Abstract
Hypothalamic magnocellular neuroendocrine cells have unique electrical properties and a remarkable capacity for morphological and synaptic plasticity. Their large somatic size, their relatively uniform and dense clustering in the supraoptic and paraventricular nuclei, and their large axon terminals in the neurohypophysis make them an attractive target for direct electrophysiological interrogation. Here, we provide a brief review of significant recent findings in the neuroplasticity and neurophysiological properties of these neurons that were presented in the symposium “Electrophysiology of Magnocellular Neurons” at the 13th World Congress on Neurohypophysial Hormones in Ein Gedi, Israel in April 2019. Magnocellular vasopressin (VP) neurons respond directly to hypertonic stimulation with membrane depolarization, which is triggered by cell shrinkage-induced opening of N terminal-truncated TRPV1 channels. New findings indicate that this mechanotransduction depends on actin and microtubule cytoskeletal networks, and that direct coupling of the TRPV1 channels to microtubules is responsible for mechanical gating of the channels. Vasopressin neurons also respond to osmostimulation by activation of epithelial Na+ channels (ENaC). It was shown recently that changes in ENaC activity modulate magnocellular neuron basal firing by generating tonic changes in membrane potential. Both oxytocin and VP neurons also undergo robust excitatory synapse plasticity during chronic osmotic stimulation. Recent findings indicate that new glutamate synapses induced during chronic salt loading express highly labile Ca2+-permeable GluA1 receptors that require continuous dendritic protein synthesis for synapse maintenance. Finally, recordings from the uniquely tractable neurohypophysial terminals recently revealed an unexpected property of activity-dependent neuropeptide release. A significant fraction of the voltage-dependent neurohypophysial neurosecretion was found to be independent of Ca2+ influx through voltage-gated Ca2+ channels. Together, these findings provide a snapshot of significant new advances in the electrophysiological signaling mechanisms and neuroplasticity of the hypothalamic-neurohypophysial system, a system that continues to make important contributions to the field of neurophysiology.
Keywords: oxytocin, vasopressin, neuroplasticity, neurosecretion, osmoregulation
Introduction
The classically defined hypothalamic-neurohypophysial system consists of magnocellular vasopressin (VP)- and oxytocin (OT)-secreting neurons and their axonal projections to the neurohypophysis, the electrical activities of which are tightly coupled to the pattern and amount of neurohypophysial hormone release (1). The system’s well described physiological functions in regulating blood pressure, water and salt balance, and parturition/lactation, combined with the ability to precisely identify neuron types in vivo and in vitro both anatomically and electrophysiologically, have made it a model system for understanding neuropeptide-secreting neurons. Here, we describe recent significant advances in the neurophysiology of the hypothalamic-neurohypophysial system that were presented at the 2019 annual World Congress on Neurohypophysial Hormones in Ein Gedi, Israel.
A unique property of the magnocellular OT and VP neurons is their intrinsic osmosensitivity (2-4), a property that involves dynamic changes in cell size and activation of a non-selective cation channel formed by an N terminal-truncated variant of the transient receptor potential vanilloid type-1 (TRPV1) channel, which serves as a mechanoreceptor (5-7). Recent work highlighted here by the contribution of Prager-Khoutorsky reveals that these changes are dependent on complex plasticity in the cytoskeletal machinery of actin filaments and microtubules, the latter of which are tethered directly to the ΔN-TRPV1 channels. Together these elements respond to changes in osmotic pressure, e.g., hypertonicity, which causes cell shrinkage leading to depolarization of the magnocellular neurons and an increase in action potential activity and hormone release.
A striking feature of the hypothalamic-neurohypophysial system is the remarkable degree of morphological and physiological plasticity exhibited under conditions of sustained hormone demand, such as during lactation and chronic dehydration, presumably to adapt its properties to afford efficient secretion as neurosecretory stores become progressively depleted. These adaptations involve dynamic morphological changes in neuronal-glial relationships in the hypothalamic magnocellular nuclei and coincident synaptic rearrangements (8-11). Early studies first demonstrated increased synaptic contacts as well as somatic and dendritic membrane close appositions coupled to the retraction of glial processes during chronic dehydration and lactation, which were followed more recently by studies of plasticity in synaptic and membrane properties (12-14). Plasticity during enhanced demand for hormone can take various forms, including changes in presynaptic facilitation during repetitive activity (15, 16), modification of transmitter receptor channels (17, 18), upregulation of intrinsic currents (19-22), and dynamic regulation of Cl− buffering to shape inhibitory synaptic responses (9, 23, 24). As highlighted in the contributions here by Teruyama, Tasker and Prager-Khoutorsky, chronic salt intake is a reliable means of osmotically stressing the system and inducing these adaptive changes, in this case changes in epithelial Na+ channels (ENaCs) that contribute to the resting membrane potential specifically in VP neurons, AMPA receptors that provide the excitatory drive to OT and VP neurons and contribute to synaptic plasticity, and microtubules that are involved in the regulation of autonomous osmosensitivity of VP neurons. Thus, plasticity in the hypothalamic-neurohypophysial system in response to chronic challenges is multi-dimensional, from morphological restructuring to changes in ion channel expression and function, and serves to shape the electrical activity needed to sustain hormone release in the face of changing physiological demand.
Unlike the great majority of mammalian neurons, the axon terminals of OT and VP neurons are large and, therefore, amenable to electrophysiological recording, which allows the high-resolution characterization of Ca2+-secretion coupling (25-28). Recordings from axon terminals in the neurohypophysis have provided detailed insight, for example, into the complex Ca2+ buffering that occurs in axons (29) and the second messenger modulation of hormone secretion (22). Recent work described here in the contribution by Lemos suggests an unexpectedly prominent role of Ca2+ release from ryanodine-sensitive internal stores in the release of OT and VP. Thus, true to form, the hypothalamic-neurohypophysial system, because of its tractability to experimental study, continues to drive new directions of neurophysiological discovery.
The role of cytoskeletal networks in the osmosensitivity of vasopressin neurons
Magnocellular neurosecretory cells are intrinsically osmosensitive. Firing activity of isolated magnocellular neurons is increased by hypertonicity and inhibited by hypotonicity in the absence of synaptic inputs and the surrounding glial cells (2, 30-32). This modulation of neuronal activity in response to changes in extracellular fluid osmolality is associated with changes in cell volume. A hypertonic extracellular environment causes water to flow out of the cell to balance the elevated concentration of solutes in the extracellular fluid, resulting in cell shrinkage, which in turn leads to cell excitation and increases the firing rate of magnocellular neurons. A hypotonic environment causes water to flow into the cell, inducing cell swelling and leading to a decrease in the firing rate of these neurons (30, 32, 33). This autonomous osmolality-induced modulation of neuronal activity is a mechanical process linked to cell shrinking and swelling since it can be achieved without alterations in solute concentration or ionic strength by changing cell volume via application of negative or positive pressure through a whole-cell patch pipette (33-35). In contrast to other cell types that strive to restore their volume in response to changes in the extracellular osmolality (36, 37), magnocellular neurons exhibit sustained osmolality-induced changes in cell volume, and these changes are inversely proportional to the extracellular fluid osmolality (33, 38). This feature is crucial, as the activity of magnocellular neurons should be modulated for as long as blood plasma osmolality is above or below the set point, to ensure an appropriate secretion of VP and OT to restore fluid balance.
The role of TRPV1 channels
The modulation of magnocellular neuron activity by osmolality or mechanical stimulation is mediated by the activation/inhibition of a non-selective cation current which causes cell depolarization/hyperpolarization, leading to an increase/decrease in the action potential firing rate. As briefly described above, recent studies demonstrated that the TRPV1 channel mediates this effect (6, 7). Supraoptic nucleus (SON) neurons express a truncated N-terminal variant of the TRPV1 (ΔN-TRPV1) (7), and magnocellular neurons isolated from TRPV1 knockout mice lack autonomous responses to hypertonicity or suction-induced shrinking (6). Moreover, TRPV1 knockout mice display chronically elevated blood osmolality under basal conditions as well as attenuated VP release in response to acute hyperosmotic stimulation in vivo (6). These results suggest that the ΔN-TRPV1 is a mechanosensitive ion channel that can be activated by a decrease in cell volume (triggered by hypertonicity or suction) to mediate osmotically-induced responses in the magnocellular neurosecretory cells (5, 7).
The role of actin
Since the autonomous responses of magnocellular neurons to hypertonicity are mediated by the mechanical activation of ΔN-TRPV1, the intracellular cytoskeleton appears to be a key element that can convert forces generated during cell volume changes into the activation of these channels. Indeed, studies showed that magnocellular neurosecretory cells feature a thin layer of actin filaments beneath the plasma membrane (Fig. 1A). Whole-cell patch clamp recordings from isolated magnocellular neurons demonstrated that hypertonicity-induced depolarization and increases in the firing rate of these neurons are blocked by cytochalasin D, a compound that depolymerizes actin filaments. In contrast, stabilization of actin filaments with jasplakinolide, a drug that promotes actin polymerization, potentiates the activation of magnocellular neurons in response to hypertonicity or suction-induced shrinking. Importantly, pharmacological modification of the actin cytoskeleton in magnocellular neurons does not affect the cell shrinking in response to osmotic or mechanical stimuli, but changes the coupling between the cell volume and neuronal excitation (34, 39). Notably, another study demonstrated that angiotensin II potentiates the autonomous responses of SON neurons to hypertonicity or suction-induced shrinking. This increase in the intrinsic osmosensitivity of magnocellular neurons is mediated by phospholipase C and a Ca2+−dependent form of protein kinase C, leading to an increase in cortical actin density in these neurons (39, 40). These findings indicate that the actin cytoskeleton plays an important role in the autonomous osmosensitivity of magnocellular neurons, and that the sensitivity of osmotic activation can be bi-directionally modulated by changing the density of the actin layer (34, 39, 40).
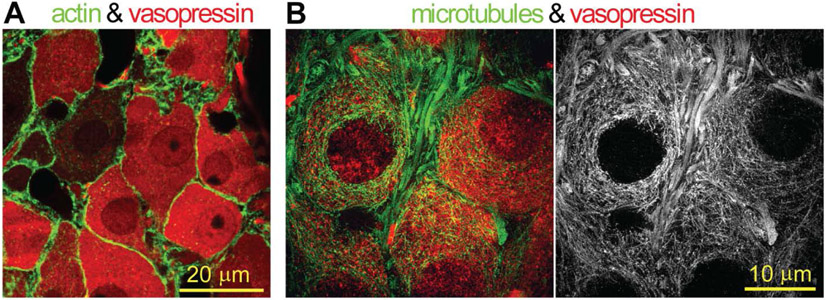
Figure 1. Cytoskeletal networks in magnocellular VP neurons.
(A) Immunostaining for actin (green) and VP (red) in a SON section of the adult rat brain analyzed by confocal imaging. (B) Immunostaining for α-tubulin (green or white) and VP (red) in a SON section of adult rat brain analyzed by super-resolution structured illumination microscopy. Note that magnocellular VP neurons feature a submembrane cortical actin layer and an interweaved cytoplasmic microtubule scaffold. Microtubules extend all the way to the plasma membrane of the neurons, where they interact with the transduction channels (ΔN-TRPV1) (41).
Despite these important findings, the molecular mechanism by which the actin cytoskeleton regulates the osmotic activation of ΔN-TRPV1 in magnocellular neurons remains unknown. It is plausible that the subcortical actin layer creates a scaffold underneath the plasma membrane that provides mechanical support during shrinkage and/or transmits forces essential for the mechanical activation of the transduction channels (41). However, the direct evidence supporting the notion that actin is physically associated with ΔN-TRPV1 channels or mediates their mechanical gating is currently missing (42, 43). Further work is required to examine the organization of the actin cytoskeleton under basal conditions and in response to osmotic stimuli, to determine how changes in the cell volume affect the organization of cortical actin to modulate the activation of ΔN-TRPV1 channels.
The role of microtubules
In addition to the actin cytoskeleton, a recent study by Prager-Khoutorsky and colleagues showed that microtubules also play an essential role in the osmotic and mechanical activation of magnocellular neurosecretory cells (44). In contrast to actin filaments, which form a thin subcortical layer in these neurons, microtubules create a highly complex three-dimensional network of filaments that occupy the entire cytoplasm of neuronal somata (Fig. 1B). Super-resolution imaging of microtubules in situ in different areas of the rodent brain revealed that magnocellular VP neurons from the SON and paraventricular nucleus (PVN) feature a unique microtubule structure that is strikingly different from the rectilinear microtubules commonly observed in other types of neurons (44-46), and from the typical pattern of centrosome-divergent microtubules found in somatic cells (47). Thus, this interwoven microtubule network appears to be a unique feature of the magnocellular neurosecretory cells. This structure is comprised of a remarkably dense scaffold of filaments that extend from the nucleus to the plasma membrane, with the microtubule tips positioned very close to the cell surface and contacting the plasma membrane at multiple points (44). Furthermore, this study demonstrated that microtubules interact with ΔN-TRPV1 channels on the plasma membrane of magnocellular neurons via two highly-conserved β-tubulin binding domains on the channel’s C-terminus (42, 48), and those interactions are essential for the autonomous cell response to osmolality (44). Disrupting microtubules with nocodazole decreases the number of interactions between microtubules and ΔN-TRPV1 at the cell surface and inhibits the activation of the neurons in response to hypertonicity or suction-induced shrinking. Stabilization of microtubules with taxol elevates microtubule density and increases the number of microtubule-to-ΔN-TRPV1 interactions and amplifies the activation of magnocellular neurons in response to shrinking. Moreover, specific disruption of these interactions by infusing isolated SON neurons with synthetic peptides mimicking the C-terminus binding domains of ΔN-TRPV1 blocks the shrinking-induced depolarization and increase in the firing rate of the neurons (44). These findings suggest that the interaction between the microtubule network and ΔN-TRPV1 channels is necessary for the autonomous activation of magnocellular neurons during cell shrinkage, and that the sensitivity of this process can be bi-directionally modulated by changing the stability of the microtubule network and the density of its interactions with the transduction channels (44).
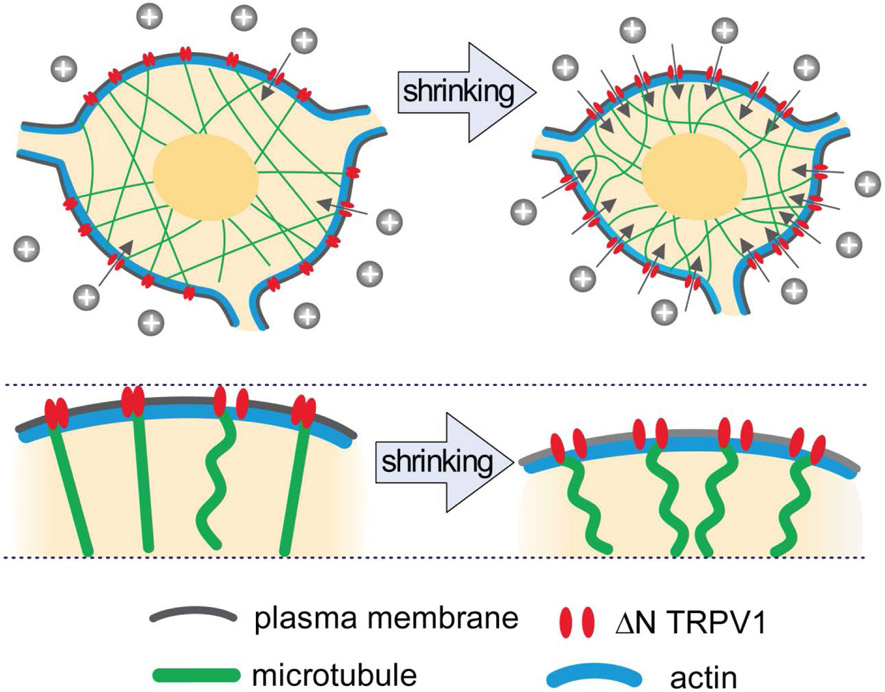
These studies propose a new model, shown in Fig. 2, to explain the autonomous mechanism underlying the activation of magnocellular neurons in response to hypertonicity-induced shrinking. This “push activation” model suggests that the activation of the transduction channel (ΔN-TRPV1) during cell shrinkage may result from a pushing force generated by microtubules attached to ΔN-TRPV1 channels as result of their compression during cell shrinking. Single-channel cell-attached recordings demonstrate that a brief positive pressure pulse applied to the membrane underneath the patch pipette is sufficient to rapidly activate channels in this membrane fragment and are consistent with this model, supporting the notion that ΔN-TRPV1 is activated directly by the application of a pushing force through the attached microtubule filament (35, 41, 44).
Figure 2. Model illustrating the role of actin and microtubules in the mechanical activation of the transduction channels in response to hypertonicity-mediated cell shrinking.
At rest, ΔN-TRPV1 channels are attached to microtubules, although only few are activated because of a lack of sufficient pushing force. As a result of hypertonicity-induced cell shrinkage, the plasma membrane shifts inward, increasing the proportion of microtubules that push onto and activate ΔN-TRPV1 channels. Cortical actin does not directly interact with the transduction channel. In response to cell shrinkage, this elastic layer transmits changes in cell volume into the adequate movement of the plasma membrane, resulting in microtubule compression and push-activation of the transduction channels, leading to cell excitation.
Additional studies are required to test this model to determine how hypertonicity-induced cell shrinkage modifies the microtubule network leading to the activation of ΔN-TRPV1 channels and to examine how hypotonicity-induced cell swelling affects microtubule organization and its interaction with the transduction channels that leads to inhibition of the channel’s activity and decreased neuronal activation.
Thus, recent studies have revealed that magnocellular neurosecretory cells are intrinsically osmosensitive and generate autonomous responses to changes in the extracellular fluid osmolality. This autonomous osmosensitivity is mediated by mechanical activation of ΔN-TRPV1 channels in response to changes in cell volume. Two major cytoskeletal elements, actin filaments and microtubules, are essential components that mediate intrinsic responses to changes in osmolality in magnocellular neurons, and modulation of the stability of these cytoskeletal networks causes proportional changes in the sensitivity of neuronal activation. The mechanism by which actin regulates ΔN-TRPV1 activation remains unclear, as previous studies suggest that actin does not bind directly to TRPV1 channels, and thus the effect of the actin network on the activity of the transduction channel is likely indirect. Conversely, microtubules directly bind to the C-terminus of the ΔN-TRPV1, and this interaction appears to be critical for the osmotic activation of the channel, presumably by providing a pushing force that causes the mechanical gating of the channel (Fig. 2). Notably, both actin filaments and microtubules are essential for the osmotic activation of the neurons, as disrupting their stability blocks the hypertonicity-mediated autonomous response. Moreover, stabilizing each of these cytoskeletal networks amplifies the activation of neurons by changes in osmolality. Interestingly, Prager-Khoutorsky and colleagues demonstrated recently that the density of microtubules in magnocellular VP neurons increases in response to salt-loading (46). The organization of microtubules in other brain areas remains unchanged following salt-loading, and only microtubule cytoskeleton in magnocellular SON and PVN neurons is modulated by this condition (46). Since enhancing microtubule density is sufficient to elevate the autonomous osmosensitivity of VP magnocellular neurons (44), the increase in microtubule density following salt-loading can facilitate the activation of magnocellular VP neurons, contributing to excessive VP release and elevated blood pressure in this condition.
Epithelial Na+ channels in magnocellular VP neurons
The epithelial Na+ channel (ENaC) is an amiloride-sensitive and non-voltage-dependent sodium-selective ion channel. A functional ENaC is composed of three homologous subunits, α, β, and γ, which are assembled into a heteromultimeric complex (49-51). Although the stoichiometry of ENaC remains uncertain, the total number of subunits varying from four to nine (50-55), it is generally agreed that each of the three ENaC subunits contributes to the formation of the functional channel complex (50, 56). ENaCs are widely expressed in aldosterone-sensitive epithelial cells, where aldosterone induces the expression of ENaC subunits via activation of the mineralocorticoid receptor (MR). In the context of blood pressure regulation, ENaCs located in the distal nephron of the kidney are known to modulate Na+ reabsorption, thereby regulating blood pressure and extracellular fluid volume (57, 58). In humans, most of the known genetic causes of hypertension are due to defects either in ENaC itself or its regulation, which results in abnormal increases in renal Na+ reabsorption (59-63).
Amiloride is a potent ENaC channel blocker, and amiloride-sensitive cells in the brain are also implicated in the regulation of blood pressure. In particular, dysregulation of amiloride-sensitive cells contributes to salt-sensitive hypertension. Intracerebroventricular (ICV) infusion of amiloride, or its analogue, benzamil, prevent 1) salt-induced hypertension in Dahl salt-sensitive rats (64, 65); 2) hypertension induced by ICV infusion of aldosterone in normal rats (66); and 3) hypertension induced by the combination of angiotensin II and a high-salt diet (67-72). These effects likely were mediated by ENaCs in the brain because the doses were lower than those having a physiological effect in the general circulation. Despite these findings, the mechanism underlying how ENaCs in the brain are involved in the development of hypertension is still not known.
All three ENaC subunits have been found in epithelial cells of the choroid plexus (73, 74) and in magnocellular neurons of the SON and PVN (75). In the SON and PVN, all three ENaC subunits were found specifically in VP, but not in OT neurons (Fig. 3A) (76). Whereas ENaCs in the epithelia of the choroid plexus regulate the [Na+] in the cerebrospinal fluid (73, 74), the regulation and function of ENaCs in VP neurons is poorly understood. Considering the role of VP in both neuroendocrine and autonomic responses, the presence of ENaCs in VP neurons suggests their involvement in the development of salt-sensitive hypertension. Here, we discuss the functional significance and regulation of ENaCs in VP neurons.
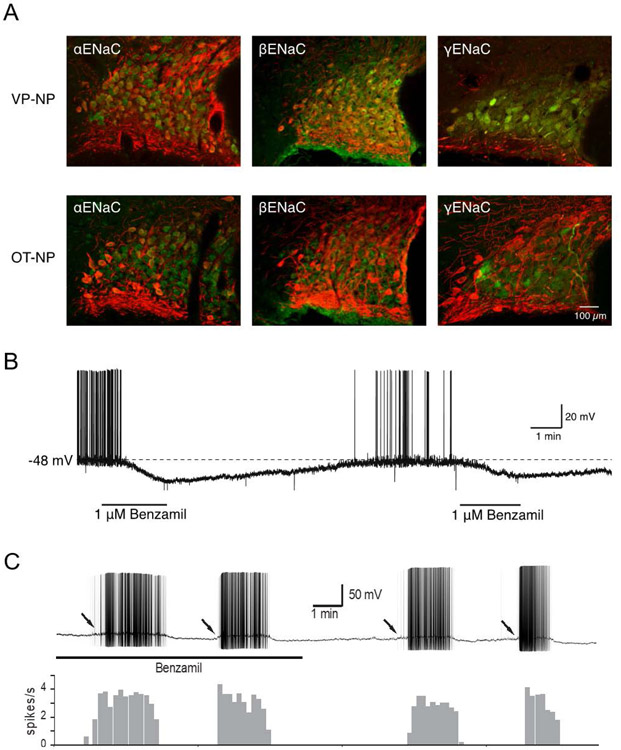
Figure 3. ENaC expression in VP neurons.
A. The three ENaC subunits, αENaC, βENaC, and γENaC, are expressed in VP neurons, but not OT neurons, of the SON. B. Blocking ENaC with bath application of benzamil caused a reversible hyperpolarization and cessation of action potentials in a spontaneously firing VP neuron recorded in a hypothalamic slice. C. Phasic bursting activity in a VP neuron (upper) and a frequency histogram (lower) during benzamil application and washout. Benzamil did not change the phasic bursting pattern, including intraburst spiking frequency, interspike interval, or burst duration. Arrows: Slow depolarizations leading to phasic bursting in the presence and absence of benzamil. Modified from (75) and (79).
ENaCs mediate a steady-state Na+ leak current that affects the resting membrane potential of VP neurons
The persistent K+ current produces a significant leak current in VP neurons that is active when the neurons are at rest (77). However, the resting membrane potential of VP neurons is about −65 mV (78), which is significantly more depolarized than the K+ reversal potential of −96 mV (79), suggesting the presence of a Na+ leak current. Teruyama and colleagues recently demonstrated that ENaC mediates a Na+ leak current that is active near the resting potential and that significantly affects the membrane potential (80). Thus, application of the ENaC inhibitor benzamil caused a hyperpolarization and cessation of firing in VP neurons, while activation of ENaC (e.g., via washout of benzamil) caused a depolarization and increased the frequency of action potentials (Fig. 3B). These results, therefore, suggest that ENaCs contribute to the VP neuron resting membrane potential.
ENaC activity does not directly affect phasic bursting activity in VP neurons
Vasopressin neurons respond to hyperosmolality, hypovolemia and hypotension by increasing their firing rate and adopting a phasic bursting pattern of spiking activity, comprising alternating periods of spiking (7-15 Hz) and silence, each phase lasting tens of seconds. The release of VP is maximized by stimulation patterns mimicking phasic bursting (81, 82). Although phasic bursting in vivo is initiated typically by synaptic inputs (83), this pattern can be observed in in vitro brain slices in the absence of synaptic inputs (84). Therefore, the intrinsic membrane properties that affect the phasic bursting that determines the release of VP are of great interest. Teruyama and colleagues found that phasic bursting was observed in the presence of benzamil and synaptic blockers (85), indicating that ENaC activity does not affect the initiation, maintenance, or termination of phasic bursting (Fig. 3C). Moreover, the application of benzamil did not affect the mean intra-burst frequency, burst duration, or interspike interval variability of phasic bursts. The repetitive firing of VP neurons is critically regulated by intrinsic membrane properties, such as the Ca2+-dependent depolarizing afterpotential and afterhyperpolarization (86). Whereas ENaC, by modulating the resting membrane potential, plays only a minor role in the regulation of the firing activity of VP neurons in the absence of synaptic inputs, these channels would nevertheless affect how synaptic potentials summate to cross the threshold for spike generation that leads to phasic bursting. This possibility must be examined in the future using an in vivo approach.
Dietary salt intake affects the expression and activity of ENaCs
The expression and activity of ENaCs in epithelial tissues are known to be affected by dietary salt intake (58), because the release of aldosterone, a known regulator of ENaC, is influenced by dietary salt. For example, deficiency in dietary salt intake increases circulating aldosterone, which promotes the abundance and activity of ENaCs in the renal collecting ducts to increase Na+ retention (87). Teruyama and colleagues investigated if dietary salt intake also affects ENaC activity in VP neurons (85). Groups of animals were fed either a salt-deficient, control, or high-salt diet for 7 days and changes in the relative amounts of ENaC subunit mRNAs were assessed. The salt-deficient diet, known to increase the circulating level of aldosterone (88), caused a significant increase in αENaC mRNA in the kidney, but not in the SON. Intriguingly, high dietary salt intake, which decreases the circulating level of aldosterone (89, 90), caused an increase in the expression of β and γENaC subunits in the SON, but not in the kidney. Moreover, translocation of αENaC immunoreactivity towards the plasma membrane was observed in VP neurons from animals on a high-salt diet, but not from animals fed a salt-deficient or control diet (Fig. 4). This is in stark contrast to the regulation of ENaC in the kidney, where Na+ deficiency in the diet or high circulating levels of aldosterone induce a selective upregulation of αENaC and redistribution of ENaC labeling to the apical membrane of the collecting duct principal cells (87, 88). These findings suggest that ENaC subunits in VP neurons are regulated independently from ENaC subunits in the kidney. Interestingly, aldosterone also induces the expression of γENaC via MR in VP neurons in in vitro slice preparations (91), suggesting that the expression of ENaC in VP neurons may be regulated by aldosterone synthesized in the hypothalamus, independent of aldosterone produced by the adrenal gland (92-94).
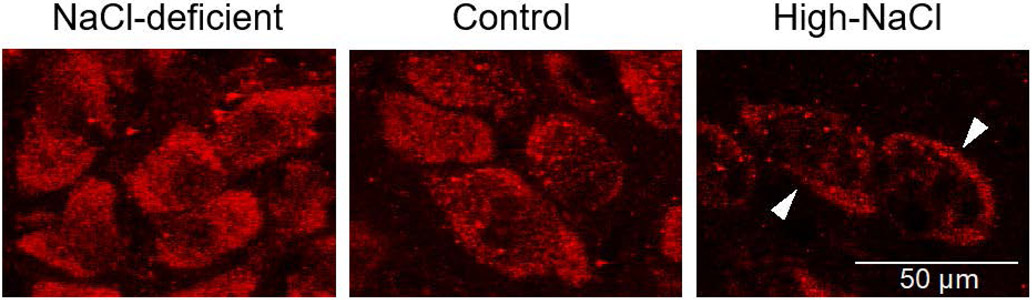
Figure 4. Dietary salt regulation of ENaC expression in VP neurons.
A low-salt diet (NaCl-deficient) caused an increase in ENaC expression in VP neurons compared to the control diet, and a high-salt diet caused a decrease in ENaC, which was localized largely at the VP neuron plasma membrane (High-NaCl). Modified from (79).
Teruyama and colleagues went on to examine the effect of the enhanced expression and subcellular translocation of ENaC in response to high dietary salt intake on the electrophysiological properties of VP neurons. VP neurons from animals fed a high-salt diet exhibited a significantly greater ENaC current compared to controls. They also confirmed that the enhanced ENaC current contributed to the more depolarized resting membrane potential observed in VP neurons in the high-salt diet group. Spontaneous firing can be observed within a relatively narrow range of membrane potentials (~−48 mV to −55 mV) in VP neurons recorded in vitro (21). The more depolarized potentials result in continuous or phasic burst firing, whereas a slightly hyperpolarized potential results in a slow irregular firing pattern or no firing at all (95). This suggests that small alterations in the resting potential are a powerful means to modulate the firing patterns that ultimately control hormone secretion according to physiological demands.
Thus, VP neurons express all three ENaC subunits to form functional ENaCs that mediate a steady state Na+ leak current that influences the resting membrane potential. High dietary salt intake causes upregulation of ENaC expression and increased activity in VP neurons. The enhanced ENaC current is responsible for the more depolarized resting membrane potential observed in VP neurons under conditions of high dietary salt intake, and may influence how synaptic potentials reach the threshold for action potential generation. Because the release of VP from the neurohypophysis is controlled by the frequency and pattern of firing in VP neurons and ENaC is regulated by salt intake, ENaC may play an important role in the increase in VP secretion known to occur during high-salt consumption.
AMPA receptor plasticity induced in magnocellular neurons by salt loading
As introduced briefly above, OT and VP neurons undergo a structural and functional plasticity in response to chronic physiological stimulation, such as salt loading, that affects both glutamatergic and GABAergic synaptic circuits (96). This plasticity is characterized by a reorganization of the SON and PVN that includes somatic hypertrophy and withdrawal of astrocytic processes from around neuronal membranes (97, 98). The glial retraction and resulting uncovering of magnocellular dendritic and somatic membrane triggers synaptogenesis of both GABA and glutamate synapses (99), alters ionic buffering, and modulates synaptic signaling in the magnocellular neurons (100). Salt loading by high-salt consumption is a strong inductive stimulus of magnocellular plasticity that leads to a hypertensive phenotype in rats (100).
Salt loading-induced GABA synapse plasticity
Salt loading leads to an increase in GABAergic synaptic contacts with the magnocellular neurons (101), as well as changes in functional GABAergic synaptic inputs (102). Additionally, the driving force on GABA inhibitory synaptic currents has also been reported to change significantly with salt loading, rendering GABA currents less inhibitory and even excitatory (100, 103). This is mediated by alterations in the transmembrane transport of Cl−, the main ion that flows through GABAA receptor channels, which is caused by salt loading-induced changes in the activity of Cl− co-transporters in magnocellular neurons (100, 103, 104). The K+-Cl− co-transporter 2 (KCC2), for example, is highly sensitive to fluid osmolality, as well as to reproductive state (105), and is largely responsible for establishing the transmembrane Cl− gradient and determining, therefore, Cl− flow across the membrane. Indeed, the baseline KCC2 expression in VP neurons is low compared to that in OT neurons, causing the Cl− equilibrium potential, and therefore the GABA reversal potential, to be shifted in VP neurons under certain conditions without a significant osmotic challenge (106-108).
Salt loading-induced glutamate synapse plasticity
In addition to the GABA synapse plasticity, glutamate synapses on magnocellular neurons also undergo a significant proliferation with chronic salt loading (14, 101, 102, 109), however it was only recently shown that the salt loading-induced plasticity in glutamate synapses includes coincident changes in glutamate receptor expression, subunit composition and signaling properties (18). An early study indicated that the increase in the number of glutamate synapses is accompanied by a correlated increase in excitatory synaptic inputs, without any change in the probability of release at glutamate synapses (102), which is consistent with the ultrastructural observation of an increase in synapse number (97, 101, 109). However, a down-regulation of NMDA receptor subunits has also been reported with salt loading (110). A recent report described an increase in excitatory synaptic inputs that was accompanied by a robust modification of glutamate receptors by chronic salt loading, including changes in AMPA receptor subunit expression and signaling (18).
Glutamate AMPA receptors are tetrameric cation channels that are usually heteromeric in nature, comprised of combinations of four AMPA receptor subunits, GluA1-GluA4, that bestow differential kinetics, voltage sensitivity, and ion permeability on the receptors. The majority of AMPA receptors in the brain contain at least one GluA2 subunit along with usually one other subunit type, often in dimer pairs (111). The GluA2 subunit prevents Ca2+ flow through the AMPA receptor channel. Therefore, unlike NMDA glutamate receptors, the majority of glutamate AMPA receptors in the brain are Ca2+ impermeable due to their GluA2-containing heteromeric structure.
In their recent study of AMPA receptor plasticity, Tasker and colleagues found that mRNA expression of the four AMPA receptor subunits in the SON did not change with chronic salt loading (18). This was surprising given the dramatic increase in the number of glutamate synapses induced by salt loading, which one might expect to be associated with an increase in AMPA receptor expression to accommodate the new synapse formation. However, they did find an increase in GluA1 subunit protein expression, by about 70%, without a coordinate increase in the GluA2-GluA4 subunits, indicating a highly targeted post-transcriptional regulation of AMPA receptor expression by chronic osmotic stimulation. The increase in GluA1 relative to GluA2 resulted in a shift in the GluA1-to-GluA2 ratio, which should increase the Ca2+ permeability of the resulting AMPA receptors due to the relative decrease in the Ca2+-blocking GluA2 subunit, and should result, therefore, in altered glutamate synaptic signaling.
Ca2+-permeable AMPA receptor induction
Calcium-permeable AMPA receptors (i.e., GluA2-lacking AMPA receptors) display a non-linear current-voltage relationship at membrane potentials positive to 0 mV due to the voltage-dependent binding of intracellular spermine in the pore of the GluA2-lacking AMPA receptors (112), which blocks the pore of the channel and reduces its ionic conductance. In their recent report, Tasker and colleagues also described a change in the current-voltage relationship of AMPA synaptic currents induced by salt loading (18). Thus, magnocellular neurons showed a linear AMPA current-voltage relationship, suggestive of GluA2-containing receptors, under control osmotic conditions, whereas the new glutamate synapses induced by salt loading generated synaptic AMPA currents in both OT and VP neurons that had highly non-linear current-voltage relations, indicative of a strong rectification caused by a lack of the GluA2 subunit (Fig. 5A-C). This suggested that excitatory synapses of OT and VP neurons are composed largely of GluA2-containing AMPA receptors under control osmotic conditions, whereas new glutamate synapses formed during salt-loading lack GluA2 subunits and are more permeable to Ca2+. This contrasted with earlier studies showing that both OT and VP neurons from normally hydrated female rats exhibited AMPA-current inward rectification, which was stronger in OT neurons, and AMPA-mediated Ca2+ influx in dissociated magnocellular neurons from male rats, suggesting that AMPA receptors are Ca2+ permeable due to a relative lack of GluA2 expression under normal conditions (113, 114). Di et al. (18) further tested this shift in AMPA receptor signaling using a GluA1-specific blocker. They found that blocking GluA1 AMPA receptors had little effect on EPSCs in magnocellular neurons from control euhydrated rats, but caused a significant inhibition of the EPSCs in both OT and VP neurons from salt-loaded rats (Fig. 5D). They concluded that salt loading resulted in a dramatic shift in the AMPA receptor subunit composition of excitatory synapses on the magnocellular neurons, significantly increasing the proportion of GluA1-containing, GluA2-lacking, putative calcium-permeable AMPA receptors. This suggested an induction by salt loading of homomeric GluA1 AMPA receptors that are capable of fluxing Ca2+ into the magnocellular neurons, providing a new source of Ca2+ for intracellular signaling that could contribute to the induction and/or maintenance of the synaptic and neuronal-glial plasticity seen during physiological stimulation. The Ca2+-permeable AMPA receptors could thus provide a new Ca2+ source for synaptic plasticity, or they could compensate for the putative loss of Ca2+influx through NMDA receptors. The NMDA receptors require the co-agonist D-serine for activation, which is derived from astrocytes surrounding glutamate synapses (115). With the glial retraction that occurs with salt loading, as that seen during parturition, the astrocytic source of D-serine may be lost, which would effectively prevent the activation of NMDA receptors (115). Ca2+-permeable AMPA receptors may compensate for the loss of NMDA receptor signaling, or may subserve new forms of synaptic plasticity.
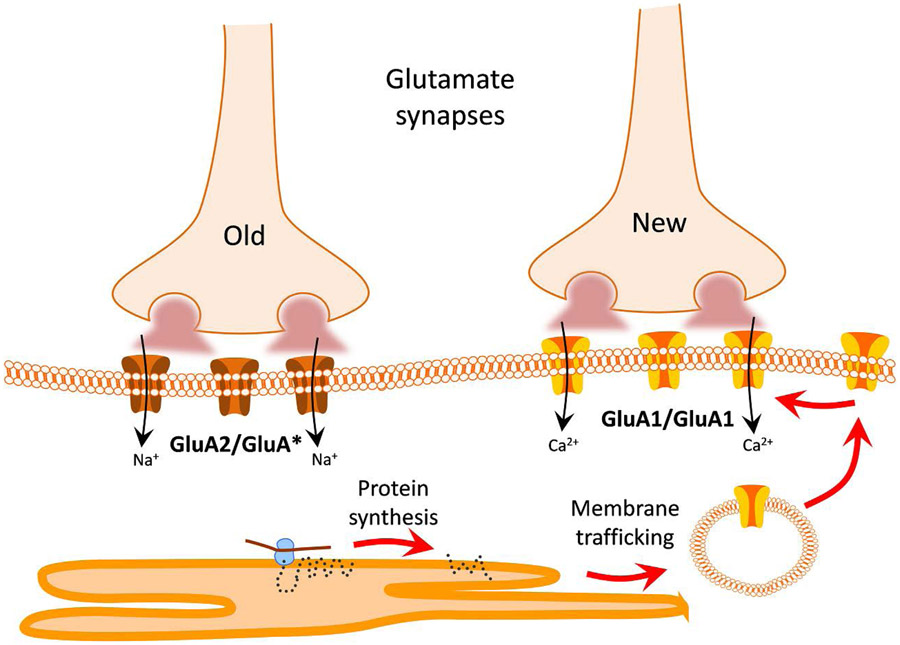
Figure 5. Salt loading induces new glutamate synapses composed of labile Ca2+-permeable AMPA receptors.
A-C. Salt loading increases inward rectification of AMPA currents, which is characterized by suppressed current positive to 0 mV and a bigger −60 mV : +40 mV rectification index. D-F. Antagonists of Ca2+-permeable AMPA receptors (NAS) and dendritic protein synthesis (rapamycin, Rapa) reverse the salt loading-induced change in AMPA current rectification. With permission. Modified from Di et al., 2019 (18).
New glutamate synapses formed during salt loading are highly labile
Interestingly, the GluA1 homomeric AMPA receptors at the glutamate synapses newly formed during salt loading require continuous protein synthesis for their maintenance (18). Preincubating brain slices in protein synthesis inhibitors silenced the new synapses with GluA2-lacking AMPA receptors, but had little effect on the old glutamate synapses with GluA2-containing AMPA receptors. The protein synthesis at new glutamate synapses formed during salt loading was also mTOR-dependent, suggesting that the new synapses are highly labile and require continuous dendritic protein synthesis to maintain AMPA receptor function (Fig. 5E, F).
Thus, as illustrated in the model in Fig. 6, chronic salt loading induces new glutamate synapses on both VP and OT magnocellular neurons that appear to be composed largely of calcium-permeable GluA1 homomeric AMPA receptors. The new synapses are labile, requiring continuous protein synthesis for the maintenance of their AMPA receptors. The lability of the synapses suggests that they are highly sensitive to osmotic conditions, undergoing functional induction and silencing based on the regulation of dendritic protein synthesis, which controls AMPA receptor trafficking into and out of the new glutamate synapses. This synaptic plasticity, therefore, is finely tuned to osmotic conditions, tying VP and OT neuron activation to changing osmotic conditions and thereby titrating VP and OT secretion as a function of the osmotic demands on the system.
Figure 6. Model of AMPA receptor plasticity induced by salt loading.
New glutamate synapses formed during chronic salt loading are composed of Ca2+-permeable GluA1 homomeric AMPA receptors, which are highly labile, dependent on continuous protein synthesis for their maintenance and the maintenance of the new, but not the old, glutamate synapses. The old glutamate synapses are composed of GluA2-containing, Ca2+-impermeable AMPA receptors.
Extracellular Ca2+-independence of neuropeptide release from hypothalamic-neurohypophysial system terminals
Depolarization-secretion coupling is historically thought to be dependent on extracellular Ca2+ influx through voltage-gated Ca2+ channels (see mechanism #1 in Fig. 7) in order to elicit transmitter release. However, a competing theory (116) invokes a distinct and separate regulation of secretion by just depolarization (see mechanism #4 in Fig. 7).
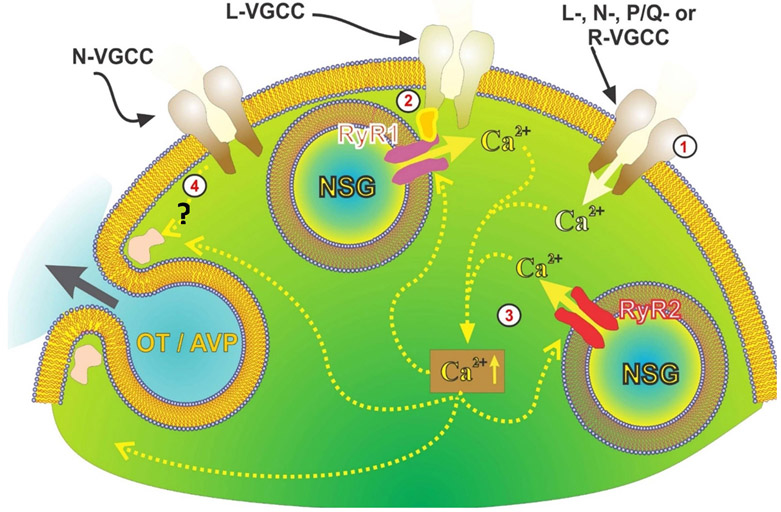
Figure 7. Model of different mechanisms of depolarization-induced neuropeptide secretion from OT/VP axon terminals.
1) Normally, secretion is activated by Ca2+ entry via different types of voltage-gated Ca2+ channels (VGCC). 2) Independent of extracellular Ca2+, ryanodine type-1 receptors are activated in response to depolarization while mechanically tethered to nifedipine-sensitive Ca2+ channels. Depolarization, thus, allows for release of Ca2+ from ryanodine-sensitive stores contributing to the overall increase in intraterminal [Ca2+]. 3) This and Ca2+ entry through VGCCs likely contribute to CICR from ryanodine type-2 receptors. Intra-terminal free Ca2+ rise triggers the exocytosis releasing OT and arginine vasopressin (AVP). 4) Finally, there is secretion directly coupled to voltage and independent of both external and internal Ca2+. Drawing courtesy of Drs. Cristina Velazquez & Hector Marrero.
Voltage-regulated sparks or “syntillas” of intracellular Ca2+ from ryanodine receptor-sensitive stores (see mechanism #2 in Fig. 7) have been described in axon terminals in the neurohypophysis (117, 118). However, a role for Ca2+ release from intracellular Ca2+ stores, such as Ca2+-induced Ca2+ release (see mechanism #3 in Fig. 7), in secretion is controversial. Lemos and colleagues showed recently that voltage-dependent release from intracellular ryanodine-sensitive stores provides another source of intracellular Ca2+ that contributes to neuropeptide secretion from neurohypophysial terminals (119).
Multiple convergent findings from their study (119) point to a dependence of neuropeptide release on voltage-dependent activation of intracellular Ca2+ mobilization. First, depolarization-induced increases in intra-terminal [Ca2+] and neuropeptide secretion from neurohypophysial terminals were maintained in the presence of voltage-gated Ca2+ channel blockers and in the absence of extracellular Ca2+. Capacitance measurements showing changes in membrane capacitance in Ca2+-free extracellular medium confirmed that depolarization of the terminals elicited a significant increase in exocytosis, which was blocked by antagonist concentrations of ryanodine. Next, they showed that depolarization of neurohypophysial terminals with high K+ in a Ca2+-free extracellular medium caused an increase in neuropeptide secretion, which was blocked by ryanodine receptor antagonists. Furthermore, such depolarizations led to increases in intracellular [Ca2+]. They then showed that depolarization of neurohypophysial terminals with high K + following pre-incubation in the membrane-permeant Ca2+ chelator BAPTA-AM in a Ca2+-free medium resulted in a partial inhibition of the depolarization-induced peptide secretion. A significant proportion of the response was not blocked, however, indicating that a component of the voltage-dependent neurosecretory response is Ca2+-independent (see mechanism #4 in Fig. 7). They also tested the depolarization-induced Ca2+ response and neuropeptide secretion for L-type Ca2+ channel dependence with nifedipine. This blocked both the intraterminal [Ca2+] increase and neuropeptide secretion and suggested the involvement of the type-1 ryanodine receptor component of voltage-induced calcium release (see mechanism #2 in Fig. 7) (117). Finally, they showed that ryanodine receptor antagonists also modulate neuropeptide release under normal physiological conditions.
Thus, as illustrated in Fig. 7, there are four different mechanisms underlying neuropeptide secretion in hypothalamic-neurohypophysial nerve terminals: 1) secretion activated by Ca2+ entry via voltage-gated Ca2+ channels; 2) secretion activated by Ca2+ released from intracellular stores via Ca2+-induced Ca2+ release; 3) secretion triggered by Ca2+ release from intracellular stores activated directly by voltage (i.e., analogous to that observed in skeletal muscle); and 4) secretion directly coupled to voltage and independent of Ca2+ (i.e., analogous to that observed in DRG somata (120)).
It appears that about 60% of the neuropeptide secretion depends on external Ca2+ (see mechanism #1 in Fig. 1), and the reduction in secretion produced by nifedipine or ryanodine in 0 Ca2+ corresponds to ~20% of the total secretion in normal external Ca2+ (see mechanism #2 & mechanism #3 in Fig. 7). The remaining ~20% of secretion appears to be Ca2+-independent, but voltage-dependent (see mechanism #4 in Fig. 7). This last component lends support to the competing theory of Parnas & Parnas (116) of Ca2+-independent secretion at nerve terminals.
Extracellular Ca2+-independent secretion could explain the efficacy of neuropeptide secretion at the end of a burst in situ, when increases in extracellular [K+] should depolarize the terminals, but extracellular [Ca2+] should be decreased within the neurohypophysial interstitial spaces, reducing Ca2+ influx (121-123). Importantly, under normal conditions, high [K+] in the interstitial spaces should increase the number of syntillas in the terminals, and this would increase intracellular [Ca2+], leading to facilitation of release and compensating for the decrease in extracellular [Ca2+]. Furthermore, during physiological stress, such as salt loading, when morphological plasticity increases interstitial space around terminals (124), Ca2+-induced Ca2+ release from intracellular stores would decrease, since smaller changes in both extracellular Ca2+ and K+ would make compensation in release less necessary. Therefore, during salt loading, there would be fewer depolarization-induced Ca2+ sparks, but also less voltage-dependent inactivation of the voltage-gated calcium channels.
Thus, these recent findings (119) demonstrate that both voltage-dependent Ca2+ release from ryanodine receptor-mediated intracellular stores and extracellular Ca2+-independent, voltage-dependent mechanisms contribute to the secretion of neuropeptides from magnocellular neuron terminals in the neurohypophysis. This does not happen at the soma/dendrites of the magnocellular neurons in the hypothalamus, where receptor-mediated intracellular Ca2+ release is, instead, mediated by inositol triphosphate receptors (125). This constitutes an important difference in the secretion mechanisms between the magnocellular cell bodies and their terminals (126). It remains to be seen whether ryanodine receptor-mediated Ca2+ release recruits neurosecretory granules at terminals to facilitate secretion as happens at dendrites (127). These mechanisms should have widespread importance, since bursting patterns of action potentials are necessary for the secretion of not only these neuropeptides but also of many other neurotransmitters in the central and peripheral nervous systems.
Conclusions
We have briefly highlighted recent studies in the authors’ labs on the neurophysiology of OT and VP neurons that were presented at the 2019 World Congress on Neurohypophysial Hormones. These studies are just a sampling of the neurophysiological investigation occurring in the hypothalamic-neurohypophysial system, presented to provide a snapshot of some of the unique physiological properties and remarkable structural and functional plasticity of this benchmark neuroendocrine system. It is clear from this work that the OT/VP neurons continue to provide a tractable model system to gain new insights into neurophysiological mechanisms that contribute to our general understanding of neuronal and hormonal signaling. We look forward to exciting new discoveries in OT and VP neuron cellular and network neurophysiology in the years to come.
Acknowledgements:
This work was supported by Canadian Institutes of Health Research grant PJT-153009 and a Heart & Stroke Foundation of Canada National New Investigator Award to MPK; and by U.S. National Institutes of Health grants R01HL115208 and R21HL093728 to RT, P20GM103642 and R01NS29470 to JRL, R01NS042081 to JGT, and R01HD072056 to WEA.
References
- 1.Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982; 7(4): 773–808. [DOI] [PubMed] [Google Scholar]
- 2.Mason WT. Supraoptic neurones of rat hypothalamus are osmosensitive. Nature. 1980; 287(5778): 154–7. [DOI] [PubMed] [Google Scholar]
- 3.Abe H, Ogata N. Ionic mechanism for the osmotically-induced depolarization in neurones of the guinea-pig supraoptic nucleus in vitro. J Physiol. 1982; 327157–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourque CW, Renaud LP. Activity patterns and osmosensitivity of rat supraoptic neurones in perfused hypothalamic explants. J Physiol. 1984; 349631–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansal V, Fisher TE. Osmotic activation of a Ca(2+)-dependent phospholipase C pathway that regulates N TRPV1-mediated currents in rat supraoptic neurons. Physiol Rep. 2017; 5(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharif-Naeini R, Witty MF, Seguela P, Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. NatNeurosci. 2006; 9(1): 93–8. [DOI] [PubMed] [Google Scholar]
- 7.Zaelzer C, Hua P, Prager-Khoutorsky M, Ciura S, Voisin DL, Liedtke W, Bourque CW. DeltaN-TRPV1: A Molecular Co-detector of Body Temperature and Osmotic Stress. Cell Rep. 2015; 13(1): 23–30. [DOI] [PubMed] [Google Scholar]
- 8.Miyata S Advances in Understanding of Structural Reorganization in the Hypothalamic Neurosecretory System. Front Endocrinol (Lausanne). 2017; 8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choe KY, Trudel E, Bourque CW. Effects of Salt Loading on the Regulation of Rat Hypothalamic Magnocellular Neurosecretory Cells by Ionotropic GABA and Glycine Receptors. J Neuroendocrinol. 2016; 28(4). [DOI] [PubMed] [Google Scholar]
- 10.Hatton GI. Dynamic neuronal-glial interactions: an overview 20 years later. Peptides. 2004; 25(3): 403–11. [DOI] [PubMed] [Google Scholar]
- 11.Theodosis DT. Oxytocin-secreting neurons: A physiological model of morphological neuronal and glial plasticity in the adult hypothalamus. Front Neuroendocrinol. 2002; 23(1): 101–35. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong WE, Wang L, Li C, Teruyama R. Performance, properties and plasticity of identified oxytocin and vasopressin neurones in vitro. Journal of neuroendocrinology. 2010; 22(5): 330–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tasker JG, Oliet SH, Bains JS, Brown CH, Stern JE. Glial regulation of neuronal function: from synapse to systems physiology. J Neuroendocrinol. 2012; 24(4): 566–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev. 2008; 88(3): 983–1008. [DOI] [PubMed] [Google Scholar]
- 15.Stern JE, Hestrin S, Armstrong WE. Enhanced neurotransmitter release at glutamatergic synapses on oxytocin neurones during lactation in the rat. J Physiol. 2000; 526109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliet SH. Functional consequences of morphological neuroglial changes in the magnocellular nuclei of the hypothalamus. J Neuroendocrinol. 2002; 14(3): 241–6. [DOI] [PubMed] [Google Scholar]
- 17.Brussaard AB, Herbison AE. Long-term plasticity of postsynaptic GABAA-receptor function in the adult brain: insights from the oxytocin neurone. Trends Neurosci. 2000; 23(5): 190–5. [DOI] [PubMed] [Google Scholar]
- 18.Di S, Jiang Z, Wang S, Harrison LM, Castro-Echeverry E, Stuart TC, Wolf ME, Tasker JG. Labile Calcium-Permeable AMPA Receptors Constitute New Glutamate Synapses Formed in Hypothalamic Neuroendocrine Cells during Salt Loading. eNeuro. 2019; 6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Chandaka GK, Foehring RC, Callaway JC, Armstrong WE. Changes in potassium channel modulation may underlie afterhyperpolarization plasticity in oxytocin neurons during late pregnancy. J Neurophysiol. 2018; 119(5): 1745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stern JE, Armstrong WE. Changes in the electrical properties of supraoptic nucleus oxytocin and vasopressin neurons during lactation. J Neurosci. 1996; 16(16): 4861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teruyama R, Armstrong WE. Enhancement of calcium-dependent afterpotentials in oxytocin neurons of the rat supraoptic nucleus during lactation. J Physiol. 2005; 566(Pt 2): 505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Star B, Rajapaksha WR, Fisher TE. Dehydration increases L-type Ca(2+) current in rat supraoptic neurons. J Physiol. 2007; 580181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balapattabi K, Farmer GE, Knapp BA, Little JT, Bachelor M, Yuan JP, Cunningham JT. Effects of salt-loading on supraoptic vasopressin neurones assessed by ClopHensorN chloride imaging. J Neuroendocrinol. 2019; 31(8): e12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim YB, Kim YS, Kim WB, Shen FY, Lee SW, Chung HJ, Kim JS, Han HC, Colwell CS, Kim YI. GABAergic excitation of vasopressin neurons: possible mechanism underlying sodium-dependent hypertension. Circ Res. 2013; 113(12): 1296–307. [DOI] [PubMed] [Google Scholar]
- 25.Bourque CW. Activity-dependent modulation of nerve terminal excitation in a mammalian peptidergic system. Trends Neurosci. 1991; 14(1): 28–30. [DOI] [PubMed] [Google Scholar]
- 26.Fisher TE, Bourque CW. Calcium-channel subtypes in the somata and axon terminals of magnocellular neurosecretory cells. Trends Neurosci. 1996; 19(10): 440–4. [DOI] [PubMed] [Google Scholar]
- 27.Jackson MB, Konnerth A, Augustine GJ. Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci U S A. 1991; 88(2): 380–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemos JR, Nowycky MC. Two types of calcium channels coexist in peptide-releasing vertebrate nerve terminals. Neuron. 1989; 2(5): 1419–26. [DOI] [PubMed] [Google Scholar]
- 29.McMahon SM, Chang CW, Jackson MB. Multiple cytosolic calcium buffers in posterior pituitary nerve terminals. J Gen Physiol. 2016; 147(3): 243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliet SH, Bourque CW. Properties of supraoptic magnocellular neurones isolated from the adult rat. J Physiol. 1992; 455291–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharif-Naeini R, Ciura S, Stachniak TJ, Trudel E, Bourque CW. Neurophysiology of supraoptic neurons in C57/BL mice studied in three acute in vitro preparations. Prog Brain Res. 2008; 170229–42. [DOI] [PubMed] [Google Scholar]
- 32.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008; 9(7): 519–31. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, Bourque CW. Osmometry in osmosensory neurons. Nat Neurosci. 2003; 6(10): 1021–2. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Kindrat AN, Sharif-Naeini R, Bourque CW. Actin filaments mediate mechanical gating during osmosensory transduction in rat supraoptic nucleus neurons. J Neurosci. 2007; 27(15): 4008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prager-Khoutorsky M, Bourque CW. Mechanical basis of osmosensory transduction in magnocellular neurosecretory neurones of the rat supraoptic nucleus. J Neuroendocrinol. 2015; 27(6): 507–15. [DOI] [PubMed] [Google Scholar]
- 36.Strange K Cellular volume homeostasis. Adv Physiol Educ. 2004; 28(1-4): 155–9. [DOI] [PubMed] [Google Scholar]
- 37.Lang F Mechanisms and significance of cell volume regulation. J Am Coll Nutr. 2007; 26(5 Suppl): 613S–23S. [DOI] [PubMed] [Google Scholar]
- 38.Oliet SH, Bourque CW. Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature. 1993; 364(6435): 341–3. [DOI] [PubMed] [Google Scholar]
- 39.Prager-Khoutorsky M, Bourque CW. Osmosensation in vasopressin neurons: changing actin density to optimize function. Trends Neurosci. 2010; 33(2): 76–83. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z, Bourque CW. Amplification of transducer gain by angiotensin II-mediated enhancement of cortical actin density in osmosensory neurons. J Neurosci. 2008; 28(38): 9536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prager-Khoutorsky M Mechanosensing in hypothalamic osmosensory neurons. Semin Cell Dev Biol. 2017; 7113–21. [DOI] [PubMed] [Google Scholar]
- 42.Goswami C, Dreger M, Jahnel R, Bogen O, Gillen C, Hucho F. Identification and characterization of a Ca2+ -sensitive interaction of the vanilloid receptor TRPV1 with tubulin. J Neurochem. 2004; 91(5): 1092–103. [DOI] [PubMed] [Google Scholar]
- 43.Goswami C, Hucho T. Submembraneous microtubule cytoskeleton: biochemical and functional interplay of TRP channels with the cytoskeleton. Febs J. 2008; 275(19): 4684–99. [DOI] [PubMed] [Google Scholar]
- 44.Prager-Khoutorsky M, Khoutorsky A, Bourque CW. Unique interweaved microtubule scaffold mediates osmosensory transduction via physical interaction with TRPV1. Neuron. 2014; 83866–78. [DOI] [PubMed] [Google Scholar]
- 45.Stiess M, Bradke F. Neuronal polarization: the cytoskeleton leads the way. Dev Neurobiol. 2011; 71(6): 430–44. [DOI] [PubMed] [Google Scholar]
- 46.Hicks AI, Barad Z, Sobrero A, Lean G, Jacob-Tomas S, Yang J, Choe KY, Prager-Khoutorsky M. Effects of Salt Loading on the Organization of Microtubules in Rat Magnocellular Vasopressin Neurons. J Neuroendocrinol. 2019e12817. [DOI] [PubMed] [Google Scholar]
- 47.Luxton GW, Gundersen GG. Orientation and function of the nuclear-centrosomal axis during cell migration. Curr Opin Cell Biol. 2011; 23(5): 579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goswami C, Hucho TB, Hucho F. Identification and characterisation of novel tubulin-binding motifs located within the C-terminus of TRPV1. J Neurochem. 2007; 101(1): 250–62. [DOI] [PubMed] [Google Scholar]
- 49.Berdiev BK, Karlson KH, Jovov B, Ripoll PJ, Morris R, Loffing-Cueni D, Halpin P, Stanton BA, Kleyman TR, Ismailov II. Subunit stoichiometry of a core conduction element in a cloned epithelial amiloride-sensitive Na+ channel. Biophys J. 1998; 75(5): 2292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Firsov D, Gautschi I, Merillat AM, Rossier BC, Schild L. The heterotetrameric architecture of the epithelial sodium channel (ENaC). EMBO J. 1998; 17(2): 344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snyder PM, Cheng C, Prince LS, Rogers JC, Welsh MJ. Electrophysiological and biochemical evidence that DEG/ENaC cation channels are composed of nine subunits. J Biol Chem. 1998; 273(2): 681–4. [DOI] [PubMed] [Google Scholar]
- 52.Kosari F, Sheng S, Li J, Mak DO, Foskett JK, Kleyman TR. Subunit stoichiometry of the epithelial sodium channel. J Biol Chem. 1998; 273(22): 13469–74. [DOI] [PubMed] [Google Scholar]
- 53.Eskandari S, Snyder PM, Kreman M, Zampighi GA, Welsh MJ, Wright EM. Number of subunits comprising the epithelial sodium channel. J Biol Chem. 1999; 274(38): 27281–6. [DOI] [PubMed] [Google Scholar]
- 54.Staruschenko A, Medina JL, Patel P, Shapiro MS, Booth RE, Stockand JD. Fluorescence resonance energy transfer analysis of subunit stoichiometry of the epithelial Na+ channel. J Biol Chem. 2004; 279(26): 27729–34. [DOI] [PubMed] [Google Scholar]
- 55.Staruschenko A, Adams E, Booth RE, Stockand JD. Epithelial Na+ channel subunit stoichiometry. Biophys J. 2005; 88(6): 3966–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994; 367(6462): 463–7. [DOI] [PubMed] [Google Scholar]
- 57.Benos DJ, Awayda MS, Ismailov II, Johnson JP. Structure and function of amiloride-sensitive Na+ channels. J Membr Biol. 1995; 143(1): 1–18. [DOI] [PubMed] [Google Scholar]
- 58.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997; 77(2): 359–96. [DOI] [PubMed] [Google Scholar]
- 59.Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR Jr., Ulick S, Milora RV, Findling JW, et al. Liddle’s syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell. 1994; 79(3): 407–14. [DOI] [PubMed] [Google Scholar]
- 60.Mune T, Rogerson FM, Nikkila H, Agarwal AK, White PC. Human hypertension caused by mutations in the kidney isozyme of 11 beta-hydroxysteroid dehydrogenase. Nat Genet. 1995; 10(4): 394–9. [DOI] [PubMed] [Google Scholar]
- 61.Lifton RP. Molecular genetics of human blood pressure variation. Science. 1996; 272(5262): 676–80. [DOI] [PubMed] [Google Scholar]
- 62.Dahlberg J, Nilsson LO, von Wowern F, Melander O. Polymorphism in NEDD4L is associated with increased salt sensitivity, reduced levels of P-renin and increased levels of Nt-proANP. PLoS ONE. 2007; 2(5): e432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou R, Patel SV, Snyder PM. Nedd4-2 catalyzes ubiquitination and degradation of cell surface ENaC. J Biol Chem. 2007; 282(28): 20207–12. [DOI] [PubMed] [Google Scholar]
- 64.Gomez-Sanchez EP, Gomez-Sanchez CE. Effect of central amiloride infusion on mineralocorticoid hypertension. Am J Physiol. 1994; 267(5 Pt 1): E754–8. [DOI] [PubMed] [Google Scholar]
- 65.Wang H, Leenen FH. Brain sodium channels mediate increases in brain “ouabain” and blood pressure in Dahl S rats. Hypertension. 2002; 40(1): 96–100. [DOI] [PubMed] [Google Scholar]
- 66.Gomez-Sanchez EP, Gomez-Sanchez CE. Effect of central infusion of benzamil on Dahl S rat hypertension. Am J Physiol. 1995; 269(3 Pt 2): H1044–7. [DOI] [PubMed] [Google Scholar]
- 67.Osborn JW, Olson DM, Guzman P, Toney GM, Fink GD. The neurogenic phase of angiotensin II-salt hypertension is prevented by chronic intracerebroventricular administration of benzamil. Physiol Rep. 2014; 2(2): e00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu J, Wang HW, Ahmad M, Keshtkar-Jahromi M, Blaustein MP, Hamlyn JM, Leenen FHH. Central and peripheral slow-pressor mechanisms contributing to Angiotensin II-salt hypertension in rats. Cardiovasc Res. 2018; 114(2): 233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang BS, White RA, Ahmad M, Leenen FH. Role of brain corticosterone and aldosterone in central angiotensin II-induced hypertension. Hypertension. 2013; 62(3): 564–71. [DOI] [PubMed] [Google Scholar]
- 70.Gabor A, Leenen FH. Central mineralocorticoid receptors and the role of angiotensin II and glutamate in the paraventricular nucleus of rats with angiotensin II-induced hypertension. Hypertension. 2013; 61(5): 1083–90. [DOI] [PubMed] [Google Scholar]
- 71.Chen A, Huang BS, Wang HW, Ahmad M, Leenen FH. Knockdown of mineralocorticoid or angiotensin II type 1 receptor gene expression in the paraventricular nucleus prevents angiotensin II hypertension in rats. J Physiol. 2014; 5923523–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen J, Gomez-Sanchez CE, Penman A, May PJ, Gomez-Sanchez E. Expression of mineralocorticoid and glucocorticoid receptors in preautonomic neurons of the rat paraventricular nucleus. American journal of physiology Regulatory, integrative and comparative physiology. 2014; 306(5): R328–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Amin MS, Reza E, Wang H, Leenen FH. Sodium transport in the choroid plexus and salt-sensitive hypertension. Hypertension. 2009; 54(4): 860–7. [DOI] [PubMed] [Google Scholar]
- 74.Nakano M, Hirooka Y, Matsukawa R, Ito K, Sunagawa K. Mineralocorticoid receptors/epithelial Na(+) channels in the choroid plexus are involved in hypertensive mechanisms in stroke-prone spontaneously hypertensive rats. Hypertens Res. 2013; 36(3): 277–84. [DOI] [PubMed] [Google Scholar]
- 75.Amin MS, Wang HW, Reza E, Whitman SC, Tuana BS, Leenen FH. Distribution of epithelial sodium channels and mineralocorticoid receptors in cardiovascular regulatory centers in rat brain. Am J Physiol Regul Integr Comp Physiol. 2005; 289(6): R1787–97. [DOI] [PubMed] [Google Scholar]
- 76.Teruyama R, Sakuraba M, Wilson LL, Wandrey NE, Armstrong WE. Epithelial Na(+) sodium channels in magnocellular cells of the rat supraoptic and paraventricular nuclei. American journal of physiology Endocrinology and metabolism. 2012; 302(3): E273–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han J, Gnatenco C, Sladek CD, Kim D. Background and tandem-pore potassium channels in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 2003; 546(Pt 3): 625–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Teruyama R, Armstrong WE. Changes in the active membrane properties of rat supraoptic neurones during pregnancy and lactation. J Neuroendocrinol. 2002; 14(12): 933–44. [DOI] [PubMed] [Google Scholar]
- 79.Bourque CW. Transient calcium-dependent potassium current in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 1988; 397331–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Teruyama R, Sakuraba M, Wilson LL, Wandrey NE, Armstrong WE. Epithelial Sodium Channels (ENaC) in Magnocellular Cells of the Rat Supraoptic and Paraventricular Nuclei. Am J Physiol Endocrinol Metab. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bicknell RJ. Optimizing release from peptide hormone secretory nerve terminals. J Exp Biol. 1988; 13951–65. [DOI] [PubMed] [Google Scholar]
- 82.Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation- coupling mechanism in the isolated rat neural lobe. J Physiol. 1985; 36945–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brown CH, Bourque CW. Mechanisms of rhythmogenesis: insights from hypothalamic vasopressin neurons. Trends Neurosci. 2006; 29(2): 108–15. [DOI] [PubMed] [Google Scholar]
- 84.Li C, Tripathi PK, Armstrong WE. Differences in spike train variability in rat vasopressin and oxytocin neurons and their relationship to synaptic activity. J Physiol. 2007; 581(Pt 1): 221–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharma K, Haque M, Guidry R, Ueta Y, Teruyama R. Effect of dietary salt intake on epithelial Na+ channels (ENaC) in vasopressin magnocellular neurosecretory neurons in the rat supraoptic nucleus. J Physiol. 2017; 595(17): 5857–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Teruyama R, Armstrong WE. Calcium-dependent fast depolarizing afterpotentials in vasopressin neurons in the rat supraoptic nucleus. J Neurophysiol. 2007; 98(5): 2612–21. [DOI] [PubMed] [Google Scholar]
- 87.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest. 1999; 104(7): R19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Masilamani S, Wang X, Kim GH, Brooks H, Nielsen J, Nielsen S, Nakamura K, Stokes JB, Knepper MA. Time course of renal Na-K-ATPase, NHE3, NKCC2, NCC, and ENaC abundance changes with dietary NaCl restriction. Am J Physiol Renal Physiol. 2002; 283(4): F648–57. [DOI] [PubMed] [Google Scholar]
- 89.Crestani S, Gasparotto Junior A, Marques MC, Sullivan JC, Webb RC, da Silva-Santos JE. Enhanced angiotensin-converting enzyme activity and systemic reactivity to angiotensin II in normotensive rats exposed to a high-sodium diet. Vascul Pharmacol. 2014; 60(2): 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kamari Y, Shimoni N, Koren F, Peleg E, Sharabi Y, Grossman E. High-salt diet increases plasma adiponectin levels independent of blood pressure in hypertensive rats: the role of the renin-angiotensin-aldosterone system. J Hypertens. 2010; 28(1): 95–101. [DOI] [PubMed] [Google Scholar]
- 91.Mills NJ, Sharma K, Haque M, Moore M, Teruyama R. Aldosterone Mediated Regulation of Epithelial Sodium Channel (ENaC) Subunits in the Rat Hypothalamus. Neuroscience. 2018; 390278–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Is aldosterone synthesized within the rat brain? Am J Physiol Endocrinol Metab. 2005; 288(2): E342–6. [DOI] [PubMed] [Google Scholar]
- 93.MacKenzie SM, Clark CJ, Fraser R, Gomez-Sanchez CE, Connell JM, Davies E. Expression of 11beta-hydroxylase and aldosterone synthase genes in the rat brain. J Mol Endocrinol. 2000; 24(3): 321–8. [DOI] [PubMed] [Google Scholar]
- 94.Wang HW, Huang BS, Chen A, Ahmad M, White RA, Leenen FH. Role of brain aldosterone and mineralocorticoid receptors in aldosterone-salt hypertension in rats. Neuroscience. 2016; 31490–105. [DOI] [PubMed] [Google Scholar]
- 95.Inenaga K, Nagatomo T, Kannan H, Yamashita H. Inward sodium current involvement in regenerative bursting activity of rat magnocellular supraoptic neurones in vitro. J Physiol. 1993; 465289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tasker JG, Voisin DL, Armstrong WE. The Cell Biology of Oxytocin and Vasopressin Cells In: Pfaff D, Joels M, eds. Hormones, Brain and Behavior: Elsevier; 2016: 305–36. [Google Scholar]
- 97.El Majdoubi M, Poulain DA, Theodosis DT. Activity-dependent morphological synaptic plasticity in an adult neurosecretory system: magnocellular oxytocin neurons of the hypothalamus. Biochem Cell Biol. 2000; 78(3): 317–27. [PubMed] [Google Scholar]
- 98.Hatton GI. Function-related plasticity in hypothalamus. Annu Rev Neurosci. 1997; 20375–97. [DOI] [PubMed] [Google Scholar]
- 99.Theodosis DT, Trailin A, Poulain DA. Remodeling of astrocytes, a prerequisite for synapse turnover in the adult brain? Insights from the oxytocin system of the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2006; 290(5): R1175–82. [DOI] [PubMed] [Google Scholar]
- 100.Choe KY, Han SY, Gaub P, Shell B, Voisin DL, Knapp BA, Barker PA, Brown CH, Cunningham JT, Bourque CW. High Salt Intake Increases Blood Pressure via BDNF-Mediated Downregulation of KCC2 and Impaired Baroreflex Inhibition of Vasopressin Neurons. Neuron. 2015; 85(3): 549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miyata S, Nakashima T, Kiyohara T. Structural dynamics of neural plasticity in the supraoptic nucleus of the rat hypothalamus during dehydration and rehydration. Brain Res Bull. 1994; 34(3): 169–75. [DOI] [PubMed] [Google Scholar]
- 102.Di S, Tasker JG. Dehydration-induced synaptic plasticity in magnocellular neurons of the hypothalamic supraoptic nucleus. Endocrinology. 2004; 145(11): 5141–9. [DOI] [PubMed] [Google Scholar]
- 103.Kim JS, Kim WB, Kim YB, Lee Y, Kim YS, Shen FY, Lee SW, Park D, Choi HJ, Hur J, Park JJ, Han HC, Colwell CS, Cho YW, Kim YI. Chronic hyperosmotic stress converts GABAergic inhibition into excitation in vasopressin and oxytocin neurons in the rat. J Neurosci. 2011; 31(37): 13312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Konopacka A, Qiu J, Yao ST, Greenwood MP, Greenwood M, Lancaster T, Inoue W, Mecawi AS, Vechiato FM, de Lima JB, Coletti R, Hoe SZ, Martin A, Lee J, Joseph M, Hindmarch C, Paton J, Antunes-Rodrigues J, Bains J, Murphy D. Osmoregulation requires brain expression of the renal Na-K-2Cl cotransporter NKCC2. J Neurosci. 2015; 35(13): 5144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee SW, Kim YB, Kim JS, Kim WB, Kim YS, Han HC, Colwell CS, Cho YW, In Kim Y. GABAergic inhibition is weakened or converted into excitation in the oxytocin and vasopressin neurons of the lactating rat. Mol Brain. 2015; 834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morton LA, Popescu IR, Haam J, Tasker JG. Short-term potentiation of GABAergic synaptic inputs to vasopressin and oxytocin neurones. J Physiol. 2014; 592(19): 4221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haam J, Halmos KC, Di S, Tasker JG. Nutritional state-dependent ghrelin activation of vasopressin neurons via retrograde trans-neuronal-glial stimulation of excitatory GABA circuits. J Neurosci. 2014; 34(18): 6201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim YB, Kim WB, Jung WW, Jin X, Kim YS, Kim B, Han HC, Block GD, Colwell CS, Kim YI. Excitatory GABAergic Action and Increased Vasopressin Synthesis in Hypothalamic Magnocellular Neurosecretory Cells Underlie the High Plasma Level of Vasopressin in Diabetic Rats. Diabetes. 2018; 67(3): 486–95. [DOI] [PubMed] [Google Scholar]
- 109.Perlmutter LS, Tweedle CD, Hatton GI. Neuronal/glial plasticity in the supraoptic dendritic zone in response to acute and chronic dehydration. Brain Res. 1985; 361(1-2): 225–32. [DOI] [PubMed] [Google Scholar]
- 110.Curras-Collazo MC, Dao J. Osmotic activation of the hypothalamo-neurohypophysial system reversibly downregulates the NMDA receptor subunit, NR2B, in the supraoptic nucleus of the hypothalamus. Brain Res Mol Brain Res. 1999; 70(2): 187–96. [DOI] [PubMed] [Google Scholar]
- 111.Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol. 2006; 16(3): 288–97. [DOI] [PubMed] [Google Scholar]
- 112.Bowie D Polyamine-mediated channel block of ionotropic glutamate receptors and its regulation by auxiliary proteins. J Biol Chem. 2018; 293(48): 18789–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992; 318(3): 329–54. [DOI] [PubMed] [Google Scholar]
- 114.Stern JE, Galarreta M, Foehring RC, Hestrin S, Armstrong WE. Differences in the properties of ionotropic glutamate synaptic currents in oxytocin and vasopressin neuroendocrine neurons. J Neurosci. 1999; 19(9): 3367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006; 125(4): 775–84. [DOI] [PubMed] [Google Scholar]
- 116.Parnas I, Parnas H. Calcium is essential but insufficient for neurotransmitter release: the calcium-voltage hypothesis. J Physiol (Paris). 1986; 81(4): 289–305. [PubMed] [Google Scholar]
- 117.De Crescenzo V, Fogarty KE, Zhuge R, Tuft RA, Lifshitz LM, Carmichael J, Bellve KD, Baker SP, Zissimopoulos S, Lai FA, Lemos JR, Walsh JV Jr. Dihydropyridine receptors and type 1 ryanodine receptors constitute the molecular machinery for voltage-induced Ca2+ release in nerve terminals. J Neurosci. 2006; 26(29): 7565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.De Crescenzo V, ZhuGe R, Velazquez-Marrero C, Lifshitz LM, Custer E, Carmichael J, Lai FA, Tuft RA, Fogarty KE, Lemos JR, Walsh JV Jr. Ca2+ syntillas, miniature Ca2+ release events in terminals of hypothalamic neurons, are increased in frequency by depolarization in the absence of Ca2+ influx. J Neurosci. 2004; 24(5): 1226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Velázquez-Marrero C. CEE, Marrero H., Ortiz-Miranda S., Lemos JR . Voltage-mediated calcium dependence of neuropeptide release from magnocellular neuronal terminals. Journal of Neuroendocrinology. 2019. in press. [Google Scholar]
- 120.Zhang C, Zhou Z. Ca(2+)-independent but voltage-dependent secretion in mammalian dorsal root ganglion neurons. Nat Neurosci. 2002; 5(5): 425–30. [DOI] [PubMed] [Google Scholar]
- 121.Marrero HG, Lemos JR. Frequency-dependent potentiation of voltage-activated responses only in the intact neurohypophysis of the rat. Pflugers Arch. 2005; 450(2): 96–110. [DOI] [PubMed] [Google Scholar]
- 122.Leng G, Shibuki K. Extracellular potassium changes in the rat neurohypophysis during activation of the magnocellular neurosecretory system. J Physiol. 1987; 39297–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Leng G, Shibuki K, Way SA. Effects of raised extracellular potassium on the excitability of, and hormone release from, the isolated rat neurohypophysis. J Physiol. 1988; 399591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Perlmutter LS, Tweedle CD, Hatton GI. Neuronal/glial plasticity in the supraoptic dendritic zone: dendritic bundling and double synapse formation at parturition. Neuroscience. 1984; 13(3): 769–79. [DOI] [PubMed] [Google Scholar]
- 125.Hamada T, Liou SY, Fukushima T, Maruyama T, Watanabe S, Mikoshiba K, Ishida N. The role of inositol trisphosphate-induced Ca2+ release from IP3-receptor in the rat suprachiasmatic nucleus on circadian entrainment mechanism. Neurosci Lett. 1999; 263(2-3): 125–8. [DOI] [PubMed] [Google Scholar]
- 126.Dayanithi G, Lemos JR. Hypothalamic Somata vs. Neurohypophysial Terminals In: Lemos JR, Dayanithi G, eds. Neurosecretion: Secretory Mechanisms: Springer Nature; 2019: in press. [Google Scholar]
- 127.Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002; 418(6893): 85–9. [DOI] [PubMed] [Google Scholar]