Abstract
Endothelial cells play essential roles in angiogenesis. Heat shock protein A12B (HSPA12B), a novel member of the multigene Hsp70 family, expresses specifically in endothelial cells. Alpha-lipoic acid (LA) has been used for the treatment of human diabetic complications for more than 20 years. However, little is known whether LA impacts endothelial proliferation and migration. To address these questions, primary human umbilical vein endothelial cells (HUVECs) were isolated and treated with LA. We found that LA reduced viable HUVECs but not caused LDH leakage and nuclear condensation, suggesting an inhibitory effect of LA on HUVEC proliferation. We also noticed that LA impeded wound closure of HUVEC monolayers. The expressions of C-Myc, VEGF, and eNOS and phosphorylation of focal adhesion kinase were reduced by LA. Moreover, LA decreased the expression of heat shock protein A12B (HSPA12B). Notably, overexpression of HSPA12B in endothelial cells prevented the LA-induced loss of VEGF. More importantly, HSPA12B overexpression attenuated the LA-induced inhibition of endothelial proliferation and migration. Collectively, the results demonstrated that LA inhibited proliferative and migratory abilities in human vascular endothelial cells through the downregulation of the HSPA12B/VEGF signaling axis. The data suggest that besides the treatment in diabetic complications, LA might represent a viable therapeutic potential for human diseases that involve high angiogenic activities such as cancers.
Keywords: Proliferation, Migration, Endothelial cells, Alpha-lipoic acid, HSPA12B
Introduction
Endothelial cells (ECs) form a monolayer that lines the interior surface of blood vessels and lymphatic vessels. Evidence has shown that ECs play important roles in maintaining vascular homeostasis and play pivotal roles in angiogenesis-related pathological events. To exert these roles, proliferation and migration of ECs are principally demanded for the angiogenesis. Angiogenesis is a critical event in response to the repair of ischemic injury; however, pathological angiogenesis and vascular malfunction that caused by excessive EC proliferation and migration are hallmarks of malignant disorders, such as diabetes-related retinopathy and nephropathy, progressed atherosclerotic plaque, and advanced cancers (Dworacka et al. 2015; Nikitenko and Boshoff 2006).
Heat shock protein A12B (HSPA12B) was initially cloned from atherosclerotic lesions in 2003 and classified as a distant member of heat shock protein 70 (HSP70) family because it contains an atypical ATPase domain (Han et al. 2003). In contrast with the ubiquitous expression of other members of the HSP70 family, we and others revealed that HSPA12B expresses specifically in endothelial cells (Han et al. 2003; Hu et al. 2006; Li et al. 2013; Vos et al. 2008). This preferential expression pattern in endothelial cells suggests that HSPA12B might play roles in endothelial-related biological events. Indeed, HSPA12B is necessary for endothelial proliferation and migration (Han et al. 2003; Hu et al. 2006). Also, we have reported that HSPA12B promotes angiogenesis following myocardial and cerebral ischemia through upregulating vascular endothelial growth factor (VEGF) and other angiogenic factors (Li et al. 2013; Zhao et al. 2018). Therefore, HSPA12B might play critical roles in the regulation of endothelial behaviors.
Alpha-lipoic acid (LA) is a compound naturally occurring in human diet including spinach, broccoli, and tomato. LA has been used as a diet supplement due to its central role in establishing and maintaining the antioxidant defense network by effectively scavenging free radicals and regenerating redox capacity (Dworacka et al. 2015). Notably, LA is licensed in most European countries as an achievable adjunct therapy for diabetic complications including retinopathy, neuropathy, and pain management (Dworacka et al. 2015; Nebbioso et al. 2013; Rochette et al. 2015). As an example, LA supplementation helps in preventing vision loss in diabetic patients (Nebbioso et al. 2013). However, both the underlying mechanisms and the indication/contraindication of LA has not been fully understood when it is employed in anti-diabetic complication treatment. When taken into account that the pathological angiogenesis contributes to the pathogenesis of some diabetic complications (e.g., retinopathy), it is possible, therefore, that LA might impact the endothelial behaviors including proliferation and migration.
To test this possibility, we treated primary human umbilical vein endothelial cells (HUVECs) with LA. We observed that LA inhibited endothelial proliferation and migration. This action of LA was mediated, at least in part, through downregulation of the HSPA12B/VEGF axis. The data suggest that besides the treatment in diabetic complications, LA might represent a viable therapeutic potential for human diseases that involve high angiogenic activities such as cancers, whereas LA might be harmful for the diseases that need angiogenesis for repair such as myocardial infarction. Our data put further insight into the indication/contraindication of LA when it is employed in anti-diabetic complication treatment.
Materials and methods
Reagents
Trypsin, basic fibroblast growth factor (bFGF), and primary antibody for α-tubulin were from Sigma-Aldrich (St. Louis, MO). M199 medium and fetal bovine serum (FBS) were obtained from GIBCO (Grand Island, NY, USA). Primary antibodies for VEGF and endothelial nitric oxide synthase (eNOS) were from Abcam (Cambridge, UK); phosphor-focal adhesion kinase (p-FAK) and total FAK were from Bioworld Technology (Louis Park, MN). The primary antibody for HSPA12B was a kind gift from Dr. Zhihua Han (East Tennessee State University, Johnson City, TN). Primary antibodies for HSP22, HSP27, HSP32, HSP47, HSP70, and HSP90 were from Stressgen (Victoria BC, Canada). Hoechst 33342 reagent was obtained from Invitrogen (Carlsbad, CA). BCA protein assay kit and SuperSignal West Pico chemiluminescent substrate were obtained from Pierce (Rockford, IL). MTT (3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) reagent was from Bio Basic Inc. (Markham, Ontario, Canada). Cell-Light™ EdU Apollo 567 In Vitro Kit was from RibBio Technology (Guangzhou, China). Protease inhibitor cocktail was from Roche (Mannheim, Germany). Trizol reagent was from Life Technology (Carlsbad, CA). Lactate dehydrogenase (LDH) activity assay kit was from Jiancheng Biotech (Nanjing, China). SYBR Green Master was from Roche (Indianapolis, IN).
Isolation and growth of human umbilical vein endothelial cells
HUVECs were isolated from the umbilical vein cords of normal pregnancies according to our previous methods (Wu et al. 2015). Briefly, umbilical veins were digested with 0.25% trypsin to dissociate endothelial cells. The harvested HUVECs were grown in M199 medium supplemented with 10% FBS, 100 U/ml penicillin-streptomycin, and 0.5 ng/ml bFGF in an atmosphere of 5% CO2 at 37 °C. The medium was refreshed at intervals of 2–3 days. The cells in passages 2 to 5 were used in the experiments. The Ethical Board of the First Affiliated Hospital of Nanjing Medical University approved these studies (#2012-SR-153). All the human study procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000.
Construction of HSPA12B adenovirus
The adenoviral vector containing the coding region of human HSPA12B (NM_052970) and green fluorescence protein (GFP) was generated by GeneChem (Shanghai, China) as described in our previous studies (Wu et al. 2015). To overexpress HSPA12B, HUVECs were infected with HSPA12B adenovirus (Ad-HSPA12B) for 24 h followed by the indicated treatment. The cells infected with empty adenovirus (Ad-GFP) served as negative controls.
MTT assay
HUVECs grown in 24-well plates were treated with LA at the indicated concentrations for the indicated durations. MTT assay was performed to evaluate cell viability as previously described (Yao et al. 2012). Briefly, 40 μl of 5 mg/ml MTT in PBS solution was added into the medium and incubated for 4 h. Subsequently, the formazan product was solubilized by the addition of 300 μl of dimethyl sulfoxide (DMSO). Absorbance was measured at a wavelength of 570 nm using Synergy HT plate reader (Synergy HT, Bio-Tek, USA).
RNA extraction and real-time PCR
After incubation with LA (500 μM) for 48 h, HUVECs were collected for total RNA extraction using Trizol reagent according to previous methods (Kong et al. 2016). Vehicle-treated HUVECs served as controls. An amount of 2 μg RNA was used for the first-strand cDNA synthesis using the oligo (dT) first-strand primer. After cDNA synthesis, the expression levels of C-myc and Cyclin-d1 mRNA were estimated by real-time PCR using the SYBR Green Master (Roche, Indianapolis, IN). The PCR results of β-actin served as internal controls. The primers used in the experiments for C-myc were GGCTCCTGGCAAAAGGTCA (forward) and CTGCGTAGTTGTGCTGATGT (reverse), for Cyclin-d1 were GCTGCGAAGTGGAAACCATC (forward) and CCTCCTTCTGCACACATTTGAA (reverse), and for β-actin were TGTTACCAA CTGGGACGACA (forward) and TCTCAGCTGTGGTGGTGAAG (reverse).
EdU incorporated assay
HUVECs grown in 24-well plates were treated with LA (500 μM) for 48 h, followed by incubation with EdU for 2 h. HUVEC proliferation was indicated using an EdU labeling that visualized by the assay kit according to the manufacturer’s instructions. Hoechst 33342 was used to counterstain the nuclei.
Cell number measurements
HUVECs grown in 24-well plates were treated with LA (500 μM) for 48 h. Following treatments, cells were counterstained with DAPI to indicate the nuclei of HUVECs. The cells were counted and expressed as the relative density to those of controls.
LDH leakage assay
Following incubation with LA (500 μM) for 48 h, HUVECs and culture medium were collected for LDH activity measurement according to the manufacturer’s instructions. The LDH leakage was expressed as the percentage of activity in medium divided by total activity in cells. Total LDH activity = (LDH activity in the medium) + (LDH activity in the lysate of cells treated with 0.2% Tween 20).
Nuclear condensation
After incubation with LA (500 μM) for 48 h, HUVECs were stained with Hoechst 33342 to evaluate nuclear condensation, a frequently used marker of apoptosis (Ma et al. 2010). Briefly, HUVECs grown in 24-well plates were fixed with 50% methanol and 50% acetone for 10 min and then incubated with Hoechst 33342 (0.4 g/ml) for 5 min at room temperature. The stained cells were examined under a phase-contrast fluorescence microscope (Axiovert 200). Three fields on each well were randomly examined with a magnification of × 200.
Immunoblotting analysis
After incubation with LA (500 μM) for 48 h, HUVECs were collected for immunoblotting analysis with the indicated antibodies according to previous methods (Kong et al. 2016). Briefly, cellular proteins were prepared, separated on 10% SDS-PAGE, and transferred onto Immobilon-P membranes (Millipore). The membranes were probed with appropriate primary antibodies followed by incubation with peroxidase-conjugated secondary antibodies. The signals were detected by enhanced Pierce chemiluminescence. The blots against α-tubulin served as loading controls. The signals were quantified by scanning densitometry and the results from each experimental group were expressed as relative integrated intensity compared with those of controls.
Monolayer wound-healing migration assay
To measure the migrating ability of HUVECs, a wound-healing assay was carried out according to our previous methods (Wu et al. 2015). In brief, HUVECs grown in 6-well plates were infected with Ad-HSPA12B or Ad-GFP and allowed to grow to monolayers. Following scratching with a 200-μl pipette tip, cells were exposed to LA (500 μM) for 48 h. Wound closure was photographed and quantified as the previous methods (Wu et al. 2015).
Statistical analysis
Results are expressed as mean ± standard deviation (X ± SD). Groups were compared using Student two-tailed unpaired t test, one-way or two-way analysis of variance analysis (ANOVA) followed by Tukey post hoc test. Statistical significance was set at P < 0.05.
Results
LA reduces proliferation of HUVECs
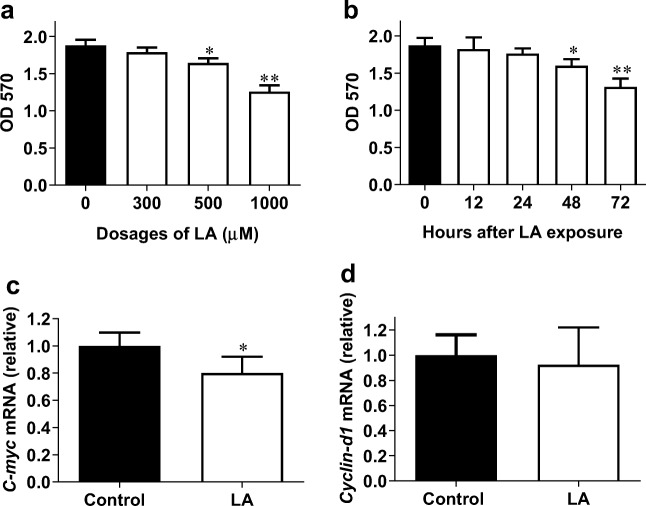
LA has been used for treating human diabetic complications for more than 20 years. Considering that angiogenesis is involved in the pathogenesis of some diabetic complications such as diabetic retinopathy and impaired wound healing (Nebbioso et al. 2013; Okonkwo and DiPietro 2017), we determined whether LA would have an impact on the vascular endothelial cell proliferation because it is the essential early step of angiogenesis. To this end, we first examined the “dose effect” of LA on HUVEC viability using MTT assay following treatment with LA at concentrations of 0, 300, 500, and 1000 μM for 48 h. As shown in Fig. 1a, 300 μM of LA showed no significant effect on cell viability compared with the untreated (0 μM) control cells. However, we found that 500 and 1000 μM of LA reduced HUVEC viability significantly by 12.7% and 33.2%, respectively, compared with the controls (P < 0.05 or 0.01). Thus, LA inhibited endothelial cell viability in a dose-dependent manner. Based on these results, 500 μM of LA was selected for the following experiments.
Fig. 1.
LA decreased viable HUVECs. a HUVECs were treated with LA at the indicated dosages for 48 h. MTT assay was performed to evaluate viable cells. **P < 0.01 and *P < 0.05 vs. untreated (0 μM) control cells, n = 3/group. b HUVECs were treated with LA (500 μM) for the indicated durations. MTT assay was performed to evaluate viable cells. **P < 0.01 and *P < 0.05 vs. 0 h control cells, n = 3/group. c HUVECs were incubated with LA (500 μM) for 48 h. Cells were collected for analysis of C-myc mRNA expression using real-time PCR. *P < 0.05 vs. untreated control cells, n = 4/group. d HUVECs were incubated with LA (500 μM) for 48 h. Cells were collected for analysis of Cyclin-D1 mRNA expression using real-time PCR. *P < 0.05 vs. untreated control cells, n = 3/group. LA, α-lipoic acid
Next, the “time effect” of LA on HUVEC viability was examined following treatment with LA (500 μM) for 0, 12, 24, 48, and 72 h. The results of the MTT assay showed that treatment with LA for 12 and 24 h did not significantly change cell viability, respectively, when compared with the untreated (0 h) control cells (Fig. 1b). However, LA treatment for 48 and 72 h significantly reduced cell viability by 17.2% and 31.9%, respectively, compared with the control cells (P < 0.05 or 0.01). Thus, LA inhibited endothelial cell viability in a time-dependent manner.
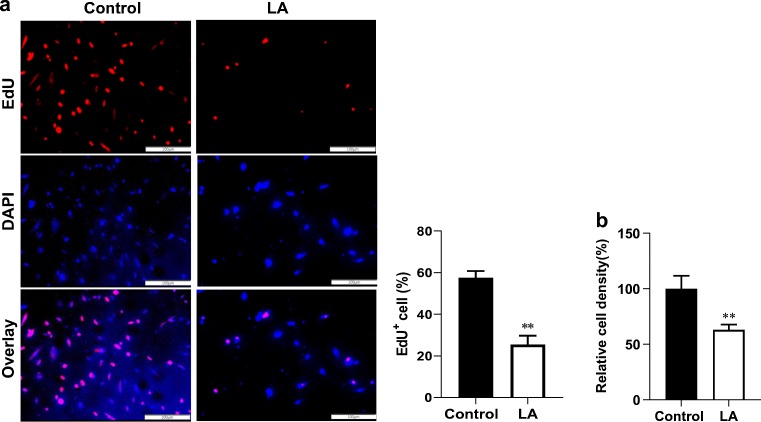
We subsequently examined the expression of C-Myc and Cyclin-D1, because they are important in regulating cell proliferation (Bretones et al. 2015). The results of real-time PCR showed that following treatment with LA (500 μM) for 48 h, C-myc mRNA level was significantly decreased by 20.0% compared with that in untreated control cells (P < 0.05, Fig. 1c). The mRNA levels of Cyclin-D1 showed no significant changes following LA treatment (Fig. 1d). To further illustrate the effect of LA on the proliferation of HUVECs, an EdU incorporative assay after was performed. As shown in Fig. 2a, after treatment with LA (500 μM) for 48 h, the EdU-positive cells decreased significantly by 55.7% compared with the control cells (P < 0.01). We also measured the effect of LA on HUVEC numbers. Following LA (500 μM) for 48 h, cell density was reduced by 36.9% compared with that of the control cells (P < 0.01, Fig. 2b).
Fig. 2.
LA decreased HUVEC proliferation. HUVECs were incubated with LA (500 μM) for 48 h. HUVEC proliferation alterations were assessed using cell counts and EdU labeling, then photographed with a fluorescence microscope at magnifications of × 100. Scale bar represents 200 μm. **P < 0.01 vs. untreated control cells, n = 3/group. LA, α-lipoic acid. Scale bar = 100 μM
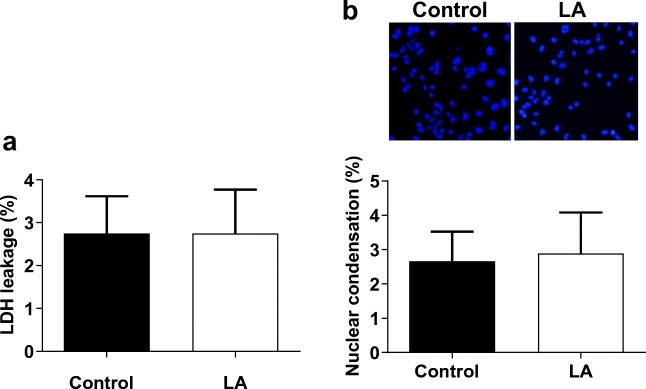
Considering that the decrease in cell viability could be attributable to either reduced proliferation or increased cell death, we investigated whether LA could induce cell injury. The LDH leakage, a marker of cell necrosis, from HUVECs into the medium was examined after treatment with LA (500 μM) for 48 h. As shown in Fig. 3a, no significant difference of LDH activities in the medium was detected between untreated control and LA-treated HUVECs. Also, the nuclear condensation, a generally used indicator of cell apoptosis, was examined by Hoechst33342 staining. We found that LA showed no significant effect on nuclear condensation compared with controls (Fig. 3b). Collectively, the data suggest that treatment with LA (500 μM) in HUVECs did not result in significant cell damage.
Fig. 3.
Effect of LA on HUVEC injury. a HUVECs were incubated with LA (500 μM) for 48 h. LDH activity in cells and culture medium were examined. The leakage of LDH was expressed as the percentage of medium LDH activity over total activities. n = 3/group. LA, α-lipoic acid; LDH, lactic acid dehydrogenase. b Following treatment with LA (500 μM) for 48 h, Hoechst33342 was stained in the HUVECs to examine nuclear condensation. n = 3/group. LA, α-lipoic acid
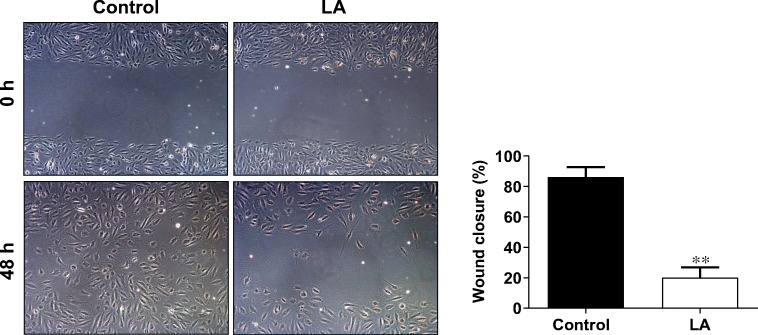
LA impedes wound closure of HUVECs
Migration of endothelial cells is another essential process for angiogenesis (Gau et al. 2017). To examine whether LA affects endothelial migration, we performed wound healing assay in HUVEC monolayers because this assay typically indicates cell migratory ability by the closure of a standard scratch in time. We found that at 48 h after scratch, the control cells without LA exposure exhibited 86.2% closure of the scratch wound (Fig. 4). However, the LA-treated cells only showed 20.0% closure of wound at 48 h after scratch, which was significantly lower than that in control cells (P < 0.01, Fig. 4), suggesting an inhibitory effect of LA on the migration of endothelial cells.
Fig. 4.
LA delayed wound closure. HUVEC layers were scratched and followed by LA (500 μM) treatment. The wound closure was measured at 48 h after scratch. **P < 0.01 vs. untreated control cells, n = 3/group. LA, α-lipoic acid
Effects of LA on the expression of HSP22, HSP32, and HSP70
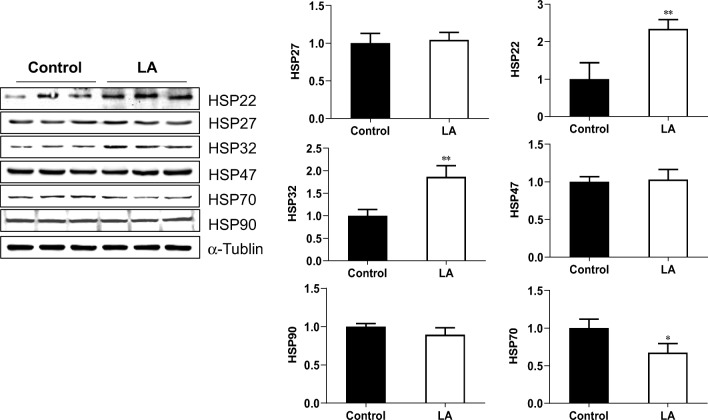
The HSPs encompass a group of structurally unrelated protein families (Saini and Sharma 2018; Vos et al. 2008). The results of immunoblotting showed that after incubation with LA (500 μM) for 48 h, HSP22 and HSP32 expressions were increased by 134.46% and 86.2%, respectively, whereas HSP70 expression was decreased by 32.7% in HUVECs, compared with that in the untreated controls (P < 0.05, P < 0.01; Fig. 5). HSP27, HSP47, and HSP90 remained unchanged following LA treatment.
Fig. 5.
Effect of LA on expression of heat shock proteins. HUVECs were incubated with LA (500 μM) for 48 h. Cells were collected for immunoblotting analysis of the indicated proteins. The blots for α-tubulin served as loading controls. **P < 0.01 and *P < 0.05 vs. untreated control cells, n = 3/group. LA, α-lipoic acid
LA decreases VEGF, eNOS, HSPA12B expression, and FAK phosphorylation
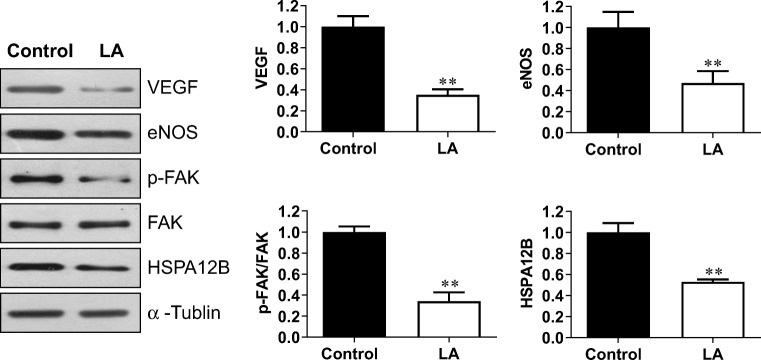
Endothelial cell proliferation is a network which is regulated by a group of signaling molecules. Among these, vascular endothelial growth factor (VEGF) and endothelial nitric oxide synthase (eNOS) have been shown to play critical roles in promoting endothelial cell proliferation for angiogenesis (Hu et al. 2006; Li et al. 2013). The results of immunoblotting demonstrated that following treatment with LA (500 μM) for 48 h, VEGF and eNOS protein expressions were decreased by 64.9% and 52.8% in HUVECs, respectively, compared with those in the untreated controls (P < 0.01, Fig. 6).
Fig. 6.
LA decreased VEGF, eNOS, and HSPA12B expression and FAK phosphorylation. HUVECs were incubated with LA (500 μM) for 48 h. Cells were collected for immunoblotting analysis of the indicated proteins. The blots for α-tubulin served as loading controls. **P < 0.01 vs. untreated control cells, n = 3–9/group. LA, α-lipoic acid
Interestingly, VEGF and eNOS also have been shown to play roles in endothelial migration through the activation of focal adhesion kinase (FAK) (Lee et al. 2010; Zhao et al. 2017). Consistent with the observation that LA decreased the migration of HUVECs, LA reduced the phosphorylation level of FAK by 66.0% compared with the control HUVECs (P < 0.01, Fig. 6). As a heat shock protein that significantly expressed in endothelial cells, HSPA12B has been shown to promote both proliferation and migration of endothelial cells for angiogenesis (Han et al. 2003; Hu et al. 2006; Li et al. 2013). Indeed, we found that compared with the control HUVECs, the LA-treated HUVECs showed significantly reduced expression of HSPA12B by 47.0% (P < 0.01, Fig. 6).
Overexpression of HSPA12B attenuates the LA-induced inhibition of VEGF expression
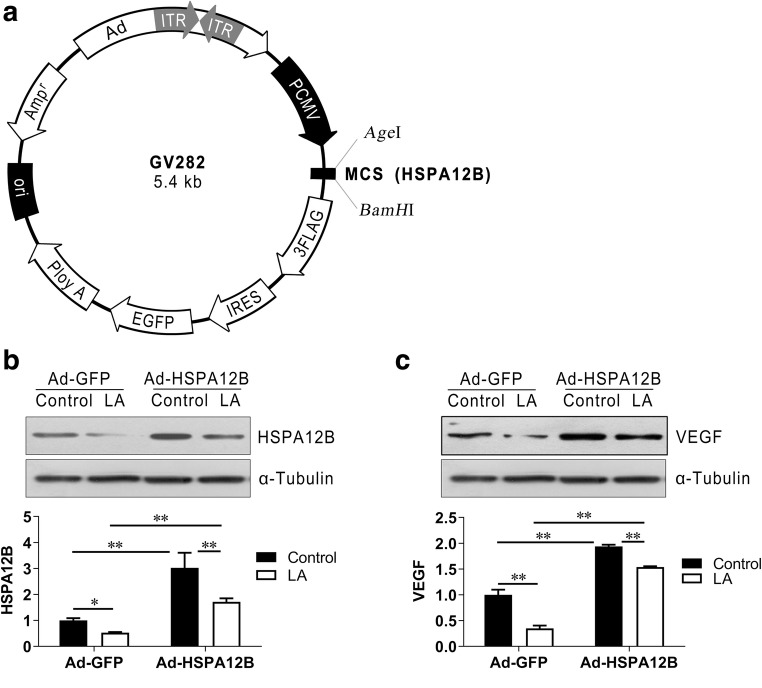
HSPA12B has been shown to play an important role in the regulation of endothelial proliferation and migration through modulating the expression of angiogenesis factors, such as VEGF (Han et al. 2003; Hu et al. 2006; Li et al. 2013). We then examined whether LA-induced VEGF downregulation was mediated by reduced HSPA12B expression. To this end, we overexpressed HSPA12B in HUVECs by infection with an adenovirus that carries HSPA12B expression sequence (Ad-HSPA12B, Fig. 7a). The HUVECs infected with empty adenovirus (Ad-GFP) served as controls. As shown in Fig. 7b, Ad-HSPA12B HUVECs showed significantly increased HSPA12B expression both in the presence and absence of LA, respectively, when compared with their Ad-GFP controls (P < 0.01). Notably, following overexpression of HSPA12B, VEGF protein expression levels were increased both in the presence and absence of LA, respectively, when compared with their Ad-GFP controls (Ad-HSPA12B/LA vs. Ad-GFP/LA, Ad-HSPA12B/Con vs. Ad-GFP/Con, P < 0.01; Fig. 7c). In addition, overexpression of HSPA12B attenuated the LA-induced downregulation of VEGF expression in HUVECs (Ad-HSPA12B/LA vs. Ad-GFP/LA, P < 0.01; Fig. 7c).
Fig. 7.
Overexpression of HSPA12B attenuated the inhibitory effects of LA on VEGF expression. HUVECs were infected with HSPA12B adenovirus (Ad-HSPA12B) or empty adenovirus (Ad-GFP). The scheme for adenovirus construction was shown (a). Twenty-four hours after infection, cells were incubated with LA (500 μM) for 48 h. Untreated cells served as controls (Con). After experiments, HUVECs were harvested for immunoblotting analysis against HSPA12B (b) or VEGF (c). The blots for α-tubulin served as loading controls. **P < 0.01 and *P < 0.05, n = 3–9/group
Overexpression of HSPA12B attenuates the LA-induced inhibition of HUVEC proliferation
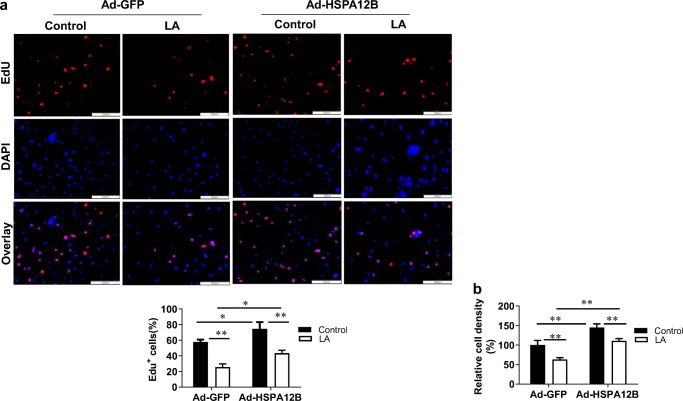
We performed an EdU incorporative assay to illustrate whether LA-induced inhibition of endothelial proliferation is mediated through the downregulation of HSPA12B. As shown in Fig. 8a, overexpression of HSPA12B in HUVECs increased EdU-positive cells compared with those in the Ad-GFP control HUVECs both in the presence and absence of LA treatment (Ad-HSPA12B/LA vs. Ad-GFP/LA, Ad-HSPA12B/Con vs. Ad-GFP/Con, P < 0.05), indicating that the LA-induced reduction of HUVEC proliferation was significantly attenuated by HSPA12B overexpression. Consistently, compared with Ad-GFP HUVECs, Ad-HSPA12B HUVECs showed an increase in relative cell density both in the presence and absence of LA treatment (Ad-HSPA12B/LA vs. Ad-GFP/LA, Ad-HSPA12B/Con vs. Ad-GFP/Con, P < 0.01 or 0.05) (Fig. 8b). To support these results, MTT assay revealed that the viability of HUVECs increased following overexpression of HSPA12B (Ad-HSPA12B/Con vs. Ad-GFP/Con, P < 0.01) (Fig. 9). However, LA inhibited the HUVEC viability by 30.6% and 32.3% with and without HSPA12B overexpression, compared with untreated controls (Ad-HSPA12B/LA vs. Ad-HSPA12B/Con, Ad-GFP/LA vs. Ad-GFP/Con; P < 0.01). Our data all suggest that overexpression of HSPA12B could significantly attenuate the inhibitory effect of LA on HUVEC proliferation.
Fig. 8.
Overexpression of HSPA12B attenuated the LA-induced inhibition of viable HUVECs. HUVECs were infected with HSPA12B adenovirus (Ad-HSPA12B) or empty adenovirus (Ad-GFP). Twenty-four hours later, cells were incubated with LA (500 μM) for 48 h. Untreated cells served as controls. HUVEC proliferation alterations were assessed using cell counts and EdU labeling, then photographed with a fluorescence microscope at magnification of × 100. Scale bar represents 200 μm. **P < 0.01 vs. untreated control cells, n = 3/group. LA, α-lipoic acid. **P < 0.01 or *P < 0.05, n = 3/group. LA, α-lipoic acid. Scale bar = 100 μM
Fig. 9.
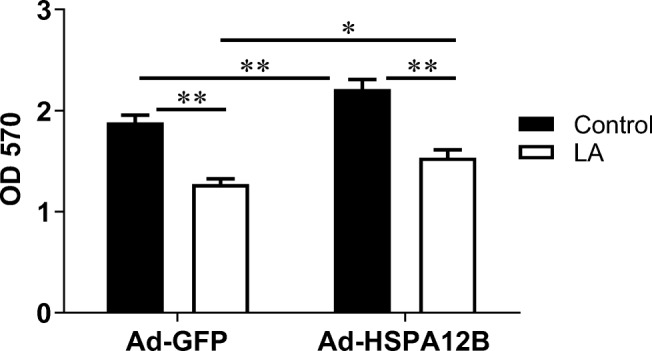
Overexpression of HSPA12B attenuated the LA-induced inhibition of proliferation. HUVECs were infected with HSPA12B adenovirus (Ad-HSPA12B) or empty adenovirus (Ad-GFP). Twenty-four hours later, cells were incubated with LA (500 μM) for 48 h. Untreated cells served as controls. MTT assay was performed subsequently. **P < 0.01 or *P < 0.05, n = 3/group. LA, α-lipoic acid
Overexpression of HSPA12B attenuates the LA-induced inhibition of HUVEC migration
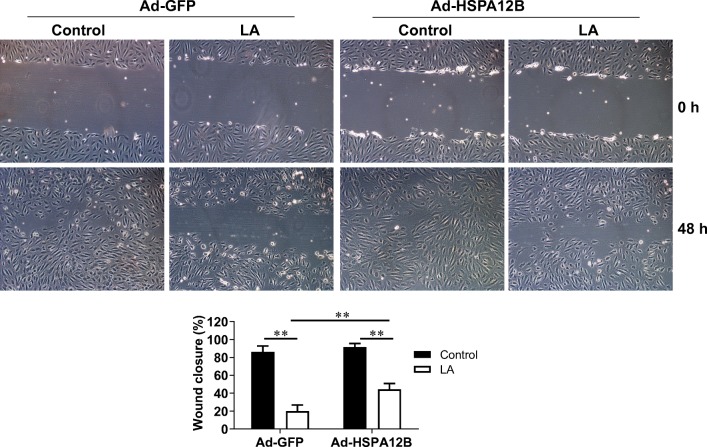
Finally, we determined whether LA-induced inhibition of endothelial migration was mediated by HSPA12B downregulation. As shown in Fig. 10, Ad-HSPA12B HUVECs showed faster wound closure than Ad-GFP HUVECs in the presence of LA (Ad-HSPA12B/LA vs. Ad-GFP/LA, P < 0.01). However, the LA-induced inhibition of migration was only partially reversed by HSPA12B overexpression (Ad-HSPA12B/LA vs. Ad-HSPA12B/Con, P < 0.01).
Fig. 10.
Overexpression of HSPA12B attenuated the LA-induced inhibition of wound closure. HUVECs were infected with HSPA12B adenovirus (Ad-HSPA12B) or empty adenovirus (Ad-GFP). Twenty-four hours later, cells were scratched in the presence or absence of LA (500 μM). The wound closure was measured at 48 h after scratch. **P < 0.01 vs. untreated control cells, n = 3/group. LA, α-lipoic acid
Discussion
The significant finding of this study is that alpha-lipoic acid, a diet supplement that has been used as a licensed therapy for diabetic complications for more than 20 years (Dworacka et al. 2015; Nebbioso et al. 2013; Rochette et al. 2015), inhibited endothelial cell proliferation and migration. This action of LA was medicated, at least in part, through the downregulation of HSPA12B/VEGF signaling axis. Our findings put further insight into the use of LA in patients with appropriate indications.
The endothelial proliferation and migration are essential for angiogenesis as well as for the endothelial repair when it was damaged by intravascular examination or treatment. However, angiogenesis is a double-edged sword that depends on the differential pathological status. As examples, the promotion of angiogenesis promotes the repair of ischemic injuries, such as myocardial infarction and cerebral ischemic stroke (Li et al. 2013). By striking contrast, anti-angiogenic therapies have been shown to improve the survival of patients with variant cancers in clinical trials (Hellstrom et al. 2001; Yonenaga et al. 2005). Therefore, it is important for the targeted regulation of angiogenesis by the modulation of endothelial proliferation and migration. Interestingly, we found that LA exerted an inhibitory effect on the proliferation and migration in human vascular endothelial cells, suggesting that LA might have an anti-angiogenesis therapeutic potential for the related human diseases.
LA is a compound found in the human diet as a naturally occurring co-actor for metabolic enzymes and has been used as an antioxidant. Studies have shown that some antioxidants such as N-acetylcysteine and epigallocatechin-3-gallate (the main component of green tea extraction), could be ideal “vasopressor” drugs because they have been proved to effectively inhibit angiogenesis both in vivo and in vitro (Fassina et al. 2004; Ono et al. 2002; Steinritz et al. 2010). Interestingly, besides being a diet supplement, LA has been clinically used in treating symptomatic peripheral neuropathy, retinopathy, pain management, cardiac autonomic neuropathy, and other complications in diabetes patients for more than 20 years (Pop-Busui et al. 2013; Ziegler and Gries 1997). Moreover, oral administration with LA shows no obvious adverse effect and even to be safe in pregnant women (Parente et al. 2017; Rochette et al. 2015; van de Mark et al. 2003; Ziegler and Gries 1997). However, it has not been fully addressed whether endothelial proliferation and migration are involved in the LA-induced protection from the abovementioned disorders. Our findings on endothelial proliferation and migration suggest that anti-pathological angiogenesis might be the potential mechanism underlying these protective effects of LA.
To examine whether LA has an anti-angiogenesis therapeutic potential, we examined the effect of LA on endothelial proliferation and migration and found that LA could inhibit the cell proliferation and impede wound closure of HUVECs. Consistently with our results, other investigators found that treatment of antioxidants, such as LA, might have an anti-angiogenic effect, which in turn prevents the onset or progression of diabetic retinopathy (Lee et al. 2012; Obrosova et al. 2001). However, Coletta et al. (2015) reported a different result of LA on cerebral endothelial cells from ours. They studied the effect of LA on the ability of 3-MP, which could stimulate angiogenesis, cellular bioenergetics, and wound healing in hyperglycemia and diabetes, and concluded that therapy with H2S donors or treatment with the combination of 3-MP and lipoic acid may be beneficial in improving angiogenesis and bioenergetics in hyperglycemia, which is in disagreement with our results. These differential results of LA suggest that the effects of LA on endothelial activation and angiogenesis may depend on the subtype of endothelial cells, pathological stimulation, and dose and duration of LA treatment.
To address how LA inhibits endothelial proliferation and migration, we performed an in vitro analysis in endothelial proliferation and migration. We found that endothelial proliferation was significantly inhibited by LA treatment in both dose- and time-dependent manners. Moreover, we assessed endothelial migration by a monolayer wound healing assay and found that LA showed an inhibitory effect on the migration of endothelial cells. To further dissect the underlying mechanism of the anti-proliferative effect of LA, we examined the expression of C-Myc and Cyclin-D1. C-Myc is a multifunctional transcription factor that regulates various processes including development, differentiation, and proliferation. Intriguingly, the expression of C-Myc could be regulated by VEGF in endothelial cells (Steiner et al. 2009). Cyclin-D1 has been shown to promote cell cycle progression during the G1 phase, a key event in G1-S transition and plays critical roles in cell biology, including cell proliferation and growth regulation, mitochondrial activity modulation, DNA repair, and cell migration control (Budhiraja et al. 2018; Luo et al. 2019; Ramos-Garcia et al. 2017). Indeed, we found that C-myc mRNA level was significantly decreased whereas Cyclin-d1 mRNA level remained unchanged in endothelial cells following LA treatment, suggesting that the downregulated C-Myc expression might be involved in the LA-induced inhibition of endothelial proliferation and migration.
HSPA12B is a recently identified proangiogenic regulator. As an endothelial cell-specifically expressed heat shock protein, we and others have shown that HSPA12B is required for endothelial cell proliferation and angiogenesis in the late phase of cardiac and cerebral infarction (Li et al. 2013; Zhao et al. 2018). Also, HSPA12B is essential for the vascular development of zebrafish (Hu et al. 2006). These actions of HSPA12B are mediated by increasing the expression of VEGF, which has been shown to play essential roles in modulating endothelial behaviors including proliferation, migration, and inflammation (Ma et al. 2015). In this study, we found that compared with the control HUVECs, the LA-treated HUVECs showed significantly reduced expression of HSPA12B and VEGF. To investigate whether LA-induced VEGF downregulation was mediated by reduced HSPA12B expression, we overexpressed HSPA12B in HUVECs and found that the decreased VEGF expression by LA could be partially reversed by HSPA12B overexpression. The data indicate that overexpression of HSPA12B in endothelial cells prevented the LA-induced loss of VEGF. More importantly, HSPA12B overexpression attenuated the LA-induced inhibition of endothelial proliferation and migration.
In summary, the data suggest that LA inhibited endothelial cell proliferation and migration. These actions were mediated, at least in part, through the downregulation of the HSPA12B/VEGF signaling axis. The data suggest that besides the treatment in diabetic complications, LA might represent a viable therapeutic potential for human diseases that involve high angiogenic activities such as cancers. However, LA might be harmful for the diseases that need angiogenesis for repair such as myocardial infarction. Our data put further insight into the indication/contraindication of LA when it is employed in anti-diabetic complications treatment.
Funding information
This work was supported by the National Natural Science Foundation of China (81870234 and 81770854), by Jiangsu Province’s Outstanding Medical Academic Leader program (LJ201124), and a project funded by Collaborative Innovation Center for Cardiovascular Disease Translational Medicine.
Compliance with ethical standards
The Ethical Board of the First Affiliated Hospital of Nanjing Medical University approved these studies (#2012-SR-153). All the human study procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaofei Cao, Email: xiaofei_cao@njmu.edu.cn.
Zhengnian Ding, Email: zhengnianding@njmu.edu.cn.
References
- Bretones G, Delgado MD, Leon J. Myc and cell cycle control. Biochim Biophys Acta. 2015;1849:506–516. doi: 10.1016/j.bbagrm.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Budhiraja G, Sahu N, Subramanian A. Low-intensity ultrasound upregulates the expression of Cyclin-D1 and promotes cellular proliferation in human mesenchymal stem cells. Biotechnol J. 2018;13:e1700382. doi: 10.1002/biot.201700382. [DOI] [PubMed] [Google Scholar]
- Coletta C, et al. Regulation of vascular tone, angiogenesis and cellular bioenergetics by the 3-mercaptopyruvate sulfurtransferase/H2S pathway: functional impairment by hyperglycemia and restoration by DL-α-lipoic acid. Mol Med. 2015;21:1–14. doi: 10.2119/molmed.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworacka M, Iskakova S, Krzyzagorska E, Wesolowska A, Kurmambayev Y, Dworacki G. Alpha-lipoic acid modifies circulating angiogenic factors in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2015;107:273–279. doi: 10.1016/j.diabres.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Fassina G, Vene R, Morini M, Minghelli S, Benelli R, Noonan DM, Albini A. Mechanisms of inhibition of tumor angiogenesis and vascular tumor growth by epigallocatechin-3-gallate. Clin Cancer Res. 2004;10:4865–4873. doi: 10.1158/1078-0432.CCR-03-0672. [DOI] [PubMed] [Google Scholar]
- Gau D, Veon W, Capasso TL, Bottcher R, Shroff S, Roman BL, Roy P. Pharmacological intervention of MKL/SRF signaling by CCG-1423 impedes endothelial cell migration and angiogenesis. Angiogenesis. 2017;20:663–672. doi: 10.1007/s10456-017-9560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Truong QA, Park S, Breslow JL. Two Hsp70 family members expressed in atherosclerotic lesions. Proc Natl Acad Sci U S A. 2003;100:1256–1261. doi: 10.1073/pnas.252764399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, et al. A novel endothelial-specific heat shock protein HspA12B is required in both zebrafish development and endothelial functions in vitro. J Cell Sci. 2006;119:4117–4126. doi: 10.1242/jcs.03179. [DOI] [PubMed] [Google Scholar]
- Kong Q, et al. HSPA12B attenuated acute myocardial ischemia/reperfusion injury via maintaining endothelial integrity in a PI3K/Akt/mTOR-dependent mechanism. Sci Rep. 2016;6:33636. doi: 10.1038/srep33636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Lee YJ, Song CH, Ahn YK, Han HJ. Role of FAK phosphorylation in hypoxia-induced hMSCS migration: involvement of VEGF as well as MAPKS and eNOS pathways. Am J Phys Cell Phys. 2010;298:C847–C856. doi: 10.1152/ajpcell.00418.2009. [DOI] [PubMed] [Google Scholar]
- Lee SG, Lee CG, Yun IH, Hur DY, Yang JW, Kim HW. Effect of lipoic acid on expression of angiogenic factors in diabetic rat retina. Clin Exp Ophthalmol. 2012;40:e47–e57. doi: 10.1111/j.1442-9071.2011.02695.x. [DOI] [PubMed] [Google Scholar]
- Li J, et al. HSPA12B attenuates cardiac dysfunction and remodelling after myocardial infarction through an eNOS-dependent mechanism. Cardiovasc Res. 2013;99:674–684. doi: 10.1093/cvr/cvt139. [DOI] [PubMed] [Google Scholar]
- Luo W, et al. C-Myc inhibits myoblast differentiation and promotes myoblast proliferation and muscle fibre hypertrophy by regulating the expression of its target genes, miRNAs and lincRNAs. Cell Death Differ. 2019;26:426–442. doi: 10.1038/s41418-018-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, et al. Dexamethasone attenuated bupivacaine-induced neuron injury in vitro through a threonine-serine protein kinase B-dependent mechanism. Neuroscience. 2010;167:329–342. doi: 10.1016/j.neuroscience.2009.12.049. [DOI] [PubMed] [Google Scholar]
- Ma H, et al. HSPA12B: a novel facilitator of lung tumor growth. Oncotarget. 2015;6:9924–9936. doi: 10.18632/oncotarget.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebbioso M, Pranno F, Pescosolido N. Lipoic acid in animal models and clinical use in diabetic retinopathy. Expert Opin Pharmacother. 2013;14:1829–1838. doi: 10.1517/14656566.2013.813483. [DOI] [PubMed] [Google Scholar]
- Nikitenko L, Boshoff C (2006) Endothelial cells and cancer. Handb Exp Pharmacol 307–334 [DOI] [PubMed]
- Obrosova IG, et al. Antioxidants attenuate early up regulation of retinal vascular endothelial growth factor in streptozotocin-diabetic rats. Diabetologia. 2001;44:1102–1110. doi: 10.1007/s001250100631. [DOI] [PubMed] [Google Scholar]
- Okonkwo UA, DiPietro LA (2017) Diabetes and Wound Angiogenesis. Int J Mol Sci 18. 10.3390/ijms18071419 [DOI] [PMC free article] [PubMed]
- Ono K, et al. Periodate-treated, non-anticoagulant heparin-carrying polystyrene (NAC-HCPS) affects angiogenesis and inhibits subcutaneous induced tumour growth and metastasis to the lung. Br J Cancer. 2002;86:1803–1812. doi: 10.1038/sj.bjc.6600307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente E, Colannino G, Picconi O, Monastra G. Safety of oral alpha-lipoic acid treatment in pregnant women: a retrospective observational study. Eur Rev Med Pharmacol Sci. 2017;21:4219–4227. [PubMed] [Google Scholar]
- Pop-Busui R, et al. Effects of triple antioxidant therapy on measures of cardiovascular autonomic neuropathy and on myocardial blood flow in type 1 diabetes: a randomised controlled trial. Diabetologia. 2013;56:1835–1844. doi: 10.1007/s00125-013-2942-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Garcia P, Gil-Montoya JA, Scully C, Ayen A, Gonzalez-Ruiz L, Navarro-Trivino FJ, Gonzalez-Moles MA. An update on the implications of cyclin D1 in oral carcinogenesis. Oral Dis. 2017;23:897–912. doi: 10.1111/odi.12620. [DOI] [PubMed] [Google Scholar]
- Rochette L, Ghibu S, Muresan A, Vergely C. Alpha-lipoic acid: molecular mechanisms and therapeutic potential in diabetes. Can J Physiol Pharmacol. 2015;93:1021–1027. doi: 10.1139/cjpp-2014-0353. [DOI] [PubMed] [Google Scholar]
- Saini J, Sharma PK. Clinical, Prognostic and therapeutic significance of heat shock proteins in cancer. Curr Drug Targets. 2018;19:1478–1490. doi: 10.2174/1389450118666170823121248. [DOI] [PubMed] [Google Scholar]
- Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104:236–244. doi: 10.1161/CIRCRESAHA.108.182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinritz D, et al. Effect of N-acetyl cysteine and alpha-linolenic acid on sulfur mustard caused impairment of in vitro endothelial tube formation. Toxicol Sci. 2010;118:521–529. doi: 10.1093/toxsci/kfq271. [DOI] [PubMed] [Google Scholar]
- van de Mark K, Chen JS, Steliou K, Perrine SP, Faller DV. Alpha-lipoic acid induces p27Kip-dependent cell cycle arrest in non-transformed cell lines and apoptosis in tumor cell lines. J Cell Physiol. 2003;194:325–340. doi: 10.1002/jcp.10205. [DOI] [PubMed] [Google Scholar]
- Vos MJ, Hageman J, Carra S, Kampinga HH. Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry. 2008;47:7001–7011. doi: 10.1021/bi800639z. [DOI] [PubMed] [Google Scholar]
- Wu J, Li X, Huang L, Jiang S, Tu F, Zhang X, Ma H, Li R, Li C, Li Y, Ding Z, Liu L. HSPA12B inhibits lipopolysaccharide-induced inflammatory response in human umbilical vein endothelial cells. J Cell Mol Med. 2015;19:544–554. doi: 10.1111/jcmm.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, et al. Alpha-lipoic acid increases tolerance of cardiomyoblasts to glucose/glucose oxidase-induced injury via ROS-dependent ERK1/2 activation. Biochim Biophys Acta. 2012;1823:920–929. doi: 10.1016/j.bbamcr.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Yonenaga Y, et al. Absence of smooth muscle actin-positive pericyte coverage of tumor vessels correlates with hematogenous metastasis and prognosis of colorectal cancer patients. Oncology. 2005;69:159–166. doi: 10.1159/000087840. [DOI] [PubMed] [Google Scholar]
- Zhao LN, et al. MiR-383 inhibits proliferation, migration and angiogenesis of glioma-exposed endothelial cells in vitro via VEGF-mediated FAK and Src signaling pathways. Cell Signal. 2017;30:142–153. doi: 10.1016/j.cellsig.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Zhao Y, et al. HSPA12B promotes functional recovery after ischaemic stroke through an eNOS-dependent mechanism. J Cell Mol Med. 2018;22:2252–2262. doi: 10.1111/jcmm.13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler D, Gries FA. Alpha-lipoic acid in the treatment of diabetic peripheral and cardiac autonomic neuropathy. Diabetes. 1997;46(Suppl 2):S62–S66. doi: 10.2337/diab.46.2.S62. [DOI] [PubMed] [Google Scholar]