Abstract
This study examined the role of cyclin-dependent kinase inhibitor 1a (CDK1A, p21) in response to exogenous stressors during mouse preimplantation embryo development. CDKN1A knockdown (KD) one-cell zygotes were exposed to 39 °C heat stress (HS) for 4 days or irradiated by 1 (1-Gy) or 3 (3-Gy) Gy X-rays, and their developmental competence and gene expression were compared with control embryos. CDKN1A KD and HS did not influence early cleavage or subsequent embryonic development; however, HS delayed cavitation and induced elevated Cdkn1a expression in control embryos. Exposure to 1- or 3-Gy had no effect on development to the morula stage; however, a significant number of morulae failed to develop to the blastocyst stage. Interestingly, under the 1-Gy condition, the blastocyst rate of CDKN1A KD embryos (77.7%) was significantly higher than that of the controls (44.4%). In summary, exposure to cellular stressors resulted in the upregulation of Cdkn1a in embryos exposed to HS or X-ray irradiation, particularly in response to heat stress or low-dose X-ray irradiation, and depleting Cdkn1a mRNA alleviated cell cycle arrest. These findings suggest that CDKN1A plays a vital role in cellular senescence during preimplantation embryo development.
Keywords: Cdkn1a, p21, Heat stress, Senescence, Irradiation, Preimplantation
Introduction
The cellular and molecular mechanisms governing early embryo development have important implications for assisted reproductive technology (ART) because the vast majority of embryos produced in vitro fail to develop to the blastocyst (Santos et al. 2010; Glujovsky et al. 2016). Early mammalian embryos exhibit a unique cell cycle length compared to somatic cells. In the mouse, the first two mitotic divisions last about 20 h, whereas the third and fourth divisions take approximately 11 h. Interestingly, during the first mitotic division, the G1 phase is very short (1–2 h), while the second mitotic division exhibits an unusually long G1 phase (12–16 h) when major zygotic gene activation occurs. Moreover, apoptosis is not detected until the early blastocyst stage, indicating that efficient DNA damage detection and repair processes are not activated until the fifth/sixth cell division (morula to blastocyst) (Kamjoo et al. 2002; Artus and Cohen-Tannoudji 2008).
Elevated expression of the cyclin-dependent kinase inhibitor 1a (CDKN1A; known alternatively as p21cip1/waf1) induces senescence, a form of cell cycle arrest, and prevents p53-independent apoptosis in cancer cells (Hoeferlin et al. 2011; Munoz-Espin et al. 2013). During the window of mouse preimplantation development, CDKN1A appears to play a protective role. For example, in vivo fertilized embryos generated using sperm derived from irradiated males undergo normal cleavage development, but at the morula stage, they exhibit increased Cdkn1a expression and developmental delay to blastocysts (Adiga et al. 2007). In contrast, Cdkn1a knockout (KO) embryos developed normally to the blastocyst stage but exhibited chromosome instability and increased apoptosis (Adiga et al. 2007). In this context, we postulated that Cdkn1a is a crucial gene that responds rapidly to exogenous stress to delay and/or block embryonic development. To determine whether Cdkn1a can be used as a marker for monitoring cellular senescence during early embryonic development, we exposed mouse embryos to two well-studied exogenous cellular stressors, heat stress (HS) and X-ray irradiation.
Here, we report that the two types of stressors can induce elevated Cdkn1a expression resulting in either developmental delay or cell cycle arrest during the morula-to-blastocyst stage transition. CDKN1A depletion using RNA interference (RNAi) counteracted this developmental restrain resulting in blastocyst formation.
Methods
Reagents
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Embryo collection and culture
All animal procedures were performed in accordance with Institutional Animal Care and Use Committee guidelines with prior approval from the Chungnam National University Animal Welfare and Ethics Review Body (license no. CNU-00702). Mouse embryos were obtained as previously described (Choi et al. 2012). Briefly, B6D2F1/F1 female mice aged 6~8 weeks (KOATECH, Pyeongtaek, Republic of Korea) were superovulated by an intraperitoneal (i.p.) injection of 5 IU of pregnant mare serum gonadotropin (PMSG; ProSpec-Tany TechnoGene, Rehovot, Israel), followed by an i.p. injection of 5 IU of hCG 48 h later; they were then allowed to mate with males of the same strain overnight. One-cell zygotes were collected 16–17 h after hCG injection from the ampullary regions of the oviducts in M2 medium and then cultured in modified KSOM medium (EMD Millipore, Billerica, USA) under mineral oil at 37 or 39 °C (heat stress condition; HS) with a humidified incubator 5% CO2, 5% O2, and 90% N2 until use. For microinjection, 100 μM Cdkn1a (p21) siRNA (siGENOME; Dharmacon, Lafayette, CO, USA) or 100 μM control scramble siRNA (Dharmacon) was injected into the cytoplasm of one-cell zygotes with two visible pronuclei using a PLI-100A Pico-Injector (Harvard Apparatus, Holliston, MA, USA) as described previously (Choi et al. 2012).
X-ray irradiation
One-cell zygotes with two visible pronuclei were transferred to an X-ray irradiator (Rainbow™ II prototype X-ray irradiator; Rayfresh Foods, Ann Arbor, MI, USA) and directly irradiated with 1 (1-Gy) or 3 (3-Gy) Gy X-rays. After irradiation, the embryos were returned to the culture medium. Embryonic development was examined under an inverted microscope (Nikon Eclipse Ti-U; Nikon, Tokyo, Japan), and images were captured using NIS-Elements BR basic research software (Nikon).
Relative quantification of gene expression
Total RNA extracted from embryos at each stage (one-, two-, four-, and eight-cell morula and blastocyst) using the PicoPure RNA isolation kit (Arcturus, Mountain View, CA, USA) was reverse transcribed into complementary DNA (cDNA) using oligo(dT) and reverse transcriptase (SuperScript II, Invitrogen, Carlsbad, CA, USA). qRT-PCR was performed on a StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA, USA) using gene-specific primers and SFC green as an interchelator (Biofact Inc., Daejeon, Korea). The relative quantification (RQ) of target genes in knockdown (KD) embryos was normalized to the expression levels of an endogenous control, Ubtf1 (Choi et al. 2012; Schmittgen and Livak 2008). To determine the developmental expression of Cdkn1a transcripts from the one-cell zygote to the blastocyst, Chuk was used as an internal normalization control (Falco et al. 2006). The PCR primer sequences were as follows: Cdkn1a (forward) 5’-CCG TTG TCT CTT CGG TCC C-3′ and (reverse) 5’-CAT GAG CGC ATC GCA ATC-3′; Hspa1 (forward) 5′-TGG TGC TGA CGA AGA TGA AG-3′ and (reverse) 5’-CGC TGA GAG TCG TTG AAG TAG-3′; Hspb1 (forward) 5′-GAG ATC ACC ATT CCG GTT ACT T-3′ and (reverse) 5’-CAG GCT GAT GGC TTC TAC TT-3′; Ubtf (forward) 5’-TCT CAC CCC TTA CTT CCG C-3′ and (reverse) 5’-ACT CCT GTT TCT CCC TCT GG-3′; and Chuk (forward) 5’-GAC CGT GAA CAT CCT CTG ACA TGT G-3′ and (reverse) 5’-GCT CTG GTC CTC ATT TGC TTC ACG-3′.
Immunofluorescence
Morula stage embryos were fixed with 3.7% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min, permeabilized with 0.1% Tween 20 in PBS for 20 min, and blocked with 0.1% bovine serum albumin for 1 h at room temperature (RT). The embryos were washed three times in PBS for 5 min and then incubated with p21 antibody (Invitrogen, Carlsbad, CA, USA) in blocking solution overnight at 4 °C, followed by treatment of Alexa Fluor 594 secondary antibody (Invitrogen) in PBS for 30 min at RT. The embryos were mounted in VECTASHIELD containing 4,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA) and imaged using an epifluorescence inverted microscope (Nikon Ti-Eclipse, Tokyo, Japan) with NIS-Elements software (Nikon).
Statistical analysis
The statistical analysis was performed using Student’s t test or analysis of variance (ANOVA) using INSTAT3 (GraphPad Software, San Diego, CA, USA). The data are presented as the mean ± SEM. A P value of < 0.05 was considered statistically significant unless otherwise stated.
Results
Developmental expression of Cdkn1a mRNA and stress-induced CDKNA1A upregulation in mouse preimplantation embryos
In a preliminary set of experiments, we examined the expression pattern of Cdkn1a mRNA during mouse preimplantation embryo development using qRT-PCR. Cdkn1a expression was detected at all stages of preimplantation development. Cdkn1a mRNA gradually increased from the four-cell stage onward, peaking at the morula stage and then decreasing at the blastocyst stage (P < 0.05; Fig. 1a). Next, we used immunofluorescence microscopy to examine the expression and subcellular localization of CDKN1A in morulae after exposure to either heat stress (HS; 39 °C) or X-ray irradiation (1-Gy). Compared to control embryos, we observed an increase CDKN1A protein expression and nuclear localization in response to both heat stress and X-ray irradiation.
Fig. 1.
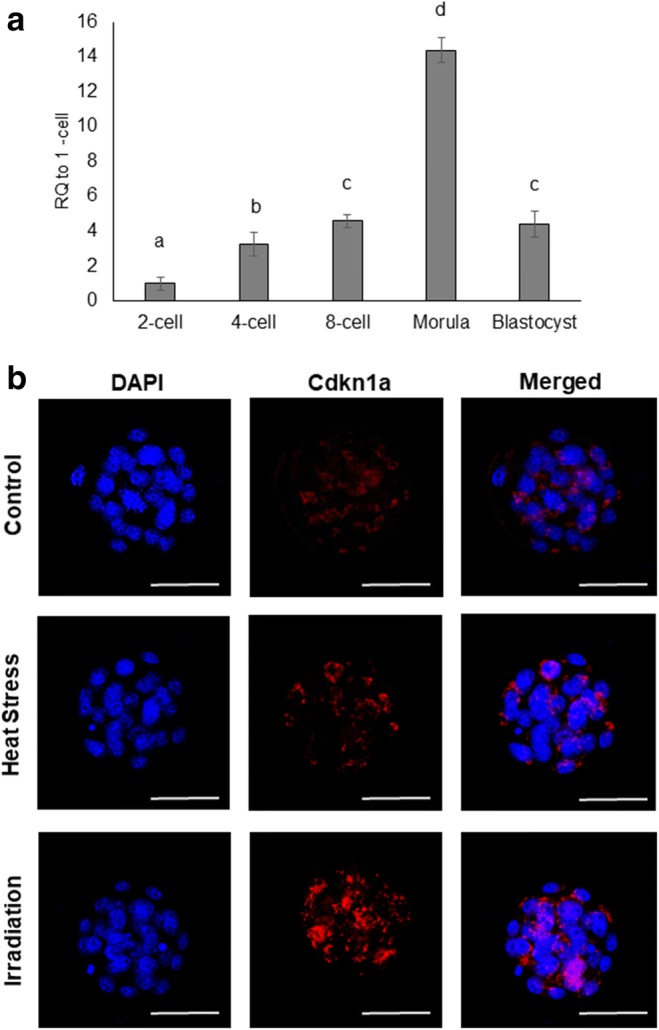
a qRT-PCR analysis of Cdkn1a transcripts in preimplantation embryos. Expression data from each stage were normalized to an internal control (Chuk) and are relative to one-cell zygotes. b Expression and subcellular localization of CDKN1A protein at morula. Error bars indicate the mean ± SEM. RQ, relative quantification. Different letters indicate significant differences (P < 0.05). Heat stress (exposed to 39 °C.); irradiation (exposed to 1-Gy X-ray)
Effects of CDKN1A knockdown (KD) on embryo development under heat stress
In the first set of experiments, we evaluated the effects of HS on mouse embryo development and gene expression. Embryos subjected to HS treatment (HS control) underwent normal cleavage development, but at 96 h post-hCG (h.p.h), the embryos exhibited a significant delay in cavitation (P < 0.05). In contrast, CDK1A KD embryos subjected to heat stress (HS-CDK1A KD) developed normally to the blastocyst stage without developmental delay (Fig. 2a, b). Regardless of the HS, CDK1A KD, or HS-CDK1A KD conditions, most embryos developed into expanded blastocysts by 112 h.p.h.
Fig. 2.

Effects of heat stress and CDKN1A KD on embryo development and gene expression. a Embryo development was examined at 96 and 112 h.p.h. Ctrl, control embryos; CDKN1A 1 KD, CDKN1A knockdown embryos; HS-Ctrl, heat-stressed control embryos; HS-CDKN1A a KD, heat-stressed CDKN1A KD embryos; 8-cell, eight-cell compacted embryos; E-Blast, early blastocyst; Ex-Blast, expanded blastocyst. b Representative images of embryos exposed to 37 or 39 °C for 4 days from one-cell zygotes at 96 and 118 h.p.h. c Expression data from CDKN1A KD, HS control, and HS-CDKN1A KD were normalized to endogenous Ubtf1, and the level of specific genes is relative to the level in the control morula (non-HS/non-KD). Error bars indicate the mean ± SEM. RQ, relative quantification. Different letters indicate significant differences (P < 0.05). Ctrl, control embryos; CDKN1A KD, CDKN1A knockdown embryos; HS, heat-stressed embryos; E-Blast, early blastocyst; Ex-Blast, expanded blastocyst
Next, we used qRT-PCR to analyze the expression of Cdkn1a and other genes associated with HS. In HS control embryos, the expression of Cdkn1a was upregulated 4.2-fold at the morula stage compared to the non-HS embryos (Fig. 2c). In parallel we evaluated the expression of two HS protein genes, Hspa1 (Hsp70) and Hspb1 (Hsp27). In CDKN1A KD and HS-CDKN1A KD embryos, we observed a significant reduction in Cdkn1a transcripts, 77.8 and 81.3%, respectively. Hspa1 (Hsp70) and Hspb1 (Hsp27) expression was upregulated in embryos treated with HS alone and HS-CDKN1A KD embryos, whereas the expression of Hspa1 and Hspb1 was unaffected in untreated CDKN1A KD embryos (Fig. 2c).
Effects of CDKN1A knockdown on embryo development with X-ray-induced DNA damage
In a second set of experiments, we examined the effects of X-ray irradiation on mouse embryo development and gene expression. Fertilized one-cell embryos were irradiated using either 0 (control), 1-Gy, or 3-Gy X-rays and then cultured to the blastocyst stage. Exposure of embryos to 1 or 3-Gy had no effect on cleavage or development to the morula stage. However, during the morula-to-blastocyst transition, there were a significant number of embryos that failed to develop into blastocysts compared to the control group. Only 44% of embryos treated with 1-Gy developed into blastocysts, while embryos exposed to 3-Gy did not develop into blastocysts at all. Notably, RNAi-mediated KD of CDKN1A rescued blastocyst formation (78%) in embryos exposed to 1-Gy irradiation, whereas CDK1A KD had not effect in embryos exposed to 3-Gy (Fig. 3a, b).
Fig. 3.
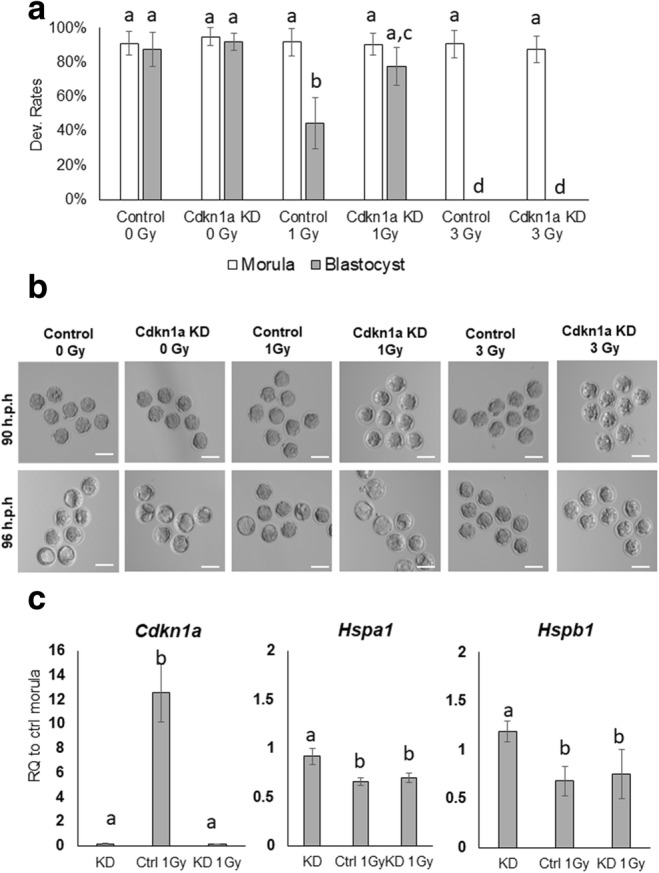
Development of preimplantation embryos irradiated with X-rays. a The development rate of control and CDKN1A KD embryos under irradiation with 0, 1, and 3-Gy X-rays. b Representative images of embryos exposed to X-ray irradiation at 90 and 96 h.p.h. c Expression data from CDKN1A KD, HS control 1-Gy, and CDKN1A KD 1-Gy were normalized to endogenous Ubtf1; the level of specific genes is relative to the level in the control morula (0 Gy/non-KD). Error bars indicate the mean ± SEM. RQ, relative quantification. Scale bars = 100 μm. Different letters indicate significant differences (P < 0.05). Ctrl, control embryos; KD, CDKN1A KD; 1-Gy, 1-Gy X-ray irradiation
Next, we examined the expression of Cdkn1a, Hspa1, and Hspb1 in control and CDKN1A KD embryos exposed to 1-Gy at the morula stage. The expression of Cdkn1a transcripts was upregulated ~12-fold in embryos treated with 1-Gy. As expected, Cdkn1a transcripts were significantly reduced in CDKN1A KD and 1-Gy treated CDKN1A KD embryos. The expression of Hspa1 and Hspb1 was similar between control and CDKN1A KD embryos. However, in the 1-Gy control and 1-Gy CDKN1A KD embryos, these genes were slightly downregulated (< 2-fold).
Discussion
In this study, we examined the developmental expression of Cdkn1a during mouse preimplantation development and evaluated its suitability as a response gene for heat stress or irradiation-induced cellular senescence. Our results in preimplantation embryos demonstrate that (1) Cdkn1a expression gradually increases during preimplantation development reaching a maximum at the morula stage, (2) exposure to moderate heat stress and low doses of irradiation trigger upregulation of CDKN1A and developmental delay and/or arrest during the morula to blastocyst transition, and (3) RNAi-mediated CDKN1A KD rescues blastocyst development in embryos exposed to heat stress and low doses of irradiation.
Consistent with Zeng et al. (2004) and Choi et al. (2012), our findings suggest that CDKN1A plays a vital role in DNA damage checkpoint and cell proliferation during the morula-to-blastocyst transition. Delayed cell cycle progression is likely due to either DNA repair or the removal of apoptotic cells that are first detected at the early blastocyst stage (Kamjoo et al. 2002).
CDKN1A was reported to induce senescence in response to different types of exogenous cellular damage (Müller and Streffer 1990; Adiga et al. 2007; Velichko et al. 2015; Hayashi et al. 2018). Therefore, to determine whether the Cdkn1a gene is responsive to exogenous stressors during early embryonic development, we subjected control and CDKN1A KD embryos to HS and different levels of X-ray irradiation. We first examined Cdkn1a expression and the timing of blastocoel formation in preimplantation embryos subjected to HS. In our previous study, we observed no differences in blastocyst development between control embryos (37 °C) and embryos subjected to moderate HS (39 °C), but delayed cavitation was observed in HS embryos (Choi et al., 2015). Interestingly, HS-treated embryos exhibited lower trophectoderm cell numbers and reduced postimplantation development after embryo transfer indicating that moderate HS treatment reduced embryo competency (Choi et al. 2015). This suggests that sublethal heat shock in preimplantation embryos affects cell proliferation and senescence. In support of this, sublethal heat shock was previously shown to negatively affect cell proliferation and induce senescence in human mesenchymal stem cells (Alekseenko et al. 2014). Consistent with our previous findings (Choi et al. 2015), delayed cavitation was observed in control embryos subjected to HS, but was not observed in the HS-CDKN1A KD embryos. This suggests that Cdkn1a is a key response gene that acts as a cellular brake during the morula-to-blastocyst transition.
Next, we examined the effects of CDKN1A KD on embryo development using a second sublethal condition where one-cell zygotes were irradiated with either 1- or 3-Gy. Previous studies reported that the threshold level of X-ray irradiation depended on the stage of preimplantation embryo development. For example, most one-cell embryos failed to develop to blastocysts at levels above 2-Gy, but 1-Gy irradiation did not affect the development of eight-cell embryos, and 2-Gy irradiation had no effect on embryos transferred to surrogates (Glenister et al. 1984; Müller et al. 1994; Hayashi et al. 2018). In accordance with Müller et al. (1994), control and CDKN1A KD embryos exposed to 3-Gy failed to develop into blastocysts, but both groups of embryos developed to the morula stage around the time Cdkn1a expression peaked (Fig. 3a, b), suggesting that CDKN1a is not directly involved in cellular senescence until the morula-to-blastocyst transition. Moreover, advanced development of 1-Gy CDKN1A KD embryos between 90 h.p.h. (morula) and 96 h.p.h. (blastocyst) and elevated Cdkn1a transcripts in 1-Gy morula embryos indicated that CDKN1A plays important roles in regulating cell cycle progression during the transition. These findings also support the notion that DNA damage responses occur in a stage-specific manner and CDKN1A is involved in apoptosis at the blastocyst stage during mouse preimplantation development (Adiga et al. 2007). As observed in the HS-CDKN1A KD condition, Hspa1 and Hspb1 mRNA were also reduced in morulae treated with 1-Gy irradiation. The mechanism has not been elucidated, but several studies have demonstrated interactions between HSP inhibitor treatments and irradiation in cancer cells, implying that HSPs are involved in cell cycle arrest and may function as molecular chaperones for DNA damage during X-ray irradiation (Ohnishi et al. 2006; Hadchity et al. 2009).
Conclusions
In summary, we found that Cdkn1a expression gradually increases during mouse preimplantation development, reaching a peak at the morula stage. CDKN1A expression was elevated in embryos exposed to HS and X-ray irradiation. Depletion of CDKN1A bypassed the cell cycle checkpoint under conditions such as long-term moderate heat stress (39 °C) or 1-Gy X-ray irradiation. Our results suggest that CDKN1A functions as a potent modulator in response to exogenous stressors and may serve as a useful marker gene for monitoring cellular senescence during mouse preimplantation embryo development.
Funding information
The work was supported by Chungnam National University (2019-0635-01 to IC), Republic of Korea, and by R01HD095371 from the National Institute of Child Health and Development to J.G.K.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jason G. Knott, Email: knottj@msu.edu
Inchul Choi, Email: icchoi@cnu.ac.kr.
References
- Adiga SK, Toyoshima M, Shiraishi K, Shimura T, Takeda J, Taga M, Nagai H, Kumar P, Niwa O. p21 provides stage specific DNA damage control to preimplantation embryos. Oncogene. 2007;26:6141–6149. doi: 10.1038/sj.onc.1210444. [DOI] [PubMed] [Google Scholar]
- Alekseenko LL, et al. Sublethal heat shock induces premature senescence rather than apoptosis in human mesenchymal stem cells. Cell Stress & Chaperones. 2014;19:355–366. doi: 10.1007/s12192-013-0463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artus J, Cohen-Tannoudji M. Cell cycle regulation during early mouse embryogenesis. Molecular and Cellular Endocrinology. 2008;282:78–86. doi: 10.1016/j.mce.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Choi I, Carey TS, Wilson CA, Knott JG. Transcription factor AP-2γ is a core regulator of tight junction biogenesis and cavity formation during mouse early embryogenesis. Development. 2012;139:4623–4632. doi: 10.1242/dev.086645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi I, Dasari A, Kim N-H, KHS C. Effects of prolonged exposure of mouse embryos to elevated temperatures on embryonic developmental competence. Reproductive BioMedicine Online. 2015;31:171–179. doi: 10.1016/j.rbmo.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Falco G, Stanghellini I, Ko MSH. Use of Chuk as an internal standard suitable for quantitative RT-PCR in mouse preimplantation embryos. Reproductive BioMedicine Online. 2006;13:394–403. doi: 10.1016/S1472-6483(10)61445-9. [DOI] [PubMed] [Google Scholar]
- Glenister PH, Whittingham DG, Lyon MF. Further studies on the effect of radiation during the storage of frozen 8-cell mouse embryos at −196 degrees C. J Reprod Fertil. 1984;70:229–234. doi: 10.1530/jrf.0.0700229. [DOI] [PubMed] [Google Scholar]
- Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D (2016) Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database of Systematic Reviews. 10.1002/14651858.CD002118.pub5 [DOI] [PubMed]
- Hadchity E, et al. Heat shock protein 27 as a new therapeutic target for radiation sensitization of head and neck squamous cell carcinoma. Molecular Therapy. 2009;17:1387–1394. doi: 10.1038/mt.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M et al (2018) Low-dose irradiation of mouse embryos increases Smad-p21 pathway activity and preserves pluripotency. Journal of Assisted Reproduction and Genetics. 10.1007/s10815-018-1156-y [DOI] [PMC free article] [PubMed]
- Hoeferlin LA, Oleinik NV, Krupenko NI, Krupenko SA. Activation of p21-dependent G1/G2 arrest in the absence of DNA damage as an antiapoptotic response to metabolic stress. Genes & Cancer. 2011;2:889–899. doi: 10.1177/1947601911432495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamjoo M, Brison DR, Kimber SJ. Apoptosis in the preimplantation mouse embryo: effect of strain difference and in vitro culture. Molecular reproduction and development. 2002;61:67–77. doi: 10.1002/mrd.1132. [DOI] [PubMed] [Google Scholar]
- Müller W-U, Streffer C, Pampfer S. The question of threshold doses for radiation damage: Malformations induced by radiation exposure of unicellular or multicellular preimplantation stages of the mouse. Radiation and Environmental Biophysics. 1994;33:63–68. doi: 10.1007/bf01255274. [DOI] [PubMed] [Google Scholar]
- Müller WU, Streffer C. Lethal and teratogenic effects after exposure to X-rays at various times of early murine gestation. Teratology. 1990;42:643–650. doi: 10.1002/tera.1420420609. [DOI] [PubMed] [Google Scholar]
- Munoz-Espin D, et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Ohnishi K, Yokota S, Takahashi A, Ohnishi T. Induction of radiation resistance by a heat shock protein inhibitor, KNK437, in human glioblastoma cells. International Journal of Radiation Biology. 2006;82:569–575. doi: 10.1080/09553000600876645. [DOI] [PubMed] [Google Scholar]
- Santos MA, Kuijk EW, Macklon NS. The impact of ovarian stimulation for IVF on the developing embryo. Reproduction. 2010;139:23–34. doi: 10.1530/rep-09-0187. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Velichko AK, Petrova NV, Razin SV, Kantidze OL. Mechanism of heat stress-induced cellular senescence elucidates the exclusive vulnerability of early S-phase cells to mild genotoxic stress. Nucleic Acids Research. 2015;43:6309–6320. doi: 10.1093/nar/gkv573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F, Baldwin DA, Schultz RM. Transcript profiling during preimplantation mouse development. Developmental Biology. 2004;272:483–496. doi: 10.1016/j.ydbio.2004.05.018. [DOI] [PubMed] [Google Scholar]


