Abstract
Low- and middle-income countries contribute to the overwhelming majority of the global perinatal and neonatal mortality. There is a growing amount of literature focused on interventions aimed at reducing the healthcare gaps and thereby reducing perinatal and neonatal mortality in low- and middle-income countries. The current review synthesizes available evidence for interventions that have shown to improve perinatal and neonatal outcomes. Reduction in important gaps in the availability and utilization of perinatal care practices is needed to end preventable deaths of newborns.
Keywords: Stillbirth, Mortality, Fetal, Perinatal mortality, Developing countries
1. Introduction
The perinatal period in life has the highest risk for mortality [1]. Annually, there is an estimated 4 million perinatal and neonatal deaths [[2], [3], [4]]. Estimates show that low- and middle-income countries contribute to approximately 98% of all perinatal deaths [2,3,5,6]. Because of the suboptimal vital registry infrastructure in low- and middle-income countries, it is likely that the total perinatal and neonatal deaths in these countries are even higher [7,8]. The contribution of neonatal mortality to the under-five mortality has been steadily rising [9] because post neonatal child mortality is decreasing faster than neonatal mortality. Home births and lack of trained neonatal care providers at birth in low- and middle-income countries are the most likely factors responsible for high perinatal and neonatal deaths [6,[10], [11], [12]]. In 2009, 60 million deliveries were estimated to occur outside health facilities [13]. Out of those 60 million deliveries, approximately 52 million deliveries happen without the presence of a trained birth attendant [11,14]. In low- and middle-income countries health facilities are inadequately equipped and lack trained neonatal care providers [6,15,16]. The limited quality of perinatal care and the neonates’ inherent low capacity to compensate in adverse conditions or diseases results in high rates of neonatal mortality in low- and middle-income countries [6,[17], [18], [19], [20], [21]]. The objective of this review is to synthesize and to evaluate the interventional and observational studies designed to assess antenatal, intrapartum, and neonatal interventions aimed to reduce perinatal and neonatal mortality in low- and middle-income countries. (see Table 1)
Table 1.
Meta-analyses focusing on interventions to reduce perinatal mortality.
| Period of intervention | Intervention n name | Year of meta-analysis | Publication year of included studies | Number of studies included | Types of trials included | Number of participants | Effects studied & size (95% CI) |
|---|---|---|---|---|---|---|---|
| Antenatal | Antenatal care [25] | 2018 | 2009–2017 | 18 | Cross sectional & case-control studies | 94,118 neonates | NM RR = 0.66 (0.54–0.80) |
| Antenatal care, sub-Saharan Africa [26] | 2019 | 2009–2019 | 12 | Cohort & cross-sectional studies | 79,990 neonates | NM RR = 0.61 (0.43–0.86) | |
| Birth interval < 18 months [28] | 2013 | 1982–2004 | 5 | Cohort studies | 32,670 neonates | SGA OR = 1.51 (1.31–1.75) Prematurity OR = 1.58 (1.19–2.10) NM OR = 1.49 (0.93–2.37) IM OR = 1.83 (1.19–2.81) |
|
| Tetanus toxoid vaccination [29] | 2010 | 1966–1998 | 2 | RCT & cohort study | 2146 neonates | NM from tetanus infection RR = 0.06 (0.02–0.2) | |
| Nutrition education [34] | 2015 | 1973–2014 | 17 | RCT | 9030 women | Prematurity RR = 0.46 (0.21–0.98) LBW RR = 0.04 (0.01–0.14) |
|
| Iron supplementation [35] | 2015 | 1947–2012 | 61 | RCT & quasi-RCT | 43,274 women | LBW RR = 0.84 (0.69–1.03) Prematurity RR = 0.93 (0.84–1.03) NM RR = 0.91 (0.71–1.18) |
|
| Vitamin A supplementation [36] | 2015 | 1993–2011 | 19 | RCT, quasi-RCT, & cluster RCT | 310,000 women | PM RR = 1.01 (0.95–1.07) NM RR = 0.98 (0.94–1.01) |
|
| Vitamin C supplementation [37] | 2015 | 1966–2014 | 29 | RCT & quasi-RCT | 24,300 women | SB RR = 1.15 (0.89–1.49) PM RR = 1.07 (0.77–1.49) NM RR = 0.79 (0.58–1.08) |
|
| Vitamin D supplementation [38] | 2019 | 1980–2017 | 30 | RCT, quasi-RCT, & cluster RCT | 7033 women | Prematurity RR = 0.66 (0.34–1.34) LBW RR = 0.55 (0.35–0.87) |
|
| Multi-micronutrient supplementation [39] | 2019 | 1975–2014 | 21 | RCT | 142,496 women | Very preterm neonates RR = 0.81(0.71–0.93) SGA RR = 0.92 (0.88–0.97) LBW RR = 0.88 (0.85–0.91) Prematurity RR = 0.95 (0.90–1.01) SB RR = 0.95 (0.86–1.04) PM RR = 1.00 (0.90–1.11) NM RR = 1.00 (0.89–1.12) |
|
| Delivery | Basic neonatal resuscitation [42] | 2011 | 1985–2011 | 24 | Observational, quasi-experimental, & cluster RCT | 392,506 neonates | Intrapartum-related deaths RR = 0.70 (0.59–0.84) |
| Basic neonatal resuscitation [43] | 2017 | 1990–2016 | 20 | RCT, quasi-RCT, interrupted time series, & before–after studies | 1,653,805 neonates | SB RR = 0.79 (0.44–1.41) 7-day NM RR = 0.53 (0.38–0.73) 28-day NM RR = 0.50 (0.37–0.68) PM RR = 0.63 (0.42–0.94) |
|
| Skilled birth attendant [45] | 2011 | 1989–2008 | 21 | Pre-post cohort studies | Not mentioned | SB RR = 0.77 (0.69–0.85) | |
| Clean delivery practices [46] | 2011 | 1991–2010 | 38 | Case-control & cohort studies | Not mentioned | Provider handwashing & NM RR = 0.19 (0.01–0.34) Maternal handwashing & NM RR = 0.44 (0.18–0.62) |
|
| Health facility delivery [59] | 2013 | 1996–2012 | 19 | Cohort & cross-sectional studies | 1,606,805 neonates | NM RR = 0.71 (0.54–0.87) | |
| Health facility delivery [60] | 2019 | 2010–2017 | 19 | Cohort & cross-sectional studies | 1,046,362 neonates | NM OR = 0.48 (0.38–0.58) | |
| Post Delivery & Neonatal | Umbilical cord chlorhexidine application [61] | 2016 | 2006–2013 | 6 | RCT, quasi-RCT, & cluster RCT | 59,179 neonates | NM RR = 0.85 (0.76–0.95) Omphalitis RR = 0.71 (0.62–0.81) |
| Exclusive breastfeeding [62] | 2015 | 1982–2011 | 11 | Observational studies | 70,976 neonates | NM OR = 3.67 (2.04–6.61) | |
| Kangaroo mother care [63] | 2016 | 1988–2016 | 21 | RCT | 3041 neonates | NM RR = 0.60 (0.39–0.92) Sepsis RR = 0.35 (0.22–0.54) Hypothermia RR = 0.28 (0.16–0.49) |
|
| Kangaroo mother care [64] | 2016 | 1988–2014 | 124 | RCT & observational studies | Not mentioned | NM RR = 0.64 (0.46–0.89) Sepsis RR = 0.53 (0.34–0.83) Hypothermia RR = 0.22 (0.12–0.41) |
|
| Combined Antenatal &Postnatal | Education [65] | 2019 | 1995–2017 | 33 | RCT, quasi-RCT, & cluster RCT | Not mentioned | NM RR = 0.87 (0.78–0.96) Early NM RR = 0.74 (0.66–0.84) Late NM RR = 0.54 (0.40–0.74) PM RR = 0.83 (0.75–0.91) |
| Education [66] | 2011 | 2008–2011 | 4 | RCT | 57270 neonates | NM OR = 0.81 (0.75–0.88) Early NM OR = 0.80 (0.70–0.91) Late NM OR = 0.79 (0.63–0.99) |
|
| Education [67] | 2017 | 2001–2017 | 17 | Cluster-RCT | Not mentioned | NM RR = 0.75 (0.69–0.80) | |
| Home based antenatal & neonatal care [69] | 2016 | 2008–2012 | 5 | Cluster-RCT | 101,655 neonates | NM RR = 0.75 (0.61–0.92) PM RR = 0.78 (0.64–0.94) |
SGA = small for gestational age, LBW = low birth weight, SB = stillbirth, PM = perinatal mortality, NM = neonatal mortality, IM = infant mortality, OR = odds ratio, RR = risk ratio.
2. Antenatal interventions
2.1. Antenatal care
Antenatal care has been shown to be associated with improved perinatal outcomes in low- and middle-income countries [22,23]. In a study based on cross-sectional data from 57 low- and middle-income countries (N = 464,728), regular antenatal care was associated with a lower risk of neonatal mortality (HR = 0.45, 95% CI = 0.42–0.48) [24]. In a meta-analysis of cross sectional and case-control studies, antenatal care was found to be associated with a lower in neonatal mortality (RR = 0.66, 95% CI 0.54–0.80) [25]. In a meta-analysis of cohort and cross-sectional studies only from sub-Saharan Africa, antenatal care was found to be associated with a lower in neonatal mortality (RR = 0.61, 95% CI 0.43–0.86) [26], There has not been a meta-analysis of comprehensive antenatal care in low- and middle-income countries. However, improved antenatal care and a program of emergency obstetric and neonatal care did not reduce perinatal or neonatal mortality in a recent large and well-conducted randomized controlled trial [27].
2.2. Birth interval
Increasing the birth interval between pregnancies is associated with less adverse neonatal outcomes. In a meta-analysis assessing cohort studies of birth interval, birth interval <18 months was associated with a higher risk of small for gestational age (aOR = 1.51, 95% CI 1.31–1.75), prematurity (aOR = 1.58, 95% CI = 1.19–2.10), neonatal mortality (aOR = 1.49, 95% CI 0.93–2.37) and infant mortality (aOR = 1.83, 95% CI = 1.19–2.81) [28].
2.3. Tetanus toxoid vaccination
In a meta-analysis based on one randomized controlled trial and one cohort study to assess the effect of tetanus vaccination on neonatal mortality, two doses of tetanus vaccine administered to the mother before delivery reduced neonatal mortality from tetanus infection (RR = 0.06 95% CI 0.02–0.2) [29].
2.4. Antenatal corticosteroids
Evidence from many well-designed randomized controlled clinical trials conducted in higher-income countries and the respective meta-analyses indicate that antenatal corticosteroids reduce neonatal mortality and major morbidities [30]. Data on the effect of antenatal corticosteroids on preterm neonatal outcomes from low-income countries are limited. In a multi-country cluster randomized controlled trial of antenatal corticosteroids in women at high risk of preterm delivery (N = 98,137), 28-day neonatal mortality increased in the antenatal steroid group compared to the control group (RR 1.12, 1.02–1.22, P = .0127). Furthermore, there was an increased incidence of suspected maternal infection in the antenatal steroid group (OR = 1.45, 95% CI = 1.33–1.58, P < .0001) [31]. The ongoing trial of antenatal corticosteroid and its impact on perinatal outcomes from low-income countries will help in further understanding the role of antenatal corticosteroids on outcomes [32].
2.5. Antenatal ultrasound
A multi-country cluster-randomized controlled trial from low-income countries of two antenatal ultrasound assessments in addition to standard care showed no significant difference in antenatal care coverage, hospital delivery of complicated pregnancies, or composite outcome of adverse perinatal outcomes including stillbirth and neonatal mortality [33].
2.6. Antenatal nutrition
A meta-analysis of randomized controlled trials of antenatal nutritional education and perinatal outcomes showed that antenatal nutrition education decreased preterm birth (RR = 0.46, 95% CI = 0.21–0.98) and low birth weight (RR = 0.04, 95% CI = 0.01–0.14). Other perinatal outcomes did not differ between the two groups [34]. A meta-analysis of randomized controlled and quasi-randomized controlled trials assessing antenatal iron supplementation and pregnancy outcomes showed that antenatal iron supplementation was associated with a trend towards the decreased risk of low birth weight (RR = 0.84, 95% CI 0.69–1.03), prematurity (RR = 0.93, 95% CI 0.84–1.03), and neonatal mortality (RR = 0.91, 95% CI 0.71–1.18) [35]. A meta-analysis of randomized controlled, quasi-randomized controlled, and cluster randomized controlled trials showed that antenatal vitamin A supplementation did not impact perinatal (RR = 1.01, 95% CI 0.95–1.07) or neonatal mortality (RR = 0.98, 95% CI 0.94–1.01) [36]. In a meta-analysis of randomized controlled and quasi-randomized controlled trials of antenatal vitamin C supplementation and perinatal outcomes, vitamin C was not found to be associated with any significant impact on stillbirth (RR = 1.15, 95% CI 0.89–1.49), perinatal mortality (RR = 1.07, 95% CI 0.77–1.49), and neonatal mortality (RR = 0.79, 95% CI 0.58–1.08) [37]. A meta-analysis of randomized controlled, quasi-randomized controlled, and cluster randomized controlled trials showed that vitamin D supplementation had no effect on preterm birth (RR = 0.66, 95% CI 0.34–1.34) but was associated with decrease in low birth weight (RR = 0.55, 95% CI 0.35–0.87). However, vitamin D with calcium supplementation was associated with increased prematurity (RR = 1.57, 95% CI 1.01–2.28) [38]. In another meta-analysis of randomized controlled trials assessing the effect of multi-micro nutrient supplementation on pregnancy outcomes, multi-micro nutrient supplementation was associated with reduction in very preterm neonates (RR = 0.81, 95% CI 0.71–0.93), small for gestation age (RR = 0.92, 95% CI 0.88–0.97), and low birth weight (RR = 0.88, 95% CI 0.85–0.91). Multi-micro nutrient supplementation was associated with a trend of reduced prematurity (RR = 0.95, 95% CI 0.90–1.01) and stillbirth (RR = 0.95, 95% CI 0.86–1.04). Multi-micro nutrient supplementation did not decrease perinatal (RR = 1.00, 95% CI 0.90–1.11) and neonatal mortality (RR = 1.00, 95% CI 0.89–1.12) [39].
2.7. Genitourinary tract infection screening and treatment
Genitourinary tract infection screening is being widely adopted in developed countries. A meta-analysis of randomized controlled trials showed that treatment of asymptomatic genitourinary infection reduced low birth weight births (adjusted RR, aRR = 0.64, 95% CI 0.45–0.93) and prematurity (RR = 0.27, 95% CI 0.11–0.62) [40]. However, the quality of evidence in this meta-analysis was deemed low for both outcomes because all fourteen studies included in the meta-analysis were from 1960 to 1987. Furthermore, the description of the methods was not clear. All studies had high or unclear risk of bias [40]. However, a recent cluster-randomized controlled trial antenatal genitourinary tract infection screening from a low-income country reported no difference in prematurity (RR = 1.07, 95% CI 0.91–1.24) [41].
3. Labor and delivery intervention
3.1. Basic neonatal resuscitation
A meta-analysis of observational, quasi-experimental, and cluster randomized controlled trials in resource-limited settings showed that basic neonatal resuscitation decreased intrapartum-related deaths (RR = 0.70, 95% CI 0.59–0.84) [42]. Another meta-analysis of randomized, quasi-randomized controlled trials, interrupted time series studies, and before–after studies showed that basic neonatal resuscitation training decreased in stillbirths (RR = 0.79, 95% CI 0.44–1.41), 7-day neonatal mortality (RR = 0.53, 95% CI 0.38–0.73), 28-day neonatal mortality (RR = 0.50, 95% CI 0.37–0.68), and perinatal mortality (RR = 0.63, 95% CI 0.42–0.94). The meta-analysis of pre- and post-neonatal resuscitation training studies including an active baseline study showed that neonatal resuscitation training was associated with lower rates of stillbirths (RR = 0.88, 95% CI 0.83–0.94), fresh stillbirths (RR = 0.74, 95% CI 0.61–0.90), 1-day neonatal mortality (RR = 0.58, 95% CI 0.42–0.82), 7-day neonatal mortality (RR = 0.82, 95% CI 0.73–0.93), and perinatal mortality (RR = 0.82, 95% CI 0.74–0.91) [43].
A systematic review of cohort pre-post design trials showed that implementation of Helping Babies Breathe was associated with a significant decrease in perinatal mortality (RR = 0.75, p < 0.001), intrapartum-related stillbirths (RR 0.31–0.76), 1-day neonatal mortality (RR 0.37–0.67), and 7-day neonatal mortality (RR 0.32) [44].
3.2. Presence of skilled birth attendant at delivery
A meta-analysis of pre-post cohort design trials showed that the presence of a skilled birth attendant in the delivery was associated with a reduction in stillbirths (RR = 0.77, 95% CI 0.69–0.85) [45].
3.3. Clean delivery and resuscitation practice
A meta-analysis of case-control and cohort studies of clean delivery and neonatal care practice, resuscitation provider handwashing (19%, 95% CI 1–34%), maternal handwashing (44%, 95% CI 18–62%), clean delivery (at home (15%, IQR 10–20%) or in a facility (27%, IQR 24–36)), and clean after birth practices (40%, IQR 25–50%) were found to be associated with a lower neonatal mortality [46].
3.4. Delayed cord clamping
Delayed cord clamping is beneficial for both term (increased iron stores, decreased risk of anemia) and preterm neonates (reduction in mortality, reduction in the risk for anemia and blood transfusion requirement, improved hemodynamic stability, and reduction in the incidence of intraventricular hemorrhage and necrotizing enterocolitis) [[47], [48], [49], [50], [51], [52]]. Being a simple and effective intervention, delayed cord clamping has been recommended in all major basic neonatal resuscitation guidelines ([[53], [54], [55], [56], [57], [58]]).
3.5. Health facility delivery
Two meta-analyses of cohort and cross-sectional studies showed that health facility delivery was associated with a lower neonatal mortality as compared to home delivery (RR = 0.71, 95% CI 0.54–0.87) (59) and (OR = 0.48, 95% CI 0.38–0.58) [60].
4. Post-delivery and neonatal interventions
4.1. Umbilical cord chlorhexidine application
A meta-analysis based on randomized controlled, quasi-randomized controlled, and cluster randomized controlled trials showed that application of chlorhexidine on the umbilical cord decreased neonatal mortality (RR = 0.85, 95% CI 0.76–0.95) and omphalitis (RR = 0.71, 95% CI 0.62–0.81) [61].
4.2. Exclusive breastfeeding
A meta-analysis of observational studies of breastfeeding and perinatal outcomes showed that exclusive breastfeeding was associated with a lower neonatal mortality (OR for non-exclusively breastfeeding = 3.67, 95% CI 2.04–6.61) [62].
4.3. Kangaroo mother care
A meta-analysis of randomized controlled trials showed that kangaroo mother care decreased neonatal mortality (RR = 0.60, 95% CI 0.39–0.92), sepsis (RR = 0.35, 95% CI 0.22–0.54), and hypothermia (RR = 0.28, 95% CI 0.16–0.49) [63]. Another meta-analysis of randomized controlled trials and observational studies showed that kangaroo mother care in low birth weight neonates decreased neonatal mortality (RR = 0.64, 95% CI 0.46–0.89), sepsis (RR = 0.53, 95% CI 0.34–0.83), and hypothermia (RR = 0.22, 95% CI 0.12–0.41) [64].
5. Interventions focusing on antenatal and postnatal periods
5.1. Education
A meta-analysis of randomized controlled, quasi-randomized, and cluster-randomized controlled trials showed that community health educational interventions decreased neonatal mortality (RR = 0.87, 95% CI 0.78–0.96), early neonatal mortality (RR = 0.74, 95% CI 0.66–0.84), late neonatal mortality (RR = 0.54, 95% CI 0.40–0.74), and perinatal mortality (RR = 0.83, 95% CI 0.75–0.91) [65]. A meta-analysis of randomized controlled trials found that community-based behavioural interventions reduced neonatal mortality (aOR = 0.81, 95% CI 0.75–0.88), early neonatal mortality (aOR = 0.80, 95% CI 0.70–0.91), and late neonatal mortality (aOR = 0.79, 95% CI 0.63–0.99) [66]. In another meta-analysis of cluster-randomized trials, community-based interventions reduced neonatal mortality (RR = 0.75, 95% CI 0.69–0.80) [67].
5.2. mHealth interventions
A meta-analysis based on randomized controlled and quasi-experimental trials showed that mHealth interventions increased antenatal care (MD = 0.67, 95% CI = 0.35–0.99) and postnatal care (OR = 1.36, 95% CI = 1.00–1.85), but their effect on neonatal mortality was not adequately reported [68].
5.3. Home based antenatal and neonatal care
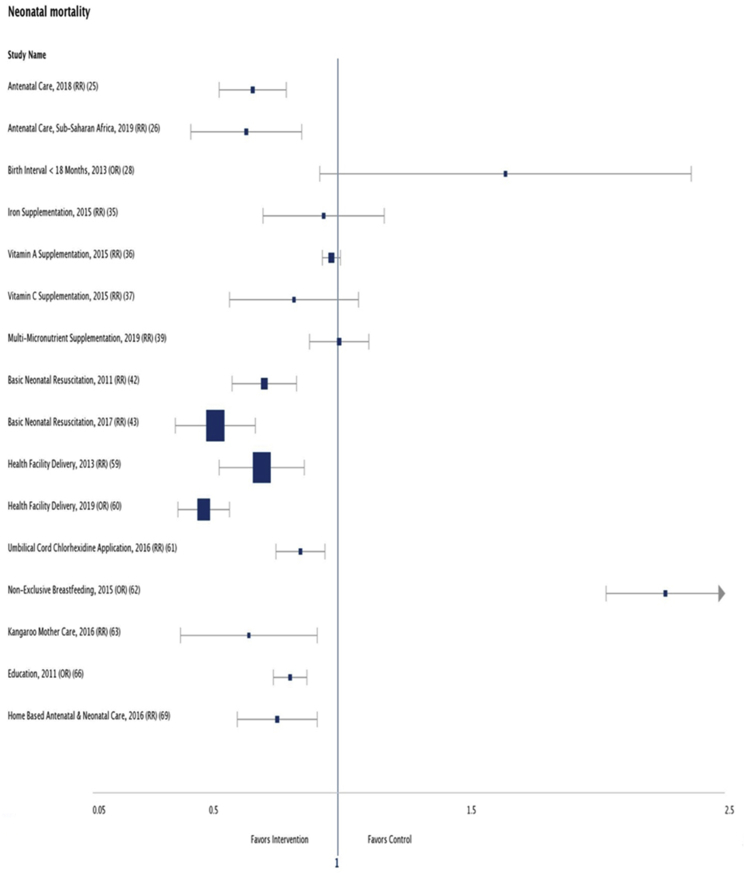
A meta-analysis of cluster-randomized trials showed that home-based antenatal and neonatal care reduced neonatal mortality (RR = 0.75, 95% CI 0.61–0.92) and perinatal mortality (RR = 0.78, 95% CI 0.64–0.94) [69]. 9 (Fig. 1).
Fig. 1.
Interventions and their effect on neonatal mortality. Not included in the Forest Plot are the meta-analysis that assessed individual cause specific mortality and those with unclear participant size. RR = risk ratio, OR = odds ratio.
6. Conclusion
Many effective interventions are associated with reduced neonatal mortality. Evidence-based interventions that improve neonatal survival should be scaled up to reduce preventable perinatal deaths. Given the magnitude of perinatal and neonatal mortality burden in low- and middle-income countries and existing gaps in healthcare availability and utilization, focused and quality interventional trials are needed to elucidate further interventions that might be of the highest benefit for improving neonatal survival. Packages of care focused on groups of interventions from pre-pregnancy to postnatal care with concurrent capacity building of local health infrastructure could identify the strategies to optimize the reduction of preventable deaths.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
References
- 1.Levels and trends in child mortality: UN Inter-agency group for child mortality estimation. 2018. pp. 1–48.https://data.unicef.org/wp-content/uploads/2018/10/Child-Mortality-Report-2018.pdf Available from: [Google Scholar]
- 2.Blencowe H., Cousens S., Jassir F.B., Say L., Chou D., Mathers C. National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health. 2016;4(2):e98–e108. doi: 10.1016/S2214-109X(15)00275-2. [DOI] [PubMed] [Google Scholar]
- 3.Liu L., Oza S., Hogan D., Perin J., Rudan I., Lawn J.E. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 4.Leisher S.H., Teoh Z., Reinebrant H., Allanson E., Blencowe H., Erwich J.J. Seeking order amidst chaos: a systematic review of classification systems for causes of stillbirth and neonatal death, 2009–2014. BMC Pregnancy Childbirth. 2016;16(1):295. doi: 10.1186/s12884-016-1071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawn J.E., Cousens S., Zupan J., Team L.N.S.S. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365(9462):891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 6.Wall S.N., Lee A.C., Niermeyer S., English M., Keenan W.J., Carlo W. Neonatal resuscitation in low-resource settings: what, who, and how to overcome challenges to scale up? Int J Gynaecol Obstet. 2009;107(Supplement):S47–S64. doi: 10.1016/j.ijgo.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L., Kalter H.D., Chu Y., Kazmi N., Koffi A.K., Amouzou A. Understanding misclassification between neonatal deaths and stillbirths: empirical evidence from Malawi. PloS One. 2016;11(12) doi: 10.1371/journal.pone.0168743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawn J., Shibuya K., Stein C. No cry at birth: global estimates of intrapartum stillbirths and intrapartum-related neonatal deaths. Bull World Health Organ. 2005;83:409–417. [PMC free article] [PubMed] [Google Scholar]
- 9.Lehtonen L., Gimeno A., Parra-Llorca A., Vento M., editors. Seminars in fetal & neonatal medicine. Elsevier; 2017. Early neonatal death: a challenge worldwide. [DOI] [PubMed] [Google Scholar]
- 10.Montagu D., Yamey G., Visconti A., Harding A., Yoong J. Where do poor women in developing countries give birth? A multi-country analysis of demographic and health survey data. PloS One. 2011;6(2) doi: 10.1371/journal.pone.0017155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darmstadt G.L., Lee A.C., Cousens S., Sibley L., Bhutta Z.A., Donnay F. 60 million non-facility births: who can deliver in community settings to reduce intrapartum-related deaths? Int J Gynaecol Obstet. 2009;107(Supplement) doi: 10.1016/j.ijgo.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sibley L.M., Sipe T.A., Barry D. Traditional birth attendant training for improving health behaviours and pregnancy outcomes. Cochrane Database Syst Rev. 2012;8 doi: 10.1002/14651858.CD005460.pub3. Epub 2012/08/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darmstadt G.L., Lee A.C., Cousens S., Sibley L., Bhutta Z.A., Donnay F. 60 Million non-facility births: who can deliver in community settings to reduce intrapartum-related deaths? Int J Gynaecol Obstet. 2009;107(Suppl 1):S89–S112. doi: 10.1016/j.ijgo.2009.07.010. Epub 2009/10/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unicef . 2009. State of the world’s children: celebrating 20 years of the convention on the rights of the child: UNICEF.https://www.unicef.org/publications/index_51772.html.Access date Available from. [Google Scholar]
- 15.Manasyan A., Saleem S., Koso-Thomas M., Althabe F., Pasha O., Chomba E. Assessment of obstetric and neonatal health services in developing country health facilities. Am J Perinatol. 2013;30(9):787. doi: 10.1055/s-0032-1333409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kouo-Ngamby M., Dissak-Delon F.N., Feldhaus I., Juillard C., Stevens K.A., Ekeke-Monono M. A cross-sectional survey of emergency and essential surgical care capacity among hospitals with high trauma burden in a Central African country. BMC Health Serv Res. 2015;15(1):478. doi: 10.1186/s12913-015-1147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierrat V., Haouari N., Liska A., Thomas D., Subtil D., Truffert P. Prevalence, causes, and outcome at 2 years of age of newborn encephalopathy: population based study. Arch Dis Child Fetal Neonatal Ed. 2005;90(3):F257–F261. doi: 10.1136/adc.2003.047985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Macki N., Miller S.P., Hall N., Shevell M. The spectrum of abnormal neurologic outcomes subsequent to term intrapartum asphyxia. Pediatr Neurol. 2009;41(6):399–405. doi: 10.1016/j.pediatrneurol.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Van Lerberghe W. 2005. The world health report 2005: make every mother and child count: world health organization.https://www.who.int/whr/2005/en/ Available from. [DOI] [PubMed] [Google Scholar]
- 20.Lee A.C., Cousens S., Wall S.N., Niermeyer S., Darmstadt G.L., Carlo W.A. Neonatal resuscitation and immediate newborn assessment and stimulation for the prevention of neonatal deaths: a systematic review, meta-analysis and Delphi estimation of mortality effect. BMC Publ Health. 2011;11(3):S12. doi: 10.1186/1471-2458-11-S3-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berkelhamer S.K., Kamath-Rayne B.D., Niermeyer S. Neonatal resuscitation in low-resource settings. Clin Perinatol. 2016;43(3):573–591. doi: 10.1016/j.clp.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Kuhnt J., Vollmer S. Antenatal care services and its implications for vital and health outcomes of children: evidence from 193 surveys in 69 low-income and middle-income countries. BMJ open. 2017;7(11) doi: 10.1136/bmjopen-2017-017122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mbuagbaw L., Medley N., Darzi A.J., Richardson M., Habiba Garga K., Ongolo-Zogo P. Health system and community level interventions for improving antenatal care coverage and health outcomes. Cochrane Database Syst Rev. 2015;(12):Cd010994. doi: 10.1002/14651858.CD010994.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doku D.T., Neupane S. Survival analysis of the association between antenatal care attendance and neonatal mortality in 57 low- and middle-income countries. Int J Epidemiol. 2017;46(5):1668–1677. doi: 10.1093/ije/dyx125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wondemagegn A.T., Alebel A., Tesema C., Abie W. The effect of antenatal care follow-up on neonatal health outcomes: a systematic review and meta-analysis. Public Health Rev. 2018;39:33. doi: 10.1186/s40985-018-0110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tekelab T., Chojenta C., Smith R., Loxton D. The impact of antenatal care on neonatal mortality in sub-Saharan Africa: a systematic review and meta-analysis. PloS One. 2019;14(9) doi: 10.1371/journal.pone.0222566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasha O., McClure E.M., Wright L.L., Saleem S., Goudar S.S., Chomba E. A combined community- and facility-based approach to improve pregnancy outcomes in low-resource settings: a Global Network cluster randomized trial. BMC Med. 2013;11:215. doi: 10.1186/1741-7015-11-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozuki N., Lee A.C., Silveira M.F., Victora C.G., Adair L., Humphrey J. The associations of birth intervals with small-for-gestational-age, preterm, and neonatal and infant mortality: a meta-analysis. BMC Publ Health. 2013;13(Suppl 3):S3. doi: 10.1186/1471-2458-13-S3-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blencowe H., Lawn J., Vandelaer J., Roper M., Cousens S. Tetanus toxoid immunization to reduce mortality from neonatal tetanus. Int J Epidemiol. 2010;39(Suppl 1):i102–i109. doi: 10.1093/ije/dyq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts D., Brown J., Medley N., Dalziel S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3 doi: 10.1002/14651858.CD004454.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Althabe F., Belizan J.M., McClure E.M., Hemingway-Foday J., Berrueta M., Mazzoni A. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet. 2015;385(9968):629–639. doi: 10.1016/S0140-6736(14)61651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The World Health Organization Action-I Antenatal CorTicosteroids for Improving Outcomes in preterm Newborns) Trial: a multi-country, multi-centre, two-arm, parallel, double-blind, placebo-controlled, individually randomized trial of antenatal corticosteroids for women at risk of imminent birth in the early preterm period in hospitals in low-resource countries. Trials. 2019;20(1):507. doi: 10.1186/s13063-019-3488-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldenberg R.L., Nathan R.O., Swanson D., Saleem S., Mirza W., Esamai F. Routine antenatal ultrasound in low- and middle-income countries: first look - a cluster randomised trial. BJOG. 2018;125(12):1591–1599. doi: 10.1111/1471-0528.15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ota E., Hori H., Mori R., Tobe-Gai R., Farrar D. Antenatal dietary education and supplementation to increase energy and protein intake. Cochrane Database Syst Rev. 2015;(6):Cd000032. doi: 10.1002/14651858.CD000032.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pena-Rosas J.P., De-Regil L.M., Garcia-Casal M.N., Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2015;7 doi: 10.1002/14651858.CD004736.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCauley M.E., van den Broek N., Dou L., Othman M. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database Syst Rev. 2015;(10) doi: 10.1002/14651858.CD008666.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rumbold A., Ota E., Nagata C., Shahrook S., Crowther C.A. Vitamin C supplementation in pregnancy. Cochrane Database Syst Rev. 2015;(9) doi: 10.1002/14651858.CD004072.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palacios C., Kostiuk L.K., Pena-Rosas J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;7 doi: 10.1002/14651858.CD008873.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keats E.C., Haider B.A., Tam E., Bhutta Z.A. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;3 doi: 10.1002/14651858.CD004905.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smaill F.M., Vazquez J.C. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2015;(8) doi: 10.1002/14651858.CD000490.pub3. [DOI] [PubMed] [Google Scholar]
- 41.Lee A.C., Mullany L.C., Quaiyum M., Mitra D.K., Labrique A., Christian P. Effect of population-based antenatal screening and treatment of genitourinary tract infections on birth outcomes in Sylhet, Bangladesh (MIST): a cluster-randomised clinical trial. Lancet Glob Health. 2019;7(1):e148–e159. doi: 10.1016/S2214-109X(18)30441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee A.C., Cousens S., Wall S.N., Niermeyer S., Darmstadt G.L., Carlo W.A. Neonatal resuscitation and immediate newborn assessment and stimulation for the prevention of neonatal deaths: a systematic review, meta-analysis and Delphi estimation of mortality effect. BMC Publ Health. 2011;11(Suppl 3):S12. doi: 10.1186/1471-2458-11-S3-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel A., Khatib M.N., Kurhe K., Bhargava S., Bang A. Impact of neonatal resuscitation trainings on neonatal and perinatal mortality: a systematic review and meta-analysis. BMJ Paediatr Open. 2017;1(1) doi: 10.1136/bmjpo-2017-000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Versantvoort J.M.D., Kleinhout M.Y., Ockhuijsen H.D.L., Bloemenkamp K., de Vries W.B., van den Hoogen A. Helping Babies Breathe and its effects on intrapartum-related stillbirths and neonatal mortality in low-resource settings: a systematic review. Arch Dis Child. 2020;105(2):127–133. doi: 10.1136/archdischild-2018-316319. [DOI] [PubMed] [Google Scholar]
- 45.Yakoob M.Y., Ali M.A., Ali M.U., Imdad A., Lawn J.E., Van Den Broek N. The effect of providing skilled birth attendance and emergency obstetric care in preventing stillbirths. BMC Publ Health. 2011;11(Suppl 3):S7. doi: 10.1186/1471-2458-11-S3-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blencowe H., Cousens S., Mullany L.C., Lee A.C., Kerber K., Wall S. Clean birth and postnatal care practices to reduce neonatal deaths from sepsis and tetanus: a systematic review and Delphi estimation of mortality effect. BMC Publ Health. 2011;11(Suppl 3):S11. doi: 10.1186/1471-2458-11-S3-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hutton E.K., Hassan E.S. Late vs early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials. J Am Med Assoc. 2007;297(11):1241–1252. doi: 10.1001/jama.297.11.1241. [DOI] [PubMed] [Google Scholar]
- 48.McDonald S.J., Middleton P., Dowswell T., Morris P.S. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2013;(7) doi: 10.1002/14651858.CD004074.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fogarty M., Osborn D.A., Askie L., Seidler A.L., Hunter K., Lui K. Delayed vs early umbilical cord clamping for preterm infants: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(1):1–18. doi: 10.1016/j.ajog.2017.10.231. [DOI] [PubMed] [Google Scholar]
- 50.Chapman J., Marfurt S., Reid J. Effectiveness of delayed cord clamping in reducing postdelivery complications in preterm infants: a systematic review. J Perinat Neonatal Nurs. 2016;30(4):372–378. doi: 10.1097/JPN.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 51.Brocato B., Holliday N., Whitehurst R.M., Jr., Lewis D., Varner S. Delayed cord clamping in preterm neonates: a review of benefits and risks. Obstet Gynecol Surv. 2016;71(1):39–42. doi: 10.1097/OGX.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 52.Rabe H., Reynolds G., Diaz-Rossello J. Early versus delayed umbilical cord clamping in preterm infants. Cochrane Database Syst Rev. 2004;(4) doi: 10.1002/14651858.CD003248.pub2. [DOI] [PubMed] [Google Scholar]
- 53.Wyckoff M.H., Aziz K., Escobedo M.B., Kapadia V.S., Kattwinkel J., Perlman J.M. Part 13: neonatal resuscitation: 2015 American heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 Suppl 2):S543–S560. doi: 10.1161/CIR.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 54.Perlman J.M., Wyllie J., Kattwinkel J., Wyckoff M.H., Aziz K., Guinsburg R. Part 7: neonatal resuscitation: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations (reprint) Pediatrics. 2015;136(Suppl 2):S120–S166. doi: 10.1542/peds.2015-3373D. [DOI] [PubMed] [Google Scholar]
- 55.Niermeyer S. From the neonatal resuscitation program to helping Babies Breathe: global impact of educational programs in neonatal resuscitation. Semin Fetal Neonatal Med. 2015;20(5):300–308. doi: 10.1016/j.siny.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 56.World Health Organization . 2012. Guidelines on basic newborn resuscitation.https://www.who.int/maternal_child_adolescent/documents/basic_newborn_resuscitation/en/ Available from. [PubMed] [Google Scholar]
- 57.World Health Organization . WHO Regional Office for the Western Pacific; Manila: 2014. Early essential newborn care: clinical practice pocket guide.https://iris.wpro.who.int/handle/10665.1/10798 Available from: [Google Scholar]
- 58.Kamath-Rayne B.D., Thukral A., Visick M.K., Schoen E., Amick E., Deorari A. Helping Babies Breathe, second edition: a model for strengthening educational programs to increase global newborn survival. Glob Health Sci Pract. 2018;6(3):538–551. doi: 10.9745/GHSP-D-18-00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tura G., Fantahun M., Worku A. The effect of health facility delivery on neonatal mortality: systematic review and meta-analysis. BMC Pregnancy Childbirth. 2013;13:18. doi: 10.1186/1471-2393-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaka E.E., Mekurie M., Abdurahman A.A., Parsaeian M., Majdzadeh R. Association between place of delivery for pregnant mothers and neonatal mortality: a systematic review and meta-analysis. Eur J Publ Health. 2019 doi: 10.1093/eurpub/ckz060. Epub 2019/04/16. [DOI] [PubMed] [Google Scholar]
- 61.Sankar M.J., Chandrasekaran A., Ravindranath A., Agarwal R., Paul V.K. Umbilical cord cleansing with chlorhexidine in neonates: a systematic review. J Perinatol. 2016;36(Suppl 1):S12–S20. doi: 10.1038/jp.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khan J., Vesel L., Bahl R., Martines J.C. Timing of breastfeeding initiation and exclusivity of breastfeeding during the first month of life: effects on neonatal mortality and morbidity--a systematic review and meta-analysis. Matern Child Health J. 2015;19(3):468–479. doi: 10.1007/s10995-014-1526-8. [DOI] [PubMed] [Google Scholar]
- 63.Conde-Agudelo A., Diaz-Rossello J.L. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2016;(8) doi: 10.1002/14651858.CD002771.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boundy E.O., Dastjerdi R., Spiegelman D., Fawzi W.W., Missmer S.A., Lieberman E. Kangaroo mother care and neonatal outcomes: a meta-analysis. Pediatrics. 2016;137(1) doi: 10.1542/peds.2015-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lassi Z.S., Kedzior S.G., Bhutta Z.A. Community-based maternal and newborn educational care packages for improving neonatal health and survival in low- and middle-income countries. Cochrane Database Syst Rev. 2019;(11) doi: 10.1002/14651858.CD007647.pub2. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tilahun D., Birhanu Z. Effect of community based behavioural change communication intervention to improve neonatal mortality in developing countries: a Systematic Review. JBI Libr Syst Rev. 2011;9(40):1650–1678. doi: 10.11124/01938924-201109400-00001. [DOI] [PubMed] [Google Scholar]
- 67.Hanson C., Kujala S., Waiswa P., Marchant T., Schellenberg J. Community-based approaches for neonatal survival: meta-analyses of randomized trial data. Bull World Health Organ. 2017;95(6) doi: 10.2471/BLT.16.175844. 453-64c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dol J., Richardson B., Tomblin Murphy G., Aston M., McMillan D., Campbell-Yeo M. Impact of mobile health (mHealth) interventions during the perinatal period for mothers in low- and middle-income countries: a systematic review. JBI Libr Syst Rev. 2019;17(8):1634–1667. doi: 10.11124/JBISRIR-2017-004022. [DOI] [PubMed] [Google Scholar]
- 69.Gogia S., Sachdev H.P. Home-based neonatal care by community health workers for preventing mortality in neonates in low- and middle-income countries: a systematic review. J Perinatol. 2016;36(Suppl 1):S55–S73. doi: 10.1038/jp.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]