Abstract
In this study, we investigated how Oroxylum indicum leaf and fruit extracts affect the viability and migration of MCF-7 breast cancer cells and the mechanisms of action responsible for these effects. MCF-7 cells treated with the extracts were examined using the sulforhodamine B, colony formation and caspase 3 activity assays, and by Western blotting. O. indicum extracts were found to inhibit MCF-7 cell growth in a concentration- and time-dependent manner, with 48 h IC50 values of 57.02 ± 2.85 μg/mL and 131.3 ± 19.2 μg/mL for leaf and fruit extracts, respectively. Further, the O. indicum leaf extract caused a reduction in MCF-7 cell viability, induction of MCF-7 cell apoptosis and ROS formation, and an increase in caspase 3 activity. Also, the two extracts inhibited MCF-7 cell migration and reduced both MMP 9 and ICAMP1 gene expression and MMP9 protein expression. Additionally, O. indicum extracts greatly reduced expression of the cell cycle regulatory protein Rac1 in the mevalonate pathway. In summary, O. indicum leaf and fruit extracts reduce breast cancer cell growth, cell viability and cell migration. O. indicum constituents could, therefore, be useful for augmenting the activity of chemotherapeutic drugs employed to treat breast cancer.
Keywords: Oroxylum indicum, Reactive oxygen species (ROS), Migration, Cell viability, Matrix metalloproteinase (MMP), Apoptosis
Graphical abstract
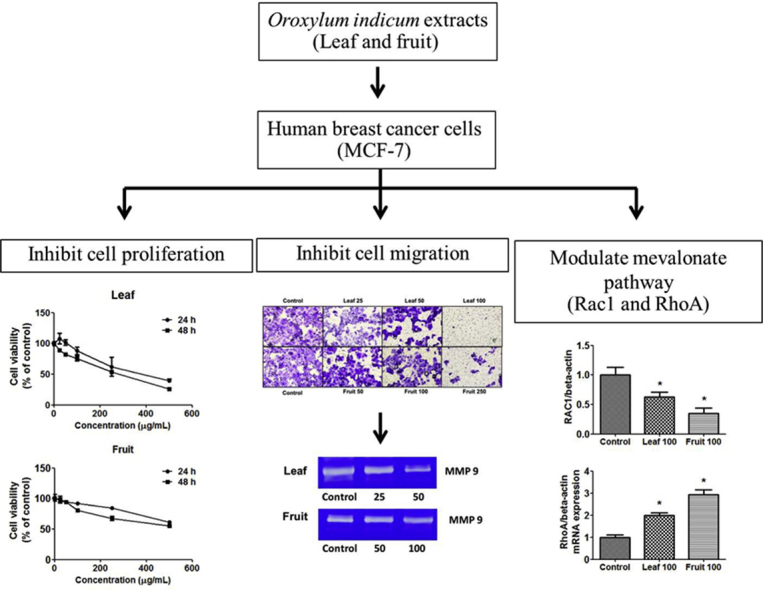
Highlights
-
•
O. indicum leaf and fruit extracts strongly inhibited breast cancer cell proliferation, with the leaf extract being the more potent of the two extracts.
-
•
O. indicum leaf extract caused cancer cell apoptosis by inducing reactive oxygen species (ROS) formation and increasing caspase 3 activity.
-
•
Both extracts inhibited cancer cell migration by decreasing MMP 9 and ICAMP1 gene expression and MMP 9 protein expression.
-
•
O. indicum extracts greatly reduced expression of the cell cycle regulatory protein Rac1 in the mevalonate pathway.
-
•
O. indicum extracts strongly inhibited breast cancer cell proliferation, induced apoptosis, and decreased migration, with reduced Rac1 in the mevalonate pathway.
1. Introduction
Oroxylum indicum belongs to the family Bignoniaceae, and is a well known food and herbal medicine in many Asian countries including Thailand [1]. Importantly, O. indicum has been extensively applied traditionally to treat many disorders such as stomach ache, rheumatism, jaundice, cough, pertussis, pharyngitis and acute and chronic bronchitis [2,3]. It has also recently been reported to have anticancer [4] activities.
Crude extracts and compounds from O. indicum have been shown to inhibit several cancer cell types including breast cancer, liver cancer, leukemia and cervical cancer cells [3,5,6]. Various parts of O. indicum including the stem bark and seed extract have been examined for their anticancer activities, but limited information is available on the activity of edible parts such as the young leaves and fruit. Kumar et al. [6] found that stem bark extract from O. indicum can induce apoptosis in ER-negative breast cancer cells as well as Hep3B and PC-3 cells. Taken together, these findings suggest O. indicum and/or its constituents could be useful in the treatment of cancer.
In this study we focused on the activity of the edible parts of O. indicum, the young leaves and fruit, against MCF-7 human breast cancer cells. In addition to measuring antiproliferative and antimigratory activity, we investigated the underlying mechanism(s) responsible for these activities.
2. Materials and methods
2.1. Reagents
Dihydroethidium (DHE), caspase 3 activity assay kits and sulforhodamine B (SRB) were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Primary antibodies and secondary antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). All cell culture reagents were purchased from Gibco BRL Life Technologies (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
2.2. Plant extraction
Young edible leaves and fruit of O. indicum were collected in May 2017 from Maha Sarakham Province, Thailand. O. indicum was identified by staff at Mahasarakham University Herbarium and a voucher specimen was deposited (MSUT_7226). Extracts were prepared as described previously, with total flavonoid content and total phenolic content determined using standardized colorimetric methods with rutin and gallic acid [7].
2.3. Cell culture and sulforhodamine B (SRB) assay
MCF-7 human breast cancer cells were grown and extract cytotoxic effects were determined by SRB assay as described previously [7]. In brief, this involved adding cells to different concentrations of extracts (0–500 μg/mL), incubating for 24–48 h, staining cells with SRB, solubilizing with Tris base solution, and measuring absorbance at 540 nm using a spectrophotometer.
2.4. Colony formation assay
Colony formation was measured using a colony formation assay described previously [7]. In brief, cells were incubated for 24 h with various concentrations of extracts (0–100 μg/mL) and then stained with 0.5% crystal violet. After this, images were captured and the colonies were counted.
2.5. Acridine orange/ethidium bromide (AO/EB) assay
Apoptosis was measured by AO/EB staining as described previously [8]. This method involved adding cells to extracts (100 μg/mL) for 24 h, staining the cells with AO and EB (1 μg/mL) for 15 min, and then capturing images using an inverted fluorescence microscope with excitation and long-pass emission filters of 480 and 535 nm (20× magnification).
2.6. Wound healing assay
Inhibition of cell migration was measured using a wound-healing assay described previously [7]. Cells were treated with various concentrations of extracts (0–100 μg/mL) and images were then captured at 0 and 48 h.
2.7. Gelatin zymography assay
Inhibition of cell migration was also measured using a gelatin zymography assay [7]. Cells were treated with various concentrations of extracts (0–100 μg/mL) for 24 h and collected using DMEM medium. Protein was then loaded in 10% SDS-PAGE-containing 0.01% gelatin (w/v). The gel was incubated with developing buffer and stained the next day with 0.5% Coomassie Brilliant Blue R-250, and washed with destaining buffer.
2.8. Matrigel migration assay
A matrigel migration assay was used to measure cell migration too. This involved seeding cells onto a 24-well Transwell chamber (8 μM pore size; Corning, Lowell, MA) at a density of 2.5 × 104 cells/well with serum-free medium containing various concentrations of extracts (0–250 μg/mL). Medium containing 10% FBS was then added to the lower part of the chamber and the cells were incubated for 24 h. After this, cells that had migrated were fixed with methanol for 30 min at –20 °C, stained with 0.5% crystal violet and images were captured by inverted microscopy (10× magnification).
2.9. Reactive oxygen species (ROS) formation assay
Extract-induced ROS formation was measured using a DHE-fluorescent probe as described previously [8]. This involved incubating cells with various concentrations of extracts (0–500 μg/mL) and DHE-fluorescent probe (25 μM) for 90 min.
2.10. Caspase 3 activity
Caspase 3 activity was measured using the caspase 3 assay kit previously described [7]. In brief, cells were treated with various concentrations of extracts (0–250 μg/mL) for 24 h. Next, cells were lysed, protein concentration was determined, and caspase activity was measured according to the assay kit manufacturer’s instructions.
2.11. Reverse transcriptase polymerase chain reaction (RT-PCR)
Extract-induced changes in gene expression were detected using RT-PCR [7]. Cells were initially exposed to the extract (100 μg/mL) for 24 h, then mRNA was extracted and converted to cDNA. Amplification via PCR was performed using target primers for each gene, Rac1, RhoA, MMP 2, MMP 9, VEGFA and ICAMP1 with ACTB as an internal control (Table 1). Differences in gene expression levels were calculated using the 2−ΔΔCq method for relative quantification.
Table 1.
Primer sequences for RT-PCR.
| Gene | Primer sequences |
|---|---|
| Rac1 | Forward primer 5’ ATG-TCC-GTG-CAA-AGT-GGT-ATC 3’ Reverse primer 5’ CTC-GGA-TCG-CTT-CGT-CAA-ACA 3’ |
| RhoA | Forward primer 5’ GGA-AAG-CAG-GTA-GAG-TTG-GCT 3’ Reverse primer 5’GGC-TGT-CGA-TGG-AAA-AAC-ACA-T 3’ |
| MMP 2 | Forward primer 5’ GAT-ACC-CCT-TTG-ACG-GTA-AGG-A 3’ Reverse primer 5’ CCT-TCT-CCC-AAG-GTC-CAT-AGC 3’ |
| MMP 9 | Forward primer 5’ GGG-ACG-CAG-ACA-TCG-TCA-TC 3’ Reverse primer 5’ TCG-TCA-TCG-TCG-AAA-TGG-GC 3’ |
| ICAM1 | Forward primer 5’ GTA-TGA-ACT-GAG-CAA-TGT-GCA-AG 3’ Reverse primer 5’ GTT-CCA-CCC-GTT-CTG-GAG-TC 3’ |
| VCAMA | Forward primer 5’ AGG-GCA-GAA-TCA-TCA-CGA-AGT 3’ Reverse primer 5’ AGG-GTC-TCG-ATT-GGA-TGG-CA 3’ |
| Beta-actin | Forward primer 5’ GTG-ACG-TTG-ACA-TCC-GTA-AAG-A 3’ Reverse primer 5’ GCC-GGA-CTC-ATC-GTA-CTC-C 3’ |
2.12. Western blotting
Extract-induced changes in protein expression were detected by Western blotting as described previously [7]. Cells were treated with extracts (100 μg/mL) for 24 h and lysed by lysis buffer prior to measurement of protein concentration. The total protein was loaded onto SDS-PAGE, transferred to a PVDF membrane, incubated with primary antibody (1:1000) overnight and then incubated with secondary antibody (1:5000) for 2 h. The membrane was washed and visualized using an enhanced ClarityTM Western ECL Substrate.
2.13. Statistical analysis
The data generated in this study are presented as mean±SEM. Differences between groups were assessed by a student unpaired t-test. Differences were deemed to be statistically significant if P values were less than 0.05 compared to the untreated control.
3. Results
3.1. Total flavonoid content and total phenolic content
The flavonoid content values were 42.92 ± 8.12 and 15.22 ± 4.23 mg/g and the phenolic compound values were 132.89 ± 25.21 and 31.23 ± 3.45 mg/g for the leaves and fruit, respectively.
3.2. Cell viability and colony formation
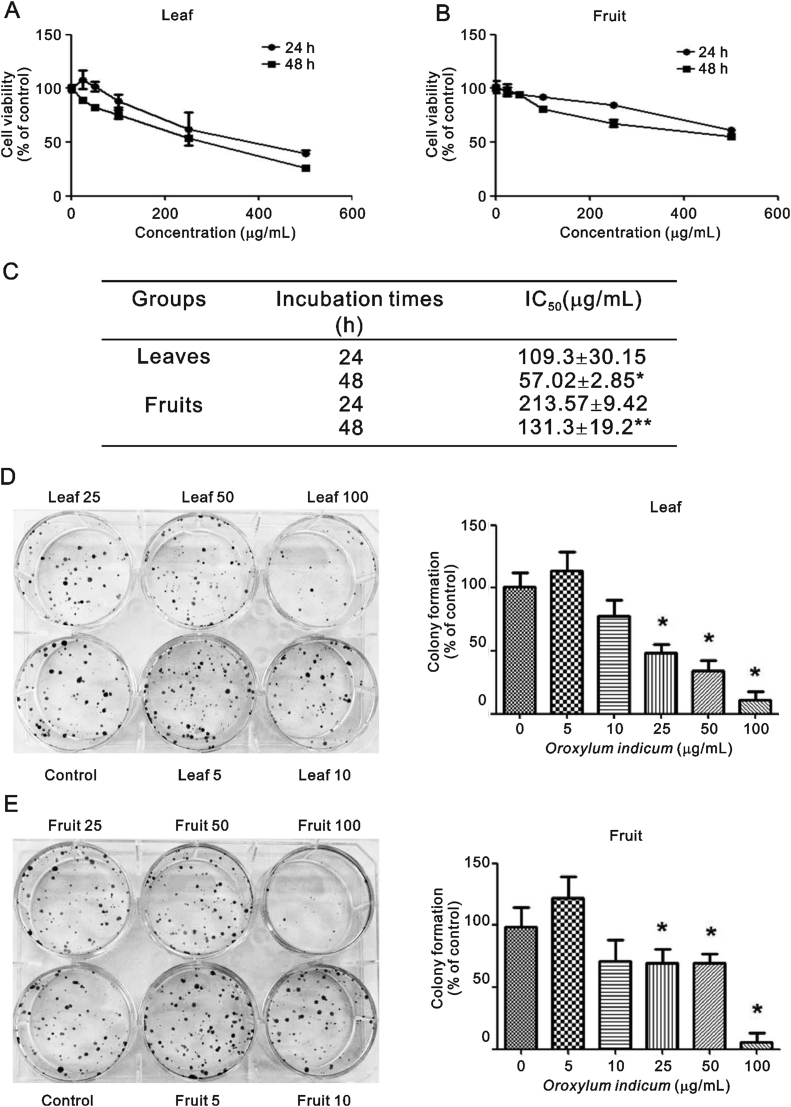
Our results showed that MCF-7 cell proliferation was inhibited in a concentration- and time-dependent manner, with 48 h IC50 values of 57.02 ± 2.85 μg/mL for the leaf extract and 131.30 ± 19.2 μg/mL for the fruit extract, respectively (Fig. 1A–C). O. indicum extracts also inhibited the colony forming ability of MCF-7 cells, with IC50 values of 29.34 ± 2.50 μg/mL for the leaf extract and 51.46 ± 6.43 μg/mL for the fruit extract (Fig. 1D and E). Both extracts displayed cytotoxic and antiproliferative effects against the MCF-7 breast cancer cells, but the leaf extract was more potent than the fruit extract.
Fig. 1.
Effect of O. indicum extracts on MCF-7 cell viability using SRB. (A and B) line graphs of cell viability, (C) IC50 values, and (D and E) colony formation. Results are presented as percentage of control and represent mean ± SEM values from three independent experiments. ∗p < 0.05 vs. control groups.
3.3. Cell apoptosis
The leaf extract caused a significant decrease in breast cancer cell viability, while also inducing cell apoptosis and necrosis compared to the untreated control group (Fig. 2A-D). The fruit extract, by comparison, induced significant apoptosis of breast cancer cells but did not alter cell viability and only slightly increased necrotic cell death (Fig. 2B-D).
Fig. 2.
Effect of O. indicum extracts on MCF-7 cell apoptosis. (A, B, C, and D) the AO/EB staining, live cells, apoptotic cells and necrotic cells, (E) ROS formation and (F) caspase 3 activity. Results are presented as percentage of control and represent mean ± SEM values from three independent experiments. ∗P < 0.05 vs. control groups.
3.4. ROS formation and caspase 3 activity
O. indicum extracts were found to induce ROS formation and stimulate caspase 3 activity (Fig. 2E and F). The leaf extract induced more ROS production and greater caspase 3 activity than the fruit extract.
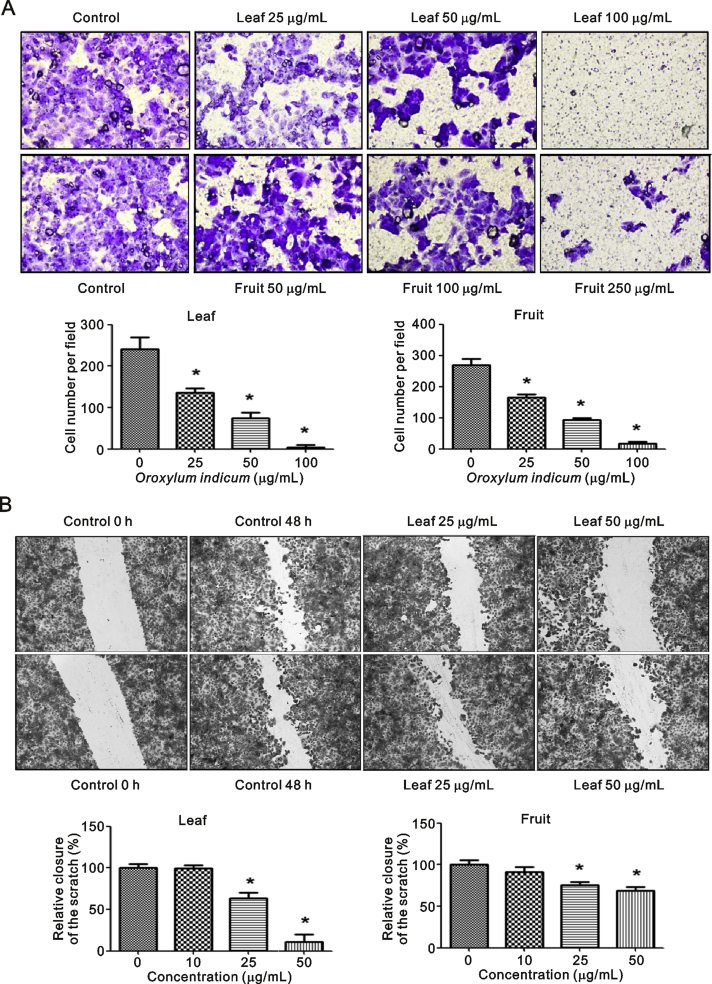
3.5. Cell migration
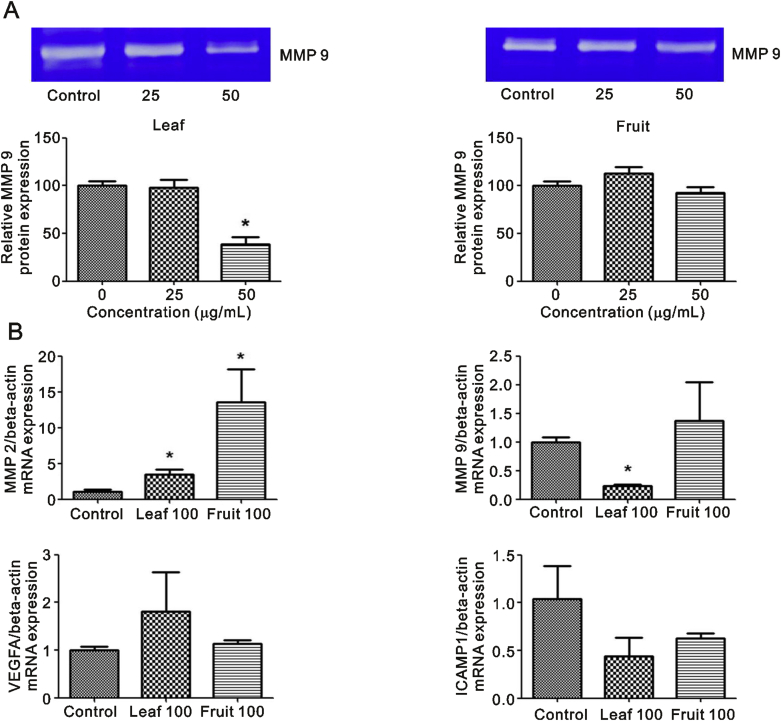
It can be seen from Fig. 3A that O. indicum extracts inhibited MCF-7 cell migration in a concentration-dependent manner. Leaf extract significantly suppressed the closing of large wounds compared with the untreated control group at a concentration of 25 μg/mL (Fig. 3B). Our results also showed that leaf extract decreased MMP 9 protein levels in the culture medium, especially at a concentration of 50 μg/mL, but that fruit extract did not (Fig. 4A). Leaf extract inhibition of cell migration was accompanied by significant suppression of MMP 9 and ICAMP1 gene expression (Fig. 4B). Taken together, these results suggested O. indicum inhibited breast cancer cell migration by suppressing MMP 9 and ICAMP1 gene expression and reducing MMP 9 protein level.
Fig. 3.
Effect of O. indicum extracts on MCF-7 cell migration by matrigel migration. (A) matrigel migration and (B) wound healing. Results are presented as percentage of control and represent mean ± SEM values from three independent experiments. ∗p < 0.05 vs. control groups.
Fig. 4.
Effects of O. indicum extracts on cell migration-related gene and protein levels. (A) gelatin zymography and (B) RT-PCR for MMP 2, MMP 9, VEGFA and ICAMP1. Results are presented as percentage of control and represent mean ± SEM values from three independent experiments. ∗p < 0.05 vs. control groups.
3.6. Rac1 and RhoA gene and protein expression levels
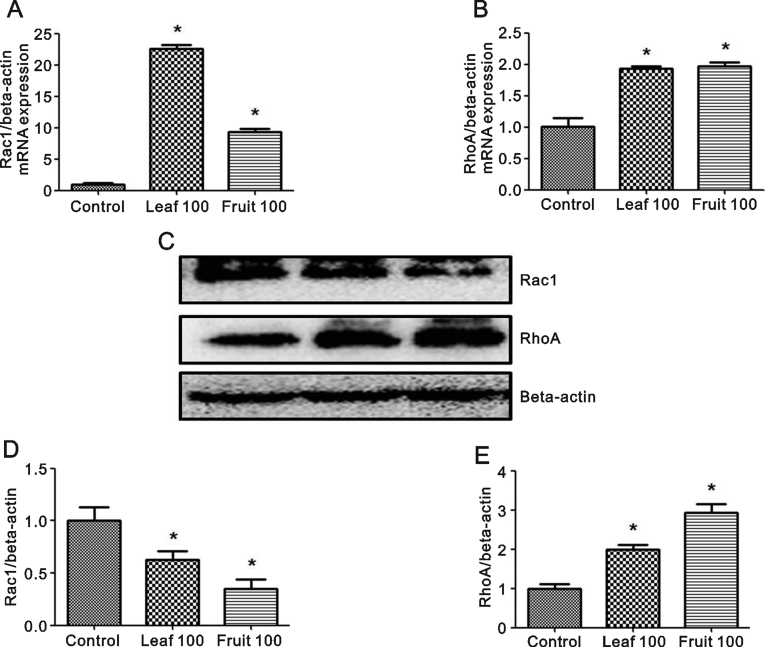
Both leaf and fruit extracts induced Rac1 and RhoA mRNA expression (Fig. 5A and B). Rac1 protein level was reduced by both extracts, but more so by the fruit extract (Fig. 5C and D). RhoA protein expression was significantly increased by both extracts (Fig. 5E), with the fruit extract causing the greater increase. Together, these results showed O. indicum extracts might inhibit cell proliferation and migration by modulating Rac1 and RhoA expression. However, more work would be needed to confirm this.
Fig. 5.
Effects of O. indicum extracts on Rac1 and RhoA gene and protein expression. (A and B) Rac1 and RhoA mRNA expression levels and (C, D and E) Rac1 and RhoA protein expression levels. Results are presented as percentage of control and represent mean ± SEM values from three independent experiments. ∗p < 0.05 vs. control groups.
4. Discussion
O. indicum has been used for centuries as a medicinal plant in a number of Asian countries including India and China. Our study showed that leaf and fruit extracts of O. indicum inhibit breast cancer cell growth and induce breast cancer cell death. Also, the leaf extract stimulates apoptotic and necrotic cell death, accelerates ROS formation and increases caspase 3 activity in a concentration-dependent manner. The leaf extract inhibits breast cancer cell migration too by reducing MMP 9 and ICAMP1 gene expression and reducing MMP 9 protein expression. These results show that extracts of the edible parts of O. indicum have good in vitro anticancer activity, that O. indicum constituents hold promise as antitumor agents, and that further research with this plant is warranted.
Little information is available on the anticancer properties of the edible parts of O. indicum. However, some studies have been conducted on the anticancer properties of other parts of O. indicum. One part of O. indicum which has been investigated quite extensively is the stem bark extract, this showing effects against several cancer cell types including leukemia, breast, cervix and prostate ones [5]. For example, stem bark ethanol extract has been shown to be highly toxic to murine melanoma, colon carcinoma and leukemia cells [9]. Stem bark extract is also toxic to cervical cancer cells with IC50 values as low as 112.3 ± 4.4 μg/mL [10]. Low IC50 values have been reported against leukemia cells too [11].
Of the previous studies that have been performed with the leaf and fruit extracts of O. indicum, activity has been detected against HeLa and HL-60 cells [1,12]. Data presented here now show that O. indicum leaf and fruit extracts have activity against MCF-7 breast cancer cells too. Both extracts induced breast cancer cell death, and inhibited cell migration by reducing MMP 9 protein levels. Having established that these O. indicum extracts are active against MCF-7 cells, we are now planning future studies to investigate how the extracts affect the mevalonate pathway.
Previous work by Kumar and colleagues has shown that O. indicum stem bark petroleum ether extract also has anti-metastatic activity [6]. This finding compares well with our study, which shows, via wound healing, matrigel migration and Western blot assays, that leaf and fruit extracts significantly inhibit cell migration and reduce MMP 9 expression. Details of the mechanism by which O. indicum constituents inhibit cell migration remain to be elucidated however.
The mevalonate pathway provides metabolites for post-translational modifications, which are critical for the activity of Rac and Rho downstream signaling, which affects cancer cell growth, differentiation, migration and apoptosis [13]. We have shown here that O. indicum extracts inhibit Rac1 protein expression, which inhibits MCF-7 cell growth and migration. However, in future we will explore other aspects of the mevalonate pathway in more detail including why, for example, RhoA increases after O. indicum extract treatment.
5. Conclusion
O. indicum leaf and fruit extracts possess powerful cytotoxic, apoptotic and antimetastatic activities, possibly by interacting with the mevalonate signaling pathway. This study provides a basis for the manipulation and application of crude extracts from the edible parts of O. indicum to be further investigated and developed for treating breast tumors.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was financially supported by Mahasarakham University (Fast Track 2019). The authors thank Dr. Tim Cushnie (MSU Faculty of Medicine) for language-editing the manuscript.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Zazali K.E., Abdullah H., Jamil N.I.N. Methanol extract of Oroxylum indicum leaves induces G1/S cell cycle arrest in HeLa cells via p53-mediated pathway. Int. J. Med. Plant Res. 2013;2:225–237. [Google Scholar]
- 2.Gokhale M., Bansal Y.K. An avowal of importance of endangered tree Oroxylum indicum (Linn.) Vent. Nat. Product. Radiance. 2006;5:112–114. [Google Scholar]
- 3.Li N.N., Meng X.S., Men W.X. Total flavonoids from Oroxylum indicum induce apoptosis via PI3K/Akt/PTEN signaling pathway in liver cancer, Evid. Based complement. Alternat. Med. 2018 doi: 10.1155/2018/3021476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar R.A., Rajkumar V., Guha G. Therapeutic potentials of Oroxylum indicum bark extract. Chin. J. Nat. Med. 2010;8:121–126. [Google Scholar]
- 5.Dinda B., SilSarma I., Dinda M. Oroxylum indicum (L.) Kurz, an important Asian traditional medicine: from traditional uses to scientific data for its commercial exploitation. J. Ethnopharmacol. 2014;161:255–278. doi: 10.1016/j.jep.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Kumar N., CijoGeorge V., Suresh P.K. Cytotoxicity, apoptosis induction and anti-metastatic potential of Oroxylum indicum in human breast cancer cells. Asian Pac. J. Cancer Prev. 2012;13:2729–2734. doi: 10.7314/apjcp.2012.13.6.2729. [DOI] [PubMed] [Google Scholar]
- 7.Buranrat B., Mairuae N., Konsue A. Cratoxy formosum leaf extract inhibits proliferation and migration of human breast cancer MCF-7 cells, Biomed. Pharma. 2017;90:77–84. doi: 10.1016/j.biopha.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 8.Buranrat B., Senggunprai L., Prawan A. Simvastatin and atorvastatin as inhibitors of proliferation and inducers of apoptosis in human cholangiocarcinoma cells. Life Sci. 2016;153:41–49. doi: 10.1016/j.lfs.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Costa-Lotufo L.V., Khan M.T., Ather A. Studies of the anticancer potential of plants used in Bangladeshi folk medicine. J. Ethnopharmacol. 2005;99:21–30. doi: 10.1016/j.jep.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 10.Siriwatanametanon N., Fiebich B.L., Efferth T. Traditionally used Thai medicinal plants: in vitro anti-inflammatory, anticancer and antioxidant activities. J. Ethnopharmacol. 2010;130:196–207. doi: 10.1016/j.jep.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Ong C.Y., Ling S.K., Ali R.M. Systematic analysis of in vitro photo-cytotoxic activity in extracts from terrestrial plants in Peninsula Malaysia for photodynamic therapy. J. Photochem. Photobiol. B. 2009;96:216–222. doi: 10.1016/j.jphotobiol.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Roy M.K., Nakahara K., Na T.V. Baicalein, a flavonoid extracted from a methanolic extract of Oroxylum indicum inhibits proliferation of a cancer cell line in vitro via induction of apoptosis. Pharmazie. 2007;62:149–153. [PubMed] [Google Scholar]
- 13.Karlic H., Thaler R., Gerner C. Inhibition of the mevalonate pathway affects epigenetic regulation in cancer cells. Cancer Genet. 2015;208:241–252. doi: 10.1016/j.cancergen.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]