Abstract
Heat shock proteins play an important role in immune inflammation and the formation and restoration of proteins. In recent years, the importance of heat shock protein 90 (Hsp90) in the activation of immune inflammation through nuclear factor kB (NFkB) has been discussed. To assess the activation of the Hsp90-NFkB system by measuring serum and urinary levels in patients with chronic glomerulonephritis (CGN). This study included 32 patients with active forms of CGN and 14 patients with Fabry nephropathy. The control group included 10 healthy individuals. Twenty-one out of 32 CGN patients had nephrotic syndrome (NS). Eleven out of 32 CGN patients had proteinuria levels from 1 to 3 g/day without nephrotic syndrome. A total of 17 patients had renal dysfunction (estimated glomerular filtration rate < 60 ml/min/1.73m2). Fourteen patients with Fabry nephropathy had proteinuria without nephrotic syndrome. Serum and urine HSP-90 and NFkB p65 levels were determined using an enzyme-linked immunosorbent assay. The levels of HSP-90 and NFkB in the serum of patients with CGN were significantly higher than in healthy individuals and patients with Fabry nephropathy. In patients with Fabry nephropathy, the HSP-90 and NFkB levels in the urine and serum did not significantly differ from those in the control subjects. Serum Hsp90 levels were significantly higher in the CGN patients with NS than in patients without NS, as well as in patients with normal renal function compared with patients with an eGFR < 60 ml/min/1.73 m2 and patients with tubulo-interstitial fibrosis. Higher levels of HSP-90 and NFkB in serum were observed in patients with nephrotic forms of CGN, including focal segmental glomerulosclerosis, minimal change disease and membranous nephropathy. There were no correlations between the clinical signs of CGN and urinary HSP90/NFkB levels. Activation of the HSP-90-NFkB system, which is directly involved in the development of immune inflammation in CGN, was found in patients with an active course of CGN, especially in those with nephrotic syndrome.
Keywords: Heat shock protein 90, NFkB, Nephrotic syndrome, Chronic glomerulonephritis
Heat shock proteins are a group of intracellular proteins that protect cells from damage by restoring and utilizing damaged protein molecules, which increases cell viability under stress (Morimoto et al. 1997; Lindquist and Craig 1998; Pockley 2003). The study of heat shock protein 90 (Hsp90) is currently a promising area of scientific research because Hsp90 plays a role in a number of key functions, including cell maintenance and tissue processes. Hsp90 is a chaperone that is involved in the regulation of cell division, proliferation and apoptosis. Hsp90 also activates numerous factors involved in inflammation, fibrosis and hypoxia (Beere 2004; Chen et al. 2005; Lanneau et al. 2008). One of the most important functions of Hsp90 is the regulation of inflammation (Binder 2014). Hsp90 complex formation is necessary for the activation of the nuclear transcription factor kB (NFkB), which is responsible for the production of proinflammatory cytokines. The binding of Hsp90 causes the inhibitory subunit of NFkB to become phosphorylated and cleaved, and the active subunits (p50 and p65) of NFkB are released (O’Neill et al. 2012). In experimental acute kidney injury, Hsp90 inhibitors prevented NFkB activation, limiting the subsequent inflammatory response and damage (Morita et al. 1995; Ohtani et al. 1995).
Given the data of experimental studies, we hypothesised that a change in the activity of Hsp90 may have pathological significance in immune-mediated renal diseases, including CGN.
The aim of our study was to evaluate the activation of Hsp90 and nuclear factor kB in patients with CGN compared with healthy individuals and patients with Fabry disease, a noninflammatory nephropathy.
Materials and methods
Study subjects
Adult patients with CGN (n = 32) and Fabry nephropathy (n = 14) were included in the study. The exclusion criteria were active urinary infection, heart failure, diabetes mellitus, diabetes insipidus, severe arterial hypertension, obesity, liver disease, diffuse connective tissue diseases and stage 5 chronic kidney disease. Venous blood samples were taken at the time of recruitment to assay serum creatinine, total protein and albumin levels. Morning urine samples were assayed for protein and red blood cells. Daily urine was collected for daily proteinuria assessment. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI formula. For controls, we studied 10 healthy subjects.
The study was approved by the Ethics Committee of the Sechenov University. All subjects provided informed written consent before participation in the study that conformed with the Declaration of Helsinki.
Determination of HSP90 and p65 NFkB in serum and urine
Ten-millilitre samples of morning urine were collected in dry plastic test tubes and were centrifuged at room temperature for 15 min with a rotational frequency of 3000 rpm. The urine supernatant was immediately frozen and stored in a freezer at − 20 °C. We used commercial enzyme-linked immunosorbent assay (ELISA) kits to determine the levels of Hsp90 (ADI-EKS-715, Enzo Life Science, USA) and p65 NFkB-RELA (BMS 21512/BMS215/2TEN, eBiosciеnce, Austria).
Statistical analysis
The data were summarized using descriptive statistics. Normality was tested by the Shapiro–Wilk test. The results are presented as the numbers and percentage for categorical variables and as the median and interquartile range (IQR) for continuous variables with a non-normal distribution. Differences in proportions and in continuous data were tested using the Pearson chi-square test and Mann–Whitney U-test, respectively. Correlations between the laboratory variables were evaluated by the Spearmen correlation coefficient. A two-sided P value < 0.05 was considered to indicate statistical significance. Statistical analysis was performed using StatSoft STATISTICA version 10.0 (StatSoft Inc., Tulsa, OK, USA).
Results
Characteristics of the study participants are shown in Table 1. Patients with CGN were distributed into two groups depending on the presence of nephrotic syndrome. In all patients, diagnosis of CGN was confirmed histologically. The diagnosis of Fabry disease was confirmed by genetic analysis and/or by determining the activity of the enzymes α-galactosidase A or globotriaosylsphingosine (lyso-GL3) in dry blood spots.
Table 1.
Characteristic of the study subjects
| CGN with NS (n = 21) | CGN without NS (n = 11) | Fabry nephropathy (n = 14) | Controls (n = 10) | |
|---|---|---|---|---|
| Age, years | 38 [28–56] | 33 [24–47] | 32 [28–50] | 29 [24–51] |
| Gender (males), n (%) | 14 (67) | 7 (63) | 5 (35.7%) | 5 (50%) |
| Kidney histology | ||||
| FSGS/MCD | 8 (38.1) | 2 (18.1) | – | – |
| MN | 5 (23.8) | 2 (18.1) | – | – |
| MPGN | 4 (19.0) | 2 (18.1) | – | – |
| IgA nephropathy | 4 (19.0) | 5 (45.5) | – | – |
| Arterial hypertension, n (%) | 12 (57) | 5 (45) | – | – |
| Proteinuria, g/24 h | 6 [3.51–9] | 1.7 [1–3] | 0.9 [0.43–1.76] | – |
| Serum albumin, g/L | 25 [21.3–28.8] | 40.9 [38–43.5] | 40.2 [36.9–42.5] | – |
| Creatinine, μmol/L | 1.31 [0.92–1.75] | 1.05 [0.85–1.71] | 0.98 [0.79–1.28] | – |
| eGFR, ml/min/1.73 m2 | 62.2 [41.2–97.1] | 73 [46–104] | 80.95 [51.2–108.13] | – |
| eGFR < 60 ml/min/1.73 m2, n (%) | 10 (48) | 7 (52) | 4 (28.6%) | – |
CGN, chronic glomerulonephritis; NS, nephrotic syndrome; MCD, minimal change disease; FSGS, focal segmental glomerular sclerosis; MN, membranous nephropathy; MPGN, membranoproliferative glomerulonephritis; eGFR, estimated glomerular filtration rate
Hsp90 in serum
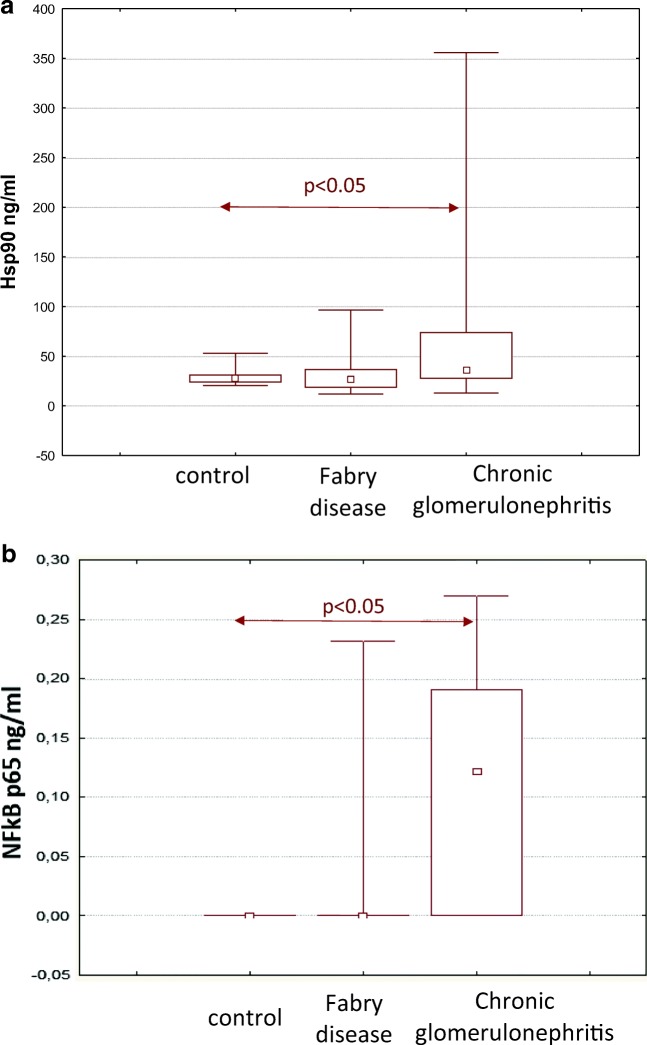
Serum Hsp90 levels in patients with CGN were higher than those in in healthy individuals and patients with Fabry nephropathy (Fig. 1).
Fig. 1.
Serum Hsp90 (a) and NFkB (b) levels in patients with CGN and Fabry disease
Hsp90 levels were significantly higher in patients with nephrotic syndrome 49.25 [33.31–77.25] ng/ml than in patients without nephrotic syndrome 22.32 [16.51–30.83] ng/ml, p < 0.05. We found a negative correlation between Hsp90 and levels of albumin (Rs = − 0.5, p = 0.001) and total serum protein (Rs = − 0.5, p = 0.002).
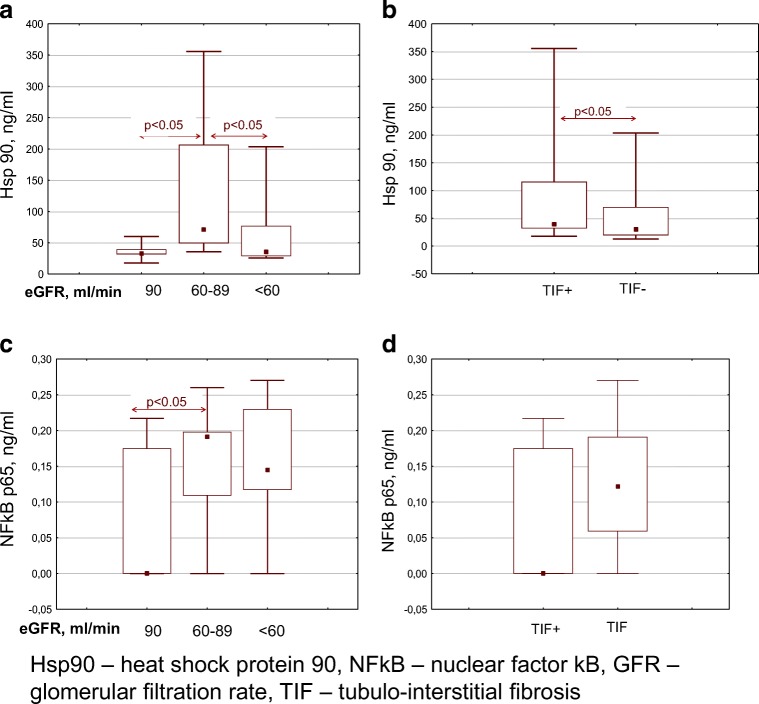
Serum Hsp90 levels tended to decrease in patients with an eGFR less than 60 ml/min and severe tubulo-interstitial fibrosis on kidney biopsies (Fig. 2).
Fig. 2.
a, b Serum Hsp90 levels in patients with chronic glomerulonephritis. c, d Serum NFkB levels in patients with chronic glomerulonephritis. Hsp90, heat shock protein 90; NFkB, nuclear factor kB; GFR, glomerular filtration rate; TIF, tubulo-interstitial fibrosis
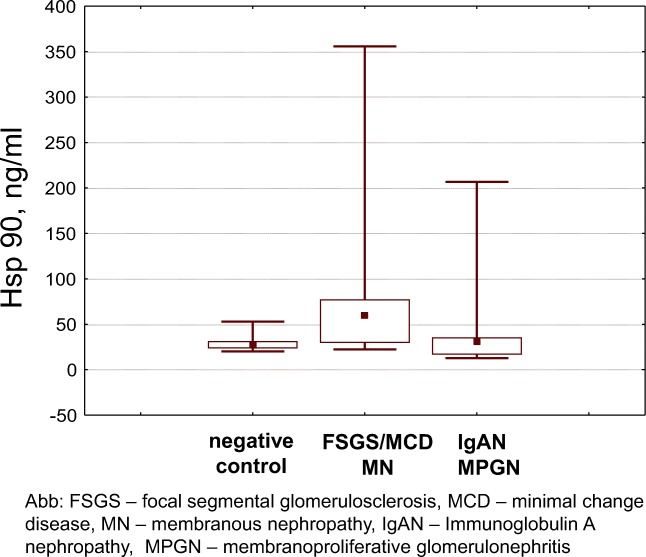
Significantly higher Hsp90 levels were noted in focal segmental glomerulosclerosis, minimal change disease and membranous nephropathy, and this occurred mainly in patients with nephrotic syndrome (Fig. 3).
Fig. 3.
Serum Hsp90 levels in patients with chronic glomerulonephritis
Hsp90 in urine
No significant correlations between urine levels of Hsp90 and proteinuria, the morphological form of nephritis or eGFR was found (Table 2). There was no direct correlation between Hsp90 levels in serum and Hsp90 levels urine.
Table 2.
Correlation of Hsp90 and NFkB p65 urinary levels with laboratory parameters in patients with CGN
| Hsp90 | NFkB p65 | |
|---|---|---|
| 24-h proteinuria, g/day | − 0.039 (p = 0.86) | 0.014 (p = 0.955) |
| Total serum protein, g/dl | − 0.180 (p = 0.412) | − 0.551 (p = 0.018) |
| Serum albumin, g/dl | − 0.128 (p = 0.559) | − 0.478 (p = 0.045) |
| Serum creatinine, mkmol/l | − 0.281 (p = 0.194) | − 0.291 (p = 0.213) |
| eGFR, ml/min/1.73 m2 | 0.145 (p = 0.384) | 0.495 (p = 0.06) |
NFkB in serum
Similar to Hsp90, the NFkB levels in serum were higher in patients with CGN than in healthy individuals and in patients with Fabry disease (Fig. 1). NFkB levels were directly correlated with the level of daily proteinuria (Rs = 0.44, p = 0.006) and were negatively correlated with the level of serum albumin (Rs = − 0.46, p = 0.004) in patients with CGN. The highest levels of NFkB were also detected in patients with nephrotic syndrome 0.145 [0–0.21] ng/ml compared with healthy individuals (below reference values). A direct correlation was found between serum Hsp90 and NFkB levels (Rs = 0.32, p < 0.05).
NFkB levels remained high in patients with an eGFR less than 60 ml/min and severe tubulo-interstitial fibrosis (Fig. 2). A negative correlation between serum NFkB levels and eGFR was observed (Rs = − 0.357, p = 0.049).
Significantly higher NFkB levels were also noted in focal segmental glomerulosclerosis, minimal change disease and membranous nephropathy 0.139 [0.105–0.175] ng/ml than in other forms of glomerulonephritis (IgA nephropathy and membranoproliferative glomerulonephritis) 0.059 [0.0–0.122] ng/ml, p < 0.05.
NFkB in urine
No significant correlations between urine levels of NFkB and proteinuria, the morphological form of nephritis or eGFR was found. Negative correlations between urine levels of NFkB and serum albumin/total serum protein (representing of severity of NS) were found (Table 2). There was no direct correlation between NFkB levels in serum and NFkB levels in urine.
Discussion
Heat shock protein 90 is a highly conserved molecular chaperone. Hsp90 fulfils its housekeeping role by contributing to cytosolic protein folding and maintaining their structural integrity and proper regulation. Hsp90 is also involved in cell cycle control and signal transduction. The conformational flexibility of Hsp90 and its variety of co-chaperone complexes contribute to its functional diversity and allow Hsp90 to assist a wide range of substrates (Picard 2002). Hsp90 assists in the maturation of a set of substrate proteins that are referred to as clients. The client proteins include tyrosine kinases (e.g. Akt and MEK), hormone receptors, structural proteins (tubulin, actin), hypoxia-inducible factor 1α (HIF-1α) (Kataria et al. 2019), HSF1 and Hsp70 (Prodromou 2016; Genest et al. 2019). It has been shown that serum Hsp90 levels were raised in patients with systemic lupus erythematosus (SLE) (Saito et al. 2015), patients with type 1 diabetes (Ocaña et al. 2019), patients with malignant tumours (Tas et al. 2017) and in patients with acute rejection after kidney transplantation (Maehana et al. 2016). In our study, the levels of Hsp90 in serum and urine were higher in patients with severe chronic glomerulonephritis. Serum Hsp90 levels were significantly higher in patients with nephrotic syndrome than in healthy individuals and patients with a less active course of nephritis. There was no direct correlation between Hsp90 levels in serum and urine. Urine levels of Hsp90 did not reflect the activity of the disease.
The mechanism for the preferential elevation of serum Hsp90 is still unknown. Saito et al. (2015) found that Hsp90 was released into the serum and its levels correlated with the severity of SLE in patients. Patients with autoimmune vasculitis also have higher serum Hsp90α levels than healthy controls (Saito et al. 2015). Maehana et al. (2016) showed that Hsp90α was released into the serum after cellular damage secondary to acute kidney transplant rejection, and can potentially be used as a biomarker to help detect acute rejection in kidney recipients.
Hsp90 is mainly expressed in the distal tubules and the cortical and medullary collecting ducts in renal tissue, which corresponds to the distribution of mineralocorticoid and glucocorticoid receptors, demonstrating the importance of Hsp90 in steroid signal transduction (Farman et al. 1991). Hsp90 expression is also observed in podocytes, parietal Bowman capsule epithelium, and in endothelial and interstitial cells (Matsubara et al. 1990), which indicates that Hsp90 has a wide range of functions in various kidney cells. Changes in Hsp90 expression are better studied in acute toxic renal injury in which Hsp90 expression increases in tubular cells during the late stage of regeneration (Beere 2004; Binder 2014). We suggest that local renal production of Hsp90 in CGN may be a source of elevated Hsp90 blood serum levels; however, the absence of a direct correlation between the amount of Hsp90 in the urine and blood serum does not support this hypothesis.
We found the highest serum Hsp90 values in patients with a primary podocytopathy and NS, such as MCD and FSGS. Approximately 40 years ago, Shalhoub et al. and later Savin et al. suggested the possible participation of T cells in the production of permeability factors in MCD and FSGS and that these factors can damage podocytes, thereby contributing to the onset of nephrotic syndrome (Shalhoub 1974; Savin et al. 1996). However, the precise nature of these factors has not been determined.
It was shown that surface Hsp90 expression was elevated in peripheral blood monocytes and may be associated with the pathogenesis of SLE (Dhillon et al. 1993). Therefore, the increased activity of Hsp90 in the blood may reflect its direct or indirect (e.g., via NFkB) activation of immune cells.
Another piece of evidence in support of the role of Hsp90 in immune inflammation is that there was no significant increase in serum Hsp90 in the control group compared with patients with Fabry disease (a noninflammatory nephropathy associated with deposition of glycosphingolipids in the kidney cells) (Alroy et al. 2002). The symptoms of kidney damage include albuminuria, proteinuria and the development of terminal chronic renal failure before the age of 40 (Ortiz et al. 2008). As opposed to patients with CGN, the levels of Hsp90 in the serum did not significantly change in patients with Fabry disease.
It has been shown that Hsp90 inhibitors can block the activity of proinflammatory mediators and can inhibit the activation of immune cells (Yun et al. 2011). Several pathways are thought to be involved in the protective effect that occurs when Hsp90 is inhibited. Since HSF1 is a client protein of Hsp90, the release of HSF1 from the total complex increases the expression of other Hsps with predominantly protective functions, including Hsp70. In particular, blocking Hsp90 leads to both the degradation of client proteins and the increased expression of Hsp70 (Zhang and Burrows 2004).
By contrast, blocking Hsp90 disrupts the IKK complex, thereby deactivating NFkB (Madrigal-Matute et al. 2010). The inhibition of the Hsp90/client interaction by antiHsp90 drugs leads to the destabilization, polyubiquitination and degradation of the client protein. Several key kinases and important intermediates of the NF-κB signalling pathway, such as RIP kinase, TAK-1 kinase and IκB kinases are Hsp90 clients (Thangjam et al. 2016). Hsp90 inhibition in most cell types leads to the disruption of NF-κB signalling and prevents the nuclear translocation of NF-κB proteins.
The findings of this study have to be seen in light of some limitations. The first is that the sample of patients was relatively small, and we did not study HSP90 and NFkB expression in renal tissue.
The second limitation is the lack of previous research studies on the topic. There is a need for further development in the area of study. Determining the role and the mechanisms of HSP-90 in immune inflammation in glomerulonephritidies requires further researches.
In conclusion, we found that serum levels of Hsp90 and NFkB in patients with CGN were higher than those in healthy individuals and in Fabry nephropathyThe closest relationship between Hsp90 and NFkB was obtained in chronic glomerulonephritis with nephrotic syndrome (FSGS/MCD and MN). But the serum level of Hsp90 decreased in patients with a lower glomerular filtration rate with tubulo-interstitial fibrosis. These changes in the later stages of the disease can be associated with either the consumption or destruction of Hsp90 during the process of immune inflammation.
Thus, Hsp90 appears to be involved in the progression of immune inflammation through its effect on the proinflammatory factor NFkB in patients with severe CGN especially in nephrotic syndrome. This data may justify the study of Hsp90 as a possible target for treating CGN in the future.
Acknowledgements
The authors thank Professor Vladimir Varshavsky for the morphological analysis. The authors also thank all patients and site staff who participated in this study.
Compliance with ethical standards
The study was approved by the Ethics Committee of the Sechenov University. All subjects provided informed written consent before participation in the study that conformed with the Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alroy J, Sabnis S, Kopp J. Renal pathology in Fabry disease. J Am Soc Nephrol. 2002;3:134–138. doi: 10.1097/01.ASN.0000016684.07368.75. [DOI] [PubMed] [Google Scholar]
- Beere HM. “The stress of dying”: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci. 2004;117:2641–2651. doi: 10.1242/jcs.01284. [DOI] [PubMed] [Google Scholar]
- Binder RJ. Functions of heat shock proteins in pathways of the innate and adaptive immune system. J Immunol. 2014;193:5765–5771. doi: 10.4049/jimmunol.1401417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Piel WH, Gui L, Bruford E, Monteiro A. The HSP90 family of genes in the human genome: insights into their divergence and evolution. Genomics. 2005;86:627–637. doi: 10.1016/j.ygeno.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Dhillon VB, McCallum S, Norton P, Twomey BM, Erkeller-Yuksel F, Lydyard P, Isenberg DA, Latchman DS. Differential heat shock protein overexpression and its clinical relevance in systemic lupus erythematosus. Ann Rheum Dis. 1993;52:436–442. doi: 10.1136/ard.52.6.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farman N, Oblin ME, Lombes M, Delahaye F, Westphal HM, Bonvalet JP, Gasc JM. Immunolocalisation of gluco- and mineralocorticoid receptors in rabbit kidney. Am J Physiol Cell Physiol. 1991;260:226–233. doi: 10.1152/ajpcell.1991.260.2.C226. [DOI] [PubMed] [Google Scholar]
- Genest O, Wickner S, Doyle SM. Hsp90 and Hsp70 chaperones: collaborators in protein remodeling. J Biol Chem. 2019;294(6):2109–2120. doi: 10.1074/jbc.REV118.002806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataria N, Martinez CA, Kerr B, Zaiter SS, Morgan M, McAlpine SR, Cook KM. C-Terminal HSP90 inhibitors block the HIF-1 hypoxic response by degrading HIF-1α through the oxygen-dependent degradation pathway. Cell Physiol Biochem. 2019;53(3):480–495. doi: 10.33594/000000152. [DOI] [PubMed] [Google Scholar]
- Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C. Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med. 2008;12(3):743–761. doi: 10.1111/j.1582-4934.2008.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1998;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Madrigal-Matute J, Lopez-Franco O, Blanco-Colio LM, Muñoz-García B, Ramos-Mozo P, Ortega L, Egido J, Martín-Ventura JL. Heat shock protein 90 inhibitors attenuate inflammatory responses in atherosclerosis. Cardiovasc Res. 2010;86:330–337. doi: 10.1093/cvr/cvq046. [DOI] [PubMed] [Google Scholar]
- Maehana T, Tanaka T, Kitamura H, Fukuzawa N, Ishida H, Harada H, Tanabe K, Masumori N (2016) Heat shock protein 90α is a potential serological biomarker of acute rejection after renal transplantation. PLoS One. 10.1371/journal.pone.0162942 [DOI] [PMC free article] [PubMed]
- Matsubara O, Kasuga T, Marumo F, Itoh H, Tashima Y. Localization of 90-kDa heat shock protein in the kidney. Kidney Int. 1990;38:830–834. doi: 10.1038/ki.1990.278. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 1997;32:17–29. [PubMed] [Google Scholar]
- Morita K, Wakui H, Komatsuda A, Ohtani H, Miura AB, Itoh H, Tashima Y. Induction of heat-shock proteins HSP73 and HSP90 in rat kidneys after ischemia. Ren Fail. 1995;17:405–419. doi: 10.3109/08860229509037605. [DOI] [PubMed] [Google Scholar]
- Ocaña GJ, Sims EK, Watkins RA, Ragg S, Mather KJ, Oram RA, Mirmira RG, DiMeglio LA, Blum JS, Evans-Molina C. Analysis of serum Hsp90 as a potential biomarker of β cell autoimmunity in type 1 diabetes. PLoS One. 2019;14(1):e0208456. doi: 10.1371/journal.pone.0208456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani H, Wakui H, Komatsuda A, Satoh K, Miura AB, Itoh H, Tashima Y. Induction and intracellular localization of 90-kDa heat shock protein in rat kidneys with acute gentamicin nephropathy. Lab Investig. 1995;72:161–165. [PubMed] [Google Scholar]
- O’Neill S, Ross JA, Wigmore SJ, Harrison EM. The role of heat shock protein 90 in modulating ischemia-reperfusion injury in the kidney. Expert Opin Investig Drugs. 2012;21(10):1535–1548. doi: 10.1517/13543784.2012.713939. [DOI] [PubMed] [Google Scholar]
- Ortiz A, Oliveira JP, Waldek S, Warnock DG, Cianciaruso B, Wanner C, Registry F. Nephropathy in males and females with Fabry disease: cross-sectional description of patients before treatment with enzyme replacement therapy. Nephrol Dial Transplantation. 2008;5(23):1600–1607. doi: 10.1093/ndt/gfm848. [DOI] [PubMed] [Google Scholar]
- Picard D. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol Life Sci. 2002;59(10):1640–1648. doi: 10.1007/pl00012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- Prodromou C. Mechanisms of Hsp90 regulation. Biochem J. 2016;473(16):2439–2452. doi: 10.1042/BCJ20160005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Kukita K, Kutomi G, Okuya K, Asanuma H, Tabeya T, Naishiro Y, Yamamoto M, Takahashi H, Torigoe T, Nakai A, Shinomura Y, Hirata K, Sato N, Tamura Y. Heat shock protein 90 associates with toll-like receptors 7/9 and mediates self-nucleic acid recognition in SLE. Eur J Immunol. 2015;45:2028–2041. doi: 10.1002/eji.201445293. [DOI] [PubMed] [Google Scholar]
- Savin VJ, Sharma R, Sharma M, McCarthy ET, Swan SK, Ellis E, Lovell H, Warady B, Gunwar S, Chonko AM, Artero M, Vincenti F. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med. 1996;334(14):878–883. doi: 10.1056/NEJM199604043341402. [DOI] [PubMed] [Google Scholar]
- Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974;2(7880):556–560. doi: 10.1016/s0140-6736(74)91880-7. [DOI] [PubMed] [Google Scholar]
- Tas F, Bilgin E, Erturk K, Duranyildiz D. Clinical significance of circulating serum cellular heat shock protein 90 (HSP90) level in patients with cutaneous malignant melanoma. Asian Pac J Cancer Prev. 2017;18(3):599–601. doi: 10.22034/APJCP.2017.18.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangjam GS, Birmpas C, Barabutis N, Gregory BW, Clemens MA, Newton JR, Fulton D, Catravas JD. Hsp90 inhibition suppresses NF-κB transcriptional activation via Sirt-2 in human lung microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2016;310(10):964–974. doi: 10.1152/ajplung.00054.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun TJ, Harning EK, Giza K, Rabah D, Li P, Arndt JW, Luchetti D, Biamonte MA, Shi J, Lundgren K, Manning A, Kehry MR. EC144, a synthetic inhibitor of heat shock protein 90, blocks innate and adaptive immune responses in models of inflammation and autoimmunity. J Immunol. 2011;186:563–575. doi: 10.4049/jimmunol.1000222. [DOI] [PubMed] [Google Scholar]
- Zhang H, Burrows F. Targeting multiple signal transduction pathways through inhibition of Hsp90. J Mol Med (Berl) 2004;82:488–499. doi: 10.1007/s00109-004-0549-9. [DOI] [PubMed] [Google Scholar]