Introduction
It has already been well recognized that guideline-recommended statin therapy is essential for the prevention of atherosclerotic cardiovascular disease (ASCVD). However, as already described by many, adherence for the statin treatment is not sufficient, and thereby beneficial effects of statin remains suboptimal. Although excellent reviews for the statin adherence has been published to highlight the clinical management of its intolerance, these are based predominantly on the observations in Western populations.
According to the growing importance of statin adherence / intolerance in ASCVD prevention and scarce information on this issue in Asian population, The Japan Atherosclerosis Society (JAS) has organized the working group on statin intolerance to survey its prevalence among Japanese individuals, and to prepare clinical guide of its management. Major concerns to diminish statin adherence are reported to be related to muscle symptom, hepatotoxicity, and glucose metabolism. Therefore, this working group was organized encompassing investigators not only lipidologists, but also neurologists, hepatologists, diabetologists, and pharmacologists, and all available data obtained in Japanese population, published in the literature or provided as post-marketing survey reports by pharmaceutical companies, regarding these concerns were enrolled for subsequent analysis.
1. Background and Significance of this Guide
Statins, originally discovered in Japan by Dr. Akira Endo, has been shown essential in primary and secondary prevention of ASCVD such as angina pectoris and myocardial infarction by means of its ability to inhibit cholesterol biosynthesis in the liver and in turn to increase the number of hepatic LDL receptors resulting in the reduction of LDL cholesterol (LDL-C) levels1). Of note, long-term administration is required especially in both secondary prevention and genetically- determined high-risk cases such as familial hypercholesterolemia (FH)2).
Good drug adherence is the basic requirement for continued statin therapy, but as is the case with drug therapies for lifestyle-related diseases, adherence is not always clinically sufficient. As a result, between 10% and 30% of cases per year find it difficult to continue therapy3). Moreover, poor adherence is known to lead to diminished preventative effects on ASCVD3). Appropriate guide of managing adverse events such as myopathy and hepatopathy, associated with statin administration, are essential to improve its adherence4). The largest concern of statin treatment is myopathy. Because of worrisome developing the most serious adverse event, rhabdomyolysis, mild cases of myopathy are considered a sign of statin intolerance. As a result of discontinuing the drug, LDL-C reduction remains insufficient, and as a result a large number of patents develop ASCVD event by insufficient treatment3).
Besides stains, a small intestine cholesterol transporter inhibitor was launched in 2007 and the PCSK9 inhibitor antibody was launched in 2016 in Japan. In Europe and United States, the ability of these drugs (the former in 2015 5) and the latter in 2017 6)) to prevent ASCVD has already been reported by large-scale clinical trials, but these were achieved in combination with statins. As for cases with difficulties in continuing statin therapy, relevant academic organizations in Europe and United States have released reports on current status and its management guide3, 4, 7–9). Despite the inclusion of promising clinical study results using PCSK9 inhibitor for statin intolerant patient10, 11), all of these reports concluded that further research and additional data are required. In Japan, alirocumab has recently been approved for statin intolerance (Nov. 2018), and evolovumab is now under review for approval (approved in Jun. 2019) (note added in proof), expecting much improvement of LDL-C lowering in statin intolerant patients.
The knowledge regarding statin intolerance among Japanese patients is limited to few research data obtained from health insurance claims12, 13). In addition, in Japanese patients, same degree of LDL-C reductions was achieved with approximately half of the dose as compared in Europe and the United States2). Therefore, it would not be appropriate to apply observations obtained in Western populations to Japanese patients, particularly in view of the fact that increased statin doses lead to increased frequency of adverse events. Furthermore, it has been reported that the genetic background of statin-associated myopathy in Japanese appears to differ from that of Euro-American descent14). Taken together with the fact that a prospective, randomized, crossover trial reported that one-third of myopathy cases had poor reproducibility and that some were due to the nocebo effect11), it would be required to understand current condition on statin intolerance in Japanese population, to identify variables linked to its mechanism, and finally to its clinical management.
Based on these, when introducing and continuing LDL-C lowering therapy for the purpose of ASCVD prevention, accurate recognition of statin intolerance and appropriate management for each individual in case of its condition would be considered as important “unmet needs” in Japanese population. In light of these current circumstances, the JAS has elucidated the frequency and the clinical features of statin intolerance and created the Statin Intolerance Clinical Guide for the purpose to enhance achievement appropriate LDL-C lowering therapy. The details of these Guidelines were mainly based on data that were publicly available as of July 2018. As new information becomes available, this Guide will need to be revised accordingly.
2. What Statin Intolerance is
As mentioned earlier, statin intolerance is a term indicating a condition in which suitable doses of statins according to a given patient ASCVD risk could not be tolerated for a variety of reasons. The main reasons include the appearance of adverse events that are associated with statin administration and laboratory test results indicating for health problems3). The National Lipid Association (NLA) defines statin intolerance as “adverse symptoms, signs and/or laboratory abnormalities attributed by the patient or provider to the statin and in most cases perceived by the patient to interfere unacceptably with activities of daily living (such as sleep, work / housework, or leisure-time activity), leading to a decision to stop or reduce statin therapy, in the individual case”15). Although these situations are not common, statin intolerant patients are included among patients in whom statin therapy is discontinued or statin dose is reduced by attending physicians or other clinicians as a result of abnormal clinical or laboratory test findings. In addition to this definition of statin intolerance for individual cases, there is also a definition of statin intolerance for the population sense. In this case, statin intolerance is defined as “clinical or laboratory adverse experiences linked to statin treatment by validated clinical evidence and presenting with pain, impairment, or risk which justifies statin cessation or dose reduction.” However, the individual definition contains elements related to the patient's subjective symptoms, resulting in the difficulty to ascertain the patient's condition according to objective and universal criteria. Therefore, the population-based definition has been considered important to apply15).
In the present Guide, statin intolerance is defined as “adverse events linked to statin administration that result in some un-tolerated impairments in daily life which justify statin cessation or dose reduction.” Taken into account the clinical use of multiple types of statins with variable doses, statin intolerance is further subdivided into “complete intolerance,” which indicates difficulty in continuing any kind of statin administration regardless of the dose, and “partial intolerance,” which indicates difficulty in continuing statin administration of some kind of statin only at certain doses.
3. Clinical Questions (CQs) Regarding Statin Intolerance
The following clinical questions (CQs) were established for the purpose of ascertaining the current status of statin intolerance among the Japanese population:
CQ1: What is the frequency of statin intolerance (difficulty in continuing statin administration) in the Japanese population?
CQ1–2: Are there any differences in the frequency of statin intolerance among various types of statin?
CQ1–3: What are the reasons for and frequency of statin intolerance (difficulty in continuing statin administration) in the Japanese population?
CQ2: Does statin intolerance (difficulty in continuing statin administration) in Japanese population influence atherosclerotic cardiovascular disease prevention and their prognosis?
CQ3: What measures are taken to deal with statin intolerance (difficulty in continuing statin administration) in the Japanese population?
Next, we used the following two approaches to identify objective data that answer the above CQs. The obtained answers are shown in the following section.
1) Systematic review of published articles.
2) Analysis of safety-related reports submitted by pharmaceutical companies that market statins in response to a request by the Working Group.
4. Answers to the CQs
4.1. Systematic Review
The period of literature search: March to June 2018.
Methods:
1. The above CQs were structured and our inclusion/exclusion criteria were determined.
2. A search form using Ichushi (Japan Medical Abstracts Society) and PubMed searches was created.
3. Reference screening (initial screening based on titles and abstracts, and secondary screening based on an assessment of the papers in full text) was performed using Ichushi (Japan Medical Abstracts Society) and MEDLINE (PubMed).
4. Data were extracted from articles that satisfied the inclusion criteria.
4.2. Results
Using PubMed, a search was conducted for comparative studies of the efficacy and safety of statins that were conducted on adult dyslipidemic patients in Japan. Eight studies met the inclusion/exclusion criteria; six were randomized comparative trials; and two were cohort studies. In spite of the fact that these studies mentioned the onset of adverse drug effects and adherence during the treatment period, they did not define intolerance or investigate its frequency and its relation to prognosis.
All articles found through the literature search in Ichushi were excluded during the secondary screening. A search for trials that were conducted in the 1970s and 1980s identified 27 papers. Of these, seven papers included 100 or more participants. Below are the responses to each CQ identified in the papers that we obtained.
CQ1: Frequency of Intolerance (cessation)
Answer: Although the frequencies reported by the studies varied from 0% to 10%, only a few studies followed up their participants for multiple years.
• Pravastatin:
1. Two comparative studies of dyslipidemic patients reported that pravastatin was discontinued in 4 out of 140 cases and 3 out of 171 cases, respectively, during the 16-week administration period16, 17).
2. A randomized controlled trial of 8,214 patients with dyslipidemia reported that pravastatin was discontinued in 7 cases during the 12-week administration period. Specifically, discontinuation was due to abnormalities in clinical test in three cases and due to adverse effects in four cases18) (Table 1).
Table 1. At five years after the start of the study 91.4% of the cases were being administered Pravastatin (ref 18).
| Year 1 | Year 3 | Year 5 | Year 7 | Year 9 | |
|---|---|---|---|---|---|
| Diet group | |||||
| Patients* | 3814 | 3627 | 2604 | 455 | 237 |
| Actual visits† | 3705 | 3354 | 2291 | 447 | 222 |
| No lipid-lowering drug | 3070 (83%) | 2398 (71%) | 1518 (66%) | 259 (58%) | 115 (52%) |
| Pravastatin | 311 (8%) | 586 (17%) | 520 (23%) | 147 (33%) | 86 (39%) |
| Other statin | 20 (1%) | 49 (1%) | 43 (2%) | 6 (1%) | 5 (2%) |
| Other lipid-lowering drug | 304 (8%) | 321 (10%) | 210 (9%) | 35 (8%) | 16 (7%) |
| Diet plus pravastatin group | |||||
| Patients* | 3678 | 3473 | 2545 | 467 | 259 |
| Actual visits† | 3574 | 3251 | 2252 | 451 | 242 |
| 20 mg pravastatin | 230 (6%) | 461 (14%) | 374 (17%) | 114 (25%) | 54 (22%) |
| 15 mg pravastatin | 4 (0.1%) | 12 (0.4%) | 10 (0.4%) | 3 (0.7%) | 3 (1%) |
| 10 mg pravastatin | 2896 (81%) | 2322 (71%) | 1522 (68%) | 285 (63%) | 152 (63%) |
| 5 mg pravastatin | 265 (7%) | 207 (6%) | 130 (6%) | 15 (3%) | 7 (3%) |
| Other statin | 6 (0.2%) | 7 (0.2%) | 17 (0.8%) | 3 (0.7%) | 5 (2%) |
| Other lipid-lowering drug | 14 (0.4%) | 29 (1%) | 22 (1%) | 8 (2%) | 5 (2%) |
| No lipid-lowering drug | 159 (4%) | 213 (7%) | 177 (8%) | 23 (5%) | 16 (7%) |
Number at risk of total mortality.
Number of patients who visited the hospital. Any patients who fitted in several categories were assigned to upper category in the table.
• Simvastatin:
(i) A cohort study of 201 dyslipidemic patients reported that nine cases suffered mild adverse drug effects but none led to cessation of statins during the 12-month follow-up period19).
(ii) A cohort study (J-LIT) of 52,421 dyslipidemic patients reported that 36,895 patients were followed up at sixth years, and 24,260 of them had continued taking Simvastatin (Table 2)20).
Table 2. At six years after the start of the study 24,260 cases were being administered Simvastatin (ref 20).
| Year |
|||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Simvastatin | n = 48,428 | 39,519 | 32,900 | 28,981 | 25,461 | 22,823 | 20,518 |
| 5 mg monotherapy | 1,729 | 2,081 | 2,069 | 2,025 | 1,966 | 1,812 | 1,668 |
| 10 mg monotherapy | 1 | 168 | 249 | 293 | 295 | 261 | 229 |
| Other monotherapy | |||||||
| Simvastatin monotherapy total | 50,158 | 41,768 | 35,218 | 31,299 | 27,722 | 24,896 | 22,415 |
| Simvastatin ± other lipid-lowering agent | 1,163 | 1,688 | 1,968 | 2,133 | 1,993 | 1,813 | 1,845 |
| Other lipid-lowering agent | 0 | 56 | 235 | 463 | 509 | 525 | 814 |
| No or Unknown medication | 0 | 6,576 | 10,419 | 11,655 | 12,074 | 12,190 | 11,821 |
| Total | 51,321 | 50,088 | 47,840 | 45,550 | 42,298 | 39,424 | 36,895 |
| Simvastatin total | 51,321 | 43,456 | 37,186 | 33,432 | 29,715 | 26,709 | 24,260 |
| 5 mg (%) | 49,495 (96.4) | 40,946 (94.2) | 34,561 (92.9) | 30,694 (91.8) | 27,065 (91.1) | 24,246 (90.8) | 21,994 (90.7) |
| 10 mg (%) | 1,825 (3.6) | 2,332 (5.4) | 2,365 (6.4) | 2,422 (7.2) | 2,334 (7.9) | 2,183 (8.2) | 2,018 (8.3) |
| Other (%) | 1 (0.0) | 178 (0.4) | 260 (0.7) | 316 (0.9) | 316 (1.1) | 280 (1.0) | 248 (1.0) |
(iii) A comparative study of dyslipidemic patients reported that simvastatin was discontinued in four out of 137 cases during the 12-week follow-up period21).
(iv) A phase II dose-finding study of MK-733 (Simvastatin) reported that one case out of 168 experienced loss of appetite and malaise during the 12-week follow-up period22).
(v) A multicenter open study of 280 dyslipidemic patients reported that simvastatin was discontinued in 4 cases due to urticaria, tightness in the chest, fatigue, and a burning sensation on the face during the 12-week administration period23).
CQ1–2: The Frequency of Statin Intolerance by Type of Statin
Answer: Although only few articles mentioned this issue, no differences were observed in the frequency of intolerance based on the type.
• Comparison of Pitavastatin and Atorvastatin
(i) A randomized controlled trial (RCT) that investigated the efficacy of Pitavastatin and Atorvastatin used on 207 dyslipidemic patients with impaired glucose tolerance reported that there was no significant difference between the two groups in the incidence of adverse drug effects and that drugs administration were discontinued due to adverse drug effects in six cases within six months in both groups. No difference between the groups was found in terms of adherence of the drugs (Table 3)24).
Table 3. Investigation of the efficacy of Pitavastatin and Atorvastatin on 207 dyslipidemia patients with impaired glucose tolerance (ref 24).
| Week 8 | Week 28 | Week 56 | |
|---|---|---|---|
| Pitavastatin | 98% (86/88) | 94% (83/88) | 93% (79/85) |
| Atorvastatin | 100% (84/84) | 96% (81/84) | 98% (80/82) |
(ii) An RCT (JAPAN-ACS Trial) comparing the effects of Pitavastatin and Atorvastatin in 307 acute coronary syndrome patients who underwent PCI with intravascular ultrasound guidance reported that there was no significant difference between the two groups in the incidence of drug discontinuation due to adverse drug effects or laboratory test abnormalities (Pitavastatin 2.7%, Atorvastatin 4.7%)25).
CQ1–3: Reasons for Intolerance
Answer: Only a limited number of articles provided the reason for intolerance, with which solid conclusion are difficult to draw.
• Pravastatin:
(i) A cohort study of 446 dyslipidemic patients reported that pravastatin administration was discontinued in seven cases during the 12-week administration period. Specifically, discontinuation was due to abnormalities in clinical tests in three cases and due to adverse drug effects in four cases26).
(ii) A cohort study of 368 dyslipidemic patients reported that pravastatin administration was discontinued in 18 cases during the 12-week administration period. Discontinuation was due to safety concerns (adverse drug effects and abnormalities in clinical tests) in 12 cases and due to non-safety-related issues (changes in attending physicians, and normalization of lipid levels) in six cases27).
• Pitavastatin:
An RCT (CHERRY Trial) that compared Pitavastatin and a combination of Pitavastatin and EPA in 241 patients with stable angina and acute coronary syndrome who underwent PCI with intravascular ultrasound guidance reported that the study drug was discontinued in 8 out of 117 patients (6.8%) in the Pitavastatin-only group. Specifically, discontinuation was due to laboratory test abnormalities in one case (AST or ALT), adverse drug effects in three cases (myopathy: 2, bleeding: 1), and “other reasons” in four cases28).
CQ2: Statin Intolerance: Effect on ASCVD Prevention and Prognosis
Answer: Although only a limited number of articles addressed this issue, it is possible that the protective effect of statins may be reduced in patients that poorly adhered to statins.
• Pravastatin:
(i) An RCT (KLIS Trial) compared Pravastatin and conventional therapy in 5,640 dyslipidemic patients for a mean follow-up period of 5 years. This study further divided the Pravastatin group into groups of good adherence and that of poor adherence; the “good adherence” group had significantly fewer cardiovascular events than the “poor adherence” group (Table 4)29).
Table 4. Good Adherence and Poor Adherence Subgroups of the Pravastatin Group in the KLIS Trial (ref 29).
| Event | Poor compliance | Coronary events |
|---|---|---|
| (n = 1,114) | (n = 1,105) | |
| Coronary events | ||
| No. of events | 33 | 32 |
| Adjusted RR* | 1.00 | 0.75 |
| (90% CI) | (0.68–1.48) | (0.50–1.11) |
| Cerebral infarction | ||
| No. of events | 20 | 27 |
| Adjusted RR | 0.83 | 0.74 |
| (90% CI) | (0.52–1.33) | (0.48–1.14) |
| Coronary events and cerebral infarction† | ||
| No. of events | 52 | 57 |
| Adjusted RR | 0.92 | 0.73 |
| (90% CI) | (0.68–1.25) | (0.54–0.98) |
RR = relative rink; CI = confidence interval.
Based on the Cox hazards model controlling for age, serum total cholesterol, serum HDL cholesterol, body mass index. angina pectoris, hypertension, diabetes mellitus, prior use of lipid-lowering drugs, smoking, and alcohol use.
The uarlier event was conted in the case of concurrent occurrences.
• Pravastatin or Simvastatin:
(i) An RCT (Japan EPA Lipid Intervention Study: JELIS) compared statins and a combination of statins and EPA in 18,645 dyslipidemic patients. When statins were administered as primary prevention, patients with complete adherence to the drugs in the control (statin alone) group had a non-significantly lower incidence of cardiac death or myocardial infarction (HR 0.79 95%CI 0.45–1.38, P = 0.409). In contrast, when statins were used as the secondary prevention, the same group had a non-significantly higher incidence of these conditions (HR 1.47, 95%ci 0.72–2.99)30).
CQ3: What measures are taken in order to deal with statin intolerance (difficulty in continuing statin administration) in the Japanese populations?
Answer: We found no articles that answered this question.
4.3. Analysis of Safety-related Reports
Utilizing the materials (mainly post-marketing surveillance studies) provided by pharmaceutical companies that dealt statins, we analyzed the period of statin discontinuation after the initiation of statin therapy as well as the reasons for statin discontinuation. The periods from the start of statin administration till the discontinuation were classified as follows; A: within 12 weeks, B: within 6 months, C: within 1 year, D: over 1 year, E: unknown.
Answer: Although the number of cases analyzed would be insufficient for estimating the incidence of discontinuation properly, the muscle symptoms, abnormal plasma CK levels or elevated plasma hepatic enzymes levels which led statin discontinuation as adverse events occurred with a frequency of between 0.3% and 1.0%, respectively. It was also found that these adverse events took place within 12 weeks following the start of statin administration in many cases.
Simvastatin
We examined 9 articles of clinical trials and post-marketing surveillance studies. There were 1,392 patients who were administered simvastatin.
Reason for discontinuation:
| • Elevated CK level alone: 2 (A, A) | 0.144% |
| • Elevated CK level with muscle pain: 2 (A, C) | |
| 0.144% | |
| • Rhabdomyolysis: None | 0% |
| • Muscle pain without elevated CK level: 3 (A, A, B) | |
| 0.216% | |
| • Muscle-related symptoms, details unknown: None | |
| 0% | |
| • Elevated liver enzymes: 3 (A, A, B) | 0.216% |
| • Other symptoms: 30 | |
| • Unknown, drop out, other reasons: 25 | |
Pravastatin
We examined 12 articles of clinical trials and post-marketing surveillance studies; there were 5,719 patients who were administered pravastatin in these articles. We also analyzed 3 articles which utilized pravastatin as a control agent in comparison with atorvastatin, fluvastatin, and pitavastatin, respectively; in these articles there were 421 patients administered with pravastatin.
Reason for discontinuation:
| • Elevated CK level alone: 2 (A, D) | 0.035% |
| • Elevated CK level with muscle pain: None | 0% |
| • Rhabdomyolysis: None | 0% |
| • Muscle pain without elevated CK level: None | 0% |
| • Muscle-related symptoms, details unknown: None | |
| 0% | |
| • Elevated liver enzymes: 6 (A, A, A, D) | 0.105% |
| • Other symptoms: 16 | |
| • Unknown, drop out, other reasons: None | |
Rosuvastatin
We examined 2 articles of clinical trial and post-marketing surveillance study. There were 149 patients who were administered rosuvastatin.
Reason for discontinuation:
| • Elevated CK level alone: 1 (B) | 0.671% |
| • Elevated CK level with muscle pain: None | 0% |
| • Rhabdomyolysis: None | 0% |
| • Muscle pain without elevated CK level: None | 0% |
| • Muscle-related symptoms, details unknown: None | |
| 0% | |
| • Elevated liver enzymes: None | 0% |
| • Other symptoms: 5 | |
| • Unknown, drop out, other reasons: 5 | |
Atorvastatin
We examined 13 articles of clinical trials and post-marketing surveillance studies. There were 29,314 patients who were administered atorvastatin. Reason for discontinuation:
| • Elevated CK level alone: 2 | 0.0068% |
| • Elevated CK level with muscle pain: None | 0% |
| • Rhabdomyolysis: 4 | 0.0137% |
| • Muscle pain without elevated CK level: 2 | |
| 0.0068% | |
| • Muscle-related symptoms, details unknown: None | |
| 0% | |
| • Elevated liver enzymes: 5 | 0.0171% |
| • Other symptoms: 33 | |
| • Unknown, drop out, other reasons: 11 | |
Fluvastatin
We examined 6 articles of clinical trials, post-marketing surveillance studies, and pharmacotherapy outcome studies. There were 26,796 who were administered fluvastatin.
Reason for discontinuation:
| • Elevated CK level alone: None | 0% |
| • Elevated CK level with muscle pain: None | 0% |
| • Rhabdomyolysis: None | 0% |
| • Muscle pain without elevated CK level: 1 | |
| 0.0037% | |
| • Muscle-related symptoms, details unknown: None | |
| 0% | |
| • Elevated liver enzymes: 7 | 0.0261% |
| • Other symptoms: 20 | |
| • Unknown, drop out, other reasons: None | |
Pitavastatin
We examined 3 articles of clinical trials and post-marketing surveillance studies. There were 6,964 patients who were administered pitavastatin.
Reason for discontinuation:
| • Elevated CK level alone: None | 0% |
| • Elevated CK level with muscle pain: 2 | 0.0287% |
| • Rhabdomyolysis: 1 | 0.0144% |
| • Muscle pain without elevated CK level: 24 | |
| 0.345% | |
| (however, whether discontinued or not was not described) | |
| • Muscle-related symptoms, details unknown: None | |
| 0% | |
| • Elevated hepatic enzymes: 85 | 1.22% |
| (however, whether discontinued or not was not described) | |
| • Other symptoms: None | |
| • Unknown, drop out, other reasons: None | |
5. The Causes of Adverse Events Associated with Statin Use and Countermeasures
The common adverse events of statin administration were myopathy (subjective symptoms of muscle pain and reduced muscle strength as well as laboratory results indicating elevated serum creatine kinase [CK]), elevated liver enzyme levels, and new-onset diabetes3), which are explained scientifically. In addition, according to a retrospective study of over 100,000 patients who were administered statins in clinical settings in the United States31), administration was discontinued due to adverse events linked to statins in 17.4% of the subjects, and the most common reasons listed for discontinuation were, in descending order, myopathy (7.2%), systemic symptoms (2.3%), and liver injury (2.1%).As indicated in the response to the previous CQ, according to randomized studies (including drug development studies) and post-marketing cohort studies, the fact that there are major discrepancies in the incidence of adverse events linked to statin administration is consistent with results obtained in Japan. The adverse events are presented as subjective symptoms and are dependent upon clinical test results that are susceptible to variation according to a variety of factors other than the drug that was administered. These factors likely contributed to the differences in frequency.
In this Clinical Guide, we identify myopathy, liver injury, and the effects on glucose tolerance as adverse events that have been linked to statin administration, and provide a summary of what is already known about issues that will become increasingly focused upon in the future, including the effect of statin administration of the central nervous system (CNS) (including cognitive function) and the thinking regarding the relationship between dose and renal dysfunction. We have created a flow chart for clinician to lead better understandings in the management of statin-associated myopathy and liver injury (Fig. 1).
Fig. 1-1.
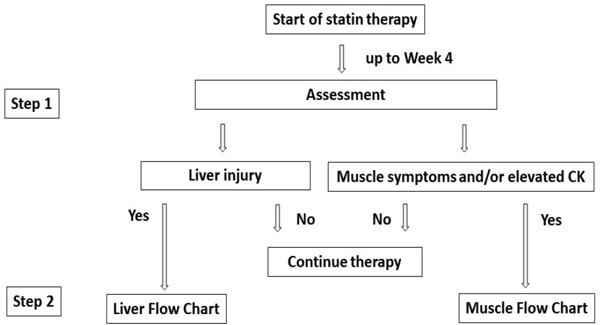
Recommended approaches to adverse events (myopathy, liver dysfunction) at initial statin
Step 1: When statin treatment was introduced according to clinical indications, subjective symptoms and laboratory test results (e.g., lipids, liver function, CK) are assessed around week 4 after its start.
Step 2: If muscle symptoms and/or elevated CK levels are observed, follow the “muscle flowchart.” If liver dysfunction is observed, follow the “liver flowchart.”
For indications of statin administration and its administered dose, see the “Atherosclerotic Disease Prevention Guidelines, 2017 Edition” and the “Dyslipidemia Examination Guide for the Purpose of Preventing Artherosclerotic Diseases, 2018 Edition” (both: Japan Atherosclerosis Society eds.), as well as the package inserts of the specific drugs utilized.
Fig. 1-2.
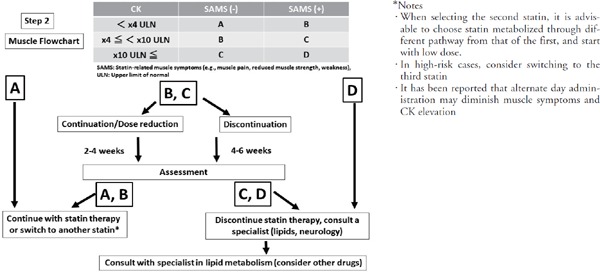
Recommended approaches to adverse events (myopathy) during statin administration
• Myopathy is categorized in the A, B, C, or D groups as shown in the chart according to SAMS and CK level. CK levels may be increased as a result of vigorous exercise and intramuscular injections, which can persist for several days. Thus, when serum CK level is found to be elevated, there is a need to confirm whether the patient experienced either of the above prior to the blood test. In such cases, it is advisable to have the patient remain in a rested state as much as possible for several days and repeat the blood test. Other risk factors associated with myopathy onset include: Being an elderly female, short stature, Asian descent, renal dysfunction, hypothyroidism, excessive alcohol consumption, and surgery. Concomitant drugs that require particular attention include: azure-based anti-fungaldrugs that are antagonists to the drug metabolism system, macrolide antibiotics, protease inhibitors (anti-viral agents), verapamil, diltiazem, amiodarone, warfarin, and cyclosporine (ref 33).
• Proceed according to the relevant flowchart. See the text of the current Guide for details regarding the specific ways of handling each category. As a general rule, the follow-up period for categories B and C are 2–4 weeks and 4–6 weeks, respectively, but following-up on patient progress more frequently may be appropriate.
Fig. 1-3.
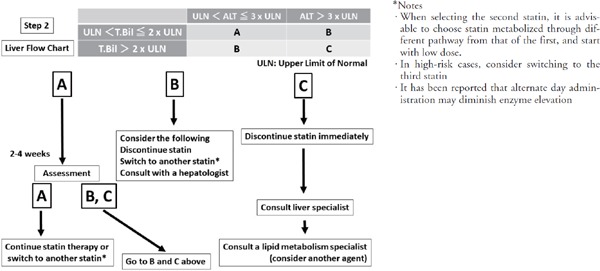
Recommended approaches for adverse events (liver dysfunction) during statin administration
• Liver dysfunction is categorized into A, B, and C groups as shown in the chart according to ALT level and T-bil level
• See the flowchart for each for approaches.
• Simultaneously check for complications with acute diseases that may cause liver dysfunction such as acute viral infection and hepatobiliary diseases as well as concomitant drugs that may cause liver dysfunction.
5.1. Myopathy
5.1.1. Muscle-Related Adverse Events Linked to Statins
Muscle-related adverse events caused by statin therapy present with a large number of muscle-related symptoms, including those identified through laboratory test abnormalities. Guidelines released by a variety of academic associations in Europe and the United States outline the various ways of dealing with muscle-related adverse events, but they are not consistent in their guidance4, 9, 32). Moreover, there are no standardized guidelines tailored to Japanese patients, and as a result issues such as statin therapy discontinuation, dose reduction, and resumption of statin therapy have been left up to each individual attending physician. As a result, it can be assumed that that are a large number of patients who have been identified as being statin intolerant due to muscle-related adverse events.
One reason for the lack of consensus on how to handle muscle-related adverse events is that there is no established definition of what constitutes a “muscle-related symptom.” For example, a term of “myopathy” sometimes indicates all symptoms linked to muscles, including muscle pain (broad sense); however, sometimes it indicates reduced muscle strength in the extremities and trunk (narrow sense) depending upon physicians. This Clinical Guide use the latter definition of the term “myopathy” (the narrow sense).
Several possible mechanisms have been associated with the cause of muscle-related adverse events, but they are focused on metabolic abnormalities of intracellular mitochondria4). In addition, a large number of risk factors have been identified, including being an elderly female, having a small physique, being of Asian descent, renal dysfunction, hypothyroidism, muscular disease, excessive alcohol consumption, overexertion, and surgery33). Those who are taking drugs related to the metabolism of the drug-metabolizing enzyme cytochrome P450 (CYP3A4) and those with genetic polymorphism of the solute carrier organic anion transporter family member 1B1 (SLCO1B1) are also at risk of muscle-related adverse events linked to statins.
Under these circumstances, clinicians who are relatively unfamiliar with muscle diseases require guidelines for easily judging in the management of statin intolerance. Based on the statement of the European Atherosclerosis Society4), we created a Guide (proposed) to use in assessing muscle-related adverse events based on symptoms and laboratory test results separately that can be combined to identify a course of action.
5.1.2. Statin-Associated Muscle Symptoms
Statin-associated muscle symptoms (SAMS) include all muscle symptoms that appear as a result of statins. They include subjective symptoms such as muscle pain, or stiffness, as well as muscle discomfort. These symptoms show no bilateral differences in the trunk and proximal extremities and appear in relatively large muscles. There are two severe forms: Rhabdomyolysis and reduced muscle strength in the trunk and extremities (the narrow sense of myopathy). It is important to identify subjective symptoms such as “head feels heavy,” “cannot raise arms,” “can't squat down and stand up.” Objective assessment is performed using manual muscle testing as a part of a neurological examination. It is likely that some patients cannot give muscle strength due to muscle pain.
SAMS appear within 4–6 weeks after the start of statin administration, but in rare cases muscle symptoms may appear several years later. People who are very physically active are susceptible. When increasing the statin dose or switching to another statin, new SAMS may appear. In many cases SAMS may appear soon after statin administration is resumed after temporary cessation4, 34).
5.1.3. Serum Creatine Kinase
CK is an enzyme that is involved in energy metabolism when muscles contract. It mediates the production of creatine phosphate and ADP from creatine and ATP. CK is a protein dimer formed from two sub-units. The three types of CK (MM, BB, MB) that are formed from combinations of B (brain-type) and M (muscle type) are isozymes. Since skeletal muscle has large amounts of MM-type and cardiac muscle has large amounts of MB-type, it is necessary to analyze the isozyme ratios in case of elevated CK.
Normal levels of serum CK that are correlated to muscle bulk are 50–200 IU/L in males and 40–170 IU/L in females, respectively. Since there are large amounts of CK in skeletal muscle, the CK level increases as a result of vigorous exercise or intramuscular injection and the effects last several days. When serum CK levels are found to be elevated, it is necessary to confirm whether the patient experienced either of these (vigorous exercise or intramuscular injection) prior to the blood test. Have the patient remain in a rested state as much as possible and repeat the blood test at a later date. However, as there is no clear criterion for determining of elevated CK levels, it is usually accepted to determine through comparison with the upper limit of normal (ULN) indicated by the guidelines published by academic associations in Europe and the US.
When using a statin for the first time or when resuming administration after a washout period, the serum CK level should be measured prior to the first administration. Hypothyroidism or undiagnosed myopathy may be cause of asymptomatic high CK levels. It is advisable to measure serum CK levels initially at four weeks after the start of statin therapy and thereafter on an intermittent basis.
As there is no clear criterion for determining whether CK levels are elevated, in many cases a standard is determined through comparison with the ULN indicated by the guidelines published by academic associations in Europe and the US. Although 4x, 10x, and 40x ULN are used as criteria4), there is no clear evidentiary support for these criteria, and as a result they are considered merely a rough guide. CK levels of under 4x ULN are considered mild elevation (less than approximately 500 IU/L), levels between ≧ 4x and under 10x ULN are considered moderate elevation (approx. ≧ 500 IU-2,000 IU/L), and levels of 10x or higher are considered severe elevation (approx. 2,000 IU/L or higher). The approach to be taken should take these divisions into account35).
5.1.4. Countermeasures
Assessment of muscle-related adverse events linked to statins are organized into four categories (A, B, C, D) based on the serum CK level and whether SAMS are present (Fig. 1).
Classification of the category
(i) Category A: Serum CK is under 4x ULN without SAMS. Normal or abnormality with test data only.
(ii) Category B: Serum CK 4x or higher but under 10x ULN without SAMS, or serum CK under 4x ULN with SAMS.
(iii) Category C: Serum CK level is 10x or more and without SAMS, or serum CK level is 4x or higher but under 10x ULN with SAMS.
(iv) Category D: Serum CK level is 10x or more ULN with SAMS.
Judgment and Management
(i) Category A: statin continuation possible.
(ii) Category B: considered a mild muscle-related adverse event. If the patient can tolerate the SAMS, continue statin therapy, or the dose may be reduced. However, after 2–4 weeks repeat assessment. If patient is in Category A or B, treatment may be continued, but if in Category C or D, treatment is discontinued. Also, if the patient is unable to tolerate the SAMS, discontinue drug administration for a period of 4–6 weeks. Repeat the assessment before resuming administration with a different statin. If Category A or B, treatment may be resumed with another statin, but if the patient is in danger of worsening to Category C or D, discontinue statin therapy.
(iii) Category C: This is considered a moderate muscle-related adverse event. In cases in which there is a great need to continue statin therapy, continue statin administration or reduce the dose. In cases in which it is determined that there is a high likelihood that the muscle-related adverse event may worsen, repeat the assessment in 2–4 weeks or sooner. If the patient improves to Category A or B, continue therapy. If the patient is in Category C or D, discontinue therapy. If the patient is unable to tolerate the SAMS, discontinue administration for 4–6 weeks. Repeat the assessment before resuming administration with a different statin. If the patient improves to Category A or B, resume administration with another statin. If at Category C or D, discontinue therapy and consult with a specialist in lipid metabolism for information on lipid management.
(iv) Category D: This is considered a severe muscle- related adverse event. Discontinue statin administration immediately and remain aware of the risk of rhabdomyolysis or myopathy. Particularly in cases in which rhabdomyolysis onset occurs, treatment must be started immediately. Consult with a specialist in lipid metabolism regarding lipid management and the possibility of using another statin.
5.1.5. Severe Muscle-Related Adverse Event
Although an infrequent muscle-related adverse event, any time an extremely severe situation is identified, treatment must commence immediately.
(i) Rhabdomyolysis: Damage to striated muscle causes the muscle tissue to breakdown and eventually develop into necrosis. One cause of this condition is statins36). While only 0.001% of patients being treated with statins develop rhabdomyolysis, it can be fatal if left untreated. The symptoms progress over the course of only a few days and symptoms include widespread muscle pain, pain when gripping, fatigue, reduced muscle strength, and fever. Often serum CK levels are 40x or more over ULN (approx. 10,000 IU/L or higher). Whether or not the patient presents with red urine (cola-colored urine), the disease presents as myoglobinuria without red blood cells in the urine sediment test results. Particular attention must be paid to the possibility of acute kidney failure. In addition to cessation of statin administration, the patient requires rest and sufficient amounts of fluid replacement.
(ii) Statin-associated myopathy: Reduced muscle strength in the extremities and trunk progresses over several months regardless of the fact that statin administration was discontinued. Statin-associated myopathy, including immune-mediated necrotizing myopathy, which is one of an inflammatory myopathy (myositis), is identified through detection in the patient's blood serum of auto-antibodies against 3-hydroxy-3-methylglutary-coenzyme A reductase (HMGCR)37). It is particularly common among elderly individuals. On average, the serum CK levels are approximately 5,000 IU/L. It is urged to consult with a neurologist and make a definitive diagnosis based on muscle MRI, needle EMG, and muscle biopsy.
Anti-HMGCR antibodies are not detected in patients who are asymptomatic with elevation of CK levels during statin administration. Therefore, it can be expected to be useful as a biomarker for the diagnosis of statin-associated myopathy (the test for this antibody is not currently covered by the Japanese health insurance system)38). Treatment of statin-associated myopathy consists of discontinuation of the culprit drug and corticosteroids. In general, the therapeutic response is favorable, and muscle strength returns gradually over several months.
5.2. Liver Injury
In Japan, six types of statins are used against hypercholesterolemia and elevated LDL cholesterol. These six stains are associated with mildly to moderately elevated transaminase levels, and according to a large-scale study conducted in Europe and the US, in many cases they appear in 0.5% to 2.0% of cases within the first three months of administration. Systematic meta-analyses of 135 RCTs that included a total of 246,000 patients reported that statins have approximately 50% higher risk of elevated transaminase levels than controls and placebos, and that in the cases of Atorvastatin, Rosuvastatin, and Simvastatin, the enzyme elevations are dose-dependent39–41). However, the overall frequency of 0.5% to 2.0% was nearly the same as that for the placebo. Since liver enzyme levels decline in many cases even if therapy is continued, it is not considered as a clinical problem42). Statin prescriptions have been increasing since the late 1990s, but no cases of severe liver injury due to statins are listed in the FDA Adverse Event Reporting System database. Recently, the US National Lipid Association's Statin Safety Task Force concluded that liver toxicity due to statins is extremely rare43).
On the other hand, in rare cases clinically clear cases of acute liver injury are presented. An analysis of 49 trials that included a total of 14,000 patients reported that incidence of ALT levels over 3x ULN was broken down as follows: Atorvastatin 10 mg: 0.1%, 80 mg: 0.6%, Placebo: 0.2%44). Although there are many reports of liver injury due to Atorvastatin and Simvastatin, there are few in case of Pravastatin and Pitavastatin. Moreover, liver injury occurs at various time points, and it has been known to occur even six months after the initiation. Liver injury can be consequence of hepatocellular injury or cholestasis, and autoimmune hepatitis has occurred in some cases. Particular care is required when administering statins to patients with type B hepatitis45) or primary biliary cholangitis (PBC; formerly known as “primary biliary cirrhosis”)46).
The mechanism of onset of idiosyncratic druginduced liver injury (IDILI) remains unknown, but it is thought that drugs or their intermediate metabolites and host proteins combine to form an adduct, which causes disease onset through an immune response triggered by T cells that detect neoantigens47). It is also thought to somehow modify the injury by transporter inhibition, mitochondria damage, endoplasmic reticulum (ER) damage and oxidative stress, inflammatory cytokines and so on. However, although diagnosis is often performed using the scoring diagnostic method, as there is no specific diagnostic method currently available, there is no alternative but to rely upon expert opinion at present47).
From the perspective of statin intolerance, when Hy's Law48–50), which estimates that there is a 10% risk of death/liver transplantation based on a large-scale drug-induced liver injury database, reaches ALT ≥ 3 X ULN, total bilirubin ≥ 2 X ULN (direct dominance) or above should be able to be set as the level at which a physician should consider consultation with a hepatologist.
Countermeasures
Although mild liver injury due to statins sometimes occurs during treatment, in many cases it is temporary and the patient recovers even if administration continues at the same dose. Although rare, liver failure and auto-antibody-positive autoimmune hepatitis sometimes does occur. Thus, using ALT ≥ 3 X ULN, total bilirubin ≥ 2 X ULN as a guide, in addition to discontinuing drug administration and consulting a hepatologist, it is also recommended that the physician consider switching to another statin.
5.3. Central Nervous System-Related Adverse Events
5.3.1. Effect on Cognitive Function
5.3.1.1. Background
In 2001, a 51-year-old man gradually developed a memory disorder after Simvastatin administration51). After switching to Pravastatin, the symptom was disappeared. A woman who was administered Atorvastatin and another woman who was administered Atorvastatin, then it was switched to Simvastatin both developed cognitive impairments temporarily52). According to the cumulative results of 60 patients of memory impairment related to statins by the US FDA MedWatch pharmaceutical Surveillance System from 1997 to 2002, 36 of the 60 patients developed the condition by Simvastatin, 23 by Atorvastatin, and 1 patient by Pravastatin. Fourteen of 25 patients (56%) showed improvement in symptoms after discontinuation of statin. In four cases, the same symptoms occurred after restart of statin. However, none of the cases underwent assessment of cognitive function53).
Upon receiving these reports, in 2012 the The U.S. Food and Drug Administration (FDA) has approved important safety label changes for the statins54). The additions were to mention the information about the potential for generally non-serious and reversible cognitive side effects (memory loss, confusion, etc.). According to the FDA, these symptoms occurs within one year after the start of statin and disappears after statin discontinuation. Thus, they do not constitute permanent and progressive forms of dementia such as Alzheimer's disease (AD)54).
When this is reported by the mass media, rumors have risen that statins cause cognitive deterioration55). However, in Japan there is no report of cognitive decline due to statin. Compared to the USA and European countries, low doses of statins are used in Japan and other differences may be involved56).
5.3.1.2. The Mechanism of Onset of Statin-Associated Cognitive Impairment
The important point to keep in mind when considering the onset mechanism of statin-related cognitive impairment is that lipid-soluble statins such as Atorvastatin and Simvastatin are more likely to pass through the blood brain barrier than water-soluble statins such as Pravastatin and Rosuvastatin. In fact, 59 out of 60 cases that developed cognitive impairment by lipid-soluble statins53).
The following two mechanism are assumed: #1 Decreased cholesterol levels by statins causes damage to the soundness of neurons and glial cells, which in turn delays neurotransmission. #2 Remyelination is delayed by statins57). In addition, reduced Coenzyme Q10 levels by statin may cause disturbed mitochondrial function, which in turn causes increased oxidative stress58).
5.3.1.3. Systematic Review Results
According to many systematic reviews evidence does not support the increase in dementia due to the use of statins. In the meta-analysis of 27 cognitive-function evaluated studies (3 RCT, 16 observational studies, 4 case controls and 4 cross-sectional studies), it was emphasized that there was at least no adverse effect on cognitive function by statin as FDA is concerned, even the evidence level is insufficient (Fig. 2)59). In addition, Swiger et al. conducted a systematic review60) based on data listed on MEDLINE, EMBASE, and the Cochrane Center Register up to April 25, 2013. Their analysis divided the patients into short-term administration (within 1 year) and long-term administration (1 or more years). On 296 individuals in the short-term group were analyzed by using the Digital Symbol Substitution Test (DSST). The results indicated that there was no clear difference between the statin group and placebo group. Analysis of the effect of long-term administration of statins (3 years–24.9 years) via three studies indicated that there was no difference between the statin administration group and the non-statin administration group in terms of the frequency of newly onset of dementia. In contrast, 5 studies reported that statin administration has a preventative effect on newly onset of dementia. An analysis of cumulative results reported that the frequency of dementia in the statin group was 29% lower than non-statin group (hazard ratio: 0.71; 95%CI 0.61–0.87). Based on these data, the authors concluded that short-term statin administration does not cause a cognitive decline and that long-term statin administration has a preventative effect on new onset of dementia. However, a randomized clinical trial LEADe (Lipitor's Effects in Alzheimer's Dementia) reported that approximately 18 months of Atorvastatin administration to the mild to moderate AD was unable to improve the cognitive function61). Therefore, it is not conclusive as to whether the statin has a prophylactic effect against AD and other types of dementia62).
Fig. 2.
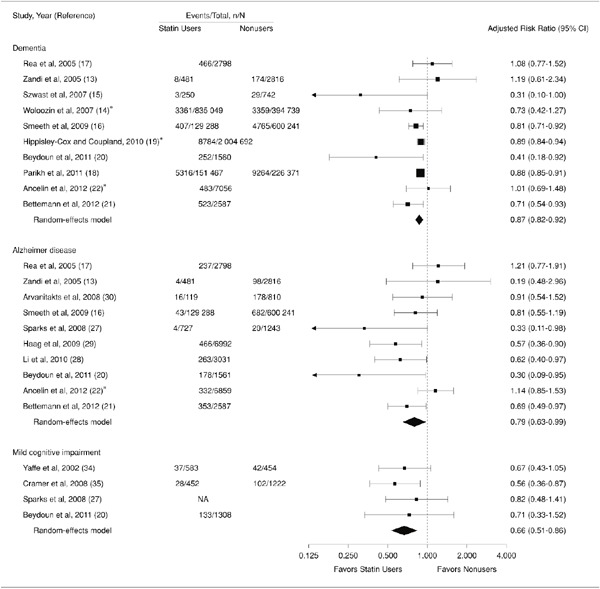
Risk ratio obtained from prospective studies of the relation between statins and Alzheimer's disease/mild cognitive impairment (ref 59)
5.3.1.4. Countermeasures when Patients taking Statins Experience Cognitive Impairment
If it was suspected that cognitive impairment is related to statins, a neuropsychological examination (e.g., Hasegawa Dementia Scale-revised (HDS-R), or Mini-Mental State Examination (MMSE)) should be conducted. If necessary, the physician should consider gradual discontinuation of statin or switching to another type of statin, e.g. a water-soluble statin. Since frequency is extremely rare, it is not necessary to perform neuropsychological examinations of all patients being administered statins63).
5.3.2. Depression
5.3.2.1. Background
In the 1990s, there were reports that increased depressed mood, aggressiveness, and suicide by diet therapy to reduce cholesterol levels and drugs other than statins. Based on these reports, studies were conducted to study the relationship between statins and depression. A randomized double-blind study reported that patients being administered Simvastatin showed increased occurrence of depression and somatization64). However, the relationship between statins and depression remains controversiale65), as it has been reported that statins protect depression as well as there is no correlation between statin and depression.
5.3.2.2. The Mechanism of Statin-Induced Depression
One hypothesis is that low cholesterol level in the serum leads reduced cholesterol level in the brain, which in turn has a negative effect on the neurotransmission function of the CNS. This then causes decreased serotonin activity, leading to depression.
5.3.2.3. Systematic Review Results
According to seven high-quality observational studies that comprised a total of 9,187 participants, statin administration prevents depression (odds ratio: 0.68; 95%CI 0.52–0.89; Fig. 3). Statins are considered to reduce several factors that inducing depression including oxidative stress and inflammatory cytokines65).
Fig. 3.
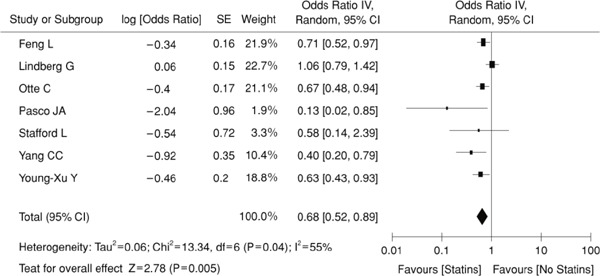
Statin and Depression (Meta-analysis) (ref 65)
5.4. Effect on Glucose Tolerance
5.4.1. The Effect of Statins on the Onset of New Diabetes
According to cardiovascular event prevention trials (e.g., PROVE-IT, JUPITER Trial), statin administration increases the incidence of new diabetes66, 67). A meta-analysis also indicated that statins increase the incidence of new diabetes68). A meta-analysis of five large-scale randomized comparative studies of the effects of statin dose reported that high statin doses increased the risk of diabetes onset more than moderate doses69). A retrospective analysis conducted in Japan70) reported the same results, which suggests that the statin dose is involved the onset of new diabetes. However, a comparison of Pitavastatin 1 mg and 4 mg did not find a significant difference in the incidence of new diabetes71).
It is not clear if there are any differences related to type of statin, but the PROVE-IT trial that compared the results obtained from administration of Pravastatin 40 mg and Atorvastatin 80 mg found that the Atorvastatin group showed significantly elevated HbA1c levels. In addition, high doses of statins caused higher incidence of new diabetes than low doses, which indicates the possibility that the effect on new onset diabetes may differ from one statin to another72, 73). Data obtained in Japan also indicates that the high-strength statin group had a significantly higher risk of onset than the low-strength statin group74). In addition, long-term statin use on patients at high risk of diabetes onset causes significantly more diabetes onset75). According to post-marketing surveillance data, fatty liver and hyperuricemia are risk factors for diabetes onset76).
5.4.2. Effect on Diabetes Patients' Blood Glucose Level Control
There have been few studies of the effect of statin drugs on diabetes patients' blood glucose level control, and no large-scale clinical trials have been conducted. A study of Japanese type 2 diabetes patients reported that in comparison to the Pravastatin 10 mg group, the Atorvastatin 10 mg group had poorer HbA1c levels (approx. 0.4%) at three months77). The same analysis indicated that Pitavastatin did not cause worsening of HbA1c levels78). This suggests that high-strength statins such as Atorvastatin are more liable to cause deterioration in glucose tolerance than lowstrength statins79).
Even among high-strength statins there may be different effects on glucose tolerance. The CHIBA Study that prospectively compared Atorvastatin and Pitavastatin reported that its sub-analysis of diabetes patients found significantly worse glycoalbumin levels in the Atorvastatin group80). Moreover, a non-blinded crossover study of Pitavastatin and Atorvastatin reported that HbA1c levels were significantly lower in the Pitavastatin group81). Thus, although statin administration to diabetes patients may have a negative effect on blood glucose levels, the data suggests that this effect on blood glucose levels differs from statin to statin.
5.4.3. Mechanism
Insulin Receptors and Insulin Secretion
The effect of statins on insulin receptors is not uniform. According to a meta-analysis conducted by Baker et al., Pravastatin significantly improves sensitivity, while Atorvastatin and Rosuvastatin reduce sensitivity slightly and Simvastatin significantly reduced sensitivity82). Also, Atorvastatin causes insulin sensitivity to worsen more than Pravastatin, but it may be that the difference between hydrophilic and hydrophobic types may cause different statins to have different tissue migrating properties, which in turn may have an effect on sensitivity77). Atorvastatin suppresses a glucose transporter (GLUT4) in fat cells, which is thought to reduce the intake of glucose83). However, the data suggest that this inhibitory action on GLUT4 may be caused by suppression of the expression of the Rab4 RhoA, which is a small G protein84). Similarly, hydrophobic Simvastatin suppresses the secretion of blood glucose dependent insulin by interfering with the Ca channel. It has also been reported that the fact that it has over 100 times the affinity for cell membranes also plays a role85).
A study of HMGCR SNP reported that HMGCR inhibition is strongly correlated to body weight gain and that this may be related to increased risk of the onset of new diabetes86).
5.4.4. The Preventative Effect of Statins on Cardiovascular Disease and Onset of New Diabetes
Although it has been shown that statins increase new diabetes onset, the absolute risk is low (over five years: placebo 1.2%, Rosuvastatin 1.5%)87). In addition, a meta-analysis of 13 randomized comparative studies reported that when statin therapy was administered to 255 individuals over a four-year period, one individual developed new onset diabetes but onset of cardiovascular events was prevented in 5.4 individuals68).
Therefore, since statin administration increases inhibitory effect on cardiovascular events, discontinuation of statin administration is not recommended for high-risk patients23).
5.4.5. Conclusion
1) Statins significantly increase new onset diabetes.
2) Increased new onset diabetes due to statins is dose-dependent, which suggests that high-strength statins are more likely to cause increases.
3) Although the effects that statins have on new onset diabetes and blood glucose levels may differ according to type of statin, the evidence for this is limited and, as a result, no consensus currently exists.
Based on the above, when administering statins, it is necessary to monitor the patient for new onset diabetes and negative effect on glucose control. Since statins protect against cardiovascular events even when new onset diabetes or poor glucose tolerance are caused, discontinuation of administration in such cases is not recommended. However, dose reduction, switching to another statin, and administration of a concomitant drug may be considered.
5.5. Statin Administration to Patients with Renal Impairment
Chronic kidney disease (CKD) is considered an important risk factor for ASCVD. Although statin administration to CKD patients is increasing, the adverse events that are caused by statin administration are known to increase as kidney function decreases. Based on previously obtained data, high-dose statin administration is not recommended for CKD patients. What follows below is the evidence for this conclusion.
The myoglobins that enter the bloodstream as a result of the breakdown of skeletal muscle cause marked declines in blood flow in the glomerulus. As a result, acute kidney injury can occur as a severe complication of rhabdomyolysis. Generally, kidney function gradually declines by age, and there are many reports of rhabdomyolysis among patients with reduced kidney function. Thus, particularly in the case of elderly patients, it is recommended that great care be practiced when administering statins (the package inserts of all types of statins include the warning to “administer with great care”).
The package inserts of some types of statins indicate that the risk and deterioration of rhabdomyolysis is increased in CKD patients (stage 3 or higher CKD, or Ccr < 30 mL/min), or cases in which there are elevated blood concentrations of statin or elevated AUC levels3, 4, 18). In general, the dose setting recommended when kidney function is reduced is linked to each stage of CKD. Admnistration of Atorvastatin and Fluvastatin in patients with renal impairment is relatively tolerable, and it has been reported that usual doses may be used even in cases of severe renal impairment of stage 4 or higher88). In contrast, comparisons with healthy volunteers have shown that Pitavastatin had a half-life that is twice as long and that Rosuvastatin causes AUC to increase three-fold in the renal impairment patients. As a result, they are not recommended for administration of Pitavastatin and Rosuvastatin to CKD patients. When they are used, the patient should be started on the minimum dose and all use should be restricted to low doses89).
The hepatic sinusoidal organic anion transporter (the aforementioned OATP1B1 that is encoded by the SLCO1B1 gene) detects a variety of compounds including hepatic metabolites contained in a variety of statins and some endogenous waste, and it mediates the uptake of these into liver cells. Investigation of the uptake rate of anionic drugs as mediated by OATP1B1 has shown that drugs that are bound to albumin have a higher uptake rate than unbound drugs in the blood due to higher affinity. Therefore, in liver cirrhosis patients and CKD patients, who have lower serum albumin concentrations, it is assumed that the rate at which statin uptake into liver cells mediated by OATP1B1 is slower. In addition, genetic polymorphism in OATP1B1 gene was found to associate with the interindividual variation of statin pharmacokinetics. For example, the frequency of the patients carrying homozygotes of *15 genotypes of OATP1B1 causing reduced function is about 3% of the Japanese population. Furthermore, whole genome analysis has elucidated the fact that SLCO1B1 (OATP1B1) genetic polymorphism is a main factor involved in the Simvastatin-induced muscle pain90–92). Some uremic toxins that accumulate in the blood of CKD patients (e.g., indoxyl sulfuric acid) have a powerful inhibitory effect on the uptake of statins into liver cells via liver OATP1B1. Thus, it is assumed that interaction between endogenous compounds contained in uremic toxins and the uptake process into liver cells causes the amount of statin exposure to increase in conjunction with elevated blood statin levels93, 94). Moreover, the functional activity of hepatic drug transporters is uniformly reduced in the presence of advanced renal dysfunction95, 96). Thus, based on an amalgamation of all these data, it is recommended that statin use on CKD patients be restricted to low doses while simultaneously performing periodic kidney function tests to determine whether intermittent dose reductions are allowable.
Based on the above, the maximum statin doses are not recommended for high LDL-C level patients with CKD.
6. Future Issues and Summary
As mentioned in the answers to the CQs, available data regarding Japanese patients with statin intolerance is insufficient. Therefore, further study is inevitably required to answer: CQ1–2: Is there any differences in this frequency among various type of statin?, CQ1–3: What are the reasons for and frequency of statin intolerance (difficulty in continuing statin administration) in Japanese patients?, CQ2: Does statin intolerance (difficulty in continuing statin administration) in Japanese patients have an effect on atherosclerotic cardiovascular disease prevention and their prognosis?, and CQ3: What measures are taken to deal with statin intolerance (difficulty in continuing statin administration) in Japanese patients?
Considering the fact that increased age is the most important risk factor for ASCVD and that Japan is rapidly becoming a “super-aging society”, it would be advisable to include a new CQ stated as, “Is there any necessity to manage statin treatment for elderly patients differently from non-elderly patients?”
Issues to be investigated as the next step are as follows. Firstly, mechanism of statin-associated myopathy, the main cause of statin intolerance, should be investigated extensively. Since the definitions of statin intolerance previously utilized in Japan were not consistent, it was difficult to obtain appropriate cases subjected to systematic survey. The establishment of uniform definition of statin intolerance by current Guide allow researchers and physicians to enroll suspected cases with consistent clinical presentation. A multidisciplinary approach applied to such cases can be expected to uncover the mechanism conferring statinassociated myopathy among Japanese. Secondly, prospective cohort study of patients treated with statins, including those utilizes the recent “big data study method”, appears to have some promise. Among Japanese population, the incidence of cardiovascular events is lower (one third to fifth) than that of Western countries, and therefore population-specific strategy should be considered. Previous study has identified the HLA genotype specific for people of Asian descent as playing an important role in statin-associated myopathy14), and therefore high expectations should be placed on joint studies conducted with other Asian countries. Thirdly, we believe it is advisable to distribute knowledge of the nocebo effect that was identified in statin-associated myopathy11). This would be an essential step toward accurate patient-physician discussion about benefit / risk of statin treatment including the issue whether the symptoms observed truly indicate a statin-induced adverse event.
In conclusion, we propose that “statin intolerance” be defined as “in the individual case treated with statins for the first time, in whom statin continuation becomes difficult due to an adverse event (e.g., elevated muscle enzymes, deteriorated renal function, abnormal liver function test results, subjective symptoms suggesting statins as the cause like myopathy) upon initial statin administration, and those adverse events were again observed by at least one another statin.” We also recommend to follow the flow charts shown in Figure 1 in the initiation of statin treatment. The current clinical guide is characterized differentially from those published from Europe and the United States4, 7, 8) by its inclusion not only myopathy, but also adverse events related to liver dysfunction (hepatopathy), which also is prevalent adverse event like myopathy. Current Guide was prepared based on data that was available as of July 2018.
Conflict of Interest
In accordance with the “COI Management Guidelines for Clinical Research” established by the Japan Association of Medical Sciences' COI committee, a conflict of interest (COI) statement has been obtained form each member of the committee involved in drafting the Statin Intolerance Clinical Guide 2018. The names of the enterprises disclosed in the COI statement are provided below. The applicable period is January 01, 2015, to December 31, 2017.
Abbvie, Astellas Amgen BioPharma, Astellas Pharma, Bayer, Boehringer Ingelheim Japan, Bristol- Myers Squibb, Central Medical, Daiichi Sankyo, Dai- Nihon Insatsu Kenko Hoken Kumiai, East Japan Institute of Technology, Eisai, Fujifilm, Gilead Sciences, Japan Data Center, Kowa, Kusatsu City Hall, Kyowa Medics, Medical Review, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, MSD, Municipal Kaizuka Hospital, Novartis Pharma, Nakamura Hospital, Novo Nordisk, Ono Pharmaceutical, Otsuka Pharmaceutical, Pfizer Japan, Rinku General Medical Center, Rohto Pharmaceutical, Sanofi, Sanwa Kagaku Kenkyusho, Skylight Biotech, St. Jude Medical, Sumitomo Dainippon Pharma, Takeda Pharmaceuctical, the City of Izumisano,.
References
- 1). Endo A. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res, 1992; 33: 1569-1582 [PubMed] [Google Scholar]
- 2). Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K. Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan — 2012 version. J Atheroscler Thromb, 2013; 20: 517-523 [DOI] [PubMed] [Google Scholar]
- 3). Fitchett DH, Hegele RA, Verma S. Cardiology patient page. Statin intolerance. Circulation, 2015; 31: 131. [DOI] [PubMed] [Google Scholar]
- 4). Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgözoğlu L, Nordestgaard BG, Bruckert E, De Backer G, Krauss RM, Laufs U, Santos RD, Hegele RA, Hovingh GK, Leiter LA, Mach F, Marz W, Newman CB, Wiklund O, Jacobson TA, Catapano AL, Chapman MJ, Ginsberg HN: European Atherosclerosis Society Consensus Panel. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J, 2015; 36: 1012-1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM, IMPROVE-IT Investigators Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med, 2015; 372: 2387-2397 [DOI] [PubMed] [Google Scholar]
- 6). Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR, FOURIER Steering Committee and Investigators Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med, 2017; 376: 1713-1722 [DOI] [PubMed] [Google Scholar]
- 7). Guyton JR, Bays HE, Grundy SM, Jacobson TA. The National Lipid Association Statin Intolerance Panel. An assessment by the Statin Intolerance Panel: 2014 update. J Clin Lipidol, 2014. May-Jun; 8 (3 Suppl): S72-81 [DOI] [PubMed] [Google Scholar]
- 8). Mancini GB, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, Gupta M, Hegele RA, Ng D, Pearson GJ, Pope J, Tashakkor AY. Diagnosis, Prevention, and Management of Statin Adverse Effects and Intolerance: Canadian Consensus Working Group Update (2016). Can J Cardiol, 2016; 32 (7 Suppl): S35-65 [DOI] [PubMed] [Google Scholar]
- 9). Rosenson RS, Baker SK, Jacobson TA, Kopecky SL, Parker BA. The National Lipid Association's Muscle Safety Expert Panel. An assessment by the Statin Muscle Safety Task Force: 2014 update. J Clin Lipidol, 2014; 8 (3 Suppl): S58-71 [DOI] [PubMed] [Google Scholar]
- 10). Moriarty PM, Thompson PD, Cannon CP, Guyton JR, Bergeron J, Zieve FJ, Bruckert E, Jacobson TA, Kopecky SL, Baccara-Dinet MT, Du Y, Pordy R, Gipe DA, ODYSSEY ALTERNATIVE Investigators Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: The ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol, 2015; Nov-Dec; 9: 758-769 [DOI] [PubMed] [Google Scholar]
- 11). Nissen SE, Stroes E, Dent-Acosta RE, Rosenson RS, Lehman SJ, Sattar N, Preiss D, Bruckert E, Ceška R, Lepor N, Ballantyne CM, Gouni-Berthold I, Elliott M, Brennan DM, Wasserman SM, Somaratne R, Scott R, Stein EA. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance. The GAUSS-3 randomized clinical trial. JAMA, 2016; 315: 1580-1590 [DOI] [PubMed] [Google Scholar]
- 12). Nagar S, Rane P, Fox K, Meyers J, Davis K, Beaubrun A, Inomata H, Qian Y, Kajinami K. Treatment patterns, statin intolerance, and subsequent cardiovascular events among Japanese patients with high cardiovascular risk initiating statin therapy. Circ J, 2018; 82: 1008-1016 [DOI] [PubMed] [Google Scholar]
- 13). Wake M, Onishi Y, Guelfucci F, Oh A, Hiroi S, Shimasaki Y, Teramoto T. Treatment patterns in hyperlipidaemia patients based on administrative claim databases in Japan. Atherosclerosis, 2018; 272: 145-152 [DOI] [PubMed] [Google Scholar]
- 14). Sai K, Kajinami K, Akao H, Iwadare M, Sato-Ishida R, Kawai Y, Takeda K, Tanimoto T, Yamano T, Akasaka T, Ishida T, Hirata KI, Saku K, Yagi S, Soeki T, Sata M, Ueno M, Miyazaki S, Shiraki A, Oyama JI, Node K, Sugamura K, Ogawa H, Kurose K, Maekawa K, Matsuzawa Y, Imatoh T, Hasegawa R, Japanese Pharmacogenomics Data Science Consortium. Saito Y. A possible role for HLA-DRB1*04: 06 in statin-related myopathy in Japanese patients. Drug Metab Pharmacokinet, 2016; 31: 467-470 [DOI] [PubMed] [Google Scholar]
- 15). Guyton JR, Bays HE, Grundy SM, Jacobson TA. The National Lipid Association Statin Intolerance Panel. An assessment by the statin intolerance panel: 2014 update. J Clin Lipidol, 2014. May-Jun; 8 (3 Suppl): S72-81 [DOI] [PubMed] [Google Scholar]
- 16). Goto Y, Yasugi T, Goto Y, Yoshida S, Saito Y, Oshima K, Takaku F, Orimo H, Hata Y, Nakaya N, Kumagai A, Takeda R, Mabuchi H, Kuzuya F, Ymamoto A, Kawai C, Kita T, Tarui S, Matsuzawa Y, Kajiyama G, Kokubu T, Nakamura M, Arakawa K. Clinical evaluation of CS-514, (pravastatin) on hyperlipidemia. Double-blind study with clinofibrate. Clin Eval, 1988; 16: 211-249 (in Japanese) [Google Scholar]
- 17). Goto Y, Ymamoto A, Matsuzawa Y, Nakaya N, Hata Y, Kita T, Mabuchi H, Yokoyama S. Journal of Clinical and Experimental Medicine, 1988; 146: 927-955 (in Japanese) [Google Scholar]
- 18). Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, Mizuno K, Ohashi Y, MEGA Study Group Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet, 2006; 368: 1155-1163 [DOI] [PubMed] [Google Scholar]
- 19). Itoh T, Matsumoto M, Hougaku H, Handa N, Tsubakihara Y, Yamada Y, Imaizumi M, Hoshi M, Shimazu Y, Hori M, Kawamori R, Ueda N, Fusamoto H, Kamada T. Effects of low-dose simvastatin therapy on serum lipid levels in patients with moderate hypercholesterolemia: a 12-month study. The Simvastatin Study Group. Clin Ther, 1997; 19: 487-497 [DOI] [PubMed] [Google Scholar]
- 20). Matsuzawa Y, Kita T, Mabuchi H, Matsuzaki M, Nakaya N, Oikawa S, Saito Y, Sasaki J, Shimamoto K, Itakura H, J-LIT Study Group Sustained reduction of serum cholesterol in low-dose 6-year simvastatin treatment with minimum side effects in 51,321 Japanese hypercholesterolemic patients. Circ J, 2003; 67: 287-294 [DOI] [PubMed] [Google Scholar]
- 21). Saito Y, Yoshida S, Nakaya N, Hata Y, Goto Y. Comparison between Morning dose and Evening dose of Simvastatin(MK-733) on Efficacy and Tolerability in Hyperlipidemic Subjects -Double-blind Comparative Study-. Journal of clinical therapeutics & medicines, 1989; 5: 2041-2074 (in Japanese) [Google Scholar]
- 22). Goto Y, Itakura H, Goto Y, Oikawa S, Hata Y, Nakaya N, Yasugi T, Yoshida S, Saito Y, Kuzuya F, Yoshimine N, Mabuchi H, Kawai C, Kita T, Ymamoto A, Arakawa K. Clinical Study of MK-733 (Simvastatin) -Dose Range Finding Study-. Journal of clinical therapeutics & medicines, 1989; 5: 2011-2040 (in Japanese) [Google Scholar]
- 23). Nakaya N, Goto Y, Oikawa S, Hata Y, Yasugi T, Itakura H, Yoshida S, Saito Y, Mabuchi H, Kuzuya F, Yoshimine N, Kawai C, Kita T, Ymamoto A, Arakawa K, Uzawa H. Clinical Effect of MK-733 (simvastatin) on hyperlipidemia -Result of Multiclinical Open Study-. Journal of clinical therapeutics & medicines, 1989; 5: 1651-1684 (in Japanese) [Google Scholar]
- 24). Sasaki J, Ikeda Y, Kuribayashi T, Kajiwara K, Biro S, Yamamoto K, Ageta M, Kobori S, Saikawa T, Otonari T, Kono S. A 52-week, randomized, open-label, parallel-group comparison of the tolerability and effects of pitavastatin and atorvastatin on high-density lipoprotein cholesterol levels and glucose metabolism in Japanese patients with elevated levels of low-density lipoprotein cholesterol and glucose intolerance. Clin Ther, 2008; 30: 1089-1101 [DOI] [PubMed] [Google Scholar]
- 25). Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M, JAPAN-ACS Investigators Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol, 2009; 54: 293-302 [DOI] [PubMed] [Google Scholar]
- 26). Goto Y, Nakaya N, Goto Y, Yoshida S, Saito Y, Oshima K, Takaku F, Orimo H, Hata Y, Yasugi T, Kumagai A, Takeda R, Mabuchi H, Kuzuya F, Ymamoto A, Kawai C, Kita T, Tarui S, Matsuzawa Y, Kajiyama G, Kokubu T, Nakamura M, Arakawa K. Clinical Effects of CS-514 (Pravastatin) on Hypercholesterolemia -Results of Multiclinic Open Study-. Journal of clinical therapeutics & medicines, 1988; 4: 201-227 (in Japanese) [Google Scholar]
- 27). Goto Y, Ymamoto A, Goto Y, Yoshida S, Saito Y, Oshima K, Yasugi T, Takaku F, Orimo H, Hata Y, Nakaya N, Kumagai A, Takeda R, Mabuchi H, Kuzuya F, Kawai C, Kita T, Tarui S, Matsuzawa Y, Kajiyama G, Kokubu T, Nakamura M, Arakawa K. Stud on Clinical Efficacy of CS-514 (Pravastatin) in Long-term Treatment on Hypercholesterolemia. Journal of clinical therapeutics & medicines, 1988; 4: 409-437 (in Japanese) [Google Scholar]
- 28). Watanabe T, Ando K, Daidoji H, Otaki Y, Sugawara S, Matsui M, Ikeno E, Hirono O, Miyawaki H, Yashiro Y, Nishiyama S, Arimoto T, Takahashi H, Shishido T, Miyashita T, Miyamoto T, Kubota I, CHERRY study investigators randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J Cardiol, 2017; 70: 537-544 [DOI] [PubMed] [Google Scholar]
- 29). The Kyushu Lipid Intervention Study Group Pravastatin Use and Risk of Coronary Events and Cerebral Infarction in Japanese Men with Moderate Hypercholesterolemia: The Kyushu Lipid Intervention Study. J Atheroscler Thromb, 2000; 7: 110-121 [DOI] [PubMed] [Google Scholar]
- 30). Origasa H, Yokoyama M, Matsuzaki M, Saito Y, Matsuzawa Y, JELIS Investigators Clinical importance of adherence to treatment with eicosapentaenoic acid by patients with hypercholesterolemia. Circ J, 2010; 74: 510-517 [DOI] [PubMed] [Google Scholar]
- 31). Zhang H, Plutzky J, Skentzos S, Morrison F, Mar P, Shubina M, Turchin A. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med, 2013; 158: 526-534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Vonbank A, Agewall S, Kjeldsen KP, Lewis BS, Torp-Pedersen C, Ceconi C, Funck-Brentano C, Kaski JC, Niessner A, Tamargo J, Walther T, Wassmann S, Rosano G, Schmidt H, Saely CH, Drexel H. Comprehensive efforts to increase adherence to statin therapy. Eur Heart J, 2017; 38: 2473-2479 [DOI] [PubMed] [Google Scholar]
- 33). Laufs U, Scharnagl H, Marz W. Statin intolerance. Curr Opin Lipidol, 2015; 26: 492-501 [DOI] [PubMed] [Google Scholar]
- 34). Parker BA, Capizzi JA, Grimaldi AS, Clarkson PM, Cole SM, Keadle J, Chipkin S, Pescatello LS, Simpson K, White CM, Thompson PD. Effect of statins on skeletal muscle function. Circulation, 2013; 127: 96-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Takagi Y: Rinshokensaguide. pp115-117. Bunkodo; Tokyo: 2015. (in Japanese) [Google Scholar]
- 36). Hamano T: Nippon Rinsho. Shinkeishoukougun (Ed 2nd) V. pp598-601. 2014. (in Japanese) [Google Scholar]
- 37). Suzuki S, Uruha A, Suzuki N, Nishino I. Integrated Diagnosis Project for Inflammatory Myopathies: An association between autoantibodies and muscle pathology. Autoimmunity reviews, 2017; 16: 693-700 [DOI] [PubMed] [Google Scholar]
- 38). Floyd JS, Brody JA, Tiniakou E, Psaty BM, Mammen A. Absence of anti-HMG-CoA reductase autoantibodies in severe self-limited statin-related myopathy. Muscle Nerve, 2016; 54: 142-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Authors/Task Force Members. Catapano AL, Graham I, Backer GD, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner ZE, Riccardi G, Taskinen MR, Tokgäzoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J, 2016; 37: 2999-3058 [DOI] [PubMed] [Google Scholar]
- 40). Mach F, Ray KK, Wiklund O, Corsini A, Catapano AL, Bruckert E, De Backer G, Hegele RA, Hovingh GK, Jacobson TA, Krauss RM, Laufs U, Leiter LA, März W, Nordestgaard BG, Raal FJ, Roden M, Santos RD, Stein EA, Stroes ES, Thompson PD, Tokgäzoglu L, Vladutiu GD, Gencer B, Stock JK, Ginsberg HN, Chapman MJ, European Atherosclerosis Society Consensus Panel Adverse effects of statin therapy: perception vs. the evidence - focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur Heart J, 2018; 39: 2526-2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Tolman KG. The liver and lovastatin. Am J Cardiol, 2002; 89: 1374-1380 [DOI] [PubMed] [Google Scholar]
- 42). Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network metaanalysis of 246 955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes, 2013; 6: 390-399 [DOI] [PubMed] [Google Scholar]
- 43). Bays H, Cohen DE, Chalasani N, Harrison SA. The National Lipid Association's Statin Safety Task F: An assessment by the Statin Liver Safety Task Force: 2014 update. J Clin Lipidol, 2014; 8 (3 Suppl): S47-57 [DOI] [PubMed] [Google Scholar]
- 44). Newman C, Tsai J, Szarek M, Luo D, Gibson E. Comparative safety of atorvastatin 80 mg versus 10 mg derived from analysis of 49 completed trials in 14,236 patients. Am J Cardiol, 2006; 97: 61-67 [DOI] [PubMed] [Google Scholar]
- 45). Herrick C, Litvin M, Goldberg AC. Lipid lowering in liver and chronic kidney disease. Best Pract Res Clin Endocrinol Metab, 2014; 28: 339-352 [DOI] [PubMed] [Google Scholar]
- 46). Sorokin A, Brown JL, Thompson PD. Primary biliary cirrhosis, hyperlipidemia, and atherosclerotic risk: a systematic review. Atherosclerosis, 2007; 194: 293-299 [DOI] [PubMed] [Google Scholar]
- 47). Kullak-Ublick GA, Andrade RJ, Merz M, End P, Benesic A, Gerbes AL, Aithal GP. Drug-induced liver injury: recent advances in diagnosis and risk assessment. Gut, 2017; 66: 1154-1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Zimmerman HJ. The spectrum of hepatotoxicity. Perspect Biol Med, 1968; 12: 135-161 [DOI] [PubMed] [Google Scholar]
- 49). Andrade RJ, Lucena MI, Fernandez MC, Pelaez G, Pachkoria K, Garcia-Ruiz E, Garcia-Munoz B, Gonzalez-Grande R, Pizarro A, Duran JA, Jiménez M, Rodrigo L, Romero-Gomez M, Navarro JM, Planas R, Costa J, Borras A, Soler A, Martin-Vivaldi R. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology, 2005; 129: 512-521 [DOI] [PubMed] [Google Scholar]
- 50). Bjornsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology, 2005; 42: 481-489 [DOI] [PubMed] [Google Scholar]
- 51). Orsi A, Sherman O, Woldeselassie Z. Simvastatin-associated memory loss. Pharmacotherapy, 2001; 21: 767-769 [DOI] [PubMed] [Google Scholar]
- 52). King DS, Wilburn AJ, Wofford MR, Harrell TK, Lindley BJ, Jones DW. Cognitive impairment associated with atorvastatin and simvastatin. Pharmacotherapy, 2003; 23: 1663-1667 [DOI] [PubMed] [Google Scholar]
- 53). Wagstaff LR, Mitton MW, Arvik BM, Doraiswamy PM. Statin-associated memory loss: analysis of 60 case reports and review of the literature. Pharmacotherapy, 2003; 23: 871-880 [DOI] [PubMed] [Google Scholar]
- 54). FDA Drug Safty Communicatoin : important safety label chages to cholesterol-lowering statin drugs. US FDA website http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm
- 55). Parker-Poke T. A heart helper may come at a price for the brain. The Well Colum. The New York Times. http//well.blogs.nytimes.com/2012/03/05/help-for-theheart-comeswith-a-price-for-the-brain/?_r=0
- 56). Sato N. Dementia and Statin. The Journal of Practical Pharmacy, 2016; 67: 116-122 (in Japanese) [Google Scholar]
- 57). Miron VE, Zehntner SP, Kuhlmann T, Ludwin SK, Owens T, Kennedy TE, Bedell BJ, Antel JP. Statin therapy inhibits remyelination in the central nervous system. Am J Pathol, 2009; 174: 1880-1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58). Langsjoen PH, Langsjoen AM. The clinical use of HMG CoA-reductase inhibitors and the associated depletion of coenzyme Q10. A review of animal and human publications. Biofactors, 2003; 18: 101-111 [DOI] [PubMed] [Google Scholar]
- 59). Richardson K, Schoen M, French B, Umscheid CA, Mitchell MD, Arnold SE, Heidenreich PA, Rader DJ, deGoma EM. Statins and cognitive function: a systematic review. Ann Intern Med, 2013; 159: 688-697 [DOI] [PubMed] [Google Scholar]
- 60). Swiger KJ, Manalac RJ, Blumenthal RS, Blaha MJ, Martin SS. Statins and cognition: a systematic review and meta- analysis of short- and long-term cognitive effects. Mayo Clin Proc, 2013; 88: 1213-1221 [DOI] [PubMed] [Google Scholar]
- 61). Feldman HH, Doody RS, Kivipelto M, Sparks DL, Waters DD, Jones RW, Schwam E, Schindler R, Hey-Hadavi J, DeMicco DA, Breazna A, LEADe Investigators Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology, 2010; 74: 956-964 [DOI] [PubMed] [Google Scholar]
- 62). JAPANESE SOCIETY OF NEUROLOGY : Ninchishousikkanshinryou Guidelines 2017. pp126-127. Igakushoin; Tokyo: 2017. (in Japanese) [Google Scholar]
- 63). Rojas-Fernandez CH, Cameron JC. Is statin-associated cognitive impairment clinically relevant? A narrative review and clinical recommendations. Ann Pharmacother, 2012; 46: 549-557 [DOI] [PubMed] [Google Scholar]
- 64). Hyyppä MT, Kronholm E, Virtanen A, Leino A, Jula A. Does simvastatin affect mood and steroid hormone levels in hypercholesterolemic men? A randomized double-blind trial. Psychoneuroendocrinology, 2003; 28: 181-194 [DOI] [PubMed] [Google Scholar]
- 65). Parsaik AK, Singh B, Murad MH, Singh K, Mascarenhas SS, Williams MD, Lapid MI, Richardson JW, West CP, Rummans TA. Statins use and risk of depression: a systematic review and meta-analysis. J Affect Disord, 2014; 160: 62-67 [DOI] [PubMed] [Google Scholar]
- 66). Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med, 2004; 350: 1495-1504 [DOI] [PubMed] [Google Scholar]
- 67). Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Jr., Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med, 2008; 359: 2195-2207 [DOI] [PubMed] [Google Scholar]
- 68). Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJM, Seshasai SRK, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Kray K, Ford I. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet, 2010; 375: 735-742 [DOI] [PubMed] [Google Scholar]
- 69). Preiss D, Seshasai SRK, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJP, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA, 2011; 305: 2556-2564 [DOI] [PubMed] [Google Scholar]
- 70). Kato S, Miura M. Risk of new-onset diabetes mellitus during treatment with low-dose statins in Japan: A retrospective cohort study. J Clin Pharm Ther, 2018. 10.1111/jcpt.12675 [published Online First: 2018/02/28] [DOI] [PubMed] [Google Scholar]
- 71). Taguchi I, Iimuro S, Iwata H, Takashima H, Abe M, Amiya E, Ogawa T, Ozaki Y, Sakuma I, Nakagawa Y, Hibi K, Hiro T, Fukumoto Y, Hokimoto S, Miyauchi K, Yamazaki T, Ito H, Otsuji Y, Kimura K, Takahashi J, Hirayama A, Yokoi H, Kitagawa K, Urabe T, Okada Y, Terayama Y, Toyoda K, Nagao T, Matsumoto M, Ohashi Y, Kaneko T, Fujita R, Ohtsu H, Ogawa H, Daida H, Shimokawa H, Saito Y, Kimura T, Inoue T, Matsuzaki M, Nagai R. High-Dose Versus Low-Dose Pitavastatin in Japanese Patients With Stable Coronary Artery Disease (REAL-CAD): A Randomized Superiority Trial. Circulation, 2018; 137: 1997-2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72). Carter AA, Gomes T, Camacho X, Juurlink DN, Shah BR, Mamdani MM. Risk of incident diabetes among patients treated with statins: population based study. BMJ, 2013; 346: f2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73). Navarese EP, Buffon A, Andreotti F, Kozinski M, Welton N, Fabiszak T, Caputo S, Grzesk G, Kubica A, Swiatkiewicz I, Sukiennik A, Kelm M, Servi SD, Kubica J. Metaanalysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol, 2013: 111: 1123-1130 [DOI] [PubMed] [Google Scholar]
- 74). Ooba N, Setoguchi S, Sato T, Kubota K. Lipid-lowering drugs and risk of new-onset diabetes: a cohort study using Japanese healthcare data linked to clinical data for health screening. BMJ Open, 2017; 7: e015935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75). Crandall JP, Mather K, Rajpathak SN, Goldberg RB, Watson K, Foo S, Ratner R, Barrett-Connor E, Temprosa M. Statin use and risk of developing diabetes: results from the Diabetes Prevention Program. BMJ Open Diabetes Res Care, 2017; 5: e000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76). Hashiguchi M, Maruyama J, Shimizu M, Takahashi D, Shiga T. Risk Factor for Diabetes Mellitus and High Blood Glucose With HMG-CoA Reductase Inhibitors Using a Post-marketing Surveillance Database in Japan. Clin Pharmacol Drug Dev, 2018; 7: 800-810 [DOI] [PubMed] [Google Scholar]
- 77). Takano T, Yamakawa T, Takahashi M, Kimura M, Okamura A. Influences of statins on glucose tolerance in patients with type 2 diabetes mellitus. J Atheroscler Thromb, 2006; 13: 95-100 [DOI] [PubMed] [Google Scholar]
- 78). Yamakawa T, Takano T, Tanaka S, Kadonosono K, Terauchi Y. Influence of pitavastatin on glucose tolerance in patients with type 2 diabetes mellitus. J Atheroscler Thromb, 2008; 15: 269-275 [DOI] [PubMed] [Google Scholar]
- 79). Mita T, Watada H, Nakayama S, Abe M, Ogihara T, Shimizu T, Uchino H, Hirose T, Kawamori R. Preferable effect of pravastatin compared to atorvastatin on beta cell function in Japanese early-state type 2 diabetes with hypercholesterolemia. Endocr J, 2007; 54: 441-447 [DOI] [PubMed] [Google Scholar]
- 80). Yokote K, Saito Y. Chiba. Influence of statins on glucose tolerance in patients with type 2 diabetes mellitus: subanalysis of the collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention (CHIBA study). J Atheroscler Thromb, 2009; 16: 297-298 [DOI] [PubMed] [Google Scholar]
- 81). Mita T, Nakayama S, Abe H, Gosho M, Iida H, Hirose T, Kawamori R, Watada H. Comparison of effects of pitavastatin and atorvastatin on glucose metabolism in type 2 diabetic patients with hypercholesterolemia. J Diabetes Investig, 2013; 4: 297-303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82). Baker WL, Talati R, White CM, Coleman CI. Differing effect of statins on insulin sensitivity in non-diabetics: a systematic review and meta-analysis. Diabetes Res Clin Pract, 2010; 87: 98-107 [DOI] [PubMed] [Google Scholar]
- 83). Nakata M, Nagasaka S, Kusaka I, Matsuoka H, Ishibashi S, Yada T. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia, 2006; 49: 1881-1892 [DOI] [PubMed] [Google Scholar]
- 84). Takaguri A, Satoh K, Itagaki M, Tokumitsu Y, Ichihara K. Effects of atorvastatin and pravastatin on signal transduction related to glucose uptake in 3T3L1 adipocytes. J Pharmacol Sci, 2008; 107: 80-89 [DOI] [PubMed] [Google Scholar]
- 85). Masters BA, Palmoski MJ, Flint OP, Gregg RE, Wangiverson D, Durham SK. In vitro myotoxicity of the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, pravastatin, lovastatin, and simvastatin, using neonatal rat skeletal myocytes. Toxicol Appl Pharmacol, 1995; 131: 163-174 [DOI] [PubMed] [Google Scholar]
- 86). S Swerdlow DI, Preiss D, Kuchenbaecker KB, Holmes MV, Engmann JE, Shah T, Sofat R, Stender S, Johnson PC, Scott RA, Leusink M, Verweij N, Sharp SJ, Guo Y, Giambartolomei C, Chung C, Peasey A, Amuzu A, Li K, Palmen J, Howard P, Cooper JA, Drenos F, Li YR, Lowe G, Gallacher J, Stewart MC, Tzoulaki I, Buxbaum SG, van der A DL, Forouhi NG, Onland-Moret NC, van der Schouw YT, Schnabel RB, Hubacek JA, Kubinova R, Baceviciene M, Tamosiunas A, Pajak A, Topor-Madry R, Stepaniak U, Malyutina S, Baldassarre D, Sennblad B, Tremoli E, de Faire U, Veglia F, Ford I, Jukema JW, Westendorp RG, de Borst GJ, de Jong PA, Algra A, Spiering W, Maitland-van der Zee AH, Klungel OH, de Boer A, Doevendans PA, Eaton CB, Robinson JG, Duggan D, DIAGRAM Consortium; MAGIC Consortium; InterAct Consortium. Kjekshus J, Downs JR, Gotto AM, Keech AC, Marchioli R, Tognoni G, Sever PS, Poulter NR, Waters DD, Pedersen TR, Amarenco P, Nakamura H, McMurray JJ, Lewsey JD, Chasman DI, Ridker PM, Maggioni AP, Tavazzi L, Ray KK, Seshasai SR, Manson JE, Price JF, Whincup PH, Morris RW, Lawlor DA, Smith GD, Ben-Shlomo Y, Schreiner PJ, Fornage M, Siscovick DS, Cushman M, Kumari M, Wareham NJ, Verschuren WM, Redline S, Patel SR, Whittaker JC, Hamsten A, Delaney JA, Dale C, Gaunt TR, Wong A, Kuh D, Hardy R, Kathiresan S, Castillo BA, van der Harst P, Brunner EJ, Tybjaerg-Hansen A, Marmot MG, Krauss RM, Tsai M, Coresh J, Hoogeveen RC, Psaty BM, Lange LA, Hakonarson H, Dudbridge F, Humphries SE, Talmud PJ, Kivimäki M, Timpson NJ, Langenberg C, Asselbergs FW, Voevoda M, Bobak M, Pikhart H, Wilson JG, Reiner AP86, Keating BJ, Hingorani AD, Sattar N. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet, 2015; 385: 351-361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87). Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn R. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet, 2012; 380: 565-571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88). Launay-Vacher V, Izzedine H, Deray G. Statins' dosage in patients with renal failure and cyclosporine drug-drug interactions in transplant recipient patients. Int J Cardiol, 2005; 101: 9-17 [DOI] [PubMed] [Google Scholar]
- 89). Pascuala V., Serranob A., Pedro-Botet J., Ascaso J., Barrios V., Millnu J., Pinto X., Cases A. Chronic kidney disease and dyslipidaemia. Clin Investig Arterioscler, 2017; 29: 22-35 [DOI] [PubMed] [Google Scholar]
- 90). Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R. SLCO1B1 variants and statin-induced myopathy--a genome wide study. N Eng J Med, 2008; 359: 789-799 [DOI] [PubMed] [Google Scholar]
- 91). Maeda K, Ieiri I, Yasuda K, Fujino A, Fujiwara H, Otsubo K, Hirano M, Watanabe T, Kitamura Y, Kusuhara H, Sugiyama Y. Effects of organic anion transporting polypeptide 1B1 haplotype on pharmacokinetics of pravastatin, valsartan, and temocapril. Clin Pharm Therap, 2006; 79: 427-439 [DOI] [PubMed] [Google Scholar]
- 92). Pasanen MK, Neuvonen PJ, Niemi M. Global analysis of genetic variation in SLCO1B1. Pharmacogenomics, 2008; 9: 19-33 [DOI] [PubMed] [Google Scholar]
- 93). Sato T, Yamaguchi H, Kogawa T, Abe T, Mano N. Organic anion transporting polypeptides 1B1 and 1B3 play an important role in uremic toxin handling and drug-uremic toxin interactions in the liver. Journal of pharmacy & pharmaceutical sciences: a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques, 2014; 17: 475-484 [DOI] [PubMed] [Google Scholar]
- 94). Tsujimoto M, Hatozaki D, Shima D, Yokota H, Furukubo T, Izumi S, Yamakawa T, Minegaki T, Nishiguchi K. Influence of serum in hemodialysis patients on the expression of intestinal and hepatic transporters for the excretion of pravastatin. Therapeutic apheresis and dialysis: official peer-reviewed journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy, 2012; 16: 580-587 [DOI] [PubMed] [Google Scholar]
- 95). Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacological reviews, 2010; 62: 1-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96). Sun H, Frassetto L, Benet LZ. Effects of renal failure on drug transport and metabolism. Pharmacology & therapeutics, 2006; 109: 1-11 [DOI] [PubMed] [Google Scholar]


