Abstract
Purpose
The aim of this cross-sectional study was to investigate the effect of scaling and root planing (SRP) on the expression of anti-inflammatory cytokines (interleukin [IL]-4, IL-9, IL-10, and IL-13) in the gingival crevicular fluid (GCF) of electronic cigarette users and non-smokers with moderate chronic periodontitis (CP).
Methods
Electronic cigarette users and non-smokers with CP were included in the study. Full-mouth plaque and gingival indices, probing depth (PD), clinical attachment loss (CAL), and marginal bone loss (MBL) were assessed. The GCF was collected, and its volume and levels of IL-4, IL-9, IL-10, and IL-13 were assessed. These parameters were evaluated at baseline and 3 months after SRP. The sample size was estimated, and comparisons between groups were performed. P<0.05 was considered to indicate statistical significance.
Results
Thirty-six electronic cigarette users (47.7±5.8 years old) and 35 non-smokers (46.5±3.4 years old) with CP were included. At baseline, there were no differences in plaque index (PI), PD, CAL, MBL, and GCF IL-4, IL-9, IL-10, and IL-13 between electronic cigarette users and non-smokers. At the 3-month follow-up, there were no significant differences in PI, gingival index (GI), PD, CAL, and MBL in electronic cigarette users compared to baseline, while there were significant reductions in PI, GI, and PD among non-smokers. At the 3-month follow-up, GCF IL-4, IL-9, IL-10, and IL-13 levels were significantly elevated in both groups (P<0.05) compared to baseline. The increases in GCF IL-4, IL-9, IL-10, and IL-13 levels were significantly higher in non-smokers (P<0.05) than in electronic cigarette users at the 3-month follow-up.
Conclusions
Levels of GCF IL-4, IL-9, IL-10, and IL-13 increased after SRP in electronic cigarette users and non-smokers with CP; however, the anti-inflammatory effect of SRP was more profound in non-smokers than in electronic cigarette users.
Keywords: Chronic periodontitis, Cytokines, Electronic nicotine delivery systems, Gingival crevicular fluid, Inflammation, Interleukins
Graphical Abstract
INTRODUCTION
Non-surgical periodontal therapy (NSPT), also known as scaling and root planing (SRP) of teeth and root surfaces, is the treatment of choice for patients with chronic periodontitis (CP) [1,2,3]. Studies have shown that SRP in CP patients helps to reduce the plaque index (PI), gingival index (GI), clinical attachment loss (CAL), and probing depth (PD) [1,2]. Pathophysiologically, studies [4,5,6] have primarily focused on the influence of SRP on the expression of destructive inflammatory cytokines in bodily fluids, including unstimulated whole saliva and gingival crevicular fluid (GCF) [7,8,9]. For instance, abundant evidence has shown that SRP reduces the expression of inflammatory biomarkers such as tumor necrosis factor alpha (TNF-α), interleukin (IL) 1-beta, and IL-6 in the GCF, improving the clinical periodontal status of patients with CP [7,8,10,11]. The habitual use of nicotine-containing products, such as cigarettes, electronic nicotine delivery systems, and electronic cigarettes, is a known risk factor that contributes to the etiopathogenesis of CP by enhancing the expression of destructive inflammatory cytokines (including IL-6, TNF-α, and IL-1β) in the GCF of smokers compared with non-smokers [12,13,14,15].
Studies [16,17,18] have reported that anti-inflammatory cytokines such as IL-4 and IL-10 help reinstate physiological health by stimulating the production of protective antibodies and lowering the levels of destructive inflammatory cytokines, such as those previously mentioned. A study by Pradeep et al. [18] showed that the GCF levels of IL-4 were significantly higher in individuals with healthy periodontal status than in patients with CP. Similarly, in a cross-sectional study, Emingil et al. [19] showed that SRP helped to increase the GCF levels of IL-4, IL-10, IL-13, and IL-17, thereby improving the clinical periodontal status of patients with CP. However, no studies have compared GCF IL-4, IL-10, IL-11, and IL-13 levels after SRP in electronic cigarette users and non-smokers. Since electronic cigarette users (who are usually former cigarette smokers) vape to assuage nicotine cravings [20], and nicotine compromises healing following surgical treatment and NSPT [21,22], the authors of the present study hypothesized that the clinical parameters and levels of anti-inflammatory cytokines in the GCF would be compromised in electronic cigarette users compared to non-smokers after SRP.
The aim of the present cross-sectional study was to investigate the effect of SRP on the expression of anti-inflammatory cytokines (IL-4, IL-9, IL-10, and IL-13) in the GCF of electronic cigarette users and non-smokers with moderate CP.
MATERIALS AND METHODS
Ethical aspects
All participants were required to read and sign a consent form. Ethical approval was obtained from the ethics research committee of the Centre for Specialist Dental Practice and Clinical Research (UDCRC/023-18) in Riyadh, Saudi Arabia. The present study was conducted in accordance with the Consolidated Standards of Reporting Trials guidelines (CTRI/2018/04/0125) [23]. Verbal and written information was provided to all individuals about the detrimental health effects of smoking and vaping at the time of patient recruitment. It was also emphasized that vaping is not a safe alternative to smoking, as electronic cigarettes jeopardize health in a manner similar to conventional smoking.
Patient selection protocol
Electronic cigarette users (individuals who vaped at least once daily for the past 12 months and did not use any other tobacco products) [12,24] and non-smokers [25] (individuals who reported never having used any form of tobacco product) diagnosed with CP were included. CP diagnosis was based on the following criteria: GI >1, CAL ≥3 mm, PD ≥4 mm, and radiographic evidence of marginal bone loss (MBL) ≥3 mm below the cementoenamel junction (CEJ) [18,25]. Current tobacco smokers (including but not limited to users of cigarettes, waterpipes, pipes, and cigars), individuals who regularly consumed alcohol, and patients with systemic diseases (including but not limited to renal and hepatic disorders, cardiac diseases, diabetes mellitus, and human immunodeficiency viruses/acquired immune deficiency syndrome) were excluded. Patients who reported the use of certain medications (including but not limited to steroids, antibiotics, probiotics, bisphosphonates, and non-steroidal anti-inflammatory drugs) were also excluded. Third molars and grossly carious teeth with embedded root remnants were not assessed. Furthermore, digital radiographs of poor quality or on which the CEJ was not clearly visible were discarded.
Clinical and radiographic periodontal parameters
In all patients, full-mouth CAL [26], GI [27], PD [28], and PI [27] were measured at baseline and at a 3-month follow-up by a trained and calibrated investigator (NAH; κ=0.88). These measurements were taken at 6 sites (distolingual/palatal, mesiolingual/palatal mesiobuccal, distobuccal, mid-lingual/palatal, and mid-buccal) for all teeth. The clinical AL and PD were measured to the nearest millimeter with a graded probe (Hu-Friedy Manufacturing, Chicago, IL, USA). All radiographs were taken and assessed by 1 trained and calibrated investigator (MA κ=0.88). The long cone paralleling technique [29,30] was used to take intraoral digital radiographs for all teeth (NOMAD Pro 2 Intraoral X-Ray Systems, Gendex, Hatfield, PA, USA). In all radiographs, the MBL was defined as the vertical distance from 2 mm below the CEJ to the alveolar crest [31]. The radiographic assessment was performed by a calibrated and experienced examiner (AA; κ=0.9).
Collection of GCF and assessment of IL-4, IL-9, IL-10, and IL-13 levels
In all patients, collection of GCF samples and assessments of IL-4, IL-9, IL-10, and IL-13 levels were performed at baseline and 3 months after SRP by a calibrated investigator (MA; κ=0.94). The GCF samples were collected according to the technique reported by Tsai et al. [32] In summary, GCF was collected from the deepest buccal pocket of the mandibular right or left first molar. The tooth was isolated using cotton rolls, and supragingival plaque was gently removed using a sterile curette. An absorbent paper strip (PerioPaper®, Proflow Inc, Amityville, NY, USA) was inserted into the periodontal pocket using sterile tweezers and gently moved down until resistance was felt. The paper strip was then held in the pocket for 30 seconds. The volume of the collected GCF sample was assessed using a digitally calibrated machine (Periotron 8000, Oraflow, Plainview, NY, USA), and the volume of GCF collected was recorded in µL. Paper strips contaminated with blood and/or saliva were discarded. The GCF samples were collected by a trained and calibrated investigator (MAD; κ=0.89). The paper strips were placed in sterile Eppendorf tubes containing 100 μL physiological saline and 0.1% Tween-20 and centrifuged for 15 minutes at 4°C and 1,500 revolutions per minute. The supernatant was collected and immediately assessed for IL-4, IL-9, IL-10, and IL-13 using enzyme-linked immunosorbent assay kits (R&D Systems Inc., Minneapolis, MN, USA). All kits were used according to the manufacturer's instructions. The concentration of each GCF sample was determined with a standard curve obtained with each standard recombinant GCF substance and recorded in pg/µL.
Statistical analysis
Data analyses were performed using SPSS version 20 (IBM Corp., Armonk, NY, USA). Data were presented as mean±standard deviation. The significance of mean differences in clinical-radiographic periodontal parameters and GCF IL-4, IL-9, IL-10, and IL-13 levels among electronic cigarette users and non-smokers was assessed using the paired t-test, where all P values were 2-tailed and P<0.05 was used to indicate statistical significance. Power analysis was performed and yielded a power of 88%. The sample size was determined using an effect size of 0.8 and an α-value of 0.05. It was estimated that the inclusion of 35 electronic cigarette users and 35 non-smokers would provide a power of 88% [33]. The statistical analysis was performed by a qualified investigator (FV).
RESULTS
Study population
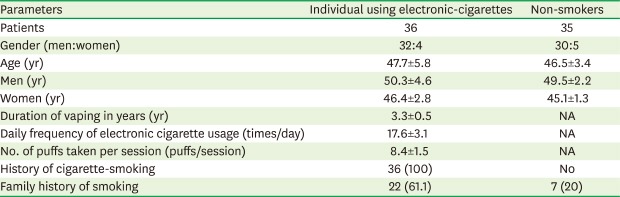
Thirty-six electronic cigarette users (32 men and 4 women) and 35 self-reported non-smokers (30 men and 5 women) were included in the study. The mean ages of the electronic cigarette users and non-smokers were 47.7±5.8 years and 46.5±3.4 years, respectively. All electronic cigarette users were former cigarette smokers, had quit smoking 3 years prior, and had been vaping for a mean duration of 3.3±0.5 years. Electronic cigarette users used their electronic nicotine-delivery systems an average of 17.6±3.1 times/day, and the mean number of puffs taken per session was 8.4±1.5 puffs. A family history of smoking was reported by 100% and 20% of the electronic cigarette users and non-smokers, respectively (Table 1). Prior to quitting cigarette smoking, the electronic cigarette users had a mean smoking history of 11.2±0.8 pack-years.
Table 1. General characteristics of patient groups.
| Parameters | Individual using electronic-cigarettes | Non-smokers |
|---|---|---|
| Patients | 36 | 35 |
| Gender (men:women) | 32:4 | 30:5 |
| Age (yr) | 47.7±5.8 | 46.5±3.4 |
| Men (yr) | 50.3±4.6 | 49.5±2.2 |
| Women (yr) | 46.4±2.8 | 45.1±1.3 |
| Duration of vaping in years (yr) | 3.3±0.5 | NA |
| Daily frequency of electronic cigarette usage (times/day) | 17.6±3.1 | NA |
| No. of puffs taken per session (puffs/session) | 8.4±1.5 | NA |
| History of cigarette-smoking | 36 (100) | No |
| Family history of smoking | 22 (61.1) | 7 (20) |
Values are presented as number (%).
NA: not applicable.
Clinical periodontal parameters at baseline and at 3-month follow-up
At baseline, there were no significant differences in PI, PD, CAL, and MBL between the electronic cigarette users and the non-smokers. At baseline, the GI was significantly higher in the non-smokers than the electronic cigarette users. At the 3-month follow-up, there were no significant differences in PI, GI, PD, CAL, and MBL among electronic cigarette users compared with their corresponding baseline values, but there was a statistically significant reduction in PI, GI, and PD among non-smokers. There was no statistically significant difference in CAL and MBL between groups at the 3-month follow-up (Table 2).
Table 2. Clinical and radiographic periodontal parameters at baseline and at the 3-month follow-up.
| Parameters | Baseline | 3-month of follow-up | |||
|---|---|---|---|---|---|
| Individual using electronic-cigarettes | Non-smokers | Individual using electronic-cigarettes | Non-smokers | ||
| Plaque index | 2.62±0.43a) | 2.25±0.16b) | 1.91±0.18c) | 0.62±0.14 | |
| Gingival index | 0.36±0.08a) | 2.55±0.08b) | 0.34±0.06c) | 0.86±0.1 | |
| Probing depth (mm) | 5.2±0.87a) | 5±0.06b) | 3.7±0.2c) | 1.8±0.07 | |
| Clinical attachment loss (mm) | 3.4±0.2 | 3.1±0.2b) | 3.2±0.1 | 2.7±0.08 | |
| Marginal bone loss (mm) | |||||
| Mesial | 4.8±0.3 | 4.7±0.2 | 4.7±0.08 | 4.6±0.03 | |
| Distal | 4.6±0.2 | 4.6±0.1 | 4.5±0.05 | 4.5±0.02 | |
Data are shown as mean±standard deviation.
a)Compared with electronic-cigarette users at 3-month of follow-up (P<0.05); b)Compared with non-smokers at 3-month of follow-up (P<0.05); c)Compared with non-smokers (P<0.05).
GCF volume and levels of IL-4, IL-10, IL-11, and IL-13 in the study groups at baseline and the 3-month follow-up
At baseline, there were no significant differences in GCF volume or IL-4, IL-10, IL-11, and IL-13 levels between electronic cigarette users and non-smokers with CP. At the 3-month follow-up, there were significant reductions in the GCF volume collected from electronic cigarette users (0.66±0.14 µL) (P<0.05) and non-smokers (0.18±0.09 µL) (P<0.05) compared with their respective baseline volumes (1.65±0.37 and 1.74±0.41 µL, respectively). The GCF volume collected at the 3-month follow-up was significantly higher in electronic cigarette users (0.66±0.14 µL) than in non-smokers (P<0.05). At the 3-month follow-up, the GCF levels of IL-4 (P<0.05), IL-10 (P<0.05), IL-11 (P<0.05), and IL-13 (P<0.05) were significantly elevated in electronic cigarette users and non-smokers compared with their respective baseline values. At the 3-month follow-up, the GCF levels of IL-4 (P<0.05), IL-10 (P<0.05), IL-11 (P<0.05), and IL-13 (P<0.05) were significantly higher in electronic cigarette users than in non-smokers (Table 3).
Table 3. GCF volume and levels of IL-4, IL-10, IL-11, and IL-13 in the study groups at baseline and at the 3-month follow-up.
| Parameters | Baseline | 3-month of follow-up | ||
|---|---|---|---|---|
| Individual using electronic-cigarettes | Non-smokers | Individual using electronic-cigarettes | Non-smokers | |
| GCF volume (µL) | 1.65±0.37a) | 1.74±0.41b) | 0.66±0.14c) | 0.18±0.09 |
| IL-4 (pg/µL) | 0.16±0.12a) | 0.15±0.13b) | 0.71±0.25c) | 1.52±0.18 |
| IL-9 (pg/µL) | 0.33±0.2a) | 0.5±0.12b) | 1.7±0.21c) | 4.21±0.3 |
| IL-10 (pg/µL) | 1.11±0.08a) | 1.34±0.15b) | 3.15±0.5c) | 6.63±0.4 |
| IL-13 (pg/µL) | 0.85±0.16a) | 0.74±0.12b) | 2.26±0.21c) | 4.63±0.47 |
Data are shown as mean±standard deviation.
GCF: gingival crevicular fluid, IL: interleukin.
a)Compared with electronic-cigarette users at 3-month of follow-up (P<0.05); b)Compared with non-smokers at 3-month of follow-up (P<0.05); c)Compared with non-smokers (P<0.05).
DISCUSSION
In clinical periodontology and related research, SRP is considered the non-surgical gold standard for the treatment of periodontal diseases such as CP [34]. In order to assess the outcomes or efficacy of SRP, clinical (CAL, GI, PI, and GI) and radiographic (MBL) parameters are traditionally measured before and after NSPT. Moreover, from an immunoinflammatory perspective, preoperative and postoperative levels of pro-inflammatory cytokines—including but not limited to IL-1β, TNF-α, and matrix metalloproteinases—are investigated in unstimulated whole saliva and GCF in patients with CP. These cytokines are usually assessed to evaluate correlations between the pathophysiological immunological events associated with the progression of CP and the clinicoradiographic periodontal inflammatory parameters in susceptible patient groups. Since SRP is known to significantly reduce the clinical parameters of periodontal inflammation (such as PD and GI) [1,10], levels of anti-inflammatory cytokines are also expected to become elevated in the GCF following the mechanical debridement of teeth and root surfaces. Nevertheless, nicotine compromises healing following surgical treatment and NSPT [21,22]; therefore, the authors of the present study hypothesized that improvements in PI, GI, CAL, PD, and MBL and levels of IL-4, IL-10, IL-11, and IL-13 in the GCF would be compromised in electronic cigarette users compared to non-smokers after SRP. The present results confirm that SRP is an effective therapeutic regimen for the treatment of CP; however, the outcomes were compromised in users of nicotine-containing products (electronic cigarette users in the present scenario) compared with non-smokers. Nicotine is a specific agonist of the α-7 nicotinic acetylcholine receptor, which regulates the expression of destructive inflammatory cytokines in tobacco smokers. Wu et al. [35] investigated the mechanisms by which nicotine influenced the proliferation of osteoclasts in human periodontal ligament cells (PLC) that were either co-cultured or not co-cultured with CD4+ T cells. The results showed that nicotine increased the secretion of IL-1β and the expression of RANKL in the serum of PLC-CD4+ T cell co-culture. Moreover, it has been shown in vitro that the flavorings used in electronic cigarettes increase oxidative stress and pro-inflammatory and pro-senescence responses (such as DNA damage and histone deacetylase reduction), which may lead to the dysfunctional regulation of repair in PLC [36]. This is one explanation for the poor clinical and immunological anti-inflammatory responses observed in electronic cigarette users with CP relative to non-smokers with CP who underwent SRP in the present study.
Notably, the present results showed no significant difference in CAL and MBL between the study groups after SRP. One potential explanation for this finding is associated with the relatively short duration (90 days) of the present study. It is worth mentioning that at the time of patient recruitment, verbal and written information was given to all individuals (irrespective of their decision to participate or withdraw from the present study) about the detrimental effects of smoking and vaping on health. It was also emphasized that vaping is not a safe alternative to smoking, as electronic cigarettes jeopardize health in a manner similar to conventional smoking. However, it is possible that electronic cigarette users continued to vape even after SRP. This factor may have contributed to the reduced expression of anti-inflammatory cytokines in the GCF and worsened clinicoradiographic periodontal inflammatory parameters. It is speculated that quitting vaping, multiple sessions of SRP every 6 months, and long-term follow-up (at least 12 months) would be associated with improvements in CAL and MBL in electronic cigarette users with CP. This possibility warrants additional research. In the present study, nearly 85% of the electronic cigarette users and non-smokers were male. The clinical, radiographic, and immunological data were stratified according to sex, but no statistically significant differences between men and women in reference to these parameters were observed. Due to this lack of a significant difference, this information was not reported in the results. Further studies are needed to assess the influence of sex on clinicoradiographic periodontal status and the expression of anti-inflammatory cytokines in the GCF of electronic cigarette users.
A limitation of the present study is that SRP was performed as the sole therapeutic strategy for the treatment of CP. Studies [1,8,37] have shown that, in patients with CP, SRP performed with adjuvant treatments such as low-level laser therapy, probiotic therapy, or antimicrobial photodynamic therapy is more efficient than SRP alone in reducing periodontal soft tissue inflammation and the levels of destructive inflammatory cytokines in the GCF and unstimulated whole saliva. It is therefore hypothesized that SRP performed with adjuvant therapies, such as those mentioned above, is more effective than SRP alone in raising the levels of anti-inflammatory cytokines (including IL-4, IL-10, IL-11, and IL-13) in the GCF, which may contribute towards restoring a satisfactory periodontal status in patients diagnosed with periodontal diseases. Another limitation of the present study is that only electronic cigarette users were included. Although the electronic cigarette users included were former cigarette smokers (who had quit smoking approximately 3 years ago), it is likely that dual smokers—that is, individuals using electronic cigarettes and smoking cigarettes—show poorer clinical and radiographic periodontal status and significantly lower levels of anti-inflammatory cytokines in the GCF than either individuals who use only electronic cigarettes or non-smokers. Furthermore, the possible presence of pro-inflammatory cytokines in the GCF of nicotine product users (as shown in other studies) may sabotage the simultaneous expression of anti-inflammatory cytokines in the GCF. Further studies are needed to assess these hypotheses.
In conclusion, levels of GCF IL-4, IL-9, IL-10, and IL-13 increased following SRP in electronic cigarette users and non-smokers with CP; however, the anti-inflammatory effect of SRP was more profound in non-smokers than electronic cigarette users.
Footnotes
Funding: The authors are grateful to the Researchers Supporting Project at King Saud University for providing funding through Researchers Supporting Project (No. RSP-2019-44).
- Conceptualization: Nawwaf Al-Hamoudi.
- Data curation: Mohammed Alrabiah.
- Formal analysis: Fahim Vohra.
- Investigation: Abdulaziz Alsahhaf, Mohammed Alrabiah.
- Methodology: Modhi Al Deeb.
- Project administration: Modhi Al Deeb.
- Software: Abdulaziz Alsahhaf.
- Writing - original draft: Nawwaf Al-Hamoudi, Abdulaziz Alsahhaf, Modhi Al Deeb, Mohammed Alrabiah, Fahim Vohra, Tariq Abduljabbar.
- Writing - review & editing: Nawwaf Al-Hamoudi, Abdulaziz Alsahhaf, Modhi Al Deeb, Mohammed Alrabiah, Fahim Vohra, Tariq Abduljabbar.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.AlAhmari F, Ahmed HB, Al-Kheraif AA, Javed F, Akram Z. Effectiveness of scaling and root planning with and without adjunct antimicrobial photodynamic therapy in the treatment of chronic periodontitis among cigarette-smokers and never-smokers: a randomized controlled clinical trial. Photodiagnosis Photodyn Ther. 2019;25:247–252. doi: 10.1016/j.pdpdt.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Jia L, Jia J, Xie M, Zhang X, Li T, Shi L, et al. Clinical attachment level gain of lasers in scaling and root planing of chronic periodontitis: a network meta-analysis of randomized controlled clinical trials. Lasers Med Sci. 2020;35:473–485. doi: 10.1007/s10103-019-02875-5. [DOI] [PubMed] [Google Scholar]
- 3.Mohan R, Varghese J, Bhat V, Chianeh YR. The effect of nonsurgical periodontal therapy on pentraxin 3 levels in smokers and nonsmokers with chronic periodontitis. Gen Dent. 2019;67:e1–6. [PubMed] [Google Scholar]
- 4.Kaushik R, Yeltiwar RK, Pushpanshu K. Salivary interleukin-1β levels in patients with chronic periodontitis before and after periodontal phase I therapy and healthy controls: a case-control study. J Periodontol. 2011;82:1353–1359. doi: 10.1902/jop.2011.100472. [DOI] [PubMed] [Google Scholar]
- 5.Kinney JS, Morelli T, Oh M, Braun TM, Ramseier CA, Sugai JV, et al. Crevicular fluid biomarkers and periodontal disease progression. J Clin Periodontol. 2014;41:113–120. doi: 10.1111/jcpe.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prakasam S, Srinivasan M. Evaluation of salivary biomarker profiles following non-surgical management of chronic periodontitis. Oral Dis. 2014;20:171–177. doi: 10.1111/odi.12085. [DOI] [PubMed] [Google Scholar]
- 7.Abduljabbar T, Vohra F, Kellesarian SV, Javed F. Efficacy of scaling and root planning with and without adjunct Nd:YAG laser therapy on clinical periodontal parameters and gingival crevicular fluid interleukin 1-beta and tumor necrosis factor-alpha levels among patients with periodontal disease: a prospective randomized split-mouth clinical study. J Photochem Photobiol B. 2017;169:70–74. doi: 10.1016/j.jphotobiol.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Qadri T, Javed F, Poddani P, Tunér J, Gustafsson A. Long-term effects of a single application of a water-cooled pulsed Nd:YAG laser in supplement to scaling and root planing in patients with periodontal inflammation. Lasers Med Sci. 2011;26:763–766. doi: 10.1007/s10103-010-0807-8. [DOI] [PubMed] [Google Scholar]
- 9.Al-Hamoudi N, Abduljabbar T, Mirza S, Al-Sowygh ZH, Vohra F, Javed F, et al. Non-surgical periodontal therapy reduces salivary adipocytokines in chronic periodontitis patients with and without obesity. J Investig Clin Dent. 2018;9:e12314. doi: 10.1111/jicd.12314. [DOI] [PubMed] [Google Scholar]
- 10.Eshghipour B, Tofighi H, Nehal F, Vohra F, Javed F, Akram Z. Effect of scaling and root planing on gingival crevicular fluid cytokine/chemokine levels in smokers with chronic periodontitis: a systematic review. J Investig Clin Dent. 2018;9:e12327. doi: 10.1111/jicd.12327. [DOI] [PubMed] [Google Scholar]
- 11.Kellesarian SV, Malignaggi VR, Majoka HA, Al-Kheraif AA, Kellesarian TV, Romanos GE, et al. Effect of laser-assisted scaling and root planing on the expression of pro-inflammatory cytokines in the gingival crevicular fluid of patients with chronic periodontitis: a systematic review. Photodiagnosis Photodyn Ther. 2017;18:63–77. doi: 10.1016/j.pdpdt.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 12.BinShabaib M, ALHarthi SS, Akram Z, Khan J, Rahman I, Romanos GE, et al. Clinical periodontal status and gingival crevicular fluid cytokine profile among cigarette-smokers, electronic-cigarette users and never-smokers. Arch Oral Biol. 2019;102:212–217. doi: 10.1016/j.archoralbio.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Abduljabbar T, Akram Z, Vohra F, Warnakulasuriya S, Javed F. Assessment of interleukin-1β, interleukin-6, and tumor necrosis factor-Α levels in the peri-implant sulcular fluid among waterpipe (narghile) smokers and never-smokers with peri-implantitis. Clin Implant Dent Relat Res. 2018;20:144–150. doi: 10.1111/cid.12557. [DOI] [PubMed] [Google Scholar]
- 14.Arias-Bujanda N, Regueira-Iglesias A, Alonso-Sampedro M, González-Peteiro MM, Mira A, Balsa-Castro C, et al. Cytokine thresholds in gingival crevicular fluid with potential diagnosis of chronic periodontitis differentiating by smoking status. Sci Rep. 2018;8:18003. doi: 10.1038/s41598-018-35920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel RP, Amirisetty R, Kalakonda B, Penumatsa NV, Koppolu P. Influence of smoking on gingival crevicular fluid interleukin 1β and interleukin-8 in patients with severe chronic periodontitis among a rural population in India. Niger Med J. 2018;59:33–38. doi: 10.4103/nmj.NMJ_142_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scarel-Caminaga RM, Trevilatto PC, Souza AP, Brito RB, Camargo LE, Line SR. Interleukin 10 gene promoter polymorphisms are associated with chronic periodontitis. J Clin Periodontol. 2004;31:443–448. doi: 10.1111/j.1600-051X.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki K, Nakajima T. Antigen specificity and T-cell clonality in periodontal disease. Periodontol 2000. 2004;35:75–100. doi: 10.1111/j.0906-6713.2004.003558.x. [DOI] [PubMed] [Google Scholar]
- 18.Pradeep AR, Roopa Y, Swati PP. Interleukin-4, a T-helper 2 cell cytokine, is associated with the remission of periodontal disease. J Periodontal Res. 2008;43:712–716. doi: 10.1111/j.1600-0765.2007.01079.x. [DOI] [PubMed] [Google Scholar]
- 19.Emingil G, Gürkan A, Atilla G, Kantarci A. Subantimicrobial-dose doxycycline and cytokine-chemokine levels in gingival crevicular fluid. J Periodontol. 2011;82:452–461. doi: 10.1902/jop.2010.100036. [DOI] [PubMed] [Google Scholar]
- 20.Browne M, Todd DG. Then and now: consumption and dependence in e-cigarette users who formerly smoked cigarettes. Addict Behav. 2018;76:113–121. doi: 10.1016/j.addbeh.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 21.Javed F, Al-Rasheed A, Almas K, Romanos GE, Al-Hezaimi K. Effect of cigarette smoking on the clinical outcomes of periodontal surgical procedures. Am J Med Sci. 2012;343:78–84. doi: 10.1097/MAJ.0b013e318228283b. [DOI] [PubMed] [Google Scholar]
- 22.Cuff MJ, McQuade MJ, Scheidt MJ, Sutherland DE, Van Dyke TE. The presence of nicotine on root surfaces of periodontally diseased teeth in smokers. J Periodontol. 1989;60:564–569. doi: 10.1902/jop.1989.60.10.564. [DOI] [PubMed] [Google Scholar]
- 23.Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alqahtani F, Alqhtani N, Alkhtani F, Divakar DD, Al-Kheraif AA, Javed F. Efficacy of mechanical debridement with and without adjunct antimicrobial photodynamic therapy in the treatment of peri-implantitis among moderate cigarette-smokers and waterpipe-users. Photodiagnosis Photodyn Ther. 2019;28:153–158. doi: 10.1016/j.pdpdt.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Javed F, Näsström K, Benchimol D, Altamash M, Klinge B, Engström PE. Comparison of periodontal and socioeconomic status between subjects with type 2 diabetes mellitus and non-diabetic controls. J Periodontol. 2007;78:2112–2119. doi: 10.1902/jop.2007.070186. [DOI] [PubMed] [Google Scholar]
- 26.Armitage GC, Dickinson WR, Jenderseck RS, Levine SM, Chambers DW. Relationship between the percentage of subgingival spirochetes and the severity of periodontal disease. J Periodontol. 1982;53:550–556. doi: 10.1902/jop.1982.53.9.550. [DOI] [PubMed] [Google Scholar]
- 27.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38(Suppl):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 28.Armitage GC, Svanberg GK, Löe H. Microscopic evaluation of clinical measurements of connective tissue attachment levels. J Clin Periodontol. 1977;4:173–190. doi: 10.1111/j.1600-051x.1977.tb02271.x. [DOI] [PubMed] [Google Scholar]
- 29.Updegrave WJ. The paralleling extension-cone technique in intraoral dental radiography. Oral Surg Oral Med Oral Pathol. 1951;4:1250–1261. doi: 10.1016/0030-4220(51)90084-9. [DOI] [PubMed] [Google Scholar]
- 30.Khocht A, Janal M, Harasty L, Chang KM. Comparison of direct digital and conventional intraoral radiographs in detecting alveolar bone loss. J Am Dent Assoc. 2003;134:1468–1475. doi: 10.14219/jada.archive.2003.0076. [DOI] [PubMed] [Google Scholar]
- 31.Javed F, Al-Kheraif AA, Al Amri MD, Mikami T, Vohra F, Warnakulasuriya S, et al. Periodontal parameters and whole salivary cytokine profiles among habitual gutka chewers and non-chewers. J Periodontol. 2015;86:689–695. doi: 10.1902/jop.2015.140556. [DOI] [PubMed] [Google Scholar]
- 32.Tsai CC, Ku CH, Ho YP, Ho KY, Wu YM, Hung CC. Changes in gingival crevicular fluid interleukin-4 and interferon-gamma in patients with chronic periodontitis before and after periodontal initial therapy. Kaohsiung J Med Sci. 2007;23:1–7. doi: 10.1016/S1607-551X(09)70367-5. [DOI] [PubMed] [Google Scholar]
- 33.Parker RI, Hagan-Burke S. Useful effect size interpretations for single case research. Behav Ther. 2007;38:95–105. doi: 10.1016/j.beth.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Cobb CM. Microbes, inflammation, scaling and root planing, and the periodontal condition. J Dent Hyg. 2008;82(Suppl 3):4–9. [PubMed] [Google Scholar]
- 35.Wu LZ, Duan DM, Liu YF, Ge X, Zhou ZF, Wang XJ. Nicotine favors osteoclastogenesis in human periodontal ligament cells co-cultured with CD4(+) T cells by upregulating IL-1β. Int J Mol Med. 2013;31:938–942. doi: 10.3892/ijmm.2013.1259. [DOI] [PubMed] [Google Scholar]
- 36.Sundar IK, Javed F, Romanos GE, Rahman I. E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget. 2016;7:77196–77204. doi: 10.18632/oncotarget.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Invernici MM, Salvador SL, Silva PH, Soares MS, Casarin R, Palioto DB, et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: a randomized clinical trial. J Clin Periodontol. 2018;45:1198–1210. doi: 10.1111/jcpe.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]


