Abstract
Hollow-fiber liquid-phase microextraction (HF-LPME) and electromembrane extraction (EME) are miniaturized extraction techniques, and have been coupled with various analytical instruments for trace analysis of heavy metals, drugs and other organic compounds, in recent years. HF-LPME and EME provide high selectivity, efficient sample cleanup and enrichment, and reduce the consumption of organic solvents to a few micro-liters per sample. HF-LPME and EME are compatible with different analytical instruments for chromatography, electrophoresis, atomic spectroscopy, mass spectrometry, and electrochemical detection. HF-LPME and EME have gained significant popularity during the recent years. This review focuses on hollow fiber based techniques (especially HF-LPME and EME) of heavy metals and pharmaceuticals (published 2017 to May 2019), and their combinations with atomic spectroscopy, UV-VIS spectrophotometry, high performance liquid chromatography, gas chromatography, capillary electrophoresis, and voltammetry.
Keywords: Hollow fiber liquid phase microextraction, Electromembrane extraction, Heavy metals, Pharmaceuticals, Instrumental techniques
Graphical abstract
Highlights
-
•
Review article focused on hollow fiber liquid-phase microextraction and electromembrane extraction.
-
•
Focus on heavy metal and pharmaceutical applications.
-
•
Covers research articles published in 2017–2019.
1. Introduction
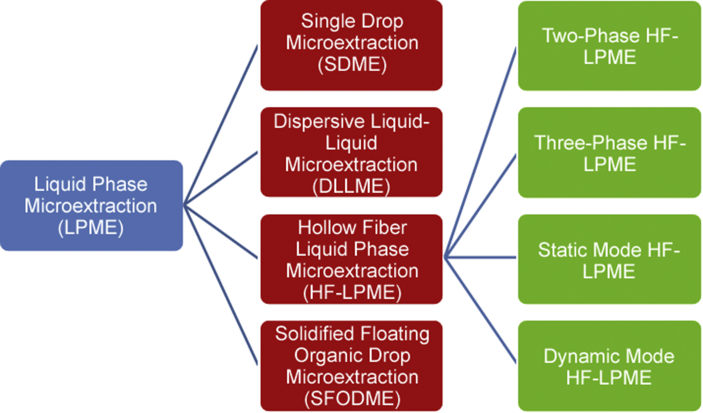
Most analytical methods include sample preparation prior to the instrumental analysis. The purpose of the sample preparation is to make the sample compatible with the analytical instrument, to remove interfering matrix components and to enrich the target analytes to a detectable level [1]. Different sample preparation techniques are available, but liquid-liquid extraction (LLE) and solid-phase extraction (SPE) have been the most popular ones [2]. For several decades, different microextraction variants of LLE and SPE have been developed. This was originally motivated by reduction or elimination of organic solvents [3] and was initiated by the invention of solid-phase microextraction (SPME) by Pawliszyn et al. [4] and liquid-phase microextraction (LPME) by Jeannot and Cantwell [5]. Since the introduction of these innovative sample preparation methods, a variety of SPME and LPME methods have been reported in the literature. Head-space solid phase microextraction (HS-SPME), in-tube solid phase microextraction (IT-SPME), thin-film solid phase microextraction (TF-SPME), head-space liquid phase microextraction (HS-LPME), liquid-liquid microextraction based on solidification of floating organic droplet (LLME-SFO), and dispersive liquid-liquid microextraction (DLLME) are examples in this direction [[6], [7], [8], [9], [10], [11]]. While SPME has become commercially available, LPME is still performed with laboratory built devices. In spite of this, LPME is still a very active area of research for reasons including 1) simplicity, 2) costs, 3) green chemistry, 4) selectivity, and 5) method sensitivity. Fig. 1 illustrates different configurations and approaches to LPME. LPME was originally performed as single drop microextraction (SDME) [12]. SDME is still under development, but in this approach the stability of the droplet of organic solvent may be an issue.
Fig. 1.
Classification of liquid phase microextraction techniques.
Considering these limitations, hollow fiber protected liquid phase microextraction (HF-LPME) was introduced in 1999 [13]. In the first paper, HF-LPME was utilized for preconcentration and extraction of pharmaceuticals prior to electrophoretic identification and determination. An aqueous extraction solvent was injected into the lumen of a porous polypropylene hollow fiber membrane, while the porous wall was impregnated by an organic supported liquid membrane (SLM). As the extraction solvent (acceptor phase) was not in direct contact with the sample solution, the extraction system was stable, and highly efficient sample cleanup was achieved with this innovative strategy. HF-LPME can be utilized for quantitative analysis from very complex environmental and biological samples [14].
A typical HF-LPME setup consists of a porous hollow fiber membrane made of polypropylene, polytetrafluoroethylene, and polyvinylidene fluoride, which is impregnated with an organic solvent. The solvent is immobilized in the pores in the wall of the hollow fiber membrane and serves as supported liquid membrane (SLM) [15]. The acceptor phase can be either an aqueous solution or an organic solvent. Due to this flexibility, HF-LPME is compatible with most analytical instruments for chromatography, electrophoresis, molecular and atomic spectrometry, and electrochemistry. The current review discusses recent applications of HF-based microextraction for pharmaceutical and heavy metal applications, and on their compatibility with different instrumental methods.
2. Principles and possible modes of the HF-LPME
In HF-based LPME methods, the extraction phase is placed in the lumen of a porous hollow fiber. In such a way, the acceptor phase is protected by the SLM. To perform HF-LPME, a polypropylene hollow fiber membrane may first be sonicated in acetone to remove polymer impurities [16]. Then the hollow fiber membrane is soaked in an impregnating solvent to fill the pores of the HF membrane by capillary forces, and excess solvent may be removed by washing with distilled water [17]. After this step, the lumen of the hollow fiber is filled with the acceptor phase. As is thoroughly discussed below, the acceptor phase can be the same organic solvent as the SLM (the two-phase mode) or can be an aqueous solution (the three-phase mode). Finally, the hollow fiber is immersed in the sample solution and the analytes are extracted from the sample, through the SLM and into the acceptor phase [18]. Four different arrangements for HF-LPME have been reported, i.e., rod like, u-shaped, hollow-fiber solvent bar, and knotted hollow-fiber. Considering the physicochemical characteristics of the desired analytes and levels of complexity of the sample, HF-LPME can be performed in a two- or three-phase mode.
2.1. Two-phase HF-LPME
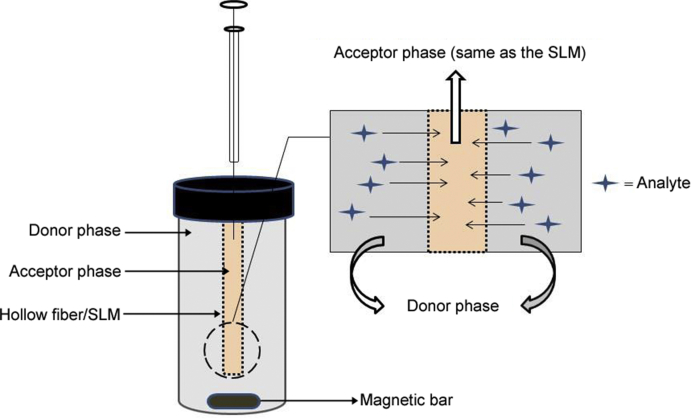
In two-phase HF-LPME, the pores in the wall of the hollow fiber and the lumen are filled with an organic solvent, which is immiscible with the aqueous sample solution. Solvents like 1-octanol and dihexyl ether are commonly used [19]. Two-phase HF-LPME is applied for extraction and preconcentration of analytes with low polarity such as polycyclic aromatic hydrocarbons (PAHs). Since the analytes are extracted into an organic solvent, two-phase LPME is compatible with gas chromatography. Fig. 2 illustrates the principle of two-phase HF-LPME. According to this the SLM and the organic acceptor phase are the same in two-phase HF-LPME.
Fig. 2.
A schematic mechanism of the two-phase HF-LPME.
2.2. Three-phase HF-LPME
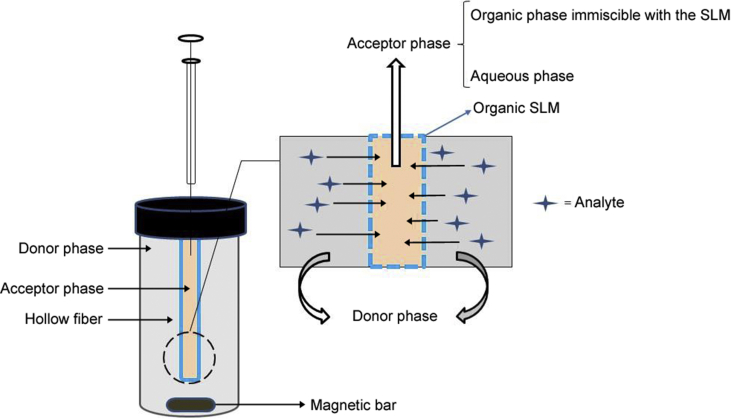
In three-phase LPME, the pores in the wall of the hollow fiber are filled with an organic solvent immiscible with water, while the lumen is filled with aqueous solution (acceptor solution). 1-Octanol and Dihexylether are common solvents [20]. The mechanism of extraction is based on pH adjustment of the sample solution and the aqueous acceptor phase. For instance, extraction of acidic compounds is achieved by acidification of the sample to suppress ionization of the target analytes. This results in successful transfer of analytes towards the acceptor phase through the organic SLM. pH of the aqueous acceptor solution should be adjusted to a pH value 2–3 units above pKa of the analyte.
Carrier mediated three-phase HF-LPME was introduced where a hydrophobic carrier was dissolved in the organic SLM before the impregnation of the HF pores [21]. The applied carrier is an organic compound capable of ion-pairing with analytes of interest. Hence, at the contact region between the sample solution and the SLM, the desired ion-pair complexes are formed, leading to successful extraction of target analytes. At the contact region between the SLM and the aqueous acceptor phase, the analyte is exchanged with a suitable inorganic counter ion dissolved in the acceptor solution, and is released into the acceptor solution.
As mentioned, three-phase HF-LPME can be applied for ionizable compounds (acids and bases). Two-phase HF-LPME offers high PFs and extraction efficiencies for non-polar analytes. However, because the SLM and the acceptor phase are the same organic solvent, there is no phase boundary. Therefore, cleanup is limited. Interestingly, Ghambarian et al. introduced a variant where n-dodecane was used as the organic SLM, while organic solvents like methanol, ethanol, or acetonitrile, which are all immiscible with n-dodecane, were used as acceptor phase [22]. This strategy provided improved sample clean-up, while extraction efficiency was not sacrificed. Fig. 3 illustrates the principles of three-phase HF-LPME.
Fig. 3.
Schematic mechanisms of two possible modes of three-phase HF-LPME.
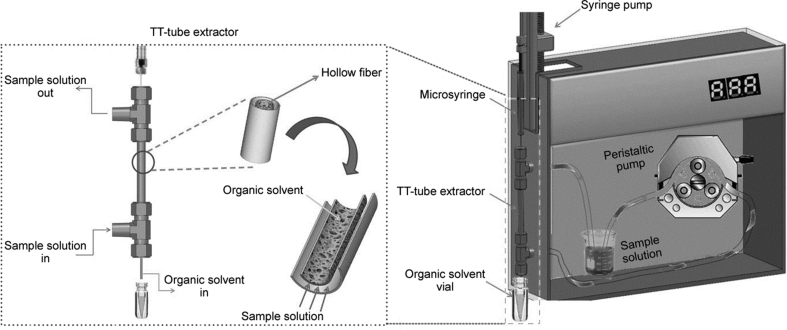
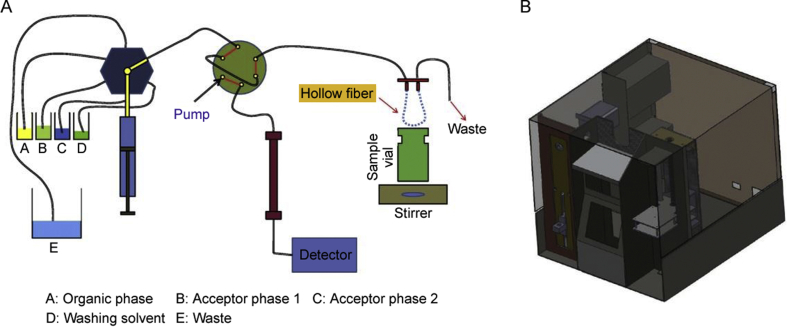
HF-LPME can be carried out through static, dynamic, and even fully automated strategies. One of the most interesting approaches for dynamic HF-LPME was introduced by Esrafili et al. called TT-extraction [23]. Between two T connectors, a stainless-steel tube was mounted, housing the hollow fiber membrane. The sample solution was pumped into the TT-extractor through the two T connectors, while the acceptor phase was injected into the hollow fiber membrane via a syringe pump. This configuration was capable of carrying out two-phase HF-LPME. Fig. 4 illustrates this innovative approach with all the details. In another strategy offered by the same research group, a fully automated HF-LPME with capability for two- and three-phase HF-LPME procedures was developed. In this setup, a syringe pump was used for washing, filling, and ejection of different solvents. Four containers were used for washing solvent, SLM, extraction solvent, and elution solvent. The extraction system was successfully connected with liquid chromatography. Fig. 5 shows the graphical design of this HF-LPME instrument [24].
Fig. 4.
Schematic configuration of the dynamic HF-LPME. Reprinted with permission from Ref. [23].
Fig. 5.
(A) Illustrative configuration and (B) schematic structure of the automated HF-LPME offered by Esrafili et al. Reprinted with permissions from Ref. [24].
2.3. Electromembrane extraction
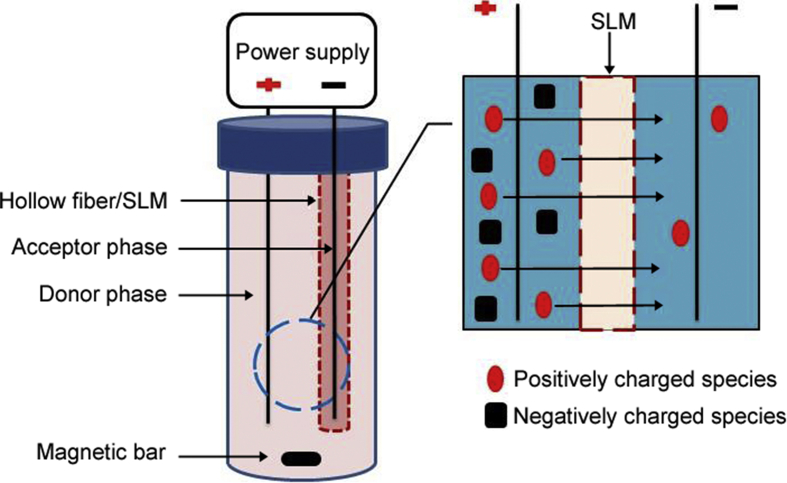
In HF-LPME, mass transfer is by passive diffusion and this results in relatively slow extraction kinetics. Alternatively, ionizable compounds can be extracted efficiently and with outstanding sample cleanup by electromembrane extraction (EME) [25]. The principle of EME is similar to that of HF-LPME. However, in EME the analytes are extracted selectively in their charged form under the influence of an electrical field. Thus mass transfer is by electrokinetic migration across the SLM, and 2-nitrophenyl octyl ether (NPOE) is often used as SLM. The setup for EME is very similar to three-phase HF-LPME, but in EME two electrodes (usually platinum wires) are utilized. One electrode is placed in the acceptor phase into the hollow fiber lumen, while the second electrode is inserted into the sample solution. Fig. 6 shows the principle of EME.
Fig. 6.
A schematic mechanism for electromembrane extraction.
For extraction of basic analytes, the cathode (-) is placed inside the acceptor phase, while for acidic analytes the direction of the electrical field is reversed [18]. After extraction, the acceptor phase is collected with a micro-syringe and injected into an analytical instrument for quantitative analysis. Due to the electrical field across the SLM, bubble formation, Joule heating, and even punctuation of SLM may occur in EME systems operated under non-optimal conditions. These phenomena occur in longer extraction times as a result of excessive migration of ions across SLM. Also, when the electrical field is applied, electrical double layers and local pH gradients are formed in the interfaces between the SLM and the aqueous solutions on both side. These may impact the mass transfer. To reduce the impact of these phenomena, application of constant direct electrical current and application of pulsed voltage have been reported [26]. Although the utilization of a stabilized and constant direct current improved the efficiency and stability of EME, this approach is still not common in EME publications. Pulsed electromembrane extraction (PEME) was introduced as a strategy to enhance the efficiency of EME using a simple and inexpensive extraction setup [27]. Pulsed voltage was found to improve extraction efficiency and system stability.
3. Operational parameters of HF-LPME and EME
The type of hollow-fiber membrane, type of extraction solvent, pH of donor and acceptor phases, extraction time, stirring rate, salt addition, and temperature are important parameters in HF-LPME and EME. Applied voltage and electrode material, thickness, and distance are additional parameters affecting EME performance.
3.1. Hollow-fiber membrane
The porous wall of the hollow-fiber serves as support for the SLM. The SLM solvent should be of low polarity and immiscible with water. For immobilization of such low-polarity solvents, the hollow-fiber should be of hydrophobic material [28]. In most cases, polypropylene hollow-fiber membranes have been used. Such hollow-fibers are commercially available at a low price. Therefore, each hollow-fiber membrane is used only for a single extraction and is then discarded. Alternatively, polyvinylidene difluoride hollow-fiber membranes can be used [14].
3.2. Supported liquid membrane (SLM)
The solvent selected for the SLM plays a major role for the stability and the efficiency of the extraction system both in HF-LPME and EME. First, the solvent should be non-volatile in order to avoid evaporation, and solvents with boiling point exceeding 200 °C are recommended. Second, the solvent should be immiscible with water in order to avoid leakage to the sample. Usually solvents with water solubility less than 0.5 mg/mL are recommended. Third, the solvent should facilitate efficient mass transfer of target analyte [14]. In HF-LPME, high efficiency can often be obtained with a variety of organic solvents. Common organic solvents for HF-LPME include n-dihexyl ether, 1-octanol, dodecyl acetate, and toluene. Ionic liquids have also been used as SLM solvent in HF-LPME [28]. In EME, the type of solvent used for the SLM is much more critical [29]. This is due to the fact that analyte molecules are charged when entering the organic SLM, and partition is voltage dependent. Common organic solvents used in EME are 2–nitrophenyloctyl ether (NPOE) and 1-octanol.
3.3. Sample and acceptor pH
Sample and acceptor pH plays a major role both in HF-LPME and EME. In HF-LPME, the analyte is extracted in neutral form into the SLM, while it is ionized in contact with the acceptor. Therefore, for extraction of basic analytes, the sample should be alkaline, while the acceptor should be acidic or neutral (depending on analyte pKa-value). For HF-LPME of acidic analytes, the pH gradient is reversed using acidic conditions in the sample and alkaline or neutral conditions in the acceptor [30,31]. In EME, the analytes are ionized in the entire extraction system. Thus, for basic analytes, both the sample and the acceptor are neutral or acidic. For acidic analytes, EME is conducted with neutral or alkaline conditions in sample and acceptor [32]. Due to local pH effects [33] at the SLM boundary layers, pH in the acceptor solution is critical in EME, and for basic analytes pH should be no less than three units above pKa. In addition, pH may change in the sample and acceptor during EME due to electrolysis [34], and therefore buffers are recommended in both sample and acceptor.
3.4. Extraction time
Both HF-LPME and EME are equilibrium techniques. This implies that extraction recovery increases rapidly versus time during initial extraction, and after a certain period of time, the systems enter equilibrium. Thus, from a theoretical point of view, recovery is constant from this point forward and extraction is terminated. Equilibrium time is typically in the range 30–60 min for HF-LPME depending on the chemical properties of the analytes and the geometry of the system [35]. For EME, equilibrium time is shorter, due to the electro-kinetic mass transfer, and ranges typically from 5 to 15 min. In EME, recoveries may drop during prolonged extraction due to pH changes in sample and acceptor, and due to instability of the SLM [36].
3.5. Stirring/agitation
HF-LPME and EME are performed under stirring or agitation conditions. By such, convection is induced in the sample and analyte mass transfer to the SLM is facilitated. In addition, stirring or agitation reduces the boundary layer thickness at the sample/SLM interface, and this is very important for efficient mass transfer [31]. The optimal stirring or agitation rate is geometry dependent, and is normally optimized during method development. Typically, stirring or agitation in the range 500–1000 rpm is used. At higher rates, air bubbles are often formed, and this can reduce extraction performance [37].
3.6. Salt addition
Addition of salt to the sample affects the efficiency of HF-LPME and EME. In HF-LPME, the addition of salt to the sample solution often increases the extraction efficiency [38,39]. The increase in extraction efficiency is due to increased ionic strength in the sample, which increases analyte partition into the organic SLM. However, negative effects of salt in the samples are also reported [40,41]. This may be due to changes in the Nernst diffusion film controlling the diffusion rate of analytes into the acceptor phase [42,43]. Typical salt additions are 5%–30% (w/v) [44]. In EME, addition of salt to the sample is normally not performed. For efficient EME, a low ion balance may be favorable under certain conditions [[45], [46], [47]]. The ion balance is defined as the ratio between the total amount of ions in the sample and the total amount of ions in the acceptor phase.
3.7. Temperature
HF-LPME and EME are normally performed at room temperature. Both extraction modes are affected by temperature. Partition coefficients decrease with the increase in temperature, while diffusion across the SLM increases. Temperatures up to 40 ͦC have been used, but above this temperature SLMs tend to be less stable [37,48].
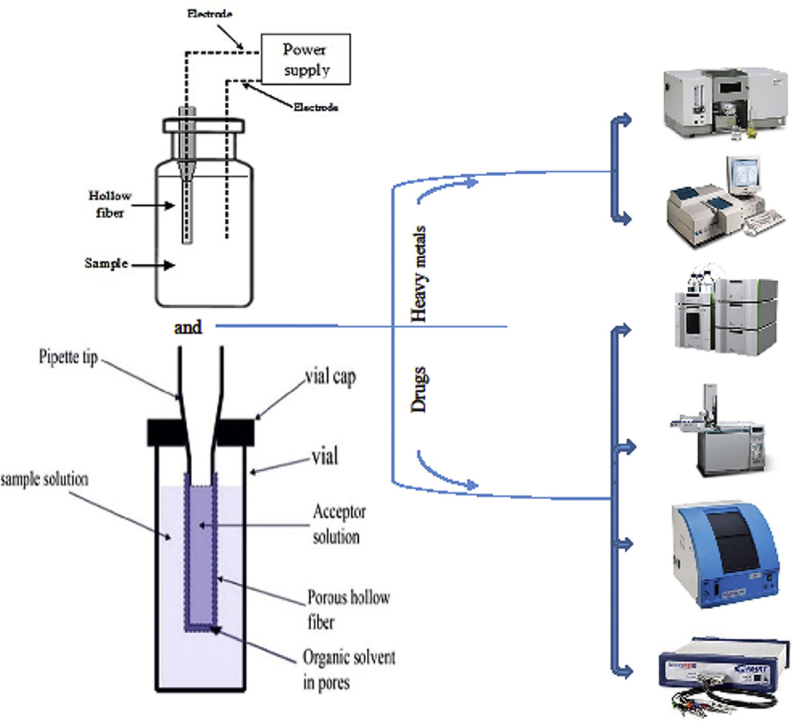
3.8. Electrodes (EME)
Platinum is normally used as inert electrode material in EME. The electrode thickness plays a key role in EME. The internal diameter of the electrodes should be substantially less than the inner diameter of the hollow fiber in order not to displace acceptor phase. The distance between the electrodes is another important parameter [49].
3.9. Applied voltage (EME)
Generally, the extraction efficiency in EME increases with the increasing voltage up to a certain level. Above this, there is no further gain in extraction efficiency, and mass transfer is no longer limited by the voltage. Voltages applied in EME normally range from 5 to 300 V. Voltages exceeding 300 V are not recommended, due to instability of the extraction system and generation of gas bubbles (electrolysis) [37].
4. Instrumental techniques coupled with HF-LPME and EME
4.1. Heavy metals determination
4.1.1. Atomic spectrometry
Combinations of hollow fiber based techniques and atomic spectroscopic techniques have mainly been used for detection of heavy metals in environmental, clinical, and biological samples, petroleum products, pharmaceuticals, and in food [50]. The hollow fiber based techniques coupled with atomic spectrometric techniques for preconcentration and determination of different heavy metals are summarized in Table 1 [[51], [52], [53], [54], [55], [56], [57], [58], [59], [60]]. HF-LPME has been coupled to flame atomic absorption spectroscopy (FAAS) and graphite furnace atomic absorption spectrometry (GFAAS) for metal analysis [[51], [52], [53], [54], [55]]. In one example, Tahmasebi et al. introduced a new approach to the extraction of Cr (VI) based on EME coupled with electrothermal atomic absorption spectrometry (ETAAS). Before using the hollow fiber membrane as SLM, polyaniline nanoparticles were coated on the surface to increase the selectivity for the extraction of Cr(VI). The polyaniline reinforced hollow fiber selectively extracted Cr (VI) from real samples via anion exchange. The surface area of the nanostructure coated polyaniline increased the preconcentration of the target analyte [56].
Table 1.
Atomic spectrophotometric and UV–Vis spectrophotometric techniques coupled with hollow fiber based extraction techniques for heavy metals determination.
| Instrumental techniques | Metal/Analyte | Preconcentratio/Separation method | Complexing agent/Carrier | SLM composition | Real applications | LODa | EF/PFb | LRc | Instrumental technique mode | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Atomic spectroscopic techniques | Pb | HFSLPME | TiO2 | TiO2 + Caprylic acid | Rice, milk, water | 0.2 μg/L | 790 | 0.6–3000 μg/mL | FAAS | [51] |
| As | HFLPME | APDC | Triton X-100 | Environmental water samples | 0.08 ng/mL | 280 | 0.4–12 ng/mL | FAAS | [52] | |
| Cr (VI) | IEME | — | 1-octanol | Environmental water samples | 3 ng/mL | 23 | 10–600 ng/mL | FAAS | [53] | |
| Pb | HFLPME | Kronenether | Kronenether + oleic acid | Blood, urine samples | 0.001–0.002 ng/mL | 18.7–21.3 | 1–50 ng/mL | GFAAS | [54] | |
| Hg | EME | DEHP | DEHP + 1-octanol | Tap water | 0.5 μg/L | 102–108 | 0.5–10 μg/L | GFAAS | [55] | |
| Cr(VI) | EME | PANI | PANI + 1-octanol | Spring, sea and distilled water | 0.02 ng/mL | 106 | 0.02–2.0 ng/mL | ETAAS | [56] | |
| Cr(VI) | HFLPME | Methyltrialkylammonium chloride | Methyltrialkylammonium chloride + 1-octanol | Drinking, mineral and tap water | 3 μg/L | — | 10–90 μg/L | RGB/ ICP-AES | [57] | |
| Pb | CM-HFLPME | CTAB | CTAB + 1-octanol | Blood samples | 0.1 ng/mL | 33 | 1–200 ng/mL | ETAAS | [58] | |
| Cr (VI) | μEME | Tetra alkyl ammonium chloride | Tetra alkyl ammonium chloride+ 1-octanol | Wastewater samples | 0.06 ng/mL | 9.1 | 0.5–14 ng/mL | ETAAS | [59] | |
| Hg | HFLPME | DDTC | DDTC + toluene | Water and freshwater fish | 0.143 | 103 | 0.5–7.5 | ETAAS | [60] | |
| UV–visible spectrometry | Cr (VI) | EME | 1,5-diphenylcarbazide | NPOE | Environmental water samples | 2.3–7 μg/L | 80 | 10–80 μg/L | UV–vis spectrometry | [61] |
| Cr (VI) | EME | Calix[4]arene | NPOE + calix[4]arene | Industrial water | — | — | — | UV–vis spectrometry | [62] | |
| Hg | EME | DEHP | 1-octanol | River, tap water and fish sample | 0.7–12 μg/kg | 130–176 | 2.3–950 μg/L for water and 40–9500 μg/kg for fish sample | UV–vis spectrometry | [63] | |
| Au | EME | PAN | 1-octanol | Tap, river and ground water | 4.5 μg/L | 200 | 20–2000 μg/L | UV–vis spectrometry | [64] | |
| Bi +3 | EME | DEHP | DEHP + 1-octanol | Plasma, water | 1.47 μg/L | 151 | 4.9–800 μg/L | UV–vis spectrometry | [65] |
EME: Electro-membrane extraction, HFLPME: Hollow fiber liquid phase microextraction, IEME: In-tube electro-membrane extraction, CM-HFLPME: carrier-mediated hollow fiber liquid phase microextraction, DEHP: bis(2-ethylhexyl) phosphate, CTAB: N,N,N-cetyltrimethyl ammonium bromide, APDC: Ammonium pyrroldinedithiocarbamate, PANI: Polyanniline, DDTC: Diethyldithiocarbamate, PAN: 1-(2-pyridylazo)-2-naphthol), NPOE: 2-nitrophenyl octyl ether, FAAS: Flame atomic absorption spectroscopy, GFAAS: Graphite furnace atomic absorption spectrometry, ETV-ICP-OES: Electrothermal vaporization inductive couple plasma optical emission spectroscopy, RGB/ ICP-AES: Red Green Blue Analysis/ inductive couple plasma atomic emission spectroscopy.
Limit of detection.
Enrichment factor/Pre-concentration factor.
Linear range.
In another example Alahmad et al. introduced an HF-LPME microfluidic paper-based analytical device (μPAD) for extraction of Cr (VI). The membrane was impregnated in a mixture of Aliquat 336 and 1-octanol for 1 min. The acceptor phase was collected for colorimetric analysis after EME, and DPC reagent was added. For quantitative analysis a scanner was used and colored images were analyzed with RGB. The results were compared with ICP-AES and found in good agreement [57].
4.1.2. UV–visible spectrophotometry
UV–visible spectrophotometry has been commonly selected as instrumental technique for quantitative analysis of heavy metals, highly conjugated organic compounds, and biological macromolecules due to easy availability, simplicity, versatility, speed, accuracy, precision, and cost-effectiveness. However, in complex samples the analytes cannot be measured directly with a UV–visible spectrophotometer due to low concentration. Therefore a sample preparation step is necessary to improve selectivity and sensitivity. Atikarnsakul et al. presented an EME procedure for preconcentration and extraction of Cr(VI). Polypropylene hollow fiber membrane was impregnated with NPOE and used for EME. For quantitative analysis, a specific volume of acceptor phase was mixed with a specific concentration of DPC and acidified with dilute H2SO4. The resultant red-violet solution was analyzed with a fiber optic ultraviolet–visible spectrophotometer with a Z-flow cell at 544 nm [61].
Onac et al. introduced a new approach to preconcentration and extraction of Cr(VI). A polymer inclusion membrane was synthesized and used as SLM for EME. The polymer inclusion membrane impregnated with NPOE contained calix [4]arene. The EME process was performed under constant current instead of constant voltage to control the extraction of charged species. The use of constant current in EME provided high reproducibility and mechanical stability. The formation of metal-calix [4]arene ion pair at the membrane-aqueous phase interface enhanced the transport of metal-complex ion through the membrane. Bath-water of chrome plating industry was used as real sample for analysis to check the selectivity of calix [4]arene towards Cr(VI) [62]. Table 1 summarizes examples on research combining hollow fiber based extraction techniques with UV–Vis spectrophotometry [[61], [62], [63], [64], [65]].
4.2. Pharmaceuticals
4.2.1. High performance liquid chromatography (HPLC)
Three-phase HF-LPME and EME are ideal for acidic and basic drugs. In three-phase HF-LPME, the acceptor phase is an aqueous solution, and this can be injected directly into HPLC systems without previous evaporation and reconstitution [30]. For extraction of basic analytes in HF-LPME, pH of donor phase must be kept alkaline to suppress the ionization and solubility of the analyte, whereas pH for acceptor phase should be acidic. In case of acidic analytes, the pH gradient across the SLM is reversed [30,31]. For extraction of basic analytes in EME, pH of donor and acceptor phases are kept acidic to ionize the analytes. The anode is placed in the donor phase, while the cathode is placed in the acceptor phase to facilitate the migration of analyte. In case of acidic analytes, the donor and acceptor phases are made alkaline and the direction of the electrical field is reversed [30,32]. Three-phase systems provide better degree of clean-up than two phase systems due to the fact that most water soluble components do not pass through the SLM.
Khan et al. presented a new strategy for selective and simultaneous extraction of three drugs with different hydrophobicity property. The EME process was carried out in a microfluidic chip device. The microfluidic device consisted of three PMMA plates having M shaped microchannels. The middle PMMA plate provided a flow path for sample solution and was in contact with two different SLMs and acceptor phases. A stainless steel electrode was embedded in the middle plate, dedicated for the flow of sample solution. Stainless steel electrodes were also embedded in the two PMMA plates dedicated for the two acceptor phases. Based on different SLMs, drugs of different hydrophobicity were extracted into two acceptor phases and analyzed with HPLC-UV [66].
In another example, Román-Hidalgo et al. introduced a new biopolymeric membrane for EME of NSAIDs and acidic compounds. The chitosan biopolymeric membrane was synthesized by mixing chitosan 60% (w/w) and Aliquat®336 40% (w/w). The synthesized membrane was characterized with scanning electron microscopy (SEM). The prepared membrane was impregnated with a suitable organic solvent and used as SLM in a home-made device. After EME, the acceptor phase was analyzed with HPLC-DAD for quantitative analysis [67]. Table 2 summarizes examples on research combining hollow fiber based extraction techniques with HPLC [66–109].
Table 2.
High performance liquid chromatography (HPLC) technique coupled with hollow fiber based extraction techniques for pharmaceutical drugs.
| Analyte | Preconcentration/Separation method | Membrane / SLM composition | Real applications | LODa | EF/PFb | LRc | Instrumental tec. | Ref. |
|---|---|---|---|---|---|---|---|---|
| Atenolol, betaxolol, propranolol | Microfluidic EME | NPOE + DEHP + TEHP | Urine, plasma | 4–10 μg/L | — | 10–850 μg/L | HPLC-UV | [66] |
| Amoxicillin, nicotinic acid, hippuric acid, salicylic acid, anthranilic acid, ketoprofen, naproxen, ibuprofen | EME | Chitosan membrane = 60% chitosan + 40% Aliquat®336 / 1-octanol | Urine | — | — | — | HPLC-DAD | [67] |
| Epinephrine, norepinephrine dopamine | Complexation mediate-EME, EME | TFPBA + DEHPi | Urine | 1.8–5 μg/L | 2.2–6 | — | HPLC-UV-MS | [68] |
| Tetracycline, chlortetracycline, doxycycline, oxytetracycline | HF-DLLME | Aliquat-336 + 1-octanol | Milk | 0.95–3.6 μg/L | — | — | HPLC-UV | [69] |
| 2-methyl hippuric acid, 3- methyl hippuric acid, 4- methyl hippuric acid | HFLPME | 1-octanol | Urine | 2–3 μg/L | 210–312 | 10–50,000 μg/L | HPLC-UV | [70] |
| Hippuric acid, mandelic acid | HF-LPME | 1-octanol + TBP | Urine | 0.007–0.009 μg/mL | 172–195 | 0.02–20 mg/L | HPLC-UV | [71] |
| 54 care products + pharmaceutical drugs | HFLSPME | 1-octanol + toluene | Fish, water | 0.31–1.61 μg/kg | 0.11–12.83 | — | HPLC-MS | [72] |
| Pramipexole | EME | RGO + NPOE | Water and urine samples | 0.04 and 0.14 ng/mL | 301 and 265 | 0.13–1000 ng/mL | HPLC-UV | [73] |
| Haloperidol, loperamide, methadone, nortriptyline, pethidine | EME | NPOE, TBP | — | — | — | — | HPLC-UV | [74] |
| Codeine, naloxone, naltrexone | PEME | DEHP + NPOE | Plasma, urine | 2–10 μg/L | — | 10.–500 μg/L | HPLC-UV | [75] |
| Rivastigmine, verapamil, amlodipine, morphine | EME | Agarose gel as SLM | Wastewater | 1.5–1.8 ng/mL | — | 5–1000 ng/mL | HPLC-UV | [76] |
| Nicotinic acid, amoxicillin, hippuric acid, salicylic acid | EME | Acrylic nanofibers membrane /Aliquat®336 + 1-octanol | Urine samples | 112–402.3 μg/L | — | 173.3–4000 μg/L | HPLC-DAD | [77] |
| Ephedrine, clonidine | On chip-EME | DEHP + NPOE | Urine, plasma | <11 μg/L | 12–19 | 10–500 μg/L | HPLC-UV | [78] |
| Imipramine, amitriptyline, chlorpromazine | μEME | Agarose film as membrane / 1-hexyl-3-methylimidazolium hexafluorophosphate | River and tap water | 0.1–0.4 μg/L | 110–150 | 1.0–1000 μg/L | HPLC-UV | [79] |
| Ibuprofen, sodium diclofenac | EME | C60 fullerene + 1-octanol | Urine | 9–10 ng/mL | 166–188 | 15–500 ng/mL | HPLC-UV | [80] |
| Ketoprofen, diclofenac, ibuprofen, mefenamic acid | EME | Agarose film / 1-octanol | River and tap water | 0.14–0.42 | 62–86 | 0.5–500 | HPLC-UV | [81] |
| Verapamil, Haloperidol, rivastigmine, clomipramine | CF-EME | 2-ethylhexanol | Wastewater and urine samples | 2.4 ng/mL | >44 | 8.0–500 ng/mL | HPLC-UV | [82] |
| Various drugs and other contaminants | HFLPME | 1-octanol | Water samples | 1.09–98.15 ng/L | 6–4177 | — | UPLC-MS | [83] |
| Ketoprofen, ibuprofen, naproxen, diclofenac | HFLPME | DHE | Urine | 1.6–4.3 μg/L | 43.2–96.8 | 5–500 μg/L | HPLC-DAD | [84] |
| Propranolol, carvedilol, verapamil, amlodipine | HFLPME | Choline chloride + 1-phenylethanol | Urine, plasma, pharmaceutical wastewater | 0.3–0.8 ng/mL | 110–135 | 0.8–500 ng/mL | HPLC-UV | [85] |
| Salicylic acid, ketoprofen, naproxen, ibuprofen, anthranilic acid, nicotinic acid, amoxicillin, hippuric acid | EME | PIM = 29% CTA + 71% Aliquat®336 / 1-octanol | Urine samples | 18–100 μg/L | — | 61–500 μg/L | HPLC-DAD | [86] |
| Pseudoephedrine, lidocaine, propranolol | EME | Polyacrylamide gel as membrane | Breast milk, wastewater | 0.3–6.0 ng/mL | 23.1–29.5 | 1–200 ng/mL | HPLC-UV | [87] |
| Zolpidem | EME | 2-ethylhexanol | Plasma, urine | 3 ng/mL | 75 | 10–1000 ng/mL | HPLC-UV | [88] |
| Indoprofen, ketoprofen, naproxen, ibuprofen | EME | 1-octanol | Urine | 0.20–0.27 μg/mL | — | 0.68–8.4 μg/mL | HPLC-UV | [89] |
| Valproic acid | EME | 1-octanol | Plasma | 0.5 μg/mL | >125 | 0.5–10 μg/mL | HPLC-UV | [90] |
| Amlodipine, verapamil, clomipramine | EME | 2-ethylhexanol | Wastewater, plasma, urine | 3.3–5 μg/L | 98.89–130.3 | 10–2000 μg/L | HPLC-UV | [91] |
| Metaraminol, benzamidine, sotalol, ephedrine, trimethoprim, pethidine hydrochloride, quetiapine, haloperidol, nortriptyline hydrochloride, methadone hydrochloride, loperamide hydrochloride | EME | Phthalate- and nitrile-based organic solvents | Plasma | — | — | — | HPLC-UV | [92] |
| Five fluoroquinolones and four parabens | μEME / LPME | 1-octanol | Urine | 16–75 μg/L | — | 0.25-10 and 0.06–5 μg/mL respectively | HPLC-UV | [93] |
| Diclofenac | HFLPME | 1-octanol | Urine and plasma | 2.8 ng/mL | 170 | 50–2000 ng/mL | HPLC-UV | [94] |
| Exemestane, letrozole, paclitaxel | HFLPME | n-dodecane + TOPO | Urine | 0.3–0.6 μg/L | 152–411 | 0.9–200 μg/L | HPLC-UV | [95] |
| Omeprazole, pantoprazole, lansoprazole | HFLPME | 1-octanol | Plasma | 0.2 μg/mL | 1.5–13 | 0.2–2.0 μg/mL | HPLC-DAD | [96] |
| Phenazopyridine | HFSLPME | 1-octanol | Urine | 0.02 μg/L | — | 0.01–10 μg/L | HPLC-DAD | [97] |
| Different sulfonamides | HFPLME | 1-octanol | Environmental water | 3.1–11.2 ng/L | 14–60 | 0.05–5 μg/L | UHPLC-FLD | [98] |
| Thiabendazole | HFM-MI-MSPE | — | Orange or lemon peel samples | 0.004 mg/kg | — | 0.02–2 mg/L | HPLC-DAD | [99] |
| Leuprolide, triptorelin | EME | 1-octanol + 2-ethyl hexanol + DEHP | Rabbit plasma | 0.15 ng/mL | — | 0.5–1000 ng/mL | HPLC-PAD | [100] |
| Salicylic acid, naproxen, ketoprofen, diclofenac, ibuprofen | EME | 1-octanol | Urine | 0.1–1.5 ng/mL | 85–133 | 0.5–750 ng/mL | HPLC-DAD/FLD | [101] |
| Naproxen, verapamil | EME | 1-octanol, 2-ethylhexanol | Lemon juice, soft drinks | — | — | — | HPLC-UV | [102] |
| Verapamil, riluzole | EME | Mixture of 2-ethyl hexanol and 1-otanol | Urine, wastewater | 1.5–2.5 ng/mL | 123.6–146.6 | 1.5–500 ng/mL | HPLC-UV | [103] |
| Barbital, phenobarbital, pentobarbital | FMLPME | 2-nonanone | Blood, urine, liver | 0.6–10 ng/g | — | 5–2500 ng/g | HPLC-MS | [104] |
| Ciprofloxacin | On chip PEME | 1-octanol | Blood | 1 μg/L | 88 | 2–500 μg/L | HPLC-UV | [105] |
| Nalmefene, diclofenac | On chip-EME | NPOE + DEHP and 1-octanol | Urine | 3–4 μg/L | 17–19 | 9.0–500 μg/L | HPLC-UV | [106] |
| Ezetimibe, simvastatin | CA–HF–SLPME | Cetyl alcohol + 1-octanol | Plasma, urine | 0.363–0.49 μg/L | — | 0.3363–25 μg/L | HPLC-DAD | [107] |
| Fosdanofloxacin, norfloxacin, enrofloxacin, ciprofloxacin | MIP-HFME | Toluene | Environmental water, urine | 0.1–10 μg/L | — | — | HPLC-MS | [108] |
| Raloxifene, ethinylestradiol | HFLPME | 1-octanol + CTAB | Pharmaceutical waste water | 5–10 μg/L | 53–86 | 20–5000 μg/L | HPLC-UV | [109] |
HFDLLME: Hollow fiber dispersive liquid-liquid microextraction, HFLSPME: Hollow fiber liquid solid phase microextraction, PEME: Pulsed electromembrane extraction, CF-EME: Continuous-flow electromembrane extraction, LPME: Liquid phase microextraction, HFM-MI-MSPE: Hollow fiber membrane-molecular imprinted-micro solid phase microextraction, FMLPE: Flat membrane based liquid phase microextraction, CA–HF–SLPME: Cetyl-alcohol-reinforced hollow fiber solid/ liquid phase microextraction, MIP-HFME: Molecular imprinted polymer hollow fiber microextraction, DEHPi: Bis(2-ethylhexyl) phosphite, TFPBA: 4-(trifluoromethyl)phenylboronic acid, RGO: Reduced graphene oxide, TBP: Tributyl phosphate, DHE: Dihexyl ether, PIM: Polymer inclusion membrane, TOPO: Trioctylphosphine oxide HPLC-UV: High performance liquid chromatography with UV detector, HPLC-MS: High performance liquid chromatography with mass spectrometry, HPLC-DAD-FLD: High performance liquid chromatography with diode array detector and fluorescence detector, HPLC-UV-MS: High performance liquid chromatography with UV detector and mass spectrometry, UHPLC-MS: Ultra high performance liquid chromatography with mass spectrometry, HPLC-DAD: High performance liquid chromatography with diode array detector, Fluorometer/RGB/ HPLC-UV: Fluorometer/ Red Green Blue analysis/ High performance liquid chromatography with UV detector.
Limit of detection.
Enrichment factor/Pre-concentration factor.
Linear range.
4.2.2. Gas chromatography (GC)
GC has been coupled with two-phase HF-LPME and EME for analysis of neutral, low and medium polar analytes. In two phase systems the acceptor solution is the same organic solvent as used for impregnation of membrane. Due to the fact that the acceptor phase is organic, it can be injected directly in GC [109]. An EME-SPME technique were introduced by Shamsayei et al. [110] for the extraction and determination of cyproheptadine and ketotifen. In this work, the surface of a stainless steel wire was electrochemically deposited with polypyrrole and manganese dioxide using cyclic voltammetry. A segment of polypropylene membrane was impregnated with a suitable organic solvent and filled with water as an acceptor phase. The prepared SPME fiber (electrochemically prepared stainless steel wire) was introduced into the membrane and used as a cathode. The cathode comprising the SLM and the acceptor solution was directed into donor solution. A platinum anode was directed into the sample solution. After EME, the SPME fiber was analyzed with GC [110].
Similarly Tabani et al. presented a new approach to preconcentration and determination of basic drugs. An agarose gel EME method was combined with dispersive liquid–liquid microextraction (DLLME) technique. For extraction of target analytes, an agarose-based gel membrane was synthesized and used for EME. After EME, acceptor phase was collected and mixed with 1 mM NaOH solution. DLLME was carried out from this solution and the final organic extract was analyzed with GC [111]. Table 3 summarizes information on research combining HF-LPME and EME with GC [[110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131]].
Table 3.
Gas chromatography, Capillary electrophoresis and Voltammetric techniques coupled with Hollow fiber based extraction techniques for determination of pharmaceutical drugs.
| Instrumental technique | Drug | Preconcentration/Separation method | SLM +/Carrier/FLM/Membrane | Real applications | LODa | EF/PF/EFb | LRc | Instrumental technique mode | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Gas chromatography | Cyproheptadine, ketotifen | EM-SPME | NPOE | Water, urine, plasma | 01–1.1 ng/mL | — | 0.3–200 and 0.5–750 ng/mL | GC-FID | [110] |
| Trimipramine, clomipramine | G-EME/DLLME | Agarose gel | Urine | 1.0–3.0 ng/L | 260–370 | 3.5–1000 ng/mL | GC-FID | [111] | |
| Clomipramine, imipramine | EME-EALLME | 2-ethylhexanol | Urine, wastewater | 0.15 ng/mL | 770.3 | 0.5–750 ng/mL | GC-FID | [112] | |
| Capillary electrophoresis | Nort [112]riptyline, papaverine, haloperidol | μEME | ENB | Urine, plasma | ≤0.15 mg/L | — | 1–20 mg/L | CE-UV | [113] |
| Nortriptyline, haloperidol, loperamide, ketoprofen, naproxen | EME | ENB | Urine | 0.075–1.5 μg/mL | — | 1–40 μg/mL | CE-DAD | [114] | |
| Imatinibmesylate | EME | NPOE + MWCNT/ZnO coated by 1-octyl-3-methylimidazolium bromide ionic liquid | Plasma | 6.24 ng/mL | 78 | 25–1500 ng/mL | CE-UV | [115] | |
| Nortriptyline, papaverine | μEME | 4-nitrocumene | Urine | ≤0.15 μg/mL | — | 0.5–10 μg/mL | CE-UV | [116] | |
| Amitriptyline, haloperidol, bupivacaine, propranolol | Pa-EME | NPOE | Plasma | — | — | — | CE-UV | [117] | |
| Methadone, methamphetamine, tramadol | EME | NPOE + MIL-110(Cr) | Urine, plasma | 0.30–0.91 μg/L | 132–190 | 1–1000 μg/L | CE-UV | [118] | |
| Tramadol, pseudoephedrine | EME | NPOE + span 80 | Urine | 2.10–4.50 μg/L | 160–188 | 5–2000 μg/L | CE-UV | [119] | |
| Capecitabine, 5-Fluorouracil | HF-SLPME | 1-octanol | Plasma | 6.4–9.3 ng/mL | — | 25–1000 ng/mL | CE-UV | [120] | |
| Tranylcypromine | S/EME | NPOE | Urine, water | 3.03–6.06 ng/mL | 166–203 | 10–1000 ng/mL | CE-UV | [121] | |
| Ibuprofen, naproxen, ketoprofen, diclofenac | μEME | 1-octanol | Urine, serum, wastewater | 4–20 ng/mL | 18–29.1 | 50–2500 ng/mL | CE-UV | [122] | |
| Nortriptyline, papaverine, haloperidol and loperamide | SLM extraction | ENB | Urine, plasma | 0.02–0.15 μg/mL | — | 0.5–30 μg/mL | CE-UV | [123] | |
| Ibuprofen, diclofenac, naproxen, ketoprofen, salicylic acid | On chip-EME | Undecanol + NPOE | Urine | 0.07–5 μg/mL | — | — | CE/HPLC-DA | [124] | |
| Voltammetry | Amlodipine | EME | NPOE | Whole blood | 0.05 ng/mL | 61 | 0.1–10 and 10–1000 ng/mL | FFSSWV | [125] |
| Diclofenec | EME | 1-octanol | Whole blood | 1 ng/mL | — | 5–1000 ng/mL | SFFTCCV | [126] | |
| Imatinib | HFSPME | — | Serum, urine | 7.39× 10−3 μmol/L | — | 0.010–200 μmol/L | DPV | [127] | |
| Estradiol valerate | EME | 1-octanol | Whole blood | 0.01 ng/mL | 2 | 0.1–1300 and 1300–10,000 ng/mL | FFTSWV | [128] | |
| Propylthiouracil | EME | Nitrobenzene + CuNPs | Urine | 0.02 μg/mL | 200 | 0.5–5 μg/mL | DPV | [129] | |
| Imipramine | EME | NPOE | Urine, whole blood | 0.001–0.01 ng/mL | 35–54 | 0.02–1000 and 0.2–1000 ng/mL | FFTSWV | [130] | |
| Vanillylmandelic acid | HFLPME | Butyl benzoate | Urine | 0.5 μmol/L | — | 0.5–100 μmol/L | DPV | [131] |
EME-EALLME: Electromembrane extraction and electro-assisted liquid-liquid microextraction, EM-SPME: Electromembrane surrounded solid phase microextraction, G-EME/DLLME: Gel-electromembrane exraction / dispersive liquid-liquid microextraction, Pa-EME: Parallel electromembrane extraction, ENB: 1-ethyl-2-nitrobenzene, MWCNTs: Multi-walled carbon nanotubes, MIL-110(Cr): Chromium terephthalate metal-organic framework, Span-80: Mono-(9Z)-9-octadecenoate, CuNPs: Copper nanoparticles, GC-FID: Gas chromatography with flame ionization detector,CE-UV: Capillary electrophoresis with UV detector, CE-DAD: Capillary electrophoresis with diode array detector, FFSSWV: Fast Fourier transform stripping square wave voltammetry, SFFTCCV: Stripping fast Fourier transform continuous cyclic voltammetry, DPV: Differential pulse voltammetry, FFTSWV: Fast Fourier transform square wave voltammetry.
Limit of detection.
Enrichment factor.
Linear range.
4.2.3. Capillary electrophoresis (CE)
CE is another analytical instrument which has frequently been coupled with three-phase HF-LPME and EME [30]. To avoid band broadening (de-stacking), the ionic strength of the acceptor phase should not exceed the ionic strength of the CE separation buffer [132]. Dvořák et al. presented a new semi-automated-μEME technique for extraction of drugs coupled with CE. A three phase disposable microextraction unit was constructed, in which a free liquid membrane was sandwiched between acceptor and donor phases. All liquid samples (acceptor phase, FLM, and donor phase) were handled with a programmable syringe pump. After EME, the acceptor phase was collected by switching the syringe pump to infusion mode. The collected acceptor phase was quantitatively analyzed with CE [113].
Ryšavá et al. investigated the effect of membrane thickness on the extraction efficiency in EME [123]. Three polypropylene membranes with different thickness were used as SLMs for EME preconcentration of basic drugs. In this experiment, it was concluded that the transfer of analytes across the membrane is inversely proportional to the membrane thickness. Recent studies of EME and HF-LPME coupled with CE are mentioned in Table 3 [[113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124]].
4.2.4. Voltammetry
One of the most important and widely used electroanalytical techniques is voltammetry [133], and for quantification of heavy metals anodic stripping voltammetry (ASV) has been combined with HF-LPME. The major advantage of ASV over other voltammetric techniques is the step of pre-concentration onto the electrode surface [134]. Anodic stripping voltammetry has low detection limits, high sensitivity and selectivity as compared to other electrochemical techniques. Detection limits with anodic stripping voltammetry of heavy metals are in the ppm range [135]. To the best of our knowledge (2017 to May-2019), there is only one research article on voltammetric instrumental technique for quantification of heavy metals using HF-LPME or EME preconcentration. For pharmaceuticals, several new approaches based on hollow fiber based techniques have been reported. In one example, Hrdlička et al. presented an HF-LPME method for extraction and determination of vanillylmandelic acid combined with DPV. In this study, a 20 mm polypropylene hollow fiber membrane was soaked in an organic solvent to impregnate the pores. The excess organic solvent was removed by air with a syringe. The lumen of the fiber was filled with acceptor phase and sealed in both ends. The prepared membrane was fully immersed in sample solution and stirred with a magnetic bar. After EME, the acceptor phase was collected and transferred to the surface of working electrode for DPV analysis [131].
Tahmasebi et al. reported a novel approach in EME for preconcentration and determination of propylthiouracil coupled with DPV. CuNPs were synthesized for selective extraction of propylthiouracil, and coated on hollow fiber membrane. The coated CuNPs increased mass transfer and selectivity of propylthiouracil due to the high selectivity of CuNPs toward thiol group/sulfhydryl compounds. The high affinity/selectivity of propylthiouracil towards CuNPs was explained by the hard and soft acid-base theory. It was concluded that nitrobenzene was the best choice for propylthiouracil extraction because alcoholic solvents such as 1-octanol and 2-ethylhexanol dissolved CuNPs. It was pointed out that this approach was not applicable for acidic compounds [129]. Research combining HF-LPME and EME with voltammetry is summarized in Table 3 [[125], [126], [127], [128], [129], [130], [131]].
5. Conclusions and looking into the future
Since 1999, a larger number of reports have been published on the development of HF-LPME. These efforts have documented HF-LPME as a green sample preparation technique requiring only a few microliters of organic solvent per sample. HF-LPME enables high enrichment and excellent sample clean-up from biological and environmental samples. In the three-phase mode, HF-LPME provides aqueous extracts which are directly injectable in HPLC. Due to the protection of the acceptor phase by the SLM, HF-LPME is amenable to highly complex samples such as plasma, whole blood, urine, saliva, breast milk, tap water, surface water, pond water, seawater, and soil slurries. HF-LPME shows great potential for routine applications, but to enter this area commercial products and automation are required. Also, generic methods have to be developed to simplify method development.
EME has been developed in parallel to HF-LPME, but EME is less mature. Although, EME provides the same advantages as HF-LPME, the electrical field open for additional perspectives. Thus, the extraction is controlled by an external electrical field, and selectivity may be tuned by the direction and magnitude of the electrical field, by the chemical composition of the SLM, and by pH in sample and acceptor phase.
Both HF-LPME and EME are highly efficient for extraction of heavy metals and pharmaceuticals. The techniques are compatible with a broad spectrum of chromatographic, spectroscopic, and electroanalytical techniques. Future work should focus on automation and commercialization. In addition, the future may see research on the use of green solvents, and SLMs modified with metal nanoparticles (MNPs) and carbon nanotubes (CNTs), which may increase the extraction efficiency of targeted analytes.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the Higher education commission of Pakistan (NRPU No.20–3925/R&D/NRPU/HEC/2014), PAK-US science and technology cooperation (Pak-US No6-4/PAK-US/HEC/2015/04) and Pakistan science foundation joint research projects with MSRT, Iran (No. PSF-MSRT/Env/KP-AWKUM).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Contributor Information
Muhammad Balal Arain, Email: bilal_ku2004@yahoo.com.
Stig Pedersen-Bjergaard, Email: stig.pedersen-bjergaard@farmasi.uio.no.
References
- 1.Ramos L. Critical overview of selected contemporary sample preparation techniques. J. Chromatogr. A. 2012;1221:84–98. doi: 10.1016/j.chroma.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Hennion M.-C. Solid-phase extraction: method development, sorbents, and coupling with liquid chromatography. J. Chromatogr. A. 1999;856:3–54. doi: 10.1016/s0021-9673(99)00832-8. [DOI] [PubMed] [Google Scholar]
- 3.Saito Y., Imaizumi M., Ban K. Development of miniaturized sample preparation with fibrous extraction media. J. Chromatogr. A. 2004;1025:27–32. doi: 10.1016/j.chroma.2003.08.098. [DOI] [PubMed] [Google Scholar]
- 4.Arthur C.L., Pawliszyn J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990;62:2145–2148. [Google Scholar]
- 5.Jeannot M.A., Cantwell F.F. Solvent microextraction into a single drop. Anal. Chem. 1996;68:2236–2240. doi: 10.1021/ac960042z. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z., Pawliszyn J. Headspace solid-phase microextraction. Anal. Chem. 1993;65:1843–1852. [Google Scholar]
- 7.Eisert R., Pawliszyn J. Automated in-tube solid-phase microextraction coupled to high-performance liquid chromatography. Anal. Chem. 1997;69:3140–3147. [Google Scholar]
- 8.Bruheim I., Liu X., Pawliszyn J. Thin-film microextraction. Anal. Chem. 2003;75:1002–1010. doi: 10.1021/ac026162q. [DOI] [PubMed] [Google Scholar]
- 9.Shen G., Lee H.K. Headspace liquid-phase microextraction of chlorobenzenes in soil with gas chromatography-electron capture detection. Anal. Chem. 2003;75:98–103. doi: 10.1021/ac020428b. [DOI] [PubMed] [Google Scholar]
- 10.Xu H., Ding Z., Lv L. A novel dispersive liquid–liquid microextraction based on solidification of floating organic droplet method for determination of polycyclic aromatic hydrocarbons in aqueous samples. Anal. Chim. Acta. 2009;636:28–33. doi: 10.1016/j.aca.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Rezaee M., Assadi Y., Milani Hosseini M.-R. Determination of organic compounds in water using dispersive liquid–liquid microextraction. J. Chromatogr. A. 2006;1116:1–9. doi: 10.1016/j.chroma.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Vera-Avila L.E., Rojo-Portillo T., Covarrubias-Herrera R. Capabilities and limitations of dispersive liquid–liquid microextraction with solidification of floating organic drop for the extraction of organic pollutants from water samples. Anal. Chim. Acta. 2013;805:60–69. doi: 10.1016/j.aca.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen-Bjergaard S., Rasmussen K.E. Liquid− liquid− liquid microextraction for sample preparation of biological fluids prior to capillary electrophoresis. Anal. Chem. 1999;71:2650–2656. doi: 10.1021/ac990055n. [DOI] [PubMed] [Google Scholar]
- 14.Alsharif A.M.A., Tan G.-H., Choo Y.-M. Efficiency of hollow fiber liquid-phase microextraction chromatography methods in the separation of organic compounds: a review. J. Chromatogr. Sci. 2017;55:378–391. doi: 10.1093/chromsci/bmw188. [DOI] [PubMed] [Google Scholar]
- 15.Ghambarian M., Yamini Y., Esrafili A. Developments in hollow fiber based liquid-phase microextraction: principles and applications. Microchimi Acta. 2012;177:271–294. [Google Scholar]
- 16.Chen H., Han J., Wang Y. Hollow fiber liquid-phase microextraction of cadmium (II) using an ionic liquid as the extractant. Microchim Acta. 2014;181:1455–1461. [Google Scholar]
- 17.Abulhassani J., Manzoori J.L., Amjadi M. Hollow fiber based-liquid phase microextraction using ionic liquid solvent for preconcentration of lead and nickel from environmental and biological samples prior to determination by electrothermal atomic absorption spectrometry. J. Hazard Mater. 2010;176:481–486. doi: 10.1016/j.jhazmat.2009.11.054. [DOI] [PubMed] [Google Scholar]
- 18.Gjelstad A., Pedersen-Bjergaard S., Seip K.F. Electromembrane extraction as a rapid and selective miniaturized sample preparation technique for biological fluids. Bioanalysis. 2015;7:2203–2209. doi: 10.4155/bio.15.150. [DOI] [PubMed] [Google Scholar]
- 19.Esrafili A., Baharfar M., Tajik M. Two-phase hollow fiber liquid-phase microextraction. TrAC Trends Anal. Chem. (Reference Ed.) 2018;108:314–322. [Google Scholar]
- 20.Folde Bårdstu K., Ho T.S., Rasmussen K.E. Supported liquid membranes in hollow fiber liquid-phase microextraction (LPME)–Practical considerations in the three-phase mode. J. Sep. Sci. 2007;30:1364–1370. doi: 10.1002/jssc.200600486. [DOI] [PubMed] [Google Scholar]
- 21.Shariati S., Yamini Y., Esrafili A. Carrier mediated hollow fiber liquid phase microextraction combined with HPLC–UV for preconcentration and determination of some tetracycline antibiotics. J. Chromatogr. B. 2009;877:393–400. doi: 10.1016/j.jchromb.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 22.Ghambarian M., Yamini Y., Esrafili A. Three-phase hollow fiber microextraction based on two immiscible organic solvents for determination of tricyclic antidepressant drugs: comparison with conventional three-phase hollow fiber microextraction. J. Chromatogr. A. 2012;1222:5–12. doi: 10.1016/j.chroma.2011.11.055. [DOI] [PubMed] [Google Scholar]
- 23.Esrafili A., Yamini Y., Ghambarian M. A novel approach to automation of dynamic hollow fiber liquid-phase microextraction. J. Sep. Sci. 2011;34:957–964. doi: 10.1002/jssc.201000913. [DOI] [PubMed] [Google Scholar]
- 24.Esrafili A., Yamini Y., Ghambarian M. Automated preconcentration and analysis of organic compounds by on-line hollow fiber liquid-phase microextraction–high performance liquid chromatography. J. Chromatogr. A. 2012;1262:27–33. doi: 10.1016/j.chroma.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen-Bjergaard S., Rasmussen K.E. Electrokinetic migration across artificial liquid membranes: new concept for rapid sample preparation of biological fluids. J. Chromatogr. A. 2006;1109:183–190. doi: 10.1016/j.chroma.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 26.Šlampová A., Kubáň P., Boček P. Electromembrane extraction using stabilized constant d.c. electric current—a simple tool for improvement of extraction performance. J. Chromatogr. A. 2012;1234:32–37. doi: 10.1016/j.chroma.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 27.Rezazadeh M., Yamini Y., Seidi S. Pulsed electromembrane extraction: a new concept of electrically enhanced extraction. J. Chromatogr., A. 2012;1262:214–218. doi: 10.1016/j.chroma.2012.08.090. [DOI] [PubMed] [Google Scholar]
- 28.Sharifi V., Abbasi A., Nosrati A. Application of hollow fiber liquid phase microextraction and dispersive liquid–liquid microextraction techniques in analytical toxicology. J. Food Drug Anal. 2016;24:264–276. doi: 10.1016/j.jfda.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishna Marothu V., Gorrepati M., Vusa R. Electromembrane extraction—a novel extraction technique for pharmaceutical, chemical, clinical and environmental analysis. J. Chromatogr. Sci. 2013;51:619–631. doi: 10.1093/chromsci/bmt041. [DOI] [PubMed] [Google Scholar]
- 30.Płotka-Wasylka J., Owczarek K., Namieśnik J. Modern solutions in the field of microextraction using liquid as a medium of extraction. TrAC Trends Anal. Chem. (Reference Ed.) 2016;85:46–64. [Google Scholar]
- 31.Rasmussen K.E., Pedersen-Bjergaard S. Developments in hollow fibre-based, liquid-phase microextraction. TrAC Trends Anal. Chem. (Reference Ed.) 2004;23:1–10. [Google Scholar]
- 32.Rezazadeh M., Yamini Y., Seidi S. Electromembrane extraction of trace amounts of naltrexone and nalmefene from untreated biological fluids. J. Chromatogr. B. 2011;879:1143–1148. doi: 10.1016/j.jchromb.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 33.Vårdal L., Øiestad E.L., Gjelstad A. Electromembrane extraction of substances with weakly basic properties: a fundamental study with benzodiazepines. Bioanalysis. 2018;10:769–781. doi: 10.4155/bio-2018-0030. [DOI] [PubMed] [Google Scholar]
- 34.Huang C., Chen Z., Gjelstad A. Electromembrane extraction. TrAC Trends Anal. Chem. (Reference Ed.) 2017;95:47–56. [Google Scholar]
- 35.Psillakis E., Kalogerakis N. Developments in liquid-phase microextraction. TrAC Trends Anal. Chem. (Reference Ed.) 2003;22:565–574. [Google Scholar]
- 36.Huang C., Seip K.F., Gjelstad A. Electromembrane extraction for pharmaceutical and biomedical analysis–Quo vadis. J. Pharm. Biomed. Anal. 2015;113:97–107. doi: 10.1016/j.jpba.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 37.Krishna Marothu V., Gorrepati M., Vusa R. Electromembrane extraction—a novel extraction technique for pharmaceutical, chemical, clinical and environmental analysis. J. Chromatogr. Sci. 2013;51:619–631. doi: 10.1093/chromsci/bmt041. [DOI] [PubMed] [Google Scholar]
- 38.Ghambarian M., Yamini Y., Esrafili A. Three-phase hollow fiber liquid-phase microextraction based on two immiscible organic solvents for determination of tramadol in urine and plasma samples. J. Pharm. Biomed. Anal. 2011;56:1041–1045. doi: 10.1016/j.jpba.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Payán M.R., López M.Á.B., Fernández-Torres R. Hollow fiber-based liquid phase microextraction (HF-LPME) as a new approach for the HPLC determination of fluoroquinolones in biological and environmental matrices. J. Pharm. Biomed. Anal. 2011;55:332–341. doi: 10.1016/j.jpba.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 40.Hadjmohammadi M.R., Soltani M. Use of hollow fiber liquid phase microextraction and HPLC for extraction and determination of apigenin in human urine after consumption of Satureja sahendica Bornm. J. Chromatogr. B. 2012;900:85–88. doi: 10.1016/j.jchromb.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 41.Al Azzam K.M., Makahleah A., Saad B. Hollow fiber liquid-phase microextraction for the determination of trace amounts of rosiglitazone (anti-diabetic drug) in biological fluids using capillary electrophoresis and high performance liquid chromatographic methods. J. Chromatogr. A. 2010;1217:3654–3659. doi: 10.1016/j.chroma.2010.03.055. [DOI] [PubMed] [Google Scholar]
- 42.Sharifi V., Abbasi A., Nosrati A. Application of hollow fiber liquid phase microextraction and dispersive liquid–liquid microextraction techniques in analytical toxicology. J. Food Drug Anal. 2016;24:264–276. doi: 10.1016/j.jfda.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiong J., Hu B. Comparison of hollow fiber liquid phase microextraction and dispersive liquid–liquid microextraction for the determination of organosulfur pesticides in environmental and beverage samples by gas chromatography with flame photometric detection. J. Chromatogr. A. 2008;1193:7–18. doi: 10.1016/j.chroma.2008.03.072. [DOI] [PubMed] [Google Scholar]
- 44.Saleh A., Yamini Y., Faraji M. Hollow fiber liquid phase microextraction followed by high performance liquid chromatography for determination of ultra-trace levels of Se (IV) after derivatization in urine, plasma and natural water samples. J. Chromatogr. B. 2009;877:1758–1764. doi: 10.1016/j.jchromb.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 45.Seidi S., Yamini Y., Heydari A. Determination of thebaine in water samples, biological fluids, poppy capsule, and narcotic drugs, using electromembrane extraction followed by high-performance liquid chromatography analysis. Anal. Chim. Acta. 2011;701:181–188. doi: 10.1016/j.aca.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 46.Middelthon-Bruer T.M., Gjelstad A., Rasmussen K.E. Parameters affecting electro membrane extraction of basic drugs. J. Sep. Sci. 2008;31:753–759. doi: 10.1002/jssc.200700502. [DOI] [PubMed] [Google Scholar]
- 47.Balchen M., Halvorsen T.G., Reubsaet L. Rapid isolation of angiotensin peptides from plasma by electromembrane extraction. J. Chromatogr. A. 2009;1216:6900–6905. doi: 10.1016/j.chroma.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 48.Kubáň P., Šlampová A., Boček P. Electric field-enhanced transport across phase boundaries and membranes and its potential use in sample pretreatment for bioanalysis. Electrophoresis. 2010;31:768–785. doi: 10.1002/elps.200900561. [DOI] [PubMed] [Google Scholar]
- 49.Yamini Y., Seidi S., Rezazadeh M. Electrical field-induced extraction and separation techniques: promising trends in analytical chemistry–a review. Anal. Chim. Acta. 2014;814:1–22. doi: 10.1016/j.aca.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 50.Sneddon J. Determination of heavy metals in food by atomic spectroscopy. Anal Endocr Disrup Comp Food. 2011:289. [Google Scholar]
- 51.Bahar S., Es’ haghi Z., Nezhadali A. An innovative method for analysis of Pb (II) in rice, milk and water samples based on TiO2 reinforced caprylic acid hollow fiber solid/liquid phase microextraction. Food Chem. 2017;221:1904–1910. doi: 10.1016/j.foodchem.2016.09.073. [DOI] [PubMed] [Google Scholar]
- 52.Zeng C., Yan Y., Tang J. Speciation of Arsenic (III) and Arsenic (V) based on Triton X-100 hollow fiber liquid phase microextraction coupled with flame atomic absorption spectrometry. Spectrosc. Lett. 2017;50:220–226. [Google Scholar]
- 53.Boutorabi L., Rajabi M., Bazregar M. Selective determination of chromium (VI) ions using in-tube electro-membrane extraction followed by flame atomic absorption spectrometry. Microchem. J. 2017;132:378–384. [Google Scholar]
- 54.Salari S., Bahrami A., Ghamari F. Multivariate optimization of the hollow fiber-based liquid phase microextraction of lead in human blood and urine samples using graphite furnace atomic absorption spectrometry. Chem. Pap. 2018;72:1945–1952. [Google Scholar]
- 55.Kamyabi M.A., Aghaei A. A simple and selective approach for determination of trace Hg (II) using electromembrane extraction followed by graphite furnace atomic absorption spectrometry. Spectrochem Acta B. 2017;128:17–21. [Google Scholar]
- 56.Tahmasebi Z., Davarani S.S.H., Ebrahimzadeh H. Ultra-trace determination of Cr (VI) ions in real water samples after electromembrane extraction through novel nanostructured polyaniline reinforced hollow fibers followed by electrothermal atomic absorption spectrometry. Microchem. J. 2018;143:212–219. [Google Scholar]
- 57.Alahmad W., Tungkijanansin N., Kaneta T. A colorimetric paper-based analytical device coupled with hollow fiber membrane liquid phase microextraction (HF-LPME) for highly sensitive detection of hexavalent chromium in water samples. Talanta. 2018;190:78–84. doi: 10.1016/j.talanta.2018.07.056. [DOI] [PubMed] [Google Scholar]
- 58.Alavi L., Seidi S., Jabbari A. Deep eutectic liquid organic salt as a new solvent for carrier-mediated hollow fiber liquid phase microextraction of lead from whole blood followed by electrothermal atomic absorption spectrometry. New J. Chem. 2017;41:7038–7044. [Google Scholar]
- 59.Nojavan S., Rahmani T., Mansouri S. Selective determination of chromium (VI) in industrial wastewater samples by micro-electromembrane extraction combined with electrothermal atomic absorption spectrometry, water, air. Soil Pollut. 2018;229:89. [Google Scholar]
- 60.Thongsaw A., Sananmuang R., Udnan Y. Speciation of mercury in water and freshwater fish samples using two-step hollow fiber liquid phase microextraction with electrothermal atomic absorption spectrometry. Spectrochem Acta B. 2019;152:102–108. doi: 10.1016/j.foodchem.2018.10.131. [DOI] [PubMed] [Google Scholar]
- 61.Atikarnsakul U., Varanusupakul P., Alahmad W. Isolation of chromium (VI) from aqueous solution by electromembrane extraction. Anal. Lett. 2018;51:983–997. [Google Scholar]
- 62.Onac C., Kaya A., Sener I. An electromembrane extraction with polymeric membrane under constant current for the recovery of Cr (VI) from industrial water. J. Electrochem. Soc. 2018;165:E76–E80. [Google Scholar]
- 63.Fashi A., Yaftian M.R., Zamani A. Electromembrane extraction-preconcentration followed by microvolume UV–Vis spectrophotometric determination of mercury in water and fish samples. Food Chem. 2017;221:714–720. doi: 10.1016/j.foodchem.2016.11.115. [DOI] [PubMed] [Google Scholar]
- 64.Khajeh M., Dahmardeh M., Bohlooli M. Determination of gold in water samples using electromembrane extraction. J. Dispersion Sci. Technol. 2018;39:311–315. [Google Scholar]
- 65.Fashi A., Yaftian M.R., Zamani A. Electromembrane-microextraction of bismuth in pharmaceutical and human plasma samples: optimization using response surface methodology. Microchem. J. 2017;130:71–78. [Google Scholar]
- 66.Khan W.A., Yamini Y., Baharfar M. A new microfluidic-chip device for selective and simultaneous extraction of drugs with various properties. New J. Chem. 2019;43:9689–9695. [Google Scholar]
- 67.Román-Hidalgo C., López-Pérez G., Martín-Valero M.J. Chitosan tailor-made membranes as biopolymeric support for electromembrane extraction. Talanta. 2019;199:290–295. doi: 10.1016/j.talanta.2019.02.079. [DOI] [PubMed] [Google Scholar]
- 68.Fernández E., Vårdal L., Vidal L. Complexation-mediated electromembrane extraction of highly polar basic drugs—a fundamental study with catecholamines in urine as model system. Anal. Bioanal. Chem. 2017;409:4215–4223. doi: 10.1007/s00216-017-0370-2. [DOI] [PubMed] [Google Scholar]
- 69.Xu H., Mi H.-Y., Guan M.-M. Residue analysis of tetracyclines in milk by HPLC coupled with hollow fiber membranes-based dynamic liquid-liquid micro-extraction. Food Chem. 2017;232:198–202. doi: 10.1016/j.foodchem.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 70.Ghamari F., Bahrami A., Yamini Y. Hollow-fiber liquid-phase microextraction based on carrier-mediated transport for determination of urinary methyl hippuric acids. Toxicol. Environ. Chem. 2017;99:760–771. [Google Scholar]
- 71.Bahrami A., Ghamari F., Yamini Y. Hollow fiber supported liquid membrane extraction combined with HPLC-UV for simultaneous preconcentration and determination of urinary hippuric acid and mandelic acid. Membranes. 2017;7:8. doi: 10.3390/membranes7010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y., Guo W., Yue Z. Rapid determination of 54 pharmaceutical and personal care products in fish samples using microwave-assisted extraction—hollow fiber—liquid/solid phase microextraction. J. Chromatogr. B. 2017;1051:41–53. doi: 10.1016/j.jchromb.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 73.Fashi A., Khanban F., Yaftian M.R. The cooperative effect of reduced graphene oxide and Triton X-114 on the electromembrane microextraction efficiency of Pramipexole as a model analyte in urine samples. Talanta. 2017;162:210–217. doi: 10.1016/j.talanta.2016.09.067. [DOI] [PubMed] [Google Scholar]
- 74.Restan M.S., Jensen H., Shen X. Comprehensive study of buffer systems and local pH effects in electromembrane extraction. Anal. Chim. Acta. 2017;984:116–123. doi: 10.1016/j.aca.2017.06.049. [DOI] [PubMed] [Google Scholar]
- 75.Karami M., Yamini Y., Asl Y.A. On-chip pulsed electromembrane extraction as a new concept for analysis of biological fluids in a small device. J. Chromatogr. A. 2017;1527:1–9. doi: 10.1016/j.chroma.2017.10.049. [DOI] [PubMed] [Google Scholar]
- 76.Tabani H., Asadi S., Nojavan S. Introduction of agarose gel as a green membrane in electromembrane extraction: an efficient procedure for the extraction of basic drugs with a wide range of polarities. J. Chromatogr. A. 2017;1497:47–55. doi: 10.1016/j.chroma.2017.03.075. [DOI] [PubMed] [Google Scholar]
- 77.Román-Hidalgo C., Martín-Valero M.J., Fernández-Torres R. New nanostructured support for carrier-mediated electromembrane extraction of high polar compounds. Talanta. 2017;162:32–37. doi: 10.1016/j.talanta.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 78.Baharfar M., Yamini Y., Seidi S. Quantitative analysis of clonidine and ephedrine by a microfluidic system: on-chip electromembrane extraction followed by high performance liquid chromatography. J. Chromatogr. B. 2017;1068:313–321. doi: 10.1016/j.jchromb.2017.10.062. [DOI] [PubMed] [Google Scholar]
- 79.Hanapi N.S.M., Sanagi M.M., Ismail A.K. Ionic liquid-impregnated agarose film two-phase micro-electrodriven membrane extraction (IL-AF-μ-EME) for the analysis of antidepressants in water samples. J. Chromatogr. B. 2017;1046:73–80. doi: 10.1016/j.jchromb.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 80.Atarodi A., Chamsaz M., Moghaddam A.Z. Introduction of fullerene as a new carrier in electromembrane extraction for the determination of ibuprofen and sodium diclofenac as model acidic drugs in real urine samples. Chromatographia. 2017;80:881–890. [Google Scholar]
- 81.Mohamad Hanapi N.S., Sanagi M.M., Ismail A.K. Rapid determination of non-steroidal anti-inflammatory drugs in aquatic matrices by two-phase micro-electrodriven membrane extraction combined with liquid chromatography. J. Chromatogr. Sci. 2017;56:166–176. doi: 10.1093/chromsci/bmx092. [DOI] [PubMed] [Google Scholar]
- 82.Nojavan S., Sirani M., Asadi S. Investigation of the continuous flow of the sample solution on the performance of electromembrane extraction: comparison with conventional procedure. J. Sep. Sci. 2017;40:3889–3897. doi: 10.1002/jssc.201700528. [DOI] [PubMed] [Google Scholar]
- 83.Salvatierra-stamp V., Muñiz-Valencia R., Jurado J.M. Hollow fiber liquid phase microextraction combined with liquid chromatography-tandem mass spectrometry for the analysis of emerging contaminants in water samples. Microchem. J. 2018;140:87–95. [Google Scholar]
- 84.Worawit C., Cocovi-Solberg D.J., Varanusupakul P. In-line carbon nanofiber reinforced hollow fiber-mediated liquid phase microextraction using a 3D printed extraction platform as a front end to liquid chromatography for automatic sample preparation and analysis: a proof of concept study. Talanta. 2018;185:611–619. doi: 10.1016/j.talanta.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 85.Rajabi M., Ghassab N., Hemmati M. Highly effective and safe intermediate based on deep eutectic medium for carrier less-three phase hollow fiber microextraction of antiarrhythmic agents in complex matrices. J. Chromatogr. B. 2019;1104:196–204. doi: 10.1016/j.jchromb.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 86.Cristina R.-H., Jesús M.-V.M., Rut F.-T. Use of polymer inclusion membranes (PIMs) as support for electromembrane extraction of non-steroidal anti-inflammatory drugs and highly polar acidic drugs. Talanta. 2018;179:601–607. doi: 10.1016/j.talanta.2017.11.066. [DOI] [PubMed] [Google Scholar]
- 87.Asadi S., Tabani H., Nojavan S. Application of polyacrylamide gel as a new membrane in electromembrane extraction for the quantification of basic drugs in breast milk and wastewater samples. J. Pharm. Biomed. Anal. 2018;151:178–185. doi: 10.1016/j.jpba.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 88.Yaripour S., Mohammadi A., Esfanjani I. Quantitation of zolpidem in biological fluids by electro-driven microextraction combined with HPLC-UV analysis. EXCLI journal. 2018;17:349. doi: 10.17179/excli2018-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim J.M., Myung S.W. Determination of non-steroidal anti-inflammatory drugs in urine by HPLC–UV/vis analysis coupled with electromembrane extraction. Bull. Korean Chem. Soc. 2018;39:335–340. [Google Scholar]
- 90.Yaripour S., Zaheri M., Mohammadi A. An electromembrane extraction–HPLC-UV analysis for the determination of valproic acid in human plasma. J. Chin. Chem. Soc. 2018;65:989–994. [Google Scholar]
- 91.Moazami H.R., Davarani S.S.H., Abrari M. Electromembrane extraction using a round-headed platinum wire as the inner electrode: a simple and practical way to enhance the performance of extraction. Chromatographia. 2018;81:1023–1033. [Google Scholar]
- 92.Huang C., Shen X., Gjelstad A. Investigation of alternative supported liquid membranes in electromembrane extraction of basic drugs from human plasma. J. Membr. Sci. 2018;548:176–183. [Google Scholar]
- 93.Ramos Payán M.a., Santigosa E., Fernández Torres R. A new microchip design. A versatile combination of electromembrane extraction and liquid-phase microextraction in a single chip device. Anal. Chem. 2018;90:10417–10424. doi: 10.1021/acs.analchem.8b02292. [DOI] [PubMed] [Google Scholar]
- 94.Saadat M.R., Qomi M., Emadzadeh S. Microextraction and determination of diclofenac in biological samples using hollow fiber liquid phase microextraction technique coupled with HPLC-UV. J Appli Chemi Resear. 2018;12:16–25. [Google Scholar]
- 95.Nazaripour A., Yamini Y., Bagheri H. Extraction and determination of trace amounts of three anticancer pharmaceuticals in urine by three-phase hollow fiber liquid-phase microextraction based on two immiscible organic solvents followed by high-performance liquid chromatography. J. Sep. Sci. 2018;41:3113–3120. doi: 10.1002/jssc.201800183. [DOI] [PubMed] [Google Scholar]
- 96.Horta R.P., do Amaral B., Peralta-Zamora P.G. Evaluation of a hollow-fiber liquid-phase microextraction technique for the simultaneous determination of PPI drugs in human plasma by LC-DAD. J. Chromatogr. Sci. 2018;56:564–573. doi: 10.1093/chromsci/bmy023. [DOI] [PubMed] [Google Scholar]
- 97.Al-Hashimi N.N., Awwad A.I., Al-Hashimi A.N. Functionalized Multi walled carbon nanotubes-reinforced hollow fiber solid/liquid phase microextraction and HPLC-DAD for determination of phenazopyridine in urine. Curr. Pharmaceut. Anal. 2019;15:447–455. [Google Scholar]
- 98.Yang L., Shi Y., Li J. In situ derivatization and hollow-fiber liquid-phase microextraction to determine sulfonamides in water using UHPLC with fluorescence detection. J. Sep. Sci. 2018;41:1651–1662. doi: 10.1002/jssc.201701041. [DOI] [PubMed] [Google Scholar]
- 99.Díaz-Álvarez M., Martín-Esteban A. Hollow fiber membrane-protected molecularly imprinted microspheres for micro solid-phase extraction and clean-up of thiabendazole in citrus samples. J. Chromatogr. A. 2018;1531:39–45. doi: 10.1016/j.chroma.2017.11.054. [DOI] [PubMed] [Google Scholar]
- 100.Hosseiny Davarani S.S., Pourahadi A., Ghasemzadeh P. Quantification of controlled release leuprolide and triptorelin in rabbit plasma using electromembrane extraction coupled with HPLC–UV. Electrophoresis. 2019;40:1074–1081. doi: 10.1002/elps.201800481. [DOI] [PubMed] [Google Scholar]
- 101.Aranda-Merino N., Ramos-Payán M., Callejon-Mochon M. Effect of counter-ions on electromembrane extraction of non-steroidal antiinflammatory drugs. J. Electroanal. Chem. 2019;840:255–262. [Google Scholar]
- 102.Nasrollahi S.S., Davarani S.S.H., Moazami H.R. Impedometric investigation of salt effects on electromembrane extraction: practical hints for pH adjustment. Electrochim. Acta. 2019;296:355–363. [Google Scholar]
- 103.Rahimi A., Nojavan S. Electromembrane extraction of verapamil and riluzole from urine and wastewater samples using a mixture of organic solvents as a supported liquid membrane: study on electric current variations. J. Sep. Sci. 2019;42:566–573. doi: 10.1002/jssc.201800741. [DOI] [PubMed] [Google Scholar]
- 104.Zhu R., Dong Y., Cai X. Determination of barbiturates in biological specimens by Flat membrane-based liquid-phase microextraction and liquid chromatography-mass spectrometry. Molecules. 2019;24:1494. doi: 10.3390/molecules24081494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seidi S., Ranjbar M.H., Baharfar M. A promising design of microfluidic electromembrane extraction coupled with sensitive colorimetric detection for colorless compounds based on quantum dots fluorescence. Talanta. 2019;194:298–307. doi: 10.1016/j.talanta.2018.10.046. [DOI] [PubMed] [Google Scholar]
- 106.Zarghampour F., Yamini Y., Baharfar M. Simultaneous extraction of acidic and basic drugs via on-chip electromembrane extraction using a single-compartment microfluidic device. Analyst. 2019;144:1159–1166. doi: 10.1039/c8an01668b. [DOI] [PubMed] [Google Scholar]
- 107.AL-Hashimi N.N., Shahin R.O., AL-Hashimi A.N. Cetyl-alcohol-reinforced hollow fiber solid/liquid-phase microextraction and HPLC–DAD analysis of ezetimibe and simvastatin in human plasma and urine. Biomed. Chromatogr. 2019;33 doi: 10.1002/bmc.4410. [DOI] [PubMed] [Google Scholar]
- 108.Barahona F., Albero B., Tadeo J.L. Molecularly imprinted polymer-hollow fiber microextraction of hydrophilic fluoroquinolone antibiotics in environmental waters and urine samples. J. Chromatogr. A. 2019;1587:42–49. doi: 10.1016/j.chroma.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 109.Seidi S., Alavi L., Jabbari A. Three-phase carrier-mediated hollow fiber microextraction based on deep eutectic solvent followed by HPLC–UV for determination of raloxifene and ethinylestradiol in pharmaceutical wastewater treatment plants. J. Iran. Chem. Soc. 2019;16:1007–1018. [Google Scholar]
- 110.Shamsayei M., Yamini Y., Asiabi H. Electromembrane surrounded solid-phase microextraction using a stainless-steel wire coated with a nanocomposite composed of polypyrrole and manganese dioxide. Microchim Acta. 2017;184:2697–2705. [Google Scholar]
- 111.Tabani H., Shokri A., Tizro S. Evaluation of dispersive liquid–liquid microextraction by coupling with green-based agarose gel-electromembrane extraction: an efficient method to the tandem extraction of basic drugs from biological fluids. Talanta. 2019;199:329–335. doi: 10.1016/j.talanta.2019.02.078. [DOI] [PubMed] [Google Scholar]
- 112.Nojavan S., Shaghaghi H., Rahmani T. Combination of electromembrane extraction and electro-assisted liquid-liquid microextraction: a tandem sample preparation method. J. Chromatogr. A. 2018;1563:20–27. doi: 10.1016/j.chroma.2018.05.068. [DOI] [PubMed] [Google Scholar]
- 113.Dvořák M., Seip K.F., Pedersen-Bjergaard S. Semi-automated set-up for exhaustive micro-electromembrane extractions of basic drugs from biological fluids. Anal. Chim. Acta. 2018;1005:34–42. doi: 10.1016/j.aca.2017.11.081. [DOI] [PubMed] [Google Scholar]
- 114.Pantůčková P., Kubáň P. In-line coupling of supported liquid membrane extraction to capillary electrophoresis for simultaneous analysis of basic and acidic drugs in urine. J. Chromatogr. A. 2017;1519:137–144. doi: 10.1016/j.chroma.2017.08.084. [DOI] [PubMed] [Google Scholar]
- 115.Forough M., Farhadi K., Eyshi A. Rapid ionic liquid-supported nano-hybrid composite reinforced hollow-fiber electromembrane extraction followed by field-amplified sample injection-capillary electrophoresis: an effective approach for extraction and quantification of Imatinib mesylate in human plasma. J. Chromatogr. A. 2017;1516:21–34. doi: 10.1016/j.chroma.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 116.Šlampová A., Kubáň P. Direct analysis of free aqueous and organic operational solutions as a tool for understanding fundamental principles of electromembrane extraction. Anal. Chem. 2017;89:12960–12967. doi: 10.1021/acs.analchem.7b03829. [DOI] [PubMed] [Google Scholar]
- 117.Drouin N., Mandscheff J.-F.o., Rudaz S. Development of a new extraction device based on parallel-electromembrane extraction. Anal. Chem. 2017;89:6346–6350. doi: 10.1021/acs.analchem.7b01284. [DOI] [PubMed] [Google Scholar]
- 118.Fakhari A.R., Asadi S., Kosalar H.M. Metal–organic framework enhanced electromembrane extraction–a conceptual study using basic drugs as model substances. Ana Methods. 2017;9:5646–5652. [Google Scholar]
- 119.Aladaghlo Z., Fakhari A.R., Hasheminasab K.S. Carrier assisted electromembrane extraction based on nonionic lipophilic surfactants for the determination of basic drugs in urine samples. Ana Methods. 2017;9:5659–5667. [Google Scholar]
- 120.Forough M., Farhadi K., Molaei R. Capillary electrophoresis with online stacking in combination with AgNPs@ MCM-41 reinforced hollow fiber solid-liquid phase microextraction for quantitative analysis of Capecitabine and its main metabolite 5-Fluorouracil in plasma samples isolated from cancer patients. J. Chromatogr. B. 2017;1040:22–37. doi: 10.1016/j.jchromb.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 121.Fakhari A.R., Mohammadi Kosalar H., Asadi S. Surfactant-assisted electromembrane extraction combined with cyclodextrin-modified capillary electrophoresis for the separation and quantification of Tranylcypromine enantiomers in biological samples. J. Sep. Sci. 2018;41:475–482. doi: 10.1002/jssc.201700488. [DOI] [PubMed] [Google Scholar]
- 122.Šlampová A., Kubáň P. Two-phase micro-electromembrane extraction across free liquid membrane for determination of acidic drugs in complex samples. Anal. Chim. Acta. 2019;1048:58–65. doi: 10.1016/j.aca.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 123.Ryšavá L., Dvořák M., Kubáň P. The effect of membrane thickness on supported liquid membrane extractions in-line coupled to capillary electrophoresis for analyses of complex samples. J. Chromatogr. A. 2019;1596:226–232. doi: 10.1016/j.chroma.2019.02.067. [DOI] [PubMed] [Google Scholar]
- 124.Roman-Hidalgo C., Santigosa-Murillo E., Ramos-Payán M. On-chip electromembrane extraction of acidic drugs. Electrophoresis. 2019 doi: 10.1002/elps.201900024. [DOI] [PubMed] [Google Scholar]
- 125.Mofidi Z., Norouzi P., Seidi S. Efficient design for in situ determination of amlodipine in whole blood samples using fast Fourier transform stripping square wave voltammetry after preconcentration by electromembrane extraction. New J. Chem. 2017;41:13567–13575. [Google Scholar]
- 126.Mofidi Z., Norouzi P., Seidi S. Determination of diclofenac using electromembrane extraction coupled with stripping FFT continuous cyclic voltammetry. Anal. Chim. Acta. 2017;972:38–45. doi: 10.1016/j.aca.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 127.Hatamluyi B., Es’ haghi Z. A layer-by-layer sensing architecture based on dendrimer and ionic liquid supported reduced graphene oxide for simultaneous hollow-fiber solid phase microextraction and electrochemical determination of anti-cancer drug imatinib in biological samples. J. Electroanal. Chem. 2017;801:439–449. [Google Scholar]
- 128.Mofidi Z., Norouzi P., Larijani B. Simultaneous determination and extraction of ultra-trace amounts of estradiol valerate from whole blood using FFT square wave voltammetry and low-voltage electrically enhanced microextraction techniques. J. Electroanal. Chem. 2018;813:83–91. [Google Scholar]
- 129.Tahmasebi Z., Davarani S.S.H., Asgharinezhad A.A. Highly efficient electrochemical determination of propylthiouracil in urine samples after selective electromembrane extraction by copper nanoparticles-decorated hollow fibers. Biosens. Bioelectron. 2018;114:66–71. doi: 10.1016/j.bios.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 130.Mofidi Z., Esmaeili C., Norouzi P. Ultra-trace determination of imipramine using a Sr (VO3) 2 doped phytic acid carbon paste electrode after preconcentration by electromembrane extraction coupled with FFT square wave voltammetry. J. Electrochem. Soc. 2018;165:H205–H212. [Google Scholar]
- 131.Hrdlička V., Navrátil T., Barek J. Application of hollow fibre based microextraction for voltammetric determination of vanillylmandelic acid in human urine. J. Electroanal. Chem. 2019;835:130–136. [Google Scholar]
- 132.Oedit A., Ramautar R., Hankemeier T. Electroextraction and electromembrane extraction: advances in hyphenation to analytical techniques. Electrophoresis. 2016;37:1170–1186. doi: 10.1002/elps.201500530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.A. Odobasic, Determination and Speciation of Trace Heavy Metals in Natural Water by DPASV, ISBN2012.
- 134.Es’haghi Z., Nezhadali A., Hosseini H.A. Pre-concentration and determination of zinc in water samples by ligand assisted pseudo stirbar hollow fiber solid/liquid phase microextraction. Arab J Chem. 2017;10:S3840–S3847. [Google Scholar]
- 135.Barón-Jaimez J., Joya M., Barba-Ortega J. Anodic stripping voltammetry–ASV for determination of heavy metals. J. Phys. Conf. Ser. 2013 IOP Publishing. [Google Scholar]