Abstract
Tumor cell plasticity exhibited as an epithelial-mesenchymal transition has been identified as a major obstacle for effective treatment of many cancers. This process, which involves the dedifferentiation of epithelial tumor cells towards a motile, metastatic, and mesenchymal tumor phenotype, mediates resistance to conventional therapies and small molecule targeted therapies. In this review, we highlight current research correlating the role of tumor plasticity with resistance to current immunotherapy approaches and discuss future and ongoing combination immunotherapy strategies to reduce tumor cell plasticity driven resistance in cancer.
Keywords: tumor plasticity, EMT, immunotherapy resistance, IL-8, TGF-β
Tumor cell plasticity remains an obstacle for breakthrough cancer immunotherapies
Cancer is a dynamic, evolving disease that exhibits remarkable heterogeneity at the molecular and phenotypic level. Tumors of the same type vary among patients and also within a given patient, where profound differences can be found among cancer cells in different lesions or within the same tumor mass [1–3]. Heterogeneity increases as the disease progresses due to genomic instability and has also been shown to increase as a result of tumor adaptation and persistence under selection pressure from drug treatments. An example of the latter is the emergence of clonal populations in lung tumors treated with EGFR-targeting therapies, which acquire additional mutations, alternative signaling pathways, or different phenotypes that confer resistance to therapy [4–6].
Immunotherapy approaches to treat cancer have revolutionized modern oncology. One of the advantages of immunotherapy in the context of tumor heterogeneity is that the adaptive immune system can recognize multiple tumor antigens within an heterogenous cancer cell population. More importantly, mutated tumor proteins or neo-antigens that appear as a consequence of genetic evolution under selection from therapies could also be effectively targeted by immune cells. However, tumors inhibit the immune system through multiple mechanisms including upregulation of suppressive ligands such as programmed death ligand 1 (PD-L1), suppressive cytokines such as TGF-β, and via the recruitment of immune-suppressive cell populations including myeloid derived suppressor cells (MDSCs) and T regulatory cells (Tregs). To overcome this immune suppression, blocking monoclonal antibodies have been developed against the immune checkpoint molecules cytotoxic T lymphocyte associated protein 4 (CTLA-4) and programmed cell death 1 receptor (PD-1) or its ligand PD-L1. Overall designated as immune checkpoint blockade therapies or ICB, these antibodies are able to reactivate anergic tumor-specific T cells in the tumor and in the periphery [7]. The clinical use of ICB has led to the successful treatment of patients with early and late stage, and even metastatic solid tumors, which result in durable clinical responses in 20–30% of patients with solid carcinomas [8]. Despite these advancements, there is a large patient population that fails to respond to monotherapy checkpoint blockade. Therefore, novel approaches aimed at targeting multiple mechanisms of immune suppression or resistance to checkpoint inhibition are being explored.
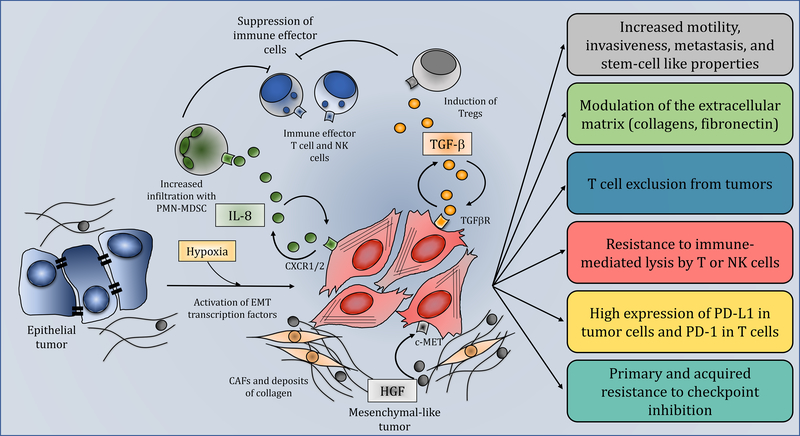
A mechanism recently implicated in resistance to ICB is the induction of tumor cell plasticity. In the context of cancer, plasticity is defined as terminally differentiated cancer cells being able to undergo profound alterations in cell phenotype as a result of activation of oncogenic drivers or in response to external stimuli. This dedifferentiation program of cancer cells, which is comparable to similar phenomena observed in embryo development, wound healing, and tissue regeneration, is termed an epithelial-mesenchymal transition (EMT). Driven by a multitude of soluble factors including TGF-β and IL-8, or transcription factors including snail, slug, twist, zeb1, zeb2, brachyury, and foxc2, tumor cells undergoing EMT downregulate expression of epithelial markers including E-cadherin, occludin, ZO-1, and keratins, while upregulating mesenchymal proteins including vimentin, fibronectin, and collagens that promote invasiveness and metastasis. There are many excellent reviews about the overarching concept of EMT in cancer and the role of this phenomenon in tumor resistance to chemotherapy, radiation, and small molecule targeted therapies [9–12]. This review will highlight current preclinical and clinical research correlating the induction of tumor cell plasticity with increased resistance to immunotherapy and discuss potential mechanisms to reduce or reverse EMT-driven resistance to ICB (Figure 1, Key Figure).
Figure 1. Role of tumor cell plasticity in resistance to immunotherapy.
Epithelial tumor cells (blue cells, left) acquire mesenchymal features through activation of EMT transcription factors or signaling initiated by soluble factors including IL-8 (circular green molecules) and TGF-β (circular orange molecules). Secreted IL-8 recruits PMN-MDSCs (green cells) to the tumor which in turn suppress immune effector T and NK cells (blue cells, top). TGF-β released by the tumor cells can induce Tregs (grey cells) which also contribute to the suppressive microenvironment. HGF (circular grey molecules) secreted by CAFs (yellow cells) signal through c-MET to promote tumor cell plasticity. Circular arrows indicate the ability of soluble factors to act in an autocrine fashion to maintain the mesenchymal phenotype of the tumor. The boxes on the right describe the various properties of mesenchymal tumor cells (red cells), including increased motility, invasiveness, and propensity to metastasize; ability to impact the tumor microenvironment (TME) via modulation of the extracellular matrix and promoting T cell exclusion from tumors; resistance to the cytotoxic activity of immune effector cells; upregulation of PD-L1. Altogether, these mechanisms synergize to promote primary and acquired resistance to immune checkpoint blockade.
Soluble factors and signaling pathways drive tumor cell plasticity and immune suppression
Many soluble factors have been implicated in the role of inducing tumor cell plasticity. These factors promote the migration, metastasis, and resistance of cancer cells to therapy and also contribute to a greater immune suppressed tumor microenvironment (TME) [13–17]. One such factor is the chemokine IL-8 [18], which was initially identified as a chemokine secreted by macrophages and epithelial cells for neutrophil recruitment to areas of inflammation, infection, or injury. The effects of IL-8 are mediated through its binding to the G protein-coupled receptors CXCR1 and CXCR2 that are normally expressed on granulocytes, monocytes, and endothelial cells [19]. However, uncharacteristic overexpression of IL-8 and its receptors has been observed in numerous types of cancer including breast, prostate, lung, colon, and others with poor clinical outcomes [20]. In tumor cells, signaling of IL-8 through CXCR1 and CXCR2 has been shown to promote the acquisition of tumor cell mesenchymal features, including vimentin, fibronectin, and transcription factors that induce the phenomenon of EMT and stemness [21], thus promoting cell migration, invasion, and metastasis. IL-8 also acts as a driver of immune suppression in the TME through the recruitment of MDSCs, which have been shown to inhibit T cell homing to lymph nodes, deplete nutrients required for T cell expansion, promote the recruitment of cancer associated fibroblasts and Tregs, and shed immune suppressive exosomes [22–24]. Not only does IL-8 chemoattract MDSCs to the TME, but also recent findings suggest that IL-8 enhances MDSC suppression. This was shown with a novel subset of MDSCs identified in gastric cancer that depend on IL-6/IL-8 derived PI3K-AKT signaling to express T cell suppressive arginase I [25]. Additionally, increasing amounts of circulating MDSC have been correlated with higher IL-8 levels in the serum of patients with prostate cancer [26]. Therefore, IL-8 plays a dual role in driving tumor progression through recruitment of suppressive immune cells and inducing tumor cell EMT.
TGF-β is another targetable, potent inducer of tumor cell plasticity and TME inflammation [27]. Yet, TGF-β has been identified as having dual roles as both tumor suppressive and tumor promoting. Early in tumorigenesis, TGF-β acts to inhibit tumor cell growth and induce apoptosis; however, in late stages of cancer it acts as a major inducer of immune suppression and as a driver of EMT in tumor cells. TGF-β can be expressed by tumor cells, immune cells, and stromal cells in three forms, TGF-β1, -β2, and -β3, which have similar but not identical biological activities [28]. TGF-β promotes tumor cell plasticity and metastasis through the activation of numerous EMT transcription factors including zeb1 [29], while driving immune suppression in the TME through multiple mechanisms, including via promotion of T cell exclusion from tumors [30, 31] or by modulating the expression of PD-1 and PD-L1 on T cells and tumor cells, respectively [32, 33]. David et al. reported that TGF-β1 was able to enhance PD-L1 gene transcription and surface expression through a Smad2 dependent mechanism in non-small cell lung cancer (NSCLC) cell lines which corresponded with high PD-L1 and phosphorylated Smad2 in patient tumor samples [33]. Park et al. also identified TGF-β1 as a mediator of PD-1 upregulation on antigen-specific T cells in the TME; the upregulation of PD-1 through the Smad3 promoter resulted in T cells with decreased cytokine production and functionality [34]. Similar to IL-8, TGF-β plays multiple roles in cancer progression through the promotion of immune suppression and tumor cell plasticity.
AXL, a member of the TAM (Tyro3, AXL, Mer) receptor tyrosine kinase family, has also been identified as a targetable inducer of tumor cell plasticity. While usually expressed ubiquitously in several organs under normal physiological conditions, AXL has been found to be overexpressed in numerous cancer types [35] and implicated in the expression of EMT transcription factors snail, slug, and twist while positively correlating with a mesenchymal phenotype in lung and breast cancers. Overall, AXL expression has been shown to increase tumor cell invasion and metastasis, directly promote tumor cell growth and survival, promote T cell exclusion from the TME, and confer resistance to tyrosine kinase inhibitors, including erlotinib [36, 37].
In addition to the factors mentioned above, several other soluble factors, signals derived from extracellular matrix (ECM) components, and environmental stresses can also promote tumor cell plasticity and drive immune suppression (Box 1).
Box 1. Factors that drive tumor cell plasticity.
In addition to IL-8, TGF-β, and AXL, several other signals originating in the TME have been shown to induce tumor cell plasticity which, in turn, could drive immune suppression and progression. Non-cellular components of the ECM including collagen, fibronectin, hyaluronan, and others have been shown to directly signal via receptors on the surface of tumor cells to induce the expression of EMT transcription factors. For example, binding of collagen I to the discoidin domain-containing receptor 2 (DDR2) has been shown to stabilize the transcription factor snail in breast cancer cells leading to an EMT [77]. Moreover, collagen can contribute to the stiffness of the ECM which in turn has been shown to induce EMT through a mechanotransduction process [78]. Among the cellular components of the ECM, cancer associated fibroblasts (CAFs) have been implicated in driving tumor progression by inducing tumor cell plasticity and stemness. CAF induction of EMT involves the secretion of numerous growth factors and cytokines including hepatocyte growth factor (HGF), fibroblast growth factor (FGF), IL-6, and others. As an example, secretion of HGF by CAFs was shown to promote tumor plasticity in gastric cancer via binding of HGF to the c-MET receptor in tumor cells and activation of the HGF/c-MET/twist1 pathway [79]. Similarly, in bladder cancer, activation of the HGF/c-MET pathway induces TGF-β signaling leading to tumor cell plasticity and invasion [80]. Regarding a role in immune suppression, the HGF/c-MET pathway has also been shown to promote the recruitment of suppressive neutrophils to the TME thus limiting T cell expansion and effector function [81].
Activation of the Wnt/β-catenin pathway is another common phenomenon in numerous cancer types including colorectal, melanoma, breast, non-small cell lung, ovarian, and others, which has been shown to be associated with the acquisition of tumor mesenchymal features. More importantly, recent studies have demonstrated the ability of β-catenin to promote tumor immune escape and resistance to checkpoint therapy, including in melanoma patients and hepatocellular carcinoma models [42, 82]. Lastly, hypoxic tumor conditions can also activate signaling pathways which enhance the recruitment and function of suppressive MDSC and Tregs while promoting the expression of EMT transcription factors, including snail, slug, twist1 and others. Interestingly, the EMT-inducing effect of hypoxia and its associated resistance to the cytotoxic effect of immune effector cells have been show to take place in subsets of cancer cells, a phenomenon that may contribute to the occurrence of heterogeneity along progression [83].
Tumor cell plasticity as a molecular phenotype correlates with immune resistance
Many recent studies have linked an overall tumor EMT phenotype with immune suppression through PD-L1 expression and resistance to therapies. For example, a positive correlation between an EMT gene signature and PD-L1 expression was reported with primary tumors of lung cancer patients. The study also showed that PD-L1 expression and metastasis were both controlled by the EMT transcription factor zeb1 [32]. In the context of colorectal cancer, a molecular subgroup of tumors characterized by the presence of EMT features and expression of TGF-β exhibited the worst overall survival and relapse-free survival [38]. Further insight was provided by a study linking an EMT gene signature and overall immune status where mesenchymal lung tumors exhibited high expression of immune checkpoint molecules [39]. Hugo et al. also described a transcriptomic signature of upregulated EMT genes that were associated with primary resistance (i.e., in patients who never responded) to anti-PD-1 therapy in metastatic melanoma patients [40]. This signature included plasticity-inducing and immune suppressive genes such as AXL, WNT, TWIST2, ROR2, IL10, VEGFA, and others. A recent study also demonstrated the upregulation of β-catenin or acquired PTEN loss as being responsible for recurrence in metastatic melanoma patients who initially responded to ICB [41]. While evaluation of tumor cell plasticity was not reported in this study, others have previously documented the role of β-catenin/Wnt signaling as a potent inducer of EMT and T cell exclusion in cancer (Box 1) [42, 43]. Overall, these studies supported a link between the occurrence of EMT or plasticity in tumors with high immune suppression and worse prognosis across numerous cancer types.
EMT transcription factors and signaling pathways that drive plasticity in cancer cells also help confer resistance against antitumor immunity and therapy. For example, melanoma tumor cells driven into an EMT via snail overexpression were shown to induce Tregs and to inhibit dendritic cell function through the secretion of TGF-β and TSP1. Snail was shown to promote tumor growth and metastasis in vivo and confer resistance against antigen-specific T cell lysis [44]. Others have identified snail as being able to shield tumor cells from T cell mediated lysis through induction of autophagy [45]. Another transcription factor, brachyury, has been shown to be a potent inducer of EMT [46, 47]. Brachyury was also shown to protect tumor cells from both caspase-dependent and caspase-independent mechanisms of immune induced cell killing via dysregulation of the CDK1 and mucin-1 pathways [48, 49]. In a more recent study, it was shown that hypoxic conditions could upregulate numerous EMT transcription factors including snail, slug, twist, and zeb2 in subsets of lung adenocarcinoma cells, resulting in highly mesenchymal cells that were resistant to both T cell and NK cell mediated killing [50].
Reversal of tumor cell plasticity and EMT sensitizes tumor cells to immune mediated cell death
Armed with the knowledge that tumor cell plasticity drives a highly inflamed and suppressive TME, novel drugs have been developed against extracellular signaling molecules and receptors or intracellular signaling kinases, which attempt to halt or reverse tumor cell plasticity [36, 51]. Due to the potent effects of IL-8 on tumor cells and the TME, neutralizing monoclonal antibodies to IL-8 were developed and tested for their ability to reverse tumor cell plasticity. Dominguez et al. utilized a neutralizing anti-IL-8 antibody, HuMax-IL8, that was able to sequester IL-8 released by human triple negative breast cancer (TNBC) cell lines. Treatment of breast cancer cells in culture with HuMax-IL8 depleted tumor cell secreted IL-8 and autocrine IL-8 signaling, which inhibited the expression of mesenchymal proteins expressed by the tumor cells, including vimentin and fibronectin, and increased expression of E-cadherin and ZO-1 [17]. In addition to this shift in tumor cell phenotype in vitro, IL-8 neutralization in vivo induced similar changes in tumor phenotype while simultaneously reducing the recruitment of polymorphonuclear (PMN)-MDSC to the tumor presumably through binding to the CXCR2 receptor expressed in these myeloid cells. Most importantly, blockade of IL-8 was able to enhance both NK and T cell mediated killing of numerous human TNBC cell lines in vitro. In a separate study, reversal of tumor cell plasticity with IL-8 neutralizing antibodies was also found to alleviate resistance of NSCLC lines to erlotinib and sensitize resistant cells to chemotherapy and immune cytotoxic NK cells [16].
Based on this data, a Phase I clinical trial was designed to evaluate increasing doses of HuMax-IL8 in patients with metastatic or unresectable solid tumors. The trial included patients with colorectal, prostate, chordoma and other types of cancer [52]. HuMax-IL8 was able to decrease serum IL-8 levels, was well tolerated, and stable disease was observed in 73.3% of total patients (n=15). Currently, HuMax-IL8 (BMS-986253) is being evaluated in a Phase II clinical study (NCT03689699) in combination with nivolumab and androgen deprivation therapy in men with hormone-sensitive prostate cancer (Table 1). Due to the relevance of IL-8 in tumor progression, a new small molecule inhibitor, termed SX-682, which simultaneously blocks CXCR1 and CXCR2, has been developed [53]. Preclinical studies have successfully used SX-682 to prevent PMN-MDSC migration to the TME and to enhance immunotherapy approaches with ICB and adoptively transferred murine NK cells [54–56]. A Phase I clinical trial (NCT03161431) of SX-682 in combination with pembrolizumab in patients with melanoma is currently ongoing (Table 1).
Table 1.
Clinical studies of the combination of ICB with agents that modulate EMT
| Checkpoint Agent | Plasticity Modifying Agent | Additional Therapy | Type of cancer | Clinical Trial Number* | Phase | Status |
|---|---|---|---|---|---|---|
| IL-8 axis | ||||||
| Nivolumab | BMS-986253 (α-IL-8) | Degarelix | Prostate | NCT03689699 | IB/II | R |
| Pembrolizumab | SX-682 (CXCR1/2 inhibitor) | Melanoma | NCT03161431 | I | R | |
| TGF-β | ||||||
| Nivolumab | Galunisertib (TGF-βRI inhibitor) | Solid tumors | NCT02423343 | I/II | A | |
| Pembrolizumab | LY3200882 (TGF-βRI inhibitor) | Advanced cancer | NCT04158700 | I/II | NR | |
| PDR001 | NIS793 (α-TGF-β) | Advanced cancer | NCT02947165 | I/IB | R | |
| Durvalumab | Vactosertib (TGF-βRI inhibitor) | Urothelial | NCT04064190 | II | NR | |
| LY3300054 | LY3200882 (TGF-βRI inhibitor) | Chemotherapy Radiotherapy | Solid tumors | NCT02937272 | I | R |
| Bintrafusp alfa (α-PDL1/TGF-βRII fusion protein) | Gemcitabine Cisplatin | Biliary tract | NCT04066491 | II/III | R | |
| Bintrafusp alfa (α-PDL1/TGF-βRII fusion protein) | Biliary tract | NCT03833661 | II | R | ||
| Bintrafusp alfa (α-PDL1/TGF-βRII fusion protein) | HER2+ breast | NCT03620201 | I | R | ||
| Bintrafusp alfa (α-PDL1/TGF-βRII fusion protein) | Radiation | HR+, HER2− breast | NCT03524170 | I | R | |
| Bintrafusp alfa (α-PDL1/TGF-βRII fusion protein) | Eribulin mesylate | TNBC | NCT03579472 | IB | R | |
| Bintrafusp alfa (α-PDL1/TGF-βRII fusion protein) | Colorectal, solid tumors w/MSI | NCT03436563 | IB/II | R | ||
| Bintrafusp alfa (α-PDL1/TGF-βRII fusion protein) | Topotecan or Temozolomide | Relapsed SCLC | NCT03554473 | I/II | R | |
| AXL | ||||||
| Nivolumab | INCB081776 (AXL inhibitor) | Solid tumors | NCT03522142 | I | R | |
| Pembrolizumab | Bemcentinib (AXL inhibitor) | NSCLC | NCT03184571 | II | R | |
| Pembrolizumab | Bemcentinib (AXL inhibitor) | Mesothelioma | NCT03654833 | II | R | |
| Durvalumab | AVB-S6-500 (AXL-Fc) | Ovarian, Fallopian tube | NCT04019288 | I/II | R | |
| Avelumab | AVB-S6-500 (AXL-Fc) | Urothelial | NCT04004442 | II | NR | |
R= recruiting; NR= not yet recruiting; A= active; as of Dec 15, 2019; α-PD-1 agents: nivolumab, pembrolizumab, PDR001; α-PD-L1 agents: durvalumab, LY3300054, avelumab.
Many small molecule inhibitors and antibodies have also been developed to target TGF-β. Galunisertib (LY2157299 monohydrate) is a small molecule inhibitor of the TGF-βRI ALK5 kinase that has been utilized in numerous preclinical studies and clinical trials with an acceptable safety profile [57]. A preclinical assessment of galunisertib revealed that it was able to regulate the phenotype of the mouse pancreatic cancer cell line KPC-M09 by preventing TGF-β mediated downregulation of E-cadherin. Galunisertib was also able to rescue effector functions of both CD8+ T cells and NK cells suppressed by TGF-β in vitro [58]. In Phase II clinical studies, galunisertib demonstrated some antitumor efficacy in patients with pancreatic cancer and in a subset of patients with advanced hepatocellular cancer [59, 60]. These studies and others have provided strong evidence that novel drugs can modulate or reverse tumor cell plasticity and EMT to potentially sensitize tumor cells to immune mediated cell death.
Reversal of tumor cell plasticity enhances ICB therapy
Because TGF-β is a major inducer of tumor cell plasticity, PD-L1 upregulation, and overall immune suppression, tumor immunotherapies combining PD-1/PD-L1 checkpoint therapy with TGF-β blockade are being extensively explored in the lab and the clinic. Recent studies have elucidated the mechanism of TGF-β inhibition plus PD-1/PD-L1 checkpoint blockade synergy in murine models of cancer. Utilizing genetically modified mice that spontaneously develop metastatic intestinal tumors, the interplay between T cell exclusion, low mutational burden, TGF-β, and PD-L1 was investigated. While anti-PD-L1 monotherapy was nonresponsive in this model, TGF-β inhibition with galunisertib reduced primary tumor growth and metastatic lesions while driving the upregulation of PD-1 on activated T cells and PD-L1 in metastatic lesions. The combination of anti-TGF-β and anti-PD-L1 was shown to induce a more potent antitumor response with T cells highly positive for T-bet, IFN-γ, and granzyme B [31]. In another study, Dodagatta-Marri et al. initially observed that numerous murine cutaneous squamous cell carcinoma cell lines with low mutational load were unresponsive to PD-1 checkpoint therapy. Resistance to ICB in these models was associated with upregulation of CD4+ Tregs in the tumor and pSmad3 expression in tumor cells driven by TGF-β. While anti-TGF-β therapy did improve tumor control, combination therapy of anti-TGFβ plus anti-PD-1 synergized to produce the most complete responses. The addition of TGF-β neutralization resulted in a decrease of tumor infiltrating Tregs, decreases in tumor cell plasticity, and tumor upregulation of antigen presentation machinery components [61]. However, it is important to emphasize that TGF-β and PD-1/PD-L1 combination therapy may not always synergize. A study utilizing the TGF-βRI inhibitor LY364947 in combination with anti-PD-L1 to treat the highly immunogenic colon MC38 and the poorly immunogenic KPC1 pancreatic tumor models demonstrated efficacy of the combination in MC38 but not in KPC1 tumor bearing mice [62]. Nevertheless, the encouraging preclinical success of combining TGF-β inhibition with ICB has led to the formulation of numerous clinical trials aimed at testing the combination in patients with advanced stage solid cancers (Table 1).
In clinical samples, Mariathasan and colleagues linked a TGF-β associated EMT gene signature and exclusion of T cells in tumors from patients with metastatic urothelial carcinoma who did not respond to atezolizumab therapy. To study a potential link between TGF-β associated EMT and T cell exclusion, the authors utilized the EMT6 murine breast cancer model that is T cell excluded, TGFβ+, and PD-L1+. While neither anti-PD-L1 nor anti-TGFβ monotherapies reduced tumor burden in EMT6 tumor bearing mice, the combination of the two drugs synergized to induce antitumor immunity [30], an effect that was shown to be driven by the reprograming of peritumoral stromal fibroblasts combined with increases in CD8+ T effector cells.
In light of the relevance of TGF-β signaling in tumor responses to anti-PD-1/PD-L1 blockade, new approaches for dual blockade of checkpoint molecules and TGF-β are being explored through the use of bifunctional fusion proteins. These novel drugs consist of a checkpoint blockade antibody (anti-PD-L1) linked to the extracellular domain of TGF-βR molecules. The rationale and functionality of these fusion proteins lies in the direct targeting of TGF-β sequestration to the TME via binding to anti-PD-L1. Many studies have been conducted with bintrafusp alfa (formerly M7824), an anti-PD-L1/TGF-βRII fusion protein. Lan et al. and Knudson et al. reported greater inhibition of tumor growth in multiple murine tumor models with bintrafusp alfa compared to either anti-PD-L1 or anti-TGF-β alone or in combination [63, 64]. Further studies conducted by David et al. reported that bintrafusp alfa was able to revert TGF-β1-induced tumor cell plasticity in NSCLC cell lines and diminish mesenchymal features in xenograft models [33]. Similar studies conducted with anti-CTLA4-TGF-βRII and anti-PDL1-TGFβ-RII fusion drugs (termed Y-traps) also demonstrated the superior efficacy of these dual agents than the combination of untethered reagents at eliminating TGF-β mediated suppression and stimulating antitumor immune responses; however, the plasticity of the tumor cells were not quantified [65]. These studies and others prompted a Phase I clinical trial of bintrafusp alfa for the treatment of heavily pretreated patients with advanced solid tumors. The completion of the dose escalation phase of this trial was recently reported [66] demonstrating a manageable safety profile with encouraging early signs of efficacy in patients. There are multiple clinical trials currently ongoing evaluating the effect of bintrafusp alfa in patients with advanced breast, NSCLC, pancreatic, biliary cancer, and HPV associated malignancies, alone or in combination with other therapeutics such as radiation or chemotherapy (Table 1).
Numerous other small molecule inhibitors, monoclonal antibodies, and recombinant fusion proteins have been developed to modulate tumor cell plasticity that are separate from the IL-8 and TGF-β pathways. Preclinical data suggest that these approaches to modulate EMT may also enhance ICB and benefit patients in the clinic. For example, several clinical trials are currently being conducted combining ICB with AXL pathway modulators (Table 1).
Novel approaches of targeting tumor cell plasticity show promise for future combination with ICB therapy
Tumor cell plasticity and EMT cannot be accurately defined or classified by one individual phenotype, genotype, or expression pattern. The pathways and factors that induce the spectrum of manifestations of mesenchymal cells derived from differentiated epithelial cells are numerous and redundant. However, numerous EMT inducing transcription factors have been identified that control these often but not always redundant expressions of tumor cell plasticity genes [67]. These include but are not limited to a core-set of factors including twist, snail, slug, zeb1 and zeb2 and others more recently described such as foxc2, brachyury and YAP1. While the relevance of EMT in metastasis has been debated in the past [68, 69], the redundancy of tumor cell plasticity pathways has proven to be a powerful obstacle in overcoming tumor immune suppression. Because EMT transcription factors are ultimately responsible for the phenotypic changes typical of tumor cell plasticity, novel therapeutic approaches involving drugs that directly target these transcription factors could be envisioned to prevent or eliminate the occurrence of tumor EMT. However, direct targeting of transcription factors has been challenging so far with conventional small molecule targeted approaches. One novel approach that is currently being investigated is the use of cancer vaccines directed against immunogenic peptides or epitopes of an EMT transcription factor for activation and expansion of T cells that could recognize and eliminate tumor cells expressing the EMT transcription factor of choice. As a model system, cancer vaccines have been developed which are currently in the clinic and directed against the transcription factor brachyury [70]. Brachyury is a T-box transcription factor that is essential for vertebrate development. In adult organisms, its expression is absent from most healthy tissues while high expression of brachyury has been demonstrated in subsets of human tumors including lung, triple negative breast, colon, and prostate cancer, and in 100% of chordomas [71]. There are currently three vaccine platforms (yeast-based, poxvirus-based, and adenovirus-based) directed against brachyury which are being evaluated in Phase I and II clinical studies in patients with various types of carcinomas. In preclinical testing, brachyury-based vaccines were able to stimulate and expand CD8+ T cells that could lyse brachyury expressing tumor cell lines and significantly reduced tumor growth and metastasis in murine tumor models [72, 73]. Analysis of peripheral blood samples from patients included in brachyury-based clinical studies also demonstrated for the first time the ability of vaccines targeting an EMT transcription factor to induce CD8+ and/or CD4+ T cell immunity specific for brachyury epitopes in a large percentage of patients [74–76]. These results are of relevance considering the future of cancer immunotherapy and the prevalence of tumor cell plasticity in therapy resistant patients. Altogether the data suggest that further combination therapies of EMT transcription factor targeting vaccines and other T cell mediated therapies with ICB and EMT modulating drugs could result in a better antitumor response.
Concluding Remarks
Tumor cell plasticity manifested as an EMT has been identified as a major obstacle in the effective treatment of cancer. This phenomenon of epithelial cells undergoing dedifferentiation to become more motile, metastatic, and resistant to therapy is common across many cancer types and stages. The induction of plasticity can be a result of a multitude of soluble factors, receptors, and transcription factors. Novel drugs have been developed to target these factors and reduce overall EMT; however, tumor cell plasticity has proven difficult to target and control due to the redundancy of the overlapping pathways at play. There are many remaining questions (see Outstanding Questions) in the field regarding which elements of EMT are necessary to target and how to target those molecules. Nevertheless, agents that reduce EMT may be needed in future combination immunotherapies to ensure positive results in all patient populations.
Outstanding Questions.
What characteristics of tumor cell plasticity drive resistance to ICB therapy and in which tumor types?
Are there additional pathways and factors involved in tumor cell plasticity and EMT that can be targeted?
What combinations of tumor cell plasticity modulators and ICB will result in optimal antitumor responses?
What additional agents could be added to the combination of tumor cell plasticity modulators and ICB to further improve antitumor responses?
Highlights.
Tumor cell plasticity in the context of an epithelial-mesenchymal transition (EMT) has been implicated in resistance to immune checkpoint blockade (ICB)
Novel drugs targeting drivers of tumor cell plasticity are able to sensitize tumors to immune mediated lysis
Tumor cell plasticity modulating drugs synergize with ICB therapy for more robust antitumor immunity
Novel drugs including cancer vaccines targeting EMT transcription factors show promising results and offer new options for patients
Acknowledgments
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute (NCI), National Institutes of Health (NIH).
References
- 1.Wood LD et al. (2007) The genomic landscapes of human breast and colorectal cancers. Science 318 (5853), 1108–13. [DOI] [PubMed] [Google Scholar]
- 2.Patel AP et al. (2014) Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344 (6190), 1396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerlinger M et al. (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366 (10), 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russo M et al. (2016) Tumor Heterogeneity and Lesion-Specific Response to Targeted Therapy in Colorectal Cancer. Cancer Discov 6 (2), 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz LA Jr. et al. (2012) The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 486 (7404), 537–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hata AN et al. (2016) Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med 22 (3), 262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei SC et al. (2018) Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov 8 (9), 1069–1086. [DOI] [PubMed] [Google Scholar]
- 8.Fares CM et al. (2019) Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am Soc Clin Oncol Educ Book 39, 147–164. [DOI] [PubMed] [Google Scholar]
- 9.Derynck R and Weinberg RA (2019) EMT and Cancer: More Than Meets the Eye. Dev Cell 49 (3), 313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brabletz T et al. (2018) EMT in cancer. Nat Rev Cancer 18 (2), 128–134. [DOI] [PubMed] [Google Scholar]
- 11.Yuan S et al. (2019) Cellular Plasticity in Cancer. Cancer Discov 9 (7), 837–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.da Silva-Diz V et al. (2018) Cancer cell plasticity: Impact on tumor progression and therapy response. Semin Cancer Biol 53, 48–58. [DOI] [PubMed] [Google Scholar]
- 13.Benoy IH et al. (2004) Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res 10 (21), 7157–62. [DOI] [PubMed] [Google Scholar]
- 14.Thiery JP et al. (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139 (5), 871–90. [DOI] [PubMed] [Google Scholar]
- 15.Fernando RI et al. (2011) IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res 71 (15), 5296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernando RI et al. (2016) IL-8 signaling is involved in resistance of lung carcinoma cells to erlotinib. Oncotarget 7 (27), 42031–42044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominguez C et al. (2017) Neutralization of IL-8 decreases tumor PMN-MDSCs and reduces mesenchymalization of claudin-low triple-negative breast cancer. JCI Insight 2 (21), e94296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palena C et al. (2012) Influence of IL-8 on the epithelial-mesenchymal transition and the tumor microenvironment. Future Oncol 8 (6), 713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waugh DJ and Wilson C (2008) The interleukin-8 pathway in cancer. Clin Cancer Res 14 (21), 6735–41. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Y et al. (2019) Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim Biophys Acta Rev Cancer 1871 (2), 289–312. [DOI] [PubMed] [Google Scholar]
- 21.David JM et al. (2016) The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance. Vaccines (Basel) 4 (3), E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geis-Asteggiante L et al. (2018) Differential Content of Proteins, mRNAs, and miRNAs Suggests that MDSC and Their Exosomes May Mediate Distinct Immune Suppressive Functions. J Proteome Res 17 (1), 486–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanson EM et al. (2009) Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol 183 (2), 937–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serafini P et al. (2008) Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res 68 (13), 5439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao FY et al. (2018) CD45(+)CD33(low)CD11b(dim) myeloid-derived suppressor cells suppress CD8(+) T cell activity via the IL-6/IL-8-arginase I axis in human gastric cancer. Cell Death Dis 9 (7), 763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chi N et al. (2014) Increased circulating myeloid-derived suppressor cells correlate with cancer stages, interleukin-8 and −6 in prostate cancer. Int J Clin Exp Med 7 (10), 3181–92. [PMC free article] [PubMed] [Google Scholar]
- 27.David CJ and Massague J (2018) Contextual determinants of TGFbeta action in development, immunity and cancer. Nat Rev Mol Cell Biol 19 (7), 419–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colak S and Ten Dijke P (2017) Targeting TGF-beta Signaling in Cancer. Trends Cancer 3 (1), 56–71. [DOI] [PubMed] [Google Scholar]
- 29.Gregory PA et al. (2011) An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol Biol Cell 22 (10), 1686–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mariathasan S et al. (2018) TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554 (7693), 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tauriello DVF et al. (2018) TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554 (7693), 538–543. [DOI] [PubMed] [Google Scholar]
- 32.Chen L et al. (2014) Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun 5, 5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.David JM et al. (2017) A novel bifunctional anti-PD-L1/TGF-beta Trap fusion protein (M7824) efficiently reverts mesenchymalization of human lung cancer cells. Oncoimmunology 6 (10), e1349589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park BV et al. (2016) TGFbeta1-Mediated SMAD3 Enhances PD-1 Expression on Antigen-Specific T Cells in Cancer. Cancer Discov 6 (12), 1366–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antony J and Huang RY (2017) AXL-Driven EMT State as a Targetable Conduit in Cancer. Cancer Res 77 (14), 3725–3732. [DOI] [PubMed] [Google Scholar]
- 36.Santamaria PG et al. (2017) EMT: Present and future in clinical oncology. Mol Oncol 11 (7), 718–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terry S et al. (2019) AXL Targeting Overcomes Human Lung Cancer Cell Resistance to NK- and CTL-Mediated Cytotoxicity. Cancer Immunol Res 7 (11), 1789–1802. [DOI] [PubMed] [Google Scholar]
- 38.Guinney J et al. (2015) The consensus molecular subtypes of colorectal cancer. Nat Med 21 (11), 1350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mak MP et al. (2016) A Patient-Derived, Pan-Cancer EMT Signature Identifies Global Molecular Alterations and Immune Target Enrichment Following Epithelial-to-Mesenchymal Transition. Clin Cancer Res 22 (3), 609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hugo W et al. (2016) Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 165 (1), 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trujillo JA et al. (2019) Secondary resistance to immunotherapy associated with beta-catenin pathway activation or PTEN loss in metastatic melanoma. J Immunother Cancer 7 (1), 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luke JJ et al. (2019) WNT/beta-catenin Pathway Activation Correlates with Immune Exclusion across Human Cancers. Clin Cancer Res 25 (10), 3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhan T et al. (2017) Wnt signaling in cancer. Oncogene 36 (11), 1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kudo-Saito C et al. (2009) Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell 15 (3), 195–206. [DOI] [PubMed] [Google Scholar]
- 45.Akalay I et al. (2013) Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Cancer Res 73 (8), 2418–27. [DOI] [PubMed] [Google Scholar]
- 46.Huang B et al. (2013) The embryonic transcription factor Brachyury blocks cell cycle progression and mediates tumor resistance to conventional antitumor therapies. Cell Death Dis 4, e682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernando RI et al. (2010) The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J Clin Invest 120 (2), 533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamilton DH et al. (2014) WEE1 inhibition alleviates resistance to immune attack of tumor cells undergoing epithelial-mesenchymal transition. Cancer Res 74 (9), 2510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.David JM et al. (2016) MUC1 upregulation promotes immune resistance in tumor cells undergoing brachyury-mediated epithelial-mesenchymal transition. Oncoimmunology 5 (4), e1117738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terry S et al. (2017) Acquisition of tumor cell phenotypic diversity along the EMT spectrum under hypoxic pressure: Consequences on susceptibility to cell-mediated cytotoxicity. Oncoimmunology 6 (2), e1271858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voon DC et al. (2017) The EMT spectrum and therapeutic opportunities. Mol Oncol 11 (7), 878–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bilusic M et al. (2019) Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J Immunother Cancer 7 (1), 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maeda DY et al. (2014) Discovery of 2-[5-(4-Fluorophenylcarbamoyl)pyridin-2-ylsulfanylmethyl]phenylboronic Acid (SX-517): Noncompetitive Boronic Acid Antagonist of CXCR1 and CXCR2. J Med Chem 57 (20), 8378–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun L et al. (2019) Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight 4 (7), 126853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liao W et al. (2019) KRAS-IRF2 Axis Drives Immune Suppression and Immune Therapy Resistance in Colorectal Cancer. Cancer Cell 35 (4), 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greene S et al. (2019) Inhibition of MDSC trafficking with SX-682, a CXCR1/2 inhibitor, enhances NK cell immunotherapy in head and neck cancer models. Clin Cancer Res. Published online January 23, 2020 10.1158/1078-0432.CCR-19-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Gramont A et al. (2017) Novel TGF-beta inhibitors ready for prime time in onco-immunology. Oncoimmunology 6 (1), e1257453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yingling JM et al. (2018) Preclinical assessment of galunisertib (LY2157299 monohydrate), a first-in-class transforming growth factor-beta receptor type I inhibitor. Oncotarget 9 (6), 6659–6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Melisi D et al. (2018) Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br J Cancer 119 (10), 1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faivre S et al. (2019) Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liver Int 39 (8), 1468–1477. [DOI] [PubMed] [Google Scholar]
- 61.Dodagatta-Marri E et al. (2019) alpha-PD-1 therapy elevates Treg/Th balance and increases tumor cell pSmad3 that are both targeted by alpha-TGFbeta antibody to promote durable rejection and immunity in squamous cell carcinomas. J Immunother Cancer 7 (1), 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sow HS et al. (2019) Combined Inhibition of TGF-beta Signaling and the PD-L1 Immune Checkpoint Is Differentially Effective in Tumor Models. Cells 8 (4), E320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knudson KM et al. (2018) M7824, a novel bifunctional anti-PD-L1/TGFbeta Trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine. Oncoimmunology 7 (5), e1426519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lan Y et al. (2018) Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-beta. Sci Transl Med 10 (424). [DOI] [PubMed] [Google Scholar]
- 65.Ravi R et al. (2018) Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFbeta enhance the efficacy of cancer immunotherapy. Nat Commun 9 (1), 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strauss J et al. (2018) Phase I Trial of M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGFbeta, in Advanced Solid Tumors. Clin Cancer Res 24 (6), 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stemmler MP et al. (2019) Non-redundant functions of EMT transcription factors. Nat Cell Biol 21 (1), 102–112. [DOI] [PubMed] [Google Scholar]
- 68.Zheng X et al. (2015) Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527 (7579), 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aiello NM et al. (2017) Upholding a role for EMT in pancreatic cancer metastasis. Nature 547 (7661), E7–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palena C et al. (2007) The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res 13 (8), 2471–8. [DOI] [PubMed] [Google Scholar]
- 71.Vujovic S et al. (2006) Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol 209 (2), 157–65. [DOI] [PubMed] [Google Scholar]
- 72.Gabitzsch ES et al. (2015) The generation and analyses of a novel combination of recombinant adenovirus vaccines targeting three tumor antigens as an immunotherapeutic. Oncotarget 6 (31), 31344–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hamilton DH et al. (2013) Immunological targeting of tumor cells undergoing an epithelial-mesenchymal transition via a recombinant brachyury-yeast vaccine. Oncotarget 4 (10), 1777–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heery CR et al. (2017) Phase I Study of a Poxviral TRICOM-Based Vaccine Directed Against the Transcription Factor Brachyury. Clin Cancer Res 23 (22), 6833–6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heery CR et al. (2015) Phase I Trial of a Yeast-Based Therapeutic Cancer Vaccine (GI-6301) Targeting the Transcription Factor Brachyury. Cancer Immunol Res 3 (11), 1248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gatti-Mays ME et al. (2019) A Phase I Trial Using a Multitargeted Recombinant Adenovirus 5 (CEA/MUC1/Brachyury)-Based Immunotherapy Vaccine Regimen in Patients with Advanced Cancer. Oncologist. Published online October 8, 2019 10.1634/theoncologist.2019-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang K et al. (2013) The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat Cell Biol 15 (6), 677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dong Y et al. (2019) Higher matrix stiffness as an independent initiator triggers epithelial-mesenchymal transition and facilitates HCC metastasis. J Hematol Oncol 12 (1), 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ding X et al. (2018) HGF-mediated crosstalk between cancer-associated fibroblasts and MET-unamplified gastric cancer cells activates coordinated tumorigenesis and metastasis. Cell Death Dis 9 (9), 867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sim WJ et al. (2019) c-Met activation leads to the establishment of a TGFbeta-receptor regulatory network in bladder cancer progression. Nat Commun 10 (1), 4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Glodde N et al. (2017) Reactive Neutrophil Responses Dependent on the Receptor Tyrosine Kinase c-MET Limit Cancer Immunotherapy. Immunity 47 (4), 789–802 e9. [DOI] [PubMed] [Google Scholar]
- 82.Ruiz de Galarreta M et al. (2019) beta-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov 9 (8), 1124–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Terry S et al. (2018) Role of Hypoxic Stress in Regulating Tumor Immunogenicity, Resistance and Plasticity. Int J Mol Sci 19 (10), E3044. [DOI] [PMC free article] [PubMed] [Google Scholar]