Abstract
Astaxanthin is a powerful carotenoid antioxidant prevalent in marine organisms and approved as a food supplement. Recent studies have demonstrated Astaxanthin’s beneficial attributes in various health states. Following initial reports of potential heat protective properties in Astaxanthin supplemented rats, we present here results of a novel study examining the effect of Astaxanthin supplementation on the heat shock response in rats in relation to core temperature (Tc) and the ensuing physiological strain. Two hours of heat stress at 41 °C during which rats developed their thermoregulatory hyperthermic plateau resulted in progressive increases in HSP72 and HSP27 in the Astaxanthin (Oleoresin)-treated group but not in the control (Olive oil) group. Enhanced elevation in HSPs suggests that Astaxanthin supplementation may augment the cellular stress protective response to heat stress.
Keywords: Astaxanthin, Heat stress, Nutritional pre-treatment, Environmental physiology
Introduction
Heat injury is a hazardous condition that may progress rapidly and potentially be fatal. Current recommendations to populations at risk focus on raising awareness to environmental conditions with respect to the individual’s state of acclimation, hydration, clothing, physical health and fitness, and supporting heat dissipation to the environment where possible (Epstein and Roberts 2011). Anticipated heat stress exposure offers an opportunity to prepare heat injury–prone populations and minimize its potentially damaging effects.
The concept of physical preconditioning has been expansively explored in the literature by utilizing a wide variety of pharmaceutical and/or neutraceutical agents (Bouchama et al. 2007; Chen et al. 2008; Lin et al. 2011; McLellan et al. 2000; Niu et al. 2003). Since the response to heat may be altered by past exposure to pharmaceuticals (Lomax and Schönbaum 1998) or environmental toxicants (Leon 2008), it may be possible to use an exogenous substance to enhance resistance to heat injury. However, to date, no supplement utilized for preconditioning to delay thermal injuries has been directly linked to improved heat tolerance (Kuennen et al. 2011; Moran et al. 1999).
The physiological, cellular, and molecular responses evoked by substantial elevation in core temperature (Tc) promote heat dissipation to the environment, together with cellular protection mechanisms, among which the heat shock response (HSR) is fundamental (Lindquist 1986). It is characterized by enhanced synthesis of heat shock proteins (HSPs) including HSP72, which is the current consensus marker of heat stress. Heat-related synthesis of HSPs was initially observed as chromosomal puffing in heat-treated Drosophila cells (Ritossa 1962). It was more than a decade later when the appearance of these chromosomal puffs after exposure to heat stress was linked with novel protein synthesis (Tissieres et al. 1974). Soon after, exposure to a single exposure of hyperthermia was shown to protect HeLa cells from recurrent heat exposure and effectively increase their thermotolerance in a dose-dependent manner (Gerner and Schneider 1975). Exposure to heat shock or trauma were later demonstrated to precede increased HSP70 protein synthesis in vivo (Currie and White 1981). An elevation in levels of HSP70 isoforms has been associated with enhanced resistance to a multitude of stressors including heat, hypoxia, and oxidative stress (Ghosh et al. 2018).
The HSPs induced during the HSR protect the proteome via chaperoning the multitude of protein forms typically prevalent during stress exposure, i.e.: unfolded, misfolded, and nascent proteins (Verghese et al. 2012). Heat protection mechanisms in the whole organism are more complex and involve alterations in other components not restricted only to the level of cellular protection. An example of that is seen in epithelial tissue, which maintains its structural integrity through heat stress and avoids the increase in permeability induced by heat stress (Dokladny et al. 2016). This mechanism of epithelial protection may contribute greatly to withstanding the damages of heat stress at the whole organism level. The rapid synthesis of inducible HSP70 isoforms in response to heat stress is due to an evolutionarily conserved structure and function of the path governing its transcription and translation through the heat shock factor one (HSF1) (Silver and Noble 2012), subsequently enhancing heat resistance (Kelty et al. 2002).
Small molecular weight HSPs are also induced by the HSR and aggregate within cells to form larger structures acting as chaperonins, which aid in maintaining protein integrity and preventing degradation. Among them, HSP27 is of particular importance as it is expressed in response to multiple stressors, such as oxidative stress, starvation, and heat stress, where its phosphorylated form protects nuclear proteins from degradation. Similarly to HSP70, it is involved in the immune response and has both pro- and anti-inflammatory actions. It is common in anti-apoptotic scenarios and serves as a biomarker for several types of cancer (Singh et al. 2017). Recently, HSP27 expression in goat cardiac tissue was shown to positively correlate with the duration of heat stress exposure, which suggests a role for HSP27 in thermal protection (Parida et al. 2019).
Astaxanthin is a xanthophyll carotenoid prevalent in marine organisms, which is approved for human consumption as a safe food supplement (Kidd 2011). Astaxanthin is considered a very potent antioxidant. Its molecular structure contains two polar ionone rings, joined by a non-polar chain of conjugated double bonds enabling it to accept or receive electrons and quench free radicals and reactive oxygen and nitrogen species, while spanning biological membranes; it thus stabilizes many processes, especially in oxidative surroundings (McNulty et al. 2007).
Many beneficial health-related traits have been attributed to Astaxanthin (Yuan et al. 2011), such as a reduction in C reactive protein and DNA damage (Park et al. 2010), reduction of exercise-induced muscle damage (Aoi et al. 2003), and improvement of cell survival after oxidative stress (Wolf et al. 2010). Astaxanthin has also been shown to confer a measure of protection against heat-related injury in rodents (Preuss et al. 2011), and to protect bovine embryos from heat stress (Namekawa et al. 2010). The aim of this study was twofold: (1) to test whether Astaxanthin pretreatment impacts the thermal response to passive heat stress and (2) to determine whether Astaxanthin pretreatment affects the HSPs profile.
Methods
Ethics
All experimental protocols were approved by the Ethics Committee for Animal Experimentation of The Hebrew University, Jerusalem, Israel (Approval numbers: MD-13-13734-4 and MD-16-14156-4) and complied with the guidelines of the National Research Council Guide for the Care and Use of Laboratory Animals (Council et al. 2010).
Study design
The study consisted of two experimental series, focusing on the thermal and cytoprotective aspects of Astaxanthin supplementation in rats. The first experiment examined core temperature dynamics during exposure to heat stress whereas the second experiment examined the dynamics of HSP levels post heat stress exposure in cardiac and hepatic tissues.
Pre-experimental conditions
Animals were housed in the animal facility at an ambient temperature of 24 ± 1 °C in a 12 h–12 h light-dark cycle, with free access to food (Teklad Global rodent diet no. 2018SC, 18% protein Harlan Teklad, USA, by Envigo, Israel) and water. Weight was monitored biweekly.
Preparation of Astaxanthin
Astaxanathin 1% v/v for gavage was prepared by dissolving Astaxanthin 10% oleoresin (Astapure®, Algatechnologies, Ktora, Israel) in extra virgin olive oil (Acidity ≤ 0.8%, Negba olive press, Revivim, Israel) by a 1:4.5 ratio and administered by gavage at a dosage of 1 mL per 100 g body weight.
Core temperature dynamics experiment
Male Sprague Dawley rats, initially 6 weeks old, weighing approximately 200 g, were peritoneally implanted with iButtons® temperature loggers (DS1921H-F5# Thermochron, Maxim Integrated Products, USA), programmed to log core temperature at 5-min intervals. The iButtons® sensors were implanted surgically by a trained veterinary surgeon under sterile conditions. Prior to implantation, the animals were anesthetized by Isofluran induction 0.2 ml\min. The peritoneal area was shaved and disinfected. Analgesia was provided subcutaneously to the back of the neck (Carprofen in normal Saline 5 mg/mL). Internal sutures were sewn with 3-0 USP monofilament absorbable sutures (Assucryl®, Assut sutures, Switzerland). External sutures were sewn with 3-0 USP Nylon blue monofilament non-absorbable (Assut sutures, Switzerland). Surgery lasted 5.5 min on average and after its completion, the animals were moved to recover under a heating lamp. No antibiotic was used in order to avoid any influence to thermoregulation. Post-surgery complications (an opening of peritoneal sutures) developed in one animal, which was euthanized. The 2-week (a total of 10 days, excluding weekends) supplementation regimen began after full recovery and consisted of four groups: (1) 100 mg/kg Astaxanthin in olive oil (ATXsd, n = 5), by gavage (2) 1 mL of olive oil (OILsd, n = 5) by gavage, (3) 1 mL normal saline by gavage (NSsd, n = 5) or (4) no treatment control animals (NTsd, n = 5) that received no treatment prior to heat stress exposure and were kept in the animal facility for the same duration prior to heat stress exposure.
The animals were then subjected to the heat stress protocol and sacrificed thereafter. iButtons® were removed posthumously and the temperature record was downloaded.
Protein dynamics post heat stress exposure experiment
Male Sabra rats initially 6 weeks old, weighing approximately 200 g were randomly divided into two groups and treated for 2 weeks (a total of ten treatment days, excluding weekends) with either Astaxanthin 100 mg/kg dissolved in olive oil, daily (ATXsa, n = 35; 4–5 animals in each group) or olive oil, in equal volume (OILsa, n = 33; 4–5 animals in each group), by gavage. The animals were exposed to the heat stress protocol and sacrificed at the following time points post heat stress: 0 min, 30 min, 1 h, 2 h, 3 h, and 4 h. Additionally, four animals of each group were designated as control and exposed to room temperature. Heart and liver were promptly removed, placed in liquid nitrogen and later stored at − 80 degrees Celsius for future analysis.
Heat stress (HS) protocol
Animals were exposed to an established controlled, passive heat stress protocol described previously (Schochina and Horowitz 1989). Briefly, heat stress exposure was conducted for 2 h in a reach-in climatic chamber (Lab-line reach in environmental chamber, Thermo Fisher Scientific Inc., USA) at a constant temperature range of 40–41 °C. Animals were monitored for weight and rectal temperature (Trec, rectal thermistor YL402, Yellow Springs industries, USA) before exposure, at 60 min and immediately after removal from the climatic chamber. Access to food and water was withdrawn at the onset of heat stress or control exposure and returned freely upon recovery. At the pre-set time points, animals were anesthetized by a mixture of 85 mg/mL Ketamine HCL and 3.6 mg/mL Xylazine, administered peritoneally at 0.1 mL per 100 g body weight and sacrificed by cardiac resection. An estimation of heat strain during the heat stress exposure was made by calculating the cumulative heat storage according to Maloyan et al. (Maloyan et al. 1999) as ΣΔTrec/min × 0.83 × body weight (normalized to 100 g of body weight). Heating rate per minute was calculated from the cumulative heat storage during the heat exposure until the peak heat storage was reached, and compared between groups.
Western immunoblotting
Left ventricle cardiac tissue and hepatic tissue were prepared as described previously (Maloyan et al. 2005) and the cytosolic fraction was frozen at − 80 °C. Protein concentration was quantified by Bradford reagent (Bio-Rad, USA), and 50-μg samples were separated using 12% SDS-PAGE gel (TGX Fast cast, Biorad, USA) and blotted onto nitrocellulose membranes, which were then blocked with 5% skim milk powder. Blots were probed overnight at 4 °C with primary antibodies against HSP72, HSP27, and HSP90. Anti-β-actin antibody was used to confirm equal protein loading. Appropriate peroxidase-coupled immunoglobulin G was used as secondary antibodies (1 h, at room temperature). Tables 1 and 2 list the primary and secondary antibodies used, respectively. Membranes were stripped for re-probing when necessary by exposing them to a guanidine thiocyanate 4 M solution for 30 s. Reactive bands were visualized by using chemiluminescence (EZ-ECL, biological industries, Israel) and detected using the ChemiDoc imaging system (Bio-rad, USA). Image lab software was used to measure band pixel density (version 5.1, BioRad, USA) which was normalized to β-actin in the same lane. Four technical repeats were performed for each sample.
Table 1.
Primary antibodies used for Western blot protein detection
| Primary antibody | ||||
|---|---|---|---|---|
| Protein detected | Manufacturer | cat. Number | Antibody origin | Dilution |
| Actin | Abcam, international | ab8227 | Rabbit | 1:5000 |
| HSP27 | Santa cruz biotechnology, USA | (C-20): sc-1048 | Goat | 1:1000 |
| HSP72 | StressMarq Biosciences Inc., Canada | SPC-103C/D | Rabbit | 1:10,000 |
| HSP90 | Abcam, international | AC88, ab13492 (monoclonal) | Mouse | 1:1250 |
Table 2.
Secondary antibodies used for Western blot protein detection
| Secondary antibody (Horeseradish peroxidase conjugated IgG) | ||||
|---|---|---|---|---|
| Protein detected | Manufacturer | cat. Number | Antibody origin | Dilution |
| Rabbit IgG | Jackson ImmunoResearch laboratories Inc., USA | 711–035-152 | Donkey | 1:5000 |
| Goat IgG | Jackson ImmunoResearch laboratories Inc., USA | 805–035-180 | Bovine | 1:10,000 |
| Mouse IgG | Jackson ImmunoResearch laboratories Inc., USA | 115–035-003 | Goat | 1:10,000 |
Animals removed from the experiment
In the protein dynamics experiment, two animals from the ATXsa group were removed from the experiment during the supplementation stage due to weight loss related to iatrogenic esophageal injury. Eight animals (four of the ATXsa group and four of the OILsa group) were removed from the climatic chamber due to high rectal temperature after 1 h and were not included in thermal or biochemical analysis. The remaining animals (29 ATXsa and 29 OILsa) were included in result analysis. Heat strain manifestations of heat stress exposure and cellular heat shock responses were compared between the groups.
Statistics
A statistical program (SPSS, version 23, USA) was used for data analysis. Biological results were averaged from the four technical repetitions for each analyzed tissue, after removal of extreme values (4.5–6%), leaving two to three averaged values for each data point. One-way ANOVA with Tukey’s post-hoc analysis was used to estimate significance of time dependent dynamic changes from the control within treatment groups. Scatter plots of individual data points as well as boxplot representations were created. The latter were used to find outliers defined by the SPSS program to be extreme or regular (3 or 1.5 times the inter quartile ratio, respectively), marked by an asterisk or a circle, respectively (see Figs. 2 and 3). Seven outlier points were removed from the database before performing one-way ANOVA within and between treatments in each tissue, followed by Tukey’s post-hoc test of significance. In the heat stress dynamics experiment, the heating rate per minute was selectively compared between groups with a two tailed t test, with equal variance assumed. P value ≤ 0.05 was considered significant.
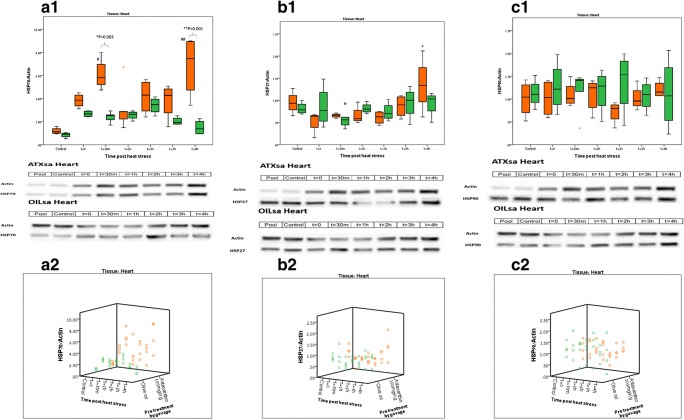
Fig. 2.
Heat shock protein dynamics post heat stress in the heart. Orange boxes—ATXsa group; Green boxes—OILsa group. n = 4–5 animals per group for each time point. Blot representations of sample membranes appear below for ATXsa and OILsa. a.1 Cardiac HSP72 protein dynamics post HS. *ATXsa t = 30 m was significantly higher than OILsa t = 30 m (p = 0.003); **ATXsa t = 4 h was significantly higher than OILsa t = 4 h (p < 0.001); #ATXsa t = 30 m, and 4 h post HS were significantly higher than the control, (p < 0.001). a.2 Cardiac HSP72 protein dynamics post HS, individual animals represented in a 3D plot. b.1 Cardiac HSP27 protein dynamics post HS. *ATXsa t = 4 h was significantly higher than ATXsa t = 0, t = 1 h, and t = 2 h (p < 0.05). b.2 Cardiac HSP27 protein dynamics post HS, individual animals represented in a 3D plot. c.1 Cardiac HSP90 protein dynamics post HS. c.2 Cardiac HSP90 protein dynamics post HS, individual animals represented in a 3D plot
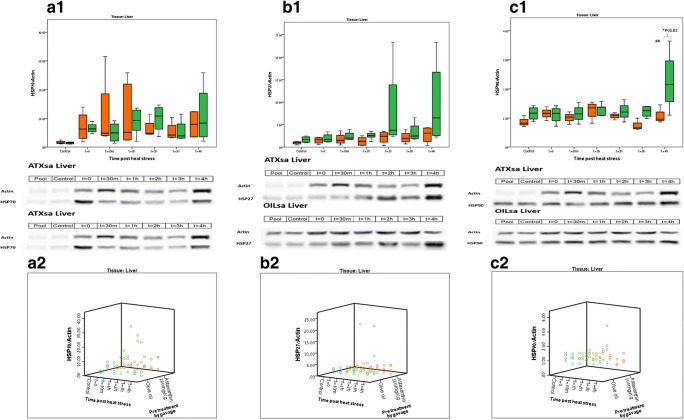
Fig. 3.
Heat shock protein dynamics post heat stress, in the liver Orange boxes—ATXsa group; green boxes—OILsa group. n = 4–5 animals per group for each time point. Blot representations of sample membranes appear below for ATXsa and OILsa. a.1 Hepatic HSP72 protein dynamics post HS. a.2 Hepatic HSP72 protein dynamics post HS, individual animals represented in a scatter plot. b.1 Hepatic HSP27 protein dynamics post HS. b.2 Hepatic HSP27 protein dynamics post HS, individual animals represented in a scatter plot. c.1 Hepatic HSP90 protein dynamics post HS. *OILsa t = 4 h is significantly higher than ATXsa t = 4 h (p < 0.02); ##OILsa t = 4 h is significantly higher than all other OILsa time points (p < 0.036). c.2 Hepatic HSP90 protein dynamics post HS, individual animals represented in a 3D plot. Graphical representations were created by using the Excel and SPSS programs
Results
Physiological heat strain during HS
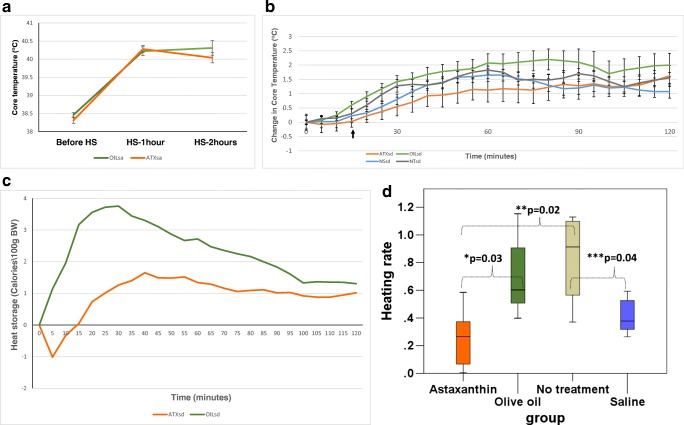
Core temperature (Tc) changes recorded during heat stress (Fig. 1a and b) displayed an overall attenuation in the increase in core temperature to the thermoregulatory hyperthermic plateau in response to passive heat stress exposure in ATXsd and ATXsa groups compared with OILsd and OILsa groups, respectively. Combined data from both experiments shows a similar average basal Tc between the Astaxanthin (38.4 °C, n = 35) and olive oil (38.3 °C, n = 35) treated animals. The normal saline (NSsd) and no treatment (NTsd) groups displayed a similar Tc profile, with an early rise in core temperature that peaked by 60 min of heat stress exposure (ΔTc at 60 min = 1.65 ± 0.15 °C and 1.825 ± 0.01 °oC, mean±S.E for the NSsd and NTsd groups, respectively), followed by a reduction to maintain the hyperthermic plateau. For the OILsd and ATXsd groups, the peak in core temperature change was reached later—at 80 min (ΔTc = 2.2 ± 0.37 °C) and 120 min (ΔTc = 1.63 ± 0.365 °C), respectively, reflecting a different rate of heating. Cumulative heat storage calculated for the heat stress exposure demonstrated development of an earlier and higher heat storage during HS exposure in the OILsd group (68.2 ± 10.9 calories/100 g BW) compared to the ATXsd group (29.2 ± 15.5 calories/100 g BW) (Fig. 1c, p < 0.04). The heating rate (Fig. 1d) was also significantly higher for the ATXsd group (0.26 ± 0.1 Calories × 100 g BW−1 × min−1), in comparison with the OILsd group (0.71 ± 0.14 Calories × 100 g BW−1 × min−1; p = 0.031), The difference between ATXsd and NTsd (0.83 ± 0.17 Calories × 100 g BW−1 × min−1) was also significant (p = 0.02), while not between ATXsd and NSsd (0.41 ± 0.06 Calories × 100 g BW−1 × min−1). The heating rate of the OILsd group was not significantly different from that of NSsd or NTsd.
Fig. 1.
a Core temperature measured before, 1 h into heat stress (HS) HS, and at the end of the 2 h HS in the protein dynamics experiment. Average temperatures are displayed with standard error bars for the OILsa (n = 25) and ATXsa (n = 25) groups. b Changes in average core temperature of the ATXsd, OILsd, NSsd, and NTsd groups, recorded during the temperature dynamics experiment with standard error bars. N = 5 in each group. Rectal temperature measured after the onset of heat strain (indicated by the black arrow) was: 39.3 ± − 0.6, 38.4 ± 0.4, 38.9 ± 0.6, and 38.9 ± 0.6 oC for the ATXsd, OILsd, NSsd, and NTsd groups, respectively (1W ANOVA ns). c Average heat strain experienced during HS by the ATX (red line) and OIL (green line) groups. Cumulative heat strain in the ATX group from 15 to 120 min (no heat storage was apparent before this point) was significantly different from that of the OIL group between 0 and 120 min (where heat storage began earlier) (t test, one tailed, equal variance, p = 0.025). d Average heating rate per minute (Calories × 100 g BW−1 × min−1) experienced by the four groups, until the peak heating rate was achieved. The heating rate for ATXsd was significantly lower than that of OILsd (*p = 0.03) and NTsd (**p = 0.02). The heating rate for NT was significantly higher than that of NSsd (***p = 0.04)
The loss in body mass post heat stress resulting from dehydration did not differ between the groups: from 313.4 ± 3.31 g before to 290.5 ± 3.31 g after the exposure in the ATXsa group and from 309.36 ± 3.67 g before to 285.44 ± 3.24 g after the exposure in the OILsa group, reflecting a total weight loss of 7.73% and 7.31% in the ATXsa and OILsa groups, respectively.
Cellular heat shock protein levels
Heart
Figure 2 displays cardiac tissue HSP levels during recovery from 2 h of HS, for the ATXsa and OILsa groups (n = 4–5 animals in each group). Significant differences in HSP72 protein level were observed between the ATXsa and OILsa treatments at two time points, 30′ (6.1 ± 0.7 and 2.4 ± 0.2, respectively) and 4 h (6.8 ± 1.3 and 1.5 ± 0.3, respectively) in the heart (p = 0.003 and p < 0.001, respectively). Within-treatment analysis revealed a significant elevation in HSP72 protein level in the ATXsa group at the 30′ (6.1 ± 0.7) and 4 h (6.8 ± 1.3) time points compared to the control (1.2 ± 0.1; p < 0.001). Contrastingly in the OILsa group, no significant difference was observed in HSP72 protein level in the heart at any of the analyzed time points.
Although changes in HSP90 and HSP27 protein levels post HS were much smaller, at 4 h post HS, HSP27 was markedly elevated in the ATXsa group (1.35 ± 0.3) and to a significantly higher level than ATXsa at t = 0 (0.5 ± 0.1), 1 h (0.7 ± 0.1) and 2 h (0.6 ± 0.1) (p < 0.05).
Liver
Figure 3 displays hepatic HSP levels, during recovery from 2 h of HS, for the ATXsa and OILsa groups (n = 4–5 animals in each group). Changes in HSP72 heat shock protein level in the liver were more dramatic than those observed in the heart: post heat stress exposure, HSP72 level in the heart tissue reached an average value of 6.64 and 3.67 in the ATXsa and OILsa groups, respectively, whereas in the liver, HSP72 protein level normalized to Actin reached 12.98 and 12.07 in the ATXsa and OILsa groups, respectively.
This elevation was apparent across all measurement time points yet was not significantly different from the control and was observed in both treatment groups, possibly indicating a tissue-specific effect which could not be attributed to the treatment.
Between-groups comparison revealed no significant difference in levels of all heat shock proteins analyzed (HSP72, HSP27, and HSP0) at all tested time points, apart from HSP90 level at t = 4 h in the OILsa group (2.3 ± 0.5) which was significantly higher than the ATXsa group (1.1 ± 0.2) at the same time point (p < 0.02). Figures 3a.1, a.2, b.1, b.2, c.1, and c.2, respectively.
Overall, no significant difference was observed within treatment groups in any of the heat shock proteins examined (HSP72, HSP90, and HSP 27), apart from t = 4 h post HS in the OILsa group (2.3 ± 0.5), where the hepatic protein level of HSP90 was significantly higher than all other time points in the OILsa group (OILsa, t = 0 min, 1.1 ± 0.1; t = 30 min, 1.15 ± 0.1; t = 1 h, 1.3 ± 0.1; t = 2 h, 1.2 ± 0.2; t = 3 h, 1.2 ± 0.1; p < 0.036).
Sample blots from each experimental group are presented in Fig. 3.
Discussion and conclusions
Core temperature dynamics during heat stress
In small rodents, exposure to heat stress induces a rise in core body temperature until the hyperthermic plateau is reached (Schwimmer et al. 2004). Pre-treatment with Astaxanthin in both rat strains attenuated the rise to the hyperthermic plateau during heat stress exposure. This fact, in combination with no significant difference in body mass loss between the experimental groups may suggest no differences in evaporative cooling (salivation) between the ATXsd and the OILsd groups during 2 h of heat stress. Tail blood flow was not measured in our experimental protocol, thus we cannot negate the possibility that the attenuated rise in Tcore was associated with cardio-vascular changes or decreased heat production. The cumulative heat strain, as well as the heating rate, was definitely lower in the ATXsd group, compared to the OILsd group.
Heat shock protein dynamics
Exposure to passive heat stress in rodents has been well documented in the past. One of the hallmarks of the heat shock response involves elevation of cellular levels of heat shock proteins (Lindquist 1986). Specifically, in rat tissue, elevated levels of HSP70 protein was found to appear as early as 2.5 h post heat shock exposure (Currie and White 1983). In addition, elevated levels of HSP72 have been correlated to the severity of injury and cellular response (Dehbi et al. 2010), but the magnitude of their elevation may delay the threshold for thermal injury. Prior elevation of HSP72 in heat acclimation, for example, leads to a delayed temperature threshold for injury in rats (Horowitz 2011).
Here we show that Astaxanthin pretreatment produced higher levels of cardiac HSP72 protein (but not hepatic) compared to the olive oil solvent. Heat stress exposure caused a detectable heat shock response manifested as an elevation in both cardiac and hepatic HSP72.
The different magnitude and timing of the physiological thermal response in the ATXsd and OILsd groups, as evident from the change in core temperature and heat storage during heat stress exposure, was reflected by a different dynamic of cardiac HSP72 during recovery, where a peak in cardiac HSP72 level was observed at 4 h post heat stress in the ATXsa group, but not in the OILsa group. Greater basal HSP level and greater induction of HSPs in the Astaxanthin pretreated group may have been linked to enhanced cellular stress response and increased cellular protection. Although Astaxanthin’s mechanism of action is attributed mainly to its stabilizing, antioxidant properties (Fassett and Coombes 2012; Kidd 2011), it has been reported, in human keratinocytes, to signal through the nuclear kinase MSK1 (Terazawa et al. 2012). Induction of MSK1 activity provides some clues as to a potential molecular-epigenetic mechanism developed during Astaxanthin supplementation prior to the heat stress. Significant up-regulation of MSK1 was found during short-term heat acclimation during stress on HSP72 and HSP90 promoters (Horowitz 2015).
The mechanism of action attributed to Astaxanthin includes other downstream signaling pathways, including:
indirect induction of anti-oxidative pathways such as: elevation of the anti-oxidant enzymes catalase, superoxide dismutase, peroxidase, and thiobarbituric acid reactive substances (TBARS) (Ranga Rao et al. 2010); activation of the phosphoinositide 3-kinase (PI3K)/AKT and extracellular signal-regulated protein kinase (ERK) pathways, which support the activation of the NRF2-anti-oxidant response elements (ARE) pathway, which up-regulates the expressions of enzymes that protect against oxygen species (OS), such as heme oxygenase-1 (HO-1), glutathione-S-transferase-α1 (GST-α1), and NAD(P)H quinine oxyreductase-1 (NQQ-1) (Fakhri et al. 2018)
induction of anti-inflammatory pathways such as inhibition of ROS-induced activation of nuclear factor-κB (NF-κB) transcription factor, which subsequently inhibits the production of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α (Speranza et al. 2012)
involvement in multiple anti-carcinogenic activities including induction of apoptotic pathways such as the PI3K/AKT pathway to facilitate cell death, and inhibition of invasiveness promoting pathways such as the JAK-2/STAT-3 pathway (Zhang and Wang 2015)
activation of anti-apoptotic effects in neuro-degenerative disorders, heart disease, ischemia, and multiple organ disorder syndromes (Fakhri et al. 2018).
The different dynamics in cardiac HSP72 and HSP27 during HS recovery suggest later induction of HSP27 production compared to HSP72, which displayed elevated levels at an earlier time post heat stress. Based on the diversity of possible downstream signaling induced by Astaxanthin, and the fact that HSP27 was also induced 4 h post HS in the Astaxanthin supplemented group, the results of altered thermoregulatory dynamics may also derive from enhanced anti-oxidant activity and indirect effects on thermal tolerance.
HSP72, HSP90, and HSP27 elevations in the liver were greater than those observed in the heart, which is consistent with previously published evidence of tissue-specific accumulation of HSP72 following heat stress in rodents (Flanagan et al. 1995). This is probably the outcome of the major thermoregulatory reflex showing splanchnic vasoconstriction coincidentally with peripheral vasodilatation, leading to higher splanchnic temperature and the resulting tissue damage (Horowitz 2011). In conclusion, our results suggest that Astaxanthin pretreatment may enhance thermo-tolerance at the whole organism level and through increased cellular protection via alteration of the threshold for thermal injury.
Abbreviations
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- HSF1
heat shock factor 1
- HSP
heat shock protein
- HSPs
heat shock poteins
- HSR
heat shock response
- IGF-1
insulin-like growth factor 1
- MSK1
mitogen- and stress-activated protein kinase-1
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- Tc
core temperature
Funding information
This work was supported by the Henry M. Jackson Foundation research fund (grant number 715817).
Footnotes
The original version of this article was revised: the top part of Figures 2 and 3 are not correctly displayed.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/16/2020
Due to an unfortunate misunderstanding, the top part of Figures 2 and 3 are not correctly displayed. The original article has been corrected and the proper version of Figures 2 and 3 is also published here.
References
- Aoi W, et al. Astaxanthin limits exercise-induced skeletal and cardiac muscle damage in mice. Antioxid Redox Signal. 2003;5:139–144. doi: 10.1089/152308603321223630. [DOI] [PubMed] [Google Scholar]
- Bouchama A et al (2007) Glucocorticoids do not protect against the lethal effects of experimental heatstroke in baboons. Shock. 10.1097/01.shk.0000246903.40142.aa [DOI] [PubMed]
- Chen YC, Liu YC, Yen DH, Wang LM, Huang CI, Lee CH, Lin MT. L-arginine causes amelioration of cerebrovascular dysfunction and brain inflammation during experimental heatstroke. Shock. 2008;29:212–216. doi: 10.1097/SHK.0b013e3180ca9ccc. [DOI] [PubMed] [Google Scholar]
- Council NR, Studies DEL, Research ILA, Animals CUGCUL. Guide for the care and use of laboratory Animals. 8. Washington, D.C.: National Academies Press; 2010. [Google Scholar]
- Currie R, White F. Trauma-induced protein in rat tissues: a physiological role for a “heat shock” protein? Science. 1981;214:72–73. doi: 10.1126/science.7280681. [DOI] [PubMed] [Google Scholar]
- Currie RW, White FP. Characterization of the synthesis and accumulation of a 71-kilodalton protein induced in rat tissues after hyperthermia. Can J Biochem Cell Biol. 1983;61:438–446. doi: 10.1139/o83-059. [DOI] [PubMed] [Google Scholar]
- Dehbi M, Baturcam E, Eldali A, Ahmed M, Kwaasi A, Chishti MA, Bouchama A. Hsp-72, a candidate prognostic indicator of heatstroke. Cell Stress Chaperones. 2010;15:593–603. doi: 10.1007/s12192-010-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokladny K, Zuhl MN, Moseley PL. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J Appl Physiol. 2016;120:692–701. doi: 10.1152/japplphysiol.00536.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein Y, Roberts WO. The pathopysiology of heat stroke: an integrative view of the final common pathway. Scand J Med Sci Sports. 2011;21:742–748. doi: 10.1111/j.1600-0838.2011.01333.x. [DOI] [PubMed] [Google Scholar]
- Fakhri S, Abbaszadeh F, Dargahi L, Jorjani M. Astaxanthin: a mechanistic review on its biological activities and health benefits. Pharmacol Res. 2018;136:1–20. doi: 10.1016/j.phrs.2018.08.012. [DOI] [PubMed] [Google Scholar]
- Fassett RG, Coombes JS. Astaxanthin in cardiovascular health and disease. Molecules. 2012;17:2030–2048. doi: 10.3390/molecules17022030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan S, Ryan A, Gisolfi C, Moseley P. Tissue-specific HSP70 response in animals undergoing heat stress. Am J Phys Regul Integr Comp Phys. 1995;268:R28–R32. doi: 10.1152/ajpregu.1995.268.1.R28. [DOI] [PubMed] [Google Scholar]
- Gerner EW, Schneider MJ. Induced thermal resistance in HeLa cells. Nature. 1975;256:500–502. doi: 10.1038/256500a0. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Sarkar P, Basak P, Mahalanobish S, Sil PC. Role of heat shock proteins in oxidative stress and stress tolerance. In: Asea AAA, Kaur P, editors. Heat shock proteins and stress. Cham: Springer International Publishing; 2018. pp. 109–126. [Google Scholar]
- Horowitz M. Heat acclimation, epigenetics, and cytoprotection memory comprehensive. Physiology. 2011;4:199–230. doi: 10.1002/cphy.c130025. [DOI] [PubMed] [Google Scholar]
- Horowitz M. Epigenetics and cytoprotection with heat acclimation. J Appl Physiol. 2015;120:702–710. doi: 10.1152/japplphysiol.00552.2015. [DOI] [PubMed] [Google Scholar]
- Kelty JD, Noseworthy PA, Feder ME, Robertson RM, Ramirez J-M. Thermal preconditioning and heat-shock protein 72 preserve synaptic transmission during thermal stress. J Neurosci. 2002;22:RC193–RC193. doi: 10.1523/JNEUROSCI.22-01-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern Med Rev. 2011;16:355–364. [PubMed] [Google Scholar]
- Kuennen M, Gillum T, Dokladny K, Bedrick E, Schneider S, Moseley P. Thermotolerance and heat acclimation may share a common mechanism in humans. Am J Phys Regul Integr Comp Phys. 2011;301:R524–R533. doi: 10.1152/ajpregu.00039.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon LR. Thermoregulatory responses to environmental toxicants: the interaction of thermal stress and toxicant exposure. Toxicol Appl Pharmacol. 2008;233:146–161. doi: 10.1016/j.taap.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Lin XJ, et al. Therapeutic effects of melatonin on heatstroke-induced multiple organ dysfunction syndrome in rats. J Pineal Res. 2011;50:436–444. doi: 10.1111/j.1600-1079X.2011.00863.x. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lomax P, Schönbaum E. Chapter 12 the effects of drugs on thermoregulation during exposure to hot environments. In: Sharma HS, Westman J, editors. Progress in brain research. Amsterdam: Elsevier; 1998. pp. 193–204. [DOI] [PubMed] [Google Scholar]
- Maloyan A, Palmon A, Horowitz M. Heat acclimation increases the basal HSP72 level and alters its production dynamics during heat stress. Am J Phys Regul Integr Comp Phys. 1999;276:R1506–R1515. doi: 10.1152/ajpregu.1999.276.5.R1506. [DOI] [PubMed] [Google Scholar]
- Maloyan A, Eli-Berchoer L, Semenza GL, Gerstenblith G, Stern MD, Horowitz M. HIF-1α-targeted pathways are activated by heat acclimation and contribute to acclimation-ischemic cross-tolerance in the heart. Physiol Genomics. 2005;23:79–88. doi: 10.1152/physiolgenomics.00279.2004. [DOI] [PubMed] [Google Scholar]
- McLellan TM, Smith IF, Gannon GA, Zamecnik J. Melatonin has no effect on tolerance to uncompensable heat stress in man. Eur J Appl Physiol. 2000;83:336–343. doi: 10.1007/s004210000291. [DOI] [PubMed] [Google Scholar]
- McNulty HP, Byun J, Lockwood SF, Jacob RF, Mason RP. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: x-ray diffraction analysis. Biochim Biophys Acta. 2007;1768:167–174. doi: 10.1016/j.bbamem.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Moran D, Epstein Y, Wiener M, Horowitz M. Dantrolene and recovery from heat stroke. Aviat Space Environ Med. 1999;70:987–989. [PubMed] [Google Scholar]
- Namekawa T, Ikeda S, Sugimoto M, Kume S. Effects of astaxanthin-containing oil on development and stress-related gene expression of bovine embryos exposed to heat stress. Reprod Domest Anim. 2010;45:e387–e391. doi: 10.1111/j.1439-0531.2010.01584.x. [DOI] [PubMed] [Google Scholar]
- Niu KC, Lin KC, Yang CY, Lin MT. Protective effects of alpha-tocopherol and mannitol in both circulatory shock and cerebral ischaemia injury in rat heatstroke. Clin Exp Pharmacol Physiol. 2003;30:745–751. doi: 10.1046/j.1440-1681.2003.03905.x. [DOI] [PubMed] [Google Scholar]
- Parida S, Mishra S, Mishra C, Dalai N, Mohapatra S, Mahapatra A, Kundu A (2019) Impact of heat stress on expression kinetics of HSP27 in cardiac cells of goats. Biol Rhythm Res:1–9 [DOI] [PubMed]
- Park JS, Chyun JH, Kim YK, Line LL, Chew BP. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab (Lond) 2010;7:18. doi: 10.1186/1743-7075-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss HG, Echard B, Yamashita E, Perricone NV. High dose astaxanthin lowers blood pressure and increases insulin sensitivity in rats: are these effects interdependent? Int J Med Sci. 2011;8:126–138. doi: 10.7150/ijms.8.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranga Rao A, Raghunath Reddy R, Baskaran V, Sarada R, Ravishankar G. Characterization of microalgal carotenoids by mass spectrometry and their bioavailability and antioxidant properties elucidated in rat model. J Agric Food Chem. 2010;58:8553–8559. doi: 10.1021/jf101187k. [DOI] [PubMed] [Google Scholar]
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia. 1962;18:571–573. doi: 10.1007/BF02172188. [DOI] [Google Scholar]
- Schochina M, Horowitz M. Central venous pressure, arterial pressure and hypovolemia: their role in adjustment during heat stress. J Therm Biol. 1989;14:109–113. doi: 10.1016/0306-4565(89)90032-6. [DOI] [Google Scholar]
- Schwimmer H, Gerstberger R, Horowitz M. Nitric oxide and angiotensin II: neuromodulation of thermoregulation during combined heat and hypohydration stress. Brain Res. 2004;1006:177–189. doi: 10.1016/j.brainres.2004.01.064. [DOI] [PubMed] [Google Scholar]
- Silver JT, Noble EG. Regulation of survival gene hsp70. Cell Stress Chaperones. 2012;17:1–9. doi: 10.1007/s12192-011-0290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Sharma B, Tiwari PK. The small heat shock protein Hsp27: present understanding and future prospects. J Therm Biol. 2017;69:149–154. doi: 10.1016/j.jtherbio.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Speranza L, Pesce M, Patruno A, Franceschelli S, de Lutiis MA, Grilli A, Felaco M. Astaxanthin treatment reduced oxidative induced pro-inflammatory cytokines secretion in U937: SHP-1 as a novel biological target. Mar Drugs. 2012;10:890–899. doi: 10.3390/md10040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terazawa S, Nakajima H, Shingo M, Niwano T, Imokawa G. Astaxanthin attenuates the UVB-induced secretion of prostaglandin E(2) and interleukin-8 in human keratinocytes by interrupting MSK1 phosphorylation in a ROS depletion-independent manner. Exp Dermatol. 2012;21(Suppl 1):11–17. doi: 10.1111/j.1600-0625.2012.01496.x. [DOI] [PubMed] [Google Scholar]
- Tissieres A, Mitchell HK, Tracy UM. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974;84:389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- Verghese J, Abrams J, Wang Y, Morano KA. Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system. Microbiol Mol Biol Rev. 2012;76:115–158. doi: 10.1128/MMBR.05018-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AM, Asoh S, Hiranuma H, Ohsawa I, Iio K, Satou A, Ishikura M, Ohta S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J Nutr Biochem. 2010;21:381–389. doi: 10.1016/j.jnutbio.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Peng J, Yin K, Wang JH. Potential health-promoting effects of astaxanthin: a high-value carotenoid mostly from microalgae. Mol Nutr Food Res. 2011;55:150–165. doi: 10.1002/mnfr.201000414. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang H. Multiple mechanisms of anti-cancer effects exerted by astaxanthin. Mar Drugs. 2015;13:4310–4330. doi: 10.3390/md13074310. [DOI] [PMC free article] [PubMed] [Google Scholar]