Abstract
Aim: A positive association between non-high-density lipoprotein cholesterol (non-HDL-C) and coronary heart disease (CHD) has been established; however, associations between non-HDL-C and stroke subtypes have not been determined.
Methods: We conducted a prospective study of 30,554 individuals aged 40–69 yrs with no history of cardiovascular disease (CVD) in Japan. Sex-specific hazard ratios (HRs) and 95% confidence intervals (CIs) for the incidence of stroke subtypes and CHD were estimated according to quintiles of non-HDL-C, using Cox proportional hazard models adjusted for other established risk factors.
Results: We identified 1,705 stroke and 296 CHD events over a median 15 yrs of follow-up. The fractional polynomials analysis revealed a U-shaped association between non-HDL-C and stroke risk in men. When analyzed for stroke subtypes, the data revealed an inverse relationship between non-HDL-C and intracerebral hemorrhage (ICH), primarily with lobar ICH, and a positive association between non-HDL-C and large-artery occlusive infarction in men [adjusted HR 0.55 (95% CI, 0.35–0.87) and 2.05 (95% CI, 1.07–3.93) for the highest and lowest quintile of non-HDL-C, respectively]. The lowest risk of ICH in women was observed in the fourth quintile, and the lowest risk of embolic infarction was observed in the third quintile. In contrast, non-HDL-C was positively associated with CHD in both sexes.
Conclusions: In Japanese men, lower non-HDL-C levels were associated with a decreased risk of large-artery occlusive infarction and an increased risk of ICH, particularly lobar ICH.
Keywords: Non-HDL-C, Coronary heart disease, Lacunar infarction, Intracerebral hemorrhage, Epidemiology
Introduction
Elevated non-high-density lipoprotein cholesterol (non-HDL-C) is an established risk factor for coronary heart disease (CHD)1). Randomized controlled trials of lipid-lowering drugs provided strong evidence of a causal connection between non-HDL-C and CHD2, 3). In contrast, the association between non-HDL-C and stroke and stroke subtypes remains unknown. Indeed, epidemiological studies in Japan showed inconsistent results, i.e., some found a positive association between non-HDL-C and atherothrombotic infarction4), while others found no associations5, 6) or even an inverse relationship with ischemic stroke7).
Unlike CHD, stroke can be subdivided into several subtypes, and the proportion of each subtype differs among populations and ethnic groups8). In Japan, intracerebral hemorrhage and lacunar infarction are major subtypes of stroke9). These subtypes are characterized by different lipid profiles10, 11). Conversely, lower lipid levels in Japanese adults are shown to be associated with an increased risk of atrial fibrillation12, 13). Together, these factors may have concealed associations between non-HDL-C and stroke risk.
Given the potentially complex associations between lipid profile and stroke risk, we conducted a large-scale prospective study to clarify these associations, focusing on stroke subtypes, in approximately 31,000 individuals in nine population-based cohorts. Non-HDL-C can be calculated simply using a well standardized algorithm regardless of the fasting triglyceride level, which is needed to estimate low density lipoprotein (LDL) cholesterol using the Friedewald equation. This study investigated the relationships between the incidence of CHD, stroke, and stroke subtypes and baseline levels of non-HDL-C over a median follow-up period of 15 yrs.
Methods
Study Population
The Japan Public Health Center-based Prospective (JPHC) Study began in 1990 (Cohort I) and 1993–1994 (Cohort II), included 140,420 Japanese residents aged 40–59 yrs (Cohort I) or 40–69 yrs (Cohort II), and it included 11 public health-centers that hold information on incident CVD events14). Participants were asked about lifestyle 5 and 10 yrs after the baseline examination. Because non-HDL-C was not available at baseline in Cohort I, we used data after 5 yrs in Cohort I (n = 45,019) and at baseline in Cohort II (n = 63,216). Excluding two communities without CVD registration, we selected individuals who received baseline blood examinations (n = 31,296). After exclusion of patients with a history of CVD or extreme hypertriglyceridemia (≥ 600 mg/dl)15), 30,554 individuals were included in the analyses.
The study protocol, including the procedure for obtaining informed consent in the JPHC study, was approved by the Human Ethics Review Committees of the National Cancer Center and Faculty of Medicine, Oita University.
Baseline Surveys
A self-administered questionnaire was used to obtain a medical history, smoking and alcohol-drinking habits, sports during leisure time, menopause (for women), and time since the last meal (≥ 8 hours or not). Sports during leisure time was determined by a validated question16, 17), i.e., “How many times did you participate in sports and physical activity other than during working hours,” with 5 predefined categories: almost never, 1–3 days per month, 1–2 days per week, 3–4 days per week, and almost daily.
Blood pressure was measured in the right arm using a standard mercury sphygmomanometer, with the patient in the sitting position after resting for at least 5 minutes. Body mass index (BMI, kg/m2) was calculated as weight divided by the square of height, and overweight was defined as BMI ≥ 25 kg/m2. Hypertension was defined as systolic and diastolic blood pressures ≥ 140/90 mmHg or the use of medication to treat hypertension. Diabetes was defined as a fasting plasma glucose ≥ 7.0 mmol/L, a non-fasting glucose ≥ 11.0 mmol/L, or the use of medication to treat diabetes. The concentrations of total cholesterol, HDL-C, triglycerides, and glucose in the blood were measured by conventional enzyme methods. External quality control for the measurement of serum lipids and plasma glucose was provided by the Standardization Program of the Japan Medical Association. For international quality control, the laboratories joined the program of the Osaka Medical Center for Health Science and Promotion, a member of the Cholesterol Reference Method Laboratory Network to standardize total cholesterol and HDL-C18).
Ascertainment of Stroke and CHD Incidence Rates
Subjects were followed-up for a median of 15.0 yrs from 1995 to December 31, 2009 (Cohort I) and from 1993 to December 31, 2012 (Cohort II). Person-yrs were calculated from the date of entry to the date of the first endpoint (death, emigration, or lost to follow-up) or to the study end-date.
CVD outcomes were determined as mentioned previously19). In brief, physicians systematically reviewed the medical records of possible stroke hospitalizations at 81 major community hospitals. Strokes were confirmed by medical records, including computed tomography scans, magnetic resonance images, and autopsy findings. All strokes met the criteria of the National Survey of Stroke20), which requires symptoms related to neurological deficits of sudden or rapid onset lasting at least 24 h or until death. Strokes were classified as subarachnoid hemorrhage, intracerebral hemorrhage (ICH), or ischemic stroke, which included lacunar infarction, large-artery occlusive infarction, embolic infarction, and unclassified21). ICH was further classified into two groups: “deep only” included ICH in the deep brain regions (basal ganglia, thalamus, internal capsules, and brainstem), and “lobar” included ICH in the cortex, subcortical white matter, and cerebellum. Mixed type (deep and lobar regions) was included in the latter group. Infarcts ≤ 1.5 cm in diameter in the deep brain regions were defined as lacunar infarctions, while infarcts > 1.5 cm involving cortical areas were classified as large-artery occlusive infarctions. The definition of embolic infarction required the same criteria as ischemic infarction plus the presence of an embolus in the brain, atrial fibrillation, or medical record evidence of a possible embolic source. A stroke that was diagnosed clinically but exhibited no lesions on CT, MRI, or autopsy was classified as a stroke of undetermined type.
Myocardial infarction meeting the criteria of the MONICA project, which required typical chest pain and evidence of infarction from an electrocardiogram, cardiac enzymes, or autopsy records22), was confirmed from medical records. Sudden cardiac death was defined as a death of unknown cause that occurred within one hour of the onset of the cardiovascular event. CHD was defined as a composite outcome of myocardial infarction and sudden cardiac death.
Statistical Analysis
Sex-specific and age-adjusted incidence rates of CHD, total stroke, subarachnoid hemorrhage, ICH, and ischemic stroke were calculated by quintile of non-HDL-C using the Poisson regression analysis stratified by study community. A Cox proportional hazards model was used to calculate sex-specific hazard ratios (HRs) and 95% confidence intervals (CIs) using data grouped according to non-HDL-C quintile, with the lowest quintile serving as a reference stratified by study community. Model 1 was adjusted for age (continuous). Model 2 was additionally adjusted for smoking status (never, ex-, < 20, or ≥ 20 cigarettes/day for men; or current smoker or not for women), alcohol intake (0, 1–150, 151–300, or ≥ 301 g/wk for men; or ≥ 1 day/week alcohol or not for women), sports at leisure time (rarely, 1–2, 3–4 times/week, or almost every day), BMI, menopause in women (yes/no), systolic blood pressure (continuous), use of antihypertensive agents (yes/no), diabetes (yes/no), HDL-C (continuous), and use of antilipemic agents (yes/no). A linear trend was examined using continuous values of non-HDL-C in the Cox regression models. We tested the non-linear trend based on the fractional polynomials analysis to determine the significance of the trend when the linearity was denied23). Analyses stratified by the presence of overweight, hypertension, and hypertriglyceridemia (fasting triglycerides ≥ 150 mg/dl or non-fasting triglycerides ≥ 300 mg/dl) were also performed. Statistical significance was assumed at p < 0.05. Analyses were performed with SAS software, version 9.4 (SAS Institute, Inc., Cary, North Carolina, USA).
Results
Table 1 shows the baseline characteristics of study participants according to sex and non-HDL-C quintile. Non-HDL-C was significantly associated with several other variables, except current smoking and mean intake of alcohol in women.
Table 1. Baseline characteristics according to non-HDL cholesterol (non-HDL-C) quintile.
| Non-HDL-C quintile |
P for difference | ||||||
|---|---|---|---|---|---|---|---|
| Sex | Variables | Q1 | Q2 | Q3 | Q4 | Q5 | |
| Men | n | 2,079 | 2,059 | 2,116 | 2,093 | 2,112 | |
| Range of non-HDL-C, mmol/L (mg/dL) | < 2.87 | 2.87–3.34 | 3.37–3.81 | 3.83–4.38 | ≥ 4.40 | ||
| (111) | (111–129) | (130–147) | (148–169) | (170) | |||
| Age | 58.3 | 57.7 | 57.5 | 57.0 | 56.6 | < 0.001 | |
| Body mass index, kg/m2 | 22.2 | 23.2 | 23.7 | 24.1 | 24.7 | < 0.001 | |
| Systolic blood pressure, mmHg | 132.2 | 132.9 | 132.9 | 133.5 | 134.5 | 0.001 | |
| Diastolic blood pressure, mmHg | 79.0 | 80.0 | 79.9 | 80.8 | 81.5 | < 0.001 | |
| Total cholesterol, mmol/L | 4.02 | 4.60 | 4.99 | 5.42 | 6.24 | < 0.001 | |
| HDL cholesterol, mmol/L | 1.58 | 1.48 | 1.40 | 1.33 | 1.27 | < 0.001 | |
| Triglycerides†, mmol/L | 0.90 | 1.08 | 1.27 | 1.51 | 1.89 | < 0.001 | |
| Overweight, % | 14.1 | 24.3 | 29.9 | 36.3 | 43.6 | < 0.001 | |
| Current smokers, % | 47.3 | 41.3 | 40.4 | 40.7 | 38.9 | < 0.001 | |
| Alcohol intake ≥ 1 day/week, % | 73.9 | 70.9 | 68.4 | 66.2 | 60.3 | < 0.001 | |
| Mean intake of alcohol‡, g/week | 325.9 | 294.5 | 278.8 | 285.4 | 271.1 | < 0.001 | |
| Sports during leisure time ≥ 1 time/week, % | 15.6 | 19.3 | 19.4 | 20.5 | 20.4 | < 0.001 | |
| Hypertension, % | 43.8 | 45.3 | 44.5 | 46.1 | 48.5 | 0.026 | |
| Diabetes, % | 5.9 | 5.9 | 5.5 | 6.8 | 8.5 | 0.001 | |
| Antilipemic agents use, % | 0.6 | 0.8 | 1.1 | 1.9 | 4.0 | < 0.001 | |
| Women | n | 3,972 | 3,979 | 4,159 | 4,004 | 3,981 | |
| Range of non-HDL-C, mmol/L (mg/dL) | < 3.16 | 3.16–3.63 | 3.65–4.12 | 4.14–4.69 | ≥ 4.71 | ||
| (122) | (122–140) | (141–159) | (160–181) | (182) | |||
| Age | 54.0 | 56.1 | 57.0 | 57.7 | 58.1 | < 0.001 | |
| Body mass index, kg/m2 | 22.7 | 23.5 | 23.9 | 24.3 | 24.7 | < 0.001 | |
| Systolic blood pressure, mmHg | 126.0 | 128.7 | 130.3 | 131.7 | 133.4 | < 0.001 | |
| Diastolic blood pressure, mmHg | 75.0 | 76.7 | 77.3 | 78.0 | 79.0 | < 0.001 | |
| Total cholesterol, mmol/L | 4.39 | 4.96 | 5.38 | 5.83 | 6.65 | < 0.001 | |
| HDL cholesterol, mmol/L | 1.65 | 1.56 | 1.50 | 1.43 | 1.36 | < 0.001 | |
| Triglycerides†, mmol/L | 0.83 | 1.02 | 1.19 | 1.37 | 1.65 | < 0.001 | |
| Overweight, % | 20.4 | 28.7 | 33.3 | 38.3 | 42.2 | < 0.001 | |
| Current smokers, % | 3.5 | 2.9 | 2.6 | 3.3 | 2.7 | 0.055 | |
| Alcohol intake ≥ 1 day/week, % | 10.4 | 8.7 | 9.3 | 7.7 | 6.5 | < 0.001 | |
| Mean intake of alcohol‡, g/week | 92.8 | 78.8 | 100.1 | 81.2 | 70.4 | 0.084 | |
| Sports during leisure time ≥ 1 time/week, % | 16.7 | 19.4 | 20.0 | 22.0 | 22.1 | < 0.001 | |
| Menopause, % | 64.4 | 64.4 | 76.0 | 76.0 | 82.8 | < 0.001 | |
| Hypertension, % | 29.4 | 36.2 | 39.6 | 43.6 | 46.7 | < 0.001 | |
| Diabetes, % | 1.9 | 2.5 | 2.7 | 3.8 | 4.8 | < 0.001 | |
| Antilipemic agents use, % | 0.6 | 1.9 | 3.6 | 4.7 | 8.2 | < 0.001 | |
P values were calculated using ANOVA or chi-square test.
Geometric mean
Among persons with alcohol intake of ≥ 1 day/week.
Incidence rates of CHD, stroke, and stroke subtype were analyzed according to sex and non-HDL-C quintile (Fig. 1). In contrast to CHD, stroke incidence rates demonstrated a U-shaped association with non-HDL-C quintile. Trends in the incidence of ICH and ischemic stroke according to non-HDL-C quintile level were significantly different.
Fig. 1.
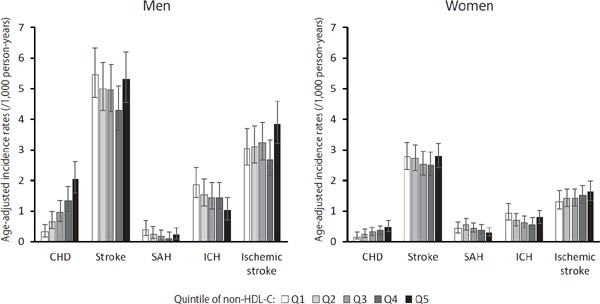
Incidence rates of CHD, stroke, and stroke subtypes according to non-HDL cholesterol quintile and sex
As for stroke occurrences in men (Table 2), the lowest multivariable adjusted HR was in the fourth non-HDL-C quintile, compared with the lowest non-HDL-C quintile [0.78 (95% CI, 0.62–0.98) in men and 0.80 (95% CI, 0.64–0.99) in women]. Furthermore, the fractional polynomial models showed significant non-linear associations between stroke risk and non-HDL-C quintile in men (p < 0.001) but not in women. The age-adjusted HRs for CHD in the highest quintile were 6.55 (95% CI, 3.45–12.4) in men and 2.88 (95% CI, 1.43–5.80) in women, stratified by the study community. Linear trend tests were significant in both sexes. After adjustment for smoking status, alcohol intake, sports during leisure time, BMI, menopause (in women), systolic blood pressure, use of antihypertensive agents, diabetes, HDL-C, and use of antilipemic agents (model 2), the association with CHD remained significant in both sexes.
Table 2. Sex-specific multivariable adjusted hazard ratios and 95% confidence intervals of non-HDL cholesterol (non-HDL-C) quintiles in relation to the incidence of coronary heart disease and stroke.
| Non-HDL-C quintile |
P for trend |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex | Q1 | Q2 | Q3 | Q4 | Q5 | Linear | Non-linear | ||
| Men | Range of non-HDL-C, mmol/L (mg/dL) | < 2.87 | 2.87–3.34 | 3.37–3.81 | 3.83–4.38 | ≥ 4.40 | |||
| (111) | (111–129) | (130–147) | (148–169) | (170) | |||||
| Stroke | Cases, n | 182 | 167 | 165 | 138 | 168 | |||
| Person-years | 30,889 | 31,776 | 32,455 | 32,269 | 32,501 | ||||
| Model 1 | 1.00 | 0.92 (0.75–1.14) | 0.90 (0.73–1.11) | 0.79 (0.63–0.99) | 0.99 (0.80–1.22) | > 0.2 | < 0.001 | ||
| Model 2 | 1.00 | 0.90 (0.73–1.11) | 0.87 (0.70–1.08) | 0.76 (0.61–0.96) | 0.92 (0.73–1.15) | > 0.2 | < 0.001 | ||
| CHD | Cases, n | 11 | 23 | 33 | 44 | 67 | |||
| Person-years | 31,724 | 32,536 | 33,237 | 32,744 | 33,009 | ||||
| Model 1 | 1.00 | 2.13 (1.04–4.37) | 3.06 (1.54–6.05) | 4.25 (2.19–8.23) | 6.55 (3.45–12.4) | < 0.001 | N/A | ||
| Model 2 | 1.00 | 1.86 (0.90–3.85) | 2.62 (1.32–5.23) | 3.42 (1.74–6.71) | 4.64 (2.39–8.98) | < 0.001 | N/A | ||
| Women | Range of non-HDLC, mmol/L (mg/dL) | < 3.16 | 3.16–3.63 | 3.65–4.12 | 4.14–4.69 | ≥ 4.71 | |||
| (122) | (122–140) | (141–159) | (160–181) | (182) | |||||
| Stroke | Cases, n | 156 | 175 | 180 | 177 | 197 | |||
| Person-years | 65,792 | 65,478 | 68,115 | 65,347 | 64,538 | ||||
| Model 1 | 1.00 | 0.95 (0.77–1.18) | 0.87 (0.70–1.08) | 0.86 (0.69–1.07) | 0.96 (0.78–1.19) | > 0.2 | N/A | ||
| Model 2 | 1.00 | 0.91 (0.73–1.14) | 0.81 (0.65–1.02) | 0.79 (0.63–0.99) | 0.86 (0.68–1.07) | > 0.2 | N/A | ||
| CHD | Cases, n | 10 | 17 | 25 | 28 | 38 | |||
| Person-years | 66,528 | 66,198 | 68,829 | 66,158 | 65,286 | ||||
| Model 1 | 1.00 | 1.47 (0.67–3.22) | 1.93 (0.93–4.04) | 2.14 (1.04–4.43) | 2.88 (1.43–5.80) | < 0.001 | N/A | ||
| Model 2 | 1.00 | 1.29 (0.59–2.84) | 1.60 (0.76–3.37) | 1.63 (0.78–3.42) | 2.02 (0.97–4.19) | 0.014 | N/A | ||
Model 1 was adjusted for age stratified by community.
Model 2 was further adjusted for smoking status (never, ex-, < 20, or ≥ 20 cigarettes/day in men; or current smoker or not in women), alcohol intake (0, 1–150, 151–300, or ≥ 301 g/week in men; or . 1 day/week alcohol drinker or not in women), sports during leisure time (rarely, 1–2, 3–4 times/week, or almost every day), body mass index, menopause (women only), systolic blood pressure, use of antihypertensive agents, diabetes, HDL-C, and use of antilipemic agents.
Linear trends were tested by using continuous values of non-HDL-C, and non-linear trends were further tested if the fractional polynomial models were fitted at a significance level of p < 0.05. N/A, not applicable.
Next, we compared the risk of stroke subtype in men according to non-HDL-C quintile, adjusted for multiple variables, relative to the lowest non-HDL-C quintile (Table 3). The HRs for SAH and ICH decreased by 0.30 (95% CI, 0.10–0.92) in the fourth quintile and by 0.57 (95% CI, 0.37–0.87) in the fifth quintile (Model 1). When adjusted for confounders, the association between non-HDL-C and SAH diminished, but the association with ICH was unchanged and suggested an inverse trend. Non-HDL-C was positively associated with an increased risk of large-artery occlusive infarction, independent of several other risk factors. The lowest risk of embolic infarction was observed in the fourth non-HDL-C quintile.
Table 3. Multivariable adjusted hazard ratios and 95% confidence intervals of non-HDL cholesterol (non-HDL-C) quintiles in relation to stroke subtypes in men.
| Non-HDL-C quintile |
|||||||
|---|---|---|---|---|---|---|---|
| Subtypes of stroke | Q1 | Q2 | Q3 | Q4 | Q5 | P for linear trend | |
| Subarachnoid hemorrhage | Cases, n | 13 | 9 | 6 | 4 | 8 | |
| Model 1 | 1 | 0.68 (0.29–1.58) | 0.44 (0.17–1.16) | 0.30 (0.10–0.92) | 0.61 (0.25–1.49) | 0.06 | |
| Model 2 | 1 | 0.79 (0.33–1.89) | 0.55 (0.20–1.49) | 0.40 (0.12–1.31) | 0.86 (0.32–2.30) | > 0.2 | |
| Intracerebral hemorrhage | Cases, n | 59 | 49 | 46 | 45 | 32 | |
| Model 1 | 1 | 0.81 (0.56–1.19) | 0.75 (0.51–1.10) | 0.78 (0.53–1.15) | 0.57 (0.37–0.87) | 0.008 | |
| Model 2 | 1 | 0.82 (0.56–1.20) | 0.74 (0.50–1.10) | 0.79 (0.53–1.19) | 0.55 (0.35–0.87) | 0.010 | |
| Ischemic stroke | Cases, n | 107 | 109 | 112 | 89 | 126 | |
| Model 1 | 1 | 1.03 (0.79–1.35) | 1.05 (0.81–1.37) | 0.88 (0.66–1.16) | 1.28 (0.99–1.66) | 0.10 | |
| Model 2 | 1 | 0.99 (0.76–1.30) | 0.99 (0.75–1.30) | 0.81 (0.60–1.09) | 1.13 (0.86–1.49) | > 0.2 | |
| Lacunar infarction | Cases, n | 40 | 49 | 43 | 38 | 53 | |
| Model 1 | 1 | 1.23 (0.81–1.87) | 1.08 (0.70–1.66) | 1.00 (0.64–1.55) | 1.41 (0.93–2.13) | 0.13 | |
| Model 2 | 1 | 1.19 (0.78–1.81) | 1.01 (0.65–1.57) | 0.92 (0.58–1.46) | 1.25 (0.80–1.94) | > 0.2 | |
| Large-artery occlusive infarction | Cases, n | 15 | 25 | 20 | 25 | 32 | |
| Model 1 | 1 | 1.68 (0.89–3.19) | 1.33 (0.68–2.59) | 1.75 (0.92–3.33) | 2.34 (1.26–4.33) | 0.005 | |
| Model 2 | 1 | 1.63 (0.86–3.11) | 1.18 (0.59–2.34) | 1.60 (0.82–3.09) | 2.05 (1.07–3.93) | 0.033 | |
| Embolic infarction | Cases, n | 43 | 30 | 41 | 24 | 37 | |
| Model 1 | 1 | 0.71 (0.45–1.13) | 0.95 (0.62–1.46) | 0.59 (0.36–0.97) | 0.94 (0.60–1.46) | > 0.2 | |
| Model 2 | 1 | 0.68 (0.42–1.09) | 0.94 (0.61–1.47) | 0.55 (0.33–0.93) | 0.84 (0.52–1.35) | > 0.2 | |
Model 1 was adjusted for age and community. Model 2 was further adjusted for smoking status (never, ex-, < 20, or ≥ 20 cigarettes/day), alcohol intake (0, 1–150, 151–300, or ≥ 301 g/week), sports at leisure time (rarely, 1–2, 3–4 times/week, or almost every day), body mass index, systolic blood pressure, use of antihypertensive agents, diabetes, HDL-C, and use of antilipemic agents. Linear trends were tested by using continuous values of non-HDL-C.
Similar to our findings in men, the age-adjusted HRs for SAH and ICH in women in the higher non-HDL-C quintiles were decreased relative to the lowest quintile (Table 4). The lowest HR for ICH was observed in the fourth quintile (HR = 0.62, 95% CI, 0.41–0.95). The association between non-HDL-C and lacunar infarction was significant in a linear manner (p for trend = 0.04), but it was substantially weakened after adjustment. In Model 2, the risk of embolic infarction was decreased by 0.50 (95% CI, 0.29–0.88) in the third non-HDL-C quintile. We examined the interaction between menopausal status and non-HDL-C in women but could not find a significant interaction with any stroke subtype.
Table 4. Mutlivaribale adjusted hazard ratios and 95% confidence intervals of non-HDL cholesterol (non-HDL-C) quintiles in relation to to stroke subtypes in women.
| Non-HDL-C quintile |
|||||||
|---|---|---|---|---|---|---|---|
| Subtypes of stroke | Q1 | Q2 | Q3 | Q4 | Q5 | P for linear trend | |
| Subarachnoid hemorrhage | Cases, n | 25 | 34 | 29 | 25 | 19 | |
| Model 1 | 1 | 1.21 (0.72–2.04) | 0.93 (0.54–1.60) | 0.82 (0.47–1.43) | 0.61 (0.34–1.12) | 0.07 | |
| Model 2 | 1 | 1.22 (0.72–2.08) | 0.93 (0.54–1.63) | 0.79 (0.44–1.42) | 0.59 (0.31–1.11) | 0.06 | |
| Intracerebral hemorrhage | Cases, n | 53 | 45 | 45 | 41 | 57 | |
| Model 1 | 1 | 0.71 (0.47–1.05) | 0.64 (0.43–0.95) | 0.58 (0.38–0.87) | 0.82 (0.56–1.19) | > 0.2 | |
| Model 2 | 1 | 0.75 (0.50–1.12) | 0.68 (0.45–1.02) | 0.62 (0.41–0.95) | 0.88 (0.59–1.32) | > 0.2 | |
| Ischemic stroke | Cases, n | 77 | 94 | 105 | 111 | 120 | |
| Model 1 | 1 | 1.04 (0.77–1.40) | 1.02 (0.76–1.37) | 1.08 (0.81–1.45) | 1.17 (0.88–1.56) | 0.15 | |
| Model 2 | 1 | 0.92 (0.68–1.26) | 0.87 (0.65–1.18) | 0.90 (0.66–1.21) | 0.93 (0.68–1.26) | > 0.2 | |
| Lacunar infarction | Cases, n | 26 | 36 | 57 | 45 | 57 | |
| Model 1 | 1 | 1.18 (0.71–1.96) | 1.66 (1.04–2.64) | 1.30 (0.80–2.12) | 1.64 (1.03–2.62) | 0.04 | |
| Model 2 | 1 | 1.01 (0.60–1.68) | 1.38 (0.86–2.22) | 1.04 (0.63–1.71) | 1.23 (0.75–2.00) | > 0.2 | |
| Large-artery occlusive infarction | Cases, n | 15 | 18 | 19 | 30 | 19 | |
| Model 1 | 1 | 1.02 (0.51–2.02) | 0.96 (0.49–1.89) | 1.52 (0.81–2.84) | 0.98 (0.49–1.94) | > 0.2 | |
| Model 2 | 1 | 0.86 (0.43–1.73) | 0.77 (0.38–1.55) | 1.20 (0.63–2.29) | 0.74 (0.36–1.51) | > 0.2 | |
| Embolic infarction | Cases, n | 31 | 35 | 23 | 28 | 37 | |
| Model 1 | 1 | 0.97 (0.60–1.58) | 0.55 (0.32–0.95) | 0.68 (0.41–1.15) | 0.90 (0.56–1.46) | > 0.2 | |
| Model 2 | 1 | 0.93 (0.57–1.52) | 0.50 (0.29–0.88) | 0.60 (0.35–1.02) | 0.76 (0.46–1.28) | 0.18 | |
Model 1 was adjusted for age and community. Model 2 was further adjusted for smoking status (current smoker or not), alcohol intake (≥ 1 day/week alcohol drinker or not), sports during leisure time (rarely, 1–2, 3–4 times/week, or almost every day), body mass index, menopause, systolic blood pressure, use of antihypertensive agents, diabetes, HDL-C, and use of antilipemic agents. Linear trends were tested by using continuous values of non-HDL-C.
Fig. 2 shows the multivariable-adjusted HRs for deep and lobar ICH in men according to non-HDL-C quintile. An inverse linear trend was observed for lobar ICH (p for trend = 0.01), and HRs were decreased by 0.40 (95% CI, 0.17–0.98) in the fourth non-HDL-C quintile. Likewise, the HR for deep ICH in the highest non-HDL-C quintile was 0.56 (95% CI, 0.32–0.98), but the linear trend was not significant (p for trend= 0.14).
Fig. 2.
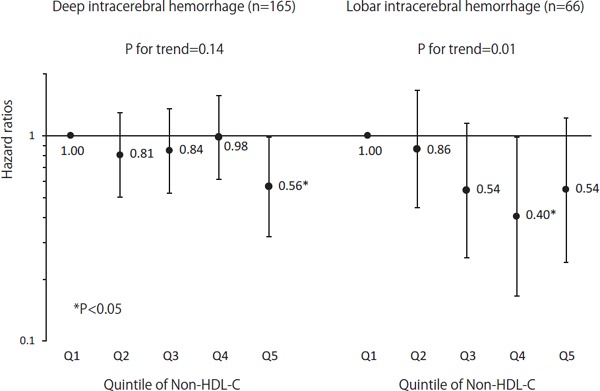
Multivariable adjusted HRs for deep and lobar ICH, grouped by non-HDL cholesterol quintile in men
HRs were adjusted for variables in Model 2 of Table 3.
Multivariable-adjusted HRs of CHD, ICH, and large-artery occlusive infarction for each standard deviation (SD) increase in non-HDL-C in men were stratified by overweight, hypertension, hypertriglyceridemia, and drinking alcohol (Table 5). The HRs for CHD and large-artery occlusive infarction increased, while the HRs for ICH decreased for each SD increase in non-HDL-C, independent of overweight, hypertension, and hypertriglyceridemia status. The association between drinking alcohol and non-HDL-C for CHD was significant (p = 0.008), with the HR being greater in patients who did not drink alcohol compared to those who did.
Table 5. Multivariable adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) of one standard deviation (SD) increase in non-HDL cholesterol related to the incidence of coronary heart disease (CHD) and stroke subtypes according to the presence/absence of overweight, hypertension, and hypertriglyceridemia in men.
| Cardiovascular disease outcomes |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| CHD |
Intracerebral hemorrhage |
Large-artery occlusive infarction |
|||||||
| Cases, n | HR (95% CI)* | P for interaction | Cases, n | HR (95% CI)* | P for interaction | Cases, n | HR (95% CI)* | P for interaction | |
| Overweight | |||||||||
| No | 111 | 1.71 (1.49–1.97) | 0.91 | 164 | 0.79 (0.66–0.94) | 0.22 | 71 | 1.22 (0.96–1.56) | 0.72 |
| Yes | 66 | 1.55 (1.21–1.98) | 67 | 0.96 (0.74–1.24) | 46 | 1.28 (0.94–1.74) | |||
| Hypertension | |||||||||
| No | 57 | 1.87 (1.56–2.23) | 0.32 | 81 | 0.90 (0.70–1.15) | 0.45 | 47 | 1.34 (1.03–1.75) | 0.34 |
| Yes | 120 | 1.55 (1.30–1.85) | 150 | 0.81 (0.68–0.96) | 70 | 1.15 (0.90–1.47) | |||
| Hypertriglyceridemia | |||||||||
| No | 143 | 1.62 (1.37–1.91) | 0.97 | 202 | 0.85 (0.73–1.00) | 0.49 | 103 | 1.16 (0.94–1.43) | 0.19 |
| Yes | 34 | 1.60 (1.22–2.09) | 29 | 0.64 (0.42–0.98) | 14 | 1.83 (1.20–2.79) | |||
| Alcohol drinking | |||||||||
| No | 91 | 1.95 (1.60–2.36) | 0.008 | 77 | 0.86 (0.68–1.10) | 0.34 | 48 | 1.19 (0.89–1.58) | 0.93 |
| Yes | 86 | 1.49 (1.24–1.78) | 154 | 0.83 (0.69–1.00) | 69 | 1.28 (1.00–1.62) | |||
Adjusted for age, smoking status (never, ex-, < 20, or ≥ 20 cigarettes/day), alcohol intake (0, 1–150, 151–300, or ≥ 301 g/week), sports at leisure time (rarely, 1–2, 3–4 times/week, or almost every day), and body mass index stratified by community. When stratified by alcohol drinking status, it was excluded from the model. HRs are expressed according to SD increase in non-HDL-C.
Finally, a sensitivity analysis was performed on the association between non-HDL-C and ICH, excluding the first 3-yr follow-up period; patients using antilipemic agents; and those who exhibited a HDL-C of ≥ 90 mg/dl. However, the inverse association remained unchanged (Supplemental Table 1).
Supplemental Table 1. Multivariable adjusted hazard ratio of non-HDLC related to CHD and stroke subtypes in men.
| Quintile of non-HDL cholesterol concentrations |
P for trend | ||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| Excluding the first 3-yr follow-up period (n = 515 for CHD and n = 613 for stroke) |
|||||||
| Men | CHD | 1 | 1.58 (0.72–3.46) | 2.81 (1.37–5.77) | 3.11 (1.52–6.36) | 4.38 (2.18–8.80) | <0.001 |
| Intracerebral hemorrhage | 1 | 0.82 (0.55–1.24) | 0.71 (0.46–1.09) | 0.83 (0.54–1.27) | 0.55 (0.34–0.89) | 0.016 | |
| Large–artery occlusive infarction | 1 | 1.63 (0.85–3.10) | 1.11 (0.55–2.23) | 1.48 (0.75–2.89) | 2.00 (1.04–3.84) | 0.045 | |
| Women | CHD | 1 | 1.26 (0.55–2.89) | 1.62 (0.74–3.53) | 1.79 (0.83–3.87) | 2.04 (0.95–4.38) | 0.013 |
| Intracerebral hemorrhage | 1 | 0.76 (0.49–1.18) | 0.81 (0.52–1.24) | 0.64 (0.41–1.02) | 0.98 (0.64–1.52) | 0.72 | |
| Large-artery occlusive infarction | 1 | 0.87 (0.42–1.78) | 0.77 (0.38–1.59) | 1.23 (0.63–2.38) | 0.78 (0.38–1.61) | 0.83 | |
| Excluding those who used antilipemic agents (n = 939) |
|||||||
| Men | CHD | 1 | 2.05 (0.97–4.34) | 2.81 (1.37–5.75) | 3.66 (1.81–7.40) | 5.14 (2.58–10.2) | < 0.001 |
| Intracerebral hemorrhage | 1 | 0.82 (0.56–1.20) | 0.74 (0.50–1.11) | 0.78 (0.52–1.18) | 0.52 (0.33–0.83) | 0.005 | |
| Large-artery occlusive infarction | 1 | 1.62 (0.85–3.10) | 1.17 (0.59–2.32) | 1.47 (0.75–2.88) | 2.02 (1.06–3.88) | 0.05 | |
| Women | CHD | 1 | 1.25 (0.57–2.78) | 1.53 (0.72–3.25) | 1.61 (0.76–3.41) | 2.07 (0.99–4.32) | 0.018 |
| Intracerebral hemorrhage | 1 | 0.75 (0.50–1.13) | 0.70 (0.46–1.06) | 0.64 (0.42–0.99) | 0.89 (0.59–1.34) | 0.92 | |
| Large–artery occlusive infarction | 1 | 0.82 (0.41–1.68) | 0.80 (0.40–1.61) | 1.19 (0.62–2.28) | 0.77 (0.37–1.58) | 0.83 | |
| Excluding those with HDL-C ≥ 90 mg/dl (n = 847) |
|||||||
| Men | CHD | 1 | 1.72 (0.83–3.57) | 2.51 (1.26–5.01) | 3.10 (1.58–6.09) | 4.42 (2.29–8.55) | < 0.001 |
| Intracerebral hemorrhage | 1 | 0.88 (0.59–1.31) | 0.78 (0.52–1.17) | 0.85 (0.56–1.29) | 0.59 (0.37–0.94) | 0.022 | |
| Large-artery occlusive infarction | 1 | 1.93 (0.96–3.87) | 1.41 (0.68–2.93) | 1.90 (0.94–3.85) | 2.44 (1.22–4.89) | 0.008 | |
| Women | CHD | 1 | 1.42 (0.63–3.20) | 1.74 (0.80–3.76) | 1.77 (0.82–3.81) | 2.12 (0.99–4.53) | 0.015 |
| Intracerebral hemorrhage | 1 | 0.67 (0.45–1.02) | 0.63 (0.42–0.96) | 0.60 (0.39–0.91) | 0.82 (0.55–1.23) | 0.66 | |
| Large-artery occlusive infarction | 1 | 0.90 (0.44–1.84) | 0.76 (0.37–1.55) | 1.25 (0.65–2.41) | 0.76 (0.37–1.57) | 0.91 | |
Sex-specified hazard ratios were adjusted for age, smoking status (never, ex-, < 20, or ≥ 20 cigarettes/day in men; or current smoker or not in women), alcohol intake (0, 1–150, 151–300, or ≥ 301 g/week in men; or ≥ 1 day/week alcohol drinker or not in women), sports during leisure time (rarely, 1–2, 3–4 times/week, or almost every day), body mass index, menopause (women only), systolic blood pressure, use of antihypertensive agents, diabetes, HDL-C, and use of antilipemic agents stratified by community.
Discussion
This long-term prospective study found a U-shaped association between total stroke incidence and non-HDL-C in men but not in women. This is in contrast to the previously identified linear association between non-HDL-C and CHD. Furthermore, high non-HDL-C strongly increased the risk of large-artery occlusive infarction and decreased the risk of ICH, particularly lobar ICH, independent of other established CVD risk factors in men. The lowest risk of embolic infarction was in the third and fourth non-HDL-C quintiles in men and women, respectively.
As expected, our study supports the association between non-HDL-C and CHD risk as reported in the Emerging Risk Factors Collaboration1) and epidemiological studies in Japan4–6, 24–26). In addition, our findings provide a new understanding of stroke risk. Non-HDL-C was eliminated as a risk factor for stroke by previous Japanese investigations4–7, 24, 27). Data from our study suggest that this finding can be explained by characteristics of the different types of ischemic stroke. A large proportion of ischemic strokes in Japan are lacunar infarctions9, 21). This fact may have weakened the measured association between non-HDL-C and ischemic stroke because the pathogenesis of lacunar infarction is quite different from that of large-artery occlusive infarction and atherothrombotic infarction11).
Consistent with this, a previous study suggested that the development of lacunar infarction was very close to that of ICH in combination with hypertension and a low cholesterol level28). Furthermore, the risk of cardioembolic infarction decreased with increased non-HDL-C4). This phenomenon was due to increased risk of atrial fibrillation among individuals with low cholesterol levels12, 13).
Low non-HDL-C, as well as low total cholesterol, was associated with an increased risk of ICH in previous studies29–31), although some studies could not identify non-HDL-C as a risk factor for ICH4, 6, 32). Two negative studies in Japan appeared to demonstrate insufficient power to detect this association4, 6). Another study in China used a large cohort in an urban area32). Our data were obtained only in local communities in Japan. It is possible these inverse associations are a characteristic of individuals living in rural areas. When ICH was classified by location (deep or lobar), the present study found that low non-HDL-C was associated with an increased risk of lobar ICH but not with deep ICH. Regarding deep ICH, the highest quintile of non-HDL-C exhibited a decreased risk of developing this condition, although a linear trend was not observed. The JPHC study demonstrated that lower intake of saturated fatty acids elevated the risk of lacunar infarction and deep ICH33). Conversely, non-HDL-C concentrations exhibited a great impact on large vessels in the brain, leading to large-artery occlusive infarction and lobar ICH events. Our findings support a previous report on the association of low cholesterol with lobar cerebral microbleeds34).
Non-HDL-C concentration is considered to reflect apolipoprotein B and triglyceride-rich lipoprotein levels, as well as levels of other atherogenic lipids35). The ability of non-HDL-C levels to predict CHD occurrence was comparable to apolipoprotein B and LDL-C measurements5, 36). Though elevated non-HDL-C increased the risk of CHD regardless of triglycerides levels, it was more predictive of large-artery occlusive infarction among individuals with hypertriglyceridemia. This stratification analysis implied that, similar to CHD, large-artery occlusive infarction is an atherogenic disease.
Drinking alcohol exhibited an impact on the association between non-HDL-C concentration and occurrence of CHD. Our JPHC study showed previously that consumption of alcohol was inversely associated with CHD in men and exhibited a protective effect against CHD37). Consistent with this finding, we observed a marked association between non-HDL-C concentration and CHD in non-alcohol drinkers, with alcohol exhibiting no beneficial effect on atherosclerosis.
In the present study, the obvious relationship between non-HDL-C and stroke subtype was limited to men. Although we examined the association of sex, there was no significant interaction between them. Thereby, whether there was a sex difference in non-HDL-C levels related to the occurrence of stroke subtypes remains unclear. Furthermore, we did not observe different results according to menopausal status in women.
The strengths of our study were that it recruited a large number of participants across Japan who were followed for 15 years and used a power of analysis that allowed for estimation of stroke subtype and CHD risk by sex. Moreover, we found a novel association between non-HDL-C and ICH in men, primarily lobar ICH rather than deep ICH. Nevertheless, several limitations must be considered. First, the study participants were limited to those who received total and HDL cholesterol values assessment during annual health checkups. This might have caused a “healthy voluntary effect” as mentioned elsewhere and thus an underestimation of CVD HR38). Second, since our populations consisted of patients living in rural areas, the results may not be representative of Japan. Third, we only used baseline non-HDL-C values in the analysis and did not evaluate changes in non-HDL-C during follow-up. Fourth, we did not gather information on electrocardiograph findings. Therefore, we were unable to adjust for atrial fibrillation as a stroke risk factor in the final analysis. Low non-HDL-C may be caused by poor nutritional status, which can increase ICH events. However, as we did not assess nutritional status, further investigations are needed to confirm our hypothesis.
In conclusion, unlike its association with CHD, the association between non-HDL-C concentration and the risk of stroke varied by stroke subtype. Lower non-HDL-C concentrations were associated with a lower risk of large-artery occlusion infarction but a higher risk of ICH, particularly lobar ICH.
Acknowledgments
Members of study groups: JPHC members are listed at the following site (as of June 2018): http://epi.ncc.go.jp/en/jphc/781/7951.html.
Grants and/or Financial Support
This study was supported by the National Cancer Center Research and Development Fund [grant number 23-A-31 [toku], 26-A-2, 29-A-4] (since 2011) and a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan (from 1989 to 2010).
References
- 1). The Emerging Risk Factors Collaboration Major lipids, apolipoproteins, and risk of vascular disease. JAMA, 2009; 302: 1993-2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, Mizuno K, Ohashi Y, Group MS. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA study): A prospective randomised controlled trial. Lancet, 2006; 368: 1155-1163 [DOI] [PubMed] [Google Scholar]
- 3). Cholesterol Treatment Trialists C. Fulcher J, O'Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C, Keech A. Efficacy and safety of ldl-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet, 2015; 385: 1397-1405 [DOI] [PubMed] [Google Scholar]
- 4). Imamura T, Doi Y, Ninomiya T, Hata J, Nagata M, Ikeda F, Mukai N, Hirakawa Y, Yoshida D, Fukuhara M, Kitazono T, Kiyohara Y. Non-high-density lipoprotein cholesterol and the development of coronary heart disease and stroke subtypes in a general Japanese population: The Hisayama study. Atherosclerosis, 2014; 233: 343-348 [DOI] [PubMed] [Google Scholar]
- 5). Okamura T, Kokubo Y, Watanabe M, Higashiyama A, Miyamoto Y, Yoshimasa Y, Okayama A. Low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol and the incidence of cardiovascular disease in an urban Japanese cohort study: The Suita study. Atherosclerosis, 2009; 203: 587-592 [DOI] [PubMed] [Google Scholar]
- 6). Tanabe N, Iso H, Okada K, Nakamura Y, Harada A, Ohashi Y, Ando T, Ueshima H, Japan Arteriosclerosis Longitudinal Study G Serum total and non-high-density lipoprotein cholesterol and the risk prediction of cardiovascular events - the JALS-ECC. Circ J, 2010; 74: 1346-1356 [DOI] [PubMed] [Google Scholar]
- 7). Kakehi E, Kotani K, Ishikawa S, Gotoh T, Kayaba K, Nakamura Y, Kajii E. Serum non-high-density lipoprotein cholesterol levels and the incidence of ischemic stroke in a Japanese population: The Jichi Medical School Cohort study. Asia Pac J Public Health, 2015; 27: 535-543 [DOI] [PubMed] [Google Scholar]
- 8). Sankai T, Miyagaki T, Iso H, Shimamoto T, Iida M, Tanigaki M, Naito Y, Sato S, Kiyama M, Kitamura A. [a population-based study of the proportion by type of stroke determined by computed tomography scan]. Nihon Koshu Eisei Zasshi, 1991; 38: 901-909 [PubMed] [Google Scholar]
- 9). Turin TC, Kita Y, Rumana N, Nakamura Y, Takashima N, Ichikawa M, Sugihara H, Morita Y, Hirose K, Okayama A, Miura K, Ueshima H. Ischemic stroke subtypes in a Japanese population: Takashima stroke registry, 1988–2004. Stroke, 2010; 41: 1871-1876 [DOI] [PubMed] [Google Scholar]
- 10). Shimamoto T, Komachi Y, Inada H, Doi M, Iso H, Sato S, Kitamura A, Iida M, Konishi M, Nakanishi N, et al. Trends for coronary heart disease and stroke and their risk factors in japan. Circulation, 1989; 79: 503-515 [DOI] [PubMed] [Google Scholar]
- 11). Konishi M, Iso H, Komachi Y, Iida M, Shimamoto T, Jacobs DR, Jr., Terao A, Baba S, Sankai T, Ito M. Associations of serum total cholesterol, different types of stroke, and stenosis distribution of cerebral arteries. The Akita pathology study. Stroke, 1993; 24: 954-964 [DOI] [PubMed] [Google Scholar]
- 12). Iguchi Y, Kimura K, Shibazaki K, Aoki J, Kobayashi K, Sakai K, Sakamoto Y. Annual incidence of atrial fibrillation and related factors in adults. Am J Cardiol, 2010; 106: 1129-1133 [DOI] [PubMed] [Google Scholar]
- 13). Watanabe H, Tanabe N, Yagihara N, Watanabe T, Aizawa Y, Kodama M. Association between lipid profile and risk of atrial fibrillation. Circ J, 2011; 75: 2767-2774 [DOI] [PubMed] [Google Scholar]
- 14). Tsugane S, Sawada N. The jphc study: Design and some findings on the typical japanese diet. Jpn J Clin Oncol, 2014; 44: 777-782 [DOI] [PubMed] [Google Scholar]
- 15). Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S, Committee for E, Clinical Management of A Japan atherosclerosis society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Suzuki R, Iwasaki M, Yamamoto S, Inoue M, Sasazuki S, Sawada N, Yamaji T, Shimazu T, Tsugane S, Japan Public Health Center-based Prospective Study G Leisure-time physical activity and breast cancer risk defined by estrogen and progesterone receptor status--the japan public health center-based prospective study. Prev Med, 2011; 52: 227-233 [DOI] [PubMed] [Google Scholar]
- 17). Inoue M, Yamamoto S, Kurahashi N, Iwasaki M, Sasazuki S, Tsugane S, Japan Public Health Center-based Prospective Study G Daily total physical activity level and total cancer risk in men and women: Results from a large-scale population-based cohort study in japan. Am J Epidemiol, 2008; 168: 391-403 [DOI] [PubMed] [Google Scholar]
- 18). Nakamura M, Sato S, Shimamoto T. Improvement in japanese clinical laboratory measurements of total cholesterol and HDL-cholesterol by the US Cholesterol Reference Method Laboratory Network. J Atheroscler Thromb, 2003; 10: 145-153 [DOI] [PubMed] [Google Scholar]
- 19). Saito I, Yamagishi K, Kokubo Y, Yatsuya H, Iso H, Sawada N, Inoue M, Tsugane S. Association between mortality and incidence rates of coronary heart disease and stroke: The Japan Public Health Center-based prospective (JPHC) study. Int J Cardiol, 2016; 222: 281-286 [DOI] [PubMed] [Google Scholar]
- 20). Walker AE, Robins M, Weinfeld FD. The national survey of stroke. Clinical findings. Stroke, 1981; 12: I13-44 [PubMed] [Google Scholar]
- 21). Cui R, Iso H, Yamagishi K, Saito I, Kokubo Y, Inoue M, Sawada N, Tsugane S, Group JS. Trends in the proportions of stroke subtypes and coronary heart disease in the Japanese men and women from 1995 to 2009. Atherosclerosis, 2016; 248: 219-223 [DOI] [PubMed] [Google Scholar]
- 22). Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA project. Registration procedures, event rates, and casefatality rates in 38 populations from 21 countries in four continents. Circulation, 1994; 90: 583-612 [DOI] [PubMed] [Google Scholar]
- 23). Sauerbreia W, Meier-Hirmerb C, Bennerc A, Roystond P. Multivariable regression model building by using fractional polynomials: Description of SAS, STATA and R programs. Computational Statistics & Data Analysis, 2006; 50: 3464-3485 [Google Scholar]
- 24). Yatsuya H, Iso H, Li Y, Yamagishi K, Kokubo Y, Saito I, Sawada N, Inoue M, Tsugane S. Development of a risk equation for the incidence of coronary artery disease and ischemic stroke for middle-aged Japanese- Japan Public Health Center-based prospective study. Circ J, 2016; 80: 1386-1395 [DOI] [PubMed] [Google Scholar]
- 25). Kitamura A, Noda H, Nakamura M, Kiyama M, Okada T, Imano H, Ohira T, Sato S, Yamagishi K, Iso H. Association between non-high-density lipoprotein cholesterol levels and the incidence of coronary heart disease among Japanese: The Circulatory Risk in Communities study (CIRCS). J Atheroscler Thromb, 2011; 18: 454-463 [DOI] [PubMed] [Google Scholar]
- 26). Noda H, Iso H, Irie F, Sairenchi T, Ohtaka E, Ohta H. Association between non-high-density lipoprotein cholesterol concentrations and mortality from coronary heart disease among Japanese men and women: The Ibaraki Prefectural Health study. J Atheroscler Thromb, 2010; 17: 30-36 [DOI] [PubMed] [Google Scholar]
- 27). Okamura T, Kokubo Y, Watanabe M, Higashiyama A, Ono Y, Miyamoto Y, Yoshimasa Y, Okayama A. Triglycerides and non-high-density lipoprotein cholesterol and the incidence of cardiovascular disease in an urban Japanese cohort: The Suita study. Atherosclerosis, 2010; 209: 290-294 [DOI] [PubMed] [Google Scholar]
- 28). Konishi M, Komachi Y, Iso H, Iida M, Naito Y, Sato S, Kiyama M, Shimamoto T, Kitamura A, Doi M, et al. Secular trends in atherosclerosis of coronary arteries and basal cerebral arteries in Japan. The Akita pathology study. Arteriosclerosis, 1990; 10: 535-540 [DOI] [PubMed] [Google Scholar]
- 29). Wang X, Dong Y, Qi X, Huang C, Hou L. Cholesterol levels and risk of hemorrhagic stroke: A systematic review and meta-analysis. Stroke, 2013; 44: 1833-1839 [DOI] [PubMed] [Google Scholar]
- 30). O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, Mondo C, Damasceno A, Lopez-Jaramillo P, Hankey GJ, Dans AL, Yusoff K, Truelsen T, Diener HC, Sacco RL, Ryglewicz D, Czlonkowska A, Weimar C, Wang X, Yusuf S, investigators I Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the Interstroke study): A case-control study. Lancet, 2010; 376: 112-123 [DOI] [PubMed] [Google Scholar]
- 31). Wieberdink RG, Poels MM, Vernooij MW, Koudstaal PJ, Hofman A, van der Lugt A, Breteler MM, Ikram MA. Serum lipid levels and the risk of intracerebral hemorrhage: The Rotterdam study. Arterioscler Thromb Vasc Biol, 2011; 31: 2982-2989 [DOI] [PubMed] [Google Scholar]
- 32). Wu J, Chen S, Zhou Y, Wang C, Wang A, Zhang Q, Gao X, Hu H, Wu S, Zhao X. Non-high-density lipoprotein cholesterol on the risks of stroke: A result from the Kailuan study. PLoS One, 2013; 8: e74634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Yamagishi K, Iso H, Kokubo Y, Saito I, Yatsuya H, Ishihara J, Inoue M, Tsugane S, Group JS. Dietary intake of saturated fatty acids and incident stroke and coronary heart disease in Japanese communities: The JPHC study. Eur Heart J, 2013; 34: 1225-1232 [DOI] [PubMed] [Google Scholar]
- 34). Romero JR, Preis SR, Beiser A, DeCarli C, Viswanathan A, Martinez-Ramirez S, Kase CS, Wolf PA, Seshadri S. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart study. Stroke, 2014; 45: 1492-1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Ramjee V, Sperling LS, Jacobson TA. Non-high-density lipoprotein cholesterol versus apolipoprotein B in cardiovascular risk stratification: Do the Math. J Am Coll Cardiol, 2011; 58: 457-463 [DOI] [PubMed] [Google Scholar]
- 36). Sondermeijer BM, Rana JS, Arsenault BJ, Shah PK, Kastelein JJ, Wareham NJ, Boekholdt SM, Khaw KT. Non-HDL cholesterol vs. Apo B for risk of coronary heart disease in healthy individuals: The EPIC-Norfolk prospective population study. Eur J Clin Invest, 2013; 43: 1009-1015 [DOI] [PubMed] [Google Scholar]
- 37). Nakamura Y, Kita Y, Iso H, Ueshima H, Okada K, Konishi M, Inoue M, Tsugane S, Group JS. Alcohol consumption, alcohol-induced flushing and incidence of acute myocardial infarction among middle-aged men in Japan: Japan Public Health Center-based prospective study. Atherosclerosis, 2007; 194: 512-516 [DOI] [PubMed] [Google Scholar]
- 38). Saito I, Yamagishi K, Kokubo Y, Yatsuya H, Iso H, Sawada N, Inoue M, Tsugane S. Association of high-density lipoprotein cholesterol concentration with different types of stroke and coronary heart disease: The Japan Public Health Center-based prospective (JPHC) study. Atherosclerosis, 2017; 265: 147-154 [DOI] [PubMed] [Google Scholar]


