Interferons (IFNs) are “warning signal” cytokines released upon pathogen sensing. IFNs control the expression of interferon-stimulated genes (ISGs), which are often crucial to restrict viral infections and establish a cellular antiviral state.1,2 Langerin (CD207), a well-known surface receptor on Langerhans cells (LC), belongs to the C-type lectin receptor (CLR) family and constitutes a major pathogen binding receptor able to regulate both innate and adaptive immune responses.3,4 Importantly, this CLR was reported as an antiviral receptor, notably able to bind and internalize incoming human immunodeficiency virus (HIV) virions in Birbeck granules for degradation.5,6 However, langerin was never viewed as a contributor to the interferon-mediated antiviral immune response. We now provide evidence that langerin is an ISG showing upregulated expression upon IFN treatment in monocyte-derived and ex vivo human skin-isolated LCs.
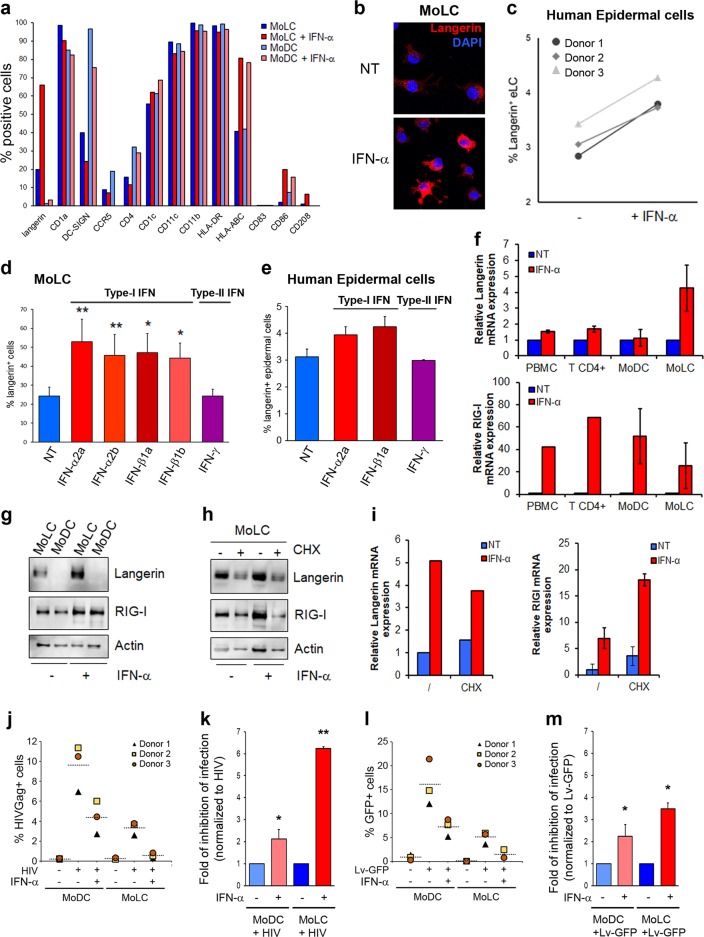
Monocyte-derived dendritic cells (MoDCs) express high levels of DC-SIGN (CD209) (>95%) but negligible levels of langerin (≤2%), while monocyte-derived Langerhans cells (MoLCs) show substantial langerin expression (≥20%) and lower DC-SIGN levels (Fig. 1a and Supplementary Fig. 1a). Upon treatment of both DC subtypes for 24 h with interferon-α (IFN-α), langerin expression was significantly increased (≥60%) in MoLCs but remained very low in MoDCs (≤2%) (Fig. 1a). Notably, langerin levels were barely increased in MoDCs treated with IFN-α, suggesting that the optimal IFN-α-mediated control of langerin expression required a preconditioned transcriptional environment similar to that observed during MoLC differentiation. Interestingly, among the markers screened, CD86 and CD208 were also positively upregulated upon IFN treatment, although at much lower levels compared to langerin expression (Fig. 1a). The enhanced expression of HLA-ABC molecules was also observed upon IFN treatment of both DC subtypes, as previously reported in lymphoid cells.7 The IFN-α-mediated upregulation of langerin expression in MoLCs was confirmed by immunofluorescence microscopy analyses of MoLCs treated or not with IFN-α for 24 h (Fig. 1b) and further validated by immunoblotting of MoLC lysates (Supplementary Fig. 1b). To expand our findings to a more relevant LC model, we isolated ex vivo human epidermal LCs (eLCs) from abdominoplasties, which were processed as previously described.8 Cells migrating from the epidermal layer were treated with or without IFN-α and stained with fluorescently coupled anti-langerin and anti-CD1a antibodies. As shown in Fig. 1c, the pool of langerin+ expressing cells from three different donors was substantially increased upon IFN-α treatment. We further demonstrated that only type-I IFNs (IFN-α2a, IFN-α2b, IFN-β1a, and IFN-β1b), but not type-II (IFN-γ), were able to upregulate langerin expression levels (Fig. 1d), reminiscent of the expression pattern of the ISG bone marrow stromal cell antigen-2 (BST-2, also named CD317 or tetherin) (Supplementary Fig. 2). Human eLCs also showed a type-I IFN-dependent increase in langerin expression (Fig. 1e). Using human PBMCs or isolated primary human CD4+ T cells in parallel with autologous MoLCs and MoDCs, we demonstrated a broad-spectrum IFN-α-mediated increase in retinoic acid-inducible gene I (RIG-I) mRNA levels in all cell types, while a significant IFN-α-mediated langerin mRNA upregulation was seemingly confined to MoLCs (Fig. 1f), as also confirmed at the protein level by immunoblotting (Fig. 1g). Cells pretreated with cycloheximide (CHX), a known protein synthesis inhibitor, showed a decrease in both langerin and RIG-I protein expression (Fig. 1h). Importantly, CHX treatment did not impede the upregulation of langerin and RIG-I gene expression upon IFN-α administration, therefore demonstrating the direct involvement of IFN-α in de novo langerin expression (Fig. 1i). Interestingly, compared to MoDCs, the treatment of MoLCs with TLR agonists induced a global reduction in TNF-α production. However, IFN-treated MoLCs (MoLC-IFN) responded efficiently to viral-mimicking TLR agonists, suggesting that these cells remain endowed with efficient viral sensing and exert a subsequent antiviral response (Supplementary Fig. 3). We thus compared the antiviral capacity of MoDCs with that of MoLCs in the presence or absence of IFN upon challenge with wild-type HIV-1 (HIV) or VSV-G pseudotyped GFP-expressing lentivectors (Lv-GFP) that are able to bypass langerin-mediated HIV entry restriction.5,8 As expected, MoDCs were more susceptible to HIV infection than autologous MoLCs were, and pretreatment with type-I IFN strongly reduced the HIV infection of both DC subtypes (Fig. 1j and k). However, the marked antiviral effect observed in HIV-infected MoLCs compared to HIV-infected MoDCs was no longer evident when both cell types were pretreated with type-I IFN and challenged with Lv-GFP (Fig. 1l), as clearly indicated by a fold reduction in the inhibition of infection between the cell types (Fig. 1m). Although the infection rate was seemingly higher in both cell types when exposed to Lv-GFP compared to HIV, the antiviral effect of IFN-α on Lv-GFP infection was diminished in MoLCs but not in MoDCs (compare Fig. 1k and 1m). This finding suggests the presence of a type-I IFN-inducible cell surface factor on MoLCs that is able to limit the entry of incoming HIV wild-type virions, a function reported for langerin. In conclusion, our study offers a novel aspect of the regulation of langerin expression and extends the list of ISGs as potential cellular effectors able to amplify the host antiviral response.
Fig. 1.
a Interferon-mediated modulation of cell surface markers in human primary monocyte-derived DCs (MoDCs) and LCs (MoLCs), pretreated or not for 24 h with 103 U/ml of IFN-α2a. b MoLCs, treated or not treated with IFN-α2a for 24 h, were seeded onto coverslips, fixed, permeabilized, and stained with anti-langerin antibodies. The nuclei were stained with DAPI. c Epidermal walkout cells treated or not with IFN-α2a for 24 h were analyzed for langerin expression levels upon staining and flow cytometry analyses. d Graph representing langerin+ MoLCs untreated or treated for 24 h with IFN-α2a or IFN-α2b or IFN-β1a or IFN-β1b or IFN-γ (all at 103 U/ml). e Same experiment as described above but with epidermal walkout cells treated as indicated. f RT-qPCR analyses of langerin and RIG-I mRNA expression in the indicated cells treated or not with IFN-α2a for 8 h (n = 2). g Lysates from MoDCs and MoLCs treated or not with IFN-α2a for 24 h were immunoblotted with anti-langerin and anti-RIG-I antibodies. Loading was controlled with anti-actin (n = 2). MoLCs pretreated with 10 μM cycloheximide (CHX) for 1 h was stimulated for 24 h to analyze the indicated protein expression levels by immunoblotting (h) or 8 h to analyze the indicated transcript levels by RT-qPCR (i). MoDCs or MoLCs were incubated or not with 103 U/ml IFN-α2a for 24 h prior to challenge with HIV-1-R5 viruses or Lv-GFP for 72 h. The cells were analyzed for HIV-Gag (j) or GFP (l) expression by flow cytometry and the results are presented in a graph in which HIV-Gag+ cells and GFP+ cells are represented by a dotted horizontal segment (n = 3). The fold inhibition of HIV infection (k) or Lv-GFP transduction (m) is presented in graphs with the data normalized to each untreated cell type (n = 3)
Supplementary information
Acknowledgements
We thank Richard O.S. Karoo and the members of Spire Cardiff Hospital (Wales, UK) for providing skin samples. We are grateful to J. Paul Mitchell, Sion A. Coulman (Cardiff, Wales, UK), and all members of the Viral Trafficking, Restriction and Innate Signaling Team for excellent technical help and/or critical reading of the manuscript. We are also grateful to Caroline Goujon and Olivier Moncorgé (IRIM, Montpellier, France) for the kind gift of reagents. Immunofluorescence imaging and some of the flow cytometry acquisitions were performed at Montpellier RIO Imaging (Montpellier, France). This work was supported in part by an ANRS grant (No. D15236) to F.P.B. This work was also supported by the Cardiff University President’s Research Scholarship to M.A.C. and grants from ISSF-WT and the Gates Foundation to V.P. G.M. was the recipient of a postdoctoral fellowship from Labex EpiGenMed (Montpellier, France) and is currently supported by the ANRS. The funding bodies had no role in the preparation of the article, design of the study, or interpretation of the data.
Author Contributions
M.A.C., G.M., and F.P.B. conceived the study. J.C.B., V.P., S.N., and F.P.B. assisted with the experimental design or provided reagents. M.A.C., G.M., J.L., M.O.I., K.F., L.P., and F.P.B. carried out the experiments; and F.P.B. wrote the manuscript. All authors read and commented on the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Ghizlane Maarifi, Magdalena A. Czubala
Supplementary information
The online version of this article (10.1038/s41423-019-0302-5) contains supplementary material.
References
- 1.Doyle T, Goujon C, Malim MH. HIV-1 and interferons: who’s interfering with whom? Nat. Rev. Microbiol. 2015;13:403–413. doi: 10.1038/nrmicro3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annu. Rev. Immunol. 2014;32:513–545. doi: 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 4.Valladeau J, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/S1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 5.de Witte L, et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 2007;13:367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 6.van der Vlist M, Geijtenbeek TB. Langerin functions as an antiviral receptor on Langerhans cells. Immunol. Cell Biol. 2010;88:410–415. doi: 10.1038/icb.2010.32. [DOI] [PubMed] [Google Scholar]
- 7.Burrone OR, Kefford RF, Gilmore D, Milstein C. Stimulation of HLA-A,B,C by IFN-alpha. The derivation of Molt 4 variants and the differential expression of HLA-A,B,C subsets. EMBO J. 1985;4:2855–2860. doi: 10.1002/j.1460-2075.1985.tb04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czubala MA, et al. TGFbeta induces a SAMHD1-independent post-entry restriction to HIV-1 infection of human epithelial Langerhans cells. J. Invest. Dermatol. 2016;136:1981–1989. doi: 10.1016/j.jid.2016.05.123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.