Abstract
Aims: Small dense low-density lipoprotein cholesterol (sdLDL-C) and remnant-like particle cholesterol (RLP-C) are the novel atherosclerotic risk factors and might be strongly associated with inflammation. The basic evidence supports that sdLDL and RLP have some different mechanisms inducing an inflammatory response. Many studies have focused on the mechanism of inflammation of sdLDL-C or RLP-C per se, with limited data on the association between sdLDL-C and RLP-C in the real-world, population-based setting. Thus, the aim of this study was to investigate the association between sdLDL-C and RLP-C with inflammation.
Methods: We examined the baseline cross-sectional data of participants from the Jichi Medical School-II Cohort Study. In total, 5,305 participants (2,439 men and 2,866 women) were included in this study.
Results: Of all quartiles of sdLDL-C, the fourth had the highest high-sensitivity C-reactive protein (hs-CRP) level. Once adjusted for age, sex, smoking status, homeostasis model assessment of insulin resistance, antidyslipidemic and antihyperglycemic medication use, and RLP-C, sdLDL-C was significantly and positively associated with hs-CRP (geometric mean, 95% confidence interval (CI), 0.36 mg/L (0.34–0.38 mg/L), 0.37 mg/L (0.35–0.39 mg/L), 0.40 mg/L (0.37–0.42 mg/L) versus 0.44 mg/L (0.42–0.47 mg/L), P < 0.001 for trend). After stratifying the participants into four sdLDL-C×four RLP-C categories, the group in the fourth sdLDL-C quartile and the forth RLP-C quartile had the highest hs-CRP level (geometric mean, 95% CI, 0.52 mg/L, 0.48–0.57 mg/L, interaction P = 0.75).
Conclusions: SdLDL-C and RLP-C had different associations with inflammation. Our results support sdLDL-C as the potential novel factor of cardiovascular disease, independently of RLP-C.
Keywords: Small dense low-density lipoprotein cholesterol, Remnant-like particle cholesterol, High-sensitivity C-reactive protein, Low-grade inflammation
Introduction
The relationship between hypercholesterolemia and cardiovascular disease (CVD) has been well established1). Guidelines for hypercholesterolemia in multiple countries recommend lowering low-density lipoprotein cholesterol (LDL-C) as the primary management target2–4). Although there is no doubt that LDL-C is one of the most important risk factors of CVD5), even when LDL-C is lowered to recommended levels, cardiovascular events continue to occur. Therefore, we need to manage the novel target as the primary management, or the residual risk of CVD beyond lowering LDL-C6).
Small dense low-density lipoprotein cholesterol (sdLDL-C) associated with triglyceride-rich lipoprotein (TRL) metabolism, and TRL-cholesterol, which is also known as remnant cholesterol, might be the better factors for the prediction of CVD than total LDL-C5, 7–11). In fact, elevated sdLDL-C and remnant cholesterol levels can be found in patients with metabolic abnormalities, such as type 2 diabetes mellitus12) and metabolic syndrome (MetS)13, 14), which have been found as highly atherogenic conditions with lowgrade inflammation15).
SdLDL particles were defined as LDL with an average diameter of < 25.5 nm and had some features related to atherogenic potential, such as increased penetration into the arterial wall by the small sizes, a longer circulation time with low affinity for the LDL receptors, and high susceptibility to oxidation by containing little antioxidative vitamins5, 16–18). The Atherosclerosis Risk in Communities study has shown that the cumulative incidence of coronary heart disease (CHD) events was higher among individuals with higher sdLDL-C (hazard ratio (HR): 1.61–1.86) regardless of LDL-C levels, and the Québec Cardiovascular Study has shown that the risk of CHD was increased among men with elevated sdLDL-C (HR:2.1–3.6)9, 10). A roughly-11-year prospective study in Japan also suggested that sdLDL-C could be an independent risk factor for CVD, stroke, and CHD5). On the other hand, remnant-like particles (RLPs) are highly heterogeneous in size and composition, and RLP cholesterol (RLP-C) is composed of the cholesterol content of very low-density lipoprotein (VLDL) and intermediate-density lipoprotein in the fasted state, and of the two lipoproteins together with chylomicron remnant in the postprandial state7). There was a causal association between RLP-C and early signs of atherosclerosis with endothelial dysfunction19, 20). Varbo et al. suggested that there was a causal association between elevated nonfasting RLP-C and low-grade inflammation, together with increased risk of CHD7). Most studies suggested that high RLP-C concentrations, especially in the postprandial state, increase the risk of atherosclerosis and CHD8).
The basic and subclinical studies support that sdLDLs and RLPs stimulate the innate and adaptive immune cells and induce the inflammatory response playing a primordial role in the pathogenesis of atherosclerosis21, 22). Basic evidence suggests that sdLDL and RLP have some different mechanisms inducing inflammation by activating these immune cells. Smaller LDL particles may have increased proteoglycan binding23); however, the interaction of sdLDLs, with endothelial cells, macrophages, T cells, and B cells is still under investigation. The high susceptibility to oxidation of sdLDLs contributes to the increased level of modified LDLs. The local inflammation might be further exacerbated by several receptors-dependent inflammatory responses induced by modified LDL24). On the other hand, RLP, not LDL (1019 < d < 1063 g/mL), increased the expression levels of adhesion molecules on endothelial cells without binding to glycoproteins that were located at the surface of the endothelium and interleukin-1β, which induced vascular inflammation20, 25, 26).
Many studies have focused on the mechanism of the inflammation of sdLDL-C or RLP-C per se, with limited data on the association between sdLDL-C and RLP-C in the real-world, population-based setting. Thus, the aim of this study was to investigate the association between sdLDL-C and RLP-C with inflammation.
Methods
Population
The present cross-sectional study was conducted as part of the Jichi Medical School (JMS)-II Cohort Study, a population-based cohort study of 6,436 participants started in 2010 to evaluate the relationship between the risk factors of atherosclerosis and CVD in ordinary Japanese people. Data were obtained from 13 rural districts in Japan between April 2010 and December 2017. In each community, a local government office sent personal invitations for mass CVD screenings to all the subjects by mail. In this study protocol, we selected Japanese people who had undertaken the screening of basic health examination that was conducted in accordance with the medical care system for the elderly and obtained informed consent for this study. The subjects were residents in the Shimotsuke, Kakara, Sue, Omori, Kamiichi, Wara, Takasu, Onabi, Nakatsu, Yame, Miwa, Ueno, and Saji areas of Japan. SdLDL-C and RLP-C were measured in samples from 5,862 participants (2,737 men and 3,125 women). After excluding 557 participants with past history of cancer and/or values of high-sensitivity C-reactive protein (hs-CRP) in excess of 10 mg/L that might represent as an acute-phase response caused by an underlying inflammatory disease, 5,305 participants (2,439 men and 2,866 women) were included in this study.
Measurements
Patients' height, weight, and waist circumference (WC) were measured using a standardized method, with 0.1 cm, 0.1 kg, and 0.1 cm units of measurement, respectively. Height was measured with stockinged feet. Weight was recorded with the subjects clothed. WC was measured at the navel level while standing, with light exhalation. WC measurements were taken once. Body mass index (BMI) was calculated as weight (kg)/height (m)2. Systolic and diastolic blood pressures (BP) were measured with a fully automated sphygmomanometer (Omron HEM-759P; Omron Healthcare Inc., Kyoto, Japan) placed on the right arm of the subjects, who had been resting while seated for five minutes before measurement. BP measurements were repeated twice in the sitting position after a 15-s interval.
Blood samples were taken after overnight fasting and were sent to an external laboratory (SRL, Tokyo, Japan) to measure total cholesterol, triglycerides (TGs), LDL-C, high-density lipoprotein cholesterol (HDL-C), fasting blood glucose, fasting insulin, hs-CRP, RLP-C, and sdLDL-C levels. Total cholesterol was measured by a cholesterol dehydrogenase-ultraviolet method. TG was measured using an enzymatic method. LDL-C and HDL-C were measured via direct methods. Fasting blood glucose was measured by a hexokinase ultraviolet absorption spectrophotometry. Fasting insulin was measured by a chemiluminescent enzyme immunoassay method. Hs-CRP was measured by a nephelometric assay (Inter-assay coefficient of variation (CV): 1.55%, intra-assay CV: 2.21%). RLP-C was measured using an enzymatic method (Until March 2016, Inter-assay CV: 1.65%, intra-assay CV: 2.81%, and since April 2016, Interassay CV: 1.80%, intra-assay CV: 1.96%). The sdLDL-C level is directly and selectively measured using the use of available kits (sdLDL-EX from Denka Seiken, Tokyo, Japan). Serum lipid markers, including sdLDL-C, were measured by the standardized program proposed by the Clinical and Laboratory Standards Institute. The homeostasis model assessment of insulin resistance (HOMA-IR) was defined as the product of fasting insulin (µU/mL) and glucose (mg/dL) divided by 405 27). Information about medical history and lifestyle was obtained with a self-reported questionnaire. Questions about past or present illness were asked, and if present, whether the subjects took medicine or not was confirmed. Smoking status was classified as smoking, ex-smoking, or never. All the participants included in the present study provided written informed consent prior to inclusion, and the ethics committees of Jichi Medical University (Tochigi, Japan) and other participating institutions approved the JMS-II Cohort Study protocol (IRB No. G09-39 [G17-64 revised]).
Definition
According to Adult Treatment Panel III guidelines28), MetS was defined as the presence of at least three of five components: abdominal obesity (given as WC [men > 102 cm, women > 88 cm]), elevated TG (≥ 150 mg/dL), low HDL-C (men < 40 mg/dL, women < 50 mg/dL), systolic BP ≥ 130 mmHg and/or diastolic BP ≥ 85 mmHg, and high fasting glucose (≥ 110 mg/dL). However, Japanese people are relatively small; therefore, we adopted WC of > 85 cm for men and > 90 cm for women as abdominal obesity, as proposed by the Japan Society for the Study of Obesity (JASSO)28, 29). We also defined BMI ≥ 25 as obesity, as proposed by JASSO. Diabetes mellitus was defined as a fasting blood glucose ≥ 126 mg/dL and/or currently being under medication.
Statistical Analysis
Data are expressed as mean ± standard deviation, except for sdLDL-C, RLP-C, hs-CRP, TG, and HOMA-IR. The distributions of these markers were highly skewed; these data were expressed as the median and interquartile range and transformed into natural logarithms before statistical analysis. P-values for trends across quartiles of sdLDL-C and RLP-C were assessed in an unadjusted model using the Jonckheere-Terpstra test and logistic regression analysis, using quartiles as ordinal variables. Adjusted geometric mean differences in hs-CRP across each quartile were calculated using linear regression analysis. A basic model (model 1) was adjusted for age (continuous), sex, and smoking status, additionally for HOMA-IR, antidyslipidemic medication use, and antihyperglycemic medication use (model 2), and additionally for RLP-C or sdLDL-C (model 3). Odds ratios (OR) and their 95% confidence interval (CI) were estimated with logistic regression models to quantify the association of quartiles of sdLDL-C or RLP-C with obesity, abdominal obesity, and MetS. Pearson's linear regression analysis was used to evaluate the relationship between sdLDL-C and RLP-C in the participants. Statistical significance was defined as p < 0.05. Statistical analyses were performed using SPSS version 24 (IBM, Chicago, IL, USA).
Results
Of the 5,305 participants (2,439 men and 2,866 women) in the JMS-II cohort, we classified each sdLDL-C and RLP-C levels into four quartiles, and the baseline characteristics are shown in Tables 1 and 2, respectively. There were significant positive associations between sdLDL-C and RLP-C levels and traditional atherosclerotic risk factors (BMI, WC, BP, serum lipid status, diabetes status, and smoking status). Subjects in the highest sdLDL-C quartile had the highest lipid levels, except for HDL-C. Increasing quartiles of sdLDL-C and RLP-C were significantly associated with increased risks of obesity, abdominal obesity, MetS, and diabetes mellitus. Once adjusted for age, sex, and smoking status, for the second, third, and fourth sdLDL-C quartiles versus the first, the OR (95% CI) for MetS were 1.26 (0.94–1.68), 2.97 (2.30–3.83), and 8.04 (6.31–10.2) (P < 0.001 for trend). For the second, third, and fourth RLP-C quartiles versus the first, the OR (95% CI) for MetS were 1.83 (1.38–2.42), 3.49 (2.69–4.52), and 8.98 (7.00–11.5) (P < 0.001 for trend) (Supplementary Table 1). Subjects with sdLDL-C and/or RLP-C levels in the highest quartile had the profiles of metabolic abnormalities.
Table 1. Baseline Characteristics and Distribution of Atherosclerotic Risk Factors across Small Dense Low-Density Lipoprotein Cholesterol (sdLDL-C) Quartiles.
| Quartiles of sdLDL-C, mg/dL |
|||||
|---|---|---|---|---|---|
| ≦ 24.2 | > 24.2 and ≦ 32.6 | > 32.6 and ≦ 44.2 | > 44.2 | ||
| N = 1,323 | N = 1,317 | N = 1,337 | N = 1,328 | P for trend | |
| Age, years | 63.0 ± 12.8 | 64.5 ± 11.4 | 64.5 ± 10.5 | 63.5 ± 10.1 | P = 0.865 |
| Male, % | 41 | 41 | 45 | 57 | |
| BMI, kg/m2 | 22.2 ± 3.3 | 22.8 ± 3.2 | 23.3 ± 3.3 | 24.1 ± 3.3 | P < 0.001 |
| Waist circumference, cm | 79.9 ± 9.1 | 81.8 ± 8.5 | 83.6 ± 8.8 | 85.8 ± 8.4 | P < 0.001 |
| Triglycerides, mg/dL | 68.0 (53.0, 88.0) | 81.0 (64.0, 104.0) | 103.0 (88.0, 134.0) | 153.0 (111.0, 207.8) | P < 0.001 |
| HDL-C, mg/dL | 63.9 ± 15.2 | 62.6 ± 14.4 | 58.4 ± 14.8 | 54.0 ± 12.5 | P < 0.001 |
| RLP-C, mg/dL | 3.7 (2.8, 5.4) | 4.2 (3.1, 6.2) | 5.0 (3.8, 7.6) | 7.3 (5.1, 12.3) | P < 0.001 |
| Total cholesterol, mg/dL | 177.9 ± 24.2 | 201.2 ± 24.7 | 211.8 ± 28.1 | 229.5 ± 30.9 | P < 0.001 |
| LDL-C, mg/dL | 94.5 ± 20.4 | 116.8 ± 21.0 | 127.4 ± 24.6 | 139.1 ± 29.9 | P < 0.001 |
| Systolic blood pressure, mmHg | 131.6 ± 20.4 | 135.0 ± 20.2 | 137.0 ± 20.5 | 139.8 ± 20.6 | P < 0.001 |
| Diastolic blood pressure, mmHg | 77.3 ± 11.3 | 79.2 ± 11.0 | 80.3 ± 11.6 | 83.0 ± 12.0 | P < 0.001 |
| Fasting glucose, mg/dL | 97.5 ± 17.4 | 98.2 ± 16.2 | 101.6 ± 20.9 | 103.4 ± 21.7 | P < 0.001 |
| HOMA-IR | 0.84 (0.55, 1.30) | 0.98 (0.65, 1.53) | 1.14 (0.76, 1.81) | 1.39 (0.88, 2.17) | P < 0.001 |
| Smoking | |||||
| Current, % | 12 | 11 | 14 | 16 | P = 0.001 |
| EX, % | 23 | 24 | 26 | 32 | P < 0.001 |
| Never, % | 65 | 65 | 60 | 52 | P < 0.001 |
| Obesity, %* | 17 | 20 | 26 | 34 | P < 0.001 |
| Metabolic syndrome, % | 7 | 9 | 19 | 39 | P < 0.001 |
| Diabetes mellitus, %** | 11 | 9 | 11 | 12 | P = 0.038 |
| Antihyperglycemic medication use, % | 8 | 6 | 8 | 7 | P = 0.467 |
| Antidyslipidemic medication use, % | 10 | 12 | 12 | 11 | P = 0.828 |
| Past history$ | |||||
| Dyslipidemia, % | 14 | 18 | 22 | 30 | P < 0.001 |
| Stroke, % | 3 | 2 | 2 | 1 | P = 0.119 |
| Myocardial infarction, % | 3 | 2 | 2 | 2 | P = 0.031 |
Data are expressed as mean ± standard deviation (SD), %, and median (25th percentile, 75th percentile). P-values for trends across quartiles of sdLDL-C were assessed in an unadjusted model using the Jonckheere–Terpstra test and logistic regression analysis using quartiles as ordinal variables. BMI = body mass index; HDL-C = high-density lipoprotein cholesterol; RLP-C = remnant-like particle cholesterol; LDL-C = low-density lipoprotein cholesterol.
Obesity was considered BMI ≥ 25.
Diabetes mellitus was fasting blood glucose . 126 mg/dL and/or currently under medication.
Data were obtained by questionnaire.
Table 2. Baseline Characteristics and Distribution of Atherosclerotic Risk Factors across Remnant-Like Particle Cholesterol (RLP-C) Quartiles.
| Quartiles of RLP-C, mg/dL |
|||||
|---|---|---|---|---|---|
| ≦ 3.4 | > 3.4 and ≦ 4.9 | > 4.9 and ≦ 7.7 | > 7.7 | ||
| N = 1,329 | N = 1,304 | N = 1,375 | N = 1,297 | P for trend | |
| Age, years | 64.1 ± 11.6 | 64.3 ± 11.3 | 64.2 ± 10.7 | 62.8 ± 11.2 | P = 0.001 |
| Male, % | 47 | 42 | 42 | 53 | |
| BMI, kg/m2 | 22.2 ± 3.1 | 22.9 ± 3.3 | 23.3 ± 3.4 | 23.9 ± 3.3 | P < 0.001 |
| Waist circumference, cm | 80.3 ± 8.6 | 82.3 ± 8.9 | 83.5 ± 9.1 | 85.0 ± 8.6 | P < 0.001 |
| Triglycerides, mg/dL | 65.0 (51.0, 82.0) | 84.0 (65.0, 106.0) | 107.0 (79.0, 138.0) | 158.0 (115.0, 220.0) | P < 0.001 |
| HDL-C, mg/dL | 63.5 ± 13.9 | 62.3 ± 14.9 | 59.8 ± 14.8 | 53.1 ± 13.4 | P < 0.001 |
| SdLDL-C, mg/dL | 25.3 (20.2, 31.7) | 31.4 (24.2, 40.8) | 36.5 (26.8, 47.2) | 42.3 (30.6, 55.7) | P < 0.001 |
| Total cholesterol, mg/dL | 187.2 ± 27.3 | 204.1 ± 28.7 | 212.9 ± 31.7 | 216.3 ± 35.6 | P < 0.001 |
| LDL-C, mg/dL | 104.9 ± 24.1 | 120.0 ± 26.0 | 127.2 ± 28.7 | 125.9 ± 32.3 | P < 0.001 |
| Systolic blood pressure, mmHg | 134.1 ± 21.3 | 135.2 ± 19.5 | 136.7 ± 21.4 | 137.4 ± 20.0 | P < 0.001 |
| Diastolic blood pressure, mmHg | 78.7 ± 11.8 | 79.3 ± 11.2 | 80.4 ± 11.5 | 81.6 ± 12.1 | P < 0.001 |
| Fasting glucose, mg/dL | 98.4 ± 16.8 | 99.0 ± 16.7 | 100.9 ± 20.5 | 102.5 ± 22.6 | P < 0.001 |
| HOMA-IR | 0.88 (0.58, 1.37) | 1.04 (0.65, 1.64) | 1.12 (0.73, 1.81) | 1.31 (0.83, 2.11) | P < 0.001 |
| Smoking | |||||
| Current, % | 11 | 12 | 12 | 17 | P < 0.001 |
| EX, % | 28 | 24 | 25 | 29 | P = 0.340 |
| Never, % | 61 | 64 | 62 | 54 | P < 0.001 |
| Obesity, %* | 16 | 21 | 27 | 33 | P < 0.001 |
| Metabolic syndrome, % | 7 | 11 | 18 | 37 | P < 0.001 |
| Diabetes mellitus, %** | 9 | 10 | 11 | 13 | P = 0.001 |
| Antihyperglycemic medication use, % | 6 | 7 | 7 | 8 | P = 0.152 |
| Antidyslipidemic medication use, % | 10 | 11 | 11 | 13 | P = 0.035 |
| Past history$ | |||||
| Dyslipidemia, % | 14 | 20 | 23 | 27 | P < 0.001 |
| Stroke, % | 3 | 1 | 2 | 2 | P = 0.283 |
| Myocardial infarction, % | 3 | 1 | 2 | 2 | P = 0.404 |
Data are expressed as mean ± standard deviation (SD), %, and median (25th percentile, 75th percentile). P-values for trends across quartiles of RLP-C were assessed in an unadjusted model using the Jonckheere–Terpstra test and logistic regression analysis using quartiles as ordinal variables. BMI = body mass index; HDL-C = high-density lipoprotein cholesterol; SdLDL-C = small dense low-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol.
Obesity was considered BMI ≥ 25.
Diabetes mellitus was fasting blood glucose ≥ 126 mg/dL and/or currently under medication.
Data were obtained by questionnaire.
Supplementary Table 1. Percentages and Adjusted Odds Ratio (95% CI) for Obesity, Abdominal Obesity, and Metabolic Syndrome for Comparison of the Three Highest Small Dense Low-Density Lipoprotein Cholesterol (sdLDL-C) Quartiles and Remnant-Like Particle Cholesterol (RLP-C) Quartiles with the First Quartile.
| Quartiles of sdLDL-C, mg/dL |
Quartiles of RLP-C, mg/dL |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P for trend | Q1 | Q2 | Q3 | Q4 | P for trend | |
| Obesity, %* | 17 | 20 | 26 | 34 | P < 0.001 | 16 | 21 | 27 | 33 | P < 0.001 |
| model 1 | 1.00 (Reference) | 1.29 (1.06–1.58) | 1.83 (1.51–2.21) | 2.55 (2.12–3.07) | 1.00 (Reference) | 1.42 (1.17–1.73) | 1.94 (1.61–2.34) | 2.50 (2.08–3.02) | ||
| Abdominal Obesity, %** | 19 | 23 | 30 | 41 | P < 0.001 | 20 | 27 | 30 | 37 | P < 0.001 |
| model 1 | 1.00 (Reference) | 1.33 (1.10–1.62) | 1.89 (1.56–2.28) | 2.69 (2.24–3.23) | 1.00 (Reference) | 1.63 (1.35–1.98) | 1.91 (1.59–2.30) | 2.40 (1.99–2.88) | ||
| Metabolic syndrome, % | 7 | 9 | 19 | 39 | P < 0.001 | 7 | 11 | 18 | 37 | P < 0.001 |
| model 1 | 1.00 (Reference) | 1.26 (0.94–1.68) | 2.97 (2.30–3.83) | 8.04 (6.31–10.2) | 1.00 (Reference) | 1.83 (1.38–2.42) | 3.49 (2.69–4.52) | 8.98 (7.00–11.5) | ||
Odds ratios are estimated from logistic regression models with adjustments for age, sex, and smoking status.
Obesity was considered BMI ≥ 25.
Abdominal obesity was considered a waist circumference of > 85 cm for men and > 90 cm for women.
Of all quartiles of sdLDL-C, the fourth had the highest hs-CRP levels. After adjustments were made for age, sex, and smoking status, sdLDL-C levels were associated with higher hs-CRP. This association also remained significant after additionally adjusting for HOMA-IR, antidyslipidemic medication use, antihyperglycemic medication use, and RLP-C (geometric mean, 95% CI, 0.36 mg/L (0.34–0.38 mg/L), 0.37 mg/L (0.35–0.39 mg/L), 0.40 mg/L (0.37–0.42 mg/L) versus 0.44 mg/L (0.42–0.47 mg/L), P < 0.001 for trend) (Table 3). Even after adjustment, RLP-C also showed that the fourth had the highest hs-CRP level (geometric mean, 95% CI, 0.35 mg/L (0.33–0.37 mg/L), 0.40 mg/L (0.38–0.43 mg/L), 0.40 mg/L (0.38–0.42 mg/L) versus 0.42 mg/L (0.40–0.45 mg/L), P < 0.001 for trend) (Table 4).
Table 3. Geometric Mean Values and Adjusted Differences (95% CI) in High-Sensitivity C-Reactive Protein (hs-CRP) across Small Dense Low-Density Lipoprotein Cholesterol (sdLDL-C) Quartiles.
| Quartiles of sdLDL-C, mg/dL |
|||||
|---|---|---|---|---|---|
| ≦ 24.2 | > 24.2 and ≦ 32.6 | > 32.6 and ≦ 44.2 | > 44.2 | ||
| N = 1,323 | N = 1,317 | N = 1,337 | N = 1,328 | P for trend | |
| SdLDL-C, mg/dL | 18.6 | 28.2 | 37.7 | 56.0 | P < 0.001 |
| Hs-CRP, mg/L | 0.322 | 0.355 | 0.407 | 0.505 | P < 0.001 |
| model 1 | 0.331 (0.312–0.351) | 0.359 (0.300–0.381) | 0.404 (0.381–0.428) | 0.490 (0.462–0.519) | P < 0.001 |
| model 2 | 0.349 (0.329–0.371) | 0.365 (0.344–0.387) | 0.397 (0.374–0.421) | 0.463 (0.436–0.492) | P < 0.001 |
| model 3 | 0.361 (0.340–0.384) | 0.371 (0.350–0.394) | 0.395 (0.372–0.418) | 0.444 (0.417–0.472) | P < 0.001 |
Data are expressed as geometric mean. Data (95% CI) in tables are estimated from linear regression models with adjustments for age, sex, and smoking status (model 1), as well as additionally for HOMA-IR (log-transformed), antidyslipidemic medication use, and antihyperglycemic medication use (model 2), and additionally for remnant-like particle cholesterol (RLP-C) (log-transformed) (model 3).
Table 4. Geometric Mean Values and Adjusted Differences (95% CI) in High-Sensitivity C-Reactive Protein (hs-CRP) across Remnant-Like Particle Cholesterol (RLP-C) Quartiles.
| Quartiles of RLP-C, mg/dL |
|||||
|---|---|---|---|---|---|
| ≦ 3.4 | > 3.4 and ≦ 4.9 | > 4.9 and ≦ 7.7 | > 7.7 | ||
| N = 1,329 | N = 1,304 | N = 1,375 | N = 1,297 | P for trend | |
| RLP-C, mg/dL | 2.6 | 4.1 | 6.0 | 13.8 | P < 0.001 |
| Hs-CRP, mg/L | 0.315 | 0.392 | 0.406 | 0.470 | P < 0.001 |
| model 1 | 0.314 (0.296–0.333) | 0.397 (0.373–0.421) | 0.409 (0.386–0.434) | 0.463 (0.436–0.491) | P < 0.001 |
| model 2 | 0.330 (0.311–0.350) | 0.401 (0.378–0.425) | 0.405 (0.383–0.429) | 0.440 (0.414–0.467) | P < 0.001 |
| model 3 | 0.345 (0.325–0.367) | 0.404 (0.381–0.428) | 0.399 (0.376–0.422) | 0.423 (0.398–0.450) | P < 0.001 |
Data are expressed as geometric mean. Data (95% CI) in tables are estimated from linear regression models with adjustments for age, sex, and smoking status (model 1), as well as additionally for HOMA-IR (log-transformed), antidyslipidemic medication use, and antihyperglycemic medication use (model 2), and additionally for small dense low-density lipoprotein cholesterol (sdLDL-C) (log-transformed) (model 3).
After stratifying the participants into four sdLDL-C × four RLP-C categories and adjusting for age, sex, and smoking status, the group in the fourth sdLDL-C quartile and the fourth RLP-C quartile had the highest hs-CRP levels (Geometric mean, 95% CI, 0.52 mg/L, 0.48–0.57 mg/L) (Fig. 1). As shown in Table 5 and Fig. 1, sdLDL-C levels were apparently not associated with hs-CRP levels in subjects with the lowest RLP-C group. The result by analysis, including all subjects with a history of cancer and/or inflammatory disease, appeared similar (data not shown). The sub-analysis showed that in the young- to middle-age group (under 65 years) with the lowest RLP-C, the group with the highest sdLDL-C had the highest values of hs-CRP. Conversely, in the elderly group (65 years and over), the group with low sdLDL-C had the highest values of hs-CRP (Supplementary Table 2 and Supplementary Fig. 1). SdLDL-C levels were positively associated with RLP-C (Pearson's r = 0.443, P < 0.001), and the interaction effect of sdLDL-C and RLP-C on low-grade inflammation was not observed (P = 0.754).
Fig. 1.
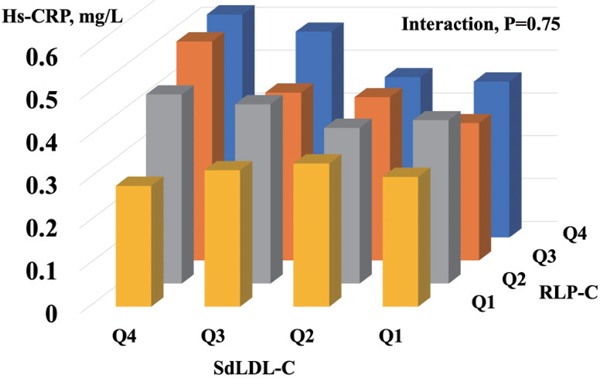
Geometric Mean Values of High-Sensitivity C-Reactive Protein (hs-CRP) After Stratifying the Participants into Four Small Dense Low-Density Lipoprotein Cholesterol (sdLDL-C) × Four Remnant-Like Particle Cholesterol (RLP-C) Categories and Adjusting for Age, Sex, and Smoking Status
Table 5. Geometric Mean Values and Adjusted Differences (95% CI) of High-Sensitivity C-Reactive Protein (hs-CRP) by Small Dense Low-Density Lipoprotein Cholesterol (sdLDL-C) and Remnant-Like Particle Cholesterol (RLP-C) Quartiles.
| SdLDL-C, mg/dL |
||||
|---|---|---|---|---|
| RLP-C, mg/dL | Q4 | Q3 | Q2 | Q1 |
| Q4 | ||||
| subjects | 605 | 319 | 223 | 149 |
| Hs-CRP, mg/L | 0.521 | 0.481 | 0.375 | 0.364 |
| (95%CI) | (0.478–0.569) | (0.427–0.542) | (0.324–0.432) | (0.305–0.433) |
| Q3 | ||||
| subjects | 441 | 396 | 276 | 261 |
| Hs-CRP, mg/L | 0.511 | 0.392 | 0.381 | 0.321 |
| (95%CI) | (0.462–0.567) | (0.352–0.437) | (0.335–0.434) | (0.281–0.366) |
| Q2 | ||||
| Subjects | 218 | 380 | 381 | 323 |
| Hs-CRP, mg/L | 0.442 | 0.419 | 0.363 | 0.381 |
| (95%CI) | (0.382–0.510) | (0.375–0.467) | (0.326–0.406) | (0.339–0.430) |
| Q1 | ||||
| Subjects | 63 | 242 | 435 | 589 |
| Hs-CRP, mg/L | 0.282 | 0.318 | 0.334 | 0.302 |
| (95%CI) | (0.215–0.369) | (0.277–0.365) | (0.301–0.370) | (0.277–0.330) |
Data are expressed as geometric mean. Data (95% CI) in tables are estimated from linear regression models with adjustments for age, sex, and smoking status.
Supplementary Table 2. Geometric Mean Values and Adjusted Differences (95% CI) of High-Sensitivity C-Reactive Protein (hs-CRP) by Small Dense Low-Density Lipoprotein Cholesterol (sdLDL-C) Quartiles in Subjects with The Lowest Remnant-Like Particle Cholesterol (RLP-C) by Age Group.
| SdLDL-C, mg/dL |
||||
|---|---|---|---|---|
| Age, years | Q4 | Q3 | Q2 | Q1 |
| 65 years ≦ | ||||
| subjects | 30 | 129 | 241 | 315 |
| Hs-CRP, mg/L | 0.325 | 0.360 | 0.426 | 0.416 |
| (95%CI) | (0.221–0.476) | (0.289–0.448) | (0.362–0.502) | (0.362–0.477) |
| < 65 years | ||||
| subjects | 44 | 134 | 242 | 347 |
| Hs-CRP, mg/L | 0.335 | 0.325 | 0.303 | 0.263 |
| (95%CI) | (0.215–0.522) | (0.262–0.403) | (0.259–0.354) | (0.229–0.302) |
Data are expressed as geometric mean. Data (95% CI) in tables are estimated from linear regression models with adjustments for age, sex, and smoking status.
Supplementary Fig. 1.
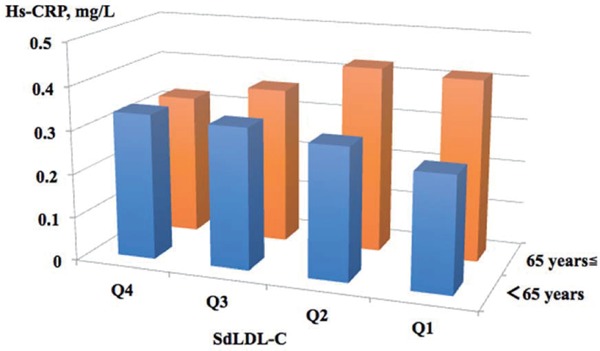
Geometric Mean Values of High-Sensitivity C-Reactive Protein (hs-CRP) by Small Dense Low-Density Lipoprotein Cholesterol (sdLDL-C) Quartiles in Subjects with The Lowest Remnant-Like Particle Cholesterol by Age Group and Adjusting for Age, Sex, and Smoking Status
Discussion
Our study of 5,305 JMS-II participants found that sdLDL-C and RLP-C were significantly and positively associated with not only metabolic abnormalities but also low-grade inflammation marked by hs-CRP. To the best of our knowledge, the present study is the first to demonstrate the association between sdLDL-C levels and low-grade inflammation, independently of RLP-C, in a real-world, population-based setting. SdLDL-C and RLP-C have different associations with low-grade inflammation, and the interaction effect of sdLDL-C and RLP-C on lowgrade inflammation was not observed. Our results partially supported the hypothesis that sdLDL-C and RLP-C mediate the association between metabolic abnormalities and low-grade inflammation5, 7–9, 30). However, both sdLDLs and remnants are regulated through multiple mechanisms, which are composed of excess adiposity, free fatty acids (FFAs), apolipoproteins such as ApoC-II, ApoC-III, and ApoE, and the other factors, including inhibition of lipoprotein lipase activity, delayed catabolism of TRLs, action of cholesterol ester transfer protein for lipoproteins, and promotion of larger VLDL secretion by increased triglyceride synthesis9, 11, 13, 31, 32). Excess adiposity and FFAs were also involved in the pathogenesis of insulin resistance and MetS. These regulations and relationships are very complex, and we require additional longitude cohorts to evaluate a causal relationship between MetS and sdLDL-C or RLP-C.
In the past era, atherosclerosis was considered to be a cholesterol storage disease characterized by the collection of cholesterol and thrombotic debris in the artery wall. Multiple studies showed that inflammation played an important role in the pathogenesis of atherosclerosis. Inflammation is involved in the initiation and progression of the plaques, and could regulate the plaque biology that triggers the thrombotic complications of atherosclerosis33–35). The lipoprotein components modified by several enzymes lead to the focal activation of endothelium and formation of several damage-associated molecular patterns, which can activate innate immune cells such as macrophage cells21). In addition to the innate immunity, multiple types of evidence support that a role for the adaptive immunity in plaque progression with inflammation. The self- and non-self-antigens can contribute to vascular inflammation by triggering the activation of T and B cells locally22). Candidate antigens for stimulation of this adaptive immune response include modified or native lipoproteins and apolipoprotein B-100. Therefore, it is likely that both sdLDLs and RLPs cooperate with the innate and adaptive immune cells and collectively induce the inflammatory response. Furthermore, several studies suggest that sdLDL and RLP had some different mechanisms activating the immune cells20, 23–25, 30). The finding of the present study that sdLDL-C and RLP-C had different associations with low-grade inflammation in a real-world, population-based setting, might reflect, in part, these different mechanisms of activating the immune cells.
Indeed, hs-CRP, which reflected systemic inflammation, was associated with future CHD events and the risk of death in a general population36, 37). The Hisayama study, which is a population-based prospective cohort study, suggested that hs-CRP levels were related linearly to future CHD events across a wide range of values in a general Japanese population36). The study also showed that the association between hs-CRP levels and CHD was strong and continuous from very low hs-CRP levels of less than about 0.2 mg/L, and a slightly elevated hs-CRP level of more than 1.0 mg/L, which was much lower than that for Western populations, was likely to be clearly associated with high risk of future cardiovascular events in Japanese. In our study, the differences in hs-CRP levels between the groups with the lowest and the highest sdLDL-C, RLP-C, and combined sdLDL-C and RLP-C were approximately 0.08, 0.08, and 0.22 mg/L (Table 3, 4, 5, and Fig. 1). Although our assessment for the risk of CVD was limited, according to these data and a linear function made by the data of the Hisayama study, the risk of CHD might be about 1.6-fold greater in the group with the highest combined sdLDL-C and RLP-C, compared with the group with the lowest combined sdLDL-C and RLP-C.
As shown in Supplementary Table 2, we found that the group with the lower sdLDL-C had the highest hs-CRP levels in elderly participants with the lowest RLP-C. For impaired lipid metabolism in patients with chronic liver diseases or some cancers, which cause tissue inflammation and increase hs-CRP levels, hypocholesterolemia states are frequently observed38). Therefore, chronic diseases being undiagnosed might be considered the causal factor. Further studies are needed to determine the association.
This study has several limitations. First, the cross-sectional design of our study cannot support the causal relationships between sdLDL-C and RLP-C and metabolic abnormalities or low-grade inflammation. Second, data regarding type and dose of medications for diabetes mellitus or dyslipidemia were not available. However, after exclusion of subjects taking medication for these diseases, the results remained identical (data not shown). Third, although the association between atherosclerotic risk markers, including hs-CRP, and sdLDL-C and/or RLP-C may explain a possible link between sdLDL-C and/or RLP-C and the risk of CVD and mortality, the true relationship is unclear. This study did not show the associations of sdLDL-C and RLP-C with prognostic outcomes. Further studies are necessary to clarify these relationships.
Conclusions
In conclusion, sdLDL-C was associated with low-grade inflammation marked by elevated hs-CRP levels. SdLDL-C and RLP-C have different associations with inflammation, and there were combined effects of sdLDL-C and RLP-C on low-grade inflammation in a real-world, population setting. These results indicate that they might increase the risk for CVD through multiple different mechanisms and support sdLDL-C as the potential novel factor of CVD in relation to inflammation, independently of RLP-C.
Acknowledgments
The authors thank the public health doctors, nurses, and officers of Shimotsuke, Kakara, Sue, Omori, Kamiichi, Wara, Takasu, Onabi, Nakatsu, Yame, Miwa, Ueno, and Saji, Japan, for their help, support, and contributions.
Sources of Funding
This research was supported by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities (S0901032); a Japanese Society for the Promotion of Science KAKENHI grant (No. 16K09141); a Grant-In-Aid from the Ministry of Health, Labour, and Welfare; and Health and Labor Sciences and Japan Comprehensive Research on Cardiovascular and Lifestyle-Related Diseases grants (H26-Junkankitou-[Seisaku]-Ippan-001 and H29-Junkankitou-Ippan-003; IRB No. G09-39 [G17-64 revised]).
Conflict of Interest
No competing interests.
References
- 1). Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and Management of the Metabolic Syndrome. Circulation, 2005; 112: 2735-2752 [DOI] [PubMed] [Google Scholar]
- 2). Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Jr, Sperling L, Virani SS, Yeboah J. 2018AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation, 2019; 139: e1082-e1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Morris PB, Ballantyne CM, Birtcher KK, Dunn SP, Urbina EM. Review of Clinical Practice Guidelines for the Management of LDL-Related Risk. Journal of the American College of Cardiology, 2014; 64: 196-206 [DOI] [PubMed] [Google Scholar]
- 4). Catapano AL, Graham I, De Backer G, Wiklund O, CHAPMAN MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen M-R, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J, 2016; 37: 2999-3058 [DOI] [PubMed] [Google Scholar]
- 5). Arai H, Kokubo Y, Watanabe M, Sawamura T, Ito Y, Minagawa A, Okamura T, Miyamato Y. Small dense lowdensity lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: the Suita study. J Atheroscler Thromb, 2013; 20: 195-203 [DOI] [PubMed] [Google Scholar]
- 6). Lawler PR, Akinkuolie AO, Harada P, Glynn RJ, Chasman DI, Ridker PM, Mora S. Residual Risk of Atherosclerotic Cardiovascular Events in Relation to Reductions in Very-Low-Density Lipoproteins. J Am Heart Assoc, 2017; 6: 1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Varbo A, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation, 2013; 128: 1298-1309 [DOI] [PubMed] [Google Scholar]
- 8). Saeed A, Feofanova EV, Yu B, Sun W, Virani SS, Nambi V, Coresh J, Guild CS, Boerwinkle E, Ballantyne CM, Hoogeveen RC. Remnant-Like Particle Cholesterol, Low-Density Lipoprotein Triglycerides, and Incident Cardiovascular Disease. Journal of the American College of Cardiology, 2018; 72: 156-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E, Ballantyne CM. Small Dense Low-Density Lipoprotein-Cholesterol Concentrations Predict Risk for Coronary Heart Disease. Arterioscler Thromb Vasc Biol, 2014; 34: 1069-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). St-Pierre AC, Cantin B, Dagenais GR, Mauriège P, Bernard P-M, Després J-P, Lamarche B. Low-Density Lipoprotein Subfractions and the Long-Term Risk of Ischemic Heart Disease in Men. Arterioscler Thromb Vasc Biol, 2005; 25: 553-559 [DOI] [PubMed] [Google Scholar]
- 11). Varbo A, Nordestgaard BG. Remnant Cholesterol and Triglyceride-Rich Lipoproteins in Atherosclerosis Progression and Cardiovascular Disease. Arterioscler Thromb Vasc Biol, 2016; 36: 2133-2135 [DOI] [PubMed] [Google Scholar]
- 12). Hirano T, Ito Y, Koba S, Toyoda M, Ikejiri A, Saegusa H, Yamazaki J-I, Yoshino G. Clinical Significance of Small Dense Low-Density Lipoprotein Cholesterol Levels Determined by the Simple Precipitation Method. Arterioscler Thromb Vasc Biol, 2004; 24: 558-563 [DOI] [PubMed] [Google Scholar]
- 13). Kathiresan S, Otvos JD, Sullivan LM, Keyes MJ, Schaefer EJ, Wilson PWF, D'Agostino RB, Vasan RS, Robins SJ. Increased Small Low-Density Lipoprotein Particle Number. Circulation, 2006; 113: 20-29 [DOI] [PubMed] [Google Scholar]
- 14). Satoh A, Adachi H, Tsuruta M, Hirai Y, Hiratsuka A, Enomoto M, Furuki K, Hino A, Takeuchi T, Imaizumi T. High plasma level of remnant-like particle cholesterol in the metabolic syndrome. Diabetes Care, 2005; 28: 2514-2518 [DOI] [PubMed] [Google Scholar]
- 15). Ishikawa S, Kayaba K, Gotoh T, Nakamura Y, Kajii E. Metabolic syndrome and C-reactive protein in the general population: JMS Cohort Study. Circ J, 2007; 71: 26-31 [DOI] [PubMed] [Google Scholar]
- 16). Ivanova EA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN. Small Dense Low-Density Lipoprotein as Biomarker for Atherosclerotic Diseases. Oxidative Medicine and Cellular Longevity, 2017; 2017: 1273042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Moriyama K, Takahashi E. Non-HDL Cholesterol is a More Superior Predictor of Small-Dense LDL Cholesterol than LDL Cholesterol in Japanese Subjects with TG Levels < 400 mg/dL. J Atheroscler Thromb, 2016; 23: 1126-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Hayashi T, Koba S, Ito Y, Hirano T. Method for estimating high sdLDL-C by measuring triglyceride and apolipoprotein B levels. Lipids in Health and Disease, 2017; 16: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Karpe F, Boquist S, Tang R, Bond GM, de Faire U, Hamsten A. Remnant lipoproteins are related to intima-media thickness of the carotid artery independently of LDL cholesterol and plasma triglycerides. J Lipid Res, 2001; 42: 17-21 [PubMed] [Google Scholar]
- 20). Twickler TB, Dallinga-Thie GM, Cohn JS, Chapman MJ. Elevated Remnant-Like Particle Cholesterol Concentration. Circulation, 2004; 109: 1918-1925 [DOI] [PubMed] [Google Scholar]
- 21). Ketelhuth DF, Hansson GK. Adaptive Response of T and B Cells in Atherosclerosis, Circulation research 2016; 118: 668-678 [DOI] [PubMed] [Google Scholar]
- 22). Hansson GK. Inflammation, Atherosclerosis, and Coronary Artery Disease. N Engl J Med, 2005; 352: 1685-1695 [DOI] [PubMed] [Google Scholar]
- 23). Tani M, Kawakami A, Mizuno Y, Imase R, Ito Y, Kondo K, Ishii H, Yoshida M. Small Dense LDL Enhances THP-1 Macrophage Foam Cell Formation. J Atheroscler Thromb, 2011; 18: 698-704 [DOI] [PubMed] [Google Scholar]
- 24). Boullier A, Li Y, Quehenberger O, Palinski W, Tabas I, Witztum JL, Miller YI. Minimally oxidized LDL offsets the apoptotic effects of extensively oxidized LDL and free cholesterol in macrophages. Arterioscler Thromb Vasc Biol, 2006; 26: 1169-1176 [DOI] [PubMed] [Google Scholar]
- 25). Kawakami A, Tanaka A, Nakajima K, Shimokado K, Yoshida M. Atorvastatin Attenuates Remnant Lipoprotein-Induced Monocyte Adhesion to Vascular Endothelium Under Flow Conditions. Circulation Research, 2002; 91: 263-271 [DOI] [PubMed] [Google Scholar]
- 26). Doi H, Kugiyama K, Oka H, Sugiyama S, Ogata N, Koide SI, Nakamura SI, Yasue H. Remnant Lipoproteins Induce Proatherothrombogenic Molecules in Endothelial Cells Through a Redox-Sensitive Mechanism. Circulation, 2000; 102: 670-676 [DOI] [PubMed] [Google Scholar]
- 27). Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia, 1985; 28: 412-419 [DOI] [PubMed] [Google Scholar]
- 28). National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Educational Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation, 2002; 106: 3143-3421 [PubMed] [Google Scholar]
- 29). Takahashi H, Mori M. Characteristics and significance of criteria for obesity disease in Japan 2011. Nihon Rinsho, 2013; 71: 257-261. [Article in Japanese] [PubMed] [Google Scholar]
- 30). Hsu SH-J, Jang M-H, Torng P-L, Su T-C. Positive Association Between Small Dense Low-Density Lipoprotein Cholesterol Concentration and Biomarkers of Inflammation, Thrombosis, and Prediabetes in Non-Diabetic Adults. J Atheroscler Thromb, 2019; 26: 624-635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Qamar A, Khetarpal SA, Khera AV, Qasim A, Rader DJ, Reilly MP. Plasma apolipoprotein C-III levels, triglycerides, and coronary artery calcification in type 2 diabetics. Arterioscler Thromb Vasc Biol, 2015; 35: 1880-1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Pechlaner R, Tsimikas S, Yin X, Willeit P, Baig F, Santer P, Oberhollenzer F, Egger G, Witztum JL, Alexander VJ, Willeit J, Kiechl S, Mayr M. Very-Low-Density Lipoprotein-Associated Apolipoproteins Predict Cardiovascular Events and Are Lowered by Inhibition of APOC-III. Journal of the American College of Cardiology, 2017; 69: 789-800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Libby P. Inflammation in Atherosclerosis. Arterioscler Thromb Vasc Biol, 2012; 32: 2045-2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Libby P. Inflammation in atherosclerosis. Nature, 2002; 420: 868-874 [DOI] [PubMed] [Google Scholar]
- 35). Jain MK, Ridker PM. Anti-Inflammatory Effects of Statins: Clinical Evidence and Basic Mechanisms. Nat Rev Drug Discov, 2005; 4: 977-987 [DOI] [PubMed] [Google Scholar]
- 36). Arima H, Kubo M, Yonemoto K, Doi Y, Ninomiya T, Tanizaki Y, Hata J, Matsumura K, Iida M, Kiyohara Y. High-Sensitivity C-Reactive Protein and Coronary Heart Disease in a General Population of Japanese. Arterioscler Thromb Vasc Biol, 2008; 28: 1385-1391 [DOI] [PubMed] [Google Scholar]
- 37). Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D'Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med, 2006; 355: 2631-2639 [DOI] [PubMed] [Google Scholar]
- 38). Chrostek L, Supronowicz L, Panasiuk A, Cylwik B, Gruszewska E, Flisiak R. The effect of the severity of liver cirrhosis on the level of lipids and lipoproteins. Clin Exp Med, 2014; 14: 417-421 [DOI] [PMC free article] [PubMed] [Google Scholar]


