Abstract
Introduction
Despite treatment with oral antidiabetic drugs (OADs), achieving effective glycaemic control in type 2 diabetes (T2D) remains a challenge. The objective of this post hoc analysis of data from the SUSTAIN 2, 3, 4 and 10 active-controlled trials was to assess the efficacy and safety of the once-weekly glucagon-like peptide 1 receptor agonist (GLP-1RA) semaglutide in patients on background treatment with metformin (MET), with or without a sulphonylurea (SU).
Methods
Data from the randomised phase 3 trials SUSTAIN 2, 3, 4 and 10 for subjects who received background MET alone or MET + SU were analysed. Change from baseline in HbA1c and body weight at the end of treatment visit (week 30 in SUSTAIN 4 and 10, week 56 in SUSTAIN 2 and 3), and rates of hypoglycaemia and adverse events leading to premature treatment discontinuation were assessed.
Results
In total, 3411 subjects were included in the full analysis set (3410 in the safety analysis set). Across the four trials, semaglutide significantly reduced HbA1c (estimated treatment difference [ETD] − 0.32 to − 0.79%-points for semaglutide 0.5 mg, and − 0.38 to − 1.07%-points for semaglutide 1.0 mg vs comparators; p < 0.01) in subjects receiving both MET and MET + SU. Regardless of background OAD, semaglutide significantly reduced body weight (ETD − 2.35 to − 4.72 kg for semaglutide 0.5 mg, and − 2.96 to − 6.76 kg for semaglutide 1.0 mg vs comparators; p < 0.0001). Across the trials, hypoglycaemic events were more common with background MET + SU than MET alone, in subjects receiving either semaglutide or a comparator. The rate of adverse events (AEs) leading to premature treatment discontinuations in subjects treated with semaglutide were generally consistent regardless of background therapy.
Conclusion
Semaglutide 0.5 mg and 1.0 mg significantly improve glycaemic control (HbA1c) and body weight in subjects with T2D, with a similar tolerability profile, regardless of whether they receive background MET or MET + SU.
Trial Registration
Clinicaltrials.gov: NCT01930188 (SUSTAIN 2), NCT01885208 (SUSTAIN 3), NCT02128932 (SUSTAIN 4) and NCT03191396 (SUSTAIN 10).
Electronic Supplementary Material
The online version of this article (10.1007/s13300-020-00796-z) contains supplementary material, which is available to authorized users.
Keywords: Diabetes care, GLP-1RA, Metformin, Oral antidiabetic agents, Randomised controlled trials, Semaglutide, Sulphonylurea, Type 2 diabetes
Key Summary Points
| Why carry out this study? |
| Current treatment guidelines recommend glucagon-like peptide 1 receptor agonists (GLP-1RAs) as second- or third-line therapies in addition to metformin (MET) and another oral antidiabetic drug (OAD), e.g. a sulphonylurea (SU), in type 2 diabetes (T2D) |
| MET and SU are some of the most commonly prescribed OADs, and it is therefore important and relevant for clinicians to establish what effect, if any, these background OADs have on the efficacy and safety of GLP-1RA therapy |
| This post hoc analysis of data from the SUSTAIN 2, 3, 4 and 10 active-controlled trials was conducted to evaluate the efficacy of semaglutide 0.5 mg and 1.0 mg vs comparators in subjects with T2D receiving either background MET or background MET + SU therapy |
| What was learned from the study? |
| After 30 weeks (SUSTAIN 4 and 10) or 56 weeks (SUSTAIN 2 and 3), significant improvements were seen with semaglutide 0.5 mg and 1.0 mg vs comparators in glycaemic control (HbA1c) and body weight, and these were generally consistent regardless of background OAD (MET or MET + SU) |
| The proportion of subjects who experienced adverse events leading to premature treatment discontinuations with semaglutide treatment was generally consistent regardless of background OAD |
| Events of hypoglycaemia (severe or blood glucose-confirmed symptomatic hypoglycaemia, minor hypoglycaemia, or American Diabetes Association (ADA)-classified severe hypoglycaemia) were more common in subjects receiving background MET + SU than those receiving MET alone, regardless of whether subjects received semaglutide or a comparator |
| These results support healthcare professionals in providing their patients with optimal care with GLP-1RAs, based on their current OAD medications |
Introduction
Type 2 diabetes (T2D) is a complex condition, with many factors affecting glycaemic control. There are several classes of oral antidiabetic drugs (OADs) available for the treatment of T2D, including metformin, dipeptidyl peptidase 4 inhibitors (DPP4is) and sodium–glucose co-transporter inhibitors (SGLT2is), and the majority of patients with T2D are treated with OADs [1, 2]. Achieving glycaemic control remains a challenge and, depending on clinical characteristics and the progression of the condition, an injectable therapy may offer benefits.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs) are an established T2D therapy, recommended by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) guidelines as the first injectable therapy; as second- or third-line treatment in addition to metformin (MET) and/or another oral therapy (e.g. sulphonylureas [SUs]) or in combination with insulin; and as a first-line treatment if MET is unsuitable for the patient [3, 4]. Furthermore, joint guidelines from the European Society of Cardiology (ESC) and EASD recommend the GLP-1RAs liraglutide, semaglutide and dulaglutide or the SGLT2is empagliflozin, canagliflozin and dapagliflozin as first-line therapy in patients with T2D and cardiovascular disease (CVD), or at very high/high cardiovascular (CV) risk, to reduce CV events [5]. In patients with T2D and established atherosclerotic CVD (ASCVD), joint ADA and EASD 2019 guidelines recommend second-line treatment with GLP-1RAs (where ASCVD predominates) or SGLT2is (where heart failure or chronic kidney disease predominate), independent of baseline HbA1c, if MET plus comprehensive lifestyle changes do not achieve glycaemic control [6]. Currently the National Institute for Health and Care Excellence (NICE) recommends GLP-1RAs as fourth-line therapy in patients for whom triple therapy with MET and two other OADs is not effective or not tolerated (and third-line if MET and two other OADs is contraindicated). Specifically, GLP-1RAs are recommended either for those patients with T2D and obesity with a body mass index (BMI) of 35 kg/m2 or more and specific physiological or any other medical problems associated with obesity, or for patients with a BMI of less than 35 kg/m2 for whom insulin would have significant occupational implications or for whom weight loss would benefit other significant obesity-related comorbidities [7].
Semaglutide is a GLP-1 analogue, with 94% amino acid homology to native GLP-1. The efficacy and safety of once-weekly subcutaneous (s.c.) administration of semaglutide has been established in the global phase 3 clinical trial programme SUSTAIN (Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes), which has included a broad range of subjects with T2D with or without background OADs or insulin [8–17].
In the SUSTAIN trials, semaglutide has demonstrated superior reductions in glycaemic control and body weight compared with placebo and active comparators. The number and type of background OAD therapies in subjects varied across the SUSTAIN trials and included MET (which is considered the backbone of T2D treatment and recommended as first-line therapy by most treatment guidelines) as a monotherapy or in combination with SU, one of the most common second-line therapies [3–5, 7]. Subjects in the SUSTAIN trials remained on stable therapy, including prior background treatment, unless rescue criteria were met [8–17].
NICE guidelines recommend following a treatment intensification approach beginning with MET, followed by MET + SU (or a DPP4i or thiazolidinedione [TZD]) if MET alone has not continued to control HbA1c levels. A second intensification of triple therapy with MET, SU and DPP4i or TZD may also be necessary if dual therapy has failed [7]. SUs (or glinide) are also recommended as part of first-line therapy by the International Diabetes Federation for rapid response where glucose levels are high, or initially where MET cannot be used [18]. MET and SU are therefore some of the most commonly recommended and prescribed OADs [19], and it is important and relevant for clinicians to establish what effect, if any, these background OADs have on the efficacy and safety of add-on GLP-1RA therapy.
In this post hoc analysis of data from across the SUSTAIN 2, 3, 4 and 10 active comparator-controlled trials, the efficacy and safety of semaglutide in combination with either MET or MET + SU was evaluated (background MET only in SUSTAIN 2).
Methods
Trial Designs
The designs of each trial have been published previously [9–11, 17] and are summarised in Table 1. SUSTAIN 2, 3, 4 and 10 were phase 3 multinational randomised controlled trials comparing once-weekly semaglutide (0.5 mg or 1.0 mg) with active comparators in subjects with uncontrolled T2D, defined as HbA1c at least 7.0% to 10.0% (SUSTAIN 4), 10.5% (SUSTAIN 2 and 3) or 11.0% (in SUSTAIN 10) [9–11, 17].
Table 1.
Trial designs for SUSTAIN 2–4 and 10
| SUSTAIN 2 | SUSTAIN 3 | SUSTAIN 4 | SUSTAIN 10 | |
|---|---|---|---|---|
| FAS, N | 1225 | 809 | 1082 | 577 |
| Semaglutide dose | 0.5 mg, 1.0 mg | 1.0 mg | 0.5 mg, 1.0 mg | 1.0 mg |
| Comparator | Sitagliptin 100 mg | Exenatide ER 2.0 mg | Insulin glargine | Liraglutide 1.2 mg |
| Duration | 56 weeks | 56 weeks | 30 weeks | 30 weeks |
| Trial design | Double-blind, double-dummy RCT | Open-label RCT | Open-label RCT | Open-label RCT |
| Permitted background therapy typea | Add-on: MET ± TZD | Add-on: 1–2 OADs (MET ± TZD ± SU) | Add-on: MET ± SU | Add-on: 1–3 OADs (SU ± MET, SGLT2i ± MET SU + SGLT2i ± MET, MET only) |
Exenatide ER exenatide extended release, FAS full analysis set, MET metformin, N number of subjects randomised, OAD oral antidiabetic drug, RCT randomised controlled trial, SGLT2i sodium–glucose co-transporter 2 inhibitor, SU sulphonylurea, TZD thiazolidinedione
aOnly subjects receiving background MET and MET + SU were included in this analysis
Semaglutide was added to existing stable background antidiabetic therapy (MET, TZD or both [SUSTAIN 2]; MET, TZD and/or SU [SUSTAIN 3]; MET or MET and SU [SUSTAIN 4]; MET and/or SU/SGLT2i, or SU and SGLT2i [SUSTAIN 10]). Subjects receiving background MET alone or MET + SU from these four studies were included in this analysis. All trials included a treatment period of at least 30 weeks, with data reported up to week 56 for SUSTAIN 2 and 3. The primary endpoint for all the trials was change from baseline to the planned end of treatment (EOT) visit in HbA1c; secondary endpoints included change from baseline to EOT in body weight.
All four trials were conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines [20] and the Declaration of Helsinki [21]. All protocols were approved by the institutional review boards and ethics committees at each participating centre, and subjects provided written informed consent before trial-related activities commenced. Links to the trial registrations can be found in the supplementary material. The four trials were registered with ClinicalTrials.gov (NCT01930188 [SUSTAIN 2], NCT01885208 [SUSTAIN 3], NCT02128932 [SUSTAIN 4] and NCT03191396 [SUSTAIN 10]).
Subgroup Analyses
Subjects receiving either background MET or background MET + SU at baseline visit were included in this post hoc analysis and reported by trial and by treatment group. In SUSTAIN 2, 94.0% of the total study full analysis set received background MET alone [9]. In SUSTAIN 3, 49.6% of subjects received MET alone and 44.9% received MET + SU [10]; in SUSTAIN 4, 48.3% received MET alone and 51.6% MET + SU [11]; and in SUSTAIN 10, 36.6% of subjects received MET alone and 35.2% MET + SU [17]. These groups were chosen for comparison as MET and SU are two of the most commonly prescribed OADs [19], and as guidelines recommend treatment intensification beginning with MET added to another OAD, followed by MET + SU and another OAD [3–5, 7]. Data are not reported here for subgroups with TZD owing to the low number of subjects receiving TZD in these trials: 5.4% and 2.3% of subjects receiving background TZD in SUSTAIN 2 and SUSTAIN 3, respectively [9, 10], and TZD was not included as a background therapy in SUSTAIN 4 or SUSTAIN 10 [11, 17].
Statistical Analyses
On-treatment without rescue medication data from all subjects contributing to the full analysis set (randomised and exposed to at least one dose of the trial product in SUSTAIN 2–4; all randomised subjects in SUSTAIN 10) in each trial were analysed. The post-baseline data were analysed by trial using the mixed model for repeated measurements (MMRM) with treatment and baseline OAD subgroup as fixed factors, and interaction between treatment and OAD subgroup, and baseline value of each parameter used as covariate, all nested within visit. Mean estimates were adjusted according to the observed baseline distribution for the parameter analysed (e.g. HbA1c or body weight) in each subgroup.
Change from baseline to EOT was analysed for HbA1c, body weight and other efficacy outcomes in the full analysis set, and values are presented as mean (standard error [SE]) unless otherwise stated. Estimated treatment differences (ETDs) for change from baseline to EOT between semaglutide and comparators were calculated with 95% confidence intervals (CIs) and p values are reported. Direct statistical comparisons between OAD subgroups were not performed.
Safety assessments were the number of subjects with adverse events leading to premature treatment discontinuation, and the number of subjects with hypoglycaemic events (defined as severe or blood glucose [BG]-confirmed symptomatic hypoglycaemia, minor hypoglycaemia or ADA-classified severe hypoglycaemia). These were analysed in the safety analysis set (subjects who were exposed to at least one dose of trial product); assessments were based on ‘on treatment’ data and were summarised descriptively.
Data from the SUSTAIN 2–4 trials by background OAD for change from baseline to EOT for HbA1c and body weight and episodes of severe or BG-confirmed symptomatic hypoglycaemia were previously presented at the Endocrine Society Annual Meeting in Orlando, FL, USA, April 1–4, 2017.
Results
Subject Disposition and Baseline Characteristics
In total, 3411 subjects were included in analyses based on the full analysis set and 3410 in the safety analysis set. Baseline characteristics for each subgroup are shown in Table 2.
Table 2.
Subject demographics and baseline characteristics by background OAD subgroup in the full analysis set
| SUSTAIN 2 | SUSTAIN 3 | SUSTAIN 4 | SUSTAIN 10 | ||||
|---|---|---|---|---|---|---|---|
| MET | MET | MET + SU | MET | MET + SU | MET | MET + SU | |
| N | 1152 | 401 | 363 | 523 | 558 | 211 | 203 |
| Age, years | 55.2 (10.0) | 55.0 (10.8) | 58.4 (10.3) | 55.4 (10.3) | 57.4 (10.5) | 58.5 (10.8) | 60.9 (9.6) |
| Male, n (%) | 579 (50.3) | 213 (53.1) | 210 (57.9) | 253 (48.4) | 320 (57.3) | 118 (55.9) | 113 (55.7) |
| Diabetes duration, years | 6.5 (5.1) | 7.8 (5.6) | 10.9 (6.9) | 7.0 (5.9) | 10.0 (6.3) | 7.7 (5.8) | 10.7 (6.1) |
| HbA1c, % | 8.1 (0.9) | 8.3 (1.0) | 8.4 (0.9) | 8.0 (0.9) | 8.3 (0.9) | 8.1 (0.9) | 8.5 (1.0) |
| Body weight, kg | 90.0 (20.1) | 96.4 (21.7) | 94.6 (21.0) | 94.7 (21.7) | 92.2 (21.8) | 98.0 (21.3) | 95.1 (21.3) |
| BMI, kg/m2 | 32.6 (6.2) | 34.2 (7.1) | 33.1 (6.4) | 33.6 (6.3) | 32.5 (6.6) | 33.9 (7.0) | 33.4 (6.7) |
| Systolic blood pressure, mmHg | 133.1 (14.8) | 132.2 (14.8) | 135.0 (14.1) | 130.9 (14.7) | 133.1 (15.8) | 136.0 (15.3) | 138.8 (14.1) |
| Total cholesterol, mmol/L | 4.9 (1.1) | 4.9 (1.1) | 4.7 (1.1) | 4.7 (1.1) | 4.5 (1.1) | 4.6 (1.1) | 4.5 (1.1) |
| LDL cholesterol, mmol/L | 2.7 (0.9) | 2.7 (1.0) | 2.6 (0.9) | 2.6 (0.9) | 2.4 (0.9) | 2.5 (0.9) | 2.4 (0.9) |
| HDL cholesterol, mmol/L | 1.2 (0.3) | 1.2 (0.3) | 1.2 (0.3) | 1.2 (0.3) | 1.2 (0.3) | 1.2 (0.3) | 1.1 (0.3) |
| Triglycerides, mmol/L | 2.2 (1.6) | 2.3 (1.7) | 2.0 (1.1) | 2.2 (1.4) | 2.1 (1.4) | 2.1 (1.3) | 2.2 (1.6) |
| eGFR, mL/min/1.73 m2 | 100.0 (23.3) | 100.5 (23.2) | 100.0 (23.8) | 98.4 (26.3) | 98.6 (27.0) | 93.3 (17.3) | 91.2 (16.8) |
| Race/ethnicity, n (%) | |||||||
| White, non-Hispanic | 622 (54.0) | 257 (64.1) | 209 (57.6) | 314 (60.0) | 322 (57.7) | 187 (88.6) | 183 (90.1) |
| Black, non-Hispanic | 56 (4.9) | 23 (5.7) | 25 (6.9) | 49 (9.4) | 48 (8.6) | 1 (0.5) | 2 (1.0) |
| Asian or Pacific Islander, non-Hispanic | 271 (23.5) | 8 (2.0) | 8 (2.2) | 35 (6.7) | 80 (14.3) | 4 (1.9) | 1 (0.5) |
| Native American, non-Hispanic | 0 (0.0) | 1 (0.2) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hispanic ethnicity (regardless of race) | 202 (17.5) | 99 (24.7) | 91 (25.1) | 117 (22.4) | 96 (17.2) | 3 (1.4) | 2 (1.0) |
| Other/missinga | 1 (0.1) | 13 (3.2) | 29 (8.0) | 8 (1.5) | 12 (2.2) | 16 (7.6) | 15 (7.4) |
Subgroup data are mean (standard deviation) unless otherwise indicated
BMI body mass index, eGFR estimated glomerular filtration rate, HDL high-density lipoprotein, LDL low-density lipoprotein, MET metformin, n number of subjects in category, N number of subjects randomised and exposed to at least one dose of trial product (full analysis set), OAD oral antidiabetic drug, SU sulphonylurea
aSubjects otherwise not included in the race/ethnicity categories listed or with missing data
Efficacy Outcomes by Background OAD Subgroup
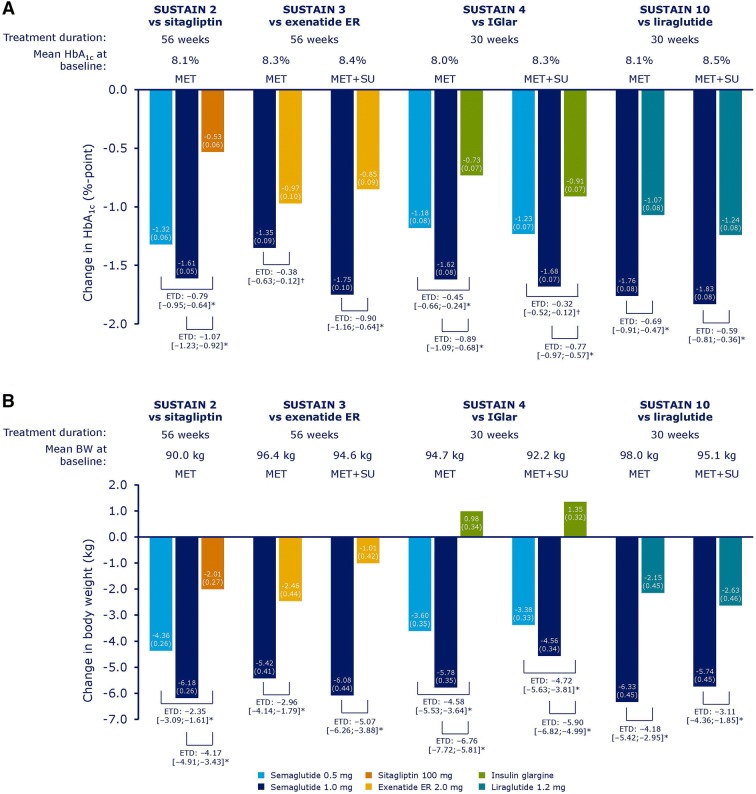
Significantly greater reductions in HbA1c from baseline were observed with subjects receiving semaglutide 0.5 mg or 1.0 mg vs comparators across all four trials at the end of treatment (week 56 for SUSTAIN 2 and 3 and week 30 for SUSTAIN 4 and 10). Semaglutide significantly reduced HbA1c from baseline to EOT vs comparators regardless of background OAD(s), and changes were generally similar in the background MET and MET + SU subgroups, except in SUSTAIN 3 where reductions with semaglutide 1.0 mg vs exenatide ER were numerically greater with background MET + SU than with background MET alone (Fig. 1a).
Fig. 1.
Mean (SE) change from baseline in HbA1c (a) and body weight (b) by background oral antidiabetic drug subgroup. Mean (SE) change from baseline and ETD [95% confidence interval] for semaglutide vs comparators shown; *p < 0.0001, †p < 0.01. BW body weight, ER extended release, ETD estimated treatment difference, IGlar insulin glargine, MET metformin, SE standard error, SU sulphonylurea
Reductions in body weight were also consistently statistically greater with semaglutide 0.5 mg and 1.0 mg vs comparators across all four trials, regardless of background OAD(s). In SUSTAIN 3, reductions with semaglutide 1.0 mg vs exenatide ER appeared to be larger in the background MET + SU subgroup than in the background MET subgroup, mainly driven by a smaller reduction in body weight with exenatide ER in subjects receiving MET + SU than in those receiving MET alone (Fig. 1b). Similarly, semaglutide also significantly reduced BMI across all four trials from baseline to end of treatment, regardless of background OAD(s), with numerically larger reductions observed with semaglutide 1.0 mg vs exenatide ER in the MET + SU group than in the MET group in SUSTAIN 3 (Fig. S1 in the supplementary material).
Semaglutide reduced systolic blood pressure from baseline to EOT across the four trials regardless of OAD subgroup, and the reductions with semaglutide were significantly greater than with comparator in SUSTAIN 2 (MET only; no MET + SU subgroup in this trial) and in SUSTAIN 4 (in the MET + SU subgroup but not in the MET alone subgroup; Fig. S2 in the supplementary material).
Across the four trials, semaglutide generally reduced levels of total and low-density lipoprotein (LDL) cholesterol and triglycerides from baseline to EOT, with little change or small increases in levels of high-density lipoprotein (HDL) cholesterol (Fig. S3A–D in the supplementary material). These trends were generally consistent regardless of background OAD therapy. In SUSTAIN 3, a significantly greater reduction in total cholesterol was observed with semaglutide vs exenatide ER in subjects with background MET + SU, but in those with background MET only there was a slightly greater (non-significant) reduction in total and LDL cholesterol with exenatide ER vs semaglutide (Fig. S3A and C in the supplementary material). For HDL cholesterol (Fig. S3B in the supplementary material), the changes from baseline were very small with no consistent pattern across the four trials; the only significant ETD was between semaglutide 1.0 mg and sitagliptin in SUSTAIN 2, which only included a MET group. There were no significant differences between semaglutide and any comparators in the change from baseline in estimated glomerular filtration rate (eGFR), and there were no observed differences based on background OAD (Fig. S4 in the supplementary material).
Safety by Baseline OAD Subgroup
A higher proportion of subjects receiving insulin glargine in SUSTAIN 4 experienced severe or BG-confirmed symptomatic hypoglycaemia or minor hypoglycaemia than those receiving either semaglutide 0.5 mg or 1.0 mg when on background MET + SU (Table 3). Across the other trials, the proportion of subjects experiencing hypoglycaemia was similar between semaglutide- and comparator-treated subjects. The proportion of subjects experiencing severe or BG-confirmed symptomatic hypoglycaemia or minor hypoglycaemia was numerically greater in those treated with background MET + SU than with background MET alone, in both the semaglutide- and comparator-treated groups (Table 3). The proportion of patients with ADA-classified severe hypoglycaemic events was low (0–2.2%), regardless of background therapy or treatment arm across most of the trials. However, the proportions of these patients were relatively higher in the background MET + SU subgroup in SUSTAIN 4 than in MET or MET + SU subgroups across the other three trials, with ADA-classified severe hypoglycaemia reported in 1.1%, 2.2% and 2.1% of subjects receiving semaglutide 0.5 mg, semaglutide 1.0 mg and insulin glargine, respectively (Table 3).
Table 3.
Hypoglycaemic episodes by classification in the safety analysis set
| SUSTAIN 2 | SUSTAIN 3 | SUSTAIN 4 | SUSTAIN 10 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MET | MET | MET + SU | MET | MET + SU | MET | MET + SU | |||||||||||
| Sema 0.5 mg |
Sema 1.0 mg |
Sita | Sema 1.0 mg |
Exe ER |
Sema 1.0 mg |
Exe ER |
Sema 0.5 mg |
Sema 1.0 mg |
IGlar | Sema 0.5 mg |
Sema 1.0 mg |
IGlar | Sema 1.0 mg |
Lira 1.2 mg |
Sema 1.0 mg |
Lira 1.2 mg |
|
| N | 382 | 388 | 382 | 213 | 188 | 167 | 196 | 176 | 175 | 172 | 186 | 185 | 187 | 106 | 104 | 104 | 99 |
| Severe or BG-confirmed symptomatic hypoglycaemiaa | |||||||||||||||||
| n (%) |
7 (1.8) |
2 (0.5) |
4 (1.0) |
7 (3.3) |
3 (1.6) |
22 (13.2) |
30 (15.3) |
1 (0.6) |
4 (2.3) |
4 (2.3) |
15 (8.1) |
16 (8.6) |
34 (18.2) |
0 (0.0) |
1 (1.0) |
5 (4.8) |
5 (5.1) |
| Number of events | 7 | 2 | 4 | 8 | 9 | 42 | 48 | 1 | 5 | 7 | 31 | 34 | 62 | 0 | 1 | 8 | 6 |
| Event rate per 100 exposure years | 1.7 | 0.5 | 0.9 | 3.7 | 4.9 | 24.3 | 23.5 | 0.9 | 4.8 | 6.2 | 26.4 | 29.8 | 51.0 | 0 | 1.5 | 12.3 | 9.5 |
| Minor hypoglycaemiab | |||||||||||||||||
| n (%) |
7 (1.8) |
2 (0.5) |
2 (0.5) |
6 (2.8) |
3 (1.6) |
22 (13.2) |
30 (15.3) |
1 (0.6) |
3 (1.7) |
3 (1.7) |
14 (7.5) |
13 (7.0) |
31 (16.6) |
11 (10.4) |
9 (8.7) |
28 (26.9) |
36 (36.4) |
| Number of events | 7 | 2 | 2 | 7 | 9 | 41 | 48 | 1 | 4 | 6 | 27 | 24 | 58 | 14 | 14 | 56 | 85 |
| Event rate per 100 exposure years | 1.7 | 0.5 | 0.5 | 3.3 | 4.9 | 23.7 | 23.5 | 0.9 | 3.8 | 5.3 | 23.0 | 21.0 | 47.7 | 21.4 | 21.1 | 86.0 | 135.2 |
| ADA-classified severe hypoglycaemia | |||||||||||||||||
| n (%) |
0 (0.0) |
0 (0.0) |
2 (0.5) |
1 (0.5) |
0 (0.0) |
1 (0.6) |
0 (0.0) |
0 (0.0) |
1 (0.6) |
1 (0.6) |
2 (1.1) |
4 (2.2) |
4 (2.1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Number of events | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 4 | 10 | 4 | 0 | 0 | 0 | 0 |
| Event rate per 100 exposure years | 0 | 0 | 0.5 | 0.5 | 0 | 0.6 | 0 | 0 | 1.0 | 0.9 | 3.4 | 8.8 | 3.3 | 0 | 0 | 0 | 0 |
Data are presented as number of subjects (% of safety analysis set), number of events and event rate
ADA American Diabetes Association, BG blood glucose, Exe ER exenatide extended release, IGlar insulin glargine, Lira liraglutide, MET metformin, n number of subjects with events, N number of subjects in the safety analysis set, Sema semaglutide, Sita sitagliptin, SU sulphonylurea
aAn episode that is severe according to ADA classification or BG-confirmed (plasma glucose of less than 3.1 mmol/L) with symptoms consistent with hypoglycaemia
bIncludes all reported hypoglycaemic episodes that were not severe or BG-confirmed
In SUSTAIN 2–4 and 10, the proportion of subjects experiencing adverse events leading to premature treatment discontinuation was higher with semaglutide than with comparators, and discontinuations were similar in the background MET and MET + SU subgroups (Table 4). The proportion of subjects who experienced serious adverse events leading to premature treatment discontinuation was less than 2% across all four trials and across all OAD subgroups (Table 4).
Table 4.
Adverse events leading to premature treatment discontinuation in the safety analysis set
| SUSTAIN 2 | SUSTAIN 3 | SUSTAIN 4 | SUSTAIN 10 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MET | MET | MET + SU | MET | MET + SU | MET | MET + SU | |||||||||||
| Sema 0.5 mg |
Sema 1.0 mg |
Sita | Sema 1.0 mg |
Exe ER |
Sema 1.0 mg |
Exe ER |
Sema 0.5 mg |
Sema 1.0 mg |
IGlar | Sema 0.5 mg |
Sema 1.0 mg |
IGlar | Sema 1.0 mg |
Lira 1.2 mg |
Sema 1.0 mg |
Lira 1.2 mg |
|
| N | 382 | 388 | 382 | 213 | 188 | 167 | 196 | 176 | 175 | 172 | 186 | 185 | 187 | 106 | 104 | 104 | 99 |
| Subjects with AEs leading to premature treatment discontinuationa, n (%) |
29 (7.6) |
35 (9.0) |
12 (3.1) |
21 (9.9) |
16 (8.5) |
16 (9.6) |
9 (4.6) |
15 (8.5) |
14 (8.0) |
3 (1.7) |
5 (2.7) |
13 (7.0) |
1 (0.5) |
15 (14.2) |
7 (6.7) |
12 (11.5) |
6 (6.1) |
| Serious AEs, n (%) |
4 (1.0) |
2 (0.5) |
4 (1.0) |
3 (1.4) |
0 (0.0) |
3 (1.8) |
0 (0.0) |
1 (0.6) |
1 (0.6) |
1 (0.6) |
2 (1.1) |
0 (0.0) |
0 (0.0) |
2 (1.9) |
2 (1.9) |
0 (0.0) |
1 (1.0) |
Data are presented as number of patients with an AE or serious AE, as a percentage of the safety analysis set
AE adverse event, Exe ER exenatide extended release, IGlar insulin glargine, Lira liraglutide, MET metformin, n number of subjects with events, N number of subjects in the safety analysis set, Sema semaglutide, Sita sitagliptin, SU sulphonylurea
aReflects subjects/events with ‘Drug withdrawn’ as action taken with trial drug
Discussion
GLP-1RAs such as semaglutide are recommended by most treatment guidelines in addition to MET and/or another OAD [3–5]. In the UK, MET and SU remain two of the most commonly prescribed therapies in clinical practice for diabetes [19], and are recommended as part of the intensification of diabetes treatment, starting with MET, followed by MET and another OAD, which is often SU [3–5, 7]. Understanding any potential impact of different background OADs such as MET and/or SU on the efficacy and safety of GLP-1RAs, and the effect of treatment intensification on the management of T2D, is key for healthcare professionals to enable them to provide their patients with optimal care.
The significant reductions in HbA1c and body weight in subjects treated with once-weekly semaglutide vs comparators (including the GLP-1RAs liraglutide and exenatide ER, the DPP4i sitagliptin, and insulin glargine) were generally consistent regardless of background OAD treatment (MET or MET + SU). This finding is likely to be clinically relevant and is supported by previous observations that the addition of a GLP-1RA to up to three OADs can be as effective as adding a GLP-1RA to fewer (1–2) OADs [22]. Additionally, previous analyses of other GLP-1RAs such as dulaglutide [23] and liraglutide [24] have also shown that GLP-1RAs are effective across a variety of background OADs, including MET, SU, MET + SU, MET + TZD and MET + SGLT2i, indicating that GLP-1RAs are effective for a broad range of patients with T2D.
In SUSTAIN 3, the estimated treatment difference (semaglutide 1.0 mg vs exenatide ER) for reduction in body weight was particularly large in the MET + SU subgroup, driven mainly by a small body weight reduction with exenatide ER in this subgroup, rather than by a difference in the semaglutide effect between subgroups. Semaglutide was more efficacious in reducing body weight than comparators in all studies, despite some differences in response to comparators between the background OAD subgroups.
Changes observed with semaglutide treatment in systolic blood pressure, lipids and eGFR were generally consistent between the two background OAD subgroups, and any differences observed were within trial and were not observed consistently across the four trials evaluated. In particular, a significantly larger reduction in systolic blood pressure was seen in SUSTAIN 4 with semaglutide vs insulin glargine in the MET + SU subgroup but not in the MET group, and this difference appeared to be driven by the smaller effect size of insulin glargine in the MET + SU subgroup than the MET only subgroup. Modest changes in lipid profile were seen across the four trials, with an observed trend for reduction in levels of total and LDL cholesterol and triglycerides, and for small increases in HDL cholesterol levels, with semaglutide and comparators. A significantly greater reduction in total cholesterol was observed in SUSTAIN 3 with semaglutide than with exenatide ER in the background MET + SU subgroup but not in the background MET subgroup, driven both by a greater reduction with semaglutide 1.0 mg and a lower reduction with exenatide ER in the MET + SU subgroup than in the MET subgroup.
The proportion of subjects who prematurely discontinued treatment because of adverse events was mostly consistent between background OAD subgroups, but the proportion was generally higher with semaglutide than with comparators, as observed across the SUSTAIN programme [8–17]. Across the four SUSTAIN trials analysed here, the event rate for severe or BG-confirmed symptomatic hypoglycaemia and minor hypoglycaemia reported with semaglutide was either comparable to or lower than the rate with active comparators (liraglutide, exenatide ER, sitagliptin or insulin glargine). This was regardless of background OAD treatment. However, there was a higher rate of hypoglycaemia in subjects receiving background MET and SU together, compared with those receiving MET alone in the same treatment arm (i.e. insulin glargine with background MET + SU compared to insulin glargine with background MET alone) across the four studies. This finding is consistent with established observations that SUs are associated with an increased risk of hypoglycaemia through excessive release of insulin, as a result of their mode of action as insulin secretagogues [25, 26], and a higher rate of hypoglycaemia was also observed in patients receiving dulaglutide with MET + SU, compared with other background therapies, in a pooled analysis of the AWARD phase 3 clinical trials [23].
A strength of this analysis is that it included a large number of subjects with T2D across four phase 3 studies. However, the analysis only evaluated subjects receiving the most commonly prescribed background therapies of MET and MET + SU and did not evaluate a wider range of background OADs, e.g. TZD or SGLT2i. The data for subjects receiving semaglutide 0.5 mg with MET + SU background therapy are based on only one trial (SUSTAIN 4), as the SUSTAIN 2 trial did not include subjects receiving background SU. This analysis was not prespecified, so results should be interpreted with caution, owing to the limitations of post hoc analyses of randomised controlled trials.
Conclusion
Improvements in glycaemic control and body weight with semaglutide were shown to be generally consistent whether subjects were taking background MET or background MET + SU. Hypoglycaemia was more common with background SU than background MET only, regardless of whether subjects received semaglutide or a comparator, and the frequency of adverse events leading to premature treatment discontinuations with semaglutide treatment was consistent regardless of background therapy. These results support healthcare professionals in providing patients with T2D with optimal care with GLP-1RAs on the basis of their background OAD medications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all the participants, investigators, and trial-site staff, as well as Ofir Frenkel and Barrie Chubb from Novo Nordisk for their review and input to the manuscript.
Funding
Novo Nordisk A/S sponsored this study and funded the Rapid Service Fee for this publication.
Medical Writing Assistance
Adam Beech, Ph.D. (AXON Communications) provided medical writing and editorial assistance for this manuscript (funded by Novo Nordisk A/S).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Matthew Capehorn reports being a partner at Clifton Medical Centre, a Clinical Manager and Partner at the Rotherham Institute for Obesity (RIO), a director at RIO Weight Management, Ltd, and a Medical Director at LighterLife (a commercial very-low-calorie diet company); he also reports research income/support for RIO from BI/Lilly, GSK, Janssen, Lighterlife, Novartis, Novo Nordisk, and Syneos; and speaker fees from BI/Lilly, GSK, Janssen, Novartis, Novo Nordisk, and Syneos. Yasmin Ghani is a Novo Nordisk employee. Charlotte Hindsberger is a consultant from S-cubed APS, Copenhagen, Denmark (contracted by Novo Nordisk A/S). Pierre Johansen is a Novo Nordisk employee and shareholder. Esteban Jódar reports being a clinical investigator for Amgen, Boehringer Ingelheim, AstraZeneca, FAES, GSK, Janssen, Lilly, MSD, Novo Nordisk, Pfizer, Sanofi; he also reports consultant fees for Amgen, AstraZeneca, FAES, Fresenius, GSK, Italfármaco, Lilly, MSD, Mundipharma, Novo Nordisk, Shire and UCB; and speaker fees from Amgen, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, FAES, Lilly, MSD, Novartis, Novo Nordisk.
Compliance with Ethics Guidelines
All four trials were conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines and the Declaration of Helsinki. All protocols were approved by the institutional review boards and ethics committees at each participating centre, and subjects provided written informed consent before trial-related activities commenced. Links to the trial registrations can be found in the supplementary material.
Data Availability
The data sets analysed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.11907837.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas, 9th edn. Brussels, Belgium: IDF; 2019. https://diabetesatlas.org/en/resources. Accessed Mar 2020.
- 2.Gavin JR, 3rd, Bohannon NJ. A review of the response to oral antidiabetes agents in patients with type 2 diabetes. Postgrad Med. 2010;122(3):43–51. doi: 10.3810/pgm.2010.05.2141. [DOI] [PubMed] [Google Scholar]
- 3.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41(12):2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S90–S102. doi: 10.2337/dc19-S009. [DOI] [PubMed] [Google Scholar]
- 5.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2019;41(2):255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 6.Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2020;63:221–228. doi: 10.1007/s00125-019-05039-w. [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence. Type 2 diabetes in adults: management [NG28] 2015. http://www.nice.org.uk/guidance/ng28. (Updated December 2015. NICE clinical guidelines). Accessed Mar 2020.
- 8.Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251–260. doi: 10.1016/S2213-8587(17)30013-X. [DOI] [PubMed] [Google Scholar]
- 9.Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341–354. doi: 10.1016/S2213-8587(17)30092-X. [DOI] [PubMed] [Google Scholar]
- 10.Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41(2):258–266. doi: 10.2337/dc17-0417. [DOI] [PubMed] [Google Scholar]
- 11.Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355–366. doi: 10.1016/S2213-8587(17)30085-2. [DOI] [PubMed] [Google Scholar]
- 12.Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291–2301. doi: 10.1210/jc.2018-00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 14.Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275–286. doi: 10.1016/S2213-8587(18)30024-X. [DOI] [PubMed] [Google Scholar]
- 15.Lingvay I, Catarig AM, Frias JP, et al. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): a double-blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(11):834–844. doi: 10.1016/S2213-8587(19)30311-0. [DOI] [PubMed] [Google Scholar]
- 16.Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356–367. doi: 10.1016/S2213-8587(19)30066-X. [DOI] [PubMed] [Google Scholar]
- 17.Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0 mg vs once-daily liraglutide 1.2 mg as add-on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10) Diabetes Metab. 2019 doi: 10.1016/j.diabet.2019.101117. [DOI] [PubMed] [Google Scholar]
- 18.International Diabetes Federation Guideline Development Group Global guideline for type 2 diabetes. Diabetes Res Clin Pract. 2014;104(1):1–52. doi: 10.1016/j.diabres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 19.National Health Service. Prescribing for diabetes: England 2007/08 to 2017/18. https://digital.nhs.uk/data-and-information/publications/statistical/prescribing-for-diabetes/2007-08---2017-18. Accessed Mar 2020.
- 20.International Conference on Harmonisation Working Group. ICH harmonised tripartite guideline: guideline for good clinical practice E6 (R1). Washington, DC: International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; 1996.
- 21.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 22.Thong K, Gupta P, Cull M, et al. GLP-1 receptor agonists in type 2 diabetes-NICE guidelines versus clinical practice. Br J Diabetes Vasc Dis. 2014;14:52–59. doi: 10.15277/bjdvd.2014.015. [DOI] [Google Scholar]
- 23.Patel H, Munir K, Sutherland S, Karanikas C, Konig M. Efficacy of dulaglutide as a first injectable option for patients with type 2 diabetes: a post hoc pooled analysis. Diabetes Ther. 2019;6(10):2321–2330. doi: 10.1007/s13300-019-00709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1-5 studies. Diabetes Obes Metab. 2009;11(Suppl 3):26–34. doi: 10.1111/j.1463-1326.2009.01075.x. [DOI] [PubMed] [Google Scholar]
- 25.Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154(9):602–613. doi: 10.7326/0003-4819-154-9-201105030-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein-Schwartz W, Stassinos G, Isbister GK. Treatment of sulfonylurea and insulin overdose. Br J Clin Pharmacol. 2016;81(3):496–504. doi: 10.1111/bcp.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets analysed during the current study are available from the corresponding author on reasonable request.