Abstract
During dental treatment, a dentist usually applies the local anesthesia. Therefore, all dentists should have expertise in local anesthesia and anesthetics. Local anesthetics have a neurotoxic effect at clinically relevant concentrations. Many studies have investigated the mechanism of neurotoxicity of local anesthetics but the precise mechanism of local anesthetic-induced neurotoxicity is still unclear. In addition, it is difficult to demonstrate the direct neurotoxic effect of local anesthetics because perioperative nerve damage is influenced by various factors, such as the anesthetic, the patient, and surgical risk factors. This review summarizes knowledge about the pharmacology of local anesthetics, nerve anatomy, and the incidence, risk factors, and possible cellular mechanisms of local anesthetic-induced neurotoxicity.
Keywords: Dentistry, Local Anesthetics, Neurotoxicity
INTRODUCTION
In the dental clinic, local anesthesia is an inevitable procedure to carry out various dental treatments. During local infiltration and peripheral nerve blocks, local anesthetics reversibly block the action potentials of neuronal voltage-gated sodium channels and induce analgesia and anesthesia [1]. However, local anesthetics may cause nerve damage and have a toxic effect on various cell types [2,3]. Furthermore, local anesthetic-induced cytotoxicity in many types of cells occurs at clinically relevant concentrations [4,5,6]. However, the ascertainment of the direct neurotoxic effect of local anesthetics is difficult and complex because perioperative nerve damage can arise from many clinical factors. The incidence of local anesthetic-induced neurotoxicity varies depending on the type of surgery, anesthetic technique, and patient factors [7,8].
In this review, we aimed to summarize knowledge about the pharmacology of local anesthetics and the incidence, risk factors, and mechanisms of neurotoxicity caused by local anesthetics.
PHARMACOLOGY OF LOCAL ANESTHETICS IN DENTISTRY
Structurally, local anesthetics consist of a lipophilic aromatic group, a hydrophilic group, and an amide or ester linkage chain; local anesthetics are divided into amino-amide or amino-ester type [9]. The amide class of local anesthetics in dental cartridges includes lidocaine, articaine, bupivacaine, mepivacaine, and prilocaine. The ester class includes benzocaine.
The onset and duration of local anesthetic action are affected by various factors. Local anesthetics are deposited extracellularly in a state of equilibrium between the unionized and ionized form after injection, which is affected by the pH of the surrounding tissue and pKa of the drug. The unionized form crosses the lipid bilayer of the neuronal membrane and blocks voltage-gated sodium channels. There are no significant differences in the pKa among the amide class of local anesthetics, except for bupivacaine, which has a slightly higher pKa, leading to a slow onset of action. High lipid solubility promotes the onset of local anesthesia to a certain degree [10,11,12]. Local anesthetics with higher degrees of protein binding have a longer duration of action. Bupivacaine provides a long duration of anesthesia in soft tissue in the arches and pulp of mandibular teeth [13].
NERVE ANATOMY
To discuss the neurotoxicity of local anesthetics, it is necessary to become well-acquainted with the anatomy of a nerve. Nerve fibers are surrounded by the endoneurium, which is a layer of loose connective tissue and Schwann cells. The endoneurium contains glial cells, fibroblasts, and blood vessel capillaries. Multiple nerve fibers are bundled into fascicles. The fascicle is surrounded by a perineurium, which is a dense layer of collagenous connective tissue. The peripheral nerve is formed with multiple fascicles and is encircled by the epineurium, which is the outermost layer of the peripheral nerve and contains arteries, arterioles, and veins. The epineurium acts as a blood-nerve barrier and protects the nerve from local anesthetics and other chemical injuries [14].
INCIDENCE OF NEUROTOXICITY OF LOCAL ANESTHETICS
It is difficult to estimate the actual incidence of neurotoxicity of local anesthetics because many confounding risk factors lead to nerve injury during the perioperative period. In large prospective studies of peripheral nerve block, the incidence of neurological complications with peripheral nerve block is < 3%. Most of these complications are transient sensory deficits, and permanent nerve injury is rare [15,16,17]. Other studies on neurological complications with peripheral nerve block have shown that the risk of nerve injury is between 0.02% and 0.5%. The incidences of neurotoxicity of local anesthetics vary among studies because the estimation of the incidence of neurotoxicity of local anesthetics is influenced by the methods used to measure anesthetic-related neurological complications [16,17]. Urban and Urquhart estimated that the incidence of neurological deficits is 3–5% after a survey of the neurological deficits 2 weeks after a brachial plexus block. However, the incidence of neurological deficits beyond 4 weeks is only 0.4% [18]. There is a higher risk of prolonged paraesthesia after the administration of 4% articaine when than after the administration of other anesthetics [19,20]. Hillerup et al. [19] reported that 4% articaine causes neurosensory disturbances to two trigeminal branches. In addition, neurosensory disturbances associated with 4% articaine are related mainly to mandibular blocks.
RISK FACTORS FOR NEUROTOXICITY OF LOCAL ANESTHETICS IN DENTISTRY
The risk factors involved in the neurotoxicity of local anesthetics can be categorized into anesthetic and patient factors.
1. Anesthetic factors
Peripheral nerve block is an independent risk factor for the neurotoxicity of local anesthetics [21]. Additionally, the location of the injection influences the incidence of local anesthetic-induced peripheral nerve injury. A recent study has shown that local anesthetic-related peripheral nerve injury is most severe with intrafascicular injection and lower with extrafascicular deposition. This suggests that the early direct exposure of nerve to a high concentration of local anesthetic can increase neurotoxicity [22,23].
Direct stimulation of the peripheral nerve with a needle during a local anesthetic injection can trigger direct nerve perforation and injury to the fascicle and perineurium. In addition, nerve injury is affected by the size and type of needle. A long-beveled needle is more likely to cause nerve punctures but more severe nerve injuries are caused by short-beveled needles [24].
High injection pressure of local anesthetics can increase the peripheral nerve injuries. Intraneural needle placement is indicated by a high injection pressure at the onset of injection, which leads to severe fascicular injury and persistent neurological deficits in dogs [25].
2. Patient factors
Patients with pre-existing neuropathies, such as diabetic peripheral neuropathy, Guillain-Barre syndrome, postpolio syndrome, and multiple sclerosis, are susceptible to local anesthetic-induced nerve injury [26]. In addition, all medical conditions that influence the microvasculature, such as peripheral vascular diseases, vasculitis, smoking, and hypertension, increase the vulnerability of nerves to ischemia and lead to an increase in local anesthetic-induced neurotoxicity during the perioperative period [22].
PATHOPHYSIOLOGY AND CELLULAR MECHANISMS OF NEUROTOXICITY OF LOCAL ANESTHETICS
Chemical nerve injury is caused by the toxicity of a solution or its additives. An in vitro study has shown that all local anesthetics have neurotoxic effects and the degree of neurotoxicity increases concentration-dependently [27]. The concentration of local anesthetics has decreased over time and the highest concentration of lidocaine and bupivacaine used currently is 2% and 0.5%, respectively. Studies that have investigated the toxicity of various local anesthetics have suggested that lidocaine is more toxic than equipotent concentrations of bupivacaine [28,29]. However, other studies have reported that there is no significant difference in toxicity among local anesthetics [27,30].
The vasoconstrictive effect of local anesthetics can aggravate nerve damage via ischemia and this damage can be aggravated further with adjuvant epinephrine. Vasoconstriction owing to local anesthetics with epinephrine prolongs the exposure of nerves to local anesthetics and reduces blood flow, which leads to a high risk of ischemic nerve damage [3]. After periods of ischemia, oxidative injury accompanied by reperfusion results in neuronal damage via the initiation of apoptosis.
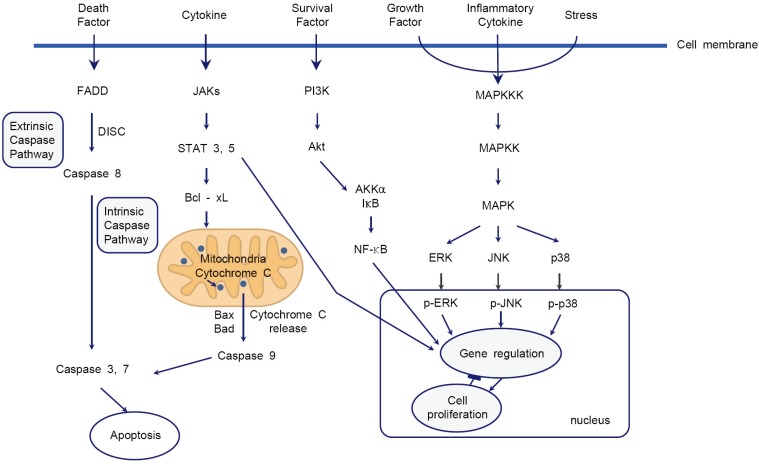
The cellular mechanisms of local anesthetic-induced neurotoxicity have not been well clarified. Some studies have reported that DNA fragmentation, mitochondrial dysfunction, and endoplasmic reticulum calcium depletion are caused by local anesthetics. These processes result in the release of cytochrome c and activation of the caspase pathway, which leads to neuronal apoptosis [31,32,33,34,35,36]. The cellular mechanisms involved in local anesthetic-induced neurotoxicity include the intrinsic caspase, phosphoinositide 3-kinase (PI3K), and mitogena-ctivated protein kinase (MAPK) pathways (Fig. 1). The voltage-gated sodium channel, a primary target of local anesthetics, and G-protein coupled receptors, a target for the systemic anti-inflammatory effect of local anesthetics, are unlikely to be involved in the pathophysiology of local anesthetic-induced neurotoxicity [37,38].
Fig. 1. Schematic diagram for the cellular mechanism of neurotoxicity of local anesthetics. The intrinsic caspase pathway, PI3K pathway, and MAPK pathway are reliable signaling pathways in the neurotoxicity of local anesthetics. Fas-associated protein with death domain (FADD); death-inducing signaling complex (DISC); phosphoinositide-3-kinase (PI3K); mitogen-activated protein kinase (MAPK).
1. Intrinsic caspase pathways
Extrinsic and intrinsic caspase pathways play a central role in apoptosis. Werdehausen et al. [39] demonstrated that lidocaine induces apoptosis via cytochrome c release and apoptosis by lidocaine is strongly reduced by B-cell lymphoma 2 (bcl-2) overexpression and caspase-9 deficiency in the Jurkat cell line. This study suggests that lidocaine induces apoptosis via the activation of the intrinsic caspase pathway. The intrinsic caspase pathway is activated by the cytochrome c release and leads to cell apoptosis. The release of cytochrome c is offset by the activation of the bcl family [40].
2. PI3K pathway
The PI3K family is involved in an intracellular signaling pathway that promotes cell survival, growth, proliferation, and metabolism and angiogenesis. The activation of PI3K phosphorylates and activates serinethreonine protein kinase B (Akt), an enzyme for protection from apoptosis [41,42]. Some studies have investigated the relevance of the PI3K pathway in the neurotoxicity of local anesthetics. Ma et al. showed that pretreatment with dexamethasone significantly attenuates bupivacaine- and lidocaine-induced cell injury, prevents the decline in mitochondrial membrane potential caused by bupivacaine, and increases Akt phosphorylation. These protective effects of dexamethasone against bupivacaine-induced cell injury are suppressed by the pharmacological inhibition of Akt, which suggests that dexamethasone has a protective effect against bupivacaine-induced neuronal cell injury through the Akt signaling pathway [43]. In another study, it was shown that lithium attenuates bupivacaine-induced neurotoxicity through the activation of the PI3K/Akt pathway in mouse neuroblastoma cells [44].
3. MAPK pathway
Wang et al. [44] demonstrated that lithium provides a protective effect against bupivacaine-induced neurotoxicity via the activation of the extracellular signal-regulated kinase (ERK) signaling pathway. ERK is a member of the MAPK family. However, other studies have reported that the inhibition of p38 MAPK or ERK has a potential therapeutic effect against a chronic constriction nerve injury model, metabolic injury, and excitotoxicity [45,46,47]. Haller et al. [48] showed that lidocaine-induced neurotoxicity is mediated by the specific activation of p38 MAPK but not that of ERK or c-Jun N-terminal kinase. In addition, the neuroprotective effect of p38 MAPK inhibitors decreases after more than 1 h of lidocaine administration, which suggests that lidocaine-induced neurotoxicity involves the specific and time-dependent activation of p38 MAPK.
CONCLUSIONS
It is difficult to discriminate the specific neurotoxic effect of local anesthetics because many factors can affect nerve damage during the perioperative period. In clinical settings, most nerve damages induced by local anesthesia are transient sensory defects and permanent nerve damage rarely occurs. However, permanent nerve damage can be fatal in a minority of patients with local anesthetic-induced permanent nerve damage. Therefore, it is essential to prevent local anesthetic-induced nerve damage. To prevent the neurotoxicity of local anesthetics, intrafascicular and high pressure injections should be avoided. Additionally, the use of the lowest effective concentration, the lowest effective volume, short-acting local anesthetics, and small needles is recommended. Finally, patients with comorbid, pre-existing neuropathies and vascular diseases require additional attention from their dentist regarding local anesthetic-induced nerve damage.
ACKNOWLEDGMENTS
This study was supported by a 2019 Clinical Research Grant, Pusan National University Dental Hospital.
Footnotes
- Eun-Jung Kim: Writing — original draft.
- Hee Young Kim: Conceptualization.
- Ji-Hye Ahn: Writing — review & editing.
COMPETING INTERESTS: The authors have declared that no competing interest exists.
References
- 1.Nau C, Wang GK. Interactions of local anesthetics with voltage-gated Na+ channels. J Membr Biol. 2004;201:1–8. doi: 10.1007/s00232-004-0702-y. [DOI] [PubMed] [Google Scholar]
- 2.Nouette-Gaulain K, Capdevila X, Rossignol R. Local anesthetic ‘in-situ’ toxicity during peripheral nerve blocks: Update on mechanisms and prevention. Curr Opin Anaesthesiol. 2012;25:589–595. doi: 10.1097/ACO.0b013e328357b9e2. [DOI] [PubMed] [Google Scholar]
- 3.Hogan QH. Pathophysiology of peripheral nerve injury during regional anesthesia. Reg Anesth Pain Med. 2008;33:435–441. doi: 10.1016/j.rapm.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai XY, Xiong LM, Yang SH, Shao ZW, Xie M, Gao F, et al. Comparison of toxicity effects of ropivacaine, bupivacaine, and lidocaine on rabbit intervertebral disc cells in vitro. Spine J. 2014;14:483–490. doi: 10.1016/j.spinee.2013.06.041. [DOI] [PubMed] [Google Scholar]
- 5.Yang S, Abrahams MS, Hurn PD, Grafe MR, Kirsch JR. Local anesthetic schwann cell toxicity is time and concentration dependent. Reg Anesth Pain Med. 2011;36:444–451. doi: 10.1097/AAP.0b013e318228c835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piper SL, Laron D, Manzano G, Pattnaik T, Liu X, Kim HT, Feeley BT. A comparison of lidocaine, ropivacaine and dexamethasone toxicity on bovine tenocytes in culture. J Bone Joint Surg Br. 2012;94:856–862. doi: 10.1302/0301-620X.94B6.29063. [DOI] [PubMed] [Google Scholar]
- 7.Kessler J, Marhofer P, Hopkins PM, Hollmann MW. Peripheral regional anaesthesia and outcome: lessons learned from the last 10 years. Br J Anaesth. 2015;114:728–745. doi: 10.1093/bja/aeu559. [DOI] [PubMed] [Google Scholar]
- 8.Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in sweden 1990–1999. Anesthesiology. 2004;101:950–959. doi: 10.1097/00000542-200410000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Lirk P, Picardi S, Hollmann MW. Local anaesthetics: 10 essentials. Eur J Anaesthesiol. 2014;31:575–585. doi: 10.1097/EJA.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 10.Yagiela JA. Local anesthetics. In: Yagiela JA, Neidle EA, Dowd FJ, editors. Pharmacology and therapeutics for dentistry. 4th ed. St. Louis: Mosby; 1998. pp. 217–234. [Google Scholar]
- 11.Yagiela JA. Local anesthetics. In: Dionne RA, Phero JC, Becker DE, editors. Pain and anxiety control in dentistry. Philadelphia: W.B. Saunders; 2002. pp. 78–96. [Google Scholar]
- 12.Haas DA. Compendium of pharmaceuticals and specialties (CPS) 37th ed. Canadian Pharmaceutical Association; 2002. Drugs in dentistry; pp. L26–L29. [Google Scholar]
- 13.Haas DA. An update on local anesthetics in dentistry. J Can Dent Assoc. 2002;68:546–551. [PubMed] [Google Scholar]
- 14.Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in sweden 1990–1999. Anesthesiology. 2004;101:950–959. doi: 10.1097/00000542-200410000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Auroy Y, Narchi P, Messiah A, Litt L, Rouvier B, Samii K. Serious complications related to regional anesthesia: results of a prospective survey in France. Anesthesiology. 1997;87:479–486. doi: 10.1097/00000542-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Brull R, McCartney CJ, Chan VW, El-Beheiry H. Neurological complications after regional anesthesia: contemporary estimates of risk. Anesth Analg. 2007;104:965–974. doi: 10.1213/01.ane.0000258740.17193.ec. [DOI] [PubMed] [Google Scholar]
- 17.Barrington MJ, Watts SA, Gledhill SR, Thomas RD, Said SA, Snyder GL, et al. Preliminary results of the australasian regional anaesthesia collaboration: a prospective audit of more than 7000 peripheral nerve and plexus blocks for neurologic and other complications. Reg Anesth Pain Med. 2009;34:534–541. doi: 10.1097/aap.0b013e3181ae72e8. [DOI] [PubMed] [Google Scholar]
- 18.Urban MK, Urquhart B. Evaluation of brachial plexus anesthesia for upper extremity surgery. Reg Anesth. 1994;19:175–182. [PubMed] [Google Scholar]
- 19.Hillerup S, Jensen RH, Ersbøll BK. Trigeminal nerve injury associated with injection of local anesthetics: needle lesion or neurotoxicity? J Am Dent Assoc. 2011;142:531–539. doi: 10.14219/jada.archive.2011.0223. [DOI] [PubMed] [Google Scholar]
- 20.Hillerup S, Jensen R. Nerve injury caused by mandibular block analgesia. Int J Oral Maxillofac Surg. 2006;35:437–443. doi: 10.1016/j.ijom.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Welch MB, et al. Perioperative peripheral nerve injuries: a retrospective study of 380,680 cases during a 10-year period at a single institution. Anesthesiology. 2009;111:490–497. doi: 10.1097/ALN.0b013e3181af61cb. [DOI] [PubMed] [Google Scholar]
- 22.Brull R, Hadzic A, Reina MA, Barrington MJ. Pathophysiology and etiology of nerve injury following peripheral nerve blockade. Reg Anesth Pain Med. 2015;40:479–490. doi: 10.1097/AAP.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 23.Yang S, Abrahams MS, Hurn PD, Grafe MR, Kirsch JR. Local anesthetic schwann cell toxicity is time and concentration dependent. Reg Anesth Pain Med. 2011;36:444–451. doi: 10.1097/AAP.0b013e318228c835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selander D, Dhunér KG, Lundborg G. Peripheral nerve injury due to injection needles used for regional anesthesia. Acta Anaesthesiol Scand. 1977;21:182–188. doi: 10.1111/j.1399-6576.1977.tb01208.x. [DOI] [PubMed] [Google Scholar]
- 25.Hadzic A, Dilberovic F, Shah S, Kulenovic A, Kapur E, Zaciragic A, et al. Combination of intraneural injection and high injection pressure leads to fascicular injury and neurologic deficits in dogs. Reg Anesth Pain Med. 2004;29:417–423. doi: 10.1016/j.rapm.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Lirk P, Birmingham B, Hogan Q. Regional anesthesia in patients with preexisting neuropathy. Int Anesthesiol Clin. 2011;49:144–165. doi: 10.1097/AIA.0b013e3182101134. [DOI] [PubMed] [Google Scholar]
- 27.Werdehausen R, Fazeli S, Braun S, Hermanns H, Essmann F, Hollmann MW, et al. Apoptosis induction by different local anaesthetics in a neuroblastoma cell line. Br J Anaesth. 2009;103:711–718. doi: 10.1093/bja/aep236. [DOI] [PubMed] [Google Scholar]
- 28.Takenami T, Yagishita S, Murase S, Hiruma H, Kawakami T, Hoka S. Neurotoxicity of intrathecally administered bupivacaine involves the posterior roots/posterior white matter and is milder than lidocaine in rats. Reg Anesth Pain Med. 2005;30:464–472. doi: 10.1016/j.rapm.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Sakura S, Kirihara Y, Muguruma T, Kishimoto T, Saito Y. The comparative neurotoxicity of intrathecal lidocaine and bupivacaine in rats. Anesth Analg. 2005;101:541–547. doi: 10.1213/01.ANE.0000155960.61157.12. [DOI] [PubMed] [Google Scholar]
- 30.Lirk P, Haller I, Colvin HP, Lang L, Tomaselli B, Klimaschewski L, et al. In vitro, inhibition of mitogenactivated protein kinase pathways protects against bupivacaine- and ropivacaine-induced neurotoxicity. Anesth Analg. 2008;106:1456–1464. doi: 10.1213/ane.0b013e318168514b. [DOI] [PubMed] [Google Scholar]
- 31.Boselli E, Duflo F, Debon R, Allaouchiche B, Chassard D, Thomas L, et al. The induction of apoptosis by local anesthetics: a comparison between lidocaine and ropivacaine. Anesth Analg. 2003;96:755–756. doi: 10.1213/01.ANE.0000047201.85815.9D. [DOI] [PubMed] [Google Scholar]
- 32.Unami A, Shinohara Y, Ichikawa T, Baba Y. Biochemical and microarray analyses of bupivacaine-induced apoptosis. J Toxicol Sci. 2003;28:77–94. doi: 10.2131/jts.28.77. [DOI] [PubMed] [Google Scholar]
- 33.Johnson ME, Uhl CB, Spittler KH, Wang H, Gores GJ. Mitochondrial injury and caspase activation by the local anesthetic lidocaine. Anesthesiology. 2004;101:1184–1194. doi: 10.1097/00000542-200411000-00019. [DOI] [PubMed] [Google Scholar]
- 34.Lu J, Xu SY, Zhang QG, Xu R, Lei HY. Bupivacaine induces apoptosis via mitochondria and p38 MAPK dependent pathways. Eur J Pharmacol. 2011;657:51–58. doi: 10.1016/j.ejphar.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 35.Li K, Han X. Endoplasmic reticulum stress is involved in the lidocaine-induced apoptosis in SH-SY5Y neuroblastoma cells. J Mol Neurosci. 2015;56:122–130. doi: 10.1007/s12031-014-0473-6. [DOI] [PubMed] [Google Scholar]
- 36.Zhao W, Liu Z, Yu X, Lai L, Li H, Liu Z, et al. iTRAQ proteomics analysis reveals that PI3K is highly associated with bupivacaine-induced neurotoxicity pathways. Proteomics. 2016;16:564–575. doi: 10.1002/pmic.201500202. [DOI] [PubMed] [Google Scholar]
- 37.Sakura S, Bollen AW, Ciriales R, Drasner K. Local anesthetic neurotoxicity does not result from blockade of voltage-gated sodium channels. Anesth Analg. 1995;81:338–346. doi: 10.1097/00000539-199508000-00023. [DOI] [PubMed] [Google Scholar]
- 38.Hollmann MW, Strumper D, Herroeder S, Durieux ME. Receptors, g proteins, and their interactions. Anesthesiology. 2005;103:1066–1078. doi: 10.1097/00000542-200511000-00022. [DOI] [PubMed] [Google Scholar]
- 39.Werdehausen R, Braun S, Essmann F, Schulze-Osthoff K, Walczak H, Lipfert P, et al. Lidocaine induces apoptosis via the mitochondrial pathway independently of death receptor signaling. Anesthesiology. 2007;107:136–143. doi: 10.1097/01.anes.0000268389.39436.66. [DOI] [PubMed] [Google Scholar]
- 40.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 41.King D, Yeomanson D, Bryant HE. PI3King the lock: targeting the PI3K/Akt/mTOR pathway as a novel therapeutic strategy in neuroblastoma. J Pediatr Hematol Oncol. 2015;37:245–251. doi: 10.1097/MPH.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 42.Dasari VR, Veeravalli KK, Saving KL, Gujrati M, Fassett D, Klopfenstein JD, et al. Neuroprotection by cord blood stem cells against glutamate-induced apoptosis is mediated by Akt pathway. Neurobiol Dis. 2008;32:486–498. doi: 10.1016/j.nbd.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Ma R, Wang X, Lu C, Li C, Cheng Y, Ding G, et al. Dexamethasone attenuated bupivacaine-induced neuron injury in vitro through a threonine-serine protein kinase B-dependent mechanism. Neuroscience. 2010;167:329–342. doi: 10.1016/j.neuroscience.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Shen J, Wang J, Lu T, Li C, Zhang X, et al. Lithium attenuates bupivacaine-induced neurotoxicity in vitro through phosphatidylinositol-3-kinase/threonineserine protein kinase B- and extracellular signal-regulated kinase-dependent mechanisms. Neuroscience. 2012;206:190–200. doi: 10.1016/j.neuroscience.2011.12.043. [DOI] [PubMed] [Google Scholar]
- 45.Obata K, Yamanaka H, Dai Y, Mizushima T, Fukuoka T, Tokunaga A, et al. Differential activation of MAPK in injured and uninjured drg neurons following chronic constriction injury of the sciatic nerve in rats. Eur J Neurosci. 2004;20:2881–2895. doi: 10.1111/j.1460-9568.2004.03754.x. [DOI] [PubMed] [Google Scholar]
- 46.Park JY, Kim EJ, Kwon KJ, Jung YS, Moon CH, Lee SH, et al. Neuroprotection by fructose-1,6-bisphosphate involves ROS alterations via p38 MAPK/ERK. Brain Res. 2004;1026:295–301. doi: 10.1016/j.brainres.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 47.Horstmann S, Kahle PJ, Borasio GD. Inhibitors of p38 mitogen-activated protein kinase promote neuronal survival in vitro. J Neurosci Res. 1998;52:483–490. doi: 10.1002/(SICI)1097-4547(19980515)52:4<483::AID-JNR12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 48.Haller I, Hausott B, Tomaselli B, Keller C, Klimaschewski L, Gerner P, et al. Neurotoxicity of lidocaine involves specific activation of the p38 mitogen-activated protein kinase, but not extracellular signal-regulated or c-jun n-terminal kinases, and is mediated by arachidonic acid metabolites. Anesthesiology. 2006;105:1024–1033. doi: 10.1097/00000542-200611000-00025. [DOI] [PubMed] [Google Scholar]