1) | |
| 2 | 4 (9) | 2 (9) | 6 (9) | |
| 3 | 11 (23) | 6 (26) | 17 (24) | |
| 4 | 31 (66) | 14 (61) | 45 (64) | |
| Bone marrow involvement at transplant - no. (%)* | 0.227 | |||
| Yes | 26 (55) | 8 (35) | 34 (49) | |
| CNS involvement at transplant - no. (%) | 0.127 | |||
| Yes | 7 (15) | 0 (0) | 7 (10) | |
| Extra nodal involvement at transplant - no. (%) | 0.388 | |||
| Yes | 23 (49) | 8 (35) | 31 (44) | |
| Admit KPS - median (range) | 70 (60,90) | 80 (60,90) | 70 (60,90) | 0.319 |
| Comorbidity index - median (range) | 2 (2,6) | 3 (2,5) | 2 (2,6) | 0.142 |
| HLA match - no. (%) | ||||
| Unrelated donor | 0.860 | |||
| 8/8 | 20 (43) | 14 (61) | 34 (49) | |
| 7/8 | 5 (11) | 3 (13) | 8 (11) | |
| Related donor | 0.389 | |||
| 8/8 | 21 (45) | 5 (22) | 26 (37) | |
| 7/8 | 0 (0) | 1 (4) | 1 (1) | |
| 5/8 | 1 (2) | 0 (0) | 1 (1) | |
| ABO mismatch - no. (%) | 0.528 | |||
| Matched | 29 (62) | 10 (43) | 39 (56) | |
| Major Mismatch | 7 (15) | 5 (22) | 12 (17) | |
| Minor Mismatch | 9 (19) | 6 (26) | 15 (21) | |
| Bidirectional | 2 (4) | 2 (9) | 4 (6) | |
| CMV serogroup status - no. (%) | 0.161 | |||
| +/+ | 17 (36) | 4 (17) | 21 (30) | |
| +/− | 4 (9) | 6 (26) | 10 (14) | |
| −/+ | 14 (30) | 7 (30) | 21 (30) | |
| −/− | 12 (26) | 6 (26) | 18 (26) | |
| Sex mismatch - no. (%)# | 0.456 | |||
| M-M | 22 (47) | 8 (35) | 30 (43) | |
| M-F | 4 (9) | 4 (17) | 8 (11) | |
| F-M | 9 (19) | 4 (17) | 13 (19) | |
| F-F | 4 (9) | 4 (17) | 8 (11) | |
| Type of transplant - no. (%) | 0.161 | |||
| Allo related | 22 (47) | 6 (26) | 28 (40) | |
| Allo unrelated | 25 (53) | 17 (74) | 42 (60) | |
| Source of stem cell - no. (%) | >0.99 | |||
| PBSC | 45 (96) | 22 (96) | 67 (96) | |
| BM | 2 (4) | 1 (4) | 3 (4) | |
| Infused CD34, million/kg - median (range) | 7.17 (2.38,22.49) | 6.51 (2.02,19.2) | 7.065 (2.02,22.49) | 0.453 |
| GVHD prophylaxis – no. (%)* | 0.240 | |||
| Thymoglobulin-based | 18 (38) | 5 (22) | 23 (33) | |
| Non-thymoglobulin-based | 28 (60) | 18 (78) | 46 (66) | |
Reduced Intensity conditioning (RIC) regimens include BU-FLU-TBI ± R (n=15) and FLUMEL-TBI ± R (n=8);
Others include two Asians
Data are not available for one patient
Data are not available for two patients
Data are not available for 11 patients
Data are not available for 32 patients
In all, the median number of pretransplant therapies was three (range, 1–6), and 17 (24%) patients had received prior autoSCT. R-BEAM group received lower median number of prior therapies (2 vs 3, p=0.008) and was less likely to have received prior autoSCT (4% vs 65%, p<0.001). Disease status at the time of alloSCT for all patients included: CR (n=21, 30%), partial remission (n=5, 7%), relapse (n=24, 34%) and refractory disease (n=20, 29%). R-BEAM group had a numerically higher percentage of patients with refractory disease (36% vs 13%), a lower percentage of CR (28% vs 35%) and relapsed patients (30% vs 43%). Bone marrow, CNS and extranodal involvement at alloSCT were noted in 35 (49%), 7 (10%) and 31 (44%) patients, respectively.
Transplant characteristics
Forty-seven patients received R-BEAM, and 23 received RIC regimens (BU-FLU-TBI± R= 15, FLU-MEL-TBI ± R= 8). Reasons for using RIC regimens were use of R-BEAM for prior autoSCT (n=14), lymphoma involving pleura or lung with a concern of higher risk of respiratory complications (n=6), presence of comorbid conditions (n=3) and exposure to some of the chemotherapeutic agents in R-BEAM (n=3). Twenty-eight patients (40%) received related and 42 (60%) received unrelated donor alloSCT. Peripheral stem cell allograft was the source of stem cells in 96% of patients. Twenty-three patients (33%) received thymoglobulin-based GVHD prophylaxis.
Engraftment and GVHD
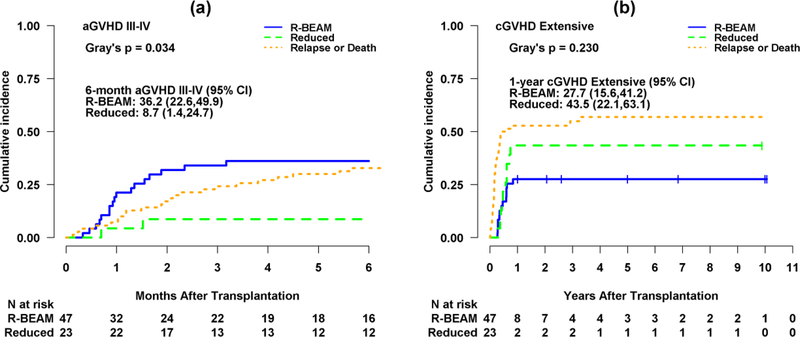
The median time to neutrophil engraftment was 10 and 11 days for R-BEAM and RIC group, respectively (p=0.34), while platelet engraftment time was 15 and 16 days for R-BEAM and RIC group, respectively (p=0.67). One patient in the R-BEAM group had primary graft failure. The cumulative incidence of grade III-IV aGVHD at 6-month was 36.2% (95% CI, 22.6–49.9%) and 8.7% (95% CI, 1.4–24.7%) for R-BEAM and RIC group, respectively (p=0.03) (Figure 1a). The 1-year cumulative incidence of chronic extensive GVHD was 27.7% (95% CI, 15.6–41.2%) and 43.5% (95% CI, 22.1–63.1%) for R-BEAM and RIC, respectively (p=0.23) (Figure 1b). Median length of hospitalization following alloSCT was 27 days (range 15–84) for R-BEAM and 26 days (18–71) for RIC (p=0.64).
Figure 1.
(a) Cumulative incidence curves for grade III-IV acute GVHD (aGVHD) with disease relapse or death as competing risks by group (R-BEAM vs. Reduced intensity).
(b) Cumulative incidence curves for extensive chronic GVHD (cGVHD) with disease relapse or death as competing risks by group.
Post-transplant toxicities
Rate of CMV reactivation was 38% for R-BEAM and 13% for RIC (p=0.05), while EBV reactivation rate was 4% for R-BEAM and 0% for RIC group (p=0.99). Systemic bacterial infections occurred in 21% of patients in R-BEAM and 30% of patients in RIC (0.55).
Survival
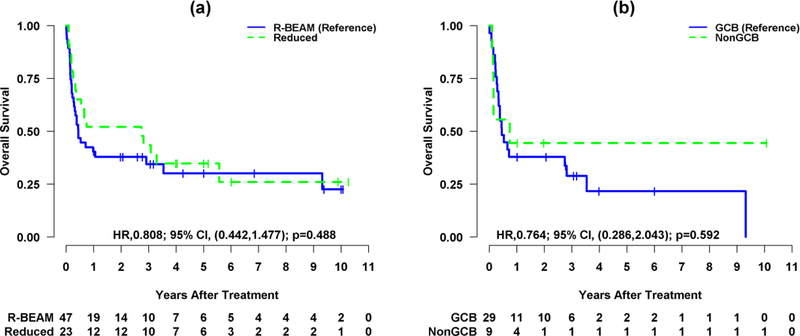
At a median follow-up of surviving patients of 3.17 and 5.58 years for R-BEAM and RIC group, respectively, 15 patients (32%) in R-BEAM and seven (30%) in RIC were alive and in remission (total n=22). The 3-year OS was 34.45% (95% CI, 22.77–52.12%) for R-BEAM and 43.48% (95% CI, 27.28–69.29%) for RIC (p=0.48) (Figure 2a). The 3-year OS was 28.9% (95% CI, 15.84–52.73%) and 44.44% (95% CI, 21.41–92.27%) for GCB and non-GCB subtypes of DLBCL, respectively (p=0.59) (Figure 2b). The disease status at alloSCT among survivors was CR + PR (n=6; 40%), relapse (n=5; 33%) and refractory disease (n=4; 27%) for R-BEAM, and CR + PR (n=4; 58%), relapse (n=3; 43%) and refractory disease (n=0) for RIC group. No difference in OS was noted in patients who received ≤3 prior lines of therapy as compared to >3 lines of therapies (p=0.67). Similarly, no impact of prior autoSCT was noted on OS (p=0.74).
Figure 2.
(a) Kaplan-Meier survival curves for overall survival (OS) by group (R-BEAM vs. Reduced intensity).
(b) Kaplan-Meier survival curves for overall survival (OS) by cell of origin (GCB vs. NonGCB).
Relapse
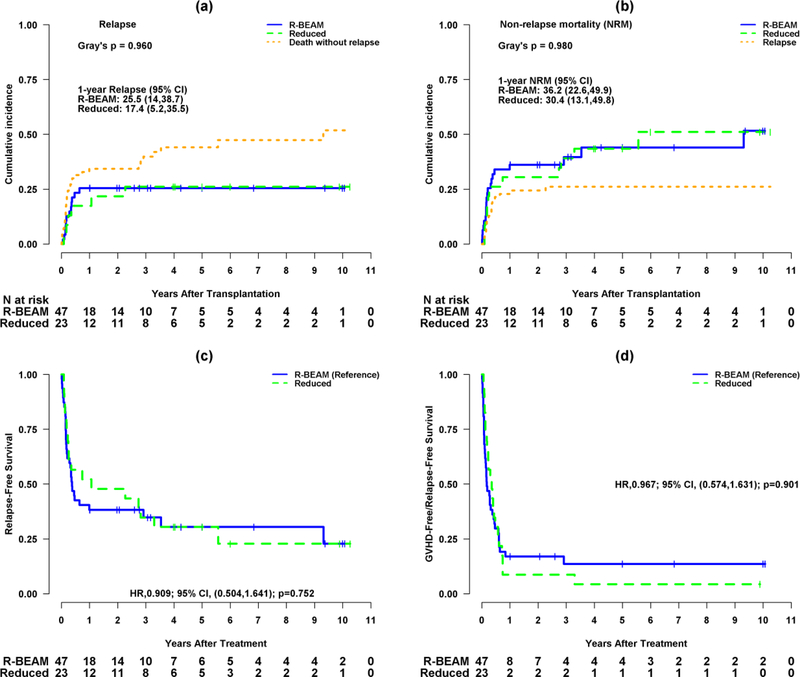
Total 18 patients relapsed: 12 (26%) in the R-BEAM group and six (26%) in the RIC group. The 3-year cumulative incidence of relapse was 25.5% (95% CI, 14–38.7%) for R-BEAM and 17.4% (95% CI, 5.2–35.5%) for RIC (p=0.96) (Figure 3a). Nine patients relapsed within 100 days, seven between 100 days and one-year, and two beyond one-year of alloSCT. At the time of this report, no patient has relapsed beyond 3 years. The disease status at transplant for patients relapsing following alloSCT was as follows: CR + PR (n=3; 25%), relapse (n=4; 33%), and refractory disease (n=5; 42%) for the R-BEAM as compared to CR + PR (n=1; 17%), relapse (n=3; 50%), and refractory disease (n=2; 33%) for RIC group. Out of 18 relapsed patients, four received donor-lymphocyte infusion. At the time of last follow up, one patient with relapse was alive and 17 died of lymphoma. No difference in relapse was observed in patients who received ≤3 prior lines of therapy as compared to >3 lines of therapies.
Figure 3.
(a) Cumulative incidence curves for relapse with death without relapse as a competing risk by group (R-BEAM vs. Reduced intensity conditioning).
(b) Cumulative incidence curves for non-relapse mortality (NRM) with relapse as a competing risk by group.
(c) Kaplan-Meier survival curves for relapse-free survival (RFS) by group.
(d) Kaplan-Meier survival curves for GVHD-free/relapse-free survival (GRFS) by group.
Non-relapse mortality and RFS
Three-year cumulative incidence of NRM was 39.7% (95% CI, 25–54%) for R-BEAM and 39.1% (95% CI, 19.2–58.6%) for RIC (p=0.98) (Figure 3b). Three-year RFS was 34.82% (95% CI, 23.15–52.37%) for R-BEAM and 34.78% (95% CI, 19.88–60.87%) for RIC (p=0.75) (Figure 3c). Three-year GRFS was 13.62% (95% CI, 6.31–29.36%) for R-BEAM and 8.7% (95% CI, 2.31–32.69%) for RIC group (p=0.90) (Figure 3d).
The common causes of death were disease relapse (35%), infection (30%), acute GVHD (15%), chronic GVHD (13%), multiorgan failure (4%) and SOS (2%).
Multivariable analyses for OS, relapse, RFS and NRM
No difference in OS, relapse, RFS or NRM was noticed between R-BEAM and RIC. Multivariable analysis revealed that lower KPS was associated with worse OS (HR 0.96; p=0.05) and marginally inferior RFS (HR 0.96; p=0.07), while no association of disease status at transplant, and donor type was observed on OS and RFS (Table 2). Chemorefractory disease at the time of alloSCT was associated with higher relapse rate (HR 8.8, p=0.04), while no impact of KPS, and donor type was observed on relapse or NRM. (Table 3).
Table 2.
Univariable and multivariable analyses of factors associated with RFS and OS
| RFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted* | Adjusted$ | Unadjusted | Adjusted | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Admit KPS | 0.963 (0.926,1.001) | 0.059 | 0.964 (0.926,1.003) | 0.07 0 | 0.960 (0.922,0.999) | 0.044 | 0.961 (0.923,1.002) | 0.059 |
| Disease status at transplant | ||||||||
| Chemo sensitivea | Reference | Reference | Reference | Reference | ||||
| Chemo refractoryb | 1.238 (0.665,2.304) | 0.501 | 1.349 (0.719,2.531) | 0.352 | 1.092 (0.585,2.038) | 0.78 2 | 1.187 (0.631,2.231) | 0.595 |
| Donor type | ||||||||
| Allo Related | Reference | Reference | Reference | Reference | ||||
| Allo Unrelated | 1.363 (0.757,2.454) | 0.302 | 1.248 (0.667,2.337) | 0.488 | 1.308 (0.724,2.364) | 0.37 3 | 1.245 (0.664,2.335) | 0.495 |
| Group | ||||||||
| R-BEAM | Reference | Reference | Reference | Reference | ||||
| Reduced intensity | 0.909 (0.504,1.641) | 0.752 | 0.878 (0.474,1.626) | 0.678 | 0.808 (0.442,1.477) | 0.48 8 | 0.773 (0.412,1.452) | 0.424 |
Abbreviations: RFS, relapse-free survival; OS, overall survival; HR, Hazard ratio; CI, Confidence interval.
CR
PR, Relapse, PIF
Univariable Cox regression analysis
Multivariable Cox regression analysis.
Table 3.
Univariable and multivariable analyses of factors associated with relapse and NRM
| Relapse | NRM | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted* | Adjusted$ | Unadjusted | Adjusted | |||||
| SHR (95% CI) | p | SHR (95% CI) | p | SHR (95% CI) | p | SHR (95% CI) | p | |
| Admit KPS | 1.005 (0.951,1.063) | 0.860 | 0.997 (0.939,1.059) | 0.930 | 0.959 (0.914,1.00 6) | 0.088 | 0.962 (0.916,1.01 1) | 0.120 |
| Disease status at transplant | ||||||||
| Chemo sensitivea | Reference | Reference | Reference | Reference | ||||
| Chemo refractoryb | 8.754 (1.177,65.090) | 0.034 | 8.866 (1.100,71.491) | 0.040 | 0.518 (0.260,1.03 2) | 0.06 2 | 0.529 (0.261,1.07 4) | 0.07 8 |
| Donor type | ||||||||
| Allo Related | Reference | Reference | Reference | Reference | ||||
| Allo Unrelated | 1.082 (0.426,2.745) | 0.870 | 1.100 (0.396,3.057) | 0.850 | 1.357 (0.657,2.801) | 0.410 | 1.289 (0.611,2.722) | 0.510 |
| Group | ||||||||
| R-BEAM | Reference | Reference | Reference | Reference | ||||
| Reduced intensity | 0.974 (0.377,2.513) | 0.960 | 1.059 (0.390,2.873) | 0.910 | 1.008 (0.499,2.033) | 0.980 | 0.968 (0.479,1.957) | 0.930 |
Abbreviations: NRM, non-relapse mortality; SHR, subdistribution hazard ratio; CI, Confidence interval.
CR
PR, Relapse, PIF
Univariable proportional subdistribution hazards regression analysis
Multivariable proportional subdistribution hazards regression analysis.
Discussion
Our study represents one of the largest series reporting outcomes of DLBCL patients undergoing alloSCT using an R-BEAM conditioning regimen. Previous studies have shown the feasibility of alloSCT in primary refractory DLBCL or failed autoSCT, and concluded that alloSCT can be an effective therapeutic modality in a subset of patients 8,11,19. These studies were limited however by small numbers and/or patient heterogeneity 9,10,20. Only the CIBMTR study included 17 patients who received BEAM 7. Thus, the information on the use of R-BEAM in this population is very limited. Like prior studies, we observed differences in certain disease related characteristics between R-BEAM and RIC group indicating selection bias. Patients receiving R-BEAM had a numerically higher percentage of primary refractory disease, a lower percentage of pretransplant therapies, and had undergone less prior autoSCT than RIC group. Moreover, R-BEAM patients had shorter interval between initial diagnosis and alloSCT compared to RIC. This study provides a retrospective comparison of patients with two different disease characteristics: one group consisting of approximately two third of patients with post-autoSCT relapse (RIC group) and the other without prior autoSCT (R-BEAM group).
No statistically significant difference in the long-term survival was observed between R-BEAM and RIC. However, caution must be taken in interpreting these data due to heterogeneity between both groups. Survival and relapse curves tend to plateau over time, suggestive of a graft versus lymphoma effect. In an EBMT study evaluating 101 DLBCL patients who received alloSCT, 3-year OS was 53% 8. The main reason for better survival in the EBMT study could be the inclusion of younger patients (median age 46 vs 53 years) than in our study. Conversely, our survival rate after R-BEAM was better than prior studies using other MAC regimens (OS, 34% vs 19%−21%) 7,19, whereas this rate was lower in comparison with prior studies involving RIC and NMA regimens (OS, 34% vs 45%−47%) 9–11. One explanation for better OS may be greater use of pretransplant rituximab compared to MAC regimens which did not incorporate rituximab 13. Furthermore, compared to studies evaluating RIC and NMA regimens, our study included higher proportion of patients with relapsed and refractory disease, which might have resulted in worse survival 9–11.
Higher non-relapse mortality remains one of the major barriers of alloSCT limiting wide applicability of this modality. NRM at 3-year was similar in R-BEAM and RIC groups (39.7% vs 39.1%, p=0.98). Our NRM rates were in line with the prior studies. The EBMT study evaluating alloSCT outcomes among 101 DLBCL patients reported 3-year NRM of 41% after MAC and 20% after RIC regimens 8. NRM after R-BEAM was lower when compared with other MAC alloSCT 7,19,21. The CIBMTR study reported alloSCT outcomes between MAC and RIC regimens in DLBCL patients and 1-year NRM was 47% and 31%, respectively 7. The CIBMTR study reported that lower Karnofsky performance status, chemotherapy resistant relapse, and unrelated donor transplant were associated with higher NRM. Because of relatively small sample size, we did not observe any factors influencing NRM. However, compared to these studies, our patient cohort was older, received higher number of pretransplant therapies and experienced higher acute and cGVHD, which might have resulted in higher NRM.
Grade III-IV acute GVHD was significantly higher in patients receiving R-BEAM regimen compared to RIC regimens (36.2% vs 8.7%, p=0.03). Possible reason for higher GVHD rate could be the intensity of the conditioning regimen. Studies have shown that MAC regimens induce intense GI mucosal injury, which could result in higher acute and chronic GVHD rates 22. The relapse rate was considerably lower at 25% in both cohorts, and the majority of the recurrences occurred early after alloSCT. The CIBMTR study showed 5-year relapse rate of 26% with MAC, 40% with NMA, and 38% with RIC 7, whereas EBMT reported 3-year relapse rate at 30% 8. Similar higher relapse rates were observed in other studies 9–11,21. The lower relapse rate in our study could be due to inclusion of rituximab prior to alloSCT.
A limitation of our study is its retrospective nature. The decision to select RIC regimens was based on individual physician choice, which may indicate selection bias. The two groups were not randomized, and RIC group was small. Moreover, information on IHC and FISH was not consistently available, which limited detailed evaluation of the impact of alloSCT on double expressor and double hit lymphoma.
In conclusion, our results indicate that alloSCT could provide durable responses in a subset of DLBCL patients. R-BEAM provided equivalent long-term outcomes in comparison with RIC. Post alloSCT relapse and high NRM still remain challenging issues. Novel approaches to reduce post alloSCT relapse, such as incorporation of ibrutinib during early post-transplant period, should be considered in this high-risk population.
No funding was obtained.
Highlights.
Allogeneic transplant is a viable treatment in diffuse large B cell lymphoma
R-BEAM regimen is associated with significantly higher grade III-IV acute GVHD
Non-relapse mortality is substantial
A few patients attain a durable response indicating graft versus lymphoma effect
Footnotes
Financial Disclosure: The authors have no existing or potential financial conflict of interest to disclose.
Conflict of interest: No relevant conflict of interests exists.
Declaration: We presented our study data at Transplantation & Cellular Therapy (TCT) annual meeting 2019. The abstract was published in Biology of Blood and Marrow Transplantation journal supplement issue in February 2019 and it is available online (Google scholar).
Contributor Information
Dipenkumar Modi, Department of Oncology, Karmanos Cancer Institute/Wayne State University 4100 John R, HW04H0, Detroit, MI 48201
Seongho Kim, Biostatistics Core, Karmanos Cancer Institute, Department of Oncology, Wayne State University, Detroit, MI 48201
Malini Surapaneni, Department of Oncology, Karmanos Cancer Institute/Wayne State University, 4100 John R, HW04H0, Detroit, MI 48201.
Lois Ayash, Department of Oncology, Blood and Marrow Stem Cell Transplant Program, Karmanos Cancer Institute/Wayne State University, 4100 John R, HW04H0, Detroit, MI 48201
Asif Alavi, Department of Oncology, Blood and Marrow Stem Cell Transplant Program, Karmanos Cancer Institute/Wayne State University, 4100 John R, HW04H0, Detroit, MI 48201
Voravit Ratanatharathorn, Department of Oncology, Blood and Marrow Stem Cell Transplant Program, Karmanos Cancer Institute/Wayne State University, 4100 John R, HW04H0, Detroit, MI 48201
Abhinav Deol, Department of Oncology, Blood and Marrow Stem Cell Transpant Program, Karmanos Cancer Institute/Wayne State University, 4100 John R, HW04H0, Detroit, MI 48201
Joseph P. Uberti, Department of Oncology, Co-Director, Blood & Marrow Stem Cell Transplant Program, Karmanos Cancer Institute/Wayne State University, 4100 John R, 4 HW04H0, Detroit, MI 48201
References
- 1.Coiffier B, Lepage E, Briere J, et al. : CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346:235–42, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Scott DW, Mottok A, Ennishi D, et al. : Prognostic Significance of Diffuse Large B-Cell Lymphoma Cell of Origin Determined by Digital Gene Expression in Formalin-Fixed Paraffin-Embedded Tissue Biopsies. J Clin Oncol 33:2848–56, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Philip T, Guglielmi C, Hagenbeek A, et al. : Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med 333:1540–5, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Van Den Neste E, Schmitz N, Mounier N, et al. : Outcomes of diffuse large B-cell lymphoma patients relapsing after autologous stem cell transplantation: an analysis of patients included in the CORAL study. Bone Marrow Transplant 52:216–221, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Nagle SJ, Woo K, Schuster SJ, et al. : Outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. Am J Hematol 88:890–4, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Ratanatharathorn V, Uberti J, Karanes C, et al. : Prospective comparative trial of autologous versus allogeneic bone marrow transplantation in patients with non-Hodgkin’s lymphoma. Blood 84:1050–5, 1994 [PubMed] [Google Scholar]
- 7.Bacher U, Klyuchnikov E, Le-Rademacher J, et al. : Conditioning regimens for allotransplants for diffuse large B-cell lymphoma: myeloablative or reduced intensity? Blood 120:4256–62, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Kampen RJ, Canals C, Schouten HC, et al. : Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin’s lymphoma relapsing after an autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. J Clin Oncol 29:1342–8, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Rezvani AR, Norasetthada L, Gooley T, et al. : Non-myeloablative allogeneic haematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: a multicentre experience. Br J Haematol 143:395–403, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sirvent A, Dhedin N, Michallet M, et al. : Low nonrelapse mortality and prolonged long-term survival after reduced-intensity allogeneic stem cell transplantation for relapsed or refractory diffuse large B cell lymphoma: report of the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Biol Blood Marrow Transplant 16:78–85, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Thomson KJ, Morris EC, Bloor A, et al. : Favorable long-term survival after reduced-intensity allogeneic transplantation for multiple-relapse aggressive non-Hodgkin’s lymphoma. J Clin Oncol 27:426–32, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Giralt S, Ballen K, Rizzo D, et al. : Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 15:367–9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratanatharathorn V, Logan B, Wang D, et al. : Prior rituximab correlates with less acute graft-versus-host disease and better survival in B-cell lymphoma patients who received allogeneic peripheral blood stem cell transplantation. Br J Haematol 145:816–24, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hans CP, Weisenburger DD, Greiner TC, et al. : Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103:275–82, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Sorror ML, Maris MB, Storb R, et al. : Hematopoietic cell transplantation (HCT)specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 106:2912–9, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheson BD, Fisher RI, Barrington SF, et al. : Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–68, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jagasia MH, Greinix HT, Arora M, et al. : National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 21:389–401 e1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glucksberg H, Storb R, Fefer A, et al. : Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 18:295–304, 1974 [DOI] [PubMed] [Google Scholar]
- 19.Hamadani M, Saber W, Ahn KW, et al. : Impact of pretransplantation conditioning regimens on outcomes of allogeneic transplantation for chemotherapy-unresponsive diffuse large B cell lymphoma and grade III follicular lymphoma. Biol Blood Marrow Transplant 19:746–53, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez R, Nademanee A, Ruel N, et al. : Comparison of reduced-intensity and conventional myeloablative regimens for allogeneic transplantation in non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant 12:1326–34, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Lazarus HM, Zhang MJ, Carreras J, et al. : A comparison of HLA-identical sibling allogeneic versus autologous transplantation for diffuse large B cell lymphoma: a report from the CIBMTR. Biol Blood Marrow Transplant 16:35–45, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Couriel DR, Saliba RM, Giralt S, et al. : Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant 10:178–85, 2004 [DOI] [PubMed] [Google Scholar]