Abstract
The Brazelton Neonatal Behavioral Assessment Scale (NBAS) evaluates a newborn infant’s autonomic, motor, state, temperament, and social-attentional systems, which can help to identify infants at risk of developmental problems. Given the prevalence of rhesus monkeys being used as an animal model for human development, here we aimed to validate a standardized test battery modelled after the NBAS for use with non-human primates called the Infant Behavioral Assessment Scale (IBAS), employing exploratory structural equation modeling using a large sample of rhesus macaque neonates (N=1056). Furthermore, we examined the repeated assessments of the common factors within the same infants to describe any changes in performance over time, taking into account two independent variables (infant sex and rearing condition) that can potentially affect developmental outcomes. Results revealed three factors (Orientation, State Control, and Motor Activity) that all increased over the first month of life. While infant sex did not have an effect on any factor, nursery-rearing led to higher scores on Orientation but lower scores on State Control and Motor Activity. These results validate the IBAS as a reliable and valuable research tool for use with rhesus macaque infants and suggest that differences in rearing conditions can affect developmental trajectories and potentially pre-expose infants to heightened levels of cognitive and emotional deficiencies.
Keywords: exploratory structural equation modeling, second-order latent growth model, motor activity, IBAS scale, orientation, state control
Introduction
It is routine practice in hospitals that each newborn baby is carefully checked for signs of health problems by doctors, nurses, and other health care providers. While some conditions can predict complications in physical health (Bateson, et al., 2004; Rees, Harding, & Walker, 2008), others may have more subtle influences e.g. on stress responsiveness or cognitive performance (Sackett, Ruppenthal, Hewitson, Simerly, & Schatten, 2006). The Neonatal Behavioral Assessment Scale (NBAS), developed in 1973 (Brazelton, 1973) and revised in 1995 (Brazelton & Nugent, 1995), has been used to evaluate health status, maturity, and temperament of neonates over the first four weeks of life (Als, Tronick, Lester, & Brazelton, 1977), and consists of a standardized battery of tests for rating normative reflexes, responses, and arousal states. Its purpose is to describe neurotypical development, to give an indication of the infant’s ability to regulate its own behavior, and to document his or her interactional capacity (Hawthorne, 2005). The NBAS is based on the idea that neonates are complexly organized, able to protect themselves from negative stimuli, in control of motor responses in order to attend to external stimuli, and capable of influencing their environment to optimize their emotional, social, and cognitive development (Als et al., 1977). The rearing environment may further enhance or suppress a neonate’s capabilities (Weinberg, Kim, & Yu, 1995), and cross-cultural differences have been noted with regard to performance on the NBAS (Brazelton, Koslowski, & Tronick, 1976; Brazelton, Robey, & Collier, 1969). Its applications have included: evaluating the effects of maternal obstetric medication; describing characteristics associated with failures in developmental outcomes; assessing the effects of maternal narcotic addiction; characterizing infants’ individual differences in interaction with caregivers; and determining the effects of intervention programs for low birth weight infants (Als et al., 1977).
The NBAS allows for comparing groups of infants, either at one point or over time, as well as describing the performance of a single infant. It consists of 27 behavioral items and 20 reflex items (Brazelton & Nugent, 1995), grouped into several a-priori subscales including Interactive Processes, Motoric Processes, State Control, and Physiological Response to Stress (Als et al., 1977). However, other statistical analyses have also been used to interpret findings including item-by-item comparison, factor analysis, overall summary scale, and type and profile analysis (Als et al., 1977).
For research purposes, the NBAS has been adapted for use with non-human primate (NHP) neonates and has been called the Infant Behavioral Assessment Scale (IBAS; Coe, Lubach, Crispen, Shirtcliff, & Schneider, 2010). NHP models are particularly useful for neurodevelopmental studies due to NHPs’ similarity to humans in physiology, neuroanatomy, development, cognition, and social complexity (Phillips et al., 2014). In addition, researchers can tightly control environmental and lifestyle variables of NHPs in a way that is not possible with humans (Schneider & Coe, 1993). Past studies have shown, for example, that chimpanzees perform remarkably similarly to human neonates in their behavioral response on the IBAS (Hallock, Worobey, & Self, 1989; Bard, Platzman, Lester, & Suomi, 1992). Other adaptations have included marmoset (Braun, Schultz‐Darken, Schneider, Moore, & Emborg, 2015) and squirrel monkey neonates (Schneider & Coe, 1993). The most widely applied use has been with rhesus macaque neonates (Schneider, Moore, Suomi, & Champoux, 1991), measuring (like the human instrument) dimensions of arousal, orientation, and neuromotor maturity, all of which have implications for later cognitive and emotional development (Schneider & Suomi, 1992). Its application has revealed, for example, that maternal stress during pregnancy (Schneider & Coe, 1993), maternal alcohol consumption during pregnancy (Schneider, Roughton, & Lubach, 1997), and genetic differences (Champoux, Suomi, & Schneider, 1994; Champoux et al., 2002) significantly impact performance on the IBAS in rhesus macaque neonates.
Analyses of the rhesus IBAS data have been similarly varied with some investigators performing principal components or common factor analyses to generate interpretable factors (e.g. Schneider et al., 1991; Coe et al., 2010), and others comparing single items between groups or over time (e.g. Ferrari et al., 2009; Dettmer, Ruggiero, Novak, Meyer, & Suomi, 2008). Both approaches can be problematic: item-by-item comparisons may suffer from the post-hoc nature of the interpretation of differences as well as the magnitude of reported differences being conceptually meaningless (Als et al., 1977). Common factor and principal components analyses may be prone to sampling error when only small sample sizes (N<50, common in NHP studies) are available, meaning that a particular solution may not be applicable to other populations. The most rigorous validation of the rhesus IBAS to date have been by Coe et al. (2010) and Kay, Marsiske, Suomi, & Higley (2010). Coe et al. (2010) used principal components analysis on the data of 413 2-week-old rhesus macaque infants, which resulted in the generation of 4 factors: state control, motor activity, orientation, and sensory sensitivity. Sex differences in state control (with females being more reactive than males) and varying with several different pregnancy manipulations were also observed. Kay et al. (2010) used data from 542 1-week-old rhesus macaque infants and 26 items hypothesized to be relevant to infant temperament. An exploratory factor analysis revealed three components, named Negative Affect, Orienting/Regulation, and Surgency/Extraversion, that resemble previously identified component of the IBAS (State Control, Orientation, and Activity) as well as factors identified in human infant temperament models (Kay et al., 2010).
The present study sought to expand on Coe et al.’s (2010) and Kay et al.’s (2010) findings by validating the rhesus IBAS scale using an exploratory structural equation modeling (ESEM) with a large sample of rhesus macaque infants. Thus, in contrast to past investigations that have performed either an exploratory or confirmatory analysis using data collected at a single point in time, we relied on a repeated measures analysis to study the underlying factor structure of the measured items across multiple points in time (Asparouhov & Muthén, 2009). We note as well that we applied common factor analysis and not principal components analysis. Common factor analysis assumes that one or more latent factors account for the patterns of correlations between measured items and that residual variance in the observed items is due to measurement error (Fabrigar, Wegener, MacCallum, & Strahan, 1999). Conversely, principal components analysis is a data reduction method that results in linear weighted combinations of the measured items that maximally account for variance in the items (Costello & Osborne, 2005). In addition to the ESEM, we applied a second-order latent curve model to further examine the repeated measures assessments of the common factors within the same infants (up to 4 within the first month of life) and describe any changes in performance of factors over time, taking into account two independent variables (infant sex: male, female; and rearing condition: mother-reared, nursery-reared) that can potentially affect developmental outcomes.
Methods
Ethical approval
Research methods were approved by the Animal Care and Use Committee, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. The study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals and complied with the Animal Welfare Act and the American Society of Primatologists Ethical Principles for the Treatment of Non-Human Primates.
Subjects
Subjects were 1056 infant rhesus macaques (Macaca mulatta), spanning 27 different birth cohorts (1989–2016). For 15 infants, rearing condition and infant sex was not documented. 541 infants (276 male) were reared by their mothers and lived in social groups comprised of 1–2 adult males, 8–12 adult females, and 2–6 infants of similar age. This type of social housing approximates rhesus macaques’ field ecology, where groups are multi-male / multi-female and can consist of 6–90 individuals (Makwana, 1978). Social groups were housed in indoor-outdoor enclosures measuring 2.44m x 3.05m x 2.21m indoors and 2.44m x 3.0m x 2.44m outdoors, and enriched with wood chips, multiple perches, swings, and other enrichment devices. Monkeys were fed Purina High Protein Monkey Chow (#5054, St. Louis, MO) and supplemental fruit and other foraging materials such as peanuts or sunflower seeds twice daily. Water was available ad libitum.
561 infants (305 male) were separated from their mothers on the day they were born (typically by 8am), and were reared in a nursery facility for ongoing, unrelated research studies (e.g. Provençal et al., 2012; Schneper, Brooks-Gunn, Notterman, & Suomi, 2016; Baker et al., 2017). All infants were individually housed in incubators (51 cm × 38 cm × 43 cm) maintained at 24–28°C for the first two weeks of life and in metal cages (61 × 61 × 76 cm) thereafter. Room temperature was maintained between 22° and 26°C, and humidity was maintained at 50 to 55%. All housing arrangements contained a moveable fleece surrogate, loose pieces of fleece fabric, and various plush, plastic, and rubber toys. For the first month of life, infants could see and hear, but not physically contact, other infants of similar age. Human caretakers were present for 13h each day and interacted with infants every 2h for feeding and cleaning purposes. Infants were bottle fed ready-to-feed Similac™ formula and as they became older, were offered water ad libitum. Starting at 16 days of age, infants were given Purina High Protein Monkey Chow (#5054, St. Louis, MO). Daily enrichment consisting of fruit, seeds, or nuts was added at 2 months old (for further details see Simpson, Miller, Ferrari, Suomi, & Paukner, 2016).
Procedure
The neonatal assessments were planned for postnatal days 7, 14, 21, and 30 (+/− 1 day). Though the majority (n = 767) of infants were measured on these days, the remainder were measured according to different subsets of these days, resulting in 15 patterns of observation (see Appendix 1). Mother–infant dyads were separated from their social group beginning at 11:00 each testing day. The mother was anesthetized (ketamine HCl, 10 mg/kg, IM); the infant was transported to the neonatal nursery for testing and reunited with the mother after completion of the test.
Each infant was evaluated with the standardized rhesus monkey test battery based on the IBAS (Schneider & Suomi, 1992) consisting of 46 items. All tests were administered by trained raters with interrater reliability determined by independently scoring the test and comparing the two sets of scores with r>.90. Ratings were based on scales ranging from 0 to 2 with half steps allowed (i.e., 0.5 and 1.5).
Data analytic strategy
The data analysis followed a two-stage approach. First, exploratory structural equation models using geomin rotation (Asparouhov & Muthén, 2009) were applied to responses on 46 items across the four waves of data collection to identify subsets of items whose correlations could be accounted for by a relatively small number of latent constructs. Infants with missing data were included in this analysis, with these animals contributing data as available. In this first stage of data analysis the full sample of n = 1056 was divided into two independent sets, of the same size, formed by random sampling. The goal was to apply ESEM to one data set (calibration sample, n = 528) and to evaluate the performance of the model using a confirmatory model applied to an independent sample (validation sample, n = 529). In ESEM, all items may have loadings on all factors; in the confirmatory model, items have loadings on specific factors and all other loadings are set equal to zero. The ESEM assumed that the factor loading of each item was invariant across the four measurement waves. Other aspects of the model were not restricted to be the same across the four waves of measurement. These included the intercepts of the measurement models for each item, the residual variances of the individual items and the variances of the latent constructs. Additionally, the residuals corresponding to the same item could covary between waves, and the latent constructs could covary within and between waves.
In the second stage of analysis, the reduced item set (based on results from the first stage) was studied using a repeated measures second-order latent growth model. This model allows for evaluation of change in the latent constructs across waves of measurement and to test if infant sex and rearing condition accounted for individual differences in change. The model was applied to both the calibration and validation samples. All models were estimated using Mplus version 8 (Muthén & Muthén, 2017) with maximum likelihood estimation with standard errors which are robust to non-normality. Missing data were assumed to be missing at random. Fifteen animals with missing values for sex and rearing condition were excluded from analyses that included these covariates in the model.
Results
From the repeated measures EFA using the calibration sample, three factors based on 19 of the set of 46 items were deemed meaningful, as judged by the estimated factor loadings that were large relative to their standard errors and that followed a factor loading pattern that was generally consistent with reports by Coe et al. (2010) and Schneider & Suomi (1992). Factor 1, Orientation, included moderate to high factor loadings for visual orientation, visual following, looking duration, attention span, and reach & grasp. Factor 2, State Control, included moderate to high factor loadings for response intensity, soothability, vocalization count, irritability, consolability, struggle during test, predominant state, cuddliness, tremulousness, and self-quieting. Factor 3, Motor activity, included moderate to high factor loadings for motor activity, passivity, coordination, and locomotion. Standardized maximum likelihood estimates from the two analyses using the reduced set of 19 items are given in Table 1, along with the root mean square error of approximation (RMSEA) and the standardized root mean square residual (SRMR) that were used to evaluate model fit. Values less than .05 for both measures are typically used to judge a model as providing a close fit to the data. The EFA yielded an acceptable level of fit, with an RMSEA value of .045 (90% CI: .043, .046). The SRMR was .059.
Table 1.
Repeated measures exploratory structural equation modeling using the calibration sample (n = 528)
| Factor 1 Orientation |
Factor 2 State Control |
Factor 3 Motor Activity |
|
|---|---|---|---|
| Item | Loading | Loading | Loading |
| Visual orientation | .84 | .03 | −.01 |
| Visual following | .75 | −.04 | −.00 |
| Looking duration | .94 | −.00 | −.00 |
| Attention span | .80 | −.10 | .02 |
| Reach and grasp | .47 | .08 | .05 |
| Response intensity | −.04 | .66 | .01 |
| Soothability | .02 | .90 | −.02 |
| Vocalization (log) | .02 | .37 | −.08 |
| Irritability | .03 | −.80 | .00 |
| Consolability | .04 | −.89 | −.03 |
| Struggle during test | −.03 | .85 | .05 |
| Predominant state | .00 | .89 | −.00 |
| Cuddliness | .10 | −.74 | −.06 |
| Tremulousness | .02 | .25 | .04 |
| Self-quieting | .07 | .47 | −.06 |
| Motor activity | −.01 | .04 | .90 |
| Passivity | −.01 | .06 | −.98 |
| Coordination | .03 | .04 | .29 |
| Locomotion | .08 | .10 | .37 |
Notes: Estimates are standardized maximum likelihood estimates assuming invariance of the factor loadings across the four repeated measurements. The variances of all factors were set equal to 1. For the calibration sample, RMSEA = .045, 90% CI of RMSEA: (0.043, 0.046).
Next, a 3-factor CFA was fit to the validation sample using the pattern of factor loadings suggested by EFA. Specifically, CFA allowed for items to differ from zero if their loadings from EFA were large relative to their standard errors and were set equal to zero if the loadings were otherwise small. Estimates from CFA using the validation sample are in Table 2, along with the RMSEA. As judged by the RMSEA, the factor structure based on CFA, as suggested by EFA using the calibration sample, provided a good fit to the validation sample (RMSEA = .047, 90% CI: .045, .048). The SRMR was .07.
Table 2.
Repeated measures confirmatory factor analysis using the validation sample (n = 528)
| Factor 1 Orientation |
Factor 2 State Control |
Factor 3 Motor Activity |
|
|---|---|---|---|
| Item | Loading | Loading | Loading |
| Visual orientation | .80 | ||
| Visual following | .70 | ||
| Looking duration | .93 | ||
| Attention span | .83 | ||
| Reach and grasp | .43 | ||
| Response intensity | .70 | ||
| Soothability | .88 | ||
| Vocalization (log) | .26 | ||
| Irritability | −.78 | ||
| Consolability | −.90 | ||
| Struggle during test | .85 | ||
| Predominant state | .86 | ||
| Cuddliness | −.78 | ||
| Tremulousness | .28 | ||
| Self-quieting | .41 | ||
| Motor activity | .99 | ||
| Passivity | −.92 | ||
| Coordination | .29 | ||
| Locomotion | .42 |
Notes: Estimates are standardized maximum likelihood estimates. The variance of each factor corresponding to the first wave of measurement was set equal to 1 to set the scale of the corresponding factor. For the validation sample, RMSEA = .046, 90% CI of RMSEA: (0.045, 0.048).
In fitting the second-order latent growth model, the form of change in the factors was evaluated before adding the covariates to the model. For these models, time was defined by the animal’s age in weeks at each measurement occasion, with time centered at one week of age (i.e., time = 0 corresponded to age = D7). Thus, the intercept of the growth model is interpreted as the factor score at 7 days of age. Time was coded to reflect change in each factor per week (i.e., time = 0, 1, 2, 3.3 [reflecting the 9 day time difference between the third and fourth measurement point] corresponded to age = D7, D14, D21, and D30). The first growth model assumed a constant rate of change for each of the three factors, and the fit of this model was compared to that of a second model that assumed quadratic change (i.e., the model included both a linear and a quadratic time effect) for each of the three factors. Based on model fit comparisons using the Akaike information criterion (AIC) and the Bayesian information criterion (BIC), first using the calibration sample and then replicating the analysis using the validation sample, a linear growth model best described change in the three factors (Factor 1 Orientation, Factor 2 State Control, Factor 3 Motor Activity). Based on the estimates of this model for both samples, the means of each factor increased over time. Estimates of this model, referred to as Model 1, are given for the calibration sample in the first column and upper part of Table 3, and those for the validation sample appear in the first column and lower part of Table 3.
Table 3.
Fixed-effects estimates of a second-order latent curve model
| Sample | Parameter | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| Calibration | Orientation, age 1 week | 0* | 0* | 0* |
| n = 528 | Male | −.04(0.05) | ||
| Nursery Reared | 0.37(0.05)a | 0.36(0.05)a | ||
| Orientation, linear change rate | .06(.01)a | 0.01(0.02) | 0.02(0.01) | |
| Male | 0.02(0.02) | |||
| Nursery Reared | 0.09(0.02)a | 0.09 (0.02)a | ||
| State Control, age 1 week | 0* | 0* | 0* | |
| Male | −0.04(0.03) | |||
| Nursery Reared | −0.55(0.04)a | −0.55 (0.04)a | ||
| State Control, linear change rate | .11(.01)a | 0.20(0.01)a | 0.21(0.01)a | |
| Male | 0.01(0.01) | |||
| Nursery Reared | −0.18(0.01)a | −0.18 (0.01)a | ||
| Motor Activity, age 1 week | 0* | 0* | 0* | |
| Male | 0.04(0.05) | |||
| Nursery Reared | −0.37(0.05)a | −0.37 (0.05)a | ||
| Motor Activity, linear change rate | .11(.01)a | 0.10(0.02)a | 0.09(0.02)a | |
| Male | −0.01(0.02) | |||
| Nursery Reared | 0.03(0.02) | 0.03(0.02) | ||
| Validation | Orientation, age 1 week | 0* | 0* | 0* |
| n = 528 | Male | 0.03 (0.05) | ||
| Nursery Reared | 0.35 (0.05)a | 0.35(0.05)a | ||
| Orientation, linear change rate | .08 (.01)a | 0.03 (0.02) | 0.03(0.01) | |
| Male | −0.01 (0.02) | |||
| Nursery Reared | 0.09 (0.02)a | 0.09 (0.02)a | ||
| State Control, age 1 week | 0* | 0* | 0* | |
| Male | −0.04 (0.03) | |||
| Nursery Reared | −0.43 (0.04)a | −0.43 (0.04)a | ||
| State Control, linear change rate | .12 (.01)a | 0.22 (0.01)a | 0.22(0.01)a | |
| Male | 0.01 (0.01) | |||
| Nursery Reared | −0.20 (0.01)a | −0.20 (0.01)a | ||
| Motor Activity, age 1 week | 0* | 0* | 0* | |
| Male | −0.09 (0.05) | |||
| Nursery Reared | −0.31 (0.05)a | −0.31 (0.05)a | ||
| Motor Activity, linear change rate | .11 (.01)a | 0.10 (0.02)a | 0.11(0.01)a | |
| Male | 0.01 (0.02) | |||
| Nursery Reared | 0.01 (0.02) | 0.01(0.02) | ||
Notes: Estimates are unstandardized maximum likelihood estimates with standard errors in parentheses.
denotes that the mean of the factor at age 1 week was set equal to 0.
denotes statistically significant effects at the .05 level.
Individual differences in the factors were assessed by examining the variances of the random effects of the growth models. The variance-covariance matrix of the random effects is given in the upper part of Table 4 for the calibration sample and in the lower part of Table 4 for the validation sample. In each matrix, the estimated variances are in the diagonal of the matrix, the covariances are given below the diagonal, and the correlations are given above the diagonal. Individual differences in each of the factors at 7 days of age is evidenced by the estimated variances of the intercepts of each growth model, all of which are large relative to their standard errors. Individual differences in the linear rates of change is revealed by the large variances of the random effects relating to change in Orientation and State Control but not Motor Activity.
Table 4.
Estimated variance-covariance matrix of the factor levels and rates of change
| Calibration sample, n = 528 | |
| Validation sample, n = 528 | |
Notes: F1 Orientation, F2 State Control, F3 Motor Activity. For the random growth coefficients, the variances are along the diagonal, covariances in the lower off-diagonal, and correlations in the upper off-diagonal. Estimates are based on Model 1. Correlations of at least .09 are statistically significant at the .05 level.
The covariates, sex (male=1, female=0) and rearing (nursery-reared=1, mother-reared=0), were added to the latent growth model to predict the factors at 7 days of age and their change over time. Estimates of this model, referred to as Model 2, for the calibration sample are in the second column and upper part of Table 3 and those for the validation sample are in the second column and lower part of Table 3. For both samples, sex was not a reliable predictor of the factors at 7 days of age or their change over the study period. Sex was dropped as a covariate and the models refitted, with estimates provided in the last column of Table 3. At 7 days of age, nursery-reared animals were relatively high on Orientation and relatively low on both State Control and Motor Activity compared to mother-reared animals. With regard to change, mother-reared animals did not change, on average, in Orientation, whereas nursery-reared animals increased, on average. Whereas mother-reared animals increased in State Control, nursery-reared animals did not change, on average. For Motor Activity, nursery-reared and mother-reared did not differ in their mean rate of change, with both groups increasing over time. Parameter estimates were comparable between the calibration and validation samples.
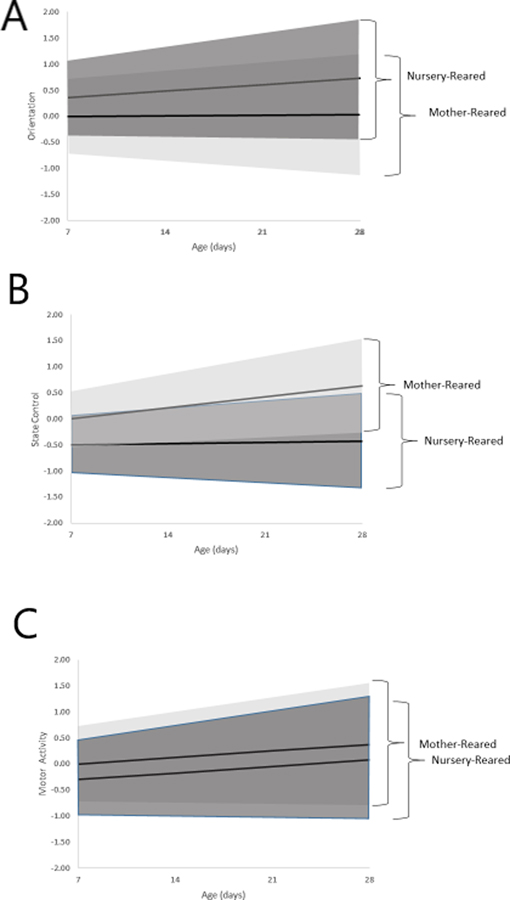
Expected mean trajectories for mother- and nursery-reared animals and corresponding 95% confidence intervals of the expected trajectories of individual animals within these groups are displayed in Figure 1. For Orientation (Figure 1a), the fitted means for the nursery-reared animals over days were such that the factor mean scores at 7 days of age were relatively high (the factor mean score for mother-reared animals was arbitrarily set equal to 0 for model identification purposes) with the estimated between-group difference in the intercept being 0.35 (SE = 0.05). For mother-reared animals, the factor mean scores remained fairly stable across days (estimated slope = 0.03, SE = 0.01); for nursery-reared animals, the factor mean scores increased at a relatively fast rate across days (the estimated between-group difference in the slope was 0.09, SE = 0.02). For State Control (Figure 1b), the fitted means for the nursery-reared animals over days were such that the factor mean scores at 7 days of age were relatively low (again, the factor mean score for mother-reared animals was arbitrarily set equal to 0 for model identification purposes) with the estimated between-group difference in the intercept being 0.43 (SE = 0.04). For mother-reared animals, the factor mean scores increased across days (estimated slope = 0.22, SE = 0.01); for nursery-reared animals, the factor mean scores remained fairly stable (the estimated between-group difference in the slope was −0.20, SE = 0.01). For Motor Activity (Figure 1c), the fitted means for the nursery-reared animals over days were such that the factor mean scores at 7 days of age were relatively low (again, the factor mean score for mother-reared animals was arbitrarily set equal to 0 for model identification purposes) with the estimated between-group difference in the intercept being −0.31 (SE 0.05). For mother-reared animals, the factor mean scores increased across days (estimated slope = 0.11, SE = 0.01); for nursery-reared animals, the factor mean scores increased at about the same rate (the estimated between-group difference in the slope was 0.01, SE = 0.02).
Figure 1.
Expected mean trajectories for mother- and nursery-reared animals and corresponding 95% confidence intervals of the expected trajectories of individual animals within these groups for Orientation (1a), State Control (1b), and Motor Activity (1c). The mean trajectories for each group are displayed using bold lines and 95% intervals of the within-group, between-animal differences in change are displayed by the shaded areas. Estimates are based on the validation sample. The variances of the random intercept and slope correspond to the between-animal variability in the factor scores at 7 days of age and in the linear rates of change, respectively. Assuming that the random effects are normally distributed, then approximately 95% of the individual intercepts and slopes are expected to range about their respective mean values by ± 1.96*SD of the corresponding random effect. For instance, the mean intercept of Orientation (1a) for nursery-reared animals was equal to 0.35 and the SD of the random intercept was 0.41. It follows that approximately 95% of intercepts for nursery-reared animals are expected to range from 0.35 ± 1.96*0.41 or −0.45 to 1.15. These values are shown for each of the three factors by the shaded areas. The lightest shading represents expected animal-level trajectories for the mother-reared animals and the darkest shading represents expected trajectories for the nursery-reared animals. The overlap between groups is represented by the medium shade of gray. As shown, there is overlap between groups in the expected range of the individual-level trajectories for each other the three factors. Thus, even though there were statistically significant differences in the mean factor scores between groups, there was considerable overlap in the expected trajectories of the individual animals.
Discussion
Our analyses of the largest-to-date sample of rhesus macaques further validated and calibrated the IBAS scale for use with rhesus macaque neonates. The large sample size (N=1056) allowed us to perform both exploratory and confirmatory factor analyses, which resulted in three robust factors: Orientation (Factor 1), State Control (Factor 2), and Motor Activity (Factor 3). Compared to previous factor analyses with much smaller sample sizes (N=23, Schneider et al., 1991; N=413, Coe et al., 2010; N=542, Kay et al., 2010), there was nonetheless surprising overlap in loadings of Orientation and State Control factors, and, perhaps to a lesser degree, the Motor Activity factor between all studies. Kay et al. (2010) found similar factors in 7 day old rhesus macaque infants, which also resemble those of the three factor model of human infant temperament. Schneider et al. (1991) differentiated between Motor Maturity and Activity, which did not emerge in the present analyses. Coe et al. (2010) obtained a fourth factor, labeled Sensory Sensitivity; none of the variables loading onto this factor were deemed meaningful in the current analyses (with the exception of Vocalization, which in the current analysis as well as Coe et al.’s (2010) analyses also loaded onto the State Control factor). Thus, we recognize all three factors as the most common and reliable constructs of the rhesus monkey IBAS scale.
It is also of interest that only 19 of the original 46 items were deemed meaningful in the construct of these factors. It may be tempting to therefore reduce the number of test items altogether in order to make the assessment faster, more streamlined, and thereby resulting in less stress to rhesus monkey neonates. However, items that did not contribute to the three factors may still be of interest to individual research studies. For example, in human infant studies individual items of the NBAS have been used to study neurobehavioral conditions in preterm infants (Alvarez-Garcia, Fornieles-Deu, Costas-Moragas, & Botet-Mussons, 2015) or the effects of the haemoconcentration on neonatal behavior (Aranda, Hernández-Martínez, Arija, Ribot, & Canals, 2017). Furthermore, some items that loaded onto the three factors, particularly those related to State Control, are assessed at the end of the test battery and evaluate the infants’ behavior throughout the test (e.g. Irritability, Consolability). Changing the structure and length of the test items may reduce the opportunities examiners have to evaluate infants on these items and introduce artificial bias to the assessment. Care should therefore be taken before considering dropping any individual test items from the test battery.
Similar to previous studies (Schneider & Suomi, 1992), the means of all three factors showed an increase over time, meaning that over the first month of life infant rhesus macaques improved in Orientation, Motor Activity, and State Control. This change is likely related to the maturation of the infants’ visual (Ordy, Latanick, Samorajski, & Massopust, 1964) and motoric (Armand, Olivier, Edgley, & Lemon, 1997) systems, as well as an increasing ability to self-sooth and self-calm. However, there were also individual differences in the linear rates of change for Orientation and State Control, but not Motor Activity. While this finding may suggest that in healthy infant macaques, postnatal motor maturation proceeds in a predictable pattern and is undisturbed by either genetic or environmental variables, others have found that stress levels during gestation can significantly affect motor development (Schneider, 1992). Maturation of Orientation and State Control appear to similarly be subject to either genetic (Champoux et al., 2002) and/or environmental (Sackett, 1972) influences, which will require further clarification in future studies.
Looking in more detail at variables that may affect neuromotor development, we found no significant effects of infant sex on any factor at 1 week old or over the first month of life. A similar lack of sex differences on the IBAS has been reported for squirrel monkey neonates (Schneider & Coe, 1993) and for a previous study on rhesus neonates (Schneider et al., 1991). In contrast, Braun et al. (2015) report that female marmosets display significantly more aggression than male marmosets at day 30 of age, and Coe et al. (2010) found that female rhesus macaques are more reactive (lower State Control) than males at 14 days of age. Human male infants are often regarded as being more vulnerable (Geschwind & Galaburda, 1985), showing higher rates of disordered regulation (Degangi, Dipietro, Greenspan, & Porges, 1991) and lower apgar scores (Singer, Westphal, & Niswander, 1968), and rhesus infants exhibit similar trends, with males reared in isolation being more aggressive, less exploratory, more stereotyped (Sackett, 1972), and being more affected by pregnancy manipulations than females (Coe et al., 2010). However, these sex differences are not universal and depend on the experimental condition employed (Morse, Beard, Azar, & Jones, 1999). While rhesus males may be more vulnerable to developmental difficulties, these susceptibilities were not apparent in the current sample. Still, latent effects such as increased risk of psychopathology in humans (Brown, 2006) or dysregulated physiology and poorer emotion regulation in rhesus monkeys (Weinstein & Capitanio, 2008; Capitanio, Mendoza, Mason, & Maninger, 2005) may persist.
Furthermore, we observed several effects of rearing condition on all three factors. Previous factor analyses of the IBAS limited the sample population to either only nursery-reared (Schneider et al., 1991), only mother-reared rhesus infants (Coe et al., 2010), or did not take rearing effects into account (Kay et al., 2010), although differences according to various forms of environmental enrichment have been previously described (Schneider et al., 1991). At 1 week of age, nursery-reared animals scored higher on Orientation and lower on both State Control and Motor Activity compared to mother-reared animals. Differences in test performance according to rearing condition may reflect differences brought about by the test conditions themselves as mother-reared animals, unlike nursery-reared animals, were not used to being handled by human caretakers. In addition, nursery-reared infants were more likely to have experienced additional behavioural experimental procedures (e.g. Nelson et al., 2011; Paukner, Simpson, Ferrari, Mrozek, & Suomi, 2014; Vanderwert et al., 2012), which may have been stressful to infants. Alternatively, nursery-rearing in rhesus macaques (without a mother as a consistent attachment figure) has been shown to lead to poor emotional and cognitive development, including poor socialization skills in adulthood (Corcoran et al., 2012; Gilmer & McKinney, 2003; Machado & Bachevalier, 2003), paralleling many features of affective disorders shown by human infants with early adverse experience and thus making rhesus macaques a good model for socio-affective development (Sclafani, Paukner, Suomi, & Ferrari, 2015). The observed differences at 1 week of age suggest that these changes may already occur after only a relatively brief period of time and during an age when infants may be particularly vulnerable, making nursery-reared animals more vigilant, more reactive, and perhaps more fearful (resulting in an increased freeze response; Kalin & Shelton, 1998). While rearing did not appear to affect Motor Activity over time, nursery-rearing influenced the developmental trajectory of both Orientation and State Control with nursery-reared animals increasing their Orientation scores over time but not their State Control scores, suggesting that they remained more vigilant than mother-reared animals and had more difficulties to self-sooth under test conditions. Both propensities further emphasize that nursery-reared animals’ developmental trajectories pre-expose them to heightened levels of cognitive and emotional deficiencies, making them ideal models to investigate how to mitigate and reverse these effects through behavioral (Sclafani et al., 2015) or pharmacological interventions (Simpson et al., 2014).
In conclusion, the IBAS for rhesus macaque neonates remains an important and valuable tool to assess neurobehavioral development in a widely-used animal model. The current analyses validated three robust factors (Orientation, State Control, and Motor Activity) and described their development over the first month of life, taking into account infant sex and rearing condition. Future studies should focus on the long-term implications of these initial behavioral tendencies, the stability of these traits throughout infancy and juvenility, and how to potentially stage interventions to reverse suboptimal trajectories.
Supplementary Material
Acknowledgements
This research was supported by the Division of Intramural Research, NICHD. We thank the technicians who looked after all animals and collected the data. The authors declare no competing interest. The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Als H, Tronick E, Lester BM, & Brazelton TB. (1977). The Brazelton neonatal behavioral assessment scale (BNBAS). Journal of Abnormal Child Psychology, 5(3), 215–229. DOI: 10.1007/BF00913693 [DOI] [PubMed] [Google Scholar]
- Alvarez-Garcia A, Fornieles-Deu A, Costas-Morgas C, & Botet-Mussons F. (2015). Neurobehavioral conditions and effects of gender, weight and severity in preterm infants according to the Neonatal Behavioral Assessment Scale. Anales De Psicología/Annals of Psychology 31(3), 818–824. DOI: 10.6018/analesps.31.3.170181 [DOI] [Google Scholar]
- Aranda N, Hernández-Martínez C, Arija V, Ribot B, & Canals J. (2017). Haemoconcentration risk at the end of pregnancy: effects on neonatal behaviour. Public Health Nutrition, 20(8), 1405–1413. DOI: 10.1017/S136898001600358X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand J, Olivier E, Edgley SA, & Lemon RN. (1997). Postnatal development of corticospinal projections from motor cortex to the cervical enlargement in the macaque monkey. Journal of Neuroscience, 17(1), 251–266. DOI: 10.1523/JNEUROSCI.17-01-00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asparouhov T, & Muthén B. (2009). Exploratory structural equation modeling. Structural Equation Modeling: A Multidisciplinary Journal, 16(3), 397–438. DOI: 10.1080/10705510903008204 [DOI] [Google Scholar]
- Baker M, Lindell SG, Driscoll CA, Zhou Z, Yuan Q, Schwandt ML, … & Sindhu RK. (2017). Early rearing history influences oxytocin receptor epigenetic regulation in rhesus macaques. Proceedings of the National Academy of Sciences, 114(44), 11769–11774. DOI: 10.1073/pnas.1706206114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard KA, Platzman KA, Lester BM, & Suomi SJ. (1992). Orientation to social and nonsocial stimuli in neonatal chimpanzees and humans. Infant Behavior and Development, 15(1), 43–56. DOI: 10.1016/0163-6383(92)90005-Q [DOI] [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D’udine B, Foley RA, … & McNamara J. (2004). Developmental plasticity and human health. Nature, 430(6998), 419 DOI: 10.1038/nature02725 [DOI] [PubMed] [Google Scholar]
- Brazelton TB. (1973). Neonatal behavioral assessment scale. Clinics in Developmental Medicine, No 50, London: William Heinemann Medical Books; Philadelphia: J.B. Lippincott. [Google Scholar]
- Brazelton TB, Koslowski B, & Tronick E. (1976). Neonatal behavior among urban Zambians and Americans. Journal of the American Academy of Child Psychiatry, 15(1), 97–107. DOI: 10.1016/S0002-7138(09)62263-9 [DOI] [PubMed] [Google Scholar]
- Brazelton TB, & Nugent JK. (1995). Neonatal behavioral assessment scale (No. 137). Cambridge University Press. [Google Scholar]
- Braun K, Schultz‐Darken N, Schneider M, Moore CF, & Emborg ME. (2015). Development of a novel postnatal neurobehavioral scale for evaluation of common marmoset monkeys. American Journal of Primatology, 77(4), 401–417. DOI: 10.1002/ajp.22356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazelton TB, Robey JS, & Collier GA. (1969). Infant development in the Zinacanteco Indians of southern Mexico. Pediatrics, 44(2), 274–290. [PubMed] [Google Scholar]
- Brown AS. (2006). Prenatal infection as a risk factor for schizophrenia. Schizophrenia Bulletin, 32(2), 200–202. DOI: 10.1093/schbul/sbj052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, & Maninger N. (2005). Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta). Developmental Psychobiology, 46, 318–330. DOI: 10.1002/dev.20067 [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, & Suomi SJ. (2002). Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Molecular Psychiatry, 7(10), 1058 DOI: 10.1038/sj.mp.4001157 [DOI] [PubMed] [Google Scholar]
- Champoux M, Suomi SJ, & Schneider ML. (1994). Temperament differences between captive Indian and Chinese-Indian hybrid rhesus macaque neonates. Laboratory Animal Science, 44(4), 351–357. [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Crispen HR, Shirtcliff EA, & Schneider ML. (2010). Challenges to maternal wellbeing during pregnancy impact temperament, attention, and neuromotor responses in the infant rhesus monkey. Developmental Psychobiology, 52(7), 625–637. DOI: 10.1002/dev.20489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran CA, Pierre PJ, Haddad T, Bice C, Suomi SJ, Grant KA, … & Bennett AJ. (2012). Long‐term effects of differential early rearing in rhesus macaques: Behavioral reactivity in adulthood. Developmental Psychobiology, 54(5), 546–555. DOI: 10.1002/dev.20613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello AB, & Osborne JW. (2005). Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Practical Assessment Research & Evaluation, 10(7). Available online: http://pareonline.net/getvn.asp?v=10&n=7 [Google Scholar]
- Degangi GA, Dipietro JA, Greenspan SI, & Porges SW. (1991). Psychophysiological characteristics of the regulatory disordered infant. Infant Behavior and Development, 14(1), 37–50. DOI: 10.1016/0163-6383(91)90053-U [DOI] [Google Scholar]
- Dettmer AM, Ruggiero AM, Novak MA, Meyer JS, & Suomi SJ. (2008). Surrogate mobility and orientation affect the early neurobehavioral development of infant rhesus macaques (Macaca mulatta). Developmental Psychobiology, 50(4), 418–422. DOI: 10.1002/dev.20296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrigar LR, Wegener DT, MacCallum RC & Strahan EJ. (1999). Evaluating the use of exploratory factor analysis in psychological research. Psychological Methods, 4, 272–299. DOI: 10.1037/1082-989X.4.3.272 [DOI] [Google Scholar]
- Ferrari PF, Paukner A, Ruggiero A, Darcey L, Unbehagen S, & Suomi SJ. (2009). Interindividual differences in neonatal imitation and the development of action chains in rhesus macaques. Child Development, 80(4), 1057–1068. DOI: 10.1111/j.1467-8624.2009.01316.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, & Galaburda AM. (1985). Cerebral lateralization: Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Archives of Neurology, 42(5), 428–459. DOI: 10.1001/archneur.1985.04060050026008 [DOI] [PubMed] [Google Scholar]
- Gilmer WS, & McKinney WT. (2003). Early experience and depressive disorders: human and non-human primate studies. Journal of Affective Disorders, 75(2), 97–113. DOI: 10.1016/S0165-0327(03)00046-6 [DOI] [PubMed] [Google Scholar]
- Hallock MB, Worobey J, & Self PA. (1989). Behavioural development in chimpanzee (Pan troglodytes) and human newborns across the first month of life. International Journal of Behavioral Development, 12(4), 527–540. DOI: 10.1177/016502548901200408 [DOI] [Google Scholar]
- Hawthorne J. (2005). Using the Neonatal Behavioural Assessment Scale to support parent-infant relationships. Infant, 1(6), 213–218. [Google Scholar]
- Kalin NH, & Shelton SE. (1998). Ontogeny and stability of separation and threat‐induced defensive behaviors in rhesus monkeys during the first year of life. American Journal of Primatology, 44(2), 125–135. DOI: [DOI] [PubMed] [Google Scholar]
- Kay DB, Marsiske M, Suomi SJ, & Higley JD. (2010). Exploratory factor analysis of human infant temperament in the rhesus monkey. Infant Behavior & Development, 33(1), 111–114. DOI: 10.1016/j.infbeh.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, ‘t Hart BA, … & Nathanielsz PW. (2014). Why primate models matter. American Journal of Primatology, 76(9), 801–827. DOI: 10.1002/ajp.22281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, & Bachevalier J. (2003). Non‐human primate models of childhood psychopathology: the promise and the limitations. Journal of Child Psychology and Psychiatry, 44(1), 64–87. DOI: 10.1111/1469-7610.00103 [DOI] [PubMed] [Google Scholar]
- Makwana SC. (1978). Field ecology and behaviour of the rhesus macaque (Macaca mulatta): I. Group composition, home range, roosting sites, and foraging routes in the Asarori Forest. Primates, 19(3), 483–492. DOI: 10.1007/BF02373310 [DOI] [Google Scholar]
- Morse AC, Beard JL, Azar MR, & Jones BC. (1999). Sex and genetics are important cofactors in assessing the impact of iron deficiency on the developing mouse brain. Nutritional Neuroscience, 2(5), 323–335. DOI: 10.1080/1028415X.1999.11747287 [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO. (1998–2017). Mplus User’s Guide. Eighth Edition. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Nelson EL, Emery MS, Babcock SM, Novak MF, Suomi SJ, & Novak MA. (2011). Head orientation and handedness trajectory in rhesus monkey infants (Macaca mulatta). Developmental Psychobiology, 53(3), 246–255. DOI: 10.1002/dev.20517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordy JM, Latanick A, Samorajski T, & Massopust LC. (1964). Visual acuity in newborn primate infants. Experimental Biology and Medicine, 115, 677–680. DOI: 10.3181/00379727-115-29004 [DOI] [PubMed] [Google Scholar]
- Paukner A, Simpson EA, Ferrari PF, Mrozek T, & Suomi SJ. (2014). Neonatal imitation predicts how infants engage with faces. Developmental Science, 17(6), 833–840. DOI: 10.1111/desc.12207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provençal N, Suderman MJ, Guillemin C, Massart R, Ruggiero A, Wang D, … & Hallett M. (2012). The signature of maternal rearing in the methylome in rhesus macaque prefrontal cortex and T cells. Journal of Neuroscience, 32(44), 15626–15642. DOI: 10.1523/JNEUROSCI.1470-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees S, Harding R, & Walker D. (2008). An adverse intrauterine environment: implications for injury and altered development of the brain. International Journal of Developmental Neuroscience, 26(1), 3–11. DOI: 10.1016/j.ijdevneu.2007.08.020 [DOI] [PubMed] [Google Scholar]
- Sackett GP. (1972). Exploratory behavior of rhesus monkeys as a function of rearing experiences and sex. Developmental Psychology, 6(2), 260 DOI: 10.1037/h0032081 [DOI] [Google Scholar]
- Sackett G, Ruppenthal G, Hewitson L, Simerly C, & Schatten G. (2006). Neonatal behavior and infant cognitive development in rhesus macaques produced by assisted reproductive technologies. Developmental Psychobiology, 48(3), 243–265. DOI: 10.1002/dev.20132 [DOI] [PubMed] [Google Scholar]
- Schneider ML. (1992). The effect of mild stress during pregnancy on birthweight and neuromotor maturation in rhesus monkey infants (Macaca mulatta). Infant Behavior and Development, 15(4), 389–403. DOI: 10.1016/0163-6383(92)80009-J [DOI] [Google Scholar]
- Schneider ML, & Coe CL. (1993). Repeated social stress during pregnancy impairs neuromotor development of the primate infant. Journal of Developmental and Behavioral Pediatrics, 14, 81–87. DOI: 10.1097/00004703-199304000-00002 [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Suomi SJ, & Champoux M. (1991). Laboratory assessment of temperament and environmental enrichment in rhesus monkey infants (Macaca mulatta). American Journal of Primatology, 25(3), 137–155. DOI: 10.1002/ajp.1350250302 [DOI] [PubMed] [Google Scholar]
- Schneider ML, Roughton EC, & Lubach GR. (1997). Moderate alcohol consumption and psychological stress during pregnancy induce attention and neuromotor impairments in primate infants. Child Development, 68(5), 747–759. DOI: 10.1111/j.1467-8624.1997.tb01959.x [DOI] [PubMed] [Google Scholar]
- Schneider ML, & Suomi SJ. (1992). Neurobehavioral assessment in rhesus monkey neonates (Macaca mulatta): developmental changes, behavioral stability, and early experience. Infant Behavior and Development, 15(2), 155–177. DOI: 10.1016/0163-6383(92)80021-L [DOI] [Google Scholar]
- Schneper LM, Brooks-Gunn J, Notterman DA, & Suomi SJ. (2016). Early life experiences and telomere length in adult rhesus monkeys: An exploratory study. Psychosomatic Medicine, 78(9), 1066 DOI: 10.1097/PSY.0000000000000402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani V, Paukner A, Suomi SJ, & Ferrari PF. (2015). Imitation promotes affiliation in infant macaques at risk for impaired social behaviors. Developmental Science, 18(4), 614–621. DOI: 10.1111/desc.12237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JE, Westphal M, & Niswander KR. (1968). Sex differences in the incidence of neonatal abnormalities and abnormal performance in early childhood. Child Development, 39(1), 103–112. DOI: 10.2307/1127362 [DOI] [PubMed] [Google Scholar]
- Simpson EA, Miller GM, Ferrari PF, Suomi SJ, & Paukner A. (2016). Neonatal imitation and early social experience predict gaze following abilities in infant monkeys. Scientific Reports, 6, 20233 DOI: 10.1038/srep20233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EA, Sclafani V, Paukner A, Hamel AF, Novak MA, Meyer JS, Suomi SJ, & Ferrari PF. (2014). Inhaled oxytocin increases positive social behaviors in newborn macaques. Proceedings of the National Academy of Sciences, 111(19), 6922–6927. DOI: 10.1073/pnas.1402471111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwert RE, Ferrari PF, Paukner A, Bower SB, Fox NA, & Suomi SJ. (2012). Spectral characteristics of the newborn rhesus macaque EEG reflect functional cortical activity. Physiology & Behavior, 107(5), 787–791. DOI: 10.1016/j.physbeh.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J, Kim CK, & Yu W. (1995). Early handling can attenuate adverse effects of fetal ethanol exposure. Alcohol, 12(4), 317–32. DOI: 10.1016/0741-8329(95)00005-C [DOI] [PubMed] [Google Scholar]
- Weinstein TA, & Capitanio JP. (2008). Individual differences in infant temperament predict social relationships of yearling rhesus monkeys, Macaca mulatta. Animal Behaviour, 76(2), 455–465. DOI: 10.1016/j.anbehav.2008.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.