Abstract
Tea polyphenols (TP) are the major ingredients in tea beverages that display health-benefits including anti-oxidation, anti-inflammation, anti-aging, attenuating blood pressure and deflating. In this study, we investigated the neuroprotective effects of TP to attenuate staurosporine (STS)-induced cytotoxicity. Rat hippocampal neurons were isolated, cultured and incubated with STS to induce neurite collapse and apoptosis, however, the medication of TP eliminated these adverse effects and maintained the morphology of neurons. STS decreased the expression of pro-BDNF, downregulated the TrkB/Akt/Bcl-2 signaling axis and promoted the activation of Erk1/2 and caspase-3. In contrast, TP rescued the expression of pro-BDNF and antagonistically restored the biochemistry of aforementioned signaling effectors. Consistently, the activity of TP can be attenuated by the inhibition of TrkB or Akt by small chemicals K252a and LY294002. Therefore, BDNF-TrkB and Akt signaling axis is essential for TP-mediated neuroprotective effects. In summary, TP showed beneficial effects to protect neurons from exogenous insults such as STS-induced neural cytotoxicity and cell death.
Abbreviations: EC, (-)-epicatechin; ECG, (-)-epigallocatechin; EGC, (-)-epicatechin-3-gallate; EGCG, (-)-epigallocatechin-3-gallate; LDH, Lactate dehydrogenase; LY, LY294002; MAP2, microtubule associated protein 2; STS, staurosporine; TP, tea polyphenols; TUNEL, terminal deoxynucleotidyl transferase mediated dUTP nick-end labeling
Keywords: Erk1/2, K252a, LY294002, MAP2, PD98059, Staurosporine
Introduction
Tea is the most consumed beverage in the world. People have long believed the health-benefit effects of tea such as reducing anxiety, alleviating blood pressure, defatting, refreshing, and prolonging life (Khan and Mukhtar, 2007). Growing analysis and research have identified the main components of tea and well-established their roles in anti-oxidation, anti-inflammation and anti-aging (Dulloo et al., 1999). Tea polyphenols (TP) are the major active components including (-)-epicatechin (EC), (-)-epigallocatechin (ECG), (-)-epicatechin-3-gallate (EGC), and (-)-epigallocatechin-3-gallate (EGCG) (Lambert and Elias, 2010). There is compelling evidence to establish the health-care functions of TP as well as single compounds. Epidemiological investigations also suggested that tea-drinking reduces the incidence of neurodegenerative diseases (Weinreb et al., 2009). Thus, TP are considered as helpful agents with neuroprotective activity. There are emerging evidence showing that TP exert as scavengers to remove reactive oxygen/nitrogen species, and toxic metal irons, respectively, resulting in the attenuation of miscellaneous pathological events induced by oxidative stress and toxic inflammation during the development of neurodegenerative disorders (Weisburger and Chung, 2002). In neurons, regulation of neurotrophins and protein kinase-mediated signaling pathways are considered to contribute to the neuroprotective activity of TP (Mandel et al., 2008; Wei et al., 2016). However, the molecular mechanisms remain to be understood.
Staurosporine (STS), a cell-permeable and prototypical alkaloid of the indolocarbazole family, is used for the establishment of neurodegenerative disease model on rat primary hippocampal neurons (Deshmukh and Johnson, 2000; Qin et al., 2012). STS exerts a broad-spectrum inhibitory activity on protein kinases and serve as an effective apoptosis inducer in cultured hippocampal neurons. In STS-induced morphological collapse and cell death, neurotrophins and the signaling effectors were significantly changed. In particular, BDNF (brain-derived neurotrophic factor), its associated receptor TrkB and downstream signaling regulators PI3K/Akt, MAPK/Erk1/2 are intensively focused on, due to their functions in regulating neural survival and plasticity (Martinowich et al., 2007; Yu et al., 2019; Zhong et al., 2019). In addition, the antagonistically expression of anti-apoptotic protein such as Bcl-2 and apoptosis promoting enzyme caspase-3 is frequently introduced in regulating cell apoptosis. In this study, we aimed to investigate the neuroprotective effects of TP in STS-induced cytotoxicity and to elucidate the potential signaling pathways. Cultured rat hippocampal neurons were incubated with STS to establish STS-induced cytotoxicity and morphological destructions model. The cell viability, morphology and protein expression were respectively examined by MTT, LDH and TUNEL assay, microscopic imaging and western blotting. With the pre-incubation of TP, STS-induced neuronal toxicity was attenuated. The expression of BDNF and associated signaling axis were examined to decipher the mechanisms of TP-mediated neuroprotective functions.
Experimental procedures
Chemicals
TP (#CAS 84650-60-2, purity ≥ 98.0 %) including 50 % EGCG, 22 % ECG, 18 % EGC, and 10 % EC was provided by Shanghai yuanye Bio-Technology Co., Ltd. K252a (#k1639), staurosporine (#19-123-M), LY294002 (#L9908), and PD98059 (#P215) were provided by Sigma-Aldrich Co. LLC. STS is a cell permeable protein kinase C (PKC) inhibitor, also inhibits kinases such as PKA, PKG, CaMKII, MLCK, and induces apoptosis at 0.2−1 μM (Couldwell et al., 1994). K252a is a staurosporine analog that inhibits protein kinases and TrkB activation as well as downstream signaling (Koizumi et al., 1988). PD98059 is a specific inhibitor of mitogen-activated protein kinase kinase (MAPKK, or Erk1/2) (Crews et al., 1992). LY294002 blocks the activity of phosphatidylinositol 3 kinase (PI3K) and its-dependent Akt phosphorylation and activation (Vlahos et al., 1994).
Culture of primary hippocampal neurons
Primary hippocampal neurons were prepared as described previously (Qin et al., 2012). In brief, newborn Sprague-Dawley rat (provided by the Experimental Animal Center of Peking University Health Science Center, Beijing) was sterilized in 70 % ethanol, and was rapidly dissected of the brain in 1x HBSS buffer. Hippocampi were isolated and dissociated. Then the cells were harvested by centrifugation and resuspended in DMEM (#11995040, Gibco) with 10 % FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin (#15140122, Gibco), seeded into culture plates or cover slips coated by poly-l-lysine (#P1399, Sigma-Aldrich) with a density of 0.5∼1 × 106 cells, respectively. Cells were maintained in 5% CO2 in air at 37 °C with saturated humidity. 10 μM cytarabine (#C3350000, Sigma-Aldrich) was added to the medium to inhibit the growth of glial cells on the first 3 days of culture. Hippocampal cells were used for the experiment after 7 days of cultivation. For drug treatments, TP were added to the culture medium and incubated with cells for 24 h, and then STS was added and incubated for 24 h to induce cytotoxicity.
Cell viability, LDH and TUNEL assay
Cell viability was examined by thiazolyl blue tetrazolium bromide (MTT, #M2128, Sigma-Aldrich). Briefly, neurons were seeded in a 96-well plate (2 × 103 cells), and treated by STS or along with TP for 24 h. Then MTT was added and incubated with the cells for 2 h. After gently discarding the medium, 150 μL DMSO was added to dissolve the formazan that yield by MTT. The absorption was recorded at 490 nm using a Bio-Rad iMark microplate reader.
Cytotoxicity was measured by LDH release assay using CytoTox 96 Non-Radioactive Cytotoxicity Assay Kit (#G1780, promega) according to the manufacturer’s instructions. TUNEL assay (TdT-mediated dUTP nick end labeling) was performed to measure apoptotic neurons according to the protocol of the manufacturer (#11767291910, Roche). Briefly, cells were fixed with 4 % paraformaldehyde, permeabilized with 0.1 % Triton X-100 containing 0.1 % sodium citrate on ice for 2 min and followed by TUNEL detection.
Immunofluorescence
Hippocampal neurons were seeded onto cover slips in the 24-well plates for experimental treatments. Then cells were fixed with 4% paraformaldehyde at room temperature for 15 min, blocked up by PBS supplemented with 5% FBS and 0.3 % Triton X-100 at room temperature for 1 h and finally incubated with primary antibody against MAP2 (dilution 1:500, #M9942, Sigma-Aldrich) at 4 °C overnight. After rinsing in PBS for 3 times to remove free antibodies, cells were incubated with donkey anti-mouse IgG (H + L) cross-adsorbed secondary antibody, dyLight 680 (dilution 1:2000, #SA5−10170, Invitrogen) at room temperature for 1.5 h. Images were captured by Leica SP8 confocal microscope with DAPI (4',6-diamidino-2-phenylindole) for staining of the nuclei.
Western blotting
5 × 106 cells were harvested after experimental treatments by centrifugation and lysis in 200 μL RIPA buffer supplemented with 1 % phenylmethanesulfonyl fluoride (PMSF) and proteinase inhibitors on ice for 30 min. Cell lysates were collected after centrifugation at 14,800 g for 15 min to discard debris. Protein concentration was measured by BCA assays and equal amounts of protein were separated by SDS-PAGE, transferred onto a PVDF membrane. Interested proteins were probed by primary antibody as described respectively after membrane blocking for 1 h in Tris-buffered saline containing 5% skim milk. Antibodies against t-Akt (#9272, dilution 1:1000), p-Akt (Ser473) (#4060, dilution 1:1000), β-actin (#4970, dilution 1:3000), Bcl-2 (#3498, dilution 1:5000), cleaved-caspase-3 (Csp3) (#9661, dilution 1:1000), t-Erk1/2(#4695, dilution 1:1000), p-Erk1/2 (Thr202/Tyr204) (#4376, dilution 1:1000), t-TrkB (#4603), p-TrkB (#4619) were provided by Cell Signaling Technology, Inc. Antibody recognize the pro-BDNF (28 kDa) (#AF1423, dilution 1:1000) was provided by Beyotime Biotechnology. IRDye 800CW-Conjugated secondary antibody (#ab216773, dilution 1:5000) was provided by abcam. The bands of target proteins were photographed in the Odyssey CLx infrared fluorescence imaging system (LI-COR Biosciences).
Statistical analysis
Results were presented as mean ± s.e.m and plotted in GraphPad 7.0. Statistical significance ∗ (#) p < 0.05, ∗∗ (##) p < 0.01, and ∗∗∗ (###) p < 0.001 represent the significance of variance between groups examined by one-way analysis of variance (ANOVA) followed by Turkey’s multiple comparisons tests.
Results
TP attenuate STS-induced cytotoxicity and morphological collapse in hippocampal neurons
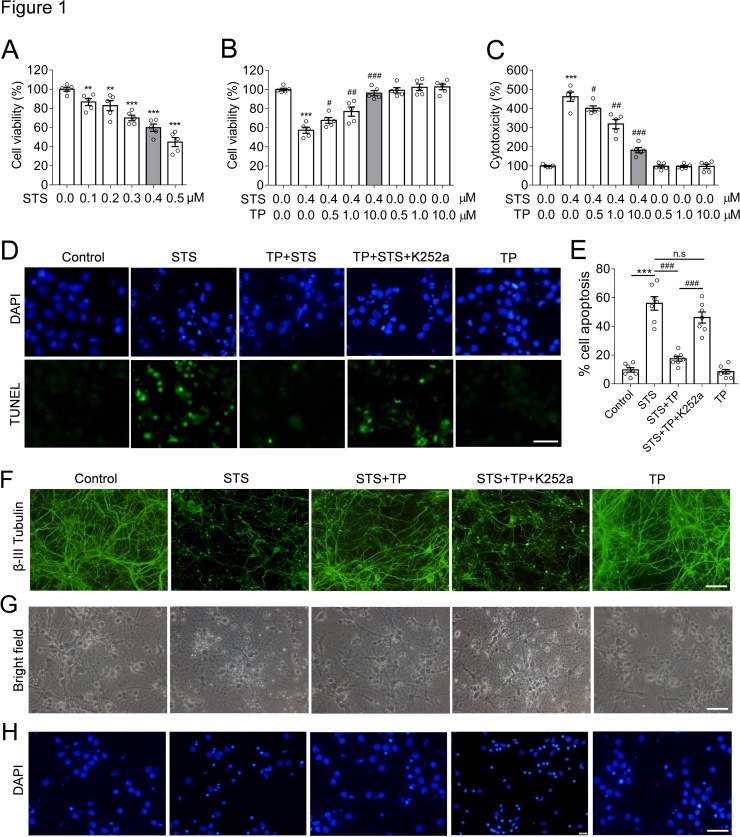
To establish the STS-induced cytotoxicity model, primary rat hippocampal neurons were incubated with a set concentration of STS from 0.1 μM to 0.5 μM. MTT assay was performed to measure the cell viability (Fig. 1A). We found that STS induced a decline of cell viability in a dose-dependent manner. The cell viability showed a decrease of over 50 % on 0.4 μM STS. As we have previously identified (Qin et al., 2012), the concentration introducing a decline of cell viability to 40 %∼50 % is ideal for neuroprotection studies. Therefore, 0.4 μM of STS was applied in the following experiments. TP were pre-incubated with the neurons for 24 h and then followed by STS treatment (Fig. 1B). The results showed that TP rescued cell viability against STS-induced toxicity. The maximal effect was observed in the experimental set treated with of 10 μM TP (Fig. 1B). Thus, 10 μM TP were used in the followed experiments. In addition, LDH cell cytotoxicity assay confirmed the protective effect of TP against STS-induced apoptosis (Fig. 1C). Besides, TP were free of observable cytotoxicity on hippocampal neurons. Furthermore, TUNEL assay was performed to confirm the effect of TP in preventing the neurons from STS-induced apoptosis, in which we found that STS significantly induced neural apoptosis. However, TP treatment effectively attenuated STS-induced apoptosis and maintained the cells in normal status in relative to the control or TP groups. Nevertheless, the apoptosis inhibitory effect of TP in neurons can be attenuated by a small chemical inhibitor K252a, a STS analogue that suppresses the activity of TrkB and its downstream signaling axis (Fig. 1D-E).
Fig. 1.
Tea polyphenols (TP) effectively attenuate STS-induced cytotoxicity and morphological collapse in hippocampal neurons. (A) STS inhibited cell viability in a dose-dependent manner. 0.4 μM of STS caused a decline of 40 % in cell viability. The scatter dot plot presented both mean ± s.e.m and individual values. ** p < 0.01 and *** p < 0.001 was examined in relative to the control group (STS = 0.0 μM). (B) TP rescued the cell viability in concentrations of 0.5–10 μM. # p < 0.05, ## p < 0.01, and ### p < 0.001 was calculated in relative to the set of STS = 0.4 μM. (C) LDH cytotoxicity assay confirmed the rescue of neurons from STS-induced toxicity by TP. (D-E) TUNEL assay verified the inhibition of STS-induced (0.4 μM) apoptosis by TP (10 μM), whereas the activity of TP was antagonized through the inhibition of TrkB activity mediated by K252a (0.2 μM). Scale bar = 50 μm. (***) or (###) p < 0.001 was calculated in relative to the control, STS, or STS + TP group, respectively; n.s, no significance. (F-H) TP (10 μM) rescued the neurons from STS-induced morphological collapse. Cells were immunofluorescence stained with β-III tubulin for visualizing the neurite (F), cell morphology (G), and DAPI for nuclei (H), Scale bar = 50 μM. STS treatment heavily destroyed the neurite and caused morphological collapse in neurons, responding to apoptotic nuclei indicated by DAPI staining. TP attenuated STS-induced toxicity and maintained the cell morphology.
There is compelling evidence shows that apoptosis is the major cellular events in STS-treated neurons (Belmokhtar et al., 2001). In addition to LDH and TUNEL assay, we performed immunofluorescence staining and microscopic imaging to observe the morphological changes of experimental cells. We found that STS-treated cells displayed morphological collapse with significantly destructive dendrites (Fig. 1F and G) and fragmented nuclei (Fig. 1H) (STS group). Luckily, TP medication prevented the occurrence of morphological collapse upon STS treatments (STS + TP group). From the results aforementioned, we concluded that TP are effective in preventing STS-induced cytotoxicity and protects neurons.
TP restore the expression of BDNF and the activity of TrkB/Akt signaling axis to attenuate STS-induced cytotoxicity
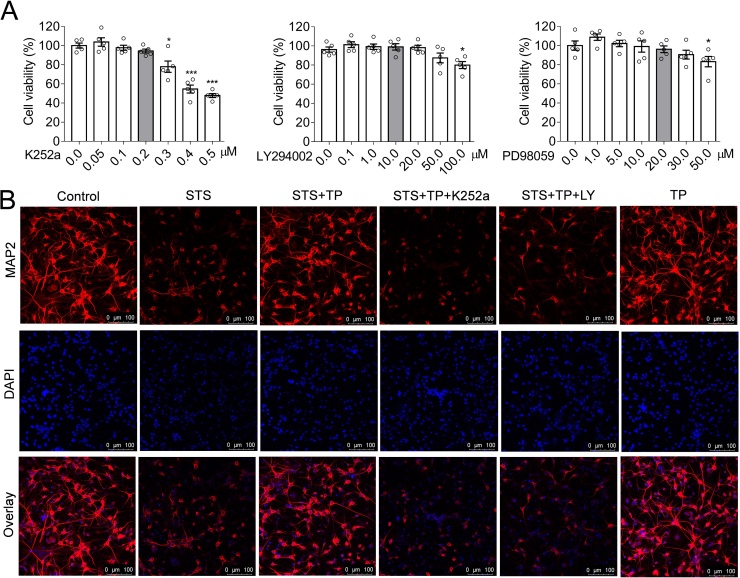
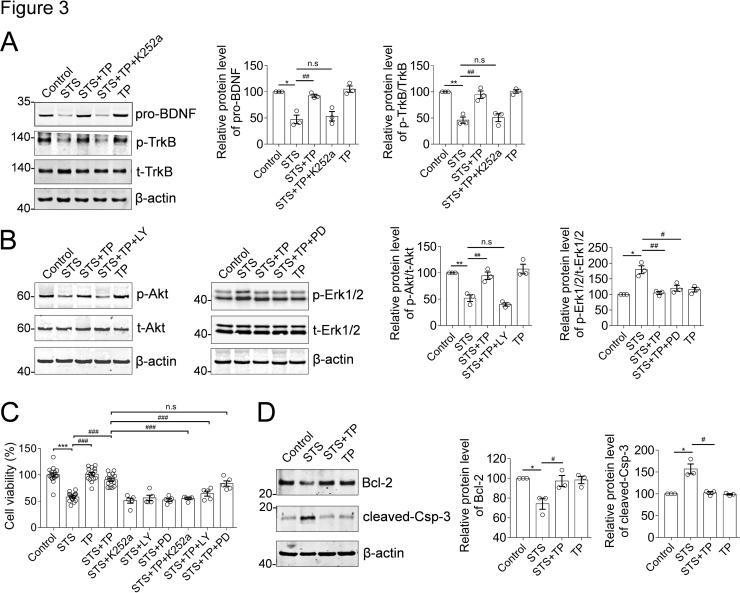
It is well established that BDNF is the critical neurotrophin essential for neural integrity and plasticity (Martinowich et al., 2007). Expression of BDNF and the moderate activity of its associated signaling axis, such as TrkB/Akt and Erk1/2 are required for neuronal survival (Kowianski et al., 2018; Zhong et al., 2019). Therefore, it is interesting to propose that TP-mediated neuroprotection is mediated by modulating TrkB/Akt and their downstream signaling pathways. In view of this, small chemical inhibitors of signaling effectors were introduced. The concentrations of the inhibitors administrating to the neurons were optimized through cell viability assay, in order to effectively inhibit the signaling pathway without hampering the neural viabilities (Fig. 2A). Accordingly, we performed immunofluorescence staining of MAP2 in neurons treated with sets of drugs (Fig. 2B). We found that STS induced neurite collapse similar to the results revealed by β-III tubulin staining (Fig. 1F). Interestingly, inhibition of TrkB with K252a (0.2 μM) or PI3K/Akt with LY294002 (10 μM), respectively, either blocked the neuroprotective effects of TP (Fig. 2B). These results strongly suggested that BDNF-TrkB/Akt signaling axis plays an essential role in TP-mediated neuroprotective effects against STS-induced cytotoxicity. Therefore, we examined the biochemistry of BDNF and its associated signaling pathways. However, it is difficult to detect the mature BDNF. The mature BDNF is produced by proteolytic removal of the propeptide from the pro-BDNF. Therefore, we proposed that the expression of mature BDNF is responding to the cellular pro-BDNF in cultured neurons. In hippocampal neurons, we found that STS induced the downregulation of pro-BDNF and phosphorylation of TrkB, suggesting the inactivation of BDNF-stimulated TrkB activation (Fig. 3A, lane of STS). However, administration of TP rescued the STS-induced decline of pro-BDNF and reactivated TrkB (Fig. 3A, lane of STS + TP). A similar situation was observed for Akt, a TrkB/PI3K downstream signaling effector. STS induced a decrease in Akt activity but restored by TP medication (Fig. 3B). In addition, cell stress response factor, Erk1/2 was activated upon STS treatment, in response to STS-induced cytotoxicity (Fig. 3B, lane of STS). Similarly, TP treatment eliminated the STS-induced Erk1/2 activation (Fig. 3B, lane of STS + TP). Inhibition of TrkB or Akt with small chemicals, such as K252a and LY294002 consistently blocked the TP-mediated rescue of TrkB/Akt signaling axis. Thus, BDNF-TrkB/Akt signaling axis plays an essential role in TP-mediated neuroprotective effects against STS-induced cytotoxicity. Furthermore, we confirmed that the inhibition of TrkB or Akt blocked the TP-mediated rescue of cell viability against STS-induced toxicity (Fig. 3C).
Fig. 2.
TP protect hippocampal neurons against STS-induced cytotoxicity and morphological collapse depending on Akt signaling axis. (A) The optimal concentrations of K252a, LY294002 and PD98059 on hippocampal neurons. (B) Immunofluroscense staining showed the TP mediated maintenance of neural morphology against STS-induced cytotoxicity. Inhibition of the activity of TrkB or Akt by small chemical inhibitor K252a (0.2 μM) or LY294002 (10 μM), respectively, significantly antagonized the cytoprotective effects of TP. Scale bar = 100 μm.
Fig. 3.
TP ameliorate BDNF-TrkB and Akt/Erk1/2 signaling axis to attenuate STS-induced cytotoxicity. (A) STS decreased the expression of pro-BDNF and downregulated the TrkB-mediated signaling axis. However, TP medication rescued the pro-BDNF expression and reactivated the TrkB-mediated signaling axis. (*) p < 0.05, (**) p < 0.01 relative to the control group; (##) p < 0.01, or (n.s) no significant relative to the STS group; n = 3. (B) STS inhibited the activity of Akt indicating by its decreased phosphorylation, and oppositely activated Erk1/2. TP rescued the activation of Akt and attenuated STS-induced Erk1/2 activation. (C) TP restored the cell viability against STS-induced toxicity and could be blocked by the inhibition of TrkB or Akt. (***) p < 0.001 is corresponding to the control group; (###) p < 0.001 or (n.s) no significant is corresponding to the STS or STS + TP group. (D) TP antagonistically restored the biochemistry of apoptosis-associated regulators Bcl-2 and cleaved-caspase 3 (Csp3) to block STS-induced apoptosis. (*) p < 0.05 relative to the control group; (#) p < 0.05 relative to the STS group, n = 3. In this figure, TP = 10.0 μM; STS = 0.4 μM; K252a = 0.2 μM; LY294002 (LY) = 10.0 μM; PD98095 (PD) =20.0 μM.
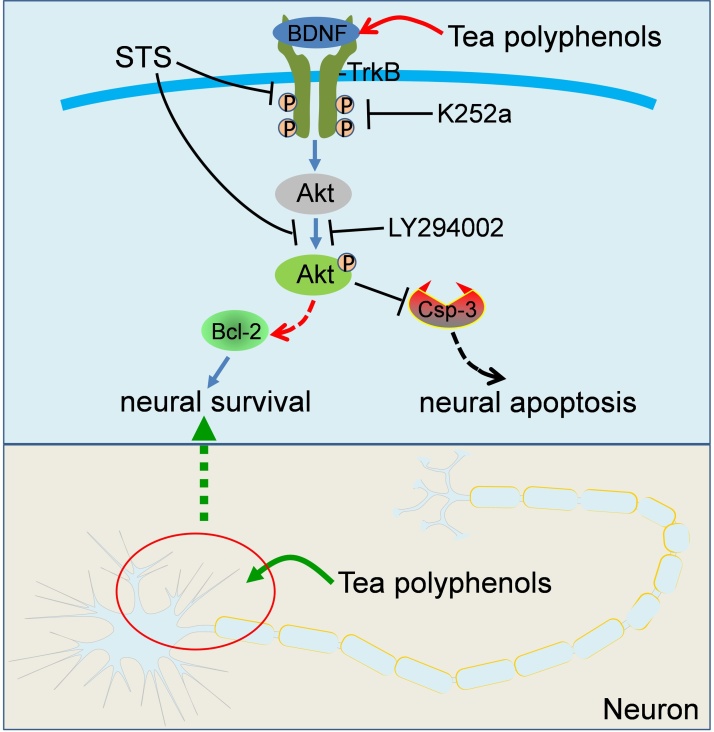
Besides, we investigated the changes of apoptosis-associated proteins in STS or STS + TP treated cells. We found that STS induced the activation of apoptosis with the yield of cleaved-caspase-3, which is the active form to promote apoptosis. In contrast, anti-apoptotic protein such as Bcl-2 was downregulated (Fig. 3D). However, TP antagonistically restored the expression of cleaved-caspase-3 and Bcl-2, to prevent STS-induced apoptosis. In summary, we established the neuroprotective effect of TP in hippocampal neurons against STS-induced cytotoxicity and morphological collapse by restoring the expression of BDNF-TrkB/Akt signaling and the biochemistry of cleaved-caspase-3 and Bcl-2 (Fig. 4).
Fig. 4.
A schematic model presents the effect of tea polyphenols (TP) in attenuating STS-induced cytotoxicity in hippocampal neurons. STS treatment induced cytotoxicity and morphological collapse in neurons, whereas TP medication rescued the neurons from STS-induced toxicity by restoring the expression of BDNF and associated TrkB-Akt and Bcl-2/caspase-3 signaling pathway to inhibit apoptosis.
Discussion
There is compelling evidence suggests the pharmaceutical effects of TP in anti-apoptosis and anti-neurotoxic inflammation in neuronal tissues (Li et al., 2004; Mandel et al., 2012). It has been well-established that TP affords effectively neuroprotective activity in vitro and in vivo models, including cleaning the ROS, inhibiting microglial activation, and alleviating inflammation. Neuronal factors such as BDNF and FGF2, and their associated signaling axis are essential regulators of neuronal survival and neurite plasticity. Deregulation of BDNF and other factors along with associated signaling axis is normally occur in various kinds of neuronal diseases (Binder and Scharfman, 2004). PI3K/Akt cascade is a pivotal signaling pathway promotes neuronal survival, migration and plasticity to provide essential protective effects of neuronal activity (Chong et al., 2012). It has been reported that PI3K/Akt interacts with autophagy signaling pathway by activating the downstream molecule, mTOR (mammalian target of rapamycin) to inhibit excessive autophagy to promote neuron survival in neurodegeneration (Heras-Sandoval et al., 2014). However, MAPK/Erk1/2 is activated is response to extracellular stress, such as oxidative stress, apoptosis-inducer. For instance, oxidative stress has been reported to enhance Erk1/2 phosphorylation and result in the injury of the primary neurons (Stanciu et al., 2000). Therefore, effectively modulation of signaling pathways in response to harmful stimuli is critical for neuronal survival. The involved regulators in the above processes may be promising targets for chemicals to exert neuroprotective functions.
In contrast to TP, STS is a broad-spectrum tyrosine kinase inhibitor that was used to induce neuronal apoptosis and death to simulate the psychological and pathological conditions in neurodegenerative diseases, such as aging, chronic inflammation and ischemia. In this study, we found that 0.4 μM STS is effective to induced hippocampal neuron apoptosis and morphological destruction (Fig. 1). Taking STS-induced cytotoxicity in neurons as models, we demonstrated that TP effectively protected hippocampal neurons from STS-induced cytotoxicity and morphological collapse. The expression of pro-BDNF, the activity of TrkB and associated downstream signaling axis were rescued. The activation of Erk1/2 in response to STS-stimuli was attenuated by TP medication. In these conditions, the apoptosis associated proteins such as Bcl-2 and cleaved-caspase-3 was antagonistically restored (Fig. 4). However, it is interesting to elucidate the mechanism that mediates the interaction of TP with cells. There is compelling evidence confirming the direct interaction of TP with the membrane phospholipid bilayer and its cytosolic and nuclear uptake (Lorenz, 2013; Sirk et al., 2009). In particular, the 67-kDa laminin receptor (67LR) was reported to mediate the binding of EGCG, the major catechin of TP, to the cell surface (Tachibana et al., 2004). The biological activities of EGCG, such as anti-oxidative stress, anti-inflammation, and neuroprotection were mediated through 67LR, although the detailed downstream signaling pathway remains to be elucidated (Khan et al., 2006; Kim et al., 2014). From the aforementioned, TP is effective to perform protective activity by restoring the expression of BDNF, and modulating two distinct protein kinase signaling TrkB/Akt and Erk1/2 against STS-induced cytotoxicity and apoptosis. TP can be promising health-care and therapeutic candidates for neurodegenerative diseases.
Conclusions
STS effectively inhibits cell viability and induces cell apoptosis in a dose-dependent manner. TP attenuate the STS-induced cytotoxicity and apoptosis and the morphological destructions, by restoring BDNF-TrkB and Akt/Bcl-2 signaling axis.
Conflicts of interest
The authors declare no conflict of interests.
Author contributions
Yang J.R.: performed the experiments and statistical analysis. Ren T.T.: performed the experiments and revised the manuscript. Prof. Qin X.Y: designed the experiments, and approved the manuscript, funding acquisition. Dr. Lan R.F.: wrote and approved the manuscript, funding acquisition.
Acknowledgments
We are grateful to the grants from the National Natural Science Foundation of China (81873088, 21778038).
Contributor Information
Rongfeng Lan, Email: lan@szu.edu.cn.
Xiao-Yan Qin, Email: bjqinxiaoyan@muc.edu.cn.
References
- Belmokhtar C.A., Hillion J., Segal-Bendirdjian E. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene. 2001;20:3354–3362. doi: 10.1038/sj.onc.1204436. [DOI] [PubMed] [Google Scholar]
- Binder D.K., Scharfman H.E. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong Z.Z., Shang Y.C., Wang S., Maiese K. A critical kinase cascade in neurological disorders: PI 3-K, Akt, and mTOR. Future Neurol. 2012;7:733–748. doi: 10.2217/fnl.12.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couldwell W.T., Hinton D.R., He S., Chen T.C., Sebat I., Weiss M.H., Law R.E. Protein kinase C inhibitors induce apoptosis in human malignant glioma cell lines. FEBS Lett. 1994;345:43–46. doi: 10.1016/0014-5793(94)00415-3. [DOI] [PubMed] [Google Scholar]
- Crews C.M., Alessandrini A., Erikson R.L. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 1992;258:478–480. doi: 10.1126/science.1411546. [DOI] [PubMed] [Google Scholar]
- Deshmukh M., Johnson E.M., Jr. Staurosporine-induced neuronal death: multiple mechanisms and methodological implications. Cell Death Differ. 2000;7:250–261. doi: 10.1038/sj.cdd.4400641. [DOI] [PubMed] [Google Scholar]
- Dulloo A.G., Duret C., Rohrer D., Girardier L., Mensi N., Fathi M., Chantre P., Vandermander J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am. J. Clin. Nutr. 1999;70:1040–1045. doi: 10.1093/ajcn/70.6.1040. [DOI] [PubMed] [Google Scholar]
- Heras-Sandoval D., Perez-Rojas J.M., Hernandez-Damian J., Pedraza-Chaverri J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell. Signal. 2014;26:2694–2701. doi: 10.1016/j.cellsig.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Khan N., Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007;81:519–533. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N., Afaq F., Saleem M., Ahmad N., Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- Kim H.S., Quon M.J., Kim J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014;2:187–195. doi: 10.1016/j.redox.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S., Contreras M.L., Matsuda Y., Hama T., Lazarovici P., Guroff G. K-252a: a specific inhibitor of the action of nerve growth factor on PC 12 cells. J. Neurosci. 1988;8:715–721. doi: 10.1523/JNEUROSCI.08-02-00715.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowianski P., Lietzau G., Czuba E., Waskow M., Steliga A., Morys J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 2018;38:579–593. doi: 10.1007/s10571-017-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J.D., Elias R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch. Biochem. Biophys. 2010;501:65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Huang Y.G., Fang D., Le W.D. (-)-Epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. J. Neurosci. Res. 2004;78:723–731. doi: 10.1002/jnr.20315. [DOI] [PubMed] [Google Scholar]
- Lorenz M. Cellular targets for the beneficial actions of tea polyphenols. Am. J. Clin. Nutr. 2013;98:1642S–1650S. doi: 10.3945/ajcn.113.058230. [DOI] [PubMed] [Google Scholar]
- Mandel S.A., Amit T., Kalfon L., Reznichenko L., Youdim M.B. Targeting multiple neurodegenerative diseases etiologies with multimodal-acting green tea catechins. J. Nutr. 2008;138:1578S–1583S. doi: 10.1093/jn/138.8.1578S. [DOI] [PubMed] [Google Scholar]
- Mandel S.A., Weinreb O., Amit T., Youdim M.B. Molecular mechanisms of the neuroprotective/neurorescue action of multi-target green tea polyphenols. Front. Biosci. (Schol. Ed.) 2012;4:581–598. doi: 10.2741/S286. [DOI] [PubMed] [Google Scholar]
- Martinowich K., Manji H., Lu B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Qin X.Y., Lv J.H., Cui J., Fang X., Zhang Y. Curcumin protects against staurosporine toxicity in rat neurons. Neurosci. Bull. 2012;28:606–610. doi: 10.1007/s12264-012-1275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirk T.W., Brown E.F., Friedman M., Sum A.K. Molecular binding of catechins to biomembranes: relationship to biological activity. J. Agric. Food Chem. 2009;57:6720–6728. doi: 10.1021/jf900951w. [DOI] [PubMed] [Google Scholar]
- Stanciu M., Wang Y., Kentor R., Burke N., Watkins S., Kress G., Reynolds I., Klann E. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J. Biol. Chem. 2000;275:12200–12206. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- Tachibana H., Koga K., Fujimura Y., Yamada K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004;11:380–381. doi: 10.1038/nsmb743. [DOI] [PubMed] [Google Scholar]
- Vlahos C.J., Matter W.F., Hui K.Y., Brown R.F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J. Biol. Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Wei J.C.C., Huang H.C., Chen W.J., Huang C.N., Peng C.H., Lin C.L. Epigallocatechin gallate attenuates amyloid beta-induced inflammation and neurotoxicity in EOC 13.31 microglia. Eur. J. Pharmacol. 2016;770:16–24. doi: 10.1016/j.ejphar.2015.11.048. [DOI] [PubMed] [Google Scholar]
- Weinreb O., Amit T., Mandel S., Youdim M.B. Neuroprotective molecular mechanisms of (-)-epigallocatechin-3-gallate: a reflective outcome of its antioxidant, iron chelating and neuritogenic properties. Genes Nutr. 2009;4:283–296. doi: 10.1007/s12263-009-0143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburger J.H., Chung F.L. Mechanisms of chronic disease causation by nutritional factors and tobacco products and their prevention by tea polyphenols. Food Chem. Toxicol. 2002;40:1145–1154. doi: 10.1016/s0278-6915(02)00044-3. [DOI] [PubMed] [Google Scholar]
- Yu Y., Lang X.Y., Li X.X., Gu R.Z., Liu Q.S., Lan R., Qin X.Y. 2,3,5,4’-Tetrahydroxystilbene-2-O-beta-d-glucoside attenuates MPP+/MPTP-induced neurotoxicity in vitro and in vivo by restoring the BDNF-TrkB and FGF2-Akt signaling axis and inhibition of apoptosis. Food Funct. 2019;10:6009–6019. doi: 10.1039/c9fo01309a. [DOI] [PubMed] [Google Scholar]
- Zhong S.J., Wang L., Wu H.T., Lan R., Qin X.Y. Coeloglossum viride var. bracteatum extract improves learning and memory of chemically-induced aging mice through upregulating neurotrophins BDNF and FGF2 and sequestering neuroinflammation. J. Funct. Foods. 2019;57:40–47. [Google Scholar]