Abstract
Protein phosphatase 5 (PP5) is a serine/threonine protein phosphatase that regulates many cellular functions including steroid hormone signaling, stress response, proliferation, apoptosis, and DNA repair. PP5 is also a co-chaperone of the heat shock protein 90 molecular chaperone machinery that assists in regulation of cellular signaling pathways essential for cell survival and growth. PP5 plays a significant role in survival and propagation of multiple cancers, which makes it a promising target for cancer therapy. Though there are several naturally occurring PP5 inhibitors, none is specific for PP5. Here, we review the roles of PP5 in cancer progression and survival and discuss the unique features of the PP5 structure that differentiate it from other phosphoprotein phosphatase (PPP) family members and make it an attractive therapeutic target.
Keywords: Serine/threonine phosphatase 5, Molecular chaperone, Co-chaperone, Heat shock protein 90, Clear cell renal cell carcinoma, Cancer, Post-translational modifications
Introduction
Protein phosphatase 5 (PP5) is a serine/threonine phosphatase and a co-chaperone of heat shock protein 90 (Hsp90) that helps regulate an array of cellular functions including stress response, proliferation, apoptosis, and DNA repair (Hinds Jr. and Sanchez 2008). It is part of the phosphoprotein phosphatase (PPP) family, which also includes PP1, PP2A, PP2B, PP4, PP6, and PP7 (Shi 2009; Swingle et al. 2004). In contrast to other family members, the catalytic and regulatory domains of PP5 are encoded on a single polypeptide (Shi 2009). This causes PP5 to have a low basal activity as it adopts an auto-inhibited conformation (Kang et al. 2001). Association of Hsp90 to the tetratricopeptide repeat (TPR) domain of PP5, binding of polyunsaturated fatty acids, or post-translational modification can all activate PP5 (Fig. 1a, b) (Chen and Cohen 1997; Dushukyan et al. 2017; Silverstein et al. 1997). PP5 substrates include the glucocorticoid receptor (GR), tumor suppressor p53, Hsp90, and the co-chaperone Cdc37 (Silverstein et al. 1997; Soroka et al. 2012; Vaughan et al. 2008; Wandinger et al. 2006; Wang et al. 2018). Elevated PP5 expression has been shown to increase proliferation in most cells and has also been linked to the progression of breast and kidney cancers (Dushukyan et al. 2017; Hinds Jr. and Sanchez 2008). In addition to cancer, through its diverse functions, PP5 has also been implicated in asthma, cardiac contractility and heart failure, diabetes, and lipid metabolism and obesity (Fransson et al. 2014; Gergs et al. 2019; Hinds Jr. et al. 2011; Krysiak et al. 2018; Pazdrak et al. 2016). The role of PP5 in proliferation and cell survival as well as its unique structure makes it a potentially attractive therapeutic target. Here, we review the current literature highlighting the distinctive properties of PP5 structure and function as well as its role in cancer and as a therapeutic target.
Fig. 1.
Mechanisms for activation of PP5. a Interaction of the extreme C-terminal MEEVD sequence of Hsp90 with the TPR domain of PP5 breaks the auto-inhibited state allowing for PP5 activity. b Alternative paradigm where mechanisms such as phosphorylation of PP5-T362 allows for activation of PP5 independent of Hsp90
PP5 structure and function
Accessing the PP5 catalytic site
Unlike other PPP family members, which are regulated by non-covalent interactions with separate regulatory proteins, the sequence and structure of PP5 include both catalytic and regulatory domains (Shi 2009). The amino-terminal regulatory domain contains three consecutive tetratricopeptide repeat (TPR) motifs used for protein-protein interaction (Fig. 2a) (Das et al. 1998). The TPR domain interacts with the extreme C-terminal alpha J helix (αJ) in the auto-inhibited state, which blocks substrate access to the catalytic groove (Fig. 2b, c) (Kang et al. 2001). The auto-inhibition can be broken by interaction of PP5 activators such as the molecular chaperone Hsp90 or fatty acids, like arachidonic acid, with the TPR domain of PP5 (Haslbeck et al. 2015b; Vaughan et al. 2008; Yang et al. 2005; Zeke et al. 2005). This releases the αJ helix and allows for substrates to access the catalytic site (Fig. 1a).
Fig. 2.
Structure of protein phosphatase-5 (PP5). a Schematic representation of the domains of PP5. b Space-filled model of the crystal structure of PP5 (PDB: 1WAO). Alpha J helix (red) contacts the TPR domain (gold) to result in auto-inhibition. The catalytic domain (blue) is connected to the TPR domain by a linker (gray). c Ribbon structure (PDB: 1WAO) demonstrating alpha helices of PP5 TPR domains (gold) with active site highlighted below by box (pink). d Detailed view of the PP5 catalytic site with essential residues D274, R275, N303, H304, and R400 highlighted in pink (PDB: 1S95). e Detailed view of the PP5 catalytic site with catalytic H304 highlighted (PDB: 1S95). f Cdc37 peptide (tan) bound to the PP5 catalytic site (PDB: 5HPE). Residues important for substrate coordination and PP5 activity R275, N308, M309, Y313, and Y451 are highlighted in pink
Within the catalytic site of PP5, there are several key residues that are essential for its activity as well as two essential metal ions (M1 and M2). PP5 has been shown to coordinate Mn+2, Zn+2, and Fe+2, but commonly prefers Mn+2 (Fig. 2d–f) (Oberoi et al. 2016; Swingle et al. 2004). These two metal ions are important for coordinating the target phosphate groups of PP5 substrates. They are often both Mn+2 ions but can be other metals as described above. An exciting new study demonstrated that different states of the manganese ion alter the structure of the active site leading to secondary changes in conformation, especially in the αJ helix, and subsequently affect PP5 activity (Wang and Yan 2019).
Based on the PP5 crystal structure, residues R275, N303, H304, and R400 are all responsible for the coordination of the target phosphate ion through direct hydrogen bond interactions with all four oxygen atoms of the phosphate group (Fig. 2d, e) (Swingle et al. 2004). N303 interacts with both M2 and the substrate phosphate group, whereas the other residues are set slightly above the metal ions and only interact with the substrate. Interestingly, though D274 does not have any direct interaction with the metal ions, water, or substrates, it is critical for PP5 activity because it forms a necessary hydrogen bond with H304 (Fig. 2d, e) (Swingle et al. 2004). These residues are essential for the catalytic activity of PP5.
In addition to the aforementioned residues, other residues crucial for PP5 function include N308, M309, Y313, and Y451 (Fig. 2f) (Oberoi et al. 2016). These residues were identified when the crystal structure of the catalytic domain in complex with a Cdc37 peptide substrate was solved (Oberoi et al. 2016). Of these four residues, the first three are involved in coordinating PP5 substrates, specifically Cdc37, through direct coordination (N308 and Y313) or through van der Waals interactions (M309). The last residue (Y451) does not directly interact with substrates, but instead forms hydrogen bonds with R275 and a water molecule that help determine substrate conformation. The substrate-contacting residues essentially form a lining along the inside of the catalytic pocket above the metal ions ensuring the proper, and stable, positioning of PP5 substrates. Although the catalytic domains of PP5 and other PPP proteins are similar enough to be inhibited by the same molecule such as okadaic acid, the abundance of key residues that are unique to PP5 makes it a druggable target (Golden et al. 2008b; Hinds Jr. and Sanchez 2008). Developing a specific PP5 inhibitor is an enticing concept as it would allow us to regulate many different cellular pathways and would also provide a possible treatment for several cancers (see below).
PP5 substrates and binding partners
PP5 plays a key regulatory role in many signaling and stress response pathways and has been shown to interact with many different transcription factors and proteins involved in the DNA damage response. One well-described interaction is with the glucocorticoid receptor (GR) through a GR-Hsp90 heterocomplex (Davies et al. 2005; Jacob et al. 2015). PP5 directly dephosphorylates GR on several residues, modulating GR activity (Wang et al. 2007). It has also been shown to directly interact and co-localize with the CDC16/CDC27 subunits of the anaphase-promoting complex (APC) (Ollendorff and Donoghue 1997). This data suggests that PP5 may play an important role in the regulation of APC activity and thus in the progression of cells from metaphase into anaphase. In addition, PP5 has been reported to regulate p53 directly and through upstream pathways showing that PP5 also plays a role in DNA damage response (Amable et al. 2011; Wang et al. 2018; Zuo et al. 1998). Other known interactions include the following: CK1ε (Partch et al. 2006); copine (Tomsig et al. 2003); cryptochrome 2 (Zhao and Sancar 1997); Hsp90-dependent heme-regulated eIF2α kinase (Shao et al. 2002); apoptosis signal–regulating kinase 1 (ASK1) (Morita et al. 2001); DNA-PKcs (DNA-dependent Ser/Thr protein kinase) (Wechsler et al. 2004); ATM (ataxia telangiectasia–mutated kinase) (Ali et al. 2004); ATR (ATM- and Rad 3–related kinase) (Zhang et al. 2005); A-regulatory subunit of protein phosphatase type 2A (Lubert et al. 2001); G12-α/G13-α subunits of heterotrimeric G proteins (Yamaguchi et al. 2002); SMAD2/3 (Bruce et al. 2012); Rac1 and Ras (Chatterjee et al. 2010; Gentile et al. 2006; Mazalouskas et al. 2014); and Raf1 (von Kriegsheim et al. 2006) (Table 1). Through these interactions, PP5 helps regulate many different pathways and checkpoints involved in the cell cycle, varying from DNA damage checkpoints to the transition to anaphase, and to apoptosis.
Table 1.
Known PP5 substrates, activators, and interactors
| References | |
|---|---|
| Substrates | |
| 53BP1 | Kang et al. (2009) |
| ABCB1 (P-glycoprotein) | Katayama et al. (2014) |
| AMPK | Chen et al. (2017); Hsieh et al. (2017a); Hsieh et al. (2017b); Hu et al. (2018) |
| Ask1 | Morita et al. (2001) |
| Cdc37 | Vaughan et al. (2008) |
| CK1ε | Partch et al. (2006) |
| DNA-PKcs | Wechsler et al. (2004); Wu et al. (2015) |
| ER | Ikeda et al. (2004) |
| FNIP1 | Sager et al. (2019) |
| GR | Davies et al. (2005); Jacob et al. (2015); Wang et al. (2007) |
| Hsf1 | Cho et al. (2014); Conde et al. (2005) |
| Hsp90 | Soroka et al. (2012); Wandinger et al. (2006) |
| p53 | Amable et al. (2011); Wang et al. (2018); Zuo et al. (1998) |
| Raf1 | Mazalouskas et al. (2014); von Kriegsheim et al. (2006) |
| PPARγ | Hinds Jr. et al. (2011) |
| SMAD2/3 | Bruce et al. (2012) |
| Tau | Gong et al. (2004); Liu et al. (2005) |
| Titin | Krysiak et al. (2018) |
| Activators | |
| Arachidonic acid | Chen and Cohen (1997); Ramsey and Chinkers (2002); Sinclair et al. (1999) |
| CK1δ | Dushukyan et al. (2017) |
| Copine I | Tomsig et al. (2003) |
| Hsp70 | Connarn et al. (2014) |
| Hsp90 | Chen et al. (1996); Ramsey and Chinkers (2002); Silverstein et al. (1997); Yang et al. (2005) |
| Rac1 | Chatterjee et al. (2010); Gentile et al. (2006); Mazalouskas et al. (2014) |
| S100 | Yamaguchi et al. (2012) |
| Interactors | |
| ATM | Ali et al. (2004); Huang et al. (2018) |
| ATR | Zhang et al. (2005) |
| AR | Schulke et al. (2010) |
| CDC16/CDC27 (APC) | Ollendorff and Donoghue (1997) |
| Cryptochrome 2 | Zhao and Sancar (1997) |
| eIF2 α | Shao et al. (2002) |
| Erk 1/2 | Mazalouskas et al. (2014) |
| FKBP51/52 | Banerjee et al. (2008); Davies et al. (2005); Gallo et al. (2007); Silverstein et al. (1997) |
| G α12 | Yamaguchi et al. (2002) |
| G α13 | Yamaguchi et al. (2002) |
| gp96 | Mkaddem et al. (2009) |
| PP2A | Lubert et al. (2001) |
| VHL | Dushukyan et al. (2017) |
Co-chaperone function of PP5
PP5 is a co-chaperone of Hsp90 and regulates the chaperone function largely through regulation of and cooperation with other Hsp90 co-chaperones. Binding to Hsp90 is also one of the mechanisms for the activation of PP5 catalytic activity. PP5 modulates the chaperoning of numerous kinases via PP5-mediated dephosphorylation of the co-chaperone Cdc37 at S13 (Vaughan et al. 2008). Cdc37 is phosphorylated by casein kinase 2 (CK2) and is then able to recruit kinases to the Hsp90 chaperone (Bandhakavi et al. 2003; Miyata and Nishida 2004; Shao et al. 2003; Vaughan et al. 2006). Binding of PP5 to this Cdc37-kinase-Hsp90 heterocomplex results in dephosphorylation of Cdc37 and leads to subsequent release of Cdc37 and mature kinase (Vaughan et al. 2008). The crystal structure of phosphomimetic Cdc37 peptide bound to the catalytic domain of PP5 revealed that despite high conservation of structure in the catalytic site, there are also elements of substrate specificity (Oberoi et al. 2016). Utilization of this structure led to generation of structure based PP5 mutants. Analysis of these mutants in vivo revealed that mutations that lead to hypoactivity of PP5 activity also result in trapping of kinases in these chaperone complexes as opposed to the usual transient interactions during an efficient chaperone cycle (Oberoi et al. 2016). Additionally, PP5 activity affects Hsp90 binding to inhibitors with varied effects. Work based on yeast ortholog of PP5 (PPT1) demonstrated that it also functions as the co-chaperone by directly dephosphorylating Hsp90 at several residues consequently modulating its chaperone function (Soroka et al. 2012; Wandinger et al. 2006).
PP5 function also modulates the effects mediated by other Hsp90 co-chaperones. PP5 has been long known to work with the co-chaperones FKBP51 and FKBP52 in the chaperoning of steroid hormone receptors (Banerjee et al. 2008; Davies et al. 2005; Gallo et al. 2007; Silverstein et al. 1997). It has additionally been shown that PP5 regulates calcium currents in inter-endothelial cell gap junctions through a PP5-FKBP51 axis (Hamilton et al. 2018). Furthermore, PP5 also dephosphorylates a relay phosphorylation of serine residues on the co-chaperone FNIP1 (Sager et al. 2019). Complete relay phosphorylation by CK2 of FNIP1 promotes FNIP1 interaction with Hsp90 and activation and stabilization of both kinase and non-kinase clients.
Activation of PP5 activity
As discussed above, PP5 exists largely in an auto-inhibited conformation that requires release for activation of PP5 catalytic activity (Kang et al. 2001; Yang et al. 2005). If the catalytic domain is artificially isolated through protein truncation or deletion of the auto-inhibitory αJ helix, the catalytic domain is constitutively active much like other PPP family members (Chen and Cohen 1997; Kang et al. 2001; Sinclair et al. 1999). One well-known mechanism of PP5 activation is through the interaction of the TPR domain of PP5 with the C-terminal MEEVD TPR-binding motif of Hsp90 (Fig. 1a) (Chen et al. 1996; Ramsey and Chinkers 2002; Silverstein et al. 1997; Yang et al. 2005). Binding of the chaperone Hsp70 to the TPR domain of PP5 also activates the phosphatase (Connarn et al. 2014). Interestingly, the MEEVD of Hsp90 binds PP5 with a higher affinity than the IEEVD of Hsp70; however, the full-length Hsp70 binding stimulates PP5 phosphatase activity more than binding of Hsp90 (Connarn et al. 2014).
In addition, PP5 can be activated by interaction with polyunsaturated fatty acids like arachidonic acid and fatty acid-CoA esters (Chen and Cohen 1997; Ramsey and Chinkers 2002; Sinclair et al. 1999). These interactions stabilize an alternate conformation of the TPR domain as compared with the interaction with Hsp90 (Yang et al. 2005). While much of this early work was performed in vitro, it has also been shown that treatment of cells with arachidonic acid or nocodazole, a microtubule-depolymerizing drug, can lead to release of PP5 from Hsp90 and Hsp70, proteolytic activation, and subsequent degradation of PP5 (Zeke et al. 2005). PP5 can also be activated by Ca+2-sensing S100 proteins (Yamaguchi et al. 2012). This ability of S100 proteins, however, is impaired by oxidative stress (Yamaguchi et al. 2016). Small molecule activators of PP5 have also been developed. The molecules bind in allosteric sites likely in the catalytic domain and data suggests they may relax the auto-inhibited state (Haslbeck et al. 2015a). Furthermore, it has also recently been demonstrated that PP5 activity can be further modulated by post-translational modifications (PTMs) on PP5 itself. Phosphorylation of PP5 on T362 leads to activation of PP5 in the absence of Hsp90 in vitro (Fig. 1b) (Dushukyan et al. 2017). T362 phosphorylation of PP5 is essential for its activity in cells despite PP5 activity in the absence of this modification in vitro. PP5 is also subjected to multi-monoubiquitination on K185 and K199, which leads to its degradation and serves as a control switch to shut off PP5 activity (Dushukyan et al. 2017). These modifications on PP5 were altered in kidney cancer suggesting that PP5 may be differentially regulated in cancer and may serve as a therapeutic target.
Role of PP5 in cancer
As mentioned above, PP5 plays a key role in several pathways important for cancer progression and survival including stress response pathways, proliferation, and DNA damage response (Fig. 3). Early work demonstrated that PP5 indirectly works upstream as a negative regulator of the tumor suppressor p53 (Zuo et al. 1998; Zuo et al. 1999). A more recent study found that PP5-deficient mice were hypersensitive to genotoxic stress, which was associated with upregulation of p53 and its downstream targets (Wang et al. 2018). PP5 was found to interact with and directly dephosphorylate p53. Furthermore, complete loss of PP5 reduced tumorigenesis and prolonged survival in p53+/– mice (Wang et al. 2018). These proteins were reciprocally regulated as p53 repressed PP5 transcription.
Fig. 3.

PP5 is involved in many normal cellular pathways and has been implicated in several cancers
There are numerous links of PP5 altering signaling networks important for such processes as metastasis, proliferation, differentiation, and oncogenesis including Rho/Rac signaling and signaling through the MAPK pathway through inactivation of Raf-1 (Gentile et al. 2006; Mazalouskas et al. 2014; Yamaguchi et al. 2002). PP5 can also directly dephosphorylate ASK1, a kinase involved in p38 and JNK kinase cascades (Morita et al. 2001). There are further reports linking p53, PP5, and ASK1; the details of the precise role of PP5 remain to be elucidated (Huang et al. 2004; Morita et al. 2001; Zhou et al. 2004). Additionally, PP5 plays other roles in DNA damage response and cell cycle control. It appears to act as a negative regulator of DNA damage–activated protein kinase, DNA-PKcs, which plays an important role in nonhomologous end-joining repair of double-strand DNA breaks (Wechsler et al. 2004). In contrast, PP5 positively regulates ATM and ATR-signaling in DNA damage cell cycle control checkpoints (Ali et al. 2004; Zhang et al. 2005). PP5 also directly binds and dephosphorylates p53-binding protein 1 (53BP1), and elevated PP5 expression was associated with reduced nonhomologous end-joining repair (Kang et al. 2009). Additionally, silencing or inhibition of PP5 is associated with G0/G1 cell cycle arrest in many cancers. Due to these many described functions as well as additional mechanisms, PP5 has been shown to be important for cancer progression and survival in a wide variety of cancers (Fig. 3).
Breast Cancer
There are numerous studies linking elevated PP5 levels to breast cancer progression. A positive correlation was found between PP5 overexpression and various malignant breast pathologies when examining tissue microarrays (TMA) for PP5 expression (Golden et al. 2008a). The same study found that PP5 overexpression was associated with increased proliferation in MCF7 cells in culture as well as increased tumor growth in a mouse xenograft model (Golden et al. 2008a). It has also been demonstrated that PP5 affects estrogen signaling. PP5 expression has been shown to be estrogen-induced; however, constitutive overexpression of PP5 allows estrogen-dependent cells to continue to proliferate in estrogen-depleted media suggesting that elevated PP5 expression may contribute to estrogen-independent growth (Urban et al. 2001). PP5 has been shown to directly interact with the estrogen receptor (ER) and to desphosphorylate Ser118 on ERα, a major phosphorylation site that has been associated with tamoxifen responsiveness (Huderson et al. 2012; Ikeda et al. 2004; Kok et al. 2009). Taken together, it is clear that PP5 expression in breast cancer may be affecting estrogen signaling and response to therapy and warrants further exploration as a therapeutic target.
Hepatocellular carcinoma and cholangiocarcinoma
Expression of PP5 has also been found to be elevated in tumor samples from patients with hepatocellular carcinoma (HCC) and was associated with worse clinical outcomes (Chen et al. 2017). This same study found that silencing or knockdown of PP5 in HCC cells inhibited growth through an AMPK-associated mechanism (Chen et al. 2017). Similarly, overexpression of PP5 in cholangiocarcinoma (CCA) cells led to increased cell growth and colony formation, whereas shRNA-mediated knockdown of PP5 in tumor xenografts decreased growth and enhanced pAMPK (Hu et al. 2018). The same group has also shown that palbociclib, a CDK4/6 inhibitor, induced autophagy, and apoptosis in HCC cells through a mechanism that involved upregulation of pAMPK and inhibition of PP5, independent of CDK4/6 (Hsieh et al. 2017a). Palbociclib also enhanced radiosensitivity of HCC and cholangiocarcinoma (CCA) cells through inhibition of ATM kinase via PP5, which would normally respond to repair double-stranded DNA breaks (Huang et al. 2018).
Non-small cell lung cancer
Similarly, PP5 expression has also been found to be elevated in human lung tumor samples relative to adjacent normal tissue (Hsieh et al. 2017b). Overexpression of PP5 in A549 lung cancer cells has also been linked to enhanced proliferation and colony formation. Inhibition of PP5 activity by cantharidin caused apoptosis and reduced tumor growth via increased pAMPK signaling (Hsieh et al. 2017b).
Colorectal carcinoma
PP5 knockdown in colorectal carcinoma (CRC) cells also inhibited cell proliferation and colony formation and caused G0/G1 arrest (Wang et al. 2015). In contrast, another study showed that overexpression of PP5 in HT-29 CRC cells led to increased sensitivity to mTOR inhibitor WAY-600. This sensitivity was found to be dependent on PP5-mediated dephosphorylation of DNA-PKcs T2609 (Wu et al. 2015). Together, these results show that PP5 is necessary for colorectal cancer growth, but it can also play an anti-tumorigenic role through DNA-PKcs dephosphorylation when it is overexpressed. Further research is required to elucidate the nuances of the role PP5 plays in colorectal cancer.
Prostate cancer
Levels of PP5 are elevated in prostate cancer cell lines when compared with normal prostate cell lines (Periyasamy et al. 2007). Although this link has not been thoroughly studied, PP5 and the androgen receptor (AR) interact in chaperone complexes (Schulke et al. 2010); however, PP5-mediated dephosphorylation of AR has yet to be established (Periyasamy et al. 2007; Schulke et al. 2010). AR associates with many of the same TPR-containing proteins that compete with PP5 for GR binding, which strengthens the likelihood that there is an as-of-yet undiscovered regulatory interaction between AR and PP5 (Schulke et al. 2010).
Kidney cancer
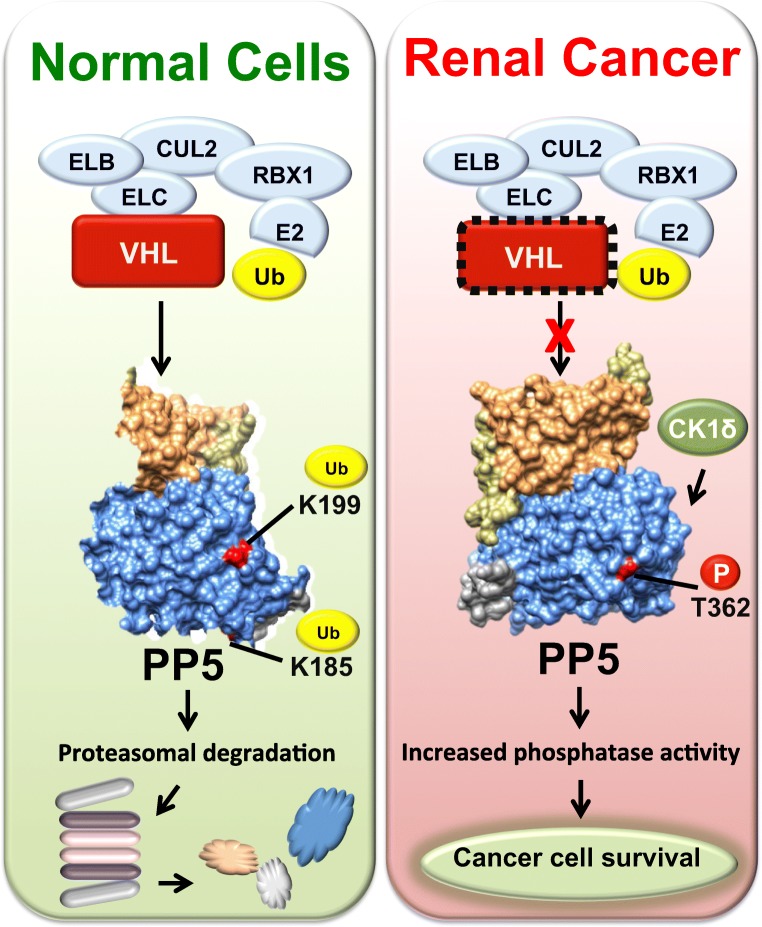
There has also been recent work demonstrating a prosurvival role for PP5 in clear cell renal cell carcinoma (ccRCC), the most common type of kidney cancer (Dushukyan et al. 2017). The majority of cases of ccRCC harbor mutations in the tumor suppressor von Hippel-Lindau (VHL), which is the recognition subunit of an E3 ubiquitin ligase complex best known for ubiquitinating HIF in a hypoxia-dependent manner (Hsieh et al. 2017c). It was demonstrated that PP5 is upregulated in VHL-null ccRCC cell lines and human tumor samples as PP5 is targeted for degradation by VHL-dependent multi-monoubiquitination of PP5 K185 and K199 (Fig. 4) (Dushukyan et al. 2017). Furthermore, PP5 activity was enhanced independent of Hsp90 activation by phosphorylation on T362 by CK1δ (Fig. 4). Targeting PP5 activity by either siRNA silencing or blocking T362 phosphorylation via IC261-mediated CK1δ inhibition led to induction of apoptosis specifically in VHL-null ccRCC cells (Dushukyan et al. 2017). This supports work to identify other important PTMs on PP5 as well as development of PP5-specific inhibitors or other mechanisms to target the activity of PP5.
Fig. 4.
Post-translational regulation of PP5 in kidney cancer. In normal cells, the VHL-containing E3 ubiquitin ligase complex ubiquitinates PP5 on K185 and K199 leading to its proteasomal degradation and controls PP5 expression level and activity. In kidney cancer with non-functional VHL, PP5 is no longer degraded, allowing it to accumulate and be phosphorylated by CK1δ. This leads to increased PP5 phosphatase activity and cancer cell survival. ELB elongin B, ELC elongin C, CUL2 cullin-2, RBX1 ring box-1 (E3 ubiquitin ligase)
Other cancers
Glioma
PP5 knockdown inhibited cell proliferation, colony formation, and cell migration and arrested cells in G0/G1 in the human glioma cell lines U251 and U373 (Zhi et al. 2015).
Ovarian Cancer
PP5 knockdown in CAOV-3 ovarian cancer cells also inhibited proliferation and colony formation as a result of G0/G1 arrest (Zheng et al. 2016).
Osteosarcoma
PP5 is also highly expressed in several human osteosarcoma cell lines. Similarly to above, lentivirus-delivered shRNA knockdown decreased viability and proliferation as a result of G0/G1 arrest (Han et al. 2017).
Inhibition of PP5
PP5 has been found to be upregulated in a variety of different cancers and silencing of PP5 or inhibition of its activity decreases tumor growth and survival. These effects are mediated by PP5 influence on diverse cellular functions including but not limited to DNA damage response, cell proliferation and cell cycle progression, and various signaling pathways including steroid hormone receptor signaling. As a result, PP5 is an attractive drug target. There are several naturally occurring PP5 inhibitors that, unfortunately, are not specific to PP5 and can also inhibit other members of the PPP family. These include but are not limited to the following: okadaic acid, microcystins, nodularin, calyculin A, tautomycin, and cantharidin (Golden et al. 2008b; Swingle et al. 2007). Due to the lack of specificity, these inhibitors are not viable as therapeutic approaches and designing specific phosphatase inhibitors is difficult due to similarities within the catalytic domains of phosphatases (McConnell and Wadzinski 2009). The antitumor drug LB-100, which has completed a phase 1 clinical trial and is actively recruiting in phase 1b/2 (NCT03886662) and phase 2 (NCT03027388) trials, was designed as a specific PPP2AC inhibitor (clinicaltrials.gov). However, it has now been shown that this drug also inhibits PP5 and some of the antitumor action may be ascribable to this role (D’Arcy et al. 2019). This highlights the difficulty in designing specific phosphatase inhibitors. Fortunately, there are several aspects of PP5 structure and function as reviewed here that make it unique and a likely druggable target. Robust fluorescent phosphatase assays for screening candidate inhibitors of PP5 have been developed (Swingle and Honkanen 2014). Recently, an ultra-high-throughput screen using this technology showed promise and identified ~ 30 new candidate PP5 inhibitors (Swingle et al. 2017). Similarly, three other new PP5 inhibitors were reported and included Ro 90-7501, which inhibited PP5 in a TPR-domain-dependent manner unlike the majority of inhibitors that bind to the phosphatase domain (Hong et al. 2017). Inhibiting PP5 by targeting the TPR domain may mediate its effects through abrogating the interaction of PP5 with Hsp90 and thereby blocking its interaction with substrates. This may provide a distinct mechanism of inhibiting PP5 activity by prohibiting its interaction with its target proteins. These recent studies suggest there may be a bright future for specific PP5 inhibitors. Additionally, we have demonstrated that targeting CK1δ, which phosphorylates T362 of PP5 and enhances its activity, provides a viable option for inducing ccRCC cell death in a PP5-specific manner (Dushukyan et al. 2017). Furthermore, this led to apoptosis selectively in VHL-null ccRCC in which PP5 is upregulated suggesting there is a therapeutic window for PP5 inhibition. This suggests there may be additional mechanisms for targeting PP5 activity aside from PP5-specific small molecule inhibitors.
Conclusions and future perspectives
Despite the vast amount of data available on PP5 structure and function, there are some outstanding questions related to PP5 regulation in cells. There is already limited information on PTMs of PP5 and their impact on activation and controlling its activity; however, it is unclear if other PTMs play a role in PP5 regulation. Additionally, although there are several reported PP5 substrates/interactors, we are still lacking a robust and comprehensive list of its substrates. This information will potentially help us in deciphering the mechanism of substrate specificity and identifying a consensus sequence or docking motif for PP5 substrates. The synthesis of this knowledge would further allow us to better understand the true physiological relevance of this phosphatase and its controlled activation. Since the role of PP5 in tumor progression and survival is firmly established, targeting PP5 is an attractive approach in treating different types of cancer.
Acknowledgments
The authors are grateful to their colleagues Dimitra Bourboulia, John D. Chisholm, Timothy A. Haystead, and Gennady Bratslavsky for their scientific contributions.
Funding information
This work was partly supported by the National Institute of General Medical Sciences of the NIH grant R01GM124256 (M.M.). This work was also supported by funds from the SUNY Upstate Medical University, the Upstate Foundation, and the Carol M. Baldwin Breast Cancer Fund (M.M.) and in part by the Urology Care Foundation Research Scholar Award Program and American Urological Association (M.M.).
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ali A, et al. Requirement of protein phosphatase 5 in DNA-damage-induced ATM activation. Genes Dev. 2004;18:249–254. doi: 10.1101/gad.1176004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amable L, Grankvist N, Largen JW, Ortsater H, Sjoholm A, Honkanen RE. Disruption of serine/threonine protein phosphatase 5 (PP5:PPP5c) in mice reveals a novel role for PP5 in the regulation of ultraviolet light-induced phosphorylation of serine/threonine protein kinase Chk1 (CHEK1) J Biol Chem. 2011;286:40413–40422. doi: 10.1074/jbc.M111.244053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandhakavi S, McCann RO, Hanna DE, Glover CV. A positive feedback loop between protein kinase CKII and Cdc37 promotes the activity of multiple protein kinases. J Biol Chem. 2003;278:2829–2836. doi: 10.1074/jbc.M206662200. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Periyasamy S, Wolf IM, Hinds TD, Jr, Yong W, Shou W, Sanchez ER. Control of glucocorticoid and progesterone receptor subcellular localization by the ligand-binding domain is mediated by distinct interactions with tetratricopeptide repeat proteins. Biochemistry. 2008;47:10471–10480. doi: 10.1021/bi8011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce DL, Macartney T, Yong W, Shou W, Sapkota GP. Protein phosphatase 5 modulates SMAD3 function in the transforming growth factor-beta pathway. Cell Signal. 2012;24:1999–2006. doi: 10.1016/j.cellsig.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Wang L, Armstrong DL, Rossie S. Activated Rac1 GTPase translocates protein phosphatase 5 to the cell membrane and stimulates phosphatase activity in vitro. J Biol Chem. 2010;285:3872–3882. doi: 10.1074/jbc.M109.088427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MX, Cohen PT. Activation of protein phosphatase 5 by limited proteolysis or the binding of polyunsaturated fatty acids to the TPR domain. FEBS Lett. 1997;400:136–140. doi: 10.1016/s0014-5793(96)01427-5. [DOI] [PubMed] [Google Scholar]
- Chen MS, Silverstein AM, Pratt WB, Chinkers M. The tetratricopeptide repeat domain of protein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes and acts as a dominant negative mutant. J Biol Chem. 1996;271:32315–32320. doi: 10.1074/jbc.271.50.32315. [DOI] [PubMed] [Google Scholar]
- Chen YL, et al. Protein phosphatase 5 promotes hepatocarcinogenesis through interaction with AMP-activated protein kinase. Biochem Pharmacol. 2017;138:49–60. doi: 10.1016/j.bcp.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Cho BR, Lee P, Hahn JS (2014) CK2-dependent inhibitory phosphorylation is relieved by Ppt1 phosphatase for the ethanol stress-specific activation of Hsf1 in Saccharomyces cerevisiae Mol Microbiol 93:306–316. 10.1111/mmi.12660 [DOI] [PubMed]
- Conde R, Xavier J, McLoughlin C, Chinkers M, Ovsenek N (2005) Protein phosphatase 5 is a negative modulator of heat shock factor 1. J Biol Chem 280:28989–28996. 10.1074/jbc.M503594200 [DOI] [PubMed]
- Connarn JN, et al. The molecular chaperone Hsp70 activates protein phosphatase 5 (PP5) by binding the tetratricopeptide repeat (TPR) domain. J Biol Chem. 2014;289:2908–2917. doi: 10.1074/jbc.M113.519421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arcy BM, Swingle MR, Papke CM, Abney KA, Bouska ES, Prakash A, Honkanen RE. The antitumor drug LB-100 is a catalytic inhibitor of protein phosphatase 2A (PPP2CA) and 5 (PPP5C) coordinating with the active-site catalytic metals in PPP5C. Mol Cancer Ther. 2019;18:556–566. doi: 10.1158/1535-7163.MCT-17-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das AK, Cohen PW, Barford D. The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein-protein interactions. EMBO J. 1998;17:1192–1199. doi: 10.1093/emboj/17.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TH, Ning YM, Sanchez ER. Differential control of glucocorticoid receptor hormone-binding function by tetratricopeptide repeat (TPR) proteins and the immunosuppressive ligand FK506. Biochemistry. 2005;44:2030–2038. doi: 10.1021/bi048503v. [DOI] [PubMed] [Google Scholar]
- Dushukyan N, et al. Phosphorylation and ubiquitination regulate protein phosphatase 5 activity and its prosurvival role in kidney cancer. Cell Rep. 2017;21:1883–1895. doi: 10.1016/j.celrep.2017.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson L, et al. Mitogen-activated protein kinases and protein phosphatase 5 mediate glucocorticoid-induced cytotoxicity in pancreatic islets and beta-cells. Mol Cell Endocrinol. 2014;383:126–136. doi: 10.1016/j.mce.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Gallo LI, Ghini AA, Pilipuk GP, Galigniana MD. Differential recruitment of tetratricorpeptide repeat domain immunophilins to the mineralocorticoid receptor influences both heat-shock protein 90-dependent retrotransport and hormone-dependent transcriptional activity. Biochemistry. 2007;46:14044–14057. doi: 10.1021/bi701372c. [DOI] [PubMed] [Google Scholar]
- Gentile S, et al. Rac GTPase signaling through the PP5 protein phosphatase. Proc Natl Acad Sci U S A. 2006;103:5202–5206. doi: 10.1073/pnas.0600080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergs U, Jahn T, Werner F, Kohler C, Kopp F, Grossmann C, Neumann J. Overexpression of protein phosphatase 5 in the mouse heart: reduced contractility but increased stress tolerance - two sides of the same coin? PLoS One. 2019;14:e0221289. doi: 10.1371/journal.pone.0221289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden T, et al. Elevated levels of Ser/Thr protein phosphatase 5 (PP5) in human breast cancer. Biochim Biophys Acta. 2008;1782:259–270. doi: 10.1016/j.bbadis.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden T, Swingle M, Honkanen RE. The role of serine/threonine protein phosphatase type 5 (PP5) in the regulation of stress-induced signaling networks and cancer. Cancer Metastasis Rev. 2008;27:169–178. doi: 10.1007/s10555-008-9125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong CX et al (2004) Dephosphorylation of microtubule-associated protein tau by protein phosphatase 5. J Neurochem 88:298-310. 10.1111/j.1471-4159.2004.02147.x [DOI] [PubMed]
- Hamilton CL, et al. Serine/threonine phosphatase 5 (PP5C/PPP5C) regulates the ISOC channel through a PP5C-FKBP51 axis. Pulm Circ. 2018;8:2045893217753156. doi: 10.1177/2045893217753156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K, Gan Z, Lin S, Hu H, Shen Z, Min D. Elevated expression of serine/threonine phosphatase type 5 correlates with malignant proliferation in human osteosarcoma. Acta Biochim Pol. 2017;64:11–16. doi: 10.18388/abp.2014_951. [DOI] [PubMed] [Google Scholar]
- Haslbeck V et al (2015a) Selective activators of protein phosphatase 5 target the auto-inhibitory mechanism. Biosci Rep 35. 10.1042/BSR20150042 [DOI] [PMC free article] [PubMed]
- Haslbeck V, et al. The activity of protein phosphatase 5 towards native clients is modulated by the middle- and C-terminal domains of Hsp90. Sci Rep. 2015;5:17058. doi: 10.1038/srep17058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds TD, Jr, Sanchez ER. Protein phosphatase 5. Int J Biochem Cell Biol. 2008;40:2358–2362. doi: 10.1016/j.biocel.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds TD, Jr, et al. Protein phosphatase 5 mediates lipid metabolism through reciprocal control of glucocorticoid receptor and peroxisome proliferator-activated receptor-gamma (PPARgamma) J Biol Chem. 2011;286:42911–42922. doi: 10.1074/jbc.M111.311662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong TJ, Park K, Choi EW, Hahn JS. Ro 90-7501 inhibits PP5 through a novel, TPR-dependent mechanism. Biochem Biophys Res Commun. 2017;482:215–220. doi: 10.1016/j.bbrc.2016.11.043. [DOI] [PubMed] [Google Scholar]
- Hsieh FS, et al. Palbociclib induces activation of AMPK and inhibits hepatocellular carcinoma in a CDK4/6-independent manner. Mol Oncol. 2017;11:1035–1049. doi: 10.1002/1878-0261.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh FS, et al. Inhibition of protein phosphatase 5 suppresses non-small cell lung cancer through AMP-activated kinase activation. Lung Cancer. 2017;112:81–89. doi: 10.1016/j.lungcan.2017.07.040. [DOI] [PubMed] [Google Scholar]
- Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, Heng DY, Larkin J, Ficarra V. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. doi: 10.1038/nrdp.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MH, et al. Serine/threonine protein phosphatase 5 is a potential therapeutic target in cholangiocarcinoma. Liver Int. 2018;38:2248–2259. doi: 10.1111/liv.13887. [DOI] [PubMed] [Google Scholar]
- Huang S, Shu L, Easton J, Harwood FC, Germain GS, Ichijo H, Houghton PJ. Inhibition of mammalian target of rapamycin activates apoptosis signal-regulating kinase 1 signaling by suppressing protein phosphatase 5 activity. J Biol Chem. 2004;279:36490–36496. doi: 10.1074/jbc.M401208200. [DOI] [PubMed] [Google Scholar]
- Huang CY, et al. Palbociclib enhances radiosensitivity of hepatocellular carcinoma and cholangiocarcinoma via inhibiting ataxia telangiectasia-mutated kinase-mediated DNA damage response. Eur J Cancer. 2018;102:10–22. doi: 10.1016/j.ejca.2018.07.010. [DOI] [PubMed] [Google Scholar]
- Huderson BP, et al. Stable inhibition of specific estrogen receptor alpha (ERalpha) phosphorylation confers increased growth, migration/invasion, and disruption of estradiol signaling in MCF-7 breast cancer cells. Endocrinology. 2012;153:4144–4159. doi: 10.1210/en.2011-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, et al. Protein phosphatase 5 is a negative regulator of estrogen receptor-mediated transcription. Mol Endocrinol. 2004;18:1131–1143. doi: 10.1210/me.2003-0308. [DOI] [PubMed] [Google Scholar]
- Jacob W, Rosenzweig D, Vazquez-Martin C, Duce SL, Cohen PT. Decreased adipogenesis and adipose tissue in mice with inactivated protein phosphatase 5. Biochem J. 2015;466:163–176. doi: 10.1042/BJ20140428. [DOI] [PubMed] [Google Scholar]
- Kang H, Sayner SL, Gross KL, Russell LC, Chinkers M. Identification of amino acids in the tetratricopeptide repeat and C-terminal domains of protein phosphatase 5 involved in autoinhibition and lipid activation. Biochemistry. 2001;40:10485–10490. doi: 10.1021/bi010999i. [DOI] [PubMed] [Google Scholar]
- Kang Y, Lee JH, Hoan NN, Sohn HM, Chang IY, You HJ. Protein phosphatase 5 regulates the function of 53BP1 after neocarzinostatin-induced DNA damage. J Biol Chem. 2009;284:9845–9853. doi: 10.1074/jbc.M809272200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama K, Yamaguchi M, Noguchi K, Sugimoto Y (2014) Protein phosphatase complex PP5/PPP2R3C dephosphorylates P-glycoprotein/ABCB1 and down- regulates the expression and function. Cancer Lett 345:124–131. 10.1016/j.canlet.2013.12.007 [DOI] [PubMed]
- Kok M, Holm-Wigerup C, Hauptmann M, Michalides R, Stal O, Linn S, Landberg G. Estrogen receptor-alpha phosphorylation at serine-118 and tamoxifen response in breast cancer. J Natl Cancer Inst. 2009;101:1725–1729. doi: 10.1093/jnci/djp412. [DOI] [PubMed] [Google Scholar]
- Krysiak J, et al. Protein phosphatase 5 regulates titin phosphorylation and function at a sarcomere-associated mechanosensor complex in cardiomyocytes. Nat Commun. 2018;9:262. doi: 10.1038/s41467-017-02483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Iqbal K, Grundke-Iqbal I, Rossie S, Gong CX (2005) Dephosphorylation of tau by protein phosphatase 5: impairment in Alzheimer's disease J Biol Chem 280:1790–1796 10.1074/jbc.M410775200 [DOI] [PubMed]
- Lubert EJ, Hong Y, Sarge KD. Interaction between protein phosphatase 5 and the A subunit of protein phosphatase 2A: evidence for a heterotrimeric form of protein phosphatase 5. J Biol Chem. 2001;276:38582–38587. doi: 10.1074/jbc.M106906200. [DOI] [PubMed] [Google Scholar]
- Mazalouskas MD, Godoy-Ruiz R, Weber DJ, Zimmer DB, Honkanen RE, Wadzinski BE. Small G proteins Rac1 and Ras regulate serine/threonine protein phosphatase 5 (PP5) extracellular signal-regulated kinase (ERK) complexes involved in the feedback regulation of Raf1. J Biol Chem. 2014;289:4219–4232. doi: 10.1074/jbc.M113.518514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell JL, Wadzinski BE. Targeting protein serine/threonine phosphatases for drug development. Mol Pharmacol. 2009;75:1249–1261. doi: 10.1124/mol.108.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata Y, Nishida E. CK2 controls multiple protein kinases by phosphorylating a kinase-targeting molecular chaperone, Cdc37. Mol Cell Biol. 2004;24:4065–4074. doi: 10.1128/MCB.24.9.4065-4074.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkaddem SB, Werts C, Goujon JM, Bens M, Pedruzzi E, Ogier-Denis E, Vandewalle A (2009) Heat shock protein gp96 interacts with protein phosphatase 5 and controls toll-like receptor 2 (TLR2)-mediated activation of extracellular signal- regulated kinase (ERK) 1/2 in post-hypoxic kidney cells. J Biol Chem 284:12541–12549. 10.1074/jbc.M808376200 [DOI] [PMC free article] [PubMed]
- Morita K, Saitoh M, Tobiume K, Matsuura H, Enomoto S, Nishitoh H, Ichijo H. Negative feedback regulation of ASK1 by protein phosphatase 5 (PP5) in response to oxidative stress. EMBO J. 2001;20:6028–6036. doi: 10.1093/emboj/20.21.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberoi J et al (2016) Structural and functional basis of protein phosphatase 5 substrate specificity. Proc Natl Acad Sci U S A. 10.1073/pnas.1603059113 [DOI] [PMC free article] [PubMed]
- Ollendorff V, Donoghue DJ. The serine/threonine phosphatase PP5 interacts with CDC16 and CDC27, two tetratricopeptide repeat-containing subunits of the anaphase-promoting complex. J Biol Chem. 1997;272:32011–32018. doi: 10.1074/jbc.272.51.32011. [DOI] [PubMed] [Google Scholar]
- Partch CL, Shields KF, Thompson CL, Selby CP, Sancar A. Posttranslational regulation of the mammalian circadian clock by cryptochrome and protein phosphatase 5. Proc Natl Acad Sci U S A. 2006;103:10467–10472. doi: 10.1073/pnas.0604138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazdrak K, Straub C, Maroto R, Stafford S, White WI, Calhoun WJ, Kurosky A. Cytokine-induced glucocorticoid resistance from eosinophil activation: protein phosphatase 5 modulation of glucocorticoid receptor phosphorylation and signaling. J Immunol. 2016;197:3782–3791. doi: 10.4049/jimmunol.1601029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periyasamy S, Warrier M, Tillekeratne MP, Shou W, Sanchez ER. The immunophilin ligands cyclosporin A and FK506 suppress prostate cancer cell growth by androgen receptor-dependent and -independent mechanisms. Endocrinology. 2007;148:4716–4726. doi: 10.1210/en.2007-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey AJ, Chinkers M. Identification of potential physiological activators of protein phosphatase 5. Biochemistry. 2002;41:5625–5632. doi: 10.1021/bi016090h. [DOI] [PubMed] [Google Scholar]
- Sager RA, et al. Post-translational regulation of FNIP1 creates a rheostat for the molecular chaperone Hsp90. Cell Rep. 2019;26:1344–1356 e1345. doi: 10.1016/j.celrep.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulke JP, et al. Differential impact of tetratricopeptide repeat proteins on the steroid hormone receptors. PLoS One. 2010;5:e11717. doi: 10.1371/journal.pone.0011717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Hartson SD, Matts RL. Evidence that protein phosphatase 5 functions to negatively modulate the maturation of the Hsp90-dependent heme-regulated eIF2alpha kinase. Biochemistry. 2002;41:6770–6779. doi: 10.1021/bi025737a. [DOI] [PubMed] [Google Scholar]
- Shao J, Prince T, Hartson SD, Matts RL. Phosphorylation of serine 13 is required for the proper function of the Hsp90 co-chaperone, Cdc37. J Biol Chem. 2003;278:38117–38120. doi: 10.1074/jbc.C300330200. [DOI] [PubMed] [Google Scholar]
- Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Silverstein AM, Galigniana MD, Chen MS, Owens-Grillo JK, Chinkers M, Pratt WB. Protein phosphatase 5 is a major component of glucocorticoid receptor.hsp90 complexes with properties of an FK506-binding immunophilin. J Biol Chem. 1997;272:16224–16230. doi: 10.1074/jbc.272.26.16224. [DOI] [PubMed] [Google Scholar]
- Sinclair C, Borchers C, Parker C, Tomer K, Charbonneau H, Rossie S (1999) The tetratricopeptide repeat domain and a C-terminal region control the activity of Ser/Thr protein phosphatase 5. 10.1074/jbc.274.33.23666 [DOI] [PubMed]
- Soroka J, Wandinger SK, Mausbacher N, Schreiber T, Richter K, Daub H, Buchner J. Conformational switching of the molecular chaperone Hsp90 via regulated phosphorylation. Mol Cell. 2012;45:517–528. doi: 10.1016/j.molcel.2011.12.031. [DOI] [PubMed] [Google Scholar]
- Swingle MR, Honkanen RE. Development and validation of a robust and sensitive assay for the discovery of selective inhibitors for serine/threonine protein phosphatases PP1alpha (PPP1C) and PP5 (PPP5C) Assay Drug Dev Technol. 2014;12:481–496. doi: 10.1089/adt.2014.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swingle MR, Honkanen RE, Ciszak EM. Structural basis for the catalytic activity of human serine/threonine protein phosphatase-5. J Biol Chem. 2004;279:33992–33999. doi: 10.1074/jbc.M402855200. [DOI] [PubMed] [Google Scholar]
- Swingle M, Ni L, Honkanen RE. Small-molecule inhibitors of ser/thr protein phosphatases: specificity, use and common forms of abuse. Methods Mol Biol. 2007;365:23–38. doi: 10.1385/1-59745-267-X:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swingle M, et al. An ultra-high-throughput screen for catalytic inhibitors of serine/threonine protein phosphatases types 1 and 5 (PP1C and PP5C) SLAS Discov. 2017;22:21–31. doi: 10.1177/1087057116668852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomsig JL, Snyder SL, Creutz CE. Identification of targets for calcium signaling through the copine family of proteins. Characterization of a coiled-coil copine-binding motif. J Biol Chem. 2003;278:10048–10054. doi: 10.1074/jbc.M212632200. [DOI] [PubMed] [Google Scholar]
- Urban G, Golden T, Aragon IV, Scammell JG, Dean NM, Honkanen RE. Identification of an estrogen-inducible phosphatase (PP5) that converts MCF-7 human breast carcinoma cells into an estrogen-independent phenotype when expressed constitutively. J Biol Chem. 2001;276:27638–27646. doi: 10.1074/jbc.M103512200. [DOI] [PubMed] [Google Scholar]
- Vaughan CK, et al. Structure of an Hsp90-Cdc37-Cdk4 complex. Mol Cell. 2006;23:697–707. doi: 10.1016/j.molcel.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CK, et al. Hsp90-dependent activation of protein kinases is regulated by chaperone-targeted dephosphorylation of Cdc37. Mol Cell. 2008;31:886–895. doi: 10.1016/j.molcel.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kriegsheim A, Pitt A, Grindlay GJ, Kolch W, Dhillon AS. Regulation of the Raf-MEK-ERK pathway by protein phosphatase 5. Nat Cell Biol. 2006;8:1011–1016. doi: 10.1038/ncb1465. [DOI] [PubMed] [Google Scholar]
- Wandinger SK, Suhre MH, Wegele H, Buchner J. The phosphatase Ppt1 is a dedicated regulator of the molecular chaperone Hsp90. EMBO J. 2006;25:367–376. doi: 10.1038/sj.emboj.7600930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yan F. Exploring the role of active site Mn(2+) ions in the binding of protein phosphatase 5 with its substrate using molecular dynamics simulations. Biochem Biophys Res Commun. 2019;511:612–618. doi: 10.1016/j.bbrc.2019.02.113. [DOI] [PubMed] [Google Scholar]
- Wang Z, Chen W, Kono E, Dang T, Garabedian MJ. Modulation of glucocorticoid receptor phosphorylation and transcriptional activity by a C-terminal-associated protein phosphatase. Mol Endocrinol. 2007;21:625–634. doi: 10.1210/me.2005-0338. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhu J, Dong M, Yu H, Dai X, Li K. Inhibition of protein phosphatase 5 (PP5) suppresses survival and growth of colorectal cancer cells. Biotechnol Appl Biochem. 2015;62:621–627. doi: 10.1002/bab.1308. [DOI] [PubMed] [Google Scholar]
- Wang J, Shen T, Zhu W, Dou L, Gu H, Zhang L, Yang Z, Chen H, Zhou Q, Sánchez ER, Field LJ, Mayo LD, Xie Z, Xiao D, Lin X, Shou W, Yong W. Protein phosphatase 5 and the tumor suppressor p53 down-regulate each other’s activities in mice. J Biol Chem. 2018;293:18218–18229. doi: 10.1074/jbc.RA118.004256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler T, et al. DNA-PKcs function regulated specifically by protein phosphatase 5. Proc Natl Acad Sci U S A. 2004;101:1247–1252. doi: 10.1073/pnas.0307765100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Zhang J, Wu H, Han E. DNA-PKcs interference sensitizes colorectal cancer cells to a mTOR kinase inhibitor WAY-600. Biochem Biophys Res Commun. 2015;466:547–553. doi: 10.1016/j.bbrc.2015.09.068. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Katoh H, Mori K, Negishi M. Galpha(12) and Galpha(13) interact with Ser/Thr protein phosphatase type 5 and stimulate its phosphatase activity. Curr Biol. 2002;12:1353–1358. doi: 10.1016/s0960-9822(02)01034-5. [DOI] [PubMed] [Google Scholar]
- Yamaguchi F, Umeda Y, Shimamoto S, Tsuchiya M, Tokumitsu H, Tokuda M, Kobayashi R. S100 proteins modulate protein phosphatase 5 function: a link between CA2+ signal transduction and protein dephosphorylation. J Biol Chem. 2012;287:13787–13798. doi: 10.1074/jbc.M111.329771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi F, Tsuchiya M, Shimamoto S, Fujimoto T, Tokumitsu H, Tokuda M, Kobayashi R. Oxidative stress impairs the stimulatory effect of S100 proteins on protein phosphatase 5 activity. Tohoku J Exp Med. 2016;240:67–78. doi: 10.1620/tjem.240.67. [DOI] [PubMed] [Google Scholar]
- Yang J, Roe SM, Cliff MJ, Williams MA, Ladbury JE, Cohen PT, Barford D. Molecular basis for TPR domain-mediated regulation of protein phosphatase 5. EMBO J. 2005;24:1–10. doi: 10.1038/sj.emboj.7600496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeke T, Morrice N, Vazquez-Martin C, Cohen PT. Human protein phosphatase 5 dissociates from heat-shock proteins and is proteolytically activated in response to arachidonic acid and the microtubule-depolymerizing drug nocodazole. Biochem J. 2005;385:45–56. doi: 10.1042/BJ20040690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Bao S, Furumai R, Kucera KS, Ali A, Dean NM, Wang XF. Protein phosphatase 5 is required for ATR-mediated checkpoint activation. Mol Cell Biol. 2005;25:9910–9919. doi: 10.1128/MCB.25.22.9910-9919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Sancar A. Human blue-light photoreceptor hCRY2 specifically interacts with protein serine/threonine phosphatase 5 and modulates its activity. Photochem Photobiol. 1997;66:727–731. doi: 10.1111/j.1751-1097.1997.tb03214.x. [DOI] [PubMed] [Google Scholar]
- Zheng X, Zhang L, Jin B, Zhang F, Zhang D, Cui L. Knockdown of protein phosphatase 5 inhibits ovarian cancer growth in vitro. Oncol Lett. 2016;11:168–172. doi: 10.3892/ol.2015.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi X, Zhang H, He C, Wei Y, Bian L, Li G. Serine/threonine protein phosphatase-5 accelerates cell growth and migration in human glioma cell. Mol Neurobiol. 2015;35:669–677. doi: 10.1007/s10571-015-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Golden T, Aragon IV, Honkanen RE. Ser/Thr protein phosphatase 5 inactivates hypoxia-induced activation of an apoptosis signal-regulating kinase 1/MKK-4/JNK signaling cascade. J Biol Chem. 2004;279:46595–46605. doi: 10.1074/jbc.M408320200. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Dean NM, Honkanen RE. Serine/threonine protein phosphatase type 5 acts upstream of p53 to regulate the induction of p21(WAF1/Cip1) and mediate growth arrest. J Biol Chem. 1998;273:12250–12258. doi: 10.1074/jbc.273.20.12250. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Urban G, Scammell JG, Dean NM, McLean TK, Aragon I, Honkanen RE. Ser/Thr protein phosphatase type 5 (PP5) is a negative regulator of glucocorticoid receptor-mediated growth arrest. Biochemistry. 1999;38:8849–8857. doi: 10.1021/bi990842e. [DOI] [PubMed] [Google Scholar]