Abstract
Nonalcoholic fatty liver disease (NAFLD) is a heterogeneous disease driven by genetic and environmental factors. MicroRNAs (miRNAs) serve as pleiotropic post‐transcriptional regulators of cellular pathways. Although several miRNAs have been associated with NAFLD and fibrosis, there are limited studies in humans examining their differential association with pathogenic factors or histological features of NAFLD. We examined the differential relationships of five of the best‐described circulating microRNAs (miR‐34a, miR‐122, miR‐191, miR‐192, and miR‐200a) with histological features and pathogenic factors of NAFLD. A cross‐sectional study was conducted to examine the relationship between relative levels of circulating microRNAs standardized by z‐scores and histological features of NAFLD, common NAFLD genetic polymorphisms, and insulin resistance measured by the enhanced lipoprotein insulin resistance index in 132 subjects with biopsy‐proven NAFLD. We found that miR‐34a, miR‐122, miR‐192, miR‐200a, but not miR‐191, strongly correlate with fibrosis in NAFLD by increases of 0.20 to 0.40 SD (P < 0.005) with each stage of fibrosis. In multivariate analysis, miR‐34a, miR‐122, and miR‐192 levels are independently associated with hepatic steatosis and fibrosis, but not lobular inflammation or ballooning degeneration, whereas miR‐200a is only associated with fibrosis. Among the four miRNAs, miR‐34a, miR‐122, and miR‐192 are associated with pathogenic factors of NAFLD, including insulin resistance measured by eLP‐IR, patatin‐like phospholipase domain containing 3 I148M, and transmembrane 6 superfamily 2 (TM6SF2) E167K polymorphisms. In contrast, miR‐200a is only associated with the TM6SF2 E167K variant. Finally, miR‐34a has the strongest predictive value for various stages of fibrosis, with C‐statistic approximates–combined predictive score for miRNAs. Conclusion: miR‐34a, miR‐122, miR‐192, and miR‐200a demonstrate strong associations with NAFLD severity by histology, but differential associations with pathogenic factors.
Although some micro RNAs have been associated with nonalcoholic liver disease, human studies are limited. We provide independent validation of four of the best described miRNA (34a, 122, 192, and 200a) in their association with NAFLD phenotype and fibrosis. We also examine differential association with pathogenesis (insulin resistance and genetics) of the four miRNA.

Abbreviations
- AUROC
area under the receiver operating characteristic curve
- BIDMC
Beth Israel Deaconess Medical Center
- BMI
body mass index
- Ct
cycle threshold
- eLP‐IR
enhanced lipoprotein insulin‐resistance index
- FIB‐4
Fibrosis‐4
- HDL‐C
high‐density lipoprotein cholesterol
- IQR
interquartile range
- LDL‐C
low‐density lipoprotein cholesterol
- miRNA
microRNA
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NMR
nuclear magnetic resonance
- PNPLA3
patatin‐like phospholipase domain containing 3
- TM6SF2
transmembrane 6 superfamily 2
Nonalcoholic fatty liver disease (NAFLD) is the most common form of chronic liver disease. NAFLD affects 10% to 30% of individuals globally and about 30% of adults within North America.( 1 , 2 ) It is projected to become the leading cause of liver transplantation and impose an increasingly significant health care burden.( 3 ) NAFLD is a heterogeneous disease manifested by a wide spectrum of disease severity that ranges from simple hepatic steatosis to nonalcoholic steatohepatitis (NASH) and progressive fibrosis leading to cirrhosis. The cause of NAFLD is multifactorial with known genetic and acquired factors playing significant roles.( 4 , 5 , 6 ) We previously proposed that genetics and insulin resistance are the main pathogenic drivers for most patients with NAFLD.( 7 ) Familial aggregation and twin studies suggest that the risk for NAFLD is approximately 50% heritable.( 8 , 9 ). Genome‐wide association studies have identified two common genetic variants associated with hepatic steatosis and accelerated fibrosis: an I148M variant in patatin‐like phospholipase domain containing protein 3 (PNPLA3) and an E167K variant in transmembrane 6 superfamily member 2 (TM6SF2).( 10 , 11 ) Both PNPLA3 and TM6SF2 are implicated in hepatic lipid and lipoprotein metabolism, a core hepatic function that is essential to the pathogenesis of NAFLD.( 12 , 13 , 14 )
MicroRNAs (miRNAs) are small, endogenous, noncoding RNAs involved in the posttranscriptional regulation of target messenger RNA.( 15 ) They are pleiotropic regulators of various cellular pathways, including metabolic stress response, cellular differentiation, and energy metabolism processes that are pivotal to the pathogenesis of NAFLD.( 16 , 17 ) Recent studies have associated several miRNAs with NAFLD and its consequent liver fibrosis.( 18 , 19 , 20 , 21 ) Higher circulating levels of miR‐34a are seen in patients with NAFLD compared to healthy controls and correlate with the degree of liver fibrosis.( 22 , 23 , 24 ) miR‐122 and miR‐192 are two liver‐predominant miRNAs that are implicated in various forms of liver injury.( 25 , 26 , 27 , 28 ) Higher levels of miR‐122 and miR‐192 are present in patients with NASH relative to those with nonalcoholic fatty liver alone.( 20 , 21 , 24 , 29 , 30 , 31 ) Additionally, miR‐200a levels are associated with hepatic steatosis in murine models and humans.( 31 ) In comparison, high levels of miR‐191 levels can be found in patients with hepatocellular carcinoma (HCC) and acute liver failure, but not in NAFLD.( 32 )
Despite the expanding body of literature on miRNA in NAFLD, most human studies are small, primarily comparing groups of patients with NAFLD or NASH to healthy controls.( 20 ) The studies examining the relationship between miRNAs and liver fibrosis have not dissected the relationship with cardinal histological features of NAFLD, which are often collinear. More importantly, it is not known how these miRNAs are implicated in the pathogenesis of NAFLD and how they are linked to pathogenic factors of NAFLD.
Herein, we focused on five of the best‐described NAFLD‐related miRNAs: miR‐34a, miR‐122, miR‐192, miR‐200a, and miR‐191 (included as a non‐NAFLD control), and aimed to perform an independent validation. Specifically, we compared the relative strength of their association with histological features of NAFLD and defined their differential associations with major pathogenic factors of NAFLD, namely genetic risk factors and insulin resistance.
Patients and Methods
Patient Population
Patients for this study were derived from a prospective NAFLD registry at Beth Israel Deaconess Medical Center (BIDMC) starting in 2009. Patients were enrolled after they had a confirmed diagnosis of NAFLD on liver biopsy. Patients who were excluded were those with other forms of chronic liver diseases or alternative causes for fatty liver, such as medication, hereditary hemochromatosis, or consumption of alcohol greater than 20 g daily. Pertinent data such as patient demographics, medical history, and physical exam findings were obtained at the enrollment of the study. Laboratory tests and serum were also collected at the time of enrollment. A liver biopsy was performed on each enrolled patient within 3 months of the index visit. Baseline whole blood and sera were stored at −80°C. The study was approved by the BIDMC institutional review board and was conducted in accordance with the Helsinki declaration of 1975, as revised in 1983. All participants consented to the study at enrollment.
Liver Biopsy
Nontargeted liver biopsy was performed under ultrasound guidance by an interventional radiologist. Staff pathologists at BIDMC who specialize in hepatopathology interpreted the biopsy results and were blinded to genetic and microRNA data. Liver biopsies were assessed and reported in a standardized fashion using the NASH Clinical Research Network scoring system, including fibrosis stages (1‐4) and NAFLD activity score (NAS; 0‐8) calculated based on the degrees of hepatic steatosis, lobular inflammation, and ballooning degeneration.( 33 )
miRNA Quantitation
miRNA quantification was performed on stored serum from 182 subjects using Exiqon’s miRCURY LNA Universal RT microRNA PCR assay (Exiqon; QIAGEN, Denmark) according to the manufacturer’s directions. The following panel of miRNAs and controls were placed on custom plates: UniSp2, 3, 4, 5, 6, miR‐34a‐5p, miR‐122‐5p, miR‐191‐5p, hsa‐miR‐103a‐3p, hsa‐miR‐30c‐5p, miR‐192‐5p, and miR‐200a‐3p. The detection of each miRNA was normalized to the average detection of two control miRNAs (hsa‐miR‐103a‐3p and hsa‐miR‐30c‐5p) and was expressed in delta cycle‐threshold values (ΔCt). We confirmed that our chosen control miRNA (hsa‐miR‐103a‐3p and hsa‐miR‐30c‐5p) did not correlate with clinical parameters of aspartate aminotransferase, alanine aminotransferase, insulin resistance, and fibrosis stage (Supporting Table S3). A total of 132 subjects had all five measurable miRNAs that met the quality control.
DNA Extraction and Single‐Nucleotide Polymorphism Genotyping
Genomic DNA was extracted from frozen human whole‐blood samples using phenol chloroform and resuspended in double distilled water. Single nucleotide polymorphism (SNP) rs738409 in the PNPLA3 gene, and rs58542926 in TM6SF2, were genotyped by TaqMan allelic discrimination using predesigned TaqMan SNP genotyping assays (Applied Biosystems, Foster City, CA).
Calculation of Enhanced Lipoprotein Insulin‐Resistance Index Scores
Nuclear magnetic resonance (NMR) spectra were collected using 1H‐NMR on a 400‐MHz Vantera Clinical Analyzer (LipoScience Inc., Morrisville, NC) as previously described.( 34 , 35 ) The LP4 algorithm, which reports concentrations of lipoprotein particles, glucose, alanine, and total branched‐chain amino acids (BCAA), was used to reanalyze stored NMR spectra and calculate the enhanced lipoprotein insulin‐resistance index (eLP‐IR) scores (1‐100). The eLP‐IR takes into account changes that occur in eight NMR‐measured lipoprotein parameters (the six parameters in LP‐IR [predecessor to eLP‐IR], medium triglyceride‐rich lipoprotein particle number, and small HDL particle number), GlycA, a marker of systemic inflammation, and BCAA, all of which are altered in patients with insulin resistance.( 36 , 37 , 38 )
Statistical Analysis
All five miRNAs (miR‐34a, miR‐122, miR‐191, miR‐192, and miR‐200a) were found have to a near‐parametric distribution among the studied subjects. The relative miRNA levels were converted to z‐scores calculated by the difference from the mean divided by the SD. This allowed the comparison of relative strength of each miRNA within the sample set. Positive numbers represented an increase in the level from the mean.
We first determined univariate associations of the five miRNAs (miR‐34a, miR‐122, miR‐191, miR‐192, and miR‐200a) with NAFLD histological phenotypes, genetics, and components of the metabolic syndrome. We examined univariate association of standardized miRNA levels with hepatic steatosis (0‐3), lobular inflammation (0‐3), and ballooning degeneration (0‐2) using bivariate normal regressions in which miRNA z‐scores were used as outcomes. We then determined the association between miRNA levels and PNPLA3 I148M carrier relative to the 148I genotype and the TM6SF2 genotype’s E167K carrier relative to the 167E genotype. Due to power limitations, homozygotes and heterozygotes were combined into single groups for analysis. We determined univariate associations between standardized miRNA levels and insulin resistance estimated by eLP‐IR (0‐100), body mass index (BMI), and fasting lipids (triglyceride, low‐density lipoprotein cholesterol [LDL‐C], and high‐density lipoprotein cholesterol [HDL‐C]) as continuous variables, as well as diabetes and hypertension as binary variables. Because the goal was to examine pathogenic associations with levels of each of the miRNA, the standardized miRNA levels were used as dependent variable in all analyses.
We then performed multivariable analysis using normal regression models for each miRNA, estimating its concentration in relation to the major pathogenic factors of insulin resistance (eLP‐IR) and genetics (PNPLA3 and TM6SF2 genotypes).
Finally, we investigated the potential value of miRNAs in predicting fibrosis stage among patients with NAFLD. Logistic regression models were constructed for stage 1+, 2+, 3+, or 4 level of fibrosis with miR‐34a alone, then four miRNAs (miR‐34a, miR‐122, miR‐192, and miR‐200a), and finally Fibrosis‐4 (FIB‐4) as predictors. Receiver operating characteristic curves were calculated to compare the C‐statistics of the three predictive models for each of the fibrosis stages. All analyses were performed using STATA 14 (StataCorp LLC, College Station, TX).
Results
Patient Characteristics
The baseline characteristics of our study population are given in Table 1. A total of 132 patients are evaluated with the mean age of 50.6 years (interquartile range [IQR] 43.0‐60.2), and 38.6% of the patients are of female gender. The mean BMI is 34 (IQR 29.6‐36.7), with 28% of the patients having diabetes and 47% having hypertension. Most of the patients have NASH with a mean NAS of 4.6 (IQR 4‐6) and stage 0‐1 fibrosis, while 34.9% and 15.9% of patients had stage 2 and stages 3‐4 fibrosis, respectively.
Table 1.
Patient Characteristics
| Total (n) | 132 |
| Age (mean, IQR) | 50.6 (43.0‐60.2) |
| Female (%) | 38.6 |
| Hispanic ethnicity (%) | 14.3 |
| BMI (mean, IQR) | 34.0 (29.6‐36.7) |
| Diabetes (%) | 28.0 |
| Hypertension (%) | 47.0 |
| Fasting lipids (mean, IQR) | |
| Triglycerides (mg/dL) | 200.8 (110‐261) |
| LDL‐C (mg/dL) | 109.4 (83‐132) |
| HDL‐C (mg/dL) | 45.9 (37‐52) |
| Fibrosis stage (%) | |
| 0‐1 | 49.2 |
| 2 | 34.9 |
| 3‐4 | 15.9 |
| NAS score (mean, IQR) | 4.6 (4‐6) |
| PNPLA3 genotype (%) | |
| CC | 34.1 |
| CG | 42.2 |
| GG | 23.5 |
| TM6SF2 genotype (%) | |
| CC | 78.0 |
| CT or TT | 22.0 |
| eLP‐IR (mean, IQR) | 67.6 (57.0‐83.2) |
Abbreviation: NAS, NAFLD activity score.
Relationships Between miRNA and Histological Features of NAFLD
The measured miRNA concentration adjusted by housekeeping genes measured in cycle threshold values and the relative concentration measured in SD via z‐score are shown by the stage of liver fibrosis in Supporting Table S1. Four miRNAs (miR‐34a, miR‐122, miR‐192, and miR‐200a) demonstrate strong associations with the stage of liver fibrosis, with an increase of each fibrosis stage associated with 0.2 to 0.4 SD in serum miRNA concentration (P < 0.001‐0.005) (Table 2). Notably, among these four miRNAs, miR‐34a has the largest β‐coefficient in association with fibrosis, and is the only miRNA that associates with all three histological features of NASH with a sizable β‐coefficient of 0.3‐0.4 SD. miR‐122 and miR‐192 are strongly associated with hepatic steatosis (~0.4 SD) and moderately with ballooning degeneration (0.2‐0.3 SD). In comparison, the association between miR‐200a and histological features of NASH is insignificant. As histological features of NASH are often colinear, we performed multivariate analysis to allow the histological scores to compete simultaneously. In this competition analysis, only the associations with fibrosis and steatosis are independent, whereas the associations with lobular inflammation and ballooning degeneration are no longer significant (Table 2). miR‐122 remains strongly associated with hepatic steatosis, while its relationship with fibrosis is weakened in this competing analysis. miR‐200a is only associated with fibrosis in multivariate analysis. In contrast, miR‐191, a miRNA linked to HCC but not NAFLD in existing literature, showed an inverse association with balloon degeneration on both univariate and multivariable analyses, but not with other histological features of NAFLD.
Table 2.
Univariate and Multivariate Associations Between miRNA and Histological Features of NAFLD
| miR‐34a | miR‐122 | miR‐192 | miR‐200a | miR‐191 | |
|---|---|---|---|---|---|
| β*, P value | β, P value | β, P value | β, P value | β, P value | |
| Univariate analysis | |||||
| Fibrosis | 0.395 | 0.199 | 0.245 | 0.267 | −0.015 |
| <0.001 | 0.005 | 0.001 | <0.001 | 0.8 | |
| Hepatic steatosis | 0.338 | 0.411 | 0.365 | 0.114 | 0.023 |
| 0.004 | <0.001 | 0.002 | 0.3 | 0.9 | |
| Lobular inflammation | 0.337 | 0.269 | 0.195 | 0.182 | 0.052 |
| 0.03 | 0.08 | 0.2 | 0.2 | 0.7 | |
| Ballooning degeneration | 0.442 | 0.361 | 0.259 | 0.317 | −0.256 |
| <0.001 | 0.002 | 0.03 | 0.007 | 0.03 | |
| miR‐34a | miR‐122 | miR‐192 | miR‐200a | miR‐191 | |
|---|---|---|---|---|---|
| β, P value | β, P value | β, P value | β, P value | β, P value | |
| Multivariate analysis | |||||
| Fibrosis | 0.352 | 0.136 | 0.233 | 0.231 | 0.059 |
| <0.001 | 0.08 | 0.003 | 0.004 | 0.5 | |
| Hepatic steatosis | 0.263 | 0.347 | 0.341 | 0.052 | 0.086 |
| 0.02 | 0.004 | 0.004 | 0.7 | 0.5 | |
| Lobular inflammation | −0.022 | −0.001 | −0.058 | −0.054 | 0.176 |
| 0.9 | 0.9 | 0.7 | 0.7 | 0.3 | |
| Ballooning degeneration | 0.120 | 0.169 | 0.014 | 0.150 | −0.374 |
| 0.3 | 0.2 | 0.9 | 0.3 | 0.008 | |
All β‐coefficients for miRNAs as outcome variables are normalized by z‐score in the unit of SD.
Relationships Between miRNA and Pathogenic Factors of NAFLD
We then examined the relationship between miRNAs and common genetic risk factors for NAFLD. The GC and GG variants at rs738409 of PNPLA3 are robustly associated with higher levels of miR‐34a, miR‐122, and miR‐192 with β‐coefficient of 0.78‐0.86 SD, but not miR‐200a (Supporting Table S2; Table 3). Instead, miR‐200a is strongly associated with the CT and TT variants at rs58542926 of TM6SF2 with a β‐coefficient of 0.45 SD. Like miR‐200a, miR‐34a demonstrates a similar extent of associations with the E167K variant at TM6SF2, whereas miR‐122 and miR‐192 demonstrate a much weaker association.
Table 3.
Univariate Associations Between MicroRNA and NAFLD Genotypes
| miR‐34a | miR‐122 | miR‐192 | miR‐200a | miR‐191 | |
|---|---|---|---|---|---|
| β*, P value | β, P value | β, P value | β, P value | β, P value | |
| Univariate analysis | |||||
| PNPLA3 rs738409 | 0.776 | 0.857 | 0.807 | 0.272 | −0.066 |
| C → G variant | <0.001 | <0.001 | <0.001 | 0.1 | 0.7 |
| TM6SF2 rs58542926 | 0.401 | 0.270 | 0.255 | 0.448 | 0.219 |
| C → T variant | 0.06 | 0.2 | 0.2 | 0.03 | 0.3 |
All β‐coefficients for miRNAs as outcome variables are normalized by z‐score in the unit of SD.
The eLP‐IR score, a measurement of insulin resistance, strongly correlates with miR‐34a and miR‐122 concentrations, with every 1% increase in eLP‐IR associated with a 1% SD increase in the levels of miRNA concentration (Table 4). Interestingly, miR‐34a is also associated with diabetes and hypertension with β‐coefficient of 0.81 and 0.54 SD, respectively, whereas miR‐122 is not. In contrast, miR‐200a is not associated with insulin resistance. Interestingly, BMI does not correlate with any of the miRNA levels, despite their relationships with insulin resistance. Not surprisingly, miR‐191 does not demonstrate associations with either genetic risk factors of NALFD or factors related to insulin resistance and metabolic syndrome.
Table 4.
Univariate Associations Between miRNA and Nongenetic Pathogenic Factors in NAFLD
| miR‐34a | miR‐122 | miR‐192 | miR‐200a | miR‐191 | |
|---|---|---|---|---|---|
| β*, P value | β, P value | β, P value | β, P value | β, P value | |
| eLP‐IR | 0.010 | 0.011 | 0.007 | 0.002 | 0.002 |
| 0.02 | 0.009 | 0.08 | 0.6 | 0.6 | |
| BMI | 0.010 | −0.002 | −0.018 | −0.008 | −0.017 |
| 0.5 | 0.9 | 0.2 | 0.6 | 0.2 | |
| Diabetes | 0.810 | 0.293 | 0.447 | 0.551 | 0.006 |
| <0.001 | 0.1 | 0.02 | 0.004 | 1.0 | |
| Dyslipidemia | |||||
| Triglyceride | 0.001 | 0.001 | 0.002 | 0.001 | −0.0001 |
| 0.3 | 0.09 | 0.02 | 0.1 | 0.9 | |
| LDL‐C | −0.002 | −0.001 | −0.0003 | −0.002 | 0.004 |
| 0.3 | 0.7 | 0.9 | 0.5 | 0.1 | |
| HDL‐C | −0.013 | −0.013 | −0.013 | 0.002 | −0.007 |
| 0.06 | 0.05 | 0.05 | 0.7 | 0.3 | |
| Hypertension | 0.537 | 0.030 | 0.102 | 0.428 | −0.030 |
| 0.002 | 0.9 | 0.6 | 0.01 | 0.9 |
All β‐coefficients for miRNAs as outcome variables are normalized by z‐score in the unit of SD.
We then constructed multivariate models of miRNA levels using both genetic risk factors of NAFLD and insulin resistance to evaluate whether these associations are collinear. The associations in multivariate models remain similar to those seen in univariate models (Table 5). miR‐34a, miR‐122, and miR‐192 demonstrate associations with both insulin resistance measured by eLP‐IR and genetic risk factors conferred by PNPLA3 and TM6SF2 variants. Of note, all three miRNAs demonstrate independent associations to these causal risk factors of NAFLD, as the β‐coefficient and P values remained largely unchanged compared with those in univariate analysis. In comparison, miR‐200a is only associated with the E167K variant in TM6SF2.
Table 5.
Multivariate Analysis Between miRNA and Key Pathogenic Factors in NAFLD
| miR‐34a | miR‐122 | miR‐192 | miR‐200a | miR‐191 | |
|---|---|---|---|---|---|
| β*, P value | β, P value | β, P value | β, P value | β, P value | |
| eLP‐IR | 0.010 | 0.011 | 0.008 | 0.003 | 0.002 |
| 0.005 | 0.003 | 0.05 | 0.5 | 0.6 | |
| PNPLA3 rs738409 | 0.763 | 0.860 | 0.814 | 0.285 | 0.039 |
| C → G variant | <0.001 | <0.001 | <0.001 | 0.1 | 0.8 |
| TM6SF2 rs58542926 | 0.559 | 0.392 | 0.357 | 0.485 | 0.089 |
| C → T variant | 0.004 | 0.04 | 0.07 | 0.02 | 0.7 |
All β‐coefficients for miRNAs as outcome variables are normalized by z‐score in the unit of SD.
Predictive Value of miRNAs for Liver Fibrosis
To test the predictive value of miRNA for liver fibrosis in NAFLD, we compared the C‐statistic of FIB‐4, a validated score for liver fibrosis, with two models: miR34a alone and a combined score from four miRNAs (Fig. 1). As expected, FIB‐4 provided great predictive value for stage 4 fibrosis with area under the curve (AUROC) of 0.92, but its predictive value for stage 1+, 2+, or even 3+ fibrosis is suboptimal (AUROC: 0.69‐0.78). In comparison, the predictive value of miR34a performs similarly across all stages of liver fibrosis, with AUROC from 0.73 to 0.76. The score derived from four miRNAs does not significantly improve the predictive value from miR34a alone for stage 1+, 2+ or 3+ fibrosis, but does moderately increase the predictive value to predict stage 4 fibrosis.
Fig. 1.
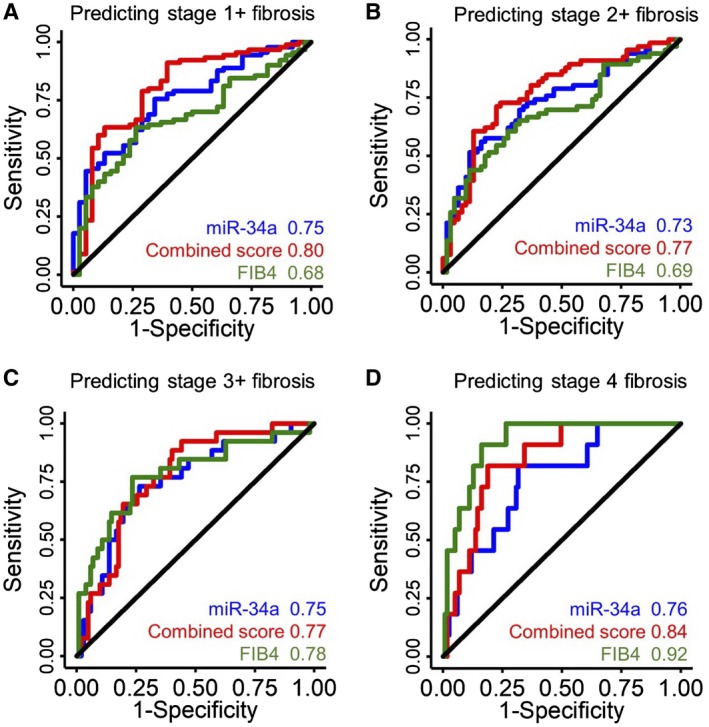
Predictive values of miRNA for liver fibrosis. Receiver operating characteristic curves of miR‐34a (blue), a combined score calculated from miR‐34a, miR‐122, miR‐192 and miR‐200a (red), and FIB‐4 in predicting stage 1+ (A), 2+ (B), 3+ (C), and 4 fibrosis (D).
Discussion
Circulating miRNAs have emerged as powerful biomarkers in recent years. MicroRNAs are readily assessable, quantifiable, and most importantly can inform tissue‐specific disease pathogenesis. In NAFLD, studies demonstrate several miRNAs being differentially associated with NAFLD.( 20 ) This study builds upon existing knowledge of miRNAs in NALFD and aims to cross‐validate four previously described miRNAs in their relative strength of association with the histological and pathogenic features that drive NAFLD. We hypothesize that if a miRNA is uniformly associated with all pathogenic factors and histological features of NAFLD, it is likely a consequence of NAFLD or NASH, whereas a differential association with such features would indicate linkage to a distinct causal pathway.
This study contributes to the growing understanding of miRNA in NAFLD. First, it provides an independent validation that miR‐34a, miR‐122, miR‐192, and miR‐200a are all robustly associated with liver fibrosis in NAFLD. miRNAs provide a moderate predictive value to determine early stages of liver fibrosis (stages 1‐3), and outperform FIB‐4 in predicting early stages of fibrosis (stages 1 and 2). This is of potential clinical utility as a minimally invasive blood‐based fibrosis staging tool. However, in‐depth analyses illustrate their differences. When measured on a uniform scale of z‐score, miR‐34a has a higher correlation with fibrosis than miRNA‐122, miR‐192, and miR‐200a measured by the β‐coefficient. In fact, miRNA34a alone provides similar predictive value for stage 1+, 2+, or 3+ fibrosis to a score that combines all four miRNAs. This may inform the development of cost‐effective miRNA‐based diagnostic strategies.
Although most of the miRNAs are associated with more than one histological feature of NAFLD, liver fibrosis is the only feature that is associated with all four miRNAs in multivariate analyses when histological features of NAFLD are competing simultaneously. Lobular inflammation and ballooning degeneration are no longer significant factors in multivariate analyses. miR‐122 and miR‐192 are strongly associated with hepatic steatosis, with a β‐coefficient higher than those with fibrosis. In comparison, miR‐200a is only associated with fibrosis. In our cohort, a control microRNA, miR‐191, is not associated with liver fibrosis or hepatic steatosis, but only inversely associated with ballooning degeneration. This is potentially in keeping with its link to cell proliferation and inhibition against apoptosis.( 39 )
The development and progression of fibrosis in most patients with NAFLD are driven by an interplay between genetic risk factors and insulin resistance, an acquired trait. On such basis, we have proposed a conceptual framework that genetics and insulin resistance are key predictors of NASH, and together with the duration of disease, they can predict liver fibrosis.( 7 ) This study reveals a differential association of these four miRNAs with pathogenic factors of NAFLD (Fig. 2). miR‐34a was associated with insulin resistance and both genetic risk factors in PNPLA3 and TM6SF2. Both miR‐192 and miR‐122 are similar to miR34a in their associations with insulin resistance and PNPLA3 I148M variant, but they have a lesser association with the TM6SF2 E167K variant with lower β‐coefficients. In contrast, miR‐200a was only associated with the TM6SF2 E167K variant.
Fig. 2.
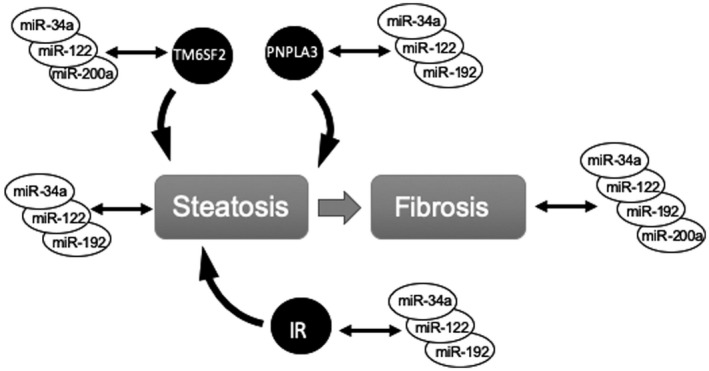
Differential associations between miRNAs and pathogenic factors in NAFLD. Abbreviation: IR, insulin resistance.
Although cross‐sectional studies do not determine causality, the observed differential associations with various histological and pathogenic factors offer insights about potential causal relationships. In this study, miR‐34a, miR‐122, and miR‐192 unexpectedly demonstrate similar patterns of associations with nearly all pathogenic factors of NAFLD, among which miR‐34a appears to have the strongest association. Hence, the elevation of these miRNAs is likely a consequence of NASH and fibrosis. In contrast, miR‐200a is associated with liver fibrosis and TM6SF2 E167K variant, but not hepatic steatosis or insulin resistance. This indicates that the relationship between miR‐200a and NASH may involve a distinct pathway.
Among the four miRNAs, miR‐122 is probably the best studied. miR‐122 is a liver‐predominant miRNA. Elevated circulating levels of free fatty acids from insulin resistance increases hepatic production of miR‐122, which in turn regulates the balance between energy storage and expenditure in the liver.( 40 ) It is regulated by grainyhead‐like transcription factor 2 (GRHL2) in the setting of alcoholic liver disease.( 41 ) A study by Wu et al. demonstrates that miR‐122 is also involved in intrahepatic lipid droplet formation through the farnesoid X receptor–small heterodimer partner signaling pathway.( 42 ) Less is known about miR‐34a, miR‐192, and miR‐200a. miR‐34a functions as a master regulator in tumor suppression and is used for cancer treatment.( 43 ) Interestingly, the induction of miR‐34a is linked to age‐related decline in the proliferative capacity of pancreatic beta cells in rats and associated with the development of diabetes in humans.( 44 ) Other studies suggest that miR‐34a promotes the activation of human hepatic stellate cells through peroxisome proliferator‐activated receptor γ in vitro.( 45 ) miR‐192 is one of the most elevated miRNAs in response to drug‐induced liver injury.( 26 , 27 ) Two clinical observational studies indicate that miR‐192 is elevated in patients with diabetic nephropathy.( 46 , 47 ) Finally, miR‐200a is a member of the miR‐200 microRNA family, and was recognized as a tumor suppressor that functions in part through the regulation of notch signaling.( 48 ) The level of miR‐200a is often reduced in association with tumor invasion and metastasis.
The current understanding of miR‐34a, miR‐192, and miR‐200a does not explain their associations with NAFLD. Further mechanistic studies are needed. Several limitations need to be considered to interpret current data and guide future investigations. This cohort reflects patients seen in a tertiary NAFLD referral center, which skews toward NASH and early fibrosis and does not include heathy controls. The sample size is moderate and limits the ability to define weaker associations. Larger prospective studies are needed to better address these issues and account for potential unadjusted confounders.
In conclusion, this study independently validates the association between liver fibrosis and four previously discovered miRNAs: miR‐34a, miR‐122, miR‐192, and miR‐200a. Among these four miRNAs, miR‐34a demonstrates the most robust associations with all histological features of NAFLD and associations with both genetic risk factors and insulin resistance. Meanwhile, miR‐200a differs from the other three miRNAs exhibiting associations with liver fibrosis and the TM6SF2 E167K variant. This study highlights important observations that help bridge the gap in understanding the underlying mechanism linking miRNA and NAFLD and that inform future strategies to use miRNA in risk stratification and personalized management of NAFLD.
Author Contributions
G.E., H.D.T., Z.G.J., and M.L. were responsible for the study design and data analysis. M.A.C. were responsible for the measurements for eLP‐IR. M.K. and M.H. were responsible for the genotyping. K.H., M.P., and M.A.C. were responsible for providing the miRNA data. I.N. was responsible for providing the histology interpretation. All authors participated in the manuscript preparation. All authors approved the final version of the manuscript.
Supporting information
Table S1‐S3
Grant Support: National Institute of General Medical Sciences, (5T32DK007760‐19); American College of Gastroenterology (Clinical Research Award); American Association for the Study of Liver Diseases (Alan Hoffman Clinical and Translational Research Award); Covance Science Council (Science and Technology Grant); and National Institute of Diabetes and Digestive and Kidney Diseases (K08DK115883, K23DK083439 and R01DK100425).
Potential conflict of interest: Dr. Afdhal consults and advises for Gilead, Echosens, Ligand, Shionogi, TRIO Healthcare, Atea, and Axcella. He owns stock in, consults, and advises Spring Bank. He owns stock in Allurion. He receives royalties from Up To Date. Dr. Jiang consults for Olix Pharma and Boehringer Ingelheim. He received grants from Gilead. Dr. Connelly is employed by LabCorp. Dr. Filozof owns stock in and is employed by Covance/LabCorp. Dr. Herman received grants from Eli Lilly & Co. Mr. Parrish owns stock in and is employed by Covance/LabCorp.
Contributor Information
Z. Gordon Jiang, Email: zgjiang@bidmc.harvard.edu.
Michelle Lai, Email: mlai@bidmc.harvard.edu.
References
Author names in bold designate shared co‐first authorship.
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 2. Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387‐1395. [DOI] [PubMed] [Google Scholar]
- 3. Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547‐555. [DOI] [PubMed] [Google Scholar]
- 4. Osterreicher CH, Brenner DA. The genetics of nonalcoholic fatty liver disease. Ann Hepatol 2007;6:83‐88. [PubMed] [Google Scholar]
- 5. Targher G, Byrne CD. A perspective on metabolic syndrome and nonalcoholic fatty liver disease. Metab Syndr Relat Disord 2015;13:235‐238. [DOI] [PubMed] [Google Scholar]
- 6. Danford CJ, Yao Z, Jiang ZG. Non‐alcoholic fatty liver disease: a narrative review of genetics. J Biomed Res 2018:32;389‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Danford CJ, Connelly MA, Shalaurova I, Kim M, Herman MA, Nasser I, et al. A pathophysiologic approach combining genetics and insulin resistance to predict the severity of nonalcoholic fatty liver disease. Hepatol Commun 2018;2:1467‐1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Makkonen J, Pietilainen KH, Rissanen A, Kaprio J, Yki‐Jarvinen H. Genetic factors contribute to variation in serum alanine aminotransferase activity independent of obesity and alcohol: a study in monozygotic and dizygotic twins. J Hepatol 2009;50:1035‐1042. [DOI] [PubMed] [Google Scholar]
- 9. Schwimmer JB, Celedon MA, Lavine JE, Salem R, Campbell N, Schork NJ, et al. Heritability of nonalcoholic fatty liver disease. Gastroenterology 2009;136:1585‐1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjærg‐Hansen A, et al. Exome‐wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2014;46:352‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461‐1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang ZG, Tapper EB, Kim M, et al. Genetic determinants of circulating lipoproteins in nonalcoholic fatty liver disease. J Clin Gastroenterol 2018;52:444‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahdessian H, Taxiarchis A, Popov S, Silveira A, Franco‐Cereceda A, Hamsten A, et al. TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content. Proc Natl Acad Sci U S A 2014;111:8913‐8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pirazzi C, Adiels M, Burza MA, Mancina RM, Levin M, Ståhlman M, et al. Patatin‐like phospholipase domain‐containing 3 (PNPLA3) I148M (rs738409) affects hepatic VLDL secretion in humans and in vitro . J Hepatol 2012;57:1276‐1282. [DOI] [PubMed] [Google Scholar]
- 15. Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell 2009;136:642‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mohr AM, Mott JL. Overview of microRNA biology. Semin Liver Dis 2015;35:3‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Su Q, Kumar V, Sud N, Mahato RI. MicroRNAs in the pathogenesis and treatment of progressive liver injury in NAFLD and liver fibrosis. Adv Drug Deliv Rev 2018;129:54‐63. [DOI] [PubMed] [Google Scholar]
- 18. Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, et al. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology 2008;48:1810‐1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soronen J, Yki‐Jarvinen H, Zhou Y, Sädevirta S, Sarin AP, Leivonen M, et al. Novel hepatic microRNAs upregulated in human nonalcoholic fatty liver disease. Physiol Rep 2016;4:e12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu CH, Ampuero J, Gil‐Gomez A, Montero‐Vallejo R, Rojas Á, Muñoz‐Hernández R, et al. miRNAs in patients with non‐alcoholic fatty liver disease: a systematic review and meta‐analysis. J Hepatol 2018;69:1335‐1348. [DOI] [PubMed] [Google Scholar]
- 21. Pirola CJ, Fernandez Gianotti T, Castano GO, Mallardi P, San Martino J, Mora Gonzalez Lopez Ledesma M, et al. Circulating microRNA signature in non‐alcoholic fatty liver disease: from serum non‐coding RNAs to liver histology and disease pathogenesis. Gut 2015;64:800‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Castro RE, Ferreira DM, Afonso MB, Borralho PM, Machado MV, Cortez‐Pinto H, et al. miR‐34a/SIRT1/p53 is suppressed by ursodeoxycholic acid in the rat liver and activated by disease severity in human non‐alcoholic fatty liver disease. J Hepatol 2013;58:119‐125. [DOI] [PubMed] [Google Scholar]
- 23. Xu Y, Zalzala M, Xu J, Li Y, Yin L, Zhang Y. A metabolic stress‐inducible miR‐34a‐HNF4alpha pathway regulates lipid and lipoprotein metabolism. Nat Commun 2015;6:7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sobolewski C, Calo N, Portius D, Foti M. MicroRNAs in fatty liver disease. Semin Liver Dis 2015;35:12‐25. [DOI] [PubMed] [Google Scholar]
- 25. Laterza OF, Lim L, Garrett‐Engele PW, Vlasakova K, Muniappa N, Tanaka WK, et al. Plasma microRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem 2009;55:1977‐1983. [DOI] [PubMed] [Google Scholar]
- 26. Starkey Lewis PJ, Dear J, Platt V, Simpson KJ, Craig DGN, Antoine DJ, et al. Circulating microRNAs as potential markers of human drug‐induced liver injury. Hepatology 2011;54:1767‐1776. [DOI] [PubMed] [Google Scholar]
- 27. Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, et al. Circulating microRNAs, potential biomarkers for drug‐induced liver injury. Proc Natl Acad Sci U S A 2009;106:4402‐4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baker LA, Lee KC, Palacios Jimenez C, Alibhai H, Chang YM, Leckie PJ, et al. Circulating microRNAs reveal time course of organ injury in a porcine model of acetaminophen‐induced acute liver failure. PLoS One 2015;10:e0128076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Afonso MB, Rodrigues PM, Simao AL, Castro RE. Circulating microRNAs as potential biomarkers in non‐alcoholic fatty liver disease and hepatocellular Carcinoma. J Clin Med 2016;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cermelli S, Ruggieri A, Marrero JA, Ioannou GN, Beretta L. Circulating microRNAs in patients with chronic hepatitis C and non‐alcoholic fatty liver disease. PLoS One 2011;6:e23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zarfeshani A, Ngo S, Sheppard AM. MicroRNA expression relating to dietary‐induced liver steatosis and NASH. J Clin Med 2015;4:1938‐1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nagpal N, Kulshreshtha R. miR‐191: an emerging player in disease biology. Front Genet 2014;5:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 34. Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med 2006;26:847‐870. [DOI] [PubMed] [Google Scholar]
- 35. Matyus SP, Braun PJ, Wolak‐Dinsmore J, Jeyarajah EJ, Shalaurova I, Xu Y, et al. NMR measurement of LDL particle number using the Vantera Clinical Analyzer. Clin Biochem 2014;47:203‐210. [DOI] [PubMed] [Google Scholar]
- 36. Otvos JD, Shalaurova I, Wolak‐Dinsmore J, Connelly MA, Mackey RH, Stein JH, et al. GlycA: a composite nuclear magnetic resonance biomarker of systemic inflammation. Clin Chem 2015;61:714‐723. [DOI] [PubMed] [Google Scholar]
- 37. Shalaurova I, Connelly MA, Garvey WT, Otvos JD. Lipoprotein insulin resistance index: a lipoprotein particle‐derived measure of insulin resistance. Metab Syndr Relat Disord 2014;12:422‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wolak‐Dinsmore J, Gruppen EG, Shalaurova I, Matyus SP, Grant RP, Gegen R, et al. A novel NMR‐based assay to measure circulating concentrations of branched‐chain amino acids: elevation in subjects with type 2 diabetes mellitus and association with carotid intima media thickness. Clin Biochem 2018;54:92‐99. [DOI] [PubMed] [Google Scholar]
- 39. Elyakim E, Sitbon E, Faerman A, Tabak S, Montia E, Belanis L, et al. hsa‐miR‐191 is a candidate oncogene target for hepatocellular carcinoma therapy. Cancer Res 2010;70:8077‐8087. [DOI] [PubMed] [Google Scholar]
- 40. Chai C, Rivkin M, Berkovits L, Simerzin A, Zorde‐Khvalevsky E, Rosenberg N, et al. Metabolic circuit involving free fatty acids, microRNA 122, and triglyceride synthesis in liver and muscle tissues. Gastroenterology 2017;153:1404‐1415. [DOI] [PubMed] [Google Scholar]
- 41. Satishchandran A, Ambade A, Rao S, Hsueh YC, Iracheta‐Vellve A, Tornai D, et al. MicroRNA 122, regulated by GRLH2, protects livers of mice and patients from ethanol‐induced liver disease. Gastroenterology 2018;154:238‐252.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu GY, Rui C, Chen JQ, Sho E, Zhan SS, Yuan XW, et al. MicroRNA‐122 inhibits lipid droplet formation and hepatic triglyceride accumulation via Yin Yang 1. Cell Physiol Biochem 2017;44:1651‐1664. [DOI] [PubMed] [Google Scholar]
- 43. Slabakova E, Culig Z, Remsik J, Soucek K. Alternative mechanisms of miR‐34a regulation in cancer. Cell Death Dis 2017;8:e3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tugay K, Guay C, Marques AC, Allagnat F, Locke JM, Harries LW, et al. Role of microRNAs in the age‐associated decline of pancreatic beta cell function in rat islets. Diabetologia 2016;59:161‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li X, Chen Y, Wu S, He J, Lou L, Ye W, Wang J. microRNA‐34a and microRNA‐34c promote the activation of human hepatic stellate cells by targeting peroxisome proliferator‐activated receptor gamma. Mol Med Rep 2015;11:1017‐1024. [DOI] [PubMed] [Google Scholar]
- 46. Al‐Kafaji G, Al‐Muhtaresh HA. Expression of microRNA377 and microRNA192 and their potential as blood‐based biomarkers for early detection of type 2 diabetic nephropathy. Mol Med Rep 2018;18:1171‐1180. [DOI] [PubMed] [Google Scholar]
- 47. Chien HY, Chen CY, Chiu YH, Lin YC, Li WC. Differential microRNA profiles predict diabetic nephropathy progression in Taiwan. Int J Med Sci 2016;13:457‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang B, Pan X, Cobb GP, Anderson TA. MicroRNAs as oncogenes and tumor suppressors. Dev Biol 2007;302:1‐12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3


