Abstract
An excessive hyperinflammatory response-caused septic shock is a major medical problem that is associated with pathogenic bacterial infections leading to high mortality rates. The intestinal microbiota and the associated elaborated metabolites such as short chain fatty acid butyrate have been shown to relieve pathogenic bacterial-caused acute inflammation. Butyrate can down-regulate inflammation by inhibiting the growth of pathobionts, increasing mucosal barrier integrity, encouraging obligate anaerobic bacterial dominance and decreasing oxygen availability in the gut. Butyrate can also decrease excessive inflammation through modulation of immune cells such as increasing functionalities of M2 macrophages and regulatory T cells and inhibiting infiltration by neutrophils. Therefore, various approaches can be used to increase butyrate to relieve pathogenic bacterial-caused hyperinflammation. In this review we summarize the roles of butyrate in attenuating pathogenic bacterial-caused hyperinflammatory responses and discuss the associated plausible mechanisms.
Keywords: Butyrate, Septic shock, Hyperinflammation, Pro-inflammatory cytokines, Macrophages, Regulatory T-cells
INTRODUCTION
The adverse health outcomes associated with systemic pathogenic bacterial invasion depend on the virulence of bacteria and host immune responses. Pathogens can cause tissue damage while host immune responses are activated that then strive to neutralize the infective agents. The process may result in the elimination of the infective agents, leading to complete recovery; or to an acute or persistant chronic infection with a hyperinflammatory response. Excessive inflammatory responses to infective agents can lead to adverse health sequalae such as septic shock, an outcome with a high morbidity and mortality (1). The rate of mortality with septic shock has been reported to be as high as 20%–50% in hospitalized patients (2). The pathogenesis of sepsis undergoes a rapid inflammatory response phase that is characterized by an increase in the level of IL-17 to an immune tolerance phase that is characterized by an increase in the level of IL-10 (3). A rapid increase in hyperinflammation is causal for mortality in the early stages of sepsis (3). Therefore, it is critical to maintain appropriate immune responses that do not reflect an excessive level of inflammatory activity following infections with pathobionts.
The gut microbiota plays a key role in the pathogenesis of pathobiont invasion. The commensal cohort of bacteria can inhibit the increased colonisation and proliferation of pathogens and pathogen-progressed inflammation (4). In contrast, disregulated gut microbiota (dysbiosis) can cause dysfunction of the gut barrier and bacterial translocation across the intestinal wall, resulting in increased local and systemic inflammatory responses which could lead to sepsis in severe cases (5). In addition, pathogenic bacterial toxins such as LPS, lipoproteins (LPP) as well as dietary Ags can also cross the leaky gut to cause various disease states (6). The anti-inflammatory effect of commensal bacteria has been identified to be mediated by short chain fatty acids (SCFAs) that include acetate, propionate and butyrate (7). Although all these SCFAs have anti-inflammatory effects, butyrate is the key mediator among the SCFAs and is the entity which has been extensively studied. In this review, we summarize important aspects of butyrate in pathogenic bacterial infections that are causal for excessive inflammation and the associated mechanisms that drive the elimination of pathogenic bacteria and pro-inflammatory effects.
INHIBITORY EFFECT OF BUTYRATE ON PATHOGENIC BACTERIAL INFECTION
Many studies have shown that butyrate supplementation can reduce the severity of pathogenic bacterial infections. For example, using a mouse model of Citrobacter rodentium infection, Jiminez et al. (8) demonstrated that supplementation with high concentrations (140 mM) of butyrate reduced the severity of a pathogenic bacterial infective insult. Further, the bacterial infection-associated intestinal inflammation was reduced and intestinal barrier permeability improved. Compared with a control group, the butyrate-treated mice had increased weight gain and feeding on day 14 at the peak phase of infectivity (8). Butyrate was also reported to reduce the abundance of an infective bacterial insult in a mouse model of Corynebacterium pseudotuberculosis infection (9). In an in vitro macrophage culture system butyrate at the concentration of 2 mM was also able to reduce the concentration of infecting bacterial counts (9).
Given that butyrate presents with an unpleasant taste, a butyrate derivative, namely phenylbutyrate has been developed. Phenylbutyrate has a phenyl group at position 4 on the butyrate molecule and exhibits similar anti-inflammatory properties (10). The compound was initially approved for the treatment of urea cycle disorders and familial cholestasis type 2 and later was found to be effective as a treatment for diseases such as spinal muscular atrophy, homozygous beta-thalassemia, neurodegenerative diseases and cancer (10). Jellbauer et al. (11) showed that phenylbutyrate decreased Salmonella enterica serovar Typhimurium in Taconic bred mice. It was reported that the first immediate immune response following infection with the bacterium was an increase in IL-23, which stimulated Th17 cells secreting IL-17. The second response was an increased secretion of IL-22, with an associated increased secretion of anti-microbial peptides (AMPs) (11). Sarker et al. (12) showed that supplementation of phenylbutyrate reduced clinical illness in infections with Shigella, with increased AMPs, cathelicidin and defensins in a rabbit model of Shigellosis. Phenylbutyrate increased cathelicidin in macrophages isolated from healthy volunteers who were administered phenylbutyrate for 8 days with dosages of 250 mg, 500 mg and 1,000 mg twice daily (13). The macrophages displayed increased eradication capacity to Mycobacterium tuberculosis. The anti-mycobacterial effect of phenylbutyrate was further potentiated by vitamin D3. The results were confirmed in patients with tuberculosis (14). Phenylbutyrate has also been shown to have a protective effect for Helicobacter pylori infection by structurally interfering with the H. pylori envelope (15).
These results suggest that butyrate and its derivative phenylbutyrate have extensive inhibitory effects on various pathogenic bacteria. These molecules can reduce bacterial virulence and as such significantly prevent hyperinflammatory responses. As the side-effects of antibiotics are causal for intestinal dysbiosis and multiple antibiotic resistance, the administrative inclusion of butyrate may be a better choice in helping resolve future anti-microbial infections. It could be further developed to be an effective approach for both inhibiting pathobiont bacteria and reducing hyperinflammation.
Effect of butyrate on intestinal epithelial-mucosal barrier integrity
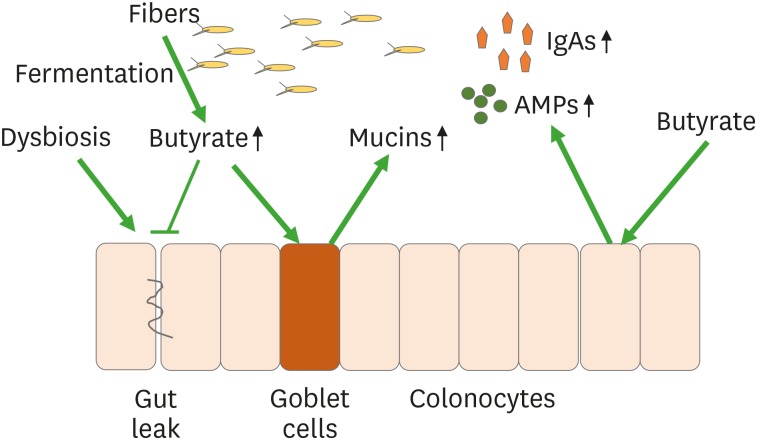
The intestinal epithelial-mucosal barrier is the first line of host defence against bacterial invasion (16). Tight junction formed by adjunct epithelial cells prevent bacteria and their toxins from crossing the barrier through size selectivity (6). Increased intestinal permeability allows larger particles to pass the gut barrier to cause bacterial translocation. It also allows bacterial toxins from live or dead bacteria to diffuse to circulatory system to cause sepsis. The intestinal epithalial-mucosal barrier is closely associated with mucins that contain AMPs and IgAs, secreted by specialized goblet epithelial cells that are composed of transmembrane and secretory glycoproteins (Fig. 1) (16). Mucins firmly attach to epithelial cells forming a thick layer that extends to the lumen where it becomes a loosened layer. The mucin layer blocks bacterial binding to epithelial cells that could enter lymphoid tissue, preventing pro-inflammatory responses. The abundance of mucins represents the main structural component of mucus that is a physical barrier to pathogenic bacterial translocations and that can be metabolised by some bacteria, which use mucins as an energy source such as Akkermansia miciniphila (17).
Figure 1. Butyrate-promoting mucin production. Butyrate produced from fibers by bacterial fermentation. Butyrate stimulates goblet cells to produce mucins. Butyrate also stimulates colonocytes to produce AMPs and IgAs.
Evidence from both in vitro and in vivo studies supports the posit that butyrate increases mucosal barrier integrity through increased secretion of mucins. In the LS174T colon cell line, butyrate promoted mucin-2 production whilst reducing cell proliferation (18). In the intestinal organoids, butyrate increased retinoic acid production and thus, increased cell maturation markers, mucin-2 and villin (19). In a chicken model, supplementation of butyrate increased the number of goblet cells in the small intestine (20). Supplementation of chickens with probiotics and prebiotics that promoted commensal bacteria increased butyrate levels with concomitantly increased production of mucins (21). Mechanistically butyrate was envisaged to increase the expression of the zinc-finger transcriptional factor Kruppel-like factor 4 (KLF4), which promotes goblet cell differentiation (22). Indeed, knockout of LKF4 resulted in a decreased number of goblet cells and altered the morphology of the cells (23,24).
Increased abundance and diversity shifts in butyrate-producing bacteria have also been associated with production of mucins. A study revealed that Bacteroides fragilis demonstrated protective effects in Clostridium difficile infections (25,26). B. fragilis increased mucin production and strengthened intestinal epithelial tight junction structure (25). The bacterium produced propionate, which is a precursor of butyrate. In a mixed culture including B. fragilis, Bifidobacterium infantis and Eubacterium limosum with Aloe vera whole leaf extract increased butyrate production (27). Two strains of commensal bacteria Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii were studied for their effects on mucins in gnotobiotic rats (22). The study showed that B. thetaiotaomicron increased goblet cell differentiation, expression of mucus-related genes and the ratio of sialylated to sulphated mucins in gnotobiotic rats. B. thetaiotaomicron is an acetate producer and acetate has been shown to up-regulate KLF4, which promotes goblet differentiation. However, F. prausnitzii attenuated the effect of this bacterium, which could be interpreted as associated with the consumption of acetate. Therefore, balanced abundance and diversity of 2 strains of bacteria are an important factor for the maintenance of mucin production and turnover. Furthermore, the interactions between gut bacteria could be important factor for mucosal barrier integrity maintenance and warrant further research attention. Decreased gut barrier permeability can reduce the transfer of LPS into the intestinal mucosa and subsequently into the circulation and thus reduce systemic inflammatory responses. This may be an important mechanistic effect for butyrate in the prevention of sepsis.
Butyrate-stimulated AMPs
AMPs include defensins, cathelicidins, and C-type lectins (such as the regenerating [Reg] islet-derived protein family). A study showed that butyrate promoted the production of AMPs, RegIIIγ and β-defensins by intestinal epithelial cells through its receptor GPR43 (28). While RegIIIγ is selective against gram-positive bacteria, the anti-microbial effect of defensins is broad, that includes viruses, fungi and protozoa (29). In a GPR43−/− mouse model, RegIIIγ and β-defensins 1, 3 and 4 were lower than that in wild-type mice. Feeding of butyrate increased production of these antimicrobial peptides in wild-type mice but not in GPR43 knockout mice, indicating GPR43 is a critical mediator. Signalling molecules mTOR and Stat3 were also demonstrated to be critically important in providing a mechanism of defence.
Butyrate has also been reported to promote cathelicidin LL37/human cationic antimicrobial protein 18 (hCAP18) through activation of MEK/ERK and JNK pathways (30,31). Hase et al. (30) revealed that butyrate increased Caco-2 and HCA-7 expressed LL37/hCAP18 while inflammatory factors that included TNF-α, IL-6, IL-1α, LPS, and IFN-γ did not affect the expression of LL37/hCAP18. S. enterica serovar Dublin or enetroinvasive Escherichia coli modestly increased the expression of the peptide (30). Schauber et al. (32) found that LL-37 only expressed in differentiated epithelial cells in the colon and ileum. In vitro, LL-37 was upregulated by butyrate, isobutyrate, propionate and trichostatin A via ERK and p38 MAPK pathways. Kang et al. (33) showed that LL-37 was effective against Staphylococcus aureus.
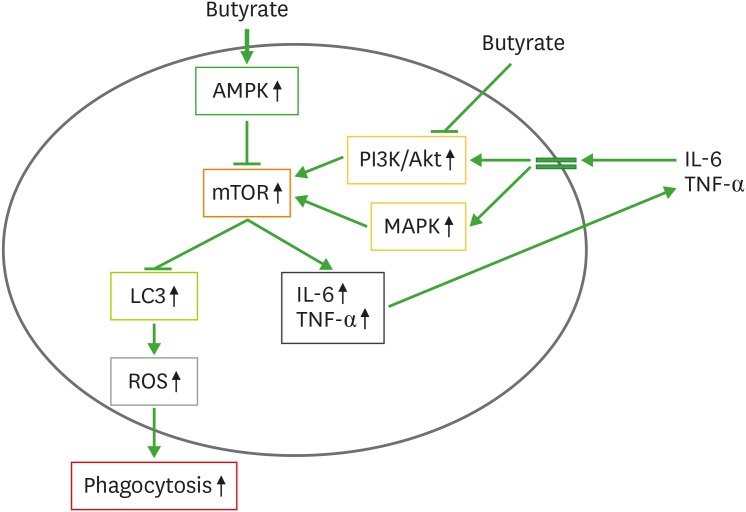
Butyrate also increases AMPs secreted by macrophages and as such increases the anti-microbial effect of gut macrophages (34,35,36). Isolated peripheral blood-derived CD14+ monocytes incubated with macrophage colony-stimulating factor, butyrate, acetate and propionate imprinted an antimicrobial program on these immune cells (35). Macrophages incubated with butyrate resulted in a superior eradicating capability against gram-negative bacteria such as S. enterica or C. rodentium and gram-positive bacteria S. aureus. This was associated with highly increased 5′adenosine monophosphate-activated protein kinase activity, which inhibited mTOR, leading to increased LC3/ROS–mediated phagocytosis (Fig. 2). In addition, butyrate also increased the expression of S100A8 and S100A9 genes, leading to increased expression of AMP calprotectin. Butyrate-increased calprotectin was regulated by histone deacetylase (HDAC) 3 inhibition (35).
Figure 2. Increased anti-microbial effect of macrophages by butyrate. Butyrate activates AMPK which blocks mTOR activity. Butyrate also blocks mTOR activity through inhibiting PI3K/Akt and MAPK activities. The mTOR blocks LC3, which stimulates ROS to increase phagocytosis. The mTOR can increase the production of IL-6 and TNF-α, which activate mTOR upsteam pathways PI3K/Akt and MAPK to form feed-forward regulation loop.
AMPK, adenosine monophosphate-activated protein kinase; LC3, microtubule-associated protein 1A/1B-light chain 3.
Butyrate controls pathogenic bacteria through regulating intestinal oxygen availability
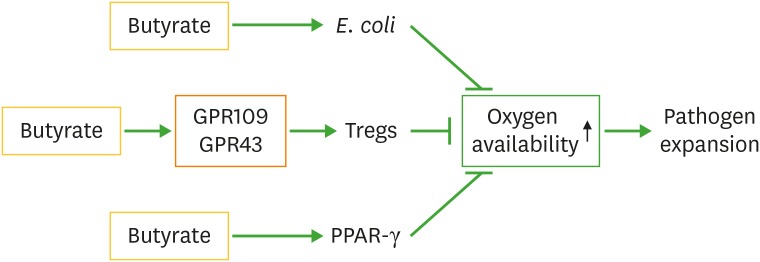
Several studies have reported another important mechanism where butyrate can indirectly control pathogenic bacterial overgrowth, i.e. contributing to intestinal oxygen availability (Fig. 3) (37,38,39). Butyrate increases peroxisome proliferator-activated receptor-γ (PPAR-γ), which in turn increases beta-oxidation of butyrate in colonocytes. This process utilises oxygen and is consumed at a higher rate, leading to decreased availability of intestinal luminal oxygen concentrations. This significantly affects pathogen replication such as happens with pathogenic E. coli, which makes an oxygen demand to produce energy, and when oxygen is in deficit replication is adversely affected. Intestinal infections with or without antibiotic treatment can increase oxygen availability, leading to intestinal dysbiosis, and thus proliferation of pathogenic bacteria that increases the risk of disease (40).
Figure 3. Butyrate limits pathogen expansion through regulating oxygen availability. Butyrate decreases gut oxygen availability though activation of PPAR-γ pathway, Tregs and commensal E. coli. Limited oxygen availability decreases pathogen expansion.
It is also established that commensal bacteria such as E. coli can inhibit the growth of the pathogen Salmonella through competition for oxygen. Litvak et al. (41) showed that neonatal chick colonization with S. enterica serovar Enteritidis relied on epithelial oxygenation and the resultant supply of oxygen for aerobic respiration and bacterial expansion. Salmonella enteritidis competes for oxygen with Enterobacteriaceae. Combining spore-forming bacteria with a probiotic E. coli protected germ-free mice from pathogen colonization but not when E. coli was genetically modified ablating the bacterium's oxygen utilising capacity. Velazquez et al. (42) also showed that mice inoculated with probiotic E. coli were resistant to Salmonella infections due to competition for oxygen utilisation.
Butyrate can promote probiotic growth through increased production of mucus as probiotic E. coli Nissle 1917 uses mucus to grow (Fig. 3). The bacterium E. coli Nissle 1917 has been shown to colonize in mucus and use 13 sugars found in mucus for growth (43). E. coli Nissle 1917 has been shown to produce butyrate. Thus it forms a beneficial feed-forward regulatory mechanism.
Interestingly, Tregs have been demonstrated to participate in the regulation of oxygen availability (38). To increase oxygen availability, both inhibition of PPAR-γ and inactivation of Tregs are required. These 2 factors co-ordinate to produce a high oxygen concentration that allows a proliferative phase to progress for pathogenic bacteria. Inhibiting only the activity of PPAR-γ is insufficient to increase oxygen availability. Therefore, through the activation of Tregs, butyrate facilitates an anaerobic environment and as such inhibits pathogenic bacterial over-growth.
INHIBITION OF INFLAMMATION BY BUTYRATE
Butyrate has been extensively studied for its anti-inflammatory effects. It inhibits pro-inflammatory immune cells such as M1 macrophages and neutrophils by reducing the production of proinflammatory cytokines whilst activating anti-inflammatory cells such as Tregs and M2 macrophages.
Effects of butyrate on macrophage development and function
Macrophages play key roles in the inflammatory status. They are developed from mononuclear cells and can be pro-inflammatory or anti-inflammatory types. Many studies have provided evidence that butyrate can act on macrophages to promote their ant-inflammatory effects in both in vitro and in vivo (44,45,46,47,48,49,50,51). Butyrate acts not only on intestinal macrophages but also circulatory macrophages.
Chang et al. (44) showed that butyrate inhibited macrophage function in vitro through inhibition of HDACs. Treatment of gut-derived macrophages with butyrate resulted in decreased production of IL-6 and IL-12, which is dependent on the inhibition of HDAC but TLRs and G-protein-coupled receptors are not involved (44). Usami et al. (45) showed that butyrate decreased TNF-α production through inhibition of HDAC-associated NF-κB activity.
S. aureus, a gram-positive pathogenic bacterium, can cause severe inflammatory responses/septic shock through TLR2 ligands such as LPP and the more potent lipoteichoic acid (46,47). Park et al. (48) have shown that butyrate inhibits macrophage response to live S. aureus and its LPP in the in vitro Raw264.7 cell culture system. The associated mechanisms were reported to involve the inhibition of NF-κB, STAT1, and HDACs, with a consequent reduction in NO production (48). However, butyrate receptors GPR43 and GPR109A were not involved, indicating the importance of HDAC inhibition in the effects of butyrate on macrophages (48).
In macrophages, mTOR regulates NF-κB by targeting the inhibitor subunit IkBa to promote inflammation. Increased mTOR activity stimulated by bacterial components leads to increased IL-6 and TNF-α, and decreased IL-10 (49) (Fig. 2). Inhibition of mTOR by rapamycin leads to decreased systemic inflammatory responses induced by the glucan, zymosan (50). Decreased mTOR activity by butyrate in macrophages has been shown to result in decreased production of TNF-α, IL-12, IL-6 as well as increased production of IL-10 (51). The effects were revealed to be mediated by HDAC3 inhibition. Given that these pro-inflammatory cytokines play key roles in progressing sepsis, metabolically persuaded decreases of these cytokines by butyrate, can reduce the severity of sepsis experienced.
Locally, butyrate has been demonstrated to affect gut macrophage differentiation to produce non-inflammatory anti-microbial macrophages. The macrophages isolated from the colons of the butyrate supplemented mice showed enhanced anti-microbial activity (35,52). These mice also had reduced bacterial load with S. enterica serovar Typhimurium or C. rodentium infection.
Systemically, butyrate affected circulatory macrophages in a study with 9 healthy and 10 obese subjects who received 4 g butyrate daily for 4 wk (53). Butyrate supplementation decreased LPS-induced IL-6 and Pam3CSK4-induced TNF-α in oxLDL-trained immunity.
Butyrate and neutrophils
Butyrate can reduce pro-inflammatory cytokines TNF-α, IL-6 and IL-12 produced by neutrophils and monocytes under concanavalin A stimulation (54). The GPR43 expressed on the surface of neutrophils has been identified as a major mediator (55). In GPR43−/− mice, inflammation-associated colon cancer mediated by neutrophils is increased through decreased activity of the downstream pathway cAMP-PKA-CREB-sfrp1/dkk3/socs1. Another report has also confirmed that butyrate reduces pro-inflammatory cytokines produced by neutrophils under stimulation of the opportunistic pathogenic bacterium Aggregatibacter actinomycetemcomitans with the identified and proposed mechanism being as HDAC inhibition rather than stimulation of GPR43 (56). The difference between the 2 studies could be explained by the butyrate dosages investigated.
Decreased levels of butyrate in inflammatory bowel diseases (IBDs) have been associated with increased levels of neutrophils as well as other immune cells (57). Simeoli et al. (58) showed that butyrate inhibited neutrophil recruitment in a mouse colitis model induced by dextran sulphate sodium. Butyrate decreased proinflammatory cytokines and increased anti-inflammatory cytokines (58). The mechanism could involve the direct inhibition of HDAC3 and HDAC9, leading to NF-κB inhibition and PPAR-γ upregulation (58).
Vinolo et al. (59) examined the effects of butyrate on LPS-stimulated neutrophils both in vitro and in vivo. Butyrate decreased neutrophil production levels of nitric oxide (NO) and proinflammatory cytokines including TNF-α and cytokine-induced neutrophil chemoattractant-2 with inhibition of HDACs and NF-κB (55). In a rat model with intraperitoneal LPS administration, butyrate decreased recruitment of neutrophils to the peritonium. Ex vivo, pro-inflammatory cytokine and NO production by neutrophils were decreased in rats that were treated with butyrate.
Effect of butyrate on Tregs
Treg cells play key roles in intestinal inflammatory process and status. Treg cells display strong anti-inflammatory effects, a necessary effect that balances immune responses and prevents chronic inflammation as well as excessive acute inflammation. Butyrate can activate and thus encourage Treg cells to exert an anti-inflammatory effect. Depletion of Treg cells has been reported to result in autoimmune diseases (60). Butyrate regulated Treg cell pool size and function provide a protective effect by Treg cells against colitis (61,62,63). Butyrate can bind and activate GRP109a receptors in macrophages/dendritic cells, phenotypically changing these cells into Treg cells and elaborating IL-10-producing T cells (64).
Cytokines have been shown to play a key role in the pathogenesis of septic shock (65). Highly increased levels of pro-inflammatory cytokines in the early stages of sepsis have been considered to be the reason for early mortality (65). As such the abolition of cytokines from the circulation by blood purification therapies has been studied for the treatment of sepsis (65). Through activation of Tregs, butyrate inhibits proinflammatory cytokines IL-6 and IL-17 (66). IL-6 can activate STAT3, which increases expression of IL-17, transcriptional factor RORγ and RORα (67,68). Consequently, IL-6 increases the blood levels of IL-17. Tregs control Th17 cells, which are highly pro-inflammatory cells, producing IL-17, IL-22, and IL-23 in responses to extracellular bacterial infections (68,69). IL-17 can exercise an anti-microbial effect through recruitment of neutrophils, macrophages and dendritic cells (70). It also facilitates production of AMPs such as beta-defensin. Even so, IL-17 has also been associated with excessive inflammatory responses.
Activation of Tregs by butyrate not only inhibits effector T cells but also produces the anti-inflammatory cytokine IL-10 (66,71). IL-10 has been demonstrated to inhibit pro-inflammatory macrophages (M1 macrophages) and to stimulate maturation of anti-inflammatory macrophages (M2 macrophages) (72,73). In addition, IL-10 from Treg cells also induces M2 macrophages through other mechanisms (74). Accordingly, butyrate can inhibit a proinflammatory biochemical process and promote an anti-inflammatory status through the encouraged production of IL-10 by M2 macrophages.
In summary, butyrate can affect above immune cells to exert anti-inflammatory effect through the various mechanisms such as HDAC inhibition and butyrate receptor activation. In addition to these cells, other immune cells are also affected by butyrate but less studied such as mast cells, dendritic cells and T cells. Mast cells are inflammatory immune cells, which produce various inflammatory factors including TNF-α, IL-6, histamine and tryptase. Butyrate can reduce mast cells to secret these pro-inflammatory factors through HDAC inhibition (75). It also inhibits dendritic cell maturation and function to secret pro-inflammatory factors IL-6 and TNF-α (76,77). Furthermore, butyrate causes T cell apoptosis through HDAC inhibition and associated cell death receptor Fas activation (78). Further studies are warranted to understand the effects of butyrate on these immune cells.
STRATEGIES TO ENRICH BUTYRATE IN THE INTESTINES AS AN ADJUNCT THERAPY
As butyrate plays a key role in ameliorating intestinal increases in the level of pathogenic bacteria and mucosal tissue translocation that triggers associated hyperinflammatory processes. Increasing production of butyrate in the intestines could have a significant therapeutic effect for the treatment of excessive inflammation caused sepsis. Such treatments could be integrated into current therapeutic regimens that target hyperinflammation elicited from pathogenic infections. Many factors are involved in the induction of high levels of intestinal microbiome elaborated butyrate, and thus multiple intervention including probiotics, prebiotics and synbiotics may show a therapeutic benefit (Table 1). Probiotics, which refer to live commensal bacteria that improve gut microbiota, have been extensively studied both in animal models and clinical trials (79). Prebiotics are fibers indigestible by humans that can be metabolically fermented by the gut microbiota to produce beneficial metabolites (79). The role of probiotics, prebiotics and synbiotics (combination of probiotics and prebiotics) in enhancing the level of gut butyrate, are scientific plausible adjunctive options to consider.
Table 1. Strategies to enrich butyrate.
| Samples of bacterium/substrate | Main findings | Ref. | |
|---|---|---|---|
| Probiotics | |||
| Faecalibacterium prausnitzii | F. prausnitzii reduced Clostridium difficile infection in a mouse model. | (83) | |
| Bacteroides fragilis | In a mouse model, B. fragilis inhibited C. difficile growth with increased butyrate and gut barrier. | (84) | |
| Butyricicoccus pullicaecorum | Administration of the bacterium decreased colon inflammation through increased production of butyrate in a rat colitis model. | (85) | |
| Roseburia hominis | Administration of the bacterium increased cecal butyrate content and reduced stress-induced visceral hypersensitivity in rats. | (86) | |
| Prebiotics | |||
| Inulin | Inulin inhibited antibiotics-induced C. difficile infection. | (104) | |
| Pectin | Fermentation of pectin increased beneficial bacterial growth. | (108) | |
| Synbiotics | |||
| Bifidobacteria + inulin | In co-culture system, the synbiotic effectively inhibited non-probiotic bacterium Bacillus cereus. | (110) | |
| F. prausnitzii + potato starch | The synbiotic was better than F. prausnitzii alone in inhibiting C. difficile growth. | (83) | |
| Bifidobacterium longum + pectin | The symbiotic was better than each component alone in inhibiting pathogenic bacteria in the Simulator of the Human Intestinal Microbial Ecosystem. | (109) | |
| Fasting mimicking diet | (113) | ||
| First day intake 50% normal caloric | It had anti-inflammatory effect and increased commensal microbiota. | ||
| Days 2–4 intake 10% normal caloric | |||
Probiotics
Many bacteria have been identified to possess the necessary metabolic ability to produce butyrate. Such bacteria predominantly are classified into 4 bacterial taxonomic families including Clostridiaceae, Eubacteriaceae, Lachnospiraceae, and Ruminococcaceae (79,80). Notwithstanding, additional butyrate-producing bacteria have also been reported in other bacterial families such as Veillonellaceae and Thermoanaerobacterales family III (81). At the species level, more than 40 butyrate-producibg bacteria have been documented (79).
Only a few of the butyrate-producing bacteria have been investigated as possible probiotics and these have included F. prausnitzii, Akkermansia muciniphila, Ruminococcus bromii, and Roseburia species. The most studied one is Faecalibacterium prauzinii which has been shown to increase butyrate levels and is of benefit to various diseases such as depression, diabetes and pathogen invasion (82,83). In animal models, administration of B. fragilis, Butyricicoccus pullicaecorum or Roseburia hominis exerted effects to increase butyrate production, inhibit bacterial infection and reduce inflammation (84,85,86).
However, in a co-culture model, F. prausnitzii did not increase epithelial barrier integrity, indicating how complicated the production of butyrate in the system is in the host and how difficult is to mimic (87).
It should be noted that butyrate-producing bacteria do not work singularly but are part of co-operative in the gut. To enable the effective production of butyrate, cross-feeding is necessary requisite. Some Bifidobacteria have been shown to possess the means to increase butyrate production through cross-feeding (88,89). Two strains of bacteria could also be competitive for sources such as Bifidobacteria angulatum and F. prausnitzii (90). In vitro models have shown that Bifidobacteria have a similar effect in the prevention of gut permeability that is induced by TNF-α in Caco-2 monolayers as a butyrate-producing bacterium (91).
Prebiotics
Prebiotics have been classified into 8 catagories that include beta-glucan, fructooligosaccharides/oligofructose/inulin, galactooligosaccharides, isomaltooligosaccharides, guar gum, lactulose, resistant starches/maltodextrin and xylooligosaccharides/arabinooliosaccharides (92). Among them indigestible polysaccharides and resistant starch are most commonly used (92,93). These prebiotics have been used to increase the abundance of butyrate-producing bacteria, leading to increased production of butyrate (92,93,94). For example woody type dietary fibers have been shown to promote the growth of Bifidobacteria, Lactobacilli, and Bacteroides genera in a culture media system (95). Tochio et al. (96) showed that 1-kestose, the smallest fructo-oliosaccharide, increased F. prausnitzii and Bifidobacteria abundances. Many studies have shown that the beneficial effects of prebiotics are mediated by SCFAs. Depending on the individual microbiota that is present, prebiotics may produce butyrate or propionate (97). Although butyrate has greater anti-inflammatory effects, propionate has also been shown to exhibit anti-inflammatory effects (98). The SCFA acetate exerts the weakest anti-inflammatory effect among all the SCFAs (99).
Inulin and pectin are 2 common fibers employed as prebiotics. Inulin is a type of fructan with beta-2:1 bonds and a linear structure. Some gut bacteria are able to metabolize inulin into butyrate to benefit the host (100,101); the butyrate that is produced results in higher concentrations of the SCFA than is produced with other fibers (101,102). Inulin has been shown to increase commensal bacterial abundance and improve colonic commensal bacterial diversity with concomitant decreases in gut pathobionts. It can stimulate both the butyrate producing species namely, Bifidobacterium adolescentis and F. prausnitzii (103). An additional study has demonstrated that inulin increases Bifidobacteria, Lactobacilli, and non-pathogenic E. coli. Inulin has been shown to decrease the infective burden of C. difficile, C. difficile-associated inflammation and gut dysbiosis through the increased production of butyrate (104). Fachi et al. (105) showed that butyrate as well as tributyrate and inulin administration reduced C. difficile-infection caused intestinal epithelial cell damage through the activation of HIF-1. C. difficle infection severity is associated with the translocation of the bacteria to the circulation system and extraintestinal organs such as liver and spleen due to increased intestinal permeability (105). Butyrate increased the barrier and thus reduced the bacterial translocation as evidenced by reduced colony-forming units from the liver and spleen. The effect of butyrate on the gut barrier was further demonstrated by decreased translocation of FITC-dextran in the mice.
Pectin, a plant cell wall component, has been shown to be utilised as an energy source substrate by F. prausnitzii with the consequent production of IL-10 in an in vitro model (106). Moreover, pectin that was fermented to butyrate attenuated atherosclerosis in an animal model (107). Using a dynamic gastrointestinal simulator, Ferreira-Lazarte et al. (108) reported that pectin remained 88% undigested after passing as starch in the small intestine but was subject to fermentative degradation in the large intestine thereby increasing local levels of butyrate. Pectin stimulated the growth of beneficial bacteria such as those from the genera Bifidobacteria spp. and Bacteroides spp. and the species F. prausnitzii (108).
Synbiotics
Synbiotic formulations are composed of both probiotic bacteria and prebiotic fibers. It has been posited that the combination may provide a better efficacious outcome for the elaboration of butyrate by intestinal commensal bacteria by either agent alone. Bianchi et al. (109) compared the effects of Bifidobacterium longum BB-46 alone and in combination with a lemon derived citric pectin in a Simulator of the Human Intestinal Microbial Ecosystem. The abundance of Firmicutes and Bacteroidetes was increased with the administration of B. longum BB-46 alone. However, a combination treatment increased Faecalibacteria, Eubacteria, Lactobacilli, and Ruminococcaceae families and reduced the abundance of proteolytic bacteria such as Bacteroides, Clostridia, Peptoniphilus, and Streptococci, as well as the production of ammonia. Importantly, BB-46 alone did not increase butyrate levels but in combination treatment it did so (109). Furthermore, it has been shown that the combination use of inulin with Bifidobacteria can substantially inhibit the pathogenic bacterium Bacillus cereus (110).
When formulating synbiotics with the inclusion of probiotics and prebiotics it has been noted that different butyrate-producing bacteria utilise different types of prebiotics. For example, F. prausnitzii and Eubacterium rectale utilise fructose, oligofrutose and inulin to produce butyrate while B. pullicaecorum and Eubacterium hallii can only utilise fructose (111).
Other approaches that can modulate the gut microbiota
It has been shown that F. prausnitzii and A. muciniphila are increased after fasting (112). Therefore, it is not surprising that fasting mimicking diets decrease dextran sulfate sodium-induced inflammation and stimulate the commensal gut microbiota, leading to reduced IBD pathological changes in the intestines (113). Fasting mimicking diets are also able to reduce neuroinflammaton and to exert a protective effect (114). Fasting mimicking diets may increase butyrate, thereby exerting anti-inflammatory effects (114). Notwithstanding additional research is needed to further confirm this impression.
CONCLUSIONS
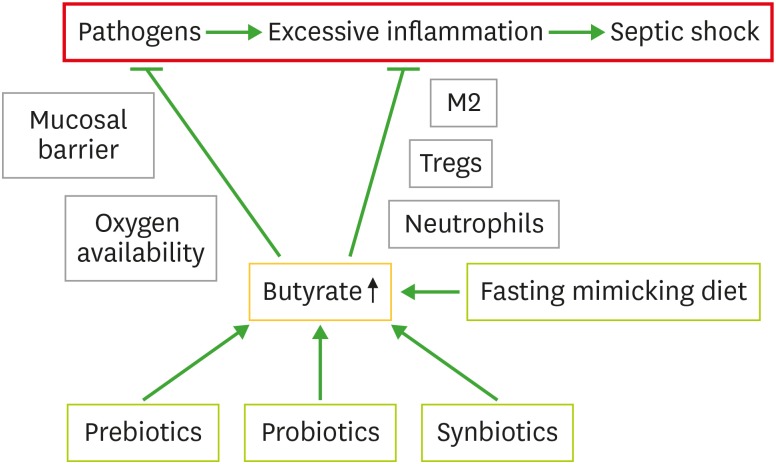
Gut microbiota plays a key role in the outcomes of pathogenic bacterial infections. Commensal bacterial metabolite butyrate not only inhibits excessive inflammation but also limits pathogenic bacterial growth (Fig. 4). Several associated mechanisms have been elucidated. Butyrate can control pathogen proliferation through increasing mucosal barrier, secretion of AMPs and oxygen availability. It inhibits pro-inflammatory immune cells and activate anti-inflammatory immune cells, and hence reduces pro-inflammatory cytokines. These important discoveries suggest supplementation of butyrate could be very helpful for the treatment of pathogenic infection and prevention of fatal consequences such as septic shock.
Figure 4. Anti-septic effect of butyrate. Pathogens can cause excessive inflammation, which can result in septic shock. Butyrate can limit both pathogens infection through mucosal barrier and oxygen availability, and excessive inflammation through activation of M2 macrophages, Tregs and inhibition of neutrophils.
Abbreviations
- AMP
anti-microbial peptide
- hCAP18
human cationic antimicrobial protein 18
- HDAC
histone deacetylase
- IBD
inflammatory bowel disease
- KLF4
Kruppel-like factor 4
- LPP
lipoproteins
- NO
nitric oxide
- PPAR
peroxisome proliferator-activated receptor
- Reg
regenerating
- SCFA
short chain fatty acid
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Chen J, Vitetta L.
- Formal analysis: Chen J, Vitetta L.
- Supervision: Vitetta L.
- Writing - original draft: Chen J.
- Writing - review & editing: Vitetta L.
References
- 1.Lappin E, Ferguson AJ. Gram-positive toxic shock syndromes. Lancet Infect Dis. 2009;9:281–290. doi: 10.1016/S1473-3099(09)70066-0. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei Y, Yang J, Wang J, Yang Y, Huang J, Gong H, Cui H, Chen D. Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis. Crit Care. 2016;20:332. doi: 10.1186/s13054-016-1491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagpal R, Yadav H. Bacterial translocation from the gut to the distant organs: an overview. Ann Nutr Metab. 2017;71(Suppl 1):11–16. doi: 10.1159/000479918. [DOI] [PubMed] [Google Scholar]
- 6.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Zhao KN, Vitetta L. Effects of intestinal microbial⁻elaborated butyrate on oncogenic signaling pathways. Nutrients. 2019;11:E1026. doi: 10.3390/nu11051026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiminez JA, Uwiera TC, Abbott DW, Uwiera RR, Inglis GD. Butyrate supplementation at high concentrations alters enteric bacterial communities and reduces intestinal inflammation in mice infected with Citrobacter rodentium . mSphere. 2017;2:e00243-17. doi: 10.1128/mSphere.00243-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Z, Yang H, Li H, Li X, Li X, Wu B, Tian S, Wu J, Wang Z, Hu S. Sodium butyrate ameliorates Corynebacterium pseudotuberculosis infection in RAW264.7 macrophages and C57BL/6 mice. Microb Pathog. 2019;131:144–149. doi: 10.1016/j.micpath.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Koren G, Rieder MJ, Amitai Y. Averting the foul taste of pediatric medicines improves adherence and can be lifesaving - Pheburane® (sodium phenylbutyrate) Patient Prefer Adherence. 2016;10:2141–2144. doi: 10.2147/PPA.S117506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jellbauer S, Perez Lopez A, Behnsen J, Gao N, Nguyen T, Murphy C, Edwards RA, Raffatellu M. Beneficial effects of sodium phenylbutyrate administration during infection with Salmonella enterica serovar Typhimurium. Infect Immun. 2016;84:2639–2652. doi: 10.1128/IAI.00132-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarker P, Ahmed S, Tiash S, Rekha RS, Stromberg R, Andersson J, Bergman P, Gudmundsson GH, Agerberth B, Raqib R. Phenylbutyrate counteracts Shigella mediated downregulation of cathelicidin in rabbit lung and intestinal epithelia: a potential therapeutic strategy. PLoS One. 2011;6:e20637. doi: 10.1371/journal.pone.0020637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mily A, Rekha RS, Kamal SM, Akhtar E, Sarker P, Rahim Z, Gudmundsson GH, Agerberth B, Raqib R. Oral intake of phenylbutyrate with or without vitamin D3 upregulates the cathelicidin LL-37 in human macrophages: a dose finding study for treatment of tuberculosis. BMC Pulm Med. 2013;13:23. doi: 10.1186/1471-2466-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mily A, Rekha RS, Kamal SM, Arifuzzaman AS, Rahim Z, Khan L, Haq MA, Zaman K, Bergman P, Brighenti S, et al. Significant effects of oral phenylbutyrate and vitamin D3 adjunctive therapy in pulmonary tuberculosis: a randomized controlled trial. PLoS One. 2015;10:e0138340. doi: 10.1371/journal.pone.0138340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yonezawa H, Osaki T, Hanawa T, Kurata S, Zaman C, Woo TD, Takahashi M, Matsubara S, Kawakami H, Ochiai K, et al. Destructive effects of butyrate on the cell envelope of Helicobacter pylori . J Med Microbiol. 2012;61:582–589. doi: 10.1099/jmm.0.039040-0. [DOI] [PubMed] [Google Scholar]
- 16.Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol. 2018;16:457–470. doi: 10.1038/s41579-018-0036-x. [DOI] [PubMed] [Google Scholar]
- 17.Belzer C, de Vos WM. Microbes inside--from diversity to function: the case of Akkermansia. ISME J. 2012;6:1449–1458. doi: 10.1038/ismej.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung TH, Park JH, Jeon WM, Han KS. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr Res Pract. 2015;9:343–349. doi: 10.4162/nrp.2015.9.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schilderink R, Verseijden C, Seppen J, Muncan V, van den Brink GR, Lambers TT, van Tol EA, de Jonge WJ. The SCFA butyrate stimulates the epithelial production of retinoic acid via inhibition of epithelial HDAC. Am J Physiol Gastrointest Liver Physiol. 2016;310:G1138–G1146. doi: 10.1152/ajpgi.00411.2015. [DOI] [PubMed] [Google Scholar]
- 20.Sikandar A, Zaneb H, Younus M, Masood S, Aslam A, Khattak F, Ashraf S, Yousaf MS, Rehman H. Effect of sodium butyrate on performance, immune status, microarchitecture of small intestinal mucosa and lymphoid organs in broiler chickens. Asian-Australas J Anim Sci. 2017;30:690–699. doi: 10.5713/ajas.16.0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onrust L, Ducatelle R, Van Driessche K, De Maesschalck C, Vermeulen K, Haesebrouck F, Eeckhaut V, Van Immerseel F. Steering endogenous butyrate production in the intestinal tract of broilers as a tool to improve gut health. Front Vet Sci. 2015;2:75. doi: 10.3389/fvets.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wrzosek L, Miquel S, Noordine ML, Bouet S, Joncquel Chevalier-Curt M, Robert V, Philippe C, Bridonneau C, Cherbuy C, Robbe-Masselot C, et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. doi: 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, Kaestner KH. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flandez M, Guilmeau S, Blache P, Augenlicht LH. KLF4 regulation in intestinal epithelial cell maturation. Exp Cell Res. 2008;314:3712–3723. doi: 10.1016/j.yexcr.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng H, Yang S, Zhang Y, Qian K, Zhang Z, Liu Y, Wang Y, Bai Y, Fan H, Zhao X, Zhi F. Bacteroides fragilis prevents Clostridium difficile infection in a mouse model by restoring gut barrier and microbiome regulation. Front Microbiol. 2018;9:2976. doi: 10.3389/fmicb.2018.02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldberg E, Amir I, Zafran M, Gophna U, Samra Z, Pitlik S, Bishara J. The correlation between Clostridium-difficile infection and human gut concentrations of Bacteroidetes phylum and clostridial species. Eur J Clin Microbiol Infect Dis. 2014;33:377–383. doi: 10.1007/s10096-013-1966-x. [DOI] [PubMed] [Google Scholar]
- 27.Pogribna M, Freeman JP, Paine D, Boudreau MD. Effect of Aloe vera whole leaf extract on short chain fatty acids production by Bacteroides fragilis, Bifidobacterium infantis and Eubacterium limosum . Lett Appl Microbiol. 2008;46:575–580. doi: 10.1111/j.1472-765X.2008.02346.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y, Chen F, Wu W, Sun M, Bilotta AJ, Yao S, Xiao Y, Huang X, Eaves-Pyles TD, Golovko G, et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018;11:752–762. doi: 10.1038/mi.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 30.Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun. 2002;70:953–963. doi: 10.1128/iai.70.2.953-963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schauber J, Svanholm C, Termén S, Iffland K, Menzel T, Scheppach W, Melcher R, Agerberth B, Lührs H, Gudmundsson GH. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut. 2003;52:735–741. doi: 10.1136/gut.52.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schauber J, Svanholm C, Termén S, Iffland K, Menzel T, Scheppach W, Melcher R, Agerberth B, Lührs H, Gudmundsson GH. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut. 2003;52:735–741. doi: 10.1136/gut.52.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang J, Dietz MJ, Li B. Antimicrobial peptide LL-37 is bactericidal against Staphylococcus aureus biofilms. PLoS One. 2019;14:e0216676. doi: 10.1371/journal.pone.0216676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flemming A. Butyrate boosts microbicidal macrophages. Nat Rev Immunol. 2019;19:135. doi: 10.1038/s41577-019-0132-9. [DOI] [PubMed] [Google Scholar]
- 35.Schulthess J, Pandey S, Capitani M, Rue-Albrecht KC, Arnold I, Franchini F, Chomka A, Ilott NE, Johnston DG, Pires E, et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. 2019;50:432–445.e7. doi: 10.1016/j.immuni.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lobel L, Garrett WS. Butyrate makes macrophages “go nuclear” against bacterial pathogens. Immunity. 2019;50:275–278. doi: 10.1016/j.immuni.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Singer-Englar T, Barlow G, Mathur R. Obesity, diabetes, and the gut microbiome: an updated review. Expert Rev Gastroenterol Hepatol. 2019;13:3–15. doi: 10.1080/17474124.2019.1543023. [DOI] [PubMed] [Google Scholar]
- 38.Byndloss MX, Olsan EE, Rivera-Chávez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y, et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357:570–575. doi: 10.1126/science.aam9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cani PD. Gut cell metabolism shapes the microbiome. Science. 2017;357:548–549. doi: 10.1126/science.aao2202. [DOI] [PubMed] [Google Scholar]
- 40.Rivera-Chávez F, Lopez CA, Bäumler AJ. Oxygen as a driver of gut dysbiosis. Free Radic Biol Med. 2017;105:93–101. doi: 10.1016/j.freeradbiomed.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 41.Litvak Y, Mon KK, Nguyen H, Chanthavixay G, Liou M, Velazquez EM, Kutter L, Alcantara MA, Byndloss MX, Tiffany CR, et al. Commensal Enterobacteriaceae protect against Salmonella colonization through oxygen competition. Cell Host Microbe. 2019;25:128–139.e5. doi: 10.1016/j.chom.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Velazquez EM, Nguyen H, Heasley KT, Saechao CH, Gil LM, Rogers AW, Miller BM, Rolston MR, Lopez CA, Litvak Y, et al. Endogenous Enterobacteriaceae underlie variation in susceptibility to Salmonella infection. Nat Microbiol. 2019;4:1057–1064. doi: 10.1038/s41564-019-0407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adediran J, Leatham-Jensen MP, Mokszycki ME, Frimodt-Møller J, Krogfelt KA, Kazmierczak K, Kenney LJ, Conway T, Cohen PS. An Escherichia coli Nissle 1917 missense mutant colonizes the streptomycin-treated mouse intestine better than the wild type but is not a better probiotic. Infect Immun. 2014;82:670–682. doi: 10.1128/IAI.01149-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Usami M, Kishimoto K, Ohata A, Miyoshi M, Aoyama M, Fueda Y, Kotani J. Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr Res. 2008;28:321–328. doi: 10.1016/j.nutres.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 46.Kim HY, Kim AR, Seo HS, Baik JE, Ahn KB, Yun CH, Han SH. Lipoproteins in Streptococcus gordonii are critical in the infection and inflammatory responses. Mol Immunol. 2018;101:574–584. doi: 10.1016/j.molimm.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 47.Bartual SG, Alcorlo M, Martínez-Caballero S, Molina R, Hermoso JA. Three-dimensional structures of Lipoproteins from Streptococcus pneumoniae and Staphylococcus aureus . Int J Med Microbiol. 2018;308:692–704. doi: 10.1016/j.ijmm.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Park JW, Kim HY, Kim MG, Jeong S, Yun CH, Han SH. Short-chain fatty acids inhibit staphylococcal lipoprotein-induced nitric oxide production in murine macrophages. Immune Netw. 2019;19:e9. doi: 10.4110/in.2019.19.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vangan N, Cao Y, Jia X, Bao W, Wang Y, He Q, Binderiya U, Feng X, Li T, Hao H, et al. mTORC1 mediates peptidoglycan induced inflammatory cytokines expression and NF-κB activation in macrophages. Microb Pathog. 2016;99:111–118. doi: 10.1016/j.micpath.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Sahan-Firat S, Temiz-Resitoglu M, Guden DS, Kucukkavruk SP, Tunctan B, Sari AN, Kocak Z, Malik KU. Protection by mTOR inhibition on zymosan-induced systemic inflammatory response and oxidative/nitrosative stress: contribution of mTOR/MEK1/ERK1/2/IKKβ/IκB-α/NF-κB signalling pathway. Inflammation. 2018;41:276–298. doi: 10.1007/s10753-017-0686-2. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Zhang X, Zhu L, Yang X, He F, Wang T, Bao T, Lu H, Wang H, Yang S. Inulin alleviates inflammation of alcoholic liver disease via SCFAs-inducing suppression of M1 and facilitation of M2 macrophages in mice. Int Immunopharmacol. 2020;78:106062. doi: 10.1016/j.intimp.2019.106062. [DOI] [PubMed] [Google Scholar]
- 52.Garrett WS. Immune recognition of microbial metabolites. Nat Rev Immunol. 2019 doi: 10.1038/s41577-019-0252-2. [DOI] [PubMed] [Google Scholar]
- 53.Cleophas MC, Ratter JM, Bekkering S, Quintin J, Schraa K, Stroes ES, Netea MG, Joosten LA. Effects of oral butyrate supplementation on inflammatory potential of circulating peripheral blood mononuclear cells in healthy and obese males. Sci Rep. 2019;9:775. doi: 10.1038/s41598-018-37246-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hazem SH, Hamed MF, Saad MA, Gameil NM. Comparison of lactate and β-hydroxybutyrate in the treatment of concanavalin-A induced hepatitis. Int Immunopharmacol. 2018;61:376–384. doi: 10.1016/j.intimp.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 55.Pan P, Oshima K, Huang YW, Agle KA, Drobyski WR, Chen X, Zhang J, Yearsley MM, Yu J, Wang LS. Loss of FFAR2 promotes colon cancer by epigenetic dysregulation of inflammation suppressors. Int J Cancer. 2018;143:886–896. doi: 10.1002/ijc.31366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corrêa RO, Vieira A, Sernaglia EM, Lancellotti M, Vieira AT, Avila-Campos MJ, Rodrigues HG, Vinolo MA. Bacterial short-chain fatty acid metabolites modulate the inflammatory response against infectious bacteria. Cell Microbiol. 2017;19:e12720. doi: 10.1111/cmi.12720. [DOI] [PubMed] [Google Scholar]
- 57.Gonçalves P, Araújo JR, Di Santo JP. A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:558–572. doi: 10.1093/ibd/izx029. [DOI] [PubMed] [Google Scholar]
- 58.Simeoli R, Mattace Raso G, Pirozzi C, Lama A, Santoro A, Russo R, Montero-Melendez T, Berni Canani R, Calignano A, Perretti M, et al. An orally administered butyrate-releasing derivative reduces neutrophil recruitment and inflammation in dextran sulphate sodium-induced murine colitis. Br J Pharmacol. 2017;174:1484–1496. doi: 10.1111/bph.13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem. 2011;22:849–855. doi: 10.1016/j.jnutbio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 60.Kim J, Lahl K, Hori S, Loddenkemper C, Chaudhry A, deRoos P, Rudensky A, Sparwasser T. Cutting edge: depletion of Foxp3+ cells leads to induction of autoimmunity by specific ablation of regulatory T cells in genetically targeted mice. J Immunol. 2009;183:7631–7634. doi: 10.4049/jimmunol.0804308. [DOI] [PubMed] [Google Scholar]
- 61.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 64.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Honore PM, Hoste E, Molnár Z, Jacobs R, Joannes-Boyau O, Malbrain ML, Forni LG. Cytokine removal in human septic shock: where are we and where are we going? Ann Intensive Care. 2019;9:56. doi: 10.1186/s13613-019-0530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen J, Vitetta L. Inflammation-modulating effect of butyrate in the prevention of colon cancer by dietary fiber. Clin Colorectal Cancer. 2018;17:e541–e544. doi: 10.1016/j.clcc.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 67.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 69.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Das S, Khader S. Yin and yang of interleukin-17 in host immunity to infection. F1000 Res. 2017;6:741. doi: 10.12688/f1000research.10862.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 73.Okeke EB, Uzonna JE. The pivotal role of regulatory T cells in the regulation of innate immune cells. Front Immunol. 2019;10:680. doi: 10.3389/fimmu.2019.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang H, Du M, Yang Q, Zhu MJ. Butyrate suppresses murine mast cell proliferation and cytokine production through inhibiting histone deacetylase. J Nutr Biochem. 2016;27:299–306. doi: 10.1016/j.jnutbio.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 76.Liu L, Li L, Min J, Wang J, Wu H, Zeng Y, Chen S, Chu Z. Butyrate interferes with the differentiation and function of human monocyte-derived dendritic cells. Cell Immunol. 2012;277:66–73. doi: 10.1016/j.cellimm.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 77.Millard AL, Mertes PM, Ittelet D, Villard F, Jeannesson P, Bernard J. Butyrate affects differentiation, maturation and function of human monocyte-derived dendritic cells and macrophages. Clin Exp Immunol. 2002;130:245–255. doi: 10.1046/j.0009-9104.2002.01977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zimmerman MA, Singh N, Martin PM, Thangaraju M, Ganapathy V, Waller JL, Shi H, Robertson KD, Munn DH, Liu K. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1405–G1415. doi: 10.1152/ajpgi.00543.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fu X, Liu Z, Zhu C, Mou H, Kong Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit Rev Food Sci Nutr. 2019;59:S130–S152. doi: 10.1080/10408398.2018.1542587. [DOI] [PubMed] [Google Scholar]
- 80.Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. MBio. 2014;5:e00889. doi: 10.1128/mBio.00889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Louis P, Duncan SH, McCrae SI, Millar J, Jackson MS, Flint HJ. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J Bacteriol. 2004;186:2099–2106. doi: 10.1128/JB.186.7.2099-2106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ganesan K, Chung SK, Vanamala J, Xu B. Causal relationship between diet-induced gut microbiota changes and diabetes: a novel strategy to transplant Faecalibacterium prausnitzii in preventing diabetes. Int J Mol Sci. 2018;19:E3720. doi: 10.3390/ijms19123720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roychowdhury S, Cadnum J, Glueck B, Obrenovich M, Donskey C, Cresci GA. Faecalibacterium prausnitzii and a prebiotic protect intestinal health in a mouse model of antibiotic and Clostridium difficile exposure. JPEN J Parenter Enteral Nutr. 2018;42:1156–1167. doi: 10.1002/jpen.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deng H, Yang S, Zhang Y, Qian K, Zhang Z, Liu Y, Wang Y, Bai Y, Fan H, Zhao X, et al. Bacteroides fragilis prevents Clostridium difficile infection in a mouse model by restoring gut barrier and microbiome regulation. Front Microbiol. 2018;9:2976. doi: 10.3389/fmicb.2018.02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eeckhaut V, Machiels K, Perrier C, Romero C, Maes S, Flahou B, Steppe M, Haesebrouck F, Sas B, Ducatelle R, et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut. 2013;62:1745–1752. doi: 10.1136/gutjnl-2012-303611. [DOI] [PubMed] [Google Scholar]
- 86.Zhang J, Song L, Wang Y, Liu C, Zhang L, Zhu S, Liu S, Duan L. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J Gastroenterol Hepatol. 2019;34:1368–1376. doi: 10.1111/jgh.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maier E, Anderson RC, Altermann E, Roy NC. Live Faecalibacterium prausnitzii induces greater TLR2 and TLR2/6 activation than the dead bacterium in an apical anaerobic co-culture system. Cell Microbiol. 2018;20:e12805. doi: 10.1111/cmi.12805. [DOI] [PubMed] [Google Scholar]
- 88.Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:979. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rios-Covian D, Gueimonde M, Duncan SH, Flint HJ, de los Reyes-Gavilan CG. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol Lett. 2015;362:fnv176. doi: 10.1093/femsle/fnv176. [DOI] [PubMed] [Google Scholar]
- 90.Moens F, Weckx S, De Vuyst L. Bifidobacterial inulin-type fructan degradation capacity determines cross-feeding interactions between bifidobacteria and Faecalibacterium prausnitzii. Int J Food Microbiol. 2016;231:76–85. doi: 10.1016/j.ijfoodmicro.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 91.Laval L, Martin R, Natividad JN, Chain F, Miquel S, Desclée de Maredsous C, Capronnier S, Sokol H, Verdu EF, van Hylckama Vlieg JE, et al. Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut Microbes. 2015;6:1–9. doi: 10.4161/19490976.2014.990784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carlson JL, Erickson JM, Lloyd BB, Slavin JL. Health effects and sources of prebiotic dietary fiber. Curr Dev Nutr. 2018;2:nzy005. doi: 10.1093/cdn/nzy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8:172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lordan C, Thapa D, Ross RP, Cotter PD. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes. 2020;11:1–20. doi: 10.1080/19490976.2019.1613124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.La Rosa SL, Kachrimanidou V, Buffetto F, Pope PB, Pudlo NA, Martens EC, Rastall RA, Gibson GR, Westereng B. Wood-derived dietary fibers promote beneficial human gut microbiota. mSphere. 2019;4:e00554-18. doi: 10.1128/mSphere.00554-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tochio T, Kadota Y, Tanaka T, Koga Y. 1-kestose, the smallest Fructooligosaccharide component, which efficiently stimulates Faecalibacterium prausnitzii as well as bifidobacteria in humans. Foods. 2018;7:E140. doi: 10.3390/foods7090140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Poeker SA, Geirnaert A, Berchtold L, Greppi A, Krych L, Steinert RE, de Wouters T, Lacroix C. Understanding the prebiotic potential of different dietary fibers using an in vitro continuous adult fermentation model (PolyFermS) Sci Rep. 2018;8:4318. doi: 10.1038/s41598-018-22438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Y, Yu K, Chen H, Su Y, Zhu W. Caecal infusion of the short-chain fatty acid propionate affects the microbiota and expression of inflammatory cytokines in the colon in a fistula pig model. Microb Biotechnol. 2018;11:859–868. doi: 10.1111/1751-7915.13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tedelind S, Westberg F, Kjerrulf M, Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13:2826–2832. doi: 10.3748/wjg.v13.i20.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pool-Zobel BL, Sauer J. Overview of experimental data on reduction of colorectal cancer risk by inulin-type fructans. J Nutr. 2007;137:2580S–2584S. doi: 10.1093/jn/137.11.2580S. [DOI] [PubMed] [Google Scholar]
- 101.Cherbut C. Inulin and oligofructose in the dietary fibre concept. Br J Nutr. 2002;87(Suppl 2):S159–S162. doi: 10.1079/BJNBJN2002532. [DOI] [PubMed] [Google Scholar]
- 102.Kilua A, Nomata R, Nagata R, Fukuma N, Shimada K, Han KH, Fukushima M. Purple sweet potato polyphenols differentially influence the microbial composition depending on the fermentability of dietary fiber in a mixed culture of swine fecal bacteria. Nutrients. 2019;11:E1495. doi: 10.3390/nu11071495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, Louis P. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii . Br J Nutr. 2009;101:541–550. doi: 10.1017/S0007114508019880. [DOI] [PubMed] [Google Scholar]
- 104.Hryckowian AJ, Van Treuren W, Smits SA, Davis NM, Gardner JO, Bouley DM, Sonnenburg JL. Microbiota-accessible carbohydrates suppress Clostridium difficile infection in a murine model. Nat Microbiol. 2018;3:662–669. doi: 10.1038/s41564-018-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fachi JL, Felipe JS, Pral LP, da Silva BK, Corrêa RO, de Andrade MC, da Fonseca DM, Basso PJ, Câmara NO, de Sales E Souza ÉL, et al. Butyrate protects mice from Clostridium difficile-induced colitis through an HIF-1-dependent mechanism. Cell Reports. 2019;27:750–761.e7. doi: 10.1016/j.celrep.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 106.Chung WSF, Meijerink M, Zeuner B, Holck J, Louis P, Meyer AS, Wells JM, Flint HJ, Duncan SH. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol Ecol. 2017;93 doi: 10.1093/femsec/fix127. [DOI] [PubMed] [Google Scholar]
- 107.Chen Y, Xu C, Huang R, Song J, Li D, Xia M. Butyrate from pectin fermentation inhibits intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E-deficient mice. J Nutr Biochem. 2018;56:175–182. doi: 10.1016/j.jnutbio.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 108.Ferreira-Lazarte A, Moreno FJ, Cueva C, Gil-Sánchez I, Villamiel M. Behaviour of citrus pectin during its gastrointestinal digestion and fermentation in a dynamic simulator (simgi®) Carbohydr Polym. 2019;207:382–390. doi: 10.1016/j.carbpol.2018.11.088. [DOI] [PubMed] [Google Scholar]
- 109.Bianchi F, Larsen N, Tieghi TM, Adorno MA, Saad SM, Jespersen L, Sivieri K. In vitro modulation of human gut microbiota composition and metabolites by Bifidobacterium longum BB-46 and a citric pectin. Food Res Int. 2019;120:595–602. doi: 10.1016/j.foodres.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 110.Karetkin BA, Guseva EV, Evdokimova SA, Mishchenko AS, Khabibulina NV, Grosheva VD, Menshutina NV, Panfilov VI. A quantitative model of Bacillus cereus ATCC 9634 growth inhibition by bifidobacteria for synbiotic effect evaluation. World J Microbiol Biotechnol. 2019;35:89. doi: 10.1007/s11274-019-2665-2. [DOI] [PubMed] [Google Scholar]
- 111.Moens F, De Vuyst L. Inulin-type fructan degradation capacity of Clostridium cluster IV and XIVa butyrate-producing colon bacteria and their associated metabolic outcomes. Benef Microbes. 2017;8:473–490. doi: 10.3920/BM2016.0142. [DOI] [PubMed] [Google Scholar]
- 112.Remely M, Hippe B, Geretschlaeger I, Stegmayer S, Hoefinger I, Haslberger A. Increased gut microbiota diversity and abundance of Faecalibacterium prausnitzii and Akkermansia after fasting: a pilot study. Wien Klin Wochenschr. 2015;127:394–398. doi: 10.1007/s00508-015-0755-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rangan P, Choi I, Wei M, Navarrete G, Guen E, Brandhorst S, Enyati N, Pasia G, Maesincee D, Ocon V, et al. Fasting-mimicking diet modulates microbiota and promotes intestinal regeneration to reduce inflammatory bowel disease pathology. Cell Reports. 2019;26:2704–2719.e6. doi: 10.1016/j.celrep.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou ZL, Jia XB, Sun MF, Zhu YL, Qiao CM, Zhang BP, Zhao LP, Yang Q, Cui C, Chen X, et al. Neuroprotection of fasting mimicking diet on MPTP-induced Parkinson's disease mice via gut microbiota and metabolites. Neurotherapeutics. 2019;16:741–760. doi: 10.1007/s13311-019-00719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]