Abstract
Introduction
The evidence on efficacy of intravitreously administered Conbercept (IVC) monotherapy for diabetic macular degeneration was still limited.
Methods
A systematic review was conducted in November 2019 to summarize the current evidence on visual acuity (VA) changes with IVC monotherapy in the treatment of diabetic macular edema (DME) from Pubmed, ClinicalTrials.gov, EMbase, China National Knowledge Infrastructure (CNKI), Wanfang Database, Chin VIP Information (VIP), and Chinese Biomedical Database (CBM). Retrospective or prospective clinical studies which used IVC injection for the treatment of DME were included. Outcomes included in the analysis were change in best-corrected visual acuity (BCVA) and central macular thickness (CMT). A meta-regression was conducted to assess 1-year BCVA and CMT changes against numbers of injections.
Results
A total of 20 studies were included in current study. At 12-month follow-up, an overall increase of 0.67 logarithm of the minimum angle of resolution (logMAR) BCVA score [95% confidence interval (CI) 0.24–1.11; P = 0.003] and 1.03 Early Treatment Diabetic Retinopathy Study (ETDRS) letters (95% CI 0.69–1.38; P < 0.001) was shown with IVC injection compared to baseline. Decrease in CMT was 142.79 μm (95% CI 112.71–172.87; P < 0.001) compared to baseline. The meta-regression showed a significant increase in effect size between number of injections and 12-month logMAR BCVA scale change as well as CMT.
Conclusion
Our findings suggest improved VA and CMT outcomes during 1-year follow-up in patients with DME who underwent IVC monotherapy. Increased injection frequency demonstrates a significant trend with improved outcomes at 12 months.
Electronic supplementary material
The online version of this article (10.1007/s13300-020-00806-0) contains supplementary material, which is available to authorized users.
Keywords: Anti-VEGF therapy, Central macular thickness, Conbercept, Diabetic macular edema, Meta-analysis, Visual acuity
Key Summary Points
| Why carry out this study? |
| The clinical efficacy of intravitreously administered Conbercept (IVC) has been reported in some prospective and retrospective studies to date. However, the evidence on efficacy of IVC monotherapy for DME was still limited |
| What was the evidence-based efficacy of IVC monotherapy for DME? |
| What was learned from the study? |
| Our findings suggest improved VA and CMT outcomes during 1-year follow-up in patients with DME who underwent IVC monotherapy |
| Increased injection frequency demonstrates a significant trend with improved outcomes at 12 months |
| IVC monotherapy as the initial treatment might be a treatment option for DME |
Introduction
Currently, the prevalence of diabetes mellitus (DM) is rapidly increasing worldwide. Among the complications of diabetes, the most common diabetic eye disease is diabetic retinopathy (DR), and DR is also the most common cause of blindness in working-age populations in developed countries [1, 2]. Among patients with DR, visual function can be severely damaged by diabetic macular edema (DME), which is one of the most common complications of DR and significantly affects the quality of life among patients with diabetes [3].
DME is caused by the destruction of the internal and external barrier functions of the retinal blood vessels, leading to the extravasation of fluid and lipoproteins into the macular area [3]. Vascular endothelial growth factor (VEGF) has been shown to be the key promoter of damage to the blood-retinal barrier by increasing vascular permeability, leakage of retinal microvessels, and accumulation of retinal fluid in the macular region, which is currently the main pathogenesis of DME [4, 5]. Hence, the treatment strategy for DME has focused on anti-VEGF therapy, and intravitreal injection with an anti-VEGF agent has emerged as the first-line therapy for DME. There are a variety of anti-VEGF agents in the clinic such as bevacizumab (Avastin®) and ranibizumab (Lucentis®), which have been found to bind VEGFA only, or both; in addition, aflibercept (Eylea®), which is composed of the second domain of human VEGF receptor (VEGFR)-1 and the third domain of VEGFR-2, fused to the Fc domain of human immunoglobulin 1 (IgG1).
Conbercept (KH902; Chengdu Kanghong Biotech Co., Ltd., Sichuan, China), as a recent novel VEGF antagonist, is a 143-kDa humanized recombinant anti-VEGF fusion protein, consisting of extracellular domain 2 of VEGFR-1 and domains 3 and 4 of VEGFR-2, which bind to the Fc domain of human IgG1, and is a soluble receptor decoy that blocks all isoforms of VEGFA, VEGFB, VEGFC, and placenta growth factor (PIGF) [6]. Conbercept can effectively antagonize the effects of VEGF. It has the advantages of multiple targets, strong affinity, and a long half-life in vitreous [7, 8]. Previous studies revealed that intravitreally administered Conbercept (IVC) could significantly improve the vision and reduce central macular thickness (CMT) of patients with DME [9, 10]. However, these studies were conducted in single centers with small sample sizes and the results have not been systematically collected, sorted, or evaluated. Recently, a meta-analysis was performed to evaluate the efficacy of intravitreally administered ranibizumab (IVR) and IVC in patients with DME [11]. To date, no systematic review has reported on the effect and safety of IVC monotherapy in patients with DME. We performed a systematic review and meta-analysis to quantify the effect of IVC monotherapy on best corrected visual acuity (BCVA) and CMT in patients with DME.
Methods
Our systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [12].
Compliance with Ethics Guidelines
The present study is based on previously reports and does not contain any studies with human participants or animals performed by any of the authors.
Search Strategy, Inclusion Criteria, Exclusion Criteria, and Data Collection
We performed a comprehensive systematic literature search using Pubmed, ClinicalTrials.gov, EMbase, Cochrane Library, China National Knowledge Infrastructure (CNKI), Chinese Biomedicine Literature Database (CBM-SinoMed), Chin VIP Information (VIP) Database for Chinese Technical Periodicals, and Wanfang Database for articles written in English or Chinese and published up to November 2019, and the references of all included studies were also traced. The search terms used were: “conbercept” OR “anti-VEGF” OR “anti-vascular endothelial growth factor” OR “Lumitin” OR “KH902” AND “diabetic macular edema” OR “diabetic macular oedema”. Two authors, TTN and JSG, assessed all eligible studies and data independently. A consensus was reached if there were any cases of disagreement.
The inclusion criteria were studies that (1) provided sufficient data for a comparison of pre- and post-treatment BCVA and CMT of patients with DME given IVC; (2) human studies available in English or Chinese.
The exclusion criteria were (1) patients with DME received more than one type of therapy rather than IVC separately; (2) no sufficient data was available on the variation change in BCVA or CMT, e.g., the mean, standard deviation, or standard error; (3) animal or cell research, non-original research (reviews, editorials, or comments), abstracts, unpublished studies, and duplicated studies.
The primary outcome of this systematic analysis focused on assessing the effect of IVC therapy on BCVA and CMT from baseline to 1, 3, 6, or 12 months of treatment for DME. Additional outcomes included the relationship between the number of IVC injections and change in outcome, as well as the complications and serious adverse events (SAEs). BCVA was obtained using the logarithm of the minimum angle of resolution (logMAR) and Early Treatment Diabetic Retinopathy Study (ETDRS). CMT was demonstrated on optical coherence tomography (OCT).
Data Extraction and Risk of Bias Assessment
The relevant data from the articles were extracted using a standard data extraction form. The extracted data included the first author(s), publication date, study design, sample size, age, sex, interventions details, and follow-up periods. The literature quality was evaluated by using the Jadad scores (ranging from 0 to 5) [13]. Studies with a score of at least 3 were considered to be “high quality” studies.
Statistical Analysis
The meta-analyses were performed using the DerSimonian–Laird random-effects method regardless of the amount of heterogeneity between studies. The standardized mean difference (SMD) or weighted mean difference (WMD) with a 95% confidence interval [CI] was used to assess continuous variable outcomes. Heterogeneity between studies was based on the size of the I2 value. Substantial heterogeneity was assumed if the I2 value was above 50%. Meta-regression was used to examine the relationship in the various studies between the number of injections, baseline CMT, and the change in outcomes. Some studies provided data on different follow-up subgroups, rather than the same follow-up time. For these studies, each subgroup was regarded as a separate study in all analyses. STATA 11.0 software was applied to integration analysis. A P value less than 0.05 was considered to be statistically significant.
Results
Study Selection
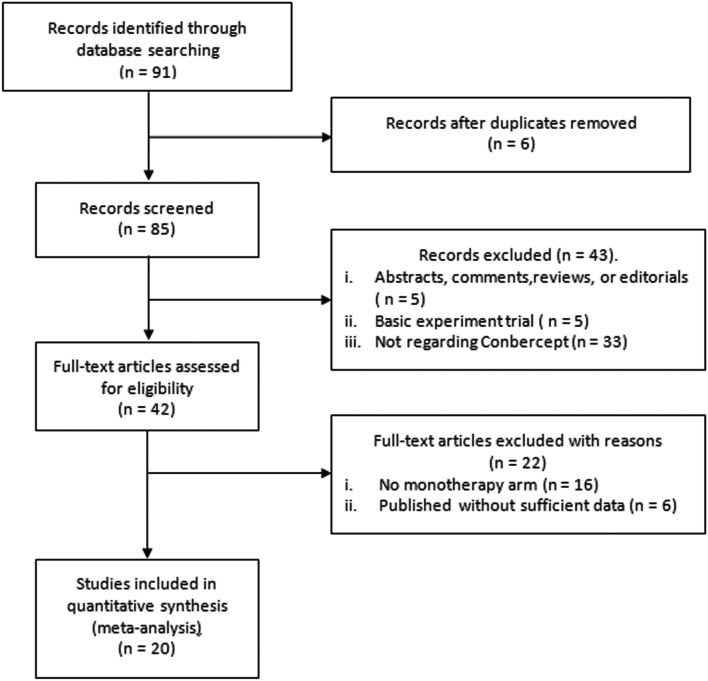
There were 91 articles identified for the initial review. After further examination of all files, 42 articles satisfied the available information on BCVA and/or CMT, then these files went through a full-text review. After excluding studies which were not original regarding articles, we included 20 studies [9, 10, 14–31] (1244 participants, 1278 eyes) in this meta-analysis. The selection of studies is shown in the PRISMA flow diagram in Fig. 1. The characteristics of the 20 included studies are provided in Table 1.
Fig. 1.
Eligibility of studies for inclusion in meta-analysis
Table 1.
Characteristics of including studies
| No. | Author (year) | Study design | DM type | DME type | Age (mean ± SD/range, years) | Male/female (N) | Patients (N) | Eyes (N) | No. of injections, mean (SD) | Treatment regimen | Jadad score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Liu et al. (2019) [14] | Retrospective | Type 2 | DME | 53.9 (34–69) | 10/3 | 13 | 26 | 3 | Monthly × 3 | 3 |
| 2 | Li et al. (2019) [15] | Retrospective | NA | DME | 50.5 ± 3.3 (44–62) | 24/26 | 50 | 50 | 3 | Monthly × 3 | 3 |
| 3 | Chang et al. (2016) [16] | Retrospective | NA | CSME | 49–62 | 11/10 | 21 | 22 | 3.05 | 1 + PRN | 3 |
| 4 | Jiang et al. (2017) [17] | Prospective | Type 2 | CSME/CME/DDME | 54.10 ± 10.65 | 12/8 | 20 | 20 | 3.20 ± 0.52 | 3 + PRN | 4 |
| 5 | Guo et al. (2018) [18] | Retrospective | NA | DME | 61.35 ± 7.58 | 28/27 | 55 | 55 | 3 | Monthly × 3 | 3 |
| 6 | Zhang et al. (2018) [19] | Prospective | NA | DDME | 51.88 ± 10.18 | 13/12 | 25 | 25 | 1 | Monthly × 1 | 4 |
| 7 | Sun et al. (2018) [20] | Prospective | NA | DME | 66.52 ± 8.39 | 13/16 | 29 | 30 | 3 | Monthly × 3 | 3 |
| 8 | Zhang et al. (2018) [21] | Prospective | NA | DDME | 51.90 ± 10.90 | NA | 17 | 17 | 3 | Monthly × 3 | 3 |
| 9 | Ren et al. (2019) [22] | Prospective | NA | DDME | 59.24 ± 8.61 | NA | 35 | 35 | 1 | Monthly × 1 | 3 |
| NA | CME | 62.51 ± 9.12 | 33 | 33 | 1 | Monthly × 1 | |||||
| NA | SRD | 60.86 ± 9.47 | 28 | 28 | 1 | Monthly × 1 | |||||
| 10 | Li et al. (2019) [23] | Retrospective | NA | DME | 54 ± 5 | 24/16 | 19 | 21 | 1 | Monthly × 1 | 4 |
| 11 | Zhang et al. (2019) [24] | Prospective | NA | DME | NA | 17/23 | 40 | 40 | 3 | Monthly × 3 | 3 |
| 12 | Xu et al. (2016) [25] | Retrospective | Type 1/Type 2 | DDME | 60.9 ± 12.9 | 12/14 | 26 | 31 | 5.6 ± 0.8 | 1 + PRN | 3 |
| 13 | Qian (2017) [26] | Retrospective | NA | DDME | NA | NA | 33 | 40 | 5.8 ± 1.7 | 1 + PRN | 3 |
| 14 | Xu et al. (2017) [27] | Retrospective | Type 1/Type 2 | CSME/DDME | 61.3 ± 14.9 | 18/14 | 32 | 36 | 6.6 ± 0.9 | 3 + PRN | 3 |
| 15 | Li et al. (2018) [28] | Retrospective | NA | DDME | NA | 28/46 | 20 | 20 | 3.20 ± 0.4 | 1 + PRN | 3 |
| CME | 36 | 36 | 3.39 ± 0.95 | 1 + PRN | |||||||
| SRD | 18 | 18 | 4.22 ± 1.55 | 1 + PRN | |||||||
| 16 | Li et al. (2017) [10] | Retrospective | Type 2 | DME | 59.8 ± 9.8 | NA | 37 | 37 | 6.74 | 1 + PRN | 3 |
| NA | DME | 54.4 ± 6.3 | NA | 25 | 25 | 6.5 | 1 + PRN | ||||
| 17 | Niu and Ji (2018) [29] | Prospective | Type 2 | DME | 52.8 ± 14.0 | 15/8 | 23 | 23 | 2 | 1 + PRN | 3 |
| 18 | Xu et al. (2019) [30] | Retrospective | NA | CSME | 60.55 ± 8.65 | 10/10 | 20 | 20 | 6.60 ± 3.02 | 3 + PRN | 3 |
| 19 | Zhou et al. (2019) [9] | Retrospective | NA | DME | 59.6 ± 8.5 | 46/43 | 60 | 60 | 4.5 ± 1.0 | 3 + PRN | 3 |
| 20 | Li et al. (2019) [31] | Prospective | NA | DME | 60.6 ± 12.3 | 19/13 | 32 | 32 | 8.58 ± 2.4 | 3 + PRN | 4 |
CSME clinical significant macular edema, CME cystoid macular edema, DME diabetic macular edema, DDME diffuse diabetic macular edema, SRD serous retinal detachment, PRN pro re nata, NA not applicable
Change in BCVA
Table 2 shows that compared with baseline data, logMAR BCVA scale was significantly improved at 1, 3, 6, and 12 months after IVC injection [SMD − 1.24 (− 2.09 to − 0.38, P < 0.001); − 2.30 (− 3.37 to − 1.23, P < 0.001); − 1.98 (− 2.88 to − 1.08, P < 0.001), and − 0.67 (− 1.11 to − 0.24, P < 0.001)]. Similar results were found to allow a quantitative synthesis that ETDRS BCVA scale was significantly improved at 1, 3, 6, 9, and 12 months after IVC injection [SMD 0.55 (0.11–0.98, P = 0.014); 0.56 (0.17–0.95, P = 0.005); 0.64 (0.31–0.97, P < 0.001); 0.51 (0.01–1.00, P = 0.046), and 1.03 (0.69–1.38, P < 0.001)]. There was still no evidence on the effects of IVC injection for DME with logMAR scale at 9 months and ETDRS scale at 2 months. Evidence of heterogeneity is shown in Table 2. A summary of the publication bias assessment using the Begg’s and Egger’s tests is provided in Table 3.
Table 2.
Pooled visual acuity changes in patients with DME by IVC injection during 1-year follow-up
| Subgroup restriction | Follow-up time | Number of studies | Visual testing scale | Pooled visual change (95% CI) | P | I2 (%) | Pheterogeneity |
|---|---|---|---|---|---|---|---|
| Retrospective | 1 month | 3 | logMAR | − 3.33 (− 5.83, − 0.84) | 0.009 | 97.2 | < 0.001 |
| Prospective | 1 month | 6 | logMAR | − 0.38 (− 0.90, 0.15) | 0.159 | 81.3 | < 0.001 |
| Overall pooling | 1 month | 9 | logMAR | − 1.24 (− 2.09, − 0.38) | 0.005 | 94.7 | < 0.001 |
| Retrospective | 2 months | 2 | logMAR | − 3.25 (− 8.07, 1.56) | 0.185 | 97.5 | < 0.001 |
| Prospective | 2 months | NA | logMAR | NA | NA | NA | NA |
| Overall pooling | 2 months | 2 | logMAR | − 3.25 (− 8.07, 1.56) | 0.185 | 97.5 | < 0.001 |
| Retrospective | 3 months | 4 | logMAR | − 6.09 (− 10.53, − 1.65) | 0.007 | 98.6 | < 0.001 |
| Prospective | 3 months | 9 | logMAR | − 0.77 (− 1.38, − 0.17) | 0.012 | 89.3 | < 0.001 |
| Overall pooling | 3 months | 13 | logMAR | − 2.30 (− 3.37, − 1.23) | < 0.001 | 97 | < 0.001 |
| Retrospective | 6 months | 3 | logMAR | − 8.58 (− 17.50, 0.34) | 0.059 | 99.2 | < 0.001 |
| Prospective | 6 months | 10 | logMAR | − 0.72 (− 1.29, − 0.15) | 0.013 | 89.3 | < 0.001 |
| Overall pooling | 6 months | 13 | logMAR | − 1.98 (− 2.88, − 1.08) | < 0.001 | 96.4 | < 0.001 |
| Retrospective | 9 months | NA | logMAR | NA | NA | NA | NA |
| Prospective | 9 months | NA | logMAR | NA | NA | NA | NA |
| Overall pooling | 9 months | NA | logMAR | NA | NA | NA | NA |
| Retrospective | 12 months | 1 | logMAR | − 0.33 (− 0.68, 0.033) | 0.075 | NA | NA |
| Prospective | 12 months | 4 | logMAR | − 0.79 (− 1.33, − 0.24) | 0.005 | 69.5 | 0.02 |
| Overall pooling | 12 months | 5 | logMAR | − 0.67 (− 1.11, − 0.24) | 0.003 | 69.5 | 0.01 |
| Retrospective | 1 month | 1 | ETDRS | 0.48 (− 0.116, 1.08) | 0.114 | NA | NA |
| Prospective | 1 month | 1 | ETDRS | 0.62 (− 0.02, 1.25) | 0.058 | NA | NA |
| Overall pooling | 1 month | 2 | ETDRS | 0.55 (0.11, 0.98) | 0.014 | 0 | 0.768 |
| Retrospective | 2 months | NA | ETDRS | NA | NA | NA | NA |
| Prospective | 2 months | NA | ETDRS | NA | NA | NA | NA |
| Overall pooling | 2 months | NA | ETDRS | NA | NA | NA | NA |
| Retrospective | 3 months | NA | ETDRS | NA | NA | NA | NA |
| Prospective | 3 months | 2 | ETDRS | 0.56 (0.17, 0.95) | 0.005 | 0 | 0.374 |
| Overall pooling | 3 months | 2 | ETDRS | 0.56 (0.17, 0.95) | 0.005 | 0 | 0.374 |
| Retrospective | 6 months | 1 | ETDRS | 0.63 (0.03, 1.23) | 0.041 | NA | NA |
| Prospective | 6 months | 2 | ETDRS | 0.64 (0.25, 1.04) | 0.001 | 0 | 0.356 |
| Overall pooling | 6 months | 3 | ETDRS | 0.64 (0.31, 0.97) | < 0.001 | 0 | 0.653 |
| Retrospective | 9 months | 0 | ETDRS | NA | NA | NA | NA |
| Prospective | 9 months | 1 | ETDRS | 0.51 (0.01, 1.00) | 0.046 | NA | NA |
| Overall pooling | 9 months | 1 | ETDRS | 0.51 (0.01, 1.00) | 0.046 | NA | NA |
| Retrospective | 12 months | 6 | ETDRS | 1.12 (0.77, 1.48) | < 0.001 | 61.4 | 0.024 |
| Prospective | 12 months | 1 | ETDRS | 0.51 (0.01, 1.01) | 0.044 | NA | NA |
| Overall pooling | 12 months | 7 | ETDRS | 1.03 (0.69, 1.38) | < 0.001 | 61.4 | 0.008 |
DME diabetic macular edema, ETDRS Early Treatment Diabetic Retinopathy Study, IVC intravitreally administered Conbercept, logMAR logarithm of the minimum angle of resolution, NA not applicable (no analysis performed because of an insufficient number of studies providing data), CI confidence interval
Table 3.
Overall publication bias testing by Begg’s test and Egger’s test
| Items | Follow-up time | Begg’s test | Egger’s test |
|---|---|---|---|
| logMAR BCVA scale | 1 month | 0.677 | 0.344 |
| 2 months | NA | 0.317 | |
| 3 months | 0.11 | 0.016 | |
| 6 months | 0.18 | 0.04 | |
| 9 months | NA | NA | |
| 12 months | 0.14 | 0.74 | |
| ETDRS BCVA scale | 1 month | 0.32 | NA |
| 2 months | NA | NA | |
| 3 months | 0.32 | NA | |
| 6 months | 0.12 | 0.36 | |
| 9 months | NA | NA | |
| 12 months | 0.65 | 0.11 | |
| CMT | 1 month | 0.48 | 0.049 |
| 2 months | 0.32 | NA | |
| 3 months | 0.24 | < 0.001 | |
| 6 months | 0.21 | 0.04 | |
| 9 months | NA | NA | |
| 12 months | 0.17 | 0.28 |
ETDRS Early Treatment Diabetic Retinopathy Study, logMAR logarithm of the minimum angle of resolution, CMT central macular thickness, NA not applicable (no analysis performed because of an insufficient number of studies providing data)
Change in CMT
The pooled results of the overall and subgroup by study design effects of IVC on changes in CMT at 1, 2, 3, 6, 9, and 12 months are shown in Table 4. The results from the various studies suggest that the overall CMT decrease was 141.48 µm (89.81–193.15, P < 0.001) at 1 month, 287.02 µm (251.71–322.34, P < 0.001) at 2 months, 182.70 µm (150.44–214.96, P < 0.001) at 3 months, 219.53 µm (165.33–273.73, P < 0.001) at 6 months, 113.40 µm (53.27–173.53, P < 0.001) at 9 months, and 142.79 µm (112.71–172.87, P < 0.001) at 12 months. Evidence of heterogeneity is shown in Table 4. A summary of the publication bias assessment using the Begg’s and Egger’s test is provided in Table 3.
Table 4.
Pooled central macular thickness changes in patients with DME by IVC injection during 1-year follow-up
| Subgroup restriction | Follow-up time | Number of studies | Pooled visual change (95% CI) | P | I2 (%) | Pheterogeneity |
|---|---|---|---|---|---|---|
| Retrospective | 1 month | 5 | − 213.92 (− 295.78, − 132.06) | < 0.001 | 92.3 | < 0.001 |
| Prospective | 1 month | 6 | − 76.565 (− 97.29, − 55.84) | < 0.001 | 20.4 | 0.28 |
| Overall pooling | 1 month | 11 | − 141.48 (− 193.15, − 89.81) | < 0.001 | 93.1 | < 0.001 |
| Retrospective | 2 months | 2 | − 287.02 (− 322.34, − 251.71) | < 0.001 | 0 | 0.951 |
| Prospective | 2 months | NA | NA | NA | NA | NA |
| Overall pooling | 2 months | 2 | − 287.02 (− 322.34, − 251.71) | < 0.001 | 0 | 0.951 |
| Retrospective | 3 months | 5 | − 212.22 (− 289.28, − 135.16) | < 0.001 | 97.7 | < 0.001 |
| Prospective | 3 months | 11 | − 168.80 (− 202.89, − 134.71) | < 0.001 | 84.5 | < 0.001 |
| Overall pooling | 3 months | 16 | − 182.70 (− 214.96, − 150.44) | < 0.001 | 94.2 | < 0.001 |
| Retrospective | 6 months | 5 | − 223.29 (− 330.63, − 115.95) | < 0.001 | 97.5 | < 0.001 |
| Prospective | 6 months | 11 | − 217.36 (− 278.02, − 156.71) | < 0.001 | 95.4 | < 0.001 |
| Overall pooling | 6 months | 16 | − 219.53 (− 273.73, − 165.33) | < 0.001 | 96.6 | < 0.001 |
| Retrospective | 9 months | NA | NA | NA | NA | NA |
| Prospective | 9 months | 1 | − 113.40 (− 173.53, − 53.27) | < 0.001 | NA | NA |
| Overall pooling | 9 months | 1 | − 113.40 (− 173.53, − 53.27) | < 0.001 | NA | NA |
| Retrospective | 12 months | 6 | − 160.82 (− 200.14, − 121.50) | < 0.001 | 77.6 | < 0.001 |
| Prospective | 12 months | 6 | − 123.89 (− 165.55, − 82.23) | < 0.001 | 79.0 | < 0.001 |
| Overall pooling | 12 months | 12 | − 142.79 (− 172.87, − 112.71) | < 0.001 | 80.8 | < 0.001 |
DME diabetic macular edema, IVC intravitreally administered Conbercept, NA not applicable (no analysis performed because of an insufficient number of studies providing data), CI confidence interval
Injection Frequency and BCVA Outcomes
Table 5 shows meta-regression results on the number of IVC injections for logMAR and ETDRS BCVA scale gain at 1, 3, 6, 9, and 12 months, which suggested a significant relationship with change in logMAR BCVA score at 12 months (Fig. S1 in the electronic supplementary material). An increase of one injection was associated with an increase of 1.19 (0.34–2.35, P = 0.04) logMAR BCVA score. However, a greater number of injections was not associated with a significant change in ETDRS BCVA scale at either 6 or 12 months. Insufficient data were available to properly evaluate this relationship at either 2 or 9 months for logMAR BCVA scale and at 1, 2, 3, or 9 months for ETDRS BCVA scale.
Table 5.
Meta-regression results for association between number of injections and visual acuity gain
| Visual acuity testing scale | Follow-up time | Number of studies | Coefficient (95% CI) | P | Adjusted R2 |
|---|---|---|---|---|---|
| logMAR | 1 month | 9 | − 0.16 (− 2.01, 1.68) | 0.84 | 17.09% |
| 2 months | NA | NA | NA | NA | |
| 3 months | 13 | 0.01 (− 1.79, 1.81) | 0.99 | 9.61% | |
| 6 months | 13 | − 0.04 (− 2.66, 2.57) | 0.97 | 10.08% | |
| 9 months | NA | NA | NA | NA | |
| 12 months | 6 | − 1.19 (− 2.35, − 0.34) | 0.04 | 100% | |
| ETDRS | 1 month | NA | NA | NA | NA |
| 2 months | NA | NA | NA | NA | |
| 3 months | NA | NA | NA | NA | |
| 6 months | 3 | 0.05 (− 0.74, 0.84) | 0.6 | 0% | |
| 9 months | NA | NA | NA | NA | |
| 12 months | 7 | 0.13 (− 0.42, 0.69) | 0.56 | 20.79% |
ETDRS Early Treatment Diabetic Retinopathy Study, logMAR logarithm of the minimum angle of resolution, NA not applicable (no analysis performed because of an insufficient number of studies providing data), CI confidence interval
Injection Frequency and CMT Outcomes
The association between the number of IVC injections and the change in CMT within patients with DME at 1, 3, 6, and 12 months was examined by meta-regression (Table 6). The number of IVC injections had a significant impact on the change in CMT at 12 months, but the association was not deemed significant at 1, 3, or 6 months. An increase of one IVC injection was associated with a mean 20.26 µm (0.87–39.66, P = 0.04) decrease in CMT (Fig. S2 in the electronic supplementary material).
Table 6.
Meta-regression results for association between number of injections and CMT gain
| Covariable | Follow-up time | Number of studies | Coefficient (95% CI) | P | Adjusted R2 |
|---|---|---|---|---|---|
| CMT | 1 month | 11 | − 19.81 (− 81.77, 42.14) | 0.49 | 6.04% |
| 2 months | NA | NA | NA | NA | |
| 3 months | 16 | − 15.04 (− 55.96, 25.87) | 0.44 | 4.14% | |
| 6 months | 16 | − 3.78 (− 65.77, 58.19) | 0.89 | 7.41% | |
| 9 months | NA | NA | NA | NA | |
| 12 months | 12 | − 20.26 (− 39.66, − 0.87) | 0.04 | 36.85% |
CMT central macular thickness, NA not applicable (no analysis performed due to an insufficient number of studies providing data), CI confidence interval
Adverse Effects (AEs)
According to systematic review, patients with DME after IVC injection experienced ocular adverse events including conjunctival hemorrhage (n = 29), intraocular pressure increase (n = 7), transient anterior chamber inflammatory activity (n = 3), vitreous floaters (n = 1), vitreous hemorrhage (n = 1), and corneal abrasion (n = 1).
Discussion
In this systematic review and meta-analysis, a total of 20 papers involving 1244 patients with DME were included. After pooled analysis, we found that IVC monotherapy led to significant visual acuity and CMT improvement in the treatment of DME. A significant relationship was found between the number of IVC injections and change in logMAR BCVA scale and CMT at 12 months. In addition, no serious AEs caused by the IVC injection were found.
Conbercept has been produced by the expression system of Chinese hamster ovary (CHO) cells and combines placental growth factor (PIGF) and all isoforms of VEGFA as well as VEGFB. Conbercept is alike in structure to aflibercept (Eylea, Regeneron Pharmaceuticals, Eastview, NY, USA). Many single-center, small-sized clinical trials showed that IVC injection for treatment of DME is significantly better than baseline in improving vision. Additionally, Conbercept has received marketing authorization in May 2019 by the China State Food and Drug Administration (CFDA) for the therapy of DME. As far as clinical practice is concerned, some patients with DME who were nonresponsive to intravitreal ranibizumab and bevacizumab therapy were still able to undergo effective treatment with Conbercept [32]. Nonetheless, large-scale, standard, and stringently controlled clinical trials are necessary to confirm these findings.
Previously, clinical trials on intravitreal anti-VEGF agent monotherapy in patients with DME showed that the mean improvement in BCVA was 0.81 logMAR after 3 months of treatment [33], and 0.3–3.85 logMAR after 12 months of treatment [34–36]. Our analysis of IVC monotherapy showed a mean logMAR BCVA scale improvement in which the largest change (6.09 logMAR) occurred at 3 months after treatment among retrospective studies (n = 4), which was higher than that previously reported. For ETDRS BCVA scale, the mean improvement with anti-VEGF agents was reported to be in the range of 5–13 letters after 12 months of treatment [34, 37–39]. Our results found that the largest mean change of ETDRS BCVA score was 1.12 (0.77–1.48) after pooling retrospective studies (n = 6), which was below the lower ends of the range of previous reports.
Additionally, there was some significant heterogeneity between included studies. This discrepancy could have occurred as a result of the heterogeneity of patient characteristics such as age and disease severity, comorbidities, methods for diagnosis and evaluation, treatment doses and interval, and study design features [40–43].
In a real-life clinical practice study, after 12-month follow-up, both IVC and IVR injection achieved similar clinical efficacy in the treatment of DME. However, in comparison to the IVR arm, IVC showed a longer treatment interval and fewer injections were needed [27]. Currently, there is still no evidence on the correlation between trends of efficacy and the number of IVC injections for DME. Hence, our meta-regression results revealed that there is a correlation between the frequency of IVC injections and efficacy (i.e., visual acuity gain and CMT decrease). We found that an increase of one injection was associated with an increase of 1.19 logMAR BCVA score at 12-month follow-up. The improvement in vision persisted till 12 months after first injection and the response had a dose–effect relationship between number of injections and visual gain. As a strong predictor of anatomical and functional outcome, CMT provides a measure of retinal recovery after treatment. Notably, our meta-analysis showed a significant decrease in CMT in patients with DME who underwent IVC injection. A significant effect was observed at 12-month follow-up, when an increase of one injection was associated with a 20.26-µm decrease in CMT.
According to the AEs reports, IVC injection was safe and well tolerated in the clinic. The ocular AEs were typical of complications with intravitreal injections such as intraocular pressure increase, conjunctival hemorrhage, etc. There were no reports of systemic AEs. The true incidence of ocular or systemic AEs requires a large-scale, real-life trial or observation for further assessment.
A strength of this study was that is was the first meta-analysis to evaluate the effect and safety of IVC injection for patients with DME. However, there were some limitations in this study: (1) As Conbercept has not yet been approved outside China, only Chinese patients were enrolled; (2) there were different follow-up times for observation; (3) only English or Chinese publications were evaluated; (4) there are no unpublished results; thus, publication bias cannot be fully excluded; (5) as the heterogeneity between study results was significant, it could be regarded as a weakness of the study. Multicenter, large-sample, double-blind randomized controlled trials are still needed to verify our findings.
Conclusions
In summary, the current systematic review and meta-analysis revealed that IVC injection alone was effective in the treatment of DME during 1-year observation. An increase of one injection was associated with an increase of 1.19 logMAR BCVA score and a decrease of 20.26 µm in CMT at 12-month follow-up. The current systematic review and meta-analysis showed that IVC monotherapy had significant visual and CMT outcomes in the treatment of DME.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study and the rapid service fee were supported by the China Postdoctoral Science Foundation (no. 2019TQ0358; no. 2019M661162), LiaoNing Revitalization Talents Program (no. XLYC1807082), Shenyang Young and Middle-aged Science and Technology Innovation Talent Support Program (RC190146) and Bethune Lang Mu Young and Middle Ophthalmologist Fund. The funders had no involvement in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Heping Wang and Jiashu Guo contributed equally to this work.
Disclosures
Heping Wang, Jiashu Guo, Shanshan Tao, Xinyu Wang, Xinshu Liu, Tingting Li, Jue Wang, Xue Yang, Tongtong Niu, and Dongning Liu declare that they have no conflict of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.11993958.
Contributor Information
Tongtong Niu, Email: ntt15840566565@163.com.
Dongning Liu, Email: liudongning1976@126.com.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Ruta LM, Magliano DJ, Lemesurier R, Taylor HR, Zimmet PZ, Shaw JE. Prevalence of diabetic retinopathy in type 2 diabetes in developing and developed countries. Diabet Med. 2013;30(4):387–398. doi: 10.1111/dme.12119. [DOI] [PubMed] [Google Scholar]
- 3.Gundogan FC, Yolcu U, Akay F, Ilhan A, Ozge G, Uzun S. Diabetic macular edema. Pak J Med Sci. 2016;32(2):505–510. doi: 10.12669/pjms.322.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daruich A, Matet A, Moulin A, et al. Mechanisms of macular edema: beyond the surface. Prog Retin Eye Res. 2018;63:20–68. doi: 10.1016/j.preteyeres.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Cohen SR, Gardner TW. Diabetic retinopathy and diabetic macular edema. Dev Ophthalmol. 2016;55:137–146. doi: 10.1159/000438970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Xu G, Wang Y, et al. Safety and efficacy of Conbercept in neovascular age-related macular degeneration: results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology. 2014;121(9):1740–1747. doi: 10.1016/j.ophtha.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Lu X, Sun X. Profile of Conbercept in the treatment of neovascular age-related macular degeneration. Drug Des Dev Ther. 2015;9:2311–2320. doi: 10.2147/DDDT.S67536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang M, Zhang J, Yan M, Li H, Yang C, Yu D. Recombinant anti-vascular endothelial growth factor fusion protein efficiently suppresses choridal neovasularization in monkeys. Mol Vis. 2008;14:37–49. [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Q, Guo C, You A, Wang D, Wang W, Zhang X. One-year outcomes of novel VEGF decoy receptor therapy with intravitreal Conbercept in diabetic retinopathy-induced macular edema. Mol Vis. 2019;25:636–644. [PMC free article] [PubMed] [Google Scholar]
- 10.Li F, Zhang L, Wang Y, et al. One-year outcome of Conbercept therapy for diabetic macular edema. Curr Eye Res. 2018;43(2):218–223. doi: 10.1080/02713683.2017.1379542. [DOI] [PubMed] [Google Scholar]
- 11.Liu W-S, Li Y-J. Comparison of Conbercept and ranibizumab for the treatment efficacy of diabetic macular edema: a meta-analysis and systematic review. Int J Ophthalmol. 2019;12(9):1479–1486. doi: 10.18240/ijo.2019.09.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Wang D, Chen F, Zhang X. Hyperreflective foci in OCT image as a biomarker of poor prognosis in diabetic macular edema patients treating with Conbercept in China. BMC Ophthalmol. 2019;19(1):157. doi: 10.1186/s12886-019-1168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, Xiang Y, Mei Z. Efficacy of intravitreal injection of Conbercept in diabetic macular edema. Chin J Gerontol. 2019;39(21):5198–5201. [Google Scholar]
- 16.Chang L, Yuan M, Wei H, Xu Z. Efficacy and frequency of intravitreal injection of Conbercept combined with retinal laser photocoagulation for diabetic macular edema. Chin J Pract Ophthalmol. 2016;34(7):712–715. [Google Scholar]
- 17.Jiang L, Li J, Nie A-Q. Clinical observation of intravitreal injection of Conbercept treating diabetic macular edema. Int Eye Sci. 2017;17(6):1105–1107. [Google Scholar]
- 18.Guo Y, Hou L-T, Hu H-X, Ren Y-Z. Comparison of curative effects of intravitreal injection of ranibizumab and Conbercept in the treatment of diabetic macular edema. Int Eye Sci. 2018;18(1):59–62. [Google Scholar]
- 19.Zhang Z-D, Guo S, Li J, Shuai T-J, Zhu M-M, Piao T-H. Comparison of efficacy between Conbercept and ranibizumab for treatment of diffuse diabetic macular edema. Guangxi Med J. 2018;40(5):217–500. [Google Scholar]
- 20.Sun H, Ren H, Zhao J, Yu X. Therapeutic effects of traditional Chinese medicine combined with Conbercept on diabetic macular edema. China J Chin Ophthalmol. 2018;28(3):170–174. [Google Scholar]
- 21.Zhang Z-D, Guo S, Shuai T-J, Piao T-H. Effect of intravitreal injection of Conbercept on diabetic diffuse macular edema. Rec Adv Ophthalmol. 2018;38(1):69–72. [Google Scholar]
- 22.Ren H, Huang D-M, Guo C-L, He Q-M. Curative effect of intravitreal injection of Conbercept on macular edema in different types of OCT diabetes mellitus. Int Eye Sci. 2019;19(7):1166–1169. [Google Scholar]
- 23.Li J, Zhu Y, Zhang L-J, Yu W. Intravitreal injection of Conbercept combined with triamcinolone acetonide for diabetic macular edema. Int Eye Sci. 2019;19(03):430–433. [Google Scholar]
- 24.Zhang C, Yang L, Ge H. Clinical observation on diabetic macular edema treated by Tangmingzu prescription combined with Conbercept. China J Chin Ophthalmol. 2019;29(03):211–424. [Google Scholar]
- 25.Xu Y, Rong A, Bi Y, Xu W. Intravitreal Conbercept injection with and without grid laser photocoagulation in the treatment of diffuse diabetic macular edema in real-life clinical practice. J Ophthalmol. 2016;2016:2143082. doi: 10.1155/2016/2143082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian T. One-year outcomes of Conbercept therapy for diffuse diabetic macular edema. 2017 APVRS. poster:EX1-085.
- 27.Xu Y, Rong A, Xu W, Niu Y, Wang Z. Comparison of 12-month therapeutic effect of Conbercept and ranibizumab for diabetic macular edema: a real-life clinical practice study. BMC Ophthalmol. 2017;17(1):158. doi: 10.1186/s12886-017-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Meng X, Wang F, Fu Y. Clinical effect of Conbercept in treatment of diabetic macular edema with different optical coherence tomography patterns. J Precis Med. 2018;33(02):143–146. [Google Scholar]
- 29.Niu H, Ji A. Observation of the efficacy of intravitreal Conbercept combined with retinal photocoagulation on diabetic macular edema. Med J Chin PLA. 2018;43(3):268–270. [Google Scholar]
- 30.Xu Y, Qu Y, Suo Y, et al. Correlation of retinal layer changes with vision gain in diabetic macular edema during Conbercept treatment. BMC Ophthalmol. 2019;19(1):123. doi: 10.1186/s12886-019-1131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, Song Y, Ding Q. The effect of Conbercept combined with 577 nm subthreshold micropulse laser photocoaguIation on diabetic maeular edema. Chin J Ocul Fundus Dis. 2019;35(2):129–134. [Google Scholar]
- 32.Qiao G, Dong W-J, Dai Y, Jiang Z-H, Guo H-K. Diabetic macular edema in proliferative stage treated with anti-vascular endothelial growth factor agent and triamcinolone acetonide by laser-based strategies. Int J Ophthalmol. 2017;10(7):1113–1119. doi: 10.18240/ijo.2017.07.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forte R, Cennamo GL, Finelli M, et al. Intravitreal bevacizumab vs intravitreal triamcinolone combined with macular laser grid for diffuse diabetic macular oedema. Eye (Lond) 2010;24(8):1325–1330. doi: 10.1038/eye.2010.23. [DOI] [PubMed] [Google Scholar]
- 34.Korobelnik J-F, Daien V, Faure C, et al. Real-world outcomes following 12 months of intravitreal aflibercept monotherapy in patients with diabetic macular edema in France: results from the APOLLON study. Graefes Arch Clin Exp Ophthalmol. 2020;258(3):521–8. [DOI] [PubMed]
- 35.Herold TR, Langer J, Vounotrypidis E, Kernt M, Liegl R, Priglinger SG. 3-year-data of combined navigated laser photocoagulation (Navilas) and intravitreal ranibizumab compared to ranibizumab monotherapy in DME patients. PLoS One. 2018;13(8):e0202483. doi: 10.1371/journal.pone.0202483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kriechbaum K, Prager S, Mylonas G, et al. Intravitreal bevacizumab (Avastin) versus triamcinolone (Volon A) for treatment of diabetic macular edema: 1-year results. Eye (Lond) 2014;28(1):9–16. doi: 10.1038/eye.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33(11):2399–2405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 39.Prünte C, Fajnkuchen F, Mahmood S, et al. Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study. Br J Ophthalmol. 2016;100(6):787–795. doi: 10.1136/bjophthalmol-2015-307249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoneoka D, Henmi M. Clinical heterogeneity in random-effect meta-analysis: between-study boundary estimate problem. Stat Med. 2019;38(21):4131–4145. doi: 10.1002/sim.8289. [DOI] [PubMed] [Google Scholar]
- 41.Sidik K, Jonkman JN. A note on the empirical Bayes heterogeneity variance estimator in meta-analysis. Stat Med. 2019;38(20):3804–3816. doi: 10.1002/sim.8197. [DOI] [PubMed] [Google Scholar]
- 42.Ainsworth C. Assessing the robustness of direct meta-analysis in the presence of heterogeneity. J Comp Eff Res. 2018;7(10):1009–1025. doi: 10.2217/cer-2018-0024. [DOI] [PubMed] [Google Scholar]
- 43.Rhodes KM, Turner RM, Savović J, Jones HE, Mawdsley D, Higgins JPT. Between-trial heterogeneity in meta-analyses may be partially explained by reported design characteristics. J Clin Epidemiol. 2018;95:45–54. doi: 10.1016/j.jclinepi.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.