Abstract
Vitamin D signaling plays an essential role in innate defense against intracellular microorganisms via the generation of the antimicrobial protein cathelicidin. In addition to directly binding to and killing a range of pathogens, cathelicidin acts as a secondary messenger driving vitamin D-mediated inflammation during infection. Recent studies have elucidated the biological and clinical functions of cathelicidin in the context of vitamin D signaling. The vitamin D-cathelicidin axis is involved in the activation of autophagy, which enhances antimicrobial effects against diverse pathogens. Vitamin D studies have also revealed positive and negative regulatory effects of cathelicidin on inflammatory responses to pathogenic stimuli. Diverse innate and adaptive immune signals crosstalk with functional vitamin D receptor signals to enhance the role of cathelicidin action in cell-autonomous effector systems. In this review, we discuss recent findings that demonstrate how the vitamin D-cathelicidin pathway regulates autophagy machinery, protective immune defenses, and inflammation, and contributes to immune cooperation between innate and adaptive immunity. Understanding how the vitamin D-cathelicidin axis operates in the host response to infection will create opportunities for the development of new therapeutic approaches against a variety of infectious diseases.
Keywords: Vitamin D, Cathelicidin, Infections, Autophagy, Immunity, Inflammation
INTRODUCTION
Vitamin D signaling and metabolism are crucial in the regulation of bone and calcium homeostasis. However, various cells and tissues, regardless of calcium metabolism, express the vitamin D receptor (VDR) and can undergo functional VDR signaling, suggesting that vitamin D plays a greater biological role than solely skeletal homeostasis (1,2). Accumulating data suggest that vitamin D and downstream receptor signaling play key roles in the ability of macrophages and other immune cells to enhance host antimicrobial defense and coordinate a variety of biological responses, including immune and inflammatory activity. The outcome of vitamin D-induced innate immune responses depends, at least partly, on the induction of gene sets encoding antimicrobial proteins (AMPs). Several AMPs are induced by vitamin D signaling, including cathelicidins, defensins, hepcidins, and neutrophil peptides, acting as principal intrinsic antibiotics given their direct antimicrobial activities against various pathogens (3). Since infections caused by drug-resistant pathogens are emerging worldwide, host defense peptides could be valuable candidates for the development of new therapeutic agents (4).
AMPs are usually cationic, binding to anionic regions (i.e., anionic lipids) of plasma membranes of various bacteria and viruses to form and eventually rupture pores (5). AMPs also function as key signaling molecules to fine-tune immune and inflammatory responses in diverse living organisms, including mammals, insects, and plants (6). Numerous previous studies have shown that vitamin D signaling leads to transcriptional activation of AMPs, including cathelicidins and defensins (7,8). Importantly, vitamin D-induced cathelicidin, the best-characterized AMP involved in VDR signaling, has multiple functions as an essential anti-infective agent against numerous pathogens, including intracellular Mycobacterium tuberculosis (Mtb), a major pathogen of human tuberculosis (TB) (7,8), and as a crucial immune modulator that impacts innate and adaptive immunity, chemotaxis, and angiogenesis (9).
In this review, we discuss the effects of the vitamin D-cathelicidin axis on antimicrobial host defense in a variety of infections caused by bacteria, viruses, and parasites, as examined in both in vitro and in vivo studies, and in clinical trials. Specifically, we review the current understanding of how VDR signaling is linked to the activation of AMP cathelicidin to modulate the autophagy machinery, cytokine/chemokine generation, and crosstalk with innate and adaptive immune responses during infection. We also speculate about the engagement of vitamin D signaling, cathelicidin, and host defense to eliminate invading pathogens without resulting in inflammatory-induced tissue injury.
OVERVIEW OF VITAMIN D METABOLISM AND SIGNALING
Vitamin D3 (hereafter mentioned as vitamin D) can be obtained from dietary intake or produced by ultraviolet light (UV)-mediated synthesis in the skin, in which UV rays promote the conversion of 7-dehydrocholesterol into vitamin D3 (10). The inactive vitamin D compound is then sequentially hydroxylated in the liver and kidney to form 25-hydroxyvitamin D3 (25D3) and active compound 1,25 dihydroxy vitamin D3 (1,25D3) by essential enzymes 25- and 1-α-hydroxylases, respectively (10). Circulating 1,25D3 levels are tightly regulated to prevent excessive VDR signaling and activity by a metabolic feedback loop involving the conversion by the inactivating enzyme 24-hydroxylase (CYP24A1) into inactive calcitroic acid, which is subsequently excreted in the bile. Immune cells also produce both activating (25- and 1α-hydroxylase) and metabolizing (24-hydroxylase) enzymes to regulate local levels of 1,25D3 in immune reaction sites (10). It is now clear that immune cells express 1-α-hydroxylase (CYP27B1), which allows them to activate functional VDR signaling pathways through the conversion of inactive 25D3 into active 1,25D3 (10). Various innate stimuli including TLR2/1 (11-13), TLR8 (14), and a combination of IL-12 and IL-18 (15) trigger local activation of functional VDR signaling to enhance cell-autonomous host defense mechanisms, which are mediated through cathelicidin induction, the autophagy pathway, and the production of IFN-γ, in host cells against intracellular pathogens.
During infection, VDR signaling activation coordinates innate immune signals for the production of AMPs, including human cathelicidin AMP (CAMP) and β-defensin 2 (HBD2) by binding to vitamin D response elements in target genes (8,16). Although antimicrobial effects have been the major function ascribed to AMPs in VDR signaling, recent studies have established AMP as a central regulator of autophagy/xenophagy, generation of cytokines, chemokines and reactive oxygen species (ROS), as well as modulation of IFN signaling, thus serving as a signaling node to regulate immune pathways (7,8,17). A number of excellent reviews have covered the antibacterial and immune regulatory roles of AMPs; therefore, this review mainly focuses on recent studies that have identified the roles of endogenous AMPs induced by VDR signaling, and on the therapeutic implications of vitamin D-AMPs in host-directed therapy against infections. The effector pathways of vitamin D-driven innate immune signaling, which will be discussed in this review.
CATHELICIDINS AND VITAMIN D-INDUCED ANTIMICROBIAL RESPONSES
The antimicrobial activity of human cathelicidin LL-37/human cationic AMP 18 (hCAP-18) has been reported in a variety of gram-positive and gram-negative pathogenic bacteria, viruses, and fungi (18,19). Vitamin D and its active analog upregulate the expression of the cathelicidin LL-37 in a wide range of cell types, including keratinocytes, epithelial cells, and human monocytes/macrophages (11,20,21). Interestingly, murine cells reportedly not to induce CAMP mRNA expression (22), suggesting that vitamin D-mediated LL-37 induction is critically involved in the human defense system. Cathelicidin is an antimicrobial weapon that eliminates intracellular mycobacteria and also plays a regulatory role in various processes of the autophagy pathway to enhance the fusion of mycobacterial phagosomes with autophagosomes and autolysosomes (11,23). In addition to its antibacterial roles, LL-37 has a pleiotropic role in a variety of biological responses, as a key immunomodulator with both pro- and anti-inflammatory functions in different cells/tissues and microenvironments in a context-dependent manner (24). The generation and detailed functional significance of cathelicidins have been expertly reviewed (24-26); therefore, we will discuss recent findings of the roles and mechanisms of cathelicidins in the context of vitamin D signaling during infection.
Antimicrobial effects of cathelicidins in host cells
VDRs are found on monocytes/macrophages, which express and induce the 1α-hydroxylase isoenzyme (CYP27B1) to produce active 1,25D3, which promotes the adherence and differentiation of normal human monocytes (10). Innate immune signaling, e.g., TLR 2 activation, results in functional activation of vitamin D signaling to enhance CYP27B1-dependent synthesis of active vitamin D and enhancement of human cathelicidin (8,12). Cathelicidins are among the well-characterized classes of human AMPs and are usually produced as a prepropeptide to be processed for the generation of anti-microbial active fragments (27). The hCAP18 is the only member of human cathelicidins; LL-37 is the C-terminal, amphipathic, alpha-helical peptide generated by cleavage of the C-terminal end of the hCAP18 protein by serine proteases and proteinase 3 (28). Due to its positive charge, LL-37 preferentially interacts with negatively charged bacterial membranes and forms pores via detergent-like effects (28). A recent study used super-resolution single-particle tracking tools to demonstrate LL-37 penetration and rigidification of bacterial cytoplasm through electrostatic linking of chromosomal DNA and a subset of ribosomes (29). Another recent study of LL-37 structure identified its core antimicrobial region for application in peptide designing of antimicrobial and antibiofilm agents (30).
Over the past 12 years, evidence has emerged for cathelicidin LL-37 as an integral regulator and effector of vitamin D-mediated anti-microbial responses in antibacterial immunity, particularly in mycobacterial infection (31). Synthetic LL-37 has shown a direct inhibitory effect on Mtb growth in broth and human macrophages (8,31). A study conducted by Liu et al. (8) showed that TLR2/1 triggering of vitamin D-mediated cathelicidin induction is crucial for anti-mycobacterial activity in human monocytes/macrophages. Numerous studies have demonstrated the beneficial effects of vitamin D-mediated cathelicidin in antimicrobial actions against Mtb infection (7,8,11). Importantly, the physiological levels of vitamin D3 contribute to the restriction of Mtb alone or Mtb/HIV replication in macrophages (11,32,33). In addition, a recent study showed that 25D3 treatment of human monocyte-derived macrophages, which are differentiated with IL-15, led to increased expression of both cathelicidin mRNA and protein, and upregulated the vitamin D-dependent antimicrobial response against intracellular Mycobacterium leprae (34). Besides mycobacterial infection, calcipotriol, a calcitriol-derivative, has been shown to inhibit the hepatitis C virus and robustly activate VDR target genes, including cathelicidin and hepcidin (35). Recent studies have shown that 1,25D3 directly activates the transcription of IL-1β, decreasing the mycobacterial burden in macrophages through IL-1β-driven epithelial production of the antimicrobial peptide DEFB4/HBD2 (36). This result suggests that, for a wide variety of infections, vitamin D-induced AMPs can serve as an antimicrobial immune effector to activate host defense.
LL-37 is internalized through an endocytotic process via the P2X7 receptor and traffics into lysosomes to enhance bactericidal activity against Staphylococcus aureus in human macrophages (37). Human cathelicidin is produced via vitamin D-mediated signaling and exhibits antimicrobial activity against intracellular pathogens through trafficking into endosomal/lysosomal compartments. Indeed, a previous study demonstrated that hCAP-18 is present in the phagolysosome with azurophil granule proteins, containing serine protease, which processes hCAP-18 to generate active AMP LL-37 (38).
Given the key roles of cathelicidin in the regulation of infection, it is not surprising that several pathogens have evolved strategies to inhibit LL-37 function (39). For example, human metapneumovirus, a major pathogen of respiratory tract infections in young children, markedly suppressed vitamin D-induced hCAP18 levels in human macrophages (40). A recent study also showed that the Clostridium difficile clnRAB operon binds directly to LL-37, thereby regulating the expression of a variety of gene sets involved in metabolism, cell signaling, and pathogenesis (41). These studies strongly suggest that microbial signals can modulate host responses through direct interaction with AMPs. These studies of vitamin D-cathelicidin during infection are summarized in Table 1. Further studies are required to clarify how pathogens modulate CAMP gene expression and/or degrade host cathelicidin proteins, to establish intracellular survival and replication during infection, and how they subvert vitamin D-mediated antimicrobial responses.
Table 1. In vivo and in vitro studies of vitamin D-cathelicidin during infection.
| Reagent | Disease/pathogen | Subject | Results | Reference | |
|---|---|---|---|---|---|
| In vitro | |||||
| TLR2/1 ligand | Mtb | Human macrophage | TLR activation upregulates VDR and the vitaminD-1-hydroxylase genes | (8) | |
| Induction of the cathelicidin | |||||
| Vitamin D | Mtb | Human monocyte and macrophage, THP-1 and RAW 264.7 cells | Upregulation of transcription of BECN1 and ATG5 through cathelicidin-dependent MAPK and C/EBPβ signaling | (11) | |
| Recruitment of cathelicidin to the autophagosomes through the Ca2+ and AMPK-dependent pathways | |||||
| Vitamin D and its analog | Mtb, BCG | Human PBMC | Vitamin D inhibits the growth of mycobacteria through VDR signaling | (31) | |
| Up-regulated the cathelicidin hCAP-18gene | |||||
| Vitamin D | Mtb, HIV | Human macrophage | Induction of autophagy and phagosomal maturation | (33) | |
| Vitamin D, IL-15 | Mycobacterium leprae | Human macrophage | Increased expression of cathelicidin | (34) | |
| Vitamin D, calcipotriol | HCV | Human hepatoma cell lines, human macrophage | Induced local structure rearrangement of VDR | (35) | |
| Vitamin D | Mtb | Murine macrophage, human macrophage, THP-1 cells | Enhanced IL-1β expression | (36) | |
| Epithelial IL1R1 signaling and DEFB4/HBD2 | |||||
| LL-37 | Staphylococcus aureus | Human macrophage, THP-1 cells | Endocytotic process via P2X7 receptor | (37) | |
| Upregulation of ROS and lysosome formation | |||||
| DrsG | Streptococcus dysgalactiae subsp. equisimilis | In vitro assay | Functions as a ligand of the cathelicidin LL-37 and inhibits the bactericidal activity of LL-37 | (39) | |
| Vitamin D | hMPV | Human macrophage | hMPV attenuates CAMP through C/EBPα | (40) | |
| In vivo | |||||
| LL-37 | Clostridium difficile | Syrian golden hamsters | C. difficile clnRAB Operon is induced by LL-37, senses and binds to LL-37 | (41) | |
| C57BL/6 mice | |||||
| - | Mtb | Patient sputum proteome | A shift of vitamin D binding protein-AMP axis in the lung of TB patients | (44) | |
| - | Mtb | Ethiopian patient blood | Patients with extrapulmonary TB in local lymph nodes show higher 25(OH)D3 levels compared with pulmonary TB patients | (45) | |
| Plasma 25(OH)D3 levels correlate with local LL-37 expression in granulomatous lesions of lymph nodes from extrapulmonary TB | |||||
| - | Mycobacterium avium complex | Patient serum | Serum hCAP18/LL-37 level and BMD are decreased in patients with MAC lung disease; No relation to serum vitamin D level. | (46) | |
| - | Urinary tract infection | Infants and young children patient serum | Serum vitamin D levels negatively correlate with age and are significantly lower in girls; Vitamin D levels positively correlate with levels of cathelicidin but not with β-defensin-2 | (47) | |
| - | Cystic fibrosis | Children | No relationship between vitamin D in sera and HBD-2 or LL-37 in bronchoalveolar lavage | (48) | |
| No differences in infective or inflammatory markers between vitamin D-sufficient and deficient groups | |||||
| Cathelicidin | Plasmodium chabaudi AS murine malaria | BALB/c mice | No curative effects by exogenous CAMP in infected mice | (49) | |
| Vitamin D | P. chabaudi AS murine malaria | BALB/c mice | Vitamin D shows antimalarial activity in the acute phase of infection | (50) | |
| Vitamin D | Influenza vaccine | Elderly person serum | No differences in cathelicidin level between vitamin D supplemented and untreated groups | (51) | |
| Vitamin D | Nontypeable Haemophilus influenzae | C57BL/6JolaH mice | Vitamin D-deficient mice resolve infection and local lung inflammation faster than vitamin D-sufficient mice, possibly through a shift of protease/anti-protease balance and upregulation of CRAMP | (52) | |
Vitamin D-cathelicidin during infection in vivo
To date, most studies of LL-37 function have been conducted using in vitro systems, which have allowed us to begin to understand the impact of LL-37 as a biomarker related to vitamin D levels in various infectious diseases, including TB and HIV infection (42,43). A study applied sputum proteomic analysis to show that vitamin D binding protein was abundant, but cathelicidin lacked in sputum samples from active pulmonary TB patients (44). In Ethiopian patients with lymph node TB, plasma 25D3 levels were significantly correlated with local LL-37 expression in granulomatous lesions in disease sites (45). Another recent study showed that serum hCAP18/LL-37 levels were significantly depressed in patients with Mycobacterium avium complex lung diseases, although they were not related to serum vitamin D levels (46); serum cathelicidin levels, which were correlated with vitamin D levels, were significantly decreased in young children and associated with urinary tract infection (47).
However, no relationship has been detected between vitamin D levels and LL-37 production in bronchoalveolar lavage fluids in children with cystic fibrosis, a genetic disorder (48). Similarly, cathelicidin may not play an important role in antimalarial effects, although vitamin D and its analogs display potent antiplasmodial activity (49,50). Vitamin D supplementation in deficient elderly persons with influenza vaccination was not found to increase serum cathelicidin levels, although vitamin D treatment drove lymphocyte polarization to the tolerogenic type (51). A recent study using an acute infection model with nontypeable Haemophilus influenzae showed a faster resolution of infection and lung inflammation in vitamin D-deficient mice, presumably due to cathelicidin-related antimicrobial peptide (CRAMP) upregulation (52). Together, these data suggest that cathelicidin production in vivo during infection is regulated by vitamin D-dependent and independent pathways, depending on the bacterial strain, cell types, and host immune status (Table 1).
THE VITAMIN D-CATHELICIDIN AXIS AND AUTOPHAGY REGULATION
Autophagy is an intracellular homeostatic process that affects diverse biological responses in the human body through quality control of cell functions. Autophagy plays an essential role in the activation of cell-autonomous immune defense against infectious agents (53). Since autophagy is an essential housekeeping function, its dysregulation is often associated with pathological conditions such as inflammation (53). Therefore, autophagy and vitamin D signaling have overlapping functions in the maintenance of homeostasis during infection and inflammation (23). In this section, we will focus on vitamin D-induced autophagy and its regulatory mechanisms, which are partly mediated by cathelicidin, in the context of infection (Fig. 1). The precise mechanisms by which vitamin D signaling activates autophagy and cathelicidin and how they interact with other signaling pathways/molecules remain to be elucidated.
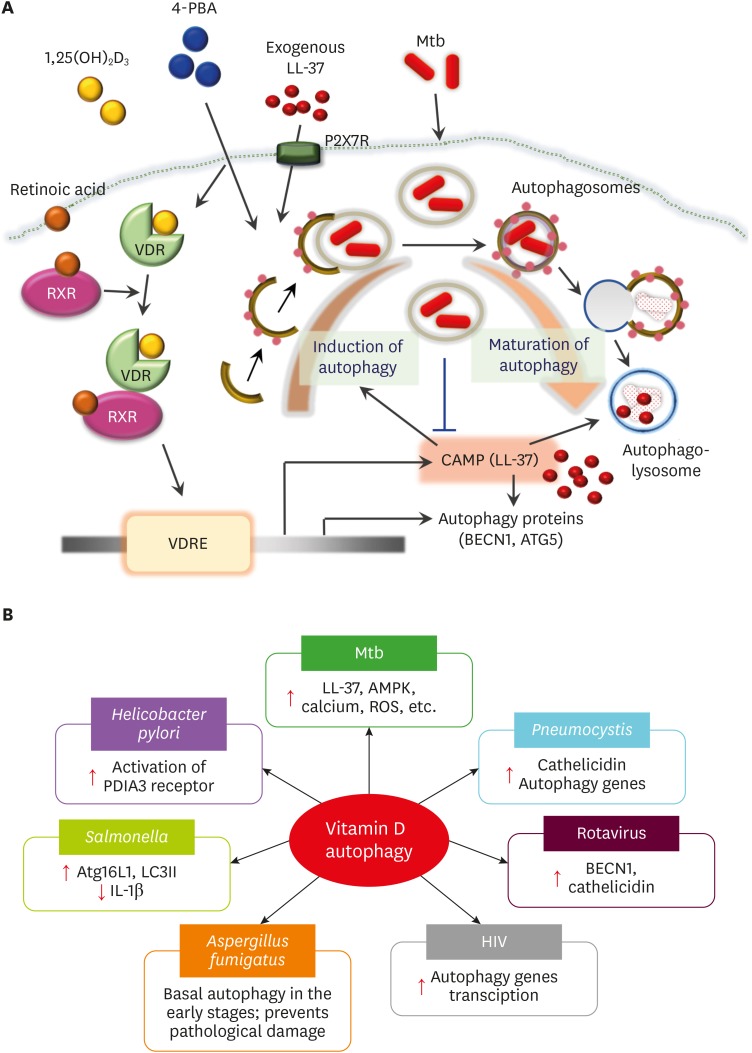
Figure 1.
Vitamin D-mediated autophagy in antimicrobial host defense during infection. (A) Vitamin D-LL-37 functions in Mtb clearance through autophagy activation. Vitamin D3 treatment alone, or combined with retinoic acid or 4-PBA, or exogenous LL-37 via P2X7R, results in the activation of functional VDR signaling to trigger expression of cathelicidin, a secondary messenger for autophagy activation through transcriptional activation of ATGs and enhancement of autophagic flux, in human monocytes/macrophages. The activation of VDR-cathelicidin-mediated autophagy is beneficial for combatting intracellular Mtb infection. (B) Vitamin D-induced autophagy in various infections. Vitamin D signaling has been found to enhance autophagy in host cells during infection by Mtb, Salmonella, Helicobacter, Aspergillus, hepatitis C virus, HIV, or Pneumocystis murina. The known mechanisms involved in vitamin D-mediated autophagy are shown in the box of each pathogen.
VDRE, vitamin D response elements.
Vitamin D-mediated autophagy in the antimicrobial effect of host cells
Upon pathogen challenge, vitamin D treatment triggers the autophagy pathway to enhance host antimicrobial responses and control excessive inflammation (11,23,54). The best examples of vitamin D-cathelicidin function in autophagy have been studied in host responses to Mtb infection. One strategy by which Mtb subverts host innate defense is the inhibition of LL-37 and ATG (beclin-1 [BECN1] and autophagy related 5 [ATG5]) expression in human macrophages (55). Previous studies have demonstrated the role of the vitamin D pathway in overcoming intracellular Mtb infection through autophagy activation and cathelicidin induction (56). Early studies identified a link between vitamin D autophagy function and Mtb clearance via LL-37 in human macrophages (11,57). The combined effects of retinoic acid and vitamin D3 have been reported to activate autophagy, to increase phagocytosis of Mtb and restrict intracellular Mtb growth in human monocytic tetrahydropalmatine (THP)-1 cells (58). It has also been reported that 4-phenylbutyrate (4-PBA) and vitamin D, separately or in combination, function as efficient inducers of autophagy through LL-37 expression and promote the co-localization of LL-37 with autophagosomes, thereby potentiating intracellular killing effects against Mtb (55). Importantly, either the cell-autonomous induction of LL-37 or exogenous synthetic LL-37 participates in vitamin D-induced autophagy activation (11,55). The detailed mechanisms by which LL-37 mediates autophagy in the context of Mtb infection is discussed in the following session.
Several studies have explored the role of vitamin D-induced autophagy in various pathogenic infections. A recent study of Helicobacter pylori infection showed that vitamin D treatment improved lysosomal acidification and degradation through activation of the protein disulfide-isomerase A3 receptor, promoting autolysosomal function and antibacterial responses, even against antibiotic-resistant bacteria (54). Vitamin D signaling also enhances the expression of Atg16L1 and LC3II, as well as the abundance of LC3 punctae in intestinal epithelial cells during Salmonella infection (59). In this context, vitamin D-mediated Atg16L1 expression counteracts IL-1β expression (59), suggesting a role for vitamin D in the activation of autophagic clearance of bacteria and prevention of excessive harmful inflammation (59). Vitamin D-mediated autophagy also contributes to antifungal immunity. In studies of Aspergillus fumigatus infection models, whose pathology is related to excessive autophagy, vitamin D deficiency has been shown to cause defective pulmonary resistance to A. fumigatus. Mechanistically, vitamin D functions in the maintenance of basal autophagy in the early stages of infection and prevents pathological damage induced by excessive autophagy (60). Together, the findings of these studies suggest that vitamin D plays a pivotal role in controlling optimal and efficient levels of autophagy to improve host defense against diverse pathogens.
In addition to bacterial and fungal infection, vitamin D-mediated autophagy plays an important role in the regulation of antiviral host defense. In human macrophages, vitamin D treatment, even at physiologically relevant concentrations, can enhance autophagy to suppress the replication of HIV infection through transcriptional activation of autophagy genes (61). In patients with hepatitis C virus infection only or combined with hepatocellular carcinoma, vitamin D and VDR levels are depressed, and correlated with serum concentrations of LC3 and caspase-3, suggesting disturbance of autophagy and apoptosis function in these patients (62). It remains unclear whether vitamin D supplementation would recover autophagy activation in such patients. In rotavirus infection, vitamin D supplementation has been reported to upregulate autophagy gene BECN1 expression and autophagic flux, and increase porcine cathelicidin levels in virus-infected porcine intestinal epithelial cells (63). Since numerous viruses subvert, block, and even hijack host xenophagy systems (64), whether vitamin D-induced autophagy restricts or favors viral replication/survival in host cells may depend on the individual virus and host cell types, as well as different pathogenic mechanisms.
Vitamin D supplementation increases the efficacy of the drug primaquine in a murine infection model with Pneumocystis murina. Treatment with vitamin D enhances the number of phagocytes, decreases inflammatory cytokine production, and enhances cathelicidin/autophagy gene expression (65). These results show the beneficial effects of vitamin D in the enhancement of host defense and amelioration of excessive inflammation during treatment of Pneumocystis infection (65), although the regulation of cathelicidin in autophagy and inflammation was not addressed in this study. Given the low vitamin D levels observed in TB, HIV, and other infectious diseases (42,43), these findings provide new insights into potential approaches to developing vitamin D-based autophagy adjunctive therapy against various infections. However, it remains largely unknown which autophagic biomarker(s) might be useful in monitoring clinical stages and/or disease outcomes. Future studies are needed to identify useful autophagic status markers that are linked to clinical parameters and vitamin D levels in individual patients during infection.
Regulatory mechanisms involved in vitamin D-induced autophagy
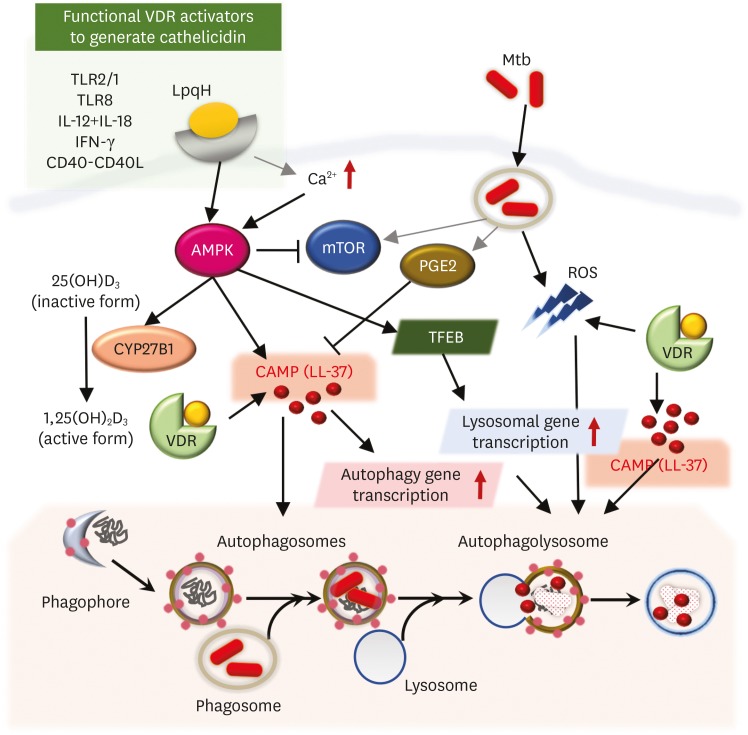
Vitamin D signaling regulates autophagy at different steps: initiation, elongation, maturation, and degradation (23). Mechanistically, vitamin D/VDR signaling activates autophagy through multiple pathways, including triggering intracellular calcium release/calcium-dependent kinases and inhibition of the mTOR, a negative regulator of autophagy (23,56). Notably, the lipoprotein LpqH, a mycobacterial TLR2/1 agonist, activates autophagy linked to functional VDR signaling and mediated by the adenosine monophosphate-activated protein kinase (AMPK) pathway (12). AMPK is an autophagy-activating kinase essential to various stages of the autophagy pathway and activation of lysosomal function through induction of transcription factor EB (66). The treatment of human macrophages with 4-PBA alone, or combined with vitamin D, has been shown to induce LL-37-mediated autophagy via the AMPK pathway to enhance the intracellular killing of Mtb (55). Since AMPK is a master regulator of anti-mycobacterial innate immune defense through multiple signaling pathways (67), the interplay between cathelicidin and AMPK signaling is likely to involve synergistic interaction through activation of several innate effector systems within phagocytic cells.
Redox status is crucial for the pathophysiology of numerous diseases, and strongly related to vitamin D-mediated signaling. For example, recent ex vivo analysis of redox potential in critically ill children showed that plasma LL-37 levels did not significantly differ from vitamin D levels; however, vitamin D sufficiency was closely associated with reduced oxidative stress in pediatric critical illness (68). Moreover, another epidemiological study found the relationship between serum 25D3 levels and plasma thiol/disulphide redox systems (69). The relevance of ROS functioning, as signaling molecules in autophagy regulation, has been suggested in the context of the vitamin D-cathelicidin axis during infection and inflammation; 1,25D3 leads to significant ROS production, which promotes autophagy (70) and antimicrobial activity (71). Indeed, TLR2-induced cathelicidin expression is mediated by ROS signaling in human monocytes/macrophages (72). Given the critical role played by ROS signals in autophagy and vice versa (73), future studies are needed to examine the function of the vitamin D-cathelicidin axis in redox homeostasis regulation and its ultimate link to autophagy activity.
Cathelicidin as a regulator of vitamin D-induced autophagy during infection
The role of the vitamin D-cathelicidin axis in antibacterial autophagy has been widely studied in mycobacterial infection. In human monocytes/macrophages, 1,25D3 treatment, through cathelicidin induction, robustly induces autophagosome formation, increases Mtb phagosomal maturation, and promotes antimicrobial responses against Mtb (11). Vitamin D treatment also enhances cathelicidin production and autophagic antimicrobial effects against Mycobacterium marinum, a pathogen causing skin lesions, in human THP-1 cells (74). Functional vitamin D signaling activation triggered by TLR2/1 leads to increased antimicrobial responses against Mtb infection through cathelicidin and autophagy activation (11-13,75). Vitamin D-mediated antimicrobial responses to coinfection by HIV and Mtb have been shown to be mediated by cathelicidin-dependent autophagy and enhancement of phagosomal maturation (32,33). Interestingly, TLR8 activation triggers gene expression for vitamin D signaling and human cathelicidin to the vitamin D-cathelicidin-mediated autophagy pathway, which is required for HIV restriction in human macrophages (14). A recent study showed that the combination of IL-12 and IL-18 enhances autophagy and anti-mycobacterial immune responses in human macrophages and pulmonary epithelial A549 cells. The protective host defense depends on the production of IFN-γ, VDR-derived antimicrobial peptide cathelicidin, and autophagy, but does not involve caspase-mediated apoptosis (15). Together, these findings suggest that numerous innate immune signals including TLR2/1, TLR8, and cytokines (IL-12+IL-18) are linked to a cell-autonomous defense mechanism, the CAMP/autophagy pathway, to promote antimicrobial action against Mtb in various human host cells.
Although it is not fully understood, the mechanisms by which cathelicidin regulates the induction of autophagy are mostly studied in mycobacterial infection, as summarized in Fig. 2. Vitamin D-induced autophagy activation is at least partly mediated by cathelicidin-mediated transcriptional activation of autophagy genes, including BECN1 and ATG5 (11). Similarly, a more recent study showed that the bioactive form of vitamin D enhanced the gene expression of ATG5, BECN1, and a mannose receptor in Mtb-infected monocytes/macrophages; these gene expression levels were lower in pulmonary TB patients (76). Importantly, a positive correlation has been reported between the expression of ATG5 and BECN1 and that of cathelicidin in monocytes/macrophages from healthy controls and TB patients (76). This result suggests that vitamin D-induced cathelicidin, as a secondary messenger, plays a role in transcriptional activation of autophagy genes, which are essential to all steps of the autophagy process (77). However, it remains an open question of how cathelicidin contributes to the transcriptional activation of autophagy genes in innate immune cells. LL-37 activates vitamin D and PBA-induced autophagy through an autocrine or paracrine pathway and that LL-37-mediated autophagy is associated with various intracellular signaling pathways, including the P2RX7 receptor, intracellular Ca2+ release, and the AMPK, and PI3K pathways (55). These findings suggest that cathelicidin-induced autophagy crosstalks with multiple intracellular signaling proteins and receptors, as well as other secondary messengers. Further studies are required to clarify the mechanism by which cathelicidin interacts with other transcription factors and/or regulates the signaling pathway, ultimately leading to the activation of autophagy at the transcriptional and post-translational levels.
Figure 2.
Regulatory mechanisms involved in vitamin D-induced autophagy. Vitamin D/VDR signaling activates autophagy through multiple pathways, including the triggering of intracellular calcium release/calcium-dependent kinases, the mTOR/AMPK pathway, ROS signaling, and cathelicidin. Cathelicidin, a key regulator of the vitamin D-autophagy pathway, is activated by functional VDR signaling downstream of TLR2/1, TLR8, CD40-CD40L, and cytokines, including IFN-γ and IL-12/IL-18. Mtb-derived PGE2 generation is known to inhibit LL-37 induction in human monocytes/macrophages.
TFEB, transcription factor EB.
To date, few mechanisms have been suggested for the regulation of vitamin D-cathelicidin signaling in host cells. A role for prostaglandin E2 (PGE2), a lipid mediator of inflammation and immunity, in vitamin D function has been identified (78). PGE2 triggers the cyclic AMP/protein kinase A pathway to induce inhibitory transcription factor signaling involving the E prostanoid (EP) 2 and EP4 receptors, thereby inhibiting cathelicidin expression and autophagy during Mtb infection. PGE2-mediated impairment of cathelicidin expression and autophagy inhibition promotes intracellular Mtb growth in human macrophages (78). Most studies have indicated a link between cathelicidin in vitamin D-induced autophagy and antimicrobial responses. However, one study reported that the vitamin D-cathelicidin axis does not trigger autophagy against HIV infection, although vitamin D-mediated induction of autophagy was found to be beneficial in the inhibition of HIV-1 replication (61). Thus, the involvement of cathelicidin in vitamin D-mediated autophagy may depend on the type of pathogen; future studies should clarify this issue in relation to a more extended range of status, cell types, pathogens, and diseases.
VITAMIN D-CATHELICIDIN SIGNALING IN THE REGULATION OF INFLAMMATION
The vitamin D-cathelicidin axis and inflammation activation
The balance between protective immunity and inflammation is vital to control intracellular bacterial growth while minimizing immune-mediated tissue damage. Numerous studies have reported anti-inflammatory effects of vitamin D in a variety of human diseases, including inflammatory bowel disease (IBD) (79), diabetes (80), atherosclerosis (81), and autoimmune diseases (82,83). However, the function of cathelicidin in the context of vitamin D signaling remains to be characterized in the regulation of inflammation, with some studies suggesting that cathelicidin promotes inflammation in other diseases (24,84). In this section, we focus on recent findings on the role of vitamin D-cathelicidin axis in the regulation of inflammatory responses during infection and inflammation (Fig. 3).
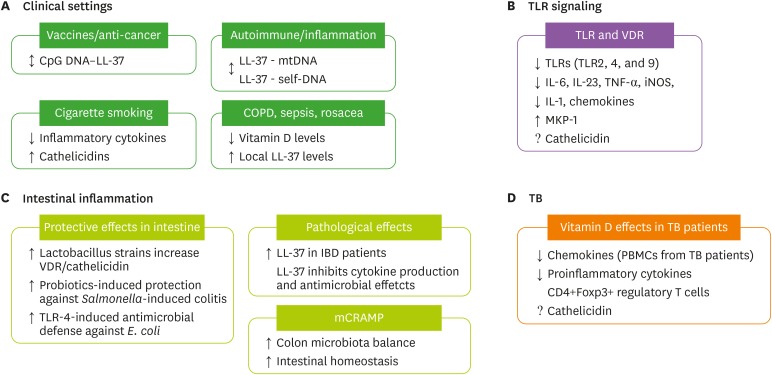
Figure 3.
Vitamin D-cathelicidin signaling in the regulation of inflammation. (A) In clinical settings, LL-37 and the DNA complex play dual roles as potential candidates as vaccines/anti-cancer agents and in pathologic inflammation and pathogenesis of various inflammatory diseases, including chronic obstructive pulmonary disease, sepsis, and rosacea (see text, section IV). In cigarette smoking models, vitamin D treatment inhibits inflammatory cytokine production and increases cathelicidin levels. (B) VDR signaling inhibits the expression of TLRs and proinflammatory cytokines/chemokines in monocytes/macrophages and can activate MKP-1 signaling to suppress inflammatory responses. (C) VDR-cathelicidin maintains intestinal homeostasis. Lactobacillus strains increase VDR protein and cathelicidin expression. Probiotic treatment confers protective effects against Salmonella-induced colitis. Cathelicidin also elevates TLR4 response and protein expression and promotes antimicrobial defenses against E. coli. Murine cathelicidin is involved in colon microbiota balance and protective effects during experimental colitis. (D) In TB patients, vitamin D inhibits chemokines and inflammatory cytokines and increases Treg abundance. Cathelicidin levels remain to be characterized in a clinical setting (↕: dual role; ↑: increase/induce; ↓: decrease/inhibit).
mtDNA, mitochondrial DNA.
Several studies have identified the potential mechanisms by which cathelicidin promotes inflammatory responses. Human cathelicidin LL-37 efficiently senses and delivers the CpG motifs of bacterial DNA to immune cells, regardless of its bactericidal properties (85), suggesting a potential avenue for the development of vaccines and anti-cancer therapies based on the ability of LL-37 to deliver CpG/TLR9 agonists. However, other studies suggested a pathogenic role for LL-37 in autoimmune and inflammatory diseases. In atherosclerotic plasma and plaques, the mtDNA-LL-37 complex is enhanced, not degraded, by DNase II, resulting in the activation of TLR9-induced inflammatory responses (86). Systemic lupus erythematosus patients have a complex of AMPs and self-DNA in their sera; these complexes can activate innate plasmacytoid dendritic cells (pDCs) through TLR9 (87). Combined with the strong implication of links between vitamin D signaling and both autoimmunity and cardiovascular diseases (81,88), future studies must clarify whether and how the LL-37—self-DNA immune complex can be degraded and resolved through vitamin D treatment. Another issue that may regulate pathogenesis is cell type-specific expression of pattern recognition receptors such as TLR9 and downstream immune functions for different cell types in the local environment, i.e., pDCs vs. inflammatory macrophages.
An immune alarm function for cathelicidin in the upregulation of proapoptosis and inflammasome activation during bacterial infection to enhance host protective immunity has been suggested (89,90). During Mtb infection, vitamin D functions in the general boosting of cytokine and chemokine levels, particularly via the induction of IL-1β, which controls the intracellular Mtb burden in macrophages through DEFB4/HBD2 production (36). The protective role of IL-1 in anti-mycobacterial responses has been reported in TB mouse models, suggesting a function for IL-1/IL-1R as a critical regulator of host defense (91). In patients with chronic obstructive pulmonary disease (COPD; n=215), high sputum hCAP18/LL-37 levels were associated with the risk of acute COPD exacerbation, suggesting that the local generation of hCAP18 is associated with airway inflammation (92). Sepsis patients have been reported to have lower vitamin D levels (93,94), but increased serum cathelicidin levels, which may be involved in the pathogenesis of systemic inflammation (93). Notably, vitamin D administration has been shown to reduce alveolar inflammation and cell damage in a sepsis model (94); however, this study did not address whether cathelicidin levels are modulated by vitamin D treatment. Several reports have indicated that high cathelicidin levels, but low vitamin D status, in chronic inflammatory diseases such as rosacea (95) represent the involvement of excessive cathelicidin in inflammatory disease pathogenesis. These reports suggest the involvement of an altered vitamin D-cathelicidin axis in the pathogenesis of a variety of acute and chronic inflammatory diseases.
In contrast, several studies have reported a role for inflammatory cytokines in vitamin D-mediated antibacterial activity. For example, TNF-α/IL-1β treatment of primary bronchial epithelial cells inhibited vitamin D-induced hCAP18/LL-37 expression and killing effects against H. influenzae; interestingly, TNF-α/IL-1β treatment led to increased expression of CYP24A1 (a vitamin D-degrading enzyme), suggesting that chronic inflammation results in the impairment of vitamin D-mediated protective responses (96). Thus, in pathological settings with altered regulation of the vitamin D-cathelicidin axis, cathelicidin initiates cell-type-specific inflammatory damage that results in inappropriate inflammation, which may, in turn, affect vitamin D-mediated innate effector responses.
The inhibitory role of vitamin D in inflammation
Since vitamin D is a well-known immunomodulatory agent, the inhibitory role of 1,25D3 in inflammation has been widely studied in innate immune responses. In TLR signaling, vitamin D can decrease the expression of various TLRs (TLR2, 4, and 9), and inhibit the production of proinflammatory cytokines, including IL-6, IL-23, TNF-α, inducible nitric oxide synthase, IL-1, and various T cell-recruiting chemokines in monocytes/macrophages (97). It has been demonstrated that vitamin D inhibits LPS-induced cytokine production through the activation of MAPK phosphatase-1 (MKP-1) signaling in monocytes and macrophages (98). The expression of a variety of chemokines has been shown to be downregulated by 1,25D3 in PBMCs from pulmonary TB patients (99). Recent studies have shown that 1,25D3 treatment of PBMCs from TB patients and healthy controls has an anti-inflammatory effect through the inhibition of proinflammatory cytokines and chemokines (100,101). Vitamin D3 treatment of PBMCs has also been shown to significantly upregulate CD4+Foxp3+ Tregs (101). However, the role of cathelicidin in vitamin D-induced inhibitory function in inflammatory responses remains unclear. In a previous study using a cigarette smoke model, vitamin D treatment was found to reduce inflammatory cytokine levels, but enhance cathelicidin expression (102), suggesting that vitamin D plays dual roles in airways, attenuating airway inflammation, and the promotion antibacterial host defense.
Importantly, vitamin D administration in human TB patients resulted in a rapid drop in inflammatory cytokine and chemokine generation during antibiotic treatment, suggesting a vitamin D-mediated resolution effect on hazardous inflammation (103). A recent meta-analysis showed that adjunctive vitamin D accelerates sputum culture conversion in multidrug-resistant TB patients however; it did not influence drug-sensitive TB (104). This finding suggests that vitamin D-adjunctive therapy can improve clinical outcomes by controlling harmful inflammation and tissue damage during infection or treatment. However, there is little evidence that cathelicidin has a general function in vitamin D-mediated inhibition of inflammation, except in intestinal inflammation, which is discussed in the next section.
Vitamin D and cathelicidin in the regulation of intestinal inflammation
A large body of evidence shows that VDR function is connected to cathelicidin in intestinal homeostasis, barrier function, and gut inflammation regulation. Studies using mouse IBD models have found that AMP production via VDR signaling is responsible for modifying the intestinal microbiota and enhancing bacterial clearance at barrier sites in addition to immune cells (17). For example, the treatment of intestinal epithelial cells with probiotic Lactobacillus rhamnosus strain GG and Lactobacillus plantarum resulted in increased VDR protein and cathelicidin expression. Probiotic treatment also confers a protective effect against Salmonella-induced colitis depending on VDR signaling and enhances the abundance of Paneth cells to increase AMPs (105). Regardless of VDR signaling, several studies have also shown beneficial roles for cathelicidin in the maintenance of intestinal homeostasis. In intestinal epithelial cells, cathelicidin amplifies TLR4 responses and protein expression and promotes epithelial antimicrobial defenses against Escherichia coli (106). Also, another recent study showed that LL-37 mRNA expression was significantly increased in mucosal tissues of subjects with ulcerative colitis and Crohn's disease and that LL-37 had antimicrobial effects interacting with LPS (107).
Murine CRAMP (mCRAMP) is induced by double-stranded RNA in intestinal epithelial cells, and intra-rectal administration of double-stranded RNA suppresses intestinal bacterial load and inflammation during Shigella infection through the induction of mCRAMP (108). Using CRAMP-deficient mouse models, it was recently reported that CRAMP plays an important role in the balance of colon microbiota and maintenance of intestinal mucosal homeostasis (109). However, it remains unknown whether and how vitamin D administration has any beneficial effect on cathelicidin-mediated protective responses against intestinal inflammation. Future studies are required to clarify the roles and mechanisms by which cathelicidin regulates host-pathogen interaction during intestinal homeostasis in the context of VDR signaling.
The vitamin D-cathelicidin axis: a key link between innate and adaptive immunity
Accumulating data suggest the function of vitamin D in adaptive immunity regulation in connection with innate immunity, in terms of mycobacterial infection. Fabri et al. (13) showed that IFN-γ-mediated antimicrobial responses in human macrophages were mediated by vitamin D, suggesting a role for vitamin D in acquired immunity against Mtb infection; notably, 25D3 treatment of human macrophages upregulated IFN-γ-induced antimicrobial responses, antimicrobial peptide expression, autophagy, and phagosome-lysosome fusion (13). Previous studies have also shown that pulmonary TB patients with vitamin D deficiency had depressed LL-37 levels in granulomatous lesions, but increased levels of IgG-secreting B cells and Tregs in TB lesions (110). Although these findings suggest that vitamin D-deficient TB patients have weak antibacterial responses as well as immunosuppression at disease sites (110), little is known about the molecular link between the vitamin D-cathelicidin pathway and Treg function in TB patients. A recent study showed that glucocorticoid-mediated cathelicidin induction is independent of the vitamin D signaling pathway, and failed to enhance antimicrobial responses against intracellular BCG mycobacteria. IFN-γ enhances, but glucocorticoid treatment decreases, lysosomal acidification of BCG via the expression of TCIRG1, a vacuolar H(+)-ATPase a3 subunit (111). Together, these findings indicate that cathelicidin production may be insufficient for host defense, requiring another key signaling of lysosomal acidification, presumably combined with IFN-γ, to augment anti-mycobacterial activity.
To support this hypothesis, a recent study showed that co-treatment with IFN-γ and IL-17A upregulated autophagy and autophagosome formation in primary monocytes from healthy controls and high-response TB patients; this effect was found to be related to anti-mycobacterial responses in human macrophages (112). However, a low-response group of TB patients exhibited a depressed autophagic response by monocytes in response to exogenous IL-17A (112). In contrast, cytokine IL-4 inhibited TLR2/1-induced, vitamin D-mediated antimicrobial responses in human monocytes (113). IL-4 alone, or combined with the TLR2/1 ligand, induced catabolism of 25D3 through vitamin D-24-hydroxylase gene expression (113). Future studies should explore the reasons for intrinsic autophagy response defects among certain populations of TB patients.
In addition to Mtb infection, IFN-γ treatment of human Langerhans cells, specialized dendritic cells in the skin, upregulates cathelicidin production and autophagy activation and promotes the fusion of M. leprae phagosomes with lysosomes. Importantly, the frequency of Langerhans cells that contain both cathelicidin and autophagic vesicles is higher in self-healing lesions than in progressive lesions. In addition, IFN-γ-induced autophagy promotes the ability of Langerhans cells to present Ags to T cells (114). A combination of CD40–CD40 ligand signaling and IFN-γ induced gene expression in CYP27b1-hydroxylase, which is essential for functional VDR signaling activation through the conversion of 25D3 to active 1,25D3, in human monocytes (115). The activation of functional VDR signaling upregulated cathelicidin and DEFB4 expression and activated autophagy in human monocytes (115). These findings suggest that vitamin D signaling contributes to a link between innate host defense and adaptive immune responses via T cell-mediated mechanisms involving CD40L and IFN-γ (13,115).
CLINICAL TRIALS OF VITAMIN D-CATHELICIDIN AXIS IN INFECTIOUS DISEASES
It is generally thought that inadequate levels of vitamin D are linked to an increased susceptibility to infectious diseases (2,116), yet clinical trials with to prevent or treat infectious diseases by vitamin D supplementation have yielded mixed results. So far, there have been many clinical studies of vitamin D supplements on human infectious diseases such as sepsis, viral infection, TB, pneumonia, peritonitis. Among the clinical studies with vitamin D treatment, several studies for patients with sepsis and HIV infection reported that the levels of cathelicidin correlate with the clinical responses and the cathelicidin levels after supplementing of vitamin D (117-119). In addition, several other studies on TB, bronchiectasis, and acute respiratory infection reported the serum cathelicidin levels for monitoring anti-infectious effects after supplementation of vitamin D (120-122). It was noted that vitamin D significantly increased the serum cathelicidin levels, which correlate with the improved clinical outcome of infectious diseases (121,122). These data provide strong evidence that vitamin D supplementation contributes to beneficial roles in human infectious diseases through the regulation of cathelicidin production. Studies showed that vitamin D supplementation in children with vitamin D deficiency markedly decreased the incidences of influenza A and acute respiratory tract infection (123,124). The most beneficial effect was observed in individuals with the lowest serum 25D3 levels, suggesting the need of new studies focused in this clinical group.
TB is the most widely studied infectious disease associated with vitamin D treatment; however, there are still debates in the effectiveness of vitamin D therapy. In patients with low baseline serum vitamin D levels, supplementation of high doses of vitamin D accelerated clinical and radiologic improvements as well as the increased immune activation (125,126). These studies indicate that the measurement of vitamin D and cathelicidin levels may be helpful for screening and selection of appropriate patients for vitamin D-based, host-directed therapy against TB. Decreased vitamin D levels are often associated with increased risk of TB in HIV-positive patients (127), suggesting that vitamin D may play an important role in the case of immune-compromised state such as HIV co-infection. Vitamin D levels have been shown to be depressed in bronchoalveolar fluids from HIV-positive individuals (128). Combined with the finding that vitamin D treatment restores innate response in HIV-positive macrophages impaired by Mtb infection (128), vitamin D supplementation may help to restore defective innate effector functions at the physiological level. These findings support the potential application of vitamin D as adjunctive therapy for TB infection in HIV-positive patients through boosting antimicrobial peptide cathelicidin LL-37 production.
Table 2 summarizes the clinical trials of vitamin D supplementation with or without measuring cathelicidin levels in patients with several infectious diseases, including TB, sepsis, respiratory infections, etc. In some studies, detailed results/outcomes are still pending. Depending on the disease categories and patient criteria, vitamin D treatment can be either beneficial or non-efficacious. Nevertheless, the arsenal of vitamin D-based therapy could potentially offer favorable immunomodulation as host-directed therapy for various infectious diseases, particularly TB. Additionally, vitamin D response during infection remains to be monitored and interpreted in terms of the levels of antimicrobial peptide cathelicidin, which may predict the response of vitamin D treatment. Therefore, innovative clinical trials should be proposed or designed with regard to the subgroup selection of patients and functional monitoring of immune and autophagic systems based on cathelicidin levels.
Table 2. Clinical studies of vitamin D-cathelicidin in infectious disease.
| Study name | Study type | Outcomes measurement & Results | Interventions | Clinicaltrials.gov identifier (references) | |
|---|---|---|---|---|---|
| Sepsis & infectious disease | |||||
| Vitamin D in ventilated ICU patients* | Interventional (phase 2) | Concentration of plasma 25(OH)D and LL-37 level | Dietary supplement: vitamin D | NCT01372995 | |
| Cholecalciferol supplementation for sepsis in the ICU | Interventional | Change in vitamin D Status and immunological profiles | Dietary supplement: vitamin D | NCT01896544 | |
| Effects of vitamin D and omega-3 fatty acids on infectious diseases and hCAP18* | Interventional (recruiting) | hCAP18 (LL-37) level | Vitamin D and omega-3 fatty acids | NCT01758081 | |
| Effect of high dose vitamin D3 in smokers and non-smokers with and without HIV* | Interventional (recruiting) | Difference in vitamin and peptide LL-37 levels | Dietary supplement: vitamin D | NCT03270709 | |
| Effects of smoking and vitamin D3 on the levels of human cathelicidin peptide LL-37* | Observational | Serum D3 vitamin and LL-37 levels | Cross-sectional observation | NCT03923218 | |
| Randomized trial of vitamin D supplement to prevent influenza A | Interventional | Vitamin D supplementation may reduce the incidence of influenza A | Dietary supplement: vitamin D | UMIN000001373 (124) | |
| Randomized trial of vitamin D supplement and risk of acute respiratory infection | Interventional | Vitamin D supplementation reduced acute respiratory infection among children with vitamin D deficiency | Dietary supplement: vitamin D | NCT00886379 (123) | |
| TB | |||||
| Clinical trial of PBA and vitamin D in TB* | Interventional (phase 2) | PBA and vitamin D promoted immunomodulation to improve TB treatment outcomes | Oral sodium Phenylbutyrate, cholecalciferol | NCT01580007 (120,122) | |
| PBA and vitamin D induced intracellular killing of MTB by macrophages with the elevation of LL-37 | |||||
| Vitamin D3 and the association with cathelicidin expression in patients with active TB* | Observational | Severe forms of intrathoracic TB may be associated with lower vitamin D3 status and lower of LL-37 | Cross-sectional observation | (45) | |
| The impact of vitamin D on TB among Koreans | Observational | Vitamin D deficiency is significantly prevalent in TB patients compared to people without TB | Cohort study | NCT01137370 | |
| Role of vitamin D in innate immunity to TB | Interventional | Vitamin D supplementation had significant favorable effects on serum 25(OH)D concentrations | Dietary supplement: vitamin D | NCT01244204 (130) | |
| Trial of adjunctive vitamin D in TB treatment | Interventional (phase 3) | Administration of vitamin D increased serum 25(OH)D in TB patients. | Adjunctive vitamin D | NCT00419068 (131) | |
| Replacement of vitamin D in patients with active TB | Interventional | Cytokine response | Intramuscular injection of cholecalciferol | NCT01130311 | |
| A study the effect of vitamin D to conventional treatment in new pulmonary TB patients | Interventional | Vitamin D supplementation did not reduce time to sputum culture conversion. | Supplemental high-dose oral vitamin D | NCT00366470 (132) | |
| Pneumonia & peritonitis | |||||
| Study of vitamin D for the prevention of acute respiratory infections in children* | Interventional | Serum of cathelicidin, 25(OD)-D levels | Dietary supplement: vitamin D | NCT02046577 | |
| Vitamin D3 supplementation in adults with bronchiectasis* | Interventional | Serum of cathelicidin, 25(OD)-D levels | Dietary supplement: vitamin D | ACTRN12607000641493 (121) | |
| Vitamin D supplementation prevent early pneumonia | Interventional | Prevalence of pneumonia, all-cause mortality | Dietary supplement: vitamin D | NCT00877422 | |
| Vitamin D supplementation and respiratory index of severity in children in pneumonia | Interventional (phase 4) | Respiratory index of severity in children | Supplemental cholecalciferol | NCT02936895 | |
| The effect of vitamin D supplement on the prevention of peritoneal dialysis-related peritonitis | Interventional | Change in serum 25(OH)-vitamin D level | Supplemental cholecalciferol | NCT03264625 | |
Further details for trial with NCT numbers can be accessed at http://clinicaltrials.gov.
ICU, intensive care unit; NCT, national clinical trial; UMIN, University hospital Medical Information Network; ACTRN, Australian New Zealand Clinical Trials Registry.
*Clinical studies that measured the level of LL-37 in patients' samples.
CONCLUSION
In this review, we highlight the roles of vitamin D-cathelicidin signaling in cell-autonomous protection, autophagy regulation, and immune response modulation in host cells against various pathogen infections and inflammatory diseases. Functional VDR signaling activation, which is associated with cathelicidin induction, is clearly the best-studied area in the field of vitamin D-mediated antimicrobial responses to eradicate a broad spectrum of pathogens. Cathelicidin is involved in shaping our immune system to promote cell-autonomous defense mechanisms, simultaneously maintaining a balance with inflammation during infection. Recent studies have revealed key roles for vitamin D-induced cathelicidin in the maintenance of homeostasis as an autophagy process that is closely regulated by other intracellular signaling pathways, including calcium and AMPK. Indeed, vitamin D-cathelicidin interacts with other effector systems such as autophagy and lysosomal function, possibly converging into a dedicated protective role by preventing excessive immune pathology against a variety of infections. Nevertheless, several aspects in this field are only beginning to be understood; many open questions remain, including how the vitamin D-cathelicidin axis interconnects with key innate effector systems (e.g., autophagy and lysosomal acidification) and which factors are critically involved in the coordinated control of innate and adaptive immunity to promote protective immune responses during various infections. Clearly, further research is needed to manipulate vitamin D-cathelicidin signaling in different biological contexts. Topics for further study include the exact function of vitamin D-antimicrobial immunity in patients with various infectious diseases at different clinical stages, considering genetic variation in VDR genes and sera vitamin D levels.
Despite continuing efforts to develop new antibiotics, multidrug-resistant infections are emerging as a major health burden worldwide, with high associated morbidity and mortality. The activation of autophagy by VDR signaling is anticipated as a promising host-directed therapeutic strategy, even against drug-resistant bacterial strains (129). Understanding the mechanisms by which vitamin D-cathelicidin regulates innate host defense systems, in the context of autophagy and immune pathways, is of prime importance for exploring strong candidates for host-directed therapeutics against diverse infectious diseases. Vitamin D is strongly clinically associated with acute respiratory tract infections, parallel to vitamin D status and incidences of airway infections (16). Current findings provide promising evidence for the protective effects of vitamin D on respiratory tract infections, including TB (16). To date, vitamin D supplemental therapy has been the most widely used potential treatment in TB clinical trials. However, further evidence of the cathelicidin levels and its efficacy as a prerequisite before vitamin D supplementation move into clinical routine in infectious diseases. Further translational knowledge about vitamin D-cathelicidin axis should be accumulated to support the clinical application of vitamin D in various acute and chronic infectious diseases for prevention and/or to offer improved outcomes.
ACKNOWLEDGEMENTS
We are indebted to current and past members of our laboratory for discussions and investigations that contributed to this article. This work was supported by the research fund of Chungnam National University. I apologize to colleagues whose work and publications could not be referenced owing to space constraints.
Abbreviations
- 1,25D3
1,25 dihydroxy vitamin D3
- 25D3
25-hydroxyvitamin D3
- AMP
antimicrobial protein
- AMPK
adenosine monophosphate-activated protein kinase
- ATG5
autophagy related 5
- BECN1
beclin-1
- CAMP
cathelicidin AMP
- CRAMP
cathelicidin-related antimicrobial peptide
- EP
E prostanoid
- HBD2
human β-defensin 2
- hCAP-18
human cationic AMP 18
- IBD
inflammatory bowel disease
- mCRAMP
murine CRAMP
- MKP-1
MAPK phosphatase-1
- Mtb
Mycobacterium tuberculosis
- PBA
phenylbutyrate
- pDC
plasmacytoid dendritic cell
- PGE2
prostaglandin E2
- ROS
reactive oxygen species
- TB
tuberculosis
- UV
ultraviolet light
- VDR
vitamin D receptor
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Chung C, Jo EK.
- Investigation: Jo EK, Chung C.
- Supervision: Jo EK, Modlin RL.
- Validation: Jo EK, Chung C, Silwal P, Kim I.
- Writing - original draft: Chung C, Kim I, Jo EK.
- Writing - review & editing: Jo EK, Chung C, Silwal P, Kim I, Modlin RL.
References
- 1.Watkins RR, Lemonovich TL, Salata RA. An update on the association of vitamin D deficiency with common infectious diseases. Can J Physiol Pharmacol. 2015;93:363–368. doi: 10.1139/cjpp-2014-0352. [DOI] [PubMed] [Google Scholar]
- 2.Korf H, Decallonne B, Mathieu C. Vitamin D for infections. Curr Opin Endocrinol Diabetes Obes. 2014;21:431–436. doi: 10.1097/MED.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 3.Shin DM, Jo EK. Antimicrobial peptides in innate immunity against mycobacteria. Immune Netw. 2011;11:245–252. doi: 10.4110/in.2011.11.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afacan NJ, Yeung AT, Pena OM, Hancock REW. Therapeutic potential of host defense peptides in antibiotic-resistant infections. Curr Pharm Des. 2012;18:807–819. doi: 10.2174/138161212799277617. [DOI] [PubMed] [Google Scholar]
- 5.Hasan M, Yamazaki M. Elementary processes and mechanisms of interactions of antimicrobial peptides with membranes-single giant unilamellar vesicle studies. Adv Exp Med Biol. 2019;1117:17–32. doi: 10.1007/978-981-13-3588-4_3. [DOI] [PubMed] [Google Scholar]
- 6.Mandal SM, Manna S, Mondal S, Ghosh AK, Chakraborty R. Transcriptional regulation of human defense peptides: a new direction in infection control. Biol Chem. 2018;399:1277–1284. doi: 10.1515/hsz-2018-0182. [DOI] [PubMed] [Google Scholar]
- 7.Dimitrov V, White JH. Species-specific regulation of innate immunity by vitamin D signaling. J Steroid Biochem Mol Biol. 2016;164:246–253. doi: 10.1016/j.jsbmb.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 9.Vandamme D, Landuyt B, Luyten W, Schoofs L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol. 2012;280:22–35. doi: 10.1016/j.cellimm.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Shin DM, Yuk JM, Lee HM, Lee SH, Son JW, Harding CV, Kim JM, Modlin RL, Jo EK. Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signalling. Cell Microbiol. 2010;12:1648–1665. doi: 10.1111/j.1462-5822.2010.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, Lee HM, Krutzik SR, Schenk M, Sieling PA, et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3:104ra102. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell GR, Spector SA. Toll-like receptor 8 ligands activate a vitamin D mediated autophagic response that inhibits human immunodeficiency virus type 1. PLoS Pathog. 2012;8:e1003017. doi: 10.1371/journal.ppat.1003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang R, Yang E, Shen L, Modlin RL, Shen H, Chen ZW. IL-12+IL-18 cosignaling in human macrophages and lung epithelial cells activates cathelicidin and autophagy, inhibiting intracellular mycobacterial growth. J Immunol. 2018;200:2405–2417. doi: 10.4049/jimmunol.1701073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zittermann A, Pilz S, Hoffmann H, März W. Vitamin D and airway infections: a European perspective. Eur J Med Res. 2016;21:14. doi: 10.1186/s40001-016-0208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark A, Mach N. Role of vitamin D in the hygiene hypothesis: the interplay between vitamin D, vitamin D receptors, gut microbiota, and immune response. Front Immunol. 2016;7:627. doi: 10.3389/fimmu.2016.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan D, Coughlin LA, Neubauer MM, Kim J, Kim MS, Zhan X, Simms-Waldrip TR, Xie Y, Hooper LV, Koh AY. Activation of HIF-1α and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat Med. 2015;21:808–814. doi: 10.1038/nm.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 20.Svensson D, Nebel D, Voss U, Ekblad E, Nilsson BO. Vitamin D-induced up-regulation of human keratinocyte cathelicidin anti-microbial peptide expression involves retinoid X receptor α. Cell Tissue Res. 2016;366:353–362. doi: 10.1007/s00441-016-2449-z. [DOI] [PubMed] [Google Scholar]
- 21.Schrumpf JA, van Sterkenburg MA, Verhoosel RM, Zuyderduyn S, Hiemstra PS. Interleukin 13 exposure enhances vitamin D-mediated expression of the human cathelicidin antimicrobial peptide 18/LL-37 in bronchial epithelial cells. Infect Immun. 2012;80:4485–4494. doi: 10.1128/IAI.06224-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 23.Wu S, Sun J. Vitamin D, vitamin D receptor, and macroautophagy in inflammation and infection. Discov Med. 2011;11:325–335. [PMC free article] [PubMed] [Google Scholar]
- 24.Kahlenberg JM, Kaplan MJ. Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J Immunol. 2013;191:4895–4901. doi: 10.4049/jimmunol.1302005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fabisiak A, Murawska N, Fichna J. LL-37: cathelicidin-related antimicrobial peptide with pleiotropic activity. Pharmacol Rep. 2016;68:802–808. doi: 10.1016/j.pharep.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Dürr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 27.Eissa A, Amodeo V, Smith CR, Diamandis EP. Kallikrein-related peptidase-8 (KLK8) is an active serine protease in human epidermis and sweat and is involved in a skin barrier proteolytic cascade. J Biol Chem. 2011;286:687–706. doi: 10.1074/jbc.M110.125310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CC, Sun Y, Qian S, Huang HW. Transmembrane pores formed by human antimicrobial peptide LL-37. Biophys J. 2011;100:1688–1696. doi: 10.1016/j.bpj.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Y, Mohapatra S, Weisshaar JC. Rigidification of the Escherichia coli cytoplasm by the human antimicrobial peptide LL-37 revealed by superresolution fluorescence microscopy. Proc Natl Acad Sci U S A. 2019;116:1017–1026. doi: 10.1073/pnas.1814924116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang G, Narayana JL, Mishra B, Zhang Y, Wang F, Wang C, Zarena D, Lushnikova T, Wang X. Design of antimicrobial peptides: progress made with human cathelicidin LL-37. Adv Exp Med Biol. 2019;1117:215–240. doi: 10.1007/978-981-13-3588-4_12. [DOI] [PubMed] [Google Scholar]
- 31.Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K, Davidson RN, Sørensen OE, Kampmann B, Griffiths CJ, et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178:7190–7198. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 32.Campbell GR, Spector SA. Autophagy induction by vitamin D inhibits both Mycobacterium tuberculosis and human immunodeficiency virus type 1. Autophagy. 2012;8:1523–1525. doi: 10.4161/auto.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell GR, Spector SA. Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS Pathog. 2012;8:e1002689. doi: 10.1371/journal.ppat.1002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim EW, Teles RM, Haile S, Liu PT, Modlin RL. Vitamin D status contributes to the antimicrobial activity of macrophages against Mycobacterium leprae . PLoS Negl Trop Dis. 2018;12:e0006608. doi: 10.1371/journal.pntd.0006608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saleh M, Welsch C, Cai C, Döring C, Gouttenoire J, Friedrich J, Haselow K, Sarrazin C, Badenhoop K, Moradpour D, et al. Differential modulation of hepatitis C virus replication and innate immune pathways by synthetic calcitriol-analogs. J Steroid Biochem Mol Biol. 2018;183:142–151. doi: 10.1016/j.jsbmb.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Verway M, Bouttier M, Wang TT, Carrier M, Calderon M, An BS, Devemy E, McIntosh F, Divangahi M, Behr MA, et al. Vitamin D induces interleukin-1β expression: paracrine macrophage epithelial signaling controls M. tuberculosis infection. PLoS Pathog. 2013;9:e1003407. doi: 10.1371/journal.ppat.1003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang X, Basavarajappa D, Haeggström JZ, Wan M. P2X7 receptor regulates internalization of antimicrobial peptide LL-37 by human macrophages that promotes intracellular pathogen clearance. J Immunol. 2015;195:1191–1201. doi: 10.4049/jimmunol.1402845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sørensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS, Borregaard N. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–3959. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- 39.Smyth D, Cameron A, Davies MR, McNeilly C, Hafner L, Sriprakash KS, McMillan DJ. DrsG from Streptococcus dysgalactiae subsp. equisimilis inhibits the antimicrobial peptide LL-37. Infect Immun. 2014;82:2337–2344. doi: 10.1128/IAI.01411-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Østerhus S, Johnsen IB. Human metapneumovirus infection inhibits cathelicidin antimicrobial peptide expression in human macrophages. Front Immunol. 2018;9:902. doi: 10.3389/fimmu.2018.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woods EC, Edwards AN, Childress KO, Jones JB, McBride SM. The C. difficile clnRAB operon initiates adaptations to the host environment in response to LL-37. PLoS Pathog. 2018;14:e1007153. doi: 10.1371/journal.ppat.1007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talat N, Perry S, Parsonnet J, Dawood G, Hussain R. Vitamin D deficiency and tuberculosis progression. Emerg Infect Dis. 2010;16:853–855. doi: 10.3201/eid1605.091693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dao CN, Patel P, Overton ET, Rhame F, Pals SL, Johnson C, Bush T, Brooks JT Study to Understand the Natural History of HIV and AIDS in the Era of Effective Therapy (SUN) Investigators. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis. 2011;52:396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- 44.Bishwal SC, Das MK, Badireddy VK, Dabral D, Das A, Mahapatra AR, Sahu S, Malakar D, Singh II, Mazumdar H, et al. Sputum proteomics reveals a shift in vitamin D-binding protein and antimicrobial protein axis in tuberculosis patients. Sci Rep. 2019;9:1036. doi: 10.1038/s41598-018-37662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ashenafi S, Mazurek J, Rehn A, Lemma B, Aderaye G, Bekele A, Assefa G, Chanyalew M, Aseffa A, Andersson J, et al. Vitamin D3 status and the association with human cathelicidin expression in patients with different clinical forms of active tuberculosis. Nutrients. 2018;10:721. doi: 10.3390/nu10060721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujita K, Ito Y, Oguma T, Mio T, Niimi A, Hirai T. Association between Mycobacterium avium complex lung disease and serum vitamin D status, antimicrobial peptide levels, and bone mineral density. Medicine (Baltimore) 2018;97:e12463. doi: 10.1097/MD.0000000000012463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Georgieva V, Kamolvit W, Herthelius M, Lüthje P, Brauner A, Chromek M. Association between vitamin D, antimicrobial peptides and urinary tract infection in infants and young children. Acta Paediatr. 2019;108:551–556. doi: 10.1111/apa.14499. [DOI] [PubMed] [Google Scholar]
- 48.Thursfield RM, Naderi K, Leaver N, Rosenthal M, Alton EW, Bush A, Davies JC. Children with cystic fibrosis demonstrate no respiratory immunological, infective or physiological, consequences of vitamin D deficiency. J Cyst Fibros. 2018;17:657–665. doi: 10.1016/j.jcf.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto K, Takahashi K, Ato M, Iwanaga S, Ohta N. Antimalarial activity of vitamin D3 (VD3) does not result from VD3-induced antimicrobial agents including nitric oxide or cathelicidin. Exp Parasitol. 2019;201:67–77. doi: 10.1016/j.exppara.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto K, Iwagami M, Seki T, Kano S, Ota N, Ato M. Dual antiplasmodial activity of vitamin D3 and its analog, 22-oxacalcitriol, by direct and indirect mechanisms. Parasitol Int. 2017;66:89–99. doi: 10.1016/j.parint.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 51.Goncalves-Mendes N, Talvas J, Dualé C, Guttmann A, Corbin V, Marceau G, Sapin V, Brachet P, Evrard B, Laurichesse H, et al. Impact of vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: a randomized placebo-controlled trial. Front Immunol. 2019;10:65. doi: 10.3389/fimmu.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serré J, Mathyssen C, Ajime TT, Korf H, Maes K, Heulens N, Gysemans C, Mathieu C, Vanaudenaerde B, Janssens W, et al. Airway infection with nontypeable Haemophilus influenzae is more rapidly eradicated in vitamin D deficient mice. J Steroid Biochem Mol Biol. 2019;187:42–51. doi: 10.1016/j.jsbmb.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 53.Deretic V, Levine B. Autophagy balances inflammation in innate immunity. Autophagy. 2018;14:243–251. doi: 10.1080/15548627.2017.1402992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu W, Zhang L, Li MX, Shen J, Liu XD, Xiao ZG, Wu DL, Ho IH, Wu JC, Cheung CK, et al. Vitamin D3 activates the autolysosomal degradation function against Helicobacter pylori through the PDIA3 receptor in gastric epithelial cells. Autophagy. 2019;15:707–725. doi: 10.1080/15548627.2018.1557835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rekha RS, Rao Muvva SS, Wan M, Raqib R, Bergman P, Brighenti S, Gudmundsson GH, Agerberth B. Phenylbutyrate induces LL-37-dependent autophagy and intracellular killing of Mycobacterium tuberculosis in human macrophages. Autophagy. 2015;11:1688–1699. doi: 10.1080/15548627.2015.1075110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu G, Wang J, Gao GF, Liu CH. Insights into battles between Mycobacterium tuberculosis and macrophages. Protein Cell. 2014;5:728–736. doi: 10.1007/s13238-014-0077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jo EK. Innate immunity to mycobacteria: vitamin D and autophagy. Cell Microbiol. 2010;12:1026–1035. doi: 10.1111/j.1462-5822.2010.01491.x. [DOI] [PubMed] [Google Scholar]
- 58.Estrella JL, Kan-Sutton C, Gong X, Rajagopalan M, Lewis DE, Hunter RL, Eissa NT, Jagannath C. A novel in vitro human macrophage model to study the persistence of Mycobacterium tuberculosis using vitamin D(3) and retinoic acid activated THP-1 macrophages. Front Microbiol. 2011;2:67. doi: 10.3389/fmicb.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang FC. Vitamin D differentially regulates Salmonella-induced intestine epithelial autophagy and interleukin-1β expression. World J Gastroenterol. 2016;22:10353–10363. doi: 10.3748/wjg.v22.i47.10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dai J, Liang Y, Li H, Zhou W, Wang B, Gong A, Zhang R. Vitamin D enhances resistance to Aspergillus fumigatus in mice via inhibition of excessive autophagy. Am J Transl Res. 2018;10:381–391. [PMC free article] [PubMed] [Google Scholar]
- 61.Campbell GR, Spector SA. Hormonally active vitamin D3 (1alpha,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. J Biol Chem. 2011;286:18890–18902. doi: 10.1074/jbc.M110.206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abdel-Mohsen MA, El-Braky AA, Ghazal AA, Shamseya MM. Autophagy, apoptosis, vitamin D, and vitamin D receptor in hepatocellular carcinoma associated with hepatitis C virus. Medicine (Baltimore) 2018;97:e0172. doi: 10.1097/MD.0000000000010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tian G, Liang X, Chen D, Mao X, Yu J, Zheng P, He J, Huang Z, Yu B. Vitamin D3 supplementation alleviates rotavirus infection in pigs and IPEC-J2 cells via regulating the autophagy signaling pathway. J Steroid Biochem Mol Biol. 2016;163:157–163. doi: 10.1016/j.jsbmb.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 64.Choi Y, Bowman JW, Jung JU. Autophagy during viral infection - a double-edged sword. Nat Rev Microbiol. 2018;16:341–354. doi: 10.1038/s41579-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lei GS, Zhang C, Cheng BH, Lee CH. Mechanisms of action of vitamin D as supplemental therapy for Pneumocystis pneumonia. Antimicrob Agents Chemother. 2017;61:e01226-17. doi: 10.1128/AAC.01226-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hipolito VE, Ospina-Escobar E, Botelho RJ. Lysosome remodelling and adaptation during phagocyte activation. Cell Microbiol. 2018;20:e12824. doi: 10.1111/cmi.12824. [DOI] [PubMed] [Google Scholar]
- 67.Jo EK, Silwal P, Yuk JM. AMPK-targeted effector networks in mycobacterial infection. Front Microbiol. 2019;10:520. doi: 10.3389/fmicb.2019.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alvarez JA, Grunwell JR, Gillespie SE, Tangpricha V, Hebbar KB. Vitamin D deficiency is associated with an oxidized plasma cysteine redox potential in critically Ill children. J Steroid Biochem Mol Biol. 2018;175:164–169. doi: 10.1016/j.jsbmb.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alvarez JA, Chowdhury R, Jones DP, Martin GS, Brigham KL, Binongo JN, Ziegler TR, Tangpricha V. Vitamin D status is independently associated with plasma glutathione and cysteine thiol/disulphide redox status in adults. Clin Endocrinol (Oxf) 2014;81:458–466. doi: 10.1111/cen.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sly LM, Lopez M, Nauseef WM, Reiner NE. 1alpha,25-dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J Biol Chem. 2001;276:35482–35493. doi: 10.1074/jbc.M102876200. [DOI] [PubMed] [Google Scholar]
- 72.Yang CS, Shin DM, Kim KH, Lee ZW, Lee CH, Park SG, Bae YS, Jo EK. NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J Immunol. 2009;182:3696–3705. doi: 10.4049/jimmunol.0802217. [DOI] [PubMed] [Google Scholar]
- 73.Yan Y, Finkel T. Autophagy as a regulator of cardiovascular redox homeostasis. Free Radic Biol Med. 2017;109:108–113. doi: 10.1016/j.freeradbiomed.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sato E, Imafuku S, Ishii K, Itoh R, Chou B, Soejima T, Nakayama J, Hiromatsu K. Vitamin D-dependent cathelicidin inhibits Mycobacterium marinum infection in human monocytic cells. J Dermatol Sci. 2013;70:166–172. doi: 10.1016/j.jdermsci.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 75.Chun RF, Liu PT, Modlin RL, Adams JS, Hewison M. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Front Physiol. 2014;5:151. doi: 10.3389/fphys.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Afsal K, Selvaraj P. Effect of 1,25-dihydroxyvitamin D3 on the expression of mannose receptor, DC-SIGN and autophagy genes in pulmonary tuberculosis. Tuberculosis (Edinb) 2016;99:1–10. doi: 10.1016/j.tube.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 77.Jiang P, Mizushima N. Autophagy and human diseases. Cell Res. 2014;24:69–79. doi: 10.1038/cr.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wan M, Tang X, Rekha RS, Muvva SS, Brighenti S, Agerberth B, Haeggström JZ. Prostaglandin E2 suppresses hCAP18/LL-37 expression in human macrophages via EP2/EP4: implications for treatment of Mycobacterium tuberculosis infection. FASEB J. 2018;32:2827–2840. doi: 10.1096/fj.201701308. [DOI] [PubMed] [Google Scholar]
- 79.Gubatan J, Moss AC. Vitamin D in inflammatory bowel disease: more than just a supplement. Curr Opin Gastroenterol. 2018;34:217–225. doi: 10.1097/MOG.0000000000000449. [DOI] [PubMed] [Google Scholar]
- 80.Berridge MJ. Vitamin D deficiency and diabetes. Biochem J. 2017;474:1321–1332. doi: 10.1042/BCJ20170042. [DOI] [PubMed] [Google Scholar]
- 81.Bennett AL, Lavie CJ. Vitamin D metabolism and the implications for atherosclerosis. Adv Exp Med Biol. 2017;996:185–192. doi: 10.1007/978-3-319-56017-5_15. [DOI] [PubMed] [Google Scholar]
- 82.Czaja AJ, Montano-Loza AJ. Evolving role of vitamin D in immune-mediated disease and its implications in autoimmune hepatitis. Dig Dis Sci. 2019;64:324–344. doi: 10.1007/s10620-018-5351-6. [DOI] [PubMed] [Google Scholar]
- 83.Lu M, Taylor BV, Körner H. Genomic effects of the vitamin D receptor: potentially the link between vitamin D, immune cells, and multiple sclerosis. Front Immunol. 2018;9:477. doi: 10.3389/fimmu.2018.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takahashi T, Kulkarni NN, Lee EY, Zhang LJ, Wong GC, Gallo RL. Cathelicidin promotes inflammation by enabling binding of self-RNA to cell surface scavenger receptors. Sci Rep. 2018;8:4032. doi: 10.1038/s41598-018-22409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hurtado P, Peh CA. LL-37 promotes rapid sensing of CpG oligodeoxynucleotides by B lymphocytes and plasmacytoid dendritic cells. J Immunol. 2010;184:1425–1435. doi: 10.4049/jimmunol.0902305. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Z, Meng P, Han Y, Shen C, Li B, Hakim MA, Zhang X, Lu Q, Rong M, Lai R. Mitochondrial DNA-LL-37 complex promotes atherosclerosis by escaping from autophagic recognition. Immunity. 2015;43:1137–1147. doi: 10.1016/j.immuni.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 87.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang CY, Leung PS, Adamopoulos IE, Gershwin ME. The implication of vitamin D and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol. 2013;45:217–226. doi: 10.1007/s12016-013-8361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McHugh BJ, Wang R, Li HN, Beaumont PE, Kells R, Stevens H, Young L, Rossi AG, Gray RD, Dorin JR, et al. Cathelicidin is a “fire alarm”, generating protective NLRP3-dependent airway epithelial cell inflammatory responses during infection with Pseudomonas aeruginosa . PLoS Pathog. 2019;15:e1007694. doi: 10.1371/journal.ppat.1007694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barlow PG, Beaumont PE, Cosseau C, Mackellar A, Wilkinson TS, Hancock RE, Haslett C, Govan JR, Simpson AJ, Davidson DJ. The human cathelicidin LL-37 preferentially promotes apoptosis of infected airway epithelium. Am J Respir Cell Mol Biol. 2010;43:692–702. doi: 10.1165/rcmb.2009-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, Jacobs M, Ryffel B, Quesniaux VF. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J Immunol. 2007;179:1178–1189. doi: 10.4049/jimmunol.179.2.1178. [DOI] [PubMed] [Google Scholar]
- 92.Persson LJ, Aanerud M, Hardie JA, Miodini Nilsen R, Bakke PS, Eagan TM, Hiemstra PS. Antimicrobial peptide levels are linked to airway inflammation, bacterial colonisation and exacerbations in chronic obstructive pulmonary disease. Eur Respir J. 2017;49:1601328. doi: 10.1183/13993003.01328-2016. [DOI] [PubMed] [Google Scholar]
- 93.Greulich T, Regner W, Branscheidt M, Herr C, Koczulla AR, Vogelmeier CF, Bals R. Altered blood levels of vitamin D, cathelicidin and parathyroid hormone in patients with sepsis-a pilot study. Anaesth Intensive Care. 2017;45:36–45. doi: 10.1177/0310057X1704500106. [DOI] [PubMed] [Google Scholar]
- 94.Parekh D, Patel JM, Scott A, Lax S, Dancer RC, D'Souza V, Greenwood H, Fraser WD, Gao F, Sapey E, et al. Vitamin D deficiency in human and murine sepsis. Crit Care Med. 2017;45:282–289. doi: 10.1097/CCM.0000000000002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park BW, Ha JM, Cho EB, Jin JK, Park EJ, Park HR, Kang HJ, Ko SH, Kim KH, Kim KJ. A study on vitamin D and cathelicidin status in patients with rosacea: serum level and tissue expression. Ann Dermatol. 2018;30:136–142. doi: 10.5021/ad.2018.30.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schrumpf JA, Amatngalim GD, Veldkamp JB, Verhoosel RM, Ninaber DK, Ordonez SR, van der Does AM, Haagsman HP, Hiemstra PS. Proinflammatory cytokines impair vitamin D-induced host defense in cultured airway epithelial cells. Am J Respir Cell Mol Biol. 2017;56:749–761. doi: 10.1165/rcmb.2016-0289OC. [DOI] [PubMed] [Google Scholar]
- 97.Korf H, Wenes M, Stijlemans B, Takiishi T, Robert S, Miani M, Eizirik DL, Gysemans C, Mathieu C. 1,25-dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology. 2012;217:1292–1300. doi: 10.1016/j.imbio.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188:2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Selvaraj P, Harishankar M, Singh B, Banurekha VV, Jawahar MS. Effect of vitamin D3 on chemokine expression in pulmonary tuberculosis. Cytokine. 2012;60:212–219. doi: 10.1016/j.cyto.2012.06.238. [DOI] [PubMed] [Google Scholar]
- 100.Harishankar M, Afsal K, Banurekha VV, Meenakshi N, Selvaraj P. 1,25-Dihydroxy vitamin D3 downregulates pro-inflammatory cytokine response in pulmonary tuberculosis. Int Immunopharmacol. 2014;23:148–152. doi: 10.1016/j.intimp.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 101.Harishankar M, Anbalagan S, Selvaraj P. Effect of vitamin D3 on chemokine levels and regulatory T-cells in pulmonary tuberculosis. Int Immunopharmacol. 2016;34:86–91. doi: 10.1016/j.intimp.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 102.Heulens N, Korf H, Mathyssen C, Everaerts S, De Smidt E, Dooms C, Yserbyt J, Gysemans C, Gayan-Ramirez G, Mathieu C, et al. 1,25-dihydroxyvitamin D modulates antibacterial and inflammatory response in human cigarette smoke-exposed macrophages. PLoS One. 2016;11:e0160482. doi: 10.1371/journal.pone.0160482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Coussens AK, Wilkinson RJ, Hanifa Y, Nikolayevskyy V, Elkington PT, Islam K, Timms PM, Venton TR, Bothamley GH, Packe GE, et al. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc Natl Acad Sci U S A. 2012;109:15449–15454. doi: 10.1073/pnas.1200072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jolliffe DA, Ganmaa D, Wejse C, Raqib R, Haq MA, Salahuddin N, Daley PK, Ralph AP, Ziegler TR, Martineau AR. Adjunctive vitamin D in tuberculosis treatment: meta-analysis of individual participant data. Eur Respir J. 2019;53:53. doi: 10.1183/13993003.02003-2018. [DOI] [PubMed] [Google Scholar]
- 105.Wu S, Yoon S, Zhang YG, Lu R, Xia Y, Wan J, Petrof EO, Claud EC, Chen D, Sun J. Vitamin D receptor pathway is required for probiotic protection in colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309:G341–G349. doi: 10.1152/ajpgi.00105.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marin M, Holani R, Shah CB, Odeón A, Cobo ER. Cathelicidin modulates synthesis of toll-like receptors (TLRs) 4 and 9 in colonic epithelium. Mol Immunol. 2017;91:249–258. doi: 10.1016/j.molimm.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 107.Kusaka S, Nishida A, Takahashi K, Bamba S, Yasui H, Kawahara M, Inatomi O, Sugimoto M, Andoh A. Expression of human cathelicidin peptide LL-37 in inflammatory bowel disease. Clin Exp Immunol. 2018;191:96–106. doi: 10.1111/cei.13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ta A, Thakur BK, Dutta P, Sinha R, Koley H, Das S. Double-stranded RNA induces cathelicidin expression in the intestinal epithelial cells through phosphatidylinositol 3-kinase-protein kinase Cζ-Sp1 pathway and ameliorates shigellosis in mice. Cell Signal. 2017;35:140–153. doi: 10.1016/j.cellsig.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 109.Yoshimura T, McLean MH, Dzutsev AK, Yao X, Chen K, Huang J, Gong W, Zhou J, Xiang Y, H Badger J, et al. The antimicrobial peptide CRAMP is essential for colon homeostasis by maintaining microbiota balance. J Immunol. 2018;200:2174–2185. doi: 10.4049/jimmunol.1602073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rahman S, Rehn A, Rahman J, Andersson J, Svensson M, Brighenti S. Pulmonary tuberculosis patients with a vitamin D deficiency demonstrate low local expression of the antimicrobial peptide LL-37 but enhanced FoxP3+ regulatory T cells and IgG-secreting cells. Clin Immunol. 2015;156:85–97. doi: 10.1016/j.clim.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 111.Steiger J, Stephan A, Inkeles MS, Realegeno S, Bruns H, Kröll P, de Castro Kroner J, Sommer A, Batinica M, Pitzler L, et al. Imatinib triggers phagolysosome acidification and antimicrobial activity against Mycobacterium bovis bacille Calmette-Guérin in glucocorticoid-treated human macrophages. J Immunol. 2016;197:222–232. doi: 10.4049/jimmunol.1502407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tateosian NL, Pellegrini JM, Amiano NO, Rolandelli A, Casco N, Palmero DJ, Colombo MI, García VE. IL17A augments autophagy in Mycobacterium tuberculosis-infected monocytes from patients with active tuberculosis in association with the severity of the disease. Autophagy. 2017;13:1191–1204. doi: 10.1080/15548627.2017.1320636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Edfeldt K, Liu PT, Chun R, Fabri M, Schenk M, Wheelwright M, Keegan C, Krutzik SR, Adams JS, Hewison M, et al. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci U S A. 2010;107:22593–22598. doi: 10.1073/pnas.1011624108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dang AT, Teles RM, Liu PT, Choi A, Legaspi A, Sarno EN, Ochoa MT, Parvatiyar K, Cheng G, Gilliet M, et al. Autophagy links antimicrobial activity with antigen presentation in Langerhans cells. JCI Insight. 2019;4:126955. doi: 10.1172/jci.insight.126955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Klug-Micu GM, Stenger S, Sommer A, Liu PT, Krutzik SR, Modlin RL, Fabri M. CD40 ligand and interferon-γ induce an antimicrobial response against Mycobacterium tuberculosis in human monocytes. Immunology. 2013;139:121–128. doi: 10.1111/imm.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 117.United States National Library of Medicine. Vitamin D in ventilated ICU patients (R21 HL-110044) [Internet] [accessed on 20 May 2019]. Available at https://ClinicalTrials.gov/show/NCT01372995.
- 118.United States National Library of Medicine. Cholecalciferol supplementation for sepsis in the ICU (CSI). [Internet] [accessed on 20 May 2019]. Available at https://ClinicalTrials.gov/show/NCT01896544.
- 119.United States National Library of Medicine. Effect of high-dose vitamin D3 in smokers and non-smokers with and without HIV. [Internet] [accessed on 20 May 2019]. Available at https://ClinicalTrials.gov/show/NCT03270709.
- 120.Rekha RS, Mily A, Sultana T, Haq A, Ahmed S, Mostafa Kamal SM, van Schadewijk A, Hiemstra PS, Gudmundsson GH, Agerberth B, et al. Immune responses in the treatment of drug-sensitive pulmonary tuberculosis with phenylbutyrate and vitamin D3 as host directed therapy. BMC Infect Dis. 2018;18:303. doi: 10.1186/s12879-018-3203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bartley J, Garrett J, Camargo CA, Jr, Scragg R, Vandal A, Sisk R, Milne D, Tai R, Jeon G, Cursons R, et al. Vitamin D3 supplementation in adults with bronchiectasis: a pilot study. Chron Respir Dis. 2018;15:384–392. doi: 10.1177/1479972318761646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mily A, Rekha RS, Kamal SM, Akhtar E, Sarker P, Rahim Z, Gudmundsson GH, Agerberth B, Raqib R. Oral intake of phenylbutyrate with or without vitamin D3 upregulates the cathelicidin LL-37 in human macrophages: a dose finding study for treatment of tuberculosis. BMC Pulm Med. 2013;13:23. doi: 10.1186/1471-2466-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Camargo CA, Jr, Ganmaa D, Frazier AL, Kirchberg FF, Stuart JJ, Kleinman K, Sumberzul N, Rich-Edwards JW. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics. 2012;130:e561–e567. doi: 10.1542/peds.2011-3029. [DOI] [PubMed] [Google Scholar]
- 124.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 125.Mily A, Rekha RS, Kamal SM, Arifuzzaman AS, Rahim Z, Khan L, Haq MA, Zaman K, Bergman P, Brighenti S, et al. Significant effects of oral phenylbutyrate and vitamin D3 adjunctive therapy in pulmonary tuberculosis: a randomized controlled trial. PLoS One. 2015;10:e0138340. doi: 10.1371/journal.pone.0138340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Salahuddin N, Ali F, Hasan Z, Rao N, Aqeel M, Mahmood F. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT study [supplementary cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis'. BMC Infect Dis. 2013;13:22. doi: 10.1186/1471-2334-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Borella E, Nesher G, Israeli E, Shoenfeld Y. Vitamin D: a new anti-infective agent? Ann N Y Acad Sci. 2014;1317:76–83. doi: 10.1111/nyas.12321. [DOI] [PubMed] [Google Scholar]
- 128.Anandaiah A, Sinha S, Bole M, Sharma SK, Kumar N, Luthra K, Li X, Zhou X, Nelson B, Han X, et al. Vitamin D rescues impaired Mycobacterium tuberculosis-mediated tumor necrosis factor release in macrophages of HIV-seropositive individuals through an enhanced Toll-like receptor signaling pathway in vitro . Infect Immun. 2013;81:2–10. doi: 10.1128/IAI.00666-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Paik S, Kim JK, Chung C, Jo EK. Autophagy: a new strategy for host-directed therapy of tuberculosis. Virulence. 2019;10:448–459. doi: 10.1080/21505594.2018.1536598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ganmaa D, Giovannucci E, Bloom BR, Fawzi W, Burr W, Batbaatar D, Sumberzul N, Holick MF, Willett WC. Vitamin D, tuberculin skin test conversion, and latent tuberculosis in Mongolian school-age children: a randomized, double-blind, placebo-controlled feasibility trial. Am J Clin Nutr. 2012;96:391–396. doi: 10.3945/ajcn.112.034967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, Packe GE, Moore-Gillon JC, Darmalingam M, Davidson RN, et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377:242–250. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Daley P, Jagannathan V, John KR, Sarojini J, Latha A, Vieth R, Suzana S, Jeyaseelan L, Christopher DJ, Smieja M, et al. Adjunctive vitamin D for treatment of active tuberculosis in India: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2015;15:528–534. doi: 10.1016/S1473-3099(15)70053-8. [DOI] [PubMed] [Google Scholar]