Abstract
This research aimed to investigate whether diabetic blood-stasis syndrome had a relationship with ROS-ERK1/2 signaling pathway in rat retina Müller cells and explore the effects of traditional Chinese drugs designed for promoting blood circulation to remove blood stasis on diabetic retinopathy (DR) treatment. Immunofluorescence was applied to determine purity of Müller cells. The diabetes was induced in rats by streptozotocin (STZ). Müller cells were stimulated by blood serum obtained from rats with blood-stasis syndrome and then treated by Xuefu Zhuyu decoction. Kits for reactive oxygen species (ROS), superoxide dismutase (SOD) and glutathione (GSH) were used for corresponding detection. Western blot analysis was used to determine the phosphorylation of ERK1/2. The results indicated that stimulation of Müller cells by blood serum of rats with diabetic blood-stasis syndrome increased the expression of ROS, inhibited SOD and GSH, and activated ERK1/2 signaling pathway. Treatment of Xuefu Zhuyu decoction could weaken this phenomenon. What’s more, similar effects of ERK1/2 inhibitor U0126 with Xuefu Zhuyu decoction proved the involvement of ERK1/2 signaling pathway. Therefore, our results suggested that traditional Chinese drugs for promoting blood circulation to remove blood stasis would be an effective therapy to treat DR.
Keywords: Diabetic retinopathy, Blood-stasis syndrome, Reactive oxygen species, ERK1/2 signaling pathway, Müller cells
Introduction
Along with the improvement of life standards, the number of patients with diabetic mellitus (DM), which is a lifelong epidemic disease, has been increasing rapidly in the recent years. Among the various complications induced by DM, diabetic retinopathy (DR) has been considered as one of the most common and serious diseases, and a leading cause of blindness in the working age population worldwide (Calcutt et al. 2009). DR is characterized by gradually progressive alterations in the retinal microvasculature, leading to loss of retinal capillary and pre-retinal neovascularization. Injury of neurons and glia also occur during DR (Simó and Hernández 2014; Valverde et al. 2013; van Dijk et al. 2012). Recently, clinical research has indicated that the development and progression of DR were closely related to blood-stasis syndrome, which is a theory in traditional Chinese medicine (Yin et al. 2009; Yue et al. 2017). Blood-stasis syndrome is characterized by the stasis formed by unsmooth blood circulation and subsequent dissipation of bleeding which were caused by deficiency and circulation stagnation of vital energy (Chen et al. 2016; Liao et al. 2016). Since blood-stasis syndrome could be related to DR, the clinical application of blood-invigorating and stasis-removing herbs in DR patients would be possible (Fang et al. 2012; Pei and Gao 2015). Moreover, the demonstration of molecular mechanism underlying the effects of traditional Chinese drugs for promoting blood circulation and removing stasis on DR could provide theoretical basis for the treatment of DR.
In the past decades, accumulating evidence has indicated that elevated oxidative stress was involved in the pathogenesis of DM and its complications (Baynes 1991). Particularly, retina is more likely to be affected by oxidative stress because of the high energy demands and exposure to light (Kumari et al. 2008). Indeed, previous research concerning the pathogenesis of DR illustrated the involvement of several molecular mechanisms, such as inflammation, the polyol pathway, the flux of hexosamine pathway and protein kinase C (PKC) pathway (Brownlee 2005; Kowluru and Chan 2007). Interestingly, all of the mechanisms seem to be associated with the over-production of reactive oxygen species (ROS) (Brownlee 2005). Further combining the relationship between blood-stasis syndrome and DR, research of the relationship between blood-stasis syndrome and ROS would be of great importance and has rarely been reported. Actually, one piece of research has revealed that the elevation of NADPH oxidase concentration increased the generation of ROS, which leads to change of blood stasis syndrome, thus exacerbating the pathological process of blood stasis syndrome with myocardial ischemia (Wang et al. 2011).
Mitogen-activated protein kinase (MAPK) is a group of protein kinases which is distributed in the cytoplasm and could be phosphorylated at both Ser and Tyr sites (Takahashi et al. 2005). It is an important signaling pathway to mediate cell response and plays a key role in the regulation of gene expression and cytoplasmic function (Takahashi et al. 2005). As a member in the MAPK family, extracellular signal-regulated kinases (ERK1/2) possesses important anti-apoptosis function (Musiolik et al. 2010). Increasing evidence proved that MAPK, especially ERK1/2, signaling pathway was involved in the ROS-induced cell injury, indicating ERK1/2 as the potential downstream of ROS (Chen et al. 1995; Guyton et al. 1996; Lander 1997). Accordingly, previous studies have indicated that the inhibition of ERK1/2 signaling pathway could increase the apoptosis induced by oxidative stress injury (Gabryel et al. 2006). Besides, our previous research reported that ERK1/2 signaling pathway mediated the over-expression of VEGF in DR and regulated the upstream of VEGF in Müller cells, indicating the key role of ERK1/2 in the development and progression of DR (Ye et al. 2012). However, we were still wondering whether ERK1/2 signaling pathway is the underlying mechanism of the effects of blood-stasis syndrome on DR through over-production of ROS.
Based on all the aforementioned background, in this study, we chose the major glia cells in the retina of rats, Müller cells, to study the effects and mechanism of diabetic blood-stasis syndrome injury. This research model was established based on normal Müller cells through the stimulation by high glucose medium or the serum of rats with induced diabetic blood-stasis syndrome (Tien et al. 2017; Zhao et al. 2012). This method has been considered as an important and credible method for studying blood-stasis syndrome in traditional Chinese medicine. This work aimed to investigate the effects of diabetic blood-stasis syndrome on production of ROS and activation of ERK1/2 signaling pathway in Müller cells. Moreover, Xuefu Zhuyu decoction is a commercially available Chinese medicine and has been reported to possess therapeutic effects on diseases such as traumatic brain injury (Xing et al. 2016), hypoxia/reoxygenation injury of cadiomyocytes (Shi et al. 2017) and primary dysmenorrhea (Zhou et al. 2014). Herein, the effects of Xuefu Zhuyu decoction on production of ROS and activation status of ERK1/2 signaling pathway was also demonstrated because of its capability of promoting blood circulation and removing blood stasis. It would provide experimental evidence for the treatment of DR by traditional Chinese drugs on the molecular level, and the development of a treatment strategy against DR in clinic.
Materials and methods
Materials
One hundred male Sprague–Dawley (SD) rats weighing 250 ± 20 g were purchased from Shanghai Jiesijie Experimental Animals Co., Ltd (Shanghai, China). Streptozotocin and U0126 were obtained from Selleck Chemicals (Houston, Texas, TX, USA). The medicinal materials of Xuefu Zhuyu decoction were acquired from Shenzhen Resources Sanjiu Modern Chinese Medicine Co., Ltd (Shenzhen, Guangdong, China). The primary antibodies used in the western blotting such as anti-ERK1/2 monoclonal antibodies (mAb), anti-p-ERK1/2 mAb, and anti-GAPDH mAb were purchased from Cell Signaling Technology (Danvers, Massachusetts, MA, USA), as well as the secondary antibodies conjugated with HRP.
Experimental diabetes induction and screening for blood-stasis syndrome rats
This study was approved by the Ethic Committee of EYE & ENT Hospital and Eye Institute of Fudan University. One hundred male Sprague–Dawley (SD) rats weighing 250 ± 20 g were assigned randomly to diabetes group or non-diabetes group (normal group), among them 10 normal rats as control in the normal group. The rats were kept in a specific pathogen-free environment (18–23 °C, indoor humidity 45–60%, water was sterilized by high temperature, sterile mattress was replaced twice a week, 12/12 day/night) and fed with normal diet purchased from LabDiet, Inc. (St. Louis, MO, USA). Diabetic rat models (DM group) were established through a single injection of streptozotocin (60 mg/kg body weight) by intraperitoneal route (Hossain et al. 2016), while citrate buffer (6 ml/kg) was injected into normal rats using the same method. Three days after injection, the rats with blood glucose levels above 16.7 mM were considered as diabetic models induced successfully. Twelve weeks after establishment, the diabetic rats with characterizations including obscure fur and feather, nigrescent ears and lips, deeply purple onyx and tails and hemangiectasis in cornea and ears were screened as the blood-stasis syndrome rats (Chen 2012). Then, these blood-stasis syndrome rats were classified into four intervention groups: (1) low dosage Xuefu Zhuyu decoction group (8 g/10 ml) (low dose group); (2) middle dosage Xuefu Zhuyu decoction group (16 g/10 ml) (middle dose group); (3) high dosage Xuefu Zhuyu decoction group (32 g/10 ml) (high dose group); (4) Placebo group (1 ml/100 g of distilled water). The dose of Xuefu Zhuyu decoction was calculated according to the human and mice dose conversion method in the second edition of “Experimental Zoology”. The conversion coefficient between human (~ 70 kg) and mice (~ 20 g) is 0.0026. Xuefu Zhuyu decoction and distilled water were lavaged to the blood-stasis syndrome rats. The recipe of Xuefu Zhuyu decoction was demonstrated as follow: 12 g of Peach seed, 9 g of Flos carthami, 9 g of Angelica sinensis, 9 g of Radix rehmanniae recen, 5 g of Rhizoma chuanxiong, 6 g of Red peony root, 9 g of Achyranthes bidentate, 3 g of Bupleurum, 5 g of Radix platycodi, 6 g of Fructus aurantii and 3 g of Radix glycyrrhizae. These medicinal materials were steeped and boiled in water, and concentrated into liquid pharmaceuticals of 2 g/ml, and then stored at 4 °C after sterilization.
After observation for 4 weeks, the diabetic blood-stasis syndrome rats were sacrificed through anesthesia of chloral hydrate, and the blood sample from the rats of different intervention groups was separated from blood in abdominal aorta and then centrifuged for 15 min at 3000 rpm at 4 °C to obtain serum. Next, the serum was inactivated for 30 min at 56 °C, filtered with 0.22 μm Millipore filter and stored at − 80 °C.
Hematoxylin–eosin staining
After all rats were sacrificed through anesthesia of chloral hydrate, the pancreas of some randomly selected rats in both diabetes and normal groups were acquired and then they were fixed with 10% paraformaldehyde (PFA). Subsequently, the paraffin-embedded sections were prepared for histological analysis. The slices were cut with 5 μm sections and next stained respectively in dye liquor of hematoxylin and eosin for histological evaluation. Finally, these slices were analyzed under a microscope (Olympus, Tokyo, Japan).
Primary Müller cell culture and treatment with the blood serum of blood-stasis syndrome rats and U0126
Retinas were isolated from the eyeball of new-born SD rats under a biological dissection microscope. Subsequently, the isolated retinas were digested with 1% trypsin for 5 min with phosphate-buffered saline (PBS) washing for 45 min and then the supernatant was removed, and the dissociated retinal cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Thermo Fisher Scientific Inc., Carlsbad, CA, USA) containing 15% fetal bovine serum (FBS) (Gibco, Thermo Fisher Scientific Inc., Carlsbad, CA, USA) and 5.5 mM glucose in a humidified atmosphere of 95% air plus 5% CO2 at 37 °C. Seven days later, the aggregates and cellular debris in the medium were dislodged and this promoted to generate a purified flat cell preparation. In the culture process, confluent cultures were passaged 3 times for all the experiments and the medium was replaced at 24 h before the treatment. The purity of these isolated cells was evaluated by immunofluorescence microscopy using glial fibrillary acidic protein (GFAP) antibodies and 4′,6-diamidino-2-phenyl-indole (DAPI) staining. The positive cells for GFAP and DAPI were used three passages and to treat with the blood serum of blood-stasis syndrome rats and U0126.
The blood serum from different groups of rats was acquired as described above and added into DMEM at a concentration of 50 ml/l respectively. The stimulation time was 48 h. Furthermore, all groups were treated with U0126 (Cell Signaling Technology, Boston, Massachusetts, MA, USA) in dosage of 0.02 mM for 24 h.
Immunofluorescence
To evaluate the purity of the isolated Müller cells and these cells were treated in 30 mM glucose for 24 h. Then, the isolated cells were fixed in 4% paraformaldehyde (PFA) with 0.01 M PBS for 20 min. Subsequently, the Müller cells were permeabilized with 0.3% Triton X-100 for 30 min and 5% goat serum for 45 min, and next incubated with GFAP antibodies overnight at 4 °C (1:200). Afterwards, the Müller cells were incubated in FITC conjugated secondary antibodies for 30 min. Eventually, the nuclei of these Müller cells were stained using DAPI. The final slides were observed under a confocal microscopy (Leica TCS SP2, Germany).
Detection of ROS, SOD and GSH
Intracellular amount of ROS was measured by ROS assay kit (Beyotime, Jiangsu, China) according to the manufacturer’s instructions through flow cytometry and analyzed by flowjo software.
The detection of SOD and GSH which was performed to detect the SOD and GSH concentration in the supernatants of Müller cells was assayed using SOD and GSH kit (Beyotime, Jiangsu, China) following the manufacturer’s instructions.
Western blot analysis
Cells were lysed by ice-cold Lysis Buffer (30 mM Tris–HCl pH 7.5, 5 mM EDTA, 1% Triton X-100, 250 mM sucrose, 1 mM Sodium vanadate and protease inhibitor cocktail), then total proteins were extracted. Proteins were respectively added to gel for electrophoresis, and then transferred to a polyvinylidene difluoride (PVDF) membrane. After blocking with 5% non-fat milk at room temperature for 1 h, primary antibodies were bound overnight at 4 °C. In the next day, the PVDF membrane was incubated with corresponding secondary antibodies at room temperature for 2 h. Each membrane was visualized with ECL-plusTM Western blotting system (Amersham, Buckinghamshire, UK), and were detected by X-ray imaging analyzer (Eastern Kodak, Rochester, NY, USA). Membranes were scanned with ImageJ to quantify band intensity.
Statistical analysis
All experiments were repeated at least three times in this study. All data are shown as mean ± SD and GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, California, CA, USA) was used for statistical analysis. Differences between two groups were determined with paired or unpaired Student’s t-tests. ANOVA was used for multiple comparisons. P < 0.05 was considered as statistical significant.
Results
Purity evaluation of Müller cells
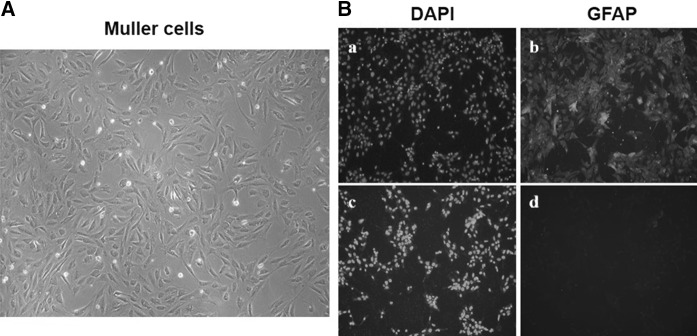
Müller glia are profoundly involved in the physiopathology of DR. It has been revealed that the activation of Müller cells, proved by over-expression of Glial Fibrillary Acidic Protein (GFAP), could be used as the first histologic hall-mark of DR (Ahmad et al. 2011; Mizutani et al. 1998). Therefore, Müller cells have been widely used in the study of DR and was also used as the research model in this study. The third generation of Müller cells isolated from the retina of new-born SD rats were cultured (Fig. 1a). Then immunofluorescence based on glial fibrillary acidic protein (GFAP) antibodies and DAPI staining was utilized to evaluate the purity of Müller cells. As shown in Fig. 1b, the blue fluorescence of DAPI staining demonstrated that the isolated Müller cells had high purity. Besides, we found that more than 95% Müller cells exhibited green fluorescence, which suggested that Müller cells had strong GFAP immunoreactivity.
Fig. 1.
Results of determining the purity of Müller cells. Morphology of Müller cells a in good condition was photographed and then the immunofluorescence b was performed to detect the purity of Müller cells: a the DAPI staining for nucleus of Müller cells; (b, d) the green fluorescence of GFAP in Müller cells; (c) the DAPI staining for nucleus of Müller cells with the secondary antibodies conjugated-FITC; d the Müller cells just with the secondary antibodies conjugated-FITC as control
Over-production of ROS in Müller cells under hyperglycemia
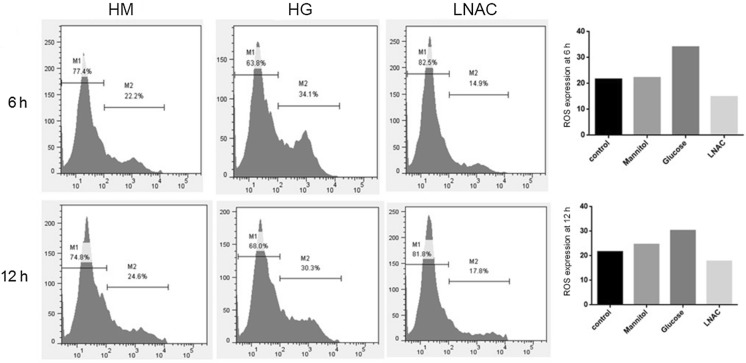
In order to confirm the ability of Müller cells to produce ROS in hyperglycemia, concentrations of ROS in Müller cells treated with high concentration of glucose (HG group), mannitol (HM group, as negative control) or both glucose and N-acetyl-l-cysteine (a commonly used anti-oxidant agent, LNAC group) were detected via flow cytometry and compared with the Müller cells cultured in normal condition. As shown in Fig. 2, the results exhibited that the treatment with high concentration of glucose distinctly increase the concentration of ROS in Müller cells compared with both control group and HM group, proving the over-production of ROS in hyperglycemia. Moreover, as expected, the increased ROS concentration could be decreased upon the treatment of LNAC which is a well-known ROS inhibitor.
Fig. 2.
Results of ROS expression in Müller cells treated with high mannitol or glucose. After treatment of Müller cells with high concentration of mannitol or glucose for 6 h or 12 h, the concentration of ROS was detected with flow cytometry
Establishment of diabetic blood-stasis syndrome rat models
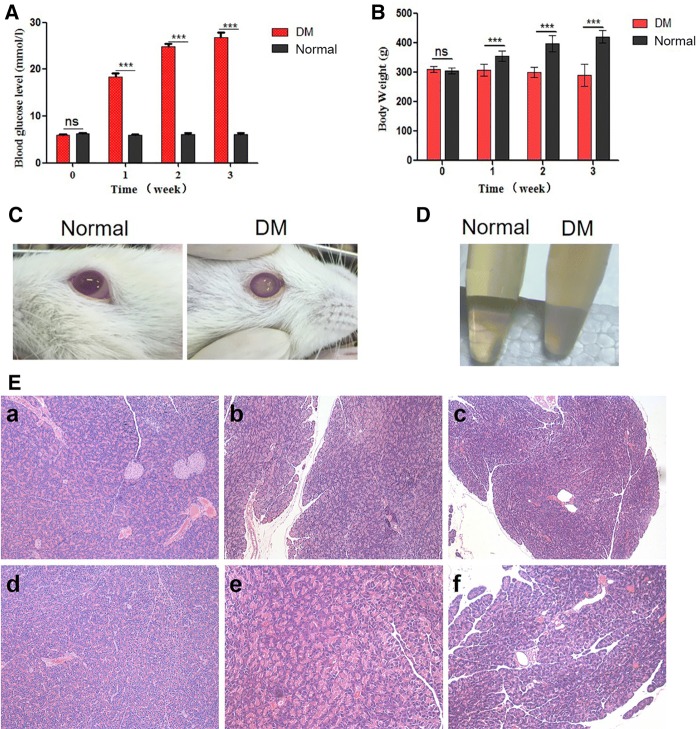
In this study, the DM rats model was induced by streptozotocin (STZ), which could selectively damage the pancreatic β cells and cause cell necrosis to induce the decrease of blood insulin level and increase of blood sugar, finally forming an insulin dependent diabetes model (Gerhardinger et al. 2005). After injection of STZ, blood glucose and body weight of SD rats were measured as the representative parameters to confirm the successful establishment of the diabetic rat models. The blood glucose level was strikingly increased in rats of diabetes group compared with normal group even in the first week of treatment (P < 0.01, Fig. 3a). Moreover, the mean body weight of rats in normal group gradually increased while that in the diabetes group slightly decreased and was always lower than that in normal group (P < 0.01, Fig. 3b). A portion of the rats in the diabetes group presented obvious cataract-related symptoms (Fig. 3c) and classic symptoms of blood-stasis syndrome such as obscure fur and feather, nigrescent ears and lips, deeply purple onyx and tails and hemangiectasis in cornea and ears. Moreover, the serum of these rats were feculent (Fig. 3d). Notably, only the rats with the symptoms of diabetic blood-stasis syndrome were investigated in this study as the DM group while the rest of the diabetic rats were excluded.
Fig. 3.
Results of screening for diabetic blood stasis syndrome rats. After injection of STZ into the SD rats by intraperitoneal route, the blood glucose levels (a) and body weight (b) of all SD rats were measured every week. Then, to screen for diabetic rats with blood-stasis syndrome, the cataract-related symptoms (c) and blood serum (d) were observed and photographed. e The pancreas of some rats in normal (a, d), diabetes (b, e) and Xuefu Zhuyu decoction treatment (c, f) groups were acquired, the pancreas islets structure was inspected by H&E staining. The magnification is 100 for a–c; 200 for d–f. The data were expressed as mean ± SD, P value less than 0.05 was considered statistically significant, ‘ns’ indicates no significance, *P < 0.05, **P < 0.01, ***P < 0.001
Subsequently, some rats were randomly selected from both DM or normal groups and eviscerated pancreas for hematoxylin–eosin (H&E) staining. The results demonstrated that, compared with the normal group, the DM group exhibited interstitial edema, necrosis, catheter expansion, incomplete cell structure accompanied by a small amount of hemorrhage (Fig. 3e), which suggested the successful establishment of diabetic rat model again. Noteworthy, only mild oedema of the glandular interstitium, mild fibrosis, mild catheter dilation accompanied by a small amount of hemorrhage could be observed in the Xuefu Zhuyu decoction treatment group (Fig. 3e), indicating the pharmaceutical effect of Xuefu Zhuyu decoction.
Diabetic blood-stasis syndrome increased the concentrations of SOD and GSH in Müller cells
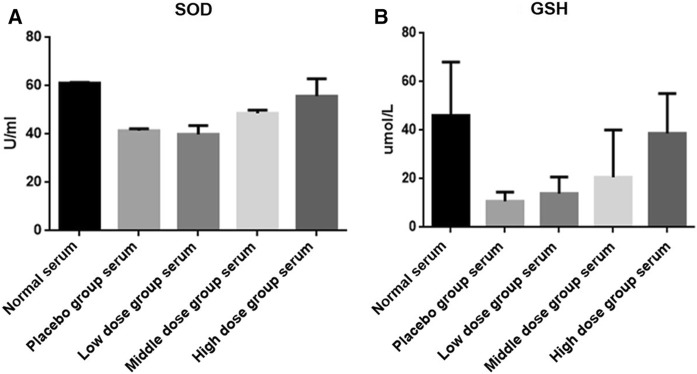
As the most important endogenous anti-oxidants, the changes of superoxide dismutase (SOD) and glutathione (GSH) could be used to evaluate the level of oxidative stress. Herein, in order to investigate the effects of diabetic blood-stasis syndrome on oxidative stress and the potential pharmaceutical effect of Xuefu Zhuyu decoction, SOD and GSH were detected in Müller cells, which were treated with the serum of the rats in DM or normal groups and followed by the treatment of Xuefu Zhuyu decoction in different doses. As shown in Fig. 4, the expression of SOD and GSH exhibited similar variation tendency: treatment of diabetic blood-stasis syndrome rats’ serum distinctly decreases concentrations of SOD and GSH compared with the serum of rats in normal group, indicating the over-production of ROS indirectly and the deepened oxidative stress; treatment of Xuefu Zhuyu decoction could reverse the inhibition of blood-stasis syndrome on SOD and GSH in a dose-dependent manner.
Fig. 4.
Diabetic blood-stasis syndrome inhibited the activity of SOD and GSH in Müller cells. After stimulated by the serum from different groups of blood-stasis syndrome rats and followed by treatment of Xuefu Zhuyu decoction in different dosages, the expressions of SOD (a) and GSH (b) were detected in Müller cells. The data were expressed as mean ± SD
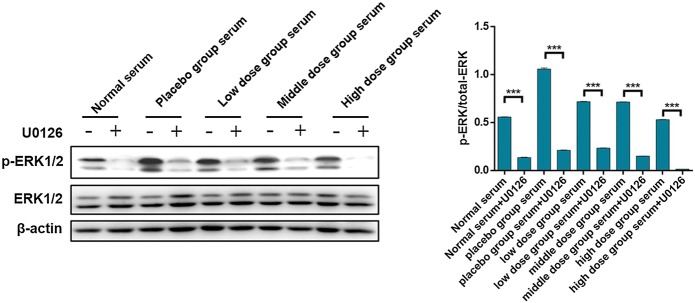
Diabetic blood-stasis syndrome activated ERK1/2 signaling pathway in Müller cells
To further explore the molecular mechanism of the effects of diabetic blood-stasis syndrome on DR, the enhanced activation of ERK1/2 was observed by western blot analysis in Müller cells treated with serum of rats in DM group. Moreover, U0126, which is a commonly used inhibitor of ERK1/2 signaling pathway, was also used for verification. As shown in Fig. 5, the phosphorylation as well as activation of ERK1/2 was observed in the DM group serum treated Müller cells which could be almost completely eliminated by U0126, indicating the role of diabetic blood-stasis syndrome in the activation of ERK1/2 signaling pathway. Moreover, treatment of Xuefu Zhuyu decoction in different doses could inhibit the blood-stasis syndrome induced activation of ERK1/2 signaling pathway to different extent. Considering that ERK1/2 is the downstream of ROS, the obtained results were in consistent with our aforementioned results in the evaluation of oxidative stress.
Fig. 5.
U0126 blocked the activation of ERK1/2 signaling pathway in Müller cells. After stimulated by the serum from different groups of blood-stasis syndrome rats, U0126 was added into the culture medium of Müller cells and incubated for 24 h. Subsequently, western blot analysis was conducted to detect the expression levels of p-ERK1/2 and ERK1/2. The data were expressed as mean ± SD, P value less than 0.05 was considered statistically significant, *P < 0.05, **P < 0.01, ***P < 0.001
U0126 inhibited the increase of ROS expression in Müller cells
Finally, for the sake of further establishing the relationship between the diabetic blood-stasis syndrome induced expression of ROS and activation of ERK1/2, ROS concentrations were measured in the serum-stimulated Müller cells upon the treatment of Xuefu Zhuyu decoction and U0126. As shown in Fig. 6, the over-production of ROS induced by diabetic blood-stasis syndrome, as well as the dose-dependent pharmaceutical effect of Xuefu Zhuyu decoction against ROS over-expression, were directly proved. Moreover, on this basis, the expression of ROS could be further inhibited upon the addition of U0126. These results showed that the blockage of ERK1/2 signaling pathway with U0126 could abolish the effects of blood-stasis syndrome on not only the phosphorylation of ERK1/2 signaling pathway, but also ROS expression in Müller cells. It indicates that the influence of blood-stasis syndrome on the development and progression of DR is through ROS-ERK1/2 signaling pathway.
Fig. 6.
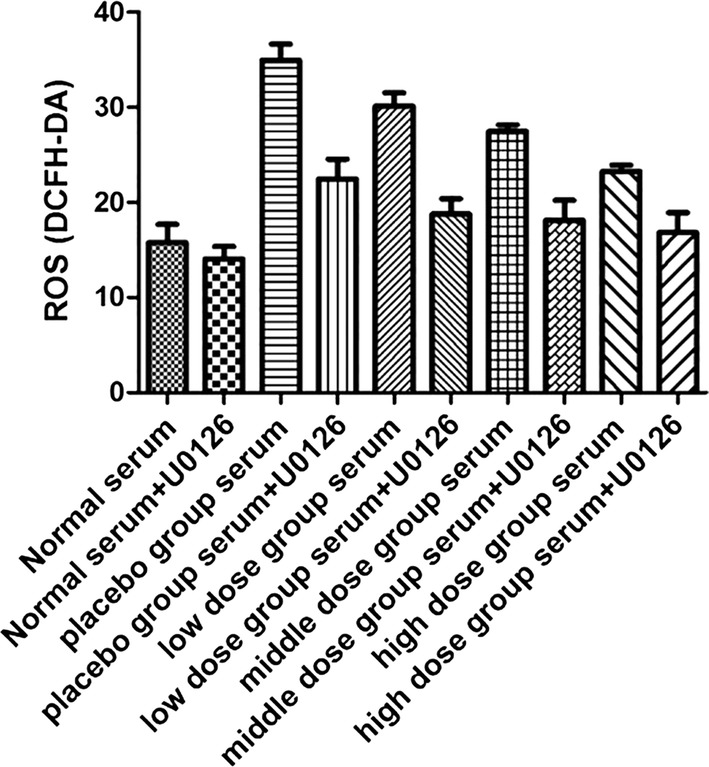
Xuefu Zhuyu decoction and U0126 blocked the expression of ROS in Müller cells. After stimulated by the serum from different groups of blood-stasis syndrome rats and followed by treatment of Xuefu Zhuyu decoction in different dosages, ROS expression was detected. Subsequently, U0126 was added into the culture medium of Müller cells and incubated for 24 h, and ROS expression was detected. The data were expressed as mean ± SD
Discussion
In this study, the function of Xuefu Zhuyu decoction, which is an ancient Chinese medicine recipe for removing blood stasis, on DR was studied for the first time. As is well known, DR is one of the most common and serious complications induced by DM that is present in nearly all patients with DM for more than 15 years (Hans-Peter et al. 2011; Klein et al. 1984). In spite of the rapid development of medical technology, traditional therapies for DR such as retinal photocoagulation and vitreous surgery could only be applied to patients in middle or late stage of disease to alleviate the progression. However, the visual function of these patients has been injured irreversibly (Hernández et al. 2017; Stewart 2017). Therefore, development of therapy strategies for early diagnosis and intervention is of great significance for DR patients. It has been observed in clinic that blood-stasis syndrome, which is a diagnosis that indicates very strong sense of traditional Chinese medicine, could be related to the development and progression of DR (Yin et al. 2009; Yue et al. 2017). Accordingly, traditional Chinese drugs designed for promoting blood circulation and removing stasis could reasonably exhibit therapeutic effect for DR which actually has been reported by several groups (Fang et al. 2012; Pei and Gao 2015; Yang and Wang 2011). For example, it has been reported that Fu Fang Xue Shuan Tong capsule combined with Calcium Dobesilate can improve retina and reduce the recurrence rate, the levels of hs-CRP, VEGF and Insulin-like Growth Factor-1 (IGF-1), thus delaying the proliferation of blood vessels in the patients with DR (Fang et al. 2012; Pei and Gao 2015). Moreover, Gegen Qinlian decoction plus Salvia miltiorrhiza displayed preventive and therapeutic effect for DR and could increase perivascular cells, reduce thickness of basement membrane of blood vessel and stop blood vessel obstruction (Yang and Wang 2011).
Herein, our results also reveal the involvement of ROS over-production in the regulation of DR by blood-stasis syndrome, which was rarely reported previously. Diabetes could induce the secretion of a large number of ROS, followed by the response of the antioxidant system (Johansen et al. 2005). The imbalance between the production and removal of ROS could lead to a series of lesions (Gao and Mann 2009). ROS such as superoxide anions (O2−) and hydrogen peroxide (H2O2) are important second messengers in intracellular signal transduction pathways such as NF-κB signaling pathway (Luo et al. 2015). Previous studies have indicated that, although ROS plays an important role in regulating cell function and immune response, its over-production could influence the growth and migration of vascular smooth muscle and inflammatory cells, change extracellular matrix, induce apoptosis of endothelial cells, activate transcription factors, mediate endothelial cell damage, and thus aggravating the occurrence of diabetic microvascular and macrovascular complications such as DR (Ding et al. 2007; Lee et al. 2003). Xu et al. reported that the pathogenic effect of ubiquitin proteasome pathway on DR involved the increase of ROS generation and NF-κB expression, which was associated with the ROS/PARP and NF-κB inflammatory factor pathways (Luo et al. 2015).
SOD and GSH are the most important endogenous antioxidants (Antonetti et al. 2006). SOD could disproportionate superoxide anion which is further reacted to generate water, thus protecting cells from oxygen free radicals. Similarly, GSH is also an important antioxidant in cells, which protects the retina against cytotoxic damage caused by ROS (Antonetti et al. 2006). In previous studies, it was found that the Müller cells in the retina are the main source of GSH, and are involved in releasing GSH to the surrounding neurons to play the defensive role in the retina (Kowluru et al. 2001). Therefore, the observation of change in concentrations of SOD and GSH could benefit for understanding the extent of oxidative stress injury.
As the major glia of the retina, Müller cells have been intensively studied and showed function of forming processes around retinal vessels, providing support to retinal neurons, controlling blood current, glucose metabolism and maintaining the blood-retinal barriers (Barber and Antonetti 2000). Therefore, Müller cells have been widely used in the study of DR and was also used as the research model. In this study, we successfully isolated Müller cells, induced diabetic rat models and screened out blood stasis rat models. The over-production of ROS was verified in Müller cells upon the stimulation of either high concentration of glucose or serum of rats in DM group, which is in consistence with the reported ability of hyperglycemia or diabetes to induce oxidative stress. Accordingly, the inhibition of the expression of SOD and GSH as well as the anti-oxidative system also proved the above results indirectly. Importantly, both the over-production of ROS and inhibition of SOD as well as GSH could be reversed via treatment by different doses of Xuefu Zhuyu decoction, indicating that the removal of blood-stasis could alleviate the consequent increase of oxidative stress.
MAPK is an important signal transduction system in organism. The family members of MAPK, including extracellular signal-regulated kinases (ERK1/2), c-Jun NH2-terminal protein kinase (JNK) and P38 MAPK, could be activated by several extracellular stimuli and mediate cell proliferation, differentiation, cell survival and apoptosis (Boldt et al. 2002). Among them, as the anti-apoptotic kinase, the activation of ERK promotes phosphorylation of cytoplasmic target protein, mediates activities of other protein kinases, promotes the phosphorylation of various transcription factors, and enhances transcriptional activity. Kuo et al. (2015) reported that, as a well-known downstream of ROS, the phosphorylation of ERK1/2 was involved in the protective effect of rhEC-SOD on diabetic nephropathy. rhEC-SOD could reverse the decrease of SOD activity, inhibit the over-production of ROS, thus inhibiting the phosphorylation of ERK1/2, protecting the progression of diabetic nephropathy. A study of Cai et al. (2017) indicated that GLP-1 could alleviate oxidative stress in DR, thus alleviating apoptosis and autophagy of retinal cells in diabetic rats through the ERK1/2 signaling pathway. These results suggested that the ROS-ERK1/2 signaling pathway may play critical role in development and progression of DR.
Conclusions
In conclusion, the outcomes of this study concluded that diabetic blood-stasis syndrome activates ERK1/2 signaling pathway through promoting expression of ROS, and Chinese medicine used to remove blood-stasis could alleviate oxidative stress, thus inhibiting activation of ERK1/2 signaling pathway and DR.
Acknowledgements
This study was supported by National Natural Science Foundation of China (Grant Number 81403245) and Bethune·Lumitin Research Funding for the young and middle-aged Ophthalmologists (Grant Number BJ-LM2017007J).
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmad I, Del Debbio CB, Das AV, Parameswaran S. Müller glia: a promising target for therapeutic regeneration. Invest Ophth Vis Sci. 2011;52:5758–5764. doi: 10.1167/iovs.11-7308. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, Kester M, Kimball SR, Krady JK, LaNoue KF, Norbury CC, Quinn PG, Sandirasegarane L, Simpson IA. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–2411. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- Barber A, Antonetti DT. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. The Penn State Retina Research Group. Invest Ophth Vis Sci. 2000;41:3561–3568. [PubMed] [Google Scholar]
- Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- Boldt S, Weidle UH, Kolch W. The role of MAPK pathways in the action of chemotherapeutic drugs. Carcinogenesis. 2002;23:1831–1838. doi: 10.1093/carcin/23.11.1831. [DOI] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Cai X, Li J, Wang M, She M, Tang Y, Li J, Li H, Hui H. GLP-1 treatment improves diabetic retinopathy by alleviating autophagy through GLP-1R-ERK1/2-HDAC6 signaling pathway. Int J Med Sci. 2017;14:1203–1212. doi: 10.7150/ijms.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcutt NA, Cooper ME, Kern TS, Schmidt AM. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov. 2009;8:417–429. doi: 10.1038/nrd2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KJ. Blood stasis syndrome and its treatment with activating blood circulation to remove blood stasis therapy. Chin J Integr Med. 2012;18:891–896. doi: 10.1007/s11655-012-1291-5. [DOI] [PubMed] [Google Scholar]
- Chen Q, Olashaw N, Wu J. Participation of reactive oxygen species in the lysophosphatidic acid-stimulated mitogen-activated protein kinase kinase activation pathway. J Biol Chem. 1995;270:28499–28502. doi: 10.1074/jbc.270.48.28499. [DOI] [PubMed] [Google Scholar]
- Chen KJ, Shi DZ, Fu CG, Gao ZY, Xu H, Lv SZ, You SJ, Huang L. Diagnostic criterion of blood stasis syndrome for coronary heart disease: activating Blood Circulation Committee of Chinese Association of Integrative Medicine. Chin J Integr Med. 2016;22:803–804. doi: 10.1007/s11655-016-2273-z. [DOI] [PubMed] [Google Scholar]
- Ding H, Hashem M, Triggle C. Increased oxidative stress in the streptozotocin-induced diabetic apoE-deficient mouse: changes in expression of NADPH oxidase subunits and eNOS. Eur J Pharmacol. 2007;561:121–128. doi: 10.1016/j.ejphar.2006.12.034. [DOI] [PubMed] [Google Scholar]
- Fang D, Wan X, Deng W, Guan H, Ke W, Xiao H, Li Y. Fufang Xue Shuan Tong capsules inhibit renal oxidative stress markers and indices of nephropathy in diabetic rats. Exp Ther Med. 2012;4:871–876. doi: 10.3892/etm.2012.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabryel B, Pudelko A, Adamczyk J, Fischer I, Malecki A. Calcineurin and Erk1/2-signaling pathways are involved in the antiapoptotic effect of cyclosporin A on astrocytes exposed to simulated ischemia in vitro. Naunyn Schmiedebergs Arch Pharmacol. 2006;374:127–139. doi: 10.1007/s00210-006-0106-x. [DOI] [PubMed] [Google Scholar]
- Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res. 2009;82:9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- Gerhardinger C, Costa MB, Coulombe MC, Toth I, Hoehn T, Grosu P. Expression of acute-phase response proteins in retinal Müller cells in diabetes. Invest Ophthalmol Vis Sci. 2005;46:349–357. doi: 10.1167/iovs.04-0860. [DOI] [PubMed] [Google Scholar]
- Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- Hans-Peter H, Feng Y, Frederick P, Michael B. Diabetic retinopathy: targeting vasoregression. Diabetes. 2011;60:9–16. doi: 10.2337/db10-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández C, Simóservat A, Bogdanov P, Simó R. Diabetic retinopathy: new therapeutic perspectives based on pathogenic mechanisms. J Endocrinol Invest. 2017;40:1–11. doi: 10.1007/s40618-017-0648-4. [DOI] [PubMed] [Google Scholar]
- Hossain A, Heron D, Davenport I, Huckaba T, Graves R, Mandal T, Muniruzzaman S, Wang S, Bhattacharjee PS. Protective effects of bestatin in the retina of streptozotocin-induced diabetic mice. Exp Eye Res. 2016;149:100–106. doi: 10.1016/j.exer.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4:5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Klein BEK, Moss SE, Davis MD, Demets DL. The Wisconsin epidemiologic study of diabetic retinopathy: II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102:520–526. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007;2007:43603. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru RA, Engerman RL, Case GL, Kern TS. Retinal glutamate in diabetes and effect of antioxidants. Neurochem Int. 2001;38:385–390. doi: 10.1016/s0197-0186(00)00112-1. [DOI] [PubMed] [Google Scholar]
- Kumari S, Panda S, Mangaraj M, Mandal MK, Mahapatra PC. Plasma MDA and antioxidant vitamins in diabetic retinopathy. Indian J Clin Biochem. 2008;23:158–162. doi: 10.1007/s12291-008-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CW, Shen CJ, Tung YT, Chen HL, Chen YH, Chang WH, Cheng KC, Yang SH, Chen CM. Extracellular superoxide dismutase ameliorates streptozotocin-induced rat diabetic nephropathy via inhibiting the ROS/ERK1/2 signaling. Life Sci. 2015;135:77–86. doi: 10.1016/j.lfs.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997;11:118–124. [PubMed] [Google Scholar]
- Lee HB, Yu MR, Yang Y, Jiang Z, Ha H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol. 2003;14:241–245. doi: 10.1097/01.asn.0000077410.66390.0f. [DOI] [PubMed] [Google Scholar]
- Liao J, Liu Y, Jie W. Erratum to: identification of more objective biomarkers for blood-stasis syndrome diagnosis. BMC Complement Altern Med. 2016;16:371. doi: 10.1186/s12906-016-1349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo DW, Zheng Z, Wang H, Fan Y, Chen F, Sun Y, Wang WJ, Sun T, Xu X. UPP mediated diabetic retinopathy via ROS/PARP and NF-κB inflammatory factor pathways. Curr Mol Med. 2015;15:790–799. doi: 10.2174/1566524015666150921110500. [DOI] [PubMed] [Google Scholar]
- Mizutani M, Gerhardinger C, Lorenzi M. Müller cell changes in human diabetic retinopathy. Diabetes. 1998;47:445. doi: 10.2337/diabetes.47.3.445. [DOI] [PubMed] [Google Scholar]
- Musiolik J, Van CP, Skyschally A, Boengler K, Gres P, Schulz R, et al. Reduction of infarct size by gentle reperfusion without activation of reperfusion injury salvage kinases in pigs. Cardiovasc Res. 2010;85:110–117. doi: 10.1093/cvr/cvp271. [DOI] [PubMed] [Google Scholar]
- Pei R, Gao H. Clinical effects and hs-CRP, VEGF and IGF-1 levels of Xueshuantong capsule combined with calcium dobesilate in treatment of early diabetic retinopathy. Modern J Integr Tradit Chin Western Med. 2015;24:3896–3907. [Google Scholar]
- Shi X, Zhu H, Zhang Y, Zhou M, Tang D, Zhang H. XuefuZhuyu decoction protected cardiomyocytes against hypoxia/reoxygenation injury by inhibiting autophagy. BMC Complem Altern Med. 2017;17:325. doi: 10.1186/s12906-017-1822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simó R, Hernández C. Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends Endocrinol Metab. 2014;25:23–33. doi: 10.1016/j.tem.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Stewart MW. A review of ranibizumab for the treatment of diabetic retinopathy. Ophthalmol Ther. 2017;6:33–47. doi: 10.1007/s40123-017-0083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Saito Y, Kuwahara K, Harada M, Tanimoto K, Nakagawa Y, Kawakami R, Nakanishi M, Yasuno S, Usami S, Yoshimura A, Nakao K. Hypertrophic responses to cardiotrophin-1 are not mediated by STAT3, but via a MEK5-ERK5 pathway in cultured cardiomyocytes. J Mol Cell Cardiol. 2005;38:185–192. doi: 10.1016/j.yjmcc.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Tien T, Zhang J, Muto T, Kim D, Sarthy VP, Roy S. High glucose induces mitochondrial dysfunction in retinal müller cells: implications for diabetic retinopathy. Invest Ophth Vis Sci. 2017;58:2915–2921. doi: 10.1167/iovs.16-21355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde AM, Soledad M, Marta GR, Águeda GR, Cristina H, Rafael S. Proapoptotic and survival signaling in the neuroretina at early stages of diabetic retinopathy. Mol Vis. 2013;19:47–53. [PMC free article] [PubMed] [Google Scholar]
- van Dijk HW, Verbraak FD, Kok PH, Stehouwer M, Garvin MK, Sonka M, DeVries JH, Schlingemann RO, Abràmoff MD. Early neurodegeneration in the retina of type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2012;53:2715–2719. doi: 10.1167/iovs.11-8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chun LI, Wen-Jing C, Guo SZ, Wang W. Experimental study on role of AngII-NADPH oxidase-ROS pathway in syndrome of blood stasis based on chronic myocardial ischemia model. China J Tradit Chin Med Pharm. 2011;26:2265–2268. [Google Scholar]
- Xing Z, Xia Z, Peng W, Li J, Zhang C, Fu C, Tao T, Luo J, Yong Z, Rong F. Xuefu Zhuyu decoction, a traditional Chinese medicine, provides neuroprotection in a rat model of traumatic brain injury via an anti-inflammatory pathway. Sci Rep. 2016;6:20040. doi: 10.1038/srep20040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MM, Wang J. Preventive and therapeutic effect of Salvia miltiorrhiza for diabetic retinopathy. J Harbin Med Univ. 2011;45:553–555. [Google Scholar]
- Ye X, Ren H, Zhang M, Sun Z, Jiang AC, Xu G. ERK1/2 signaling pathway in the release of VEGF from Müller cells in diabetes. Invest Ophthalmol Vis Sci. 2012;53:3481–3489. doi: 10.1167/iovs.11-9076. [DOI] [PubMed] [Google Scholar]
- Yin DH, Liang XC, Piao YL. Analysis of chinese medicine syndrome pattern in patients with type 2 diabetes mellitus and its relationship with diabetic chronic complications. Chin J Integr Tradit Western Med. 2009;29:506–510. [PubMed] [Google Scholar]
- Yue SJ, Xin LT, Fan YC, Li SJ, Tang YP, Duan JA, Guan HS, Wang CY. Herb pair Danggui-Honghua: mechanisms underlying blood stasis syndrome by system pharmacology approach. Sci Rep. 2017;7:40318. doi: 10.1038/srep40318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Chen J, Shi Q, Ma X, Yang Y, Luo L, Guo S, Wang Y, Han J, Wang W. Metabolomics-based study of clinical and animal plasma samples in coronary heart disease with blood stasis syndrome. Evid Based Compl Alt. 2012;2012:638723. doi: 10.1155/2012/638723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YN, Sun MY, Mu YP, Yang T, Ning BB, Ren S, Chen JM, Liu P. Xuefuzhuyu decoction inhibition of angiogenesis attenuates liver fibrosis induced by CCl4 in mice. J Ethnopharmacol. 2014;153:659–666. doi: 10.1016/j.jep.2014.03.019. [DOI] [PubMed] [Google Scholar]