Abstract
Objective:
Rates of psychiatric comorbidity are elevated in adolescents with anorexia nervosa, but little is known about how psychiatric comorbidity changes following family-based treatment (FBT).
Methods:
Adolescents with anorexia nervosa (N = 107) enrolled in a randomized controlled trial comparing two forms of FBT completed the Mini International Neuropsychiatric Interview for Children and Adolescents at baseline and end of treatment. Analyses tested whether baseline comorbid diagnoses predicted the presence of comorbid diagnoses at end of treatment and if baseline eating disorder psychopathology impacted this association.
Results:
Rates of comorbid diagnoses decreased from 54% at baseline to 26% at end of treatment. Logistic regression analyses indicated that individuals with multiple comorbid diagnoses at baseline were more likely to meet criteria for a comorbid condition at end of treatment (b = 2.00, p < .05). Individuals with reported psychotropic medication use were less likely to meet criteria for a comorbid condition at end of treatment (b = −1.63, p = .04). Diagnostic rates for major depressive disorder, generalized anxiety disorder, and panic disorder/agoraphobia decreased following FBT.
Conclusions:
Findings suggest that FBT for adolescent anorexia nervosa may aid in the resolution of some co-occurring psychiatric diagnoses. Continued research is needed to understand factors contributing to comorbid symptom improvement throughout treatment.
Keywords: adolescents, anorexia nervosa, family-based treatment, psychiatric comorbidity, recovery
1 |. INTRODUCTION
Anorexia nervosa (AN) is a debilitating psychiatric illness that is associated with high rates of mortality, morbidity, and health care costs when compared to other psychiatric illnesses (Bühren et al., 2014; Salbach-Andrae et al., 2008; Schaumberg et al., 2017). Comorbid psychiatric conditions are common in AN and are associated with more severe eating disorder (ED) psychopathology, increased suicidality, and hospitalization (Brand-Gothelf, Leor, Apter, & Fennig, 2014; Bühren et al., 2014; Fennig & Hadas, 2010), as well as ongoing psychiatric problems requiring continuing treatment (Hjern, Lindberg, & Lindbald, 2006).
Between one half and two thirds of adolescents with AN have a comorbid psychiatric diagnosis (Bühren et al., 2014; Salbach-Andrae et al., 2008). Among the most common is major depressive disorder (MDD) ranging from 31.8% to 85.6% (Bühren et al., 2014; Carrot et al., 2017), with MDD predating the ED in nearly half of all cases (Carrot et al., 2017). Reported rates of comorbid obsessive–compulsive disorder (OCD) range from 3.4% to 44.7% for girls and 35.7% for boys (Strober, Freeman, Lampert, & Diamond, 2007). One study found that 51% of adolescents with AN experienced clinically significant levels of OCD symptoms. In that study, OCD was premorbid to AN in 47.1% of these adolescents and contributed to poorer outcome at follow-up (Carrot et al., 2017). Adolescents with AN also present with significant rates of comorbid generalized anxiety disorder (GAD) and social anxiety disorder (SAD) (Bühren et al., 2014; Salbach-Andrae et al., 2008).
Specialized treatment is recommended for AN, for which more than three decades of research supports the efficacy of a specific family-focused approach for adolescents, that is, family-based treatment (FBT) or family therapy for AN (FT-AN; Eisler et al., 1997; Le Grange et al., 2016; Lock et al., 2010; Russell, Szmukler, Dare, & Eisler, 1987). Although research supports the efficacy of a focused family intervention for adolescents with AN, little is known about the impact of this treatment on comorbid psychiatric conditions. There is evidence that depressive symptoms improve after FBT for adolescents with AN, perhaps in part due to improved nutrition and weight gain during FBT (Accurso, Ciao, Fitzsimmons-Craft, Lock, & Le Grange, 2014; Bean, Louks, Kay, Cornella-Carlson, & Weltzin, 2010; Le Grange et al., 2016). Similarly, increases in positive affect and decreases in negative affect are evident after both FBT and a separated format of FBT, parent-focused treatment (PFT; Murray, Pila, Le Grange, Sawyer, & Hughes, 2017). While research by Accurso et al. (2014) and Murray et al. (2017) demonstrated meaningful improvement in symptoms associated with comorbid conditions following FBT for adolescent AN, these authors did not examine differences in rates of formally diagnosed comorbid conditions.
A recent randomized clinical trial (RCT) of FBT for adolescent AN (Le Grange et al., 2016) provides an opportunity to investigate changes in psychiatric comorbidity given that standardized clinical interviews were utilized at baseline and end of treatment. We hypothesized that fewer participants would meet diagnostic criteria for comorbid diagnoses at the end of treatment than at baseline. Given evidence that the severity of baseline ED pathology is associated with psychiatric comorbidity (Bühren et al., 2014) and psychiatric severity is positively associated with eating pathology at follow-up (Herpertz-Dahlmann et al., 2001), we also examined baseline ED severity as a potential moderating variable. We hypothesized that those with greater baseline ED psychopathology would be more likely to have a comorbid diagnosis at end of treatment and that the association between baseline and end-of-treatment comorbidity would be stronger among those with elevated baseline ED pathology.
2 |. METHODS
2.1 |. Participants and procedure
A detailed description of the study sample can be found in the main outcome report (c.f., Le Grange et al., 2016). Participants were 107 adolescents (ages 12–18 years) who met criteria for DSM-IV AN (excluding amenorrhea) or ED not otherwise specified, ENDOS-AN (i.e., individuals who experienced cognitive symptoms of AN and were ≤90% median body mass index [mBMI] for adolescents ≤75th percentile for height or < 95% mBMI for adolescents ≥75th percentile for height), and their families. A small subset of the 107 patients who enrolled in the trial never began treatment following baseline assessment (n = 3), and an additional group of patients (n = 9) withdrew from the study prior to Session 9 (for more information regarding attrition procedures, see Le Grange et al., 2016). These 12 individuals, who did not differ from the final sample on measures of baseline psychiatric comorbidity, ED psychopathology, psychotropic medication use, age, percentage of median BMI (%mBMI), or duration of illness (all ps > .05), were excluded from analyses to yield a final sample of 95 adolescents. Families were randomized to receive either conjoint FBT or PFT and were allotted 18 sessions over 6 months in the research study. Assessments were administered at baseline, end of treatment, and at further follow-up time points (the latter are not included in these analyses).
The Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-Kid; Sheehan et al., 2010), a semi-structured clinical interview, was used to determine the diagnosis of comorbid psychiatric disorders. The Eating Disorder Examination (EDE; Fairburn, Cooper, & O’Connor, 1993), another semi-structured clinical interview, was used to determine the severity of ED psychopathology in the 4 weeks prior to treatment. Research coordinators administered the MINI-Kid and EDE to participants at baseline and end of treatment. Participants were weighed in a gown using a calibrated digital scale.
2.2 |. Analytical plan
All baseline diagnoses were coded categorically such that 0 = no comorbid diagnoses, 1 = one comorbid diagnosis, and 2 = two or more comorbid diagnoses. End-of-treatment diagnoses were coded such that 0 = no comorbid diagnosis, and 1 = one or more comorbid diagnoses. A logistic regression tested the main effects of baseline comorbid diagnoses and baseline ED psychopathology (i.e., EDE global score) on end-of-treatment diagnosis status. The model also tested for moderation effects of baseline ED psychopathology on comorbidity outcome. Psychotropic medication usage was entered into the model as a covariate, as was type of FBT. Additional logistic regressions explored the main effects of individual baseline diagnoses on the presence or absence of the same diagnosis, for example, baseline MDD diagnosis predicting end-of-treatment MDD diagnosis. These models included psychotropic medication use as a covariate. As treatment type was not a significant covariate in the main model, it was not included in further analyses. Due to low numbers of participants meeting criteria for OCD, SAD, and substance use disorder at baseline, further analyses did not include these diagnoses.
3 |. RESULTS
Demographic characteristics (n = 95) are shown in Table 1. Participants received an average of 16.02 treatment sessions (SD = 2.93) over an average of 21.49 weeks (SD = 4.58). Psychotropic medication use was 7.4% at baseline and 33.7% at end of treatment. Of note, all medications were prescribed by a psychiatrist. At baseline, 51 (53.7%) participants met criteria for at least one comorbid diagnosis. By the end of treatment, this number had reduced to 25 (26.3%). Of these 25 individuals, four did not meet criteria for a comorbid diagnosis at baseline, but did meet criteria at end of treatment for varied diagnoses. The rates of specific diagnoses at baseline and end of treatment are shown in Figure 1.
TABLE 1.
Participant demographics at baseline and rates of comorbid diagnoses at baseline and end of treatment
| n (%) | M (SD) | |
|---|---|---|
| Sex | ||
| Female | 82 (86.3) | |
| Male | 13 (13.7) | |
| Age (years) | 15.81 (1.46) | |
| BMI | 16.50 (1.32) | |
| %mBMI | 81.98 (6.06) | |
| Duration of Illness (months) | 10.45 (9.83) | |
| Diagnosis | ||
| AN-restricting type | 73 (76.8) | |
| AN-binge purge type | 1 (1.1) | |
| Eating disorder not otherwise specified, AN | 21 (22.1) | |
| Rates of comorbid diagnoses | Baseline n (%) | End of treatment n (%) |
| Any diagnosis | 51 (53.7) | 25 (26.3) |
| Major depressive disorder | 39 (41.1) | 16 (16.8) |
| Panic disorder/agoraphobia | 18 (18.9) | 12 (12.6) |
| Generalized anxiety disorder | 18 (18.9) | 13 (13.7) |
| Obsessive compulsive disorder | 9 (9.5) | 8 (8.4) |
| Social anxiety disorder | 9 (9.5) | 4 (4.2) |
Note: %mBMI = percent of median body mass index.
Abbreviations: AN, anorexia nervosa; BMI, body mass index; SD, standard deviation.
FIGURE 1.
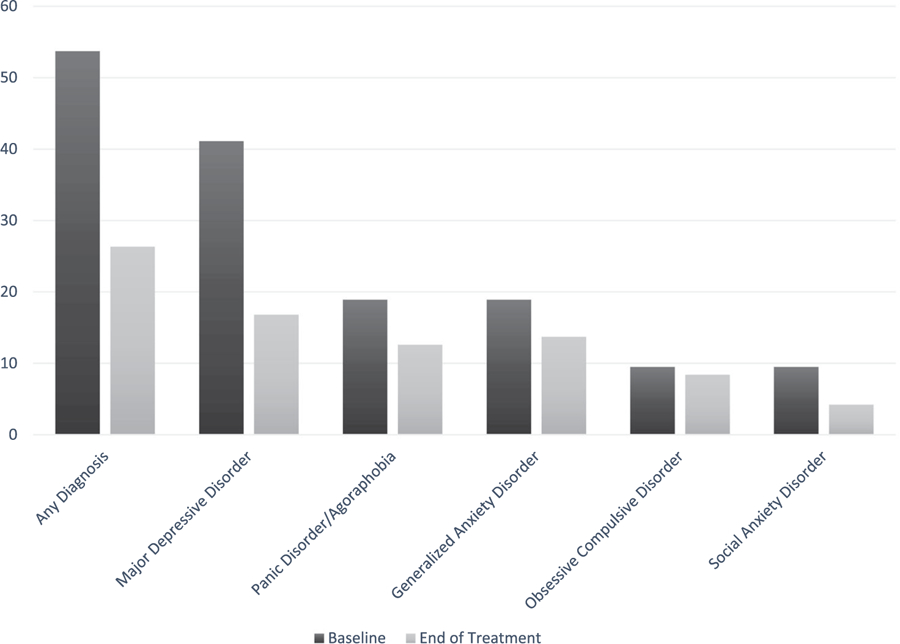
Rates of comorbid diagnoses at baseline and end of treatment
3.1 |. Regression analyses
There were significant main effects of baseline diagnosis (b = 2.00, p > .05), indicating that having a greater number of baseline comorbid diagnoses was predictive of still having a comorbid psychiatric condition at end of treatment. Main effects of baseline ED pathology on end-of-treatment comorbidities were not significant and neither was the interaction of baseline comorbid diagnoses and baseline ED pathology. Medication use while in treatment was a significant covariate (b = −1.63, p = .04), such that those who reported medication use at baseline or end of treatment were less likely to meet criteria for a comorbid diagnosis at end of treatment (see Table 2).
TABLE 2.
Binary logistic regression analyses predicting the presence of posttreatment comorbid diagnoses in patients with AN (n = 95)
| B | SE | Wald | p | OR | 95% CI | |
|---|---|---|---|---|---|---|
| Baseline comorbidity | 2.00 | 1.02 | 3.79 | .049 | 7.40 | 1.01, 54.30 |
| Baseline EDE global | 0.56 | 0.43 | 1.69 | .194 | 1.75 | 0.75, 4.08 |
| Baseline comorbidity × Baseline EDE global | −0.31 | 0.30 | 1.08 | .298 | 0.73 | 0.41, 1.31 |
| Major depressive disorder | −0.82 | 0.75 | 1.50 | .221 | 0.40 | 0.09, 1.74 |
| Panic disorder/Agoraphobia | −1.80 | 0.82 | 4.81 | .028 | 0.17 | 0.03, 0.83 |
| Generalized anxiety disorder | −1.76 | 0.77 | 5.27 | .022 | 0.17 | 0.04, 0.77 |
Note: Baseline comorbidity is categorical: 0 = none, 1 = 1 diagnosis, 2 = 2 or more diagnoses; EDE global = Eating Disorder Examination global score;
End-of-treatment comorbidity is categorical: 0 = none, 1 = 1 or more.
Abbreviations: CI, confidence interval; EDE, Eating Disorder Examination; OR, odds ratio; SE, standard error.
Associations between baseline MDD diagnosis and likelihood of an end-of-treatment MDD diagnosis were not significant. However, these associations were significant for those with a baseline diagnosis of GAD (b = −1.76, p = .02) and panic disorder/agoraphobia (b = −1.80, p = .03), indicating that those who met criteria for one of these disorders at baseline were less likely to meet criteria at end of treatment. Only for GAD was medication use at any time in treatment a significant covariate (b = −1.82, p = .02), indicating that those reporting medication use were less likely to meet criteria for GAD at end of treatment. Medication use was not a significant covariate for an end-of-treatment MDD or panic/agoraphobia diagnosis.
4 |. DISCUSSION
The current study found a marked reduction in participants with a comorbid psychiatric disorder following FBT for AN (57.6% at baseline vs. 29% at end of treatment). Those with fewer baseline comorbidities or who reported psychotropic medication use were less likely to meet criteria for a comorbid disorder at the end of treatment. Rates of MDD, GAD, and panic/agoraphobia had reduced from baseline to end of treatment, with the extent of decrease differing notably by diagnostic category. Nearly half of those with a baseline diagnosis of MDD no longer met criteria at end of treatment, while around three quarters of those with panic/agoraphobia or GAD at baseline had a persisting diagnosis at end of treatment. Overall, these findings suggest that for a large proportion of adolescents, a number of comorbid psychiatric disorders improve following FBT.
Unlike some treatment for EDs, FBT does not explicitly focus on the cognitive components of the disorder nor does it directly target comorbidities. Rather, the emphasis in FBT is first to restore the patient to a healthy weight, as malnutrition makes it challenging to benefit from psychotropic medication (Kaye et al., 2001) and to engage in psychotherapy due to severity of AN cognitions. Further, previous research suggests that depression tends to result from starvation rather than a preexisting condition and often reverses with weight restoration (Hughes, 2012; Meehan, Loeb, Roberto, & Attia, 2006). This study did not assess the duration of MDD in relation to AN. Therefore, it is possible that within the sample, there were two separate, but closely related, groups of patients: those who experienced MDD prior to the onset of AN and those who developed MDD in the context of eating pathology. As such, it could be that MDD remitted in patients for whom MDD was secondary to malnutrition but persisted in those who had MDD prior to developing AN. Future lines of inquiry may consider directly assessing the duration of MDD prior to the onset of AN to determine whether there is a difference in post-FBT comorbid outcomes between these two groups.
In contrast, anxiety disorders frequently predate the onset of AN and may be a dispositional trait that is less likely to reverse with weight gain (Hughes, 2012). This might provide some explanation of the differential reduction observed in depressive and anxiety disorders in this sample. Alternatively, the different rates of recovery between MDD and anxiety disorders following 6 months of FBT in the current study raise the question of whether this might reflect a lagged effect of weight restoration on recovery of anxiety versus depression.
Of note, taking a psychotropic medication may also increase the likelihood of psychiatric condition remitting, such as in those with GAD. This may be unexpected given that clinical experience and evidence from existing literature suggest that psychiatric medications are less effective among those with EDs who are not weight restored (Holtkamp et al., 2005). However, in the context of FBT where weight restoration is the primary focus, particularly at the start of treatment, it may be that medication effects are expedited by weight gain early in treatment. As the current study focused on assessment of comorbidity at pretreatment and at the end of treatment, the data do not allow for determination of whether comorbidities remitted before or after beginning medication or whether individuals were weight restored at this time. Determining when comorbid improvement occurs, relative to weight restoration and medication use, warrants further inquiry.
While weight restoration may play a significant role in the improvement of comorbid symptoms and medication efficacy, it is also important to consider whether the mechanisms used to promote weight gain within FBT may influence comorbid conditions. Distress tolerance, behavioural activation, and increasing families’ and individuals’ understanding of the context of their health issues are the components of FBT that may additionally influence comorbid conditions.
While we examined differences in comorbid diagnostic rates at end of treatment, we did not examine whether AN outcomes (i.e., %mBMI and ED psychopathology) differed for those with persistent MDD versus GAD or panic disorder/agoraphobia. This is an important question in determining treatment recommendations following FBT. It is possible that some individuals who were weight restored and experienced a reduction in symptoms associated with AN continued to experience significant depressive symptoms following treatment, perhaps because they are in the subgroup of individuals for whom MDD predated the onset of AN. For these individuals, treatment for depression may be indicated post-FBT.
Treatment recommendations for those with GAD or panic disorder may be different. Although the current study did not examine changes in specific symptoms during treatment, one may hypothesize that for some individuals, anxiety symptoms increase throughout treatment as the ED is challenged. For these patients, the heightened anxiety symptoms may interfere with treatment for AN and even perhaps result in worse outcomes following FBT. In these cases, directly targeting anxiety symptoms in the context of FBT may be beneficial at reducing both anxiety and ED symptoms (Hildebrandt, Bacow, Markella, & Loeb, 2012).
Additionally, future research may explore whether comorbid diagnoses at end of treatment impact longer term outcomes for AN (i.e., 12 months post-FBT). Psychiatric treatment immediately following FBT may not only be beneficial for persisting depression and anxiety, but may also decrease the risk of ED relapse. Examination of rates of comorbidities at later time points, with and without additional treatment (e.g., cognitive behaviour therapy and medication) would be informative. As the presence of residual comorbidities appears to influence whether subsequent treatment is indicated, the current study suggests that formal clinical assessment of comorbidities at end of treatment may be helpful, both to determine what further treatment is necessary and to better understand how persistent comorbid diagnoses affect longer term recovery from AN.
4.1 |. Strengths and limitations
While past research has examined changes in comorbid mood symptoms following FBT (e.g., Accurso et al., 2014; Murray et al., 2017), this is the first study to examine changes in comorbid psychiatric diagnoses as assessed by clinical interviews at baseline and end of treatment. The sample size precluded a fuller examination of OCD, SAD, and substance use disorder. Although we were not able to establish at what time point during treatment any comorbid symptom changes occurred, prior work using the same clinical sample has shown linear changes in positive and negative affect throughout FBT (Murray et al., 2017). This suggests that improvement in affect may support a broader trajectory of recovery from comorbid disorders over the course of treatment. It should be noted that, by definition, comorbid diagnoses are categorical and do not capture more nuanced improvement in symptom severity. As such, it is possible that some individuals who met criteria at both baseline and end of treatment experienced a considerable reduction in comorbid symptoms that may have been clinically meaningful but not to the extent that they no longer met full criteria. Future research may consider including regular measures of symptom severity, in addition to formal measures of comorbid diagnoses, in an effort to gain a more nuanced understanding of comorbid symptom improvement in the context of specialized ED treatment.
Additionally, we were unable to determine the validity of an MDD diagnosis while participants were in a starvation state. In order to verify whether participants were experiencing a depressive episode independent of malnutrition, it would be necessary to establish the onset of MDD in relation to the onset of AN. Doing so would allow a deeper understanding of whether the MDD truly remits following FBT or if the symptoms that initially presented as MDD were a function of malnutrition.
A strength of this study is that it allows examination of rates of comorbid psychiatric diagnoses during FBT in the context of psychotropic medication use. Rates in this sample were similar to rates of medication use in other U.S. samples (Lock et al., 2010; Monge et al., 2015), suggesting the findings are generalizable to other research and clinical samples. At least for some patients, medication may be used to effectively manage psychiatric comorbidities during FBT for AN. As the current sample size was not adequately powered to test for interactions between baseline comorbidities, treatment type, and medication use, we did not assess for these interactions. Further, in the current study sample, we were unable to determine when individuals started medication and whether starting a course of medication was subsequently associated with either reduction in symptoms associated with comorbid disorders or weight restoration. We also did not examine long-term outcomes of comorbidities and engagement in treatment post-FBT. Future research is recommended to further explore the impact of these on improvement in comorbidities during and post-FBT for adolescent AN and to determine when medication use may be helpful in the treatment of comorbid disorders.
5 |. CONCLUSION
The current study indicates reduction in rates of comorbid psychiatric diagnoses following FBT, which was most pronounced in those with a baseline diagnosis of MDD but also for panic disorder/agoraphobia and GAD. These findings are encouraging given that FBT does not directly address comorbid conditions. Further, the trajectory of recovery for comorbid disorders may differ based on diagnosis. For clinicians providing FBT, assessment of comorbid disorders over the course of treatment and at end of treatment may be helpful in determining the value of specific treatment at the end of FBT.
Highlights.
Comorbid diagnoses decreased after family-based treatment (FBT) for adolescent eating disorders
Diagnostic rates for major depressive disorder, generalized anxiety disorder, and panic disorder/agoraphobia decreased following FBT.
Adolescents with reported psychotropic medication usage were less likely to meet criteria for a comorbid condition following FBT for adolescent eating disorders
ACKNOWLEDGMENTS
This research was funded by the Baker Foundation (Australia), which supported Dr Le Grange and Dr Hughes. The Murdoch Children’s Research Institute is supported by the Victorian Government’s Operational Infrastructure Support Program. Dr Gorrell is funded by the National Institutes of Health (T32MH0118261).
Dr Le Grange receives royalties from Guilford Press and Routledge and is the co-director of the Training Institute for Child and Adolescent Eating Disorders, LLC.
Funding information
Baker Foundation; National Institutes of Health, Grant/Award Number: T32MH0118261; Victorian Government; Baker Foundation (Australia)
REFERENCES
- Accurso EC, Ciao AC, Fitzsimmons-Craft EE, Lock JD, & Le Grange D. (2014). Is weight gain really a catalyst for broader recovery?: The impact of weight gain on psychological symptoms in the treatment of adolescent anorexia nervosa. Behaviour Research and Therapy, 56, 1–6. 10.1016/j.brat.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean P, Louks H, Kay B, Cornella-Carlson T, & Weltzin T. (2010). Clinical observations of the impact of maudsley therapy in improving eating disorder symptoms, weight, and depression in adolescents receiving treatment for anorexia nervosa. Journal of Groups in Addiction and Recovery, 5(1), 70–82. 10.1080/15560350903550142 [DOI] [Google Scholar]
- Brand-Gothelf A, Leor S, Apter A, & Fennig S. (2014). The impact of comorbid depressive and anxiety disorders on severity of anorexia nervosa in adolescent girls. The Journal of Nervous and Mental Disease, 202(10), 759–762. 10.1097/NMD.0000000000000194 [DOI] [PubMed] [Google Scholar]
- Bühren K, Schwarte R, Fluck F, Timmesfeld N, Krei M, Egberts K, … Herpertz-Dahlmann B. (2014). Comorbid psychiatric disorders in female adolescents with first-onset anorexia nervosa. European Eating Disorders Review, 22(1), 39–44. 10.1002/erv.2254 [DOI] [PubMed] [Google Scholar]
- Carrot B, Radon L, Hubert T, Vibert S, Duclos J, Curt F, & Godart N. (2017). Are lifetime affective disorders predictive of long-term outcome in severe adolescent anorexia nervosa? European Child & Adolescent Psychiatry, 26(8), 969–978. 10.1007/s00787-017-0963-5 [DOI] [PubMed] [Google Scholar]
- Eisler I, Dare C, Russell GF, Szmukler G, le Grange D, & Dodge E. (1997). Family and individual therapy in anorexia nervosa. A 5-year follow-up. Archives of General Psychiatry, 54 (11), 1025–1030. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z, & O’connor M. (1993). The Eating Disorder Examination (Twelfth Edition). In Fairburn GTWCG. (Ed.), Binge eating: Nature, assessment, and treatment (pp. 317–360). New York: Guilford Press. [Google Scholar]
- Fennig S, & Hadas A. (2010). Suicidal behavior and depression in adolescents with eating disorders. Nordic Journal of Psychiatry, 64(1), 32–39. 10.3109/08039480903265751 [DOI] [PubMed] [Google Scholar]
- Herpertz-Dahlmann B, Hebebrand J, Müller B, Herpertz S, Heussen N, & Remschmidt H. (2001). Prospective 10-year follow-up in adolescent anorexia nervosa—Course, outcome, psychiatric comorbidity, and psychosocial adaptation. Journal of Child Psychology and Psychiatry, 42(5), S0021963001007326 10.1017/S0021963001007326 [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Bacow T, Markella M, & Loeb KL. (2012). Anxiety in anorexia nervosa and its management using family-based treatment. European Eating Disorders Review, 20(1), e1–e16. 10.1002/erv.1071 [DOI] [PubMed] [Google Scholar]
- Hjern A, Lindberg L, & Lindbald F. (2006). Outcome and prognostic factors for adolescent female in-patients with anorexia nervosa: 9- to 14-year follow-up. British Journal of Psychiattry, 189, 428–432. Retrieved from. https://www-cambridge-org.ucsf.idm.oclc.org/core/services/aop-cambridge-core/content/view/F63488951AFDE22D48EAD32FC49A0353/S000712500023290Xa.pdf/div-class-title-outcome-and-prognostic-factors-for-adolescent-female-in-patients-with-anorexia-nervosa-9-to-4 [DOI] [PubMed] [Google Scholar]
- Holtkamp K, Konrad K, Kaiser N, Ploenes Y, Heussen N, Grzella I, & Herpertz-Dahlmann B. (2005). A retrospective study of SSRI treatment in adolescent anorexia nervosa: Insufficient evidence for efficacy. Journal of Psychiatric Research, 39(3), 303–310. 10.1016/j.jpsychires.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Hughes EK. (2012). Comorbid depression and anxiety in childhood and adolescent anorexia nervosa: Prevalence and implications for outcome. Clinical Psychologist, 16(1), 15–24. 10.1111/j.1742-9552.2011.00034.x [DOI] [Google Scholar]
- Kaye WH, Nagata T, Weltzin TE, Hsu LKG, Sokol MS, McConaha C, … Deep D. (2001). Double-blind placebo-controlled administration of fluoxetine in restricting- and restricting-purging-type anorexia nervosa. Biological Psychiatry2, 49(7), 644–652. [DOI] [PubMed] [Google Scholar]
- Le Grange D, Hughes EK, Court A, Yeo M, Crosby RD, & Sawyer SM. (2016). Randomized clinical trial of parent-focused treatment and family-based treatment for adolescent anorexia nervosa. Journal of the American Academy of Child & Adolescent Psychiatry, 55(8), 683–692. 10.1016/j.jaac.2016.05.007 [DOI] [PubMed] [Google Scholar]
- Lock J, Le Grange D, Agras WS, Moye A, Bryson SW, & Jo B. (2010). Randomized clinical trial comparing family-based treatment with adolescent-focused individual therapy for adolescents with anorexia nervosa. Archives of General Psychiatry, 67(10), 1025–1032. 10.1001/archgenpsychiatry.2010.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan KG, Loeb KL, Roberto CA, & Attia E. (2006). Mood change during weight restoration in patients with anorexia nervosa. International Journal of Eating Disorders, 39(7), 587–589. 10.1002/eat.20337 [DOI] [PubMed] [Google Scholar]
- Monge M, Forman S, McKenzie N, Rosen D, Mammel K, Callahan S, … Woods E. (2015). Use of psychopharmacologic medications in adolescents with restrictive eating disorders: Analysis of data from the National Eating Disorder Quality Improvement Collaborative. Journal of Adolescent Health, 57 (1), 66–72. [DOI] [PubMed] [Google Scholar]
- Murray SB, Pila E, Le Grange D, Sawyer SM, & Hughes EK. (2017). Symptom trajectories throughout two family therapy treatments for adolescent anorexia nervosa. International Journal of Eating Disorders, 50(11), 1323–1327. 10.1002/eat.22776 [DOI] [PubMed] [Google Scholar]
- Russell GF, Szmukler G, Dare C, & Eisler I. (1987). An evaluation of family therapy in anorexia nervosa and bulimia nervosa. Archives of General Psychiatry1, 44(12), 1047–1056. [DOI] [PubMed] [Google Scholar]
- Salbach-Andrae H, Klinkowski N, Lenz K, Pfeiffer E, Lehmkuhl U, & Ehrlich S. (2008). Correspondence between self-reported and parent-reported psychopathology in adoelscents with eating disorders. Psychopathology, 41, 307–312. 10.1159/000146068 [DOI] [PubMed] [Google Scholar]
- Schaumberg K, Welch E, Breithaupt L, Hübel C, Baker JH, Munn-Chernoff MA, … Bulik CM. (2017). The science behind the academy for eating disorders’ nine truths about eating disorders. European Eating Disorders Review: The Journal of the Eating Disorders Association, 25(6), 432–450. 10.1002/erv.2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, … Wilkinson B. (2010). Reliability and validity of the Mini International Neuropsychiatric Interview for children and adolescents (MINI-KID). The Journal of Clinical Psychiatry, 71(03), 313–326. 10.4088/JCP.09m05305whi [DOI] [PubMed] [Google Scholar]
- Strober M, Freeman R, Lampert C, & Diamond J. (2007). The association of anxiety disorders and obsessive compulsive personality disorder with anorexia nervosa: Evidence from a family study with discussion of nosological and neurodevelopmental implications. International Journal of Eating Disorders, 40(S3), S46–S51. 10.1002/eat.20429 [DOI] [PubMed] [Google Scholar]


