Abstract
Although drug‐induced liver injury (DILI) is a rare clinical event, it carries significant morbidity and mortality, leaving it as the leading cause of acute liver failure in the United States. It is one of the most challenging diagnoses encountered by gastroenterologists. The development of various drug injury networks has played a vital role in expanding our knowledge regarding drug‐related and herbal and dietary supplement–related liver injury. In this review, we discuss what defines liver injury, epidemiology of DILI, its biochemical and pathologic patterns, and management.
Although drug‐induced liver injury (DILI) is a rare clinical event, it carries significant morbidity and mortality, leaving it as the leading cause of acute liver failure in the United States. It is one of the most challenging diagnoses encountered by gastroenterologists. DILI is also the most common single adverse event that has led to withdrawal of drugs from the marketplace, drug attrition, and failure of implicated drugs to obtain Food and Drug Administration approval. The development of various drug injury networks have played a vital role in expanding our knowledge regarding drug‐related and herbal and dietary supplement–related liver injury. In this review, we discuss what defines liver injury, epidemiology of DILI, its biochemical and pathologic patterns, and management.

Abbreviations
- AIH
autoimmune hepatitis
- ALF
acute liver failure
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- APAP
acetaminophen
- AST
aspartate aminotransferase
- DILI
drug‐induced liver injury
- DILIN
Drug‐Induced Liver Injury Network
- FDA
U.S. Food and Drug Administration
- GTE
green tea extract
- GWAS
genome‐wide association
- HDS
herbal and dietary supplement
- HBV
hepatitis B
- HCV
hepatitis C
- HLA
human leukocyte antigen
- ICI
immune checkpoint inhibitor
- IDILI
idiosyncratic DILI
- NAC
N‐acetylcysteine
- NAFLD
nonalcoholic fatty liver disease
- RUCAM
Roussel Uclaf Causality Assessment Method
- ULN
upper limit of normal
- VBDS
vanishing bile duct syndrome
- VOD/SOS
veno‐occlusive disease/sinusoidal obstruction syndrome
Although drug‐induced liver injury (DILI) is a rare clinical event, it carries significant morbidity and mortality, leaving it as the leading cause of acute liver failure (ALF) in the United States.( 1 ) It is one of the most challenging diagnoses encountered by gastroenterologists. DILI is also the most common single adverse event that has led to withdrawal of drugs from the marketplace, drug attrition, and failure of implicated drugs to obtain U.S. Food and Drug Administration (FDA) approval.( 2 )
Defining DILI
Liver injury is recognized by abnormal liver biochemistries with or without associated clinical symptoms. Using liver biochemistry criteria, which will increase the specificity of hepatotoxicity causality assessment and eliminate false positives, is key.( 3 ) This aids in early detection, prediction, and risk stratification of suspected cases of DILI. The updated Roussel Uclaf Causality Assessment Method (RUCAM) uses an alanine aminotransferase (ALT) >5‐times the upper limit of normal (ULN) and/or alkaline phosphatase (ALP) >2‐times ULN to identify liver injury.( 4 ) Conventionally, liver biochemistry elevations to this degree, lesser elevations that are sustained over time, rapidly rising tests, or any elevation combined with signs of liver dysfunction, such as increase in the international normalized ratio or encephalopathy, are clinically significant and worthy of investigation.
Burden of DILI in the United States and Abroad
In western countries, acetaminophen (APAP)‐related liver injury remains one of the leading causes of DILI.( 5 ) Given the challenges in detection and reporting, the incidence of DILI is difficult to ascertain. Annual incidence of DILI ranges from 2.3‐13.9/100,000 inhabitants in population‐based studies from Europe.( 6 , 7 , 8 ) The highest incidence of non‐APAP‐related DILI was reported at 19.1/100,000 inhabitants/year with a steady increase in age‐standardized incidence of DILI, in an Icelandic population‐based study.( 9 ) Although most of the cases reported in the western countries are DILI secondary to prescription medications, traditional/complimentary and dietary supplements are the main causative agents of DILI in Asia.( 10 , 11 ) In the only U.S. population–based study, the yearly incidence of DILI was found to be approximately 3/100,000 residents.( 12 )
U.S. DILI Network
The National Institute of Diabetes and Digestive and Kidney Diseases established the U.S. DILI Network (DILIN) in 2003 to identify, enroll, and characterize cases of non‐APAP DILI and herbal and dietary supplements (HDSs).( 13 ) The U.S. DILIN has two registry studies at eight different academic centers across the United States. The network has expanded our understanding of DILI. Antimicrobials were noted to be the most common causative agents, accounting for 45% of cases in a 2004 study, followed by HDSs, cardiovascular drugs, central nervous system agents, antineoplastic agents, and analgesics.( 14 ) Of the antimicrobials, amoxicillin‐clavulanate (22%), isoniazid (11.7%), and nitrofurantoin (10.2%) were the top three implicated agents.( 14 ) The network has also noted an increasing proportion of HDS‐related liver injury, from 7% in 2004‐2005 to 20% in 2013‐2014.( 15 , 16 )
Patterns and Outcomes of DILI
Recognizing the pattern of liver injury at the initial presentation is vital. It provides a useful foundation to establish a differential diagnosis and guides the diagnostic evaluation accordingly. Assessing the pattern of liver injury as hepatocellular, cholestatic, or mixed is based on which liver enzyme elevation predominates. For example, hepatocellular injury suggests that an elevation of ALT and/or AST is more prominent than ALP. Conversely, cholestatic injury suggests a predominant elevation of ALP. The R‐ratio is a quantitative expression of the injury pattern; it is defined as the ratio of serum ALT to ALP values, both expressed as multiples of ULN, obtained at the onset of injury. An R‐ratio of >5 indicates hepatocellular injury, <2 indicates cholestatic injury, and 2‐5 indicates mixed injury.( 17 , 18 ) Table 1 lists the drugs and pattern of associated liver injury.
Table 1.
Drugs and Pattern of Liver Injury
| Pattern of Liver Injury | |||||||
|---|---|---|---|---|---|---|---|
| Hepatocellular | Cholestatic | Mixed | |||||
| Drug |

|
|
|
|
|||
Abbreviation: NSAIDs, nonsteroidal anti‐inflammatory drugs.
Hepatocellular injury is the most common pattern of DILI across various networks, with 52%‐75% of cases reported from Spanish, Latin, and U.S. DILIN, and in the Swedish Adverse Drug Reaction Advisory Committee (SADRAC).( 14 , 19 , 20 , 21 ) Patients with hepatocellular injury tend to be younger, less likely to be clinically jaundiced, but have higher frequency of liver‐related deaths. Hepatocellular injury is 2‐3 times more likely to lead to liver transplantation. The clinical course tends to be more protracted in those with cholestatic injury.( 14 )
Hepatocellular DILI with jaundice is an important pattern of biochemical injury to recognize, commonly referred to as “Hy’s Law.”( 22 , 23 ) It was first observed by Hyman Zimmerman in an analysis of 114 patients taking isoniazid, patients with an increased ALT >3‐times ULN, and total bilirubin >2‐times ULN without initial findings of cholestasis and after excluding other potential causes experienced a case fatality rate of 10%.( 24 ) This observation has been used by the FDA to identify drugs with the potential of severe liver injury. Similar fatality rates from DILI with hepatocellular jaundice have been seen in the Spanish DILIN (11.7%) and SADRAC (12.7%).( 13 , 25 , 26 )
Adaptation is a transient and modest rise of liver tests due to a drug that persists without progression, or regresses back to normal despite continued use of a drug thought to be the cause. The common examples of liver adaptation are statins and isoniazid, although both agents have also been implicated in cases of idiosyncratic DILI.( 27 )
From a clinical standpoint, the development of low‐level liver enzyme elevation (ALT < 3‐times ULN or direct bilirubin <2‐times ULN) in an otherwise asymptomatic patient presents an important challenge. The clinician must also weigh the risks and benefits of a drug for the patient, as the benefit of a medication may outweigh the risk of hepatic injury. In this context, if low‐level enzymes are detected that, in the clinician’s assessment, are due to a drug, the patient has no liver‐related symptoms, and the drug is of evidence‐based clinical value to the patient, the enzymes should be followed monthly for at least 3 months to confirm their nonprogressive nature. A concurrent hepatic ultrasound to detect drug‐related morphological alterations such as steatosis is also wise. If the enzymes’ direct bilirubin rises, or new steatosis is detected, an alternative agent should be sought, even if within the same class of drugs. If no better alternative exists, such as may be the case with some chemotherapies, dose reduction or drug continuation with close enzyme and imaging follow‐up is recommended.
Most patients with DILI have both clinical and biochemical recovery. However, a small proportion of cases may develop chronic liver disease or chronic DILI, conventionally defined as persistence of liver‐enzyme elevations for more than 6 months after withdrawal of an offending drug. The incidence of chronic DILI varies from 5%‐20% in various DILIN registries and population‐based studies.( 9 , 28 , 29 )
Types of DILI
Conventionally, DILI is classified as intrinsic and idiosyncratic. Intrinsic DILI is predictable, occurring in a dose‐dependent manner. The typical example of an intrinsic DILI is APAP‐related liver injury. The injury is usually within a short duration of time with a predictable latency period after drug exposure. APAP‐related liver injury alone is responsible for about 46% of the cases of ALF in the United States. Pharmacodynamic studies have demonstrated a dose‐response relationship between the extent of biochemical abnormalities and dose of APAP ingested. At higher doses of APAP, the conjugation pathways for its metabolization are saturated, thereby generating N‐acetyl‐p‐benzoquinone‐imine, a highly reactive toxic metabolite leading to hepatocellular necrosis.( 30 )
Idiosyncratic DILI (IDILI) is unpredictable and, arguably, may be the most common type of drug‐associated hepatotoxicity. It has a variable latency period and is difficult to replicate in experimental models. Historically, IDILI has been considered to be dose‐independent. In the last decade, however, there have been studies showing an association of IDILI with drugs prescribed at a daily dose of more than 50 mg.( 31 , 32 ) Whether there is true association between dose and IDILI is not fully clear. The pathogenesis of idiosyncratic DILI can either be related to the pathway of metabolism of the drug and/or activation of the immune system, as will be discussed subsequently.
Mechanism of Action
Direct hepatotoxicity occurs in the setting of a known hepatotoxic agent, which leads to cell death through necrosis or apoptosis. However, the mechanism of IDILI involves a complicated process involving the drug, its metabolites, and the host immune system. Most drugs implicated in DILI are lipophilic and metabolized by the liver. They undergo phase 1 reaction, which is usually mediated by the hepatic cytochrome p450 system. The generation of intermediate bioactive or reactive metabolites is an important step, leading to both intrinsic and idiosyncratic DILI.( 33 ) These toxic intermediate products are usually inactivated by phase 2 reactions, such as glutathione or sulfate conjugation. If conjugation pathways are overwhelmed by the excess production of the toxic reactive metabolites or caused by depletion of the conjugating factors, covalent binding of the reactive metabolites to mitochondrial proteins results. This leads to production of reactive oxygen species and ATP depletion, causing cellular organelle dysfunction from activation of stress kinase signaling pathways and disruption of membrane permeability pores. The result is hepatocyte dysfunction, necrosis, and/or cell death.( 33 , 34 )
Immune‐mediated injury is an important mechanism for IDILI. Both the innate and the adaptive immune system play a vital role. Over the years, there have been various hypotheses for the mechanism of immune‐mediated DILI. Under the hapten hypothesis, reactive metabolites irreversibly bind with cellular proteins to form neoantigens (also referred to as “haptens”), which then are presented to major histocompatibility complex molecules on antigen‐presenting cells, triggering an immune response against the hepatocyte by recruitment of cytotoxic T lymphocytes, natural killer cells, and B cells. In some instances, the haptens can induce development of autoantibodies against cytochrome p450 enzymes, leading to cellular injury and death, as in the case of halothane‐related liver injury.( 34 , 35 , 36 ) The pharmacological interaction concept proposes that a drug or its metabolite can bind directly to the human leukocyte antigen (HLA) molecule to trigger a T cell–mediated injury, especially in genetically susceptible individuals.( 37 )
The liver has a remarkable capacity for immune tolerance. This is a necessary adaptation that serves as a barrier to hepatocyte dysfunction and injury from an inflammatory state, which develops in response to the constant exposure to ingested antigens. This is managed by induction of peripheral immune tolerance to incoming antigens.( 38 , 39 ) The adaptive immune response can be up‐regulated in the setting of a sublethal stress from a hapten. HLA associations are indicative of the adaptive immune response in IDILI, and in genetically susceptible individuals, overt liver injury may occur as a result of “defective adaptation.”( 40 ) Conceivably, this complex interplay between the immune and nonimmune pathways is responsible for the unpredictability of idiosyncratic DILI. Figure 1 details the mechanism of direct and immune‐mediated DILI.
Fig. 1.
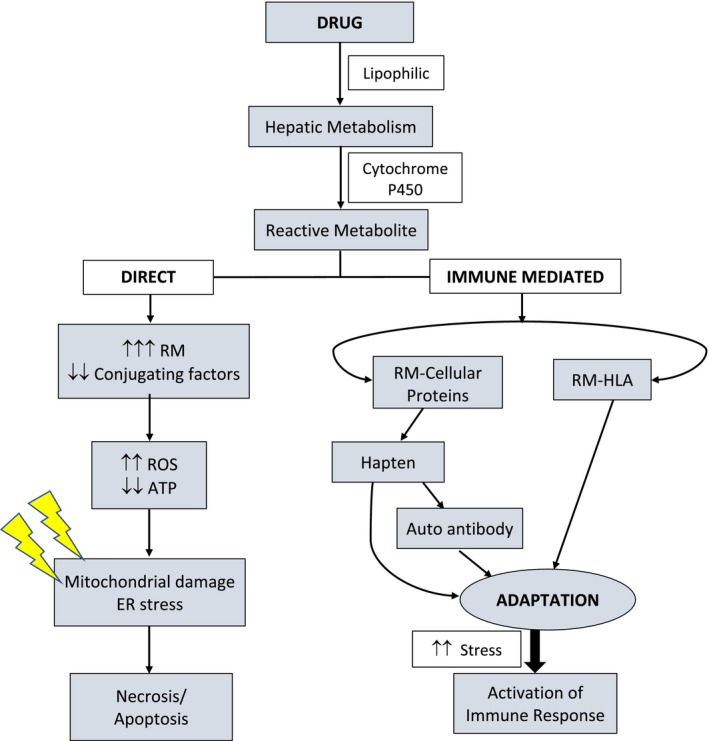
Mechanism of direct and immune mediated pathways of DILI. Abbreviations: RM, reactive metabolite; ROS, reactive oxygen species.
Risk Factors for DILI
Patient‐Related Factors
Susceptibility to DILI is influenced by modifiable and nonmodifiable host demographic, clinical, and environmental factors.
Age
Various large prospective DILI registries differ on advancing age as a risk factor for DILI. A population‐based Icelandic study showed increasing incidence of DILI with age: 5 times higher for patients over 70 years than those between 15 and 29 years. The former group was also noted to have a higher prescription rate of drugs,( 9 ) which could be a potential reason for increased DILI in this group as opposed to advanced age itself. The U.S. and Spanish networks did not identify advancing age as a risk factor for all‐cause DILI.( 14 , 33 ) Increasing age does pose as a risk factor for liver injury from medications like isoniazid, amoxicillin/clavulanate, and nitrofurantoin.( 41 ) Cholestatic DILI is more common in the elderly as compared with hepatocellular injury in younger individuals.( 14 ) Thus, age may confer a susceptibility to DILI in a drug‐specific manner.
Gender
Although epidemiological data from Spain, the United States, and Iceland do not suggest a heightened risk of DILI in women,( 9 , 14 , 33 ) others have found women to be more susceptible to DILI from some drugs. These agents include minocycline and nitrofurantoin, in which DILI is typically characterized by autoimmune features.( 42 , 43 ) Women with acute liver injury are more likely to progress to acute liver failure, as demonstrated in various registries.( 44 , 45 , 46 )
Race
Race and ethnicity are potential factors influencing DILI frequency, liver injury patterns, and outcomes. In a U.S. DILIN cohort, African‐Americans with DILI tended to be younger with higher rates of chronic DILI. The most common agents in African‐Americans were trimethoprim/sulfamethoxazole, methyldopa and phenytoin, as compared with amoxicillin/clavulanate in Caucasians. The pattern of liver injury as well as the time of recovery of DILI does not appear to be have significant differences when comparing African‐Americans and Caucasians, the predominant pattern being that of a mixed injury. African‐Americans were twice as likely to develop severe liver injury, either leading to death or liver transplantation, despite absence of disparity in attaining health care.( 47 ) The reasons for worse liver‐related outcomes and chronicity of DILI in African‐Americans in unclear. Agents associated with hypersensitivity reactions are known to be the common causes for DILI in African‐Americans, which can be as a result of genetic factors. African‐Americans have higher risk of severe cutaneous reactions to allopurinol due to higher frequency of HLA‐B*5801.( 48 )
Pregnancy
Data on DILI during pregnancy is limited and limited to therapeutic agents used to treat gestational hypertension and hyperthyroidism. The most common drugs are methyldopa, hydralazine, propylthiouracil, and antimicrobial agents like tetracycline.( 33 , 49 ) It is imperative to differentiate DILI during pregnancy from other more common etiologies of abnormal liver tests including viral hepatitis, gallstone disease, or pregnancy‐related complications such as intrahepatic cholestasis of pregnancy.
Alcohol
The pathophysiology of APAP‐associated liver injury in the setting of alcohol use is complex and driven by the manner in which alcohol is consumed. Acute alcohol co‐ingestion with APAP may be protective, as they compete with each other for use of CYP2E1 substrate, in turn reducing the byproduct N‐acetyl‐p‐benzoquinone‐imine. In contrast, chronic alcohol use augments APAP hepatotoxicity by acting as a CYP2E1 inducer. Chronic malnutrition, as may occur in patients with alcoholism, may further augment risk for DILI due to glutathione depletion in the malnourished state. In IDILI, there are no data to suggest increased risk of all‐cause DILI in the setting of chronic alcohol use. Chronic alcohol consumption is a risk factor for DILI from isoniazid, methotrexate, and halothane.( 50 )
Chronic Liver Disease and Comorbid Conditions
In a 2015 U.S. DILIN study, patients with pre‐existing liver disease were noted to have higher rates of severe DILI and 3‐times higher risk of mortality in comparison to those without prior liver disease.( 14 ) Nonalcoholic fatty liver disease (NAFLD) is also a potential factor to increased risk of DILI( 34 ); however, it should not preclude the use of statins in patients with NAFLD. In a 2010 post hoc analysis of the prospective Greek Atorvastain and Coronary Heart Disease Evaluation study, those receiving statin therapy had significantly lower rates of cardiovascular events and significant improvement in their liver chemistries.( 51 ) In several prospective studies, similar results of improvement of liver enzymes in patients with NAFLD on statin therapy have been demonstrated.( 52 , 53 , 54 ) The data from these studies provide evidence that statin use is safe for dyslipidemia with NAFLD.
Genetics
Single nucleotide polymorphisms in numerous genes and HLA regions have been shown to have an increased risk for DILI. A U.K. genome‐wide association (GWAS) identified HLA‐B*5701 genotype as a major determinant of flucloxacillin DILI.( 55 ) Another European and U.S. GWAS showed evidence of HLA genotypes HLA DRB1*15:01 and DQB1*0602 playing a significant role in amoxicillin‐clavulanate DILI susceptibility.( 56 ) HLA‐B*35:02 has been associated with increased risk of minocycline DILI, with a 16% carrier frequency in DILI cases in comparison to 0.6% in the population control in a GWAS study.( 57 ) The rarity of occurrence of DILI in relation to a given drug confers a negative predictive value greater than 95% to these HLA alleles. HLA genotyping can potentially increase the accuracy and confidence in diagnosing DILI.( 33 )
Drug‐Related Factors
Dose
There are data to suggest that drugs with daily oral dosing of ≥50 mg account for 70%‐80% of DILI cases in multiple DILI registries and population‐based studies.( 9 , 32 , 58 )
Drug Metabolism
Ghabril in 2010 noted oral medications with more than 50% hepatic metabolism had significantly higher frequency of ALT >3‐times ULN, liver failure, and fatal DILI. In the same study, drugs with biliary excretion had significantly higher frequency of jaundice. Medications with more than 50% hepatic metabolism and dose more than 50 mg/day were noted to have an additive effect, leading to higher risk of hepatotoxicity.( 59 )
Lipophilicity
Oral medication with high lipophilicity measured as LogP is known to influence drug pharmacokinetics and toxicity. Drugs with higher lipophilicity have an increased volume of distribution, higher chances of off‐target binding, and increased risk of toxicity with subsequent generation of increased reactive metabolites.( 33 ) A drug’s hepatotoxic potential has been conceptualized as the “rule of two,” characterized by high lipophilicity (LogP > 3) and daily dosing (>100 mg). These parameters have been associated with an increased risk of DILI.( 60 )
Making a Diagnosis
DILI is a challenging and complex diagnosis. Given the overlap between presentation of DILI with both acute and chronic liver diseases, it remains a diagnosis of exclusion. Obtaining a detailed history and recognition of the pattern of liver injury is key. Using the R‐ratio at presentation assists in a careful selection of diagnostic tests to evaluate for competing diagnoses: hepatic or systemic. In addition to a patient’s demographic variables, obtaining a full medication history is vital. Given the potential for prolonged latency periods, it is imperative to corroborate a patient’s medication list from their pharmacy and investigate over‐the‐counter medication/HDS use. This process can provide crucial information to establish a temporal relationship between drug exposure and development of signs/symptoms of liver disease. The spectrum of clinical presentation with DILI is broad, ranging from asymptomatic elevation in liver enzymes to nonspecific symptoms characterized by malaise, abdominal pain, and nausea to jaundice, pruritis, and encephalopathy. Establishing a timeline of onset of symptoms with respect to exposure of a culprit drug can be helpful, as the pattern of liver injury can change over the course of evolution of DILI. Figure 2 shows a stepwise approach to diagnosing DILI.
Fig. 2.
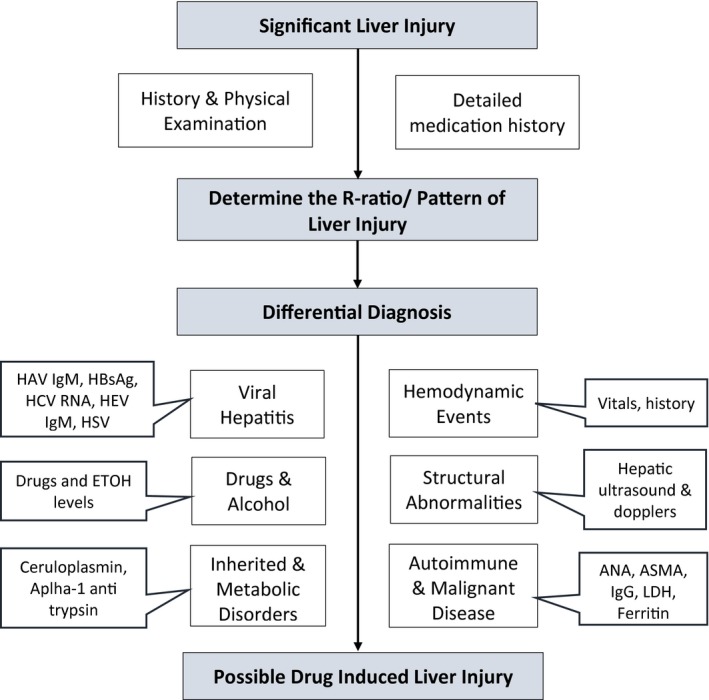
Approach to diagnosis of DILI. Abbreviations: ANA, antinuclear antibody; ASMA, anti–smooth muscle actin; EtOH, ethanol; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HEV, hepatitis E virus; HSV, herpes simplex virus; IgG, immunoglobulin G; IgM, immunoglobulin M; LDH, lactate dehydrogenase.
Causality Assessment
A physician’s awareness of a drug’s hepatotoxic potential and associated phenotypic pattern is useful when considering a diagnosis of DIL. LiverTox (https://www.ncbi.nlm.nih.gov/books/NBK547852/) provides a comprehensive characterization of drugs and HDSs, describing their typical patterns and presentations.
A number of DILI‐specific causality assessment methods have been developed. These include general scales, algorithms, and expert opinions. The assessment provided by an algorithm or scale primarily depends on the weight given to each criterion. The validity of the method can vary as a result of differences in prioritizing the parameters. Table 2 details three liver‐specific causality assessment scales.( 4 , 61 , 62 ) Causality scales are confounded by the absence of a true “gold standard” test for DILI. There is also varied interobserver reliability and reproducibility. Hence, these scales do not substitute a physician’s clinical judgement.
Table 2.
Specific Causality Assessment Scales for DILI
| Scale | Description | Comments |
|---|---|---|
| CIOMS‐RUCAM |
|
|
| Maria & Victorino |
|
|
| DDW‐J |
|
|
Abbreviations: CDS, Clinical Diagnostic Scale; CIOMS, Council for the International Organization of Medical Sciences; DDW‐J, Digestive Disease Week–Japan; M&V, Maria & Victorino.
The DILIN uses a structured expert opinion process for categorization of probability of DILI. They describe the likelihood of DILI based on percent probability of diagnosis of DILI: definite (>95%), highly likely (75%‐95%), probable (50%‐74%), possible (25%‐49%), or unlikely (<25%).( 13 ) An assessment for the first 300 patients enrolled to U.S. DILIN in 2003 determined the RUCAM approach was more conservative in assigning a high level of causality than the DILIN strategy.( 63 ) Although the two methods have been compared, the DILIN method has not been externally validated.
Liver Biopsy
Typically, a liver biopsy is not necessary to establish a diagnosis of DILI; however, it can prove useful in excluding other etiologies for liver injury and to assess the degree of inflammation and necrosis. The lack of resolution of liver injury serves as a strong rationale to obtain a liver biopsy.
Phenotypes
Clinicopathologic Phenotypes
Kleiner described 18 histopathologic patterns on liver biopsies of patients with suspected DILI. Most of the cases could be classified into one of five patterns: acute hepatitis, chronic hepatitis, acute cholestasis, chronic cholestasis, and cholestatic hepatitis.( 64 )
Necroinflammatory
Inflammation, necrosis, and apoptosis are typical findings in hepatocellular DILI. The pattern of necrosis can be zonal versus nonzonal, and acute versus chronic hepatitis–like.( 65 ) Acute hepatitis–like injury affects the hepatic parenchyma predominantly. Severe acute hepatitis is characterized by lobular disarray, from extensive sinusoidal architectural disruption, which correlates with a higher degree of hepatocyte apoptosis and confluent necrosis.( 64 ) The main differential for acute hepatitis–like DILI includes acute viral hepatitis and fulminant presentation of autoimmune hepatitis (AIH). Confluent necrosis with acute hepatitis has been seen in some cases of diclofenac related DILI.( 66 ) Chronic hepatitis–like DILI has a predominant portal infiltrate with interface hepatitis. Pure zonal necrosis, typically zone 3 necrosis, can mimic hypoxic‐ischemic liver injury, typical of APAP hepatotoxicity. This usually signifies cytotoxicity secondary to toxic drug metabolites, as opposed to necrosis with immune cell infiltration suggestive of involvement of the adaptive immune system.( 65 )
Cholestatic
Acute cholestasis from DILI can be categorized as bland cholestasis or cholestatic hepatitis. Bland cholestasis is classically associated with injury caused by anabolic steroids and estrogens.( 67 ) Both forms of acute cholestasis share common features of zone 3 hepatocellular and canalicular cholestasis. Concurrent granulomatous or eosinophilic inflammation in cholestatic hepatitis is suggestive of an immuno‐allergic or hypersensitivity‐type immune reaction.( 68 ) Penicillins, especially with beta‐lactamase inhibitors (amoxicillin/clavulanate), sulfonylureas, methimazole and cephalosporins, have been implicated in causing cholestatic hepatitis.( 69 , 70 , 71 , 72 ) Azathioprine and mercaptopurine can cause liver injury, resembling both forms of acute cholestasis.( 73 ) Vanishing bile duct syndrome (VBDS) is an ominous finding in cholestatic DILI, commonly identified by a paucity of bile ducts in over 50% of portal areas in a biopsy with at least 10 portal areas. A DILIN study confirmed the increased likelihood of development of chronic DILI in 94% of patients with VBDS in comparison to 47% in those without.( 74 ) Amoxicillin/clavulanate, azithromycin, and fluroquinolones were major causes of VBDS in this study cohort.
Steatosis and Steatohepatitis
Historically, the risk of drug‐associated fatty liver disease related to tamoxifen or methotrexate use increased with the presence of obesity or diabetes.( 33 ) Steatohepatitis has been associated with amiodarone, methotrexate, and tamoxifen. Microvesicular steatosis may indicate mitochondrial injury, which has a higher risk of severe liver injury, seen in DILI secondary to aspirin, valproate, amiodarone, and fialuridine.( 75 , 76 , 77 , 78 , 79 , 80 )
Vascular Injury
DILI from a vascular injury can present acutely with abdominal pain, hepatomegaly, abnormal liver enzymes, and acute onset portal hypertension. This usually occurs in the setting of Budd‐Chiari syndrome and veno‐occlusive disease/sinusoidal obstruction syndrome (VOD/SOS). VOD/SOS affects small veins and sinusoids, and typically presents as zone 3 necrosis. VOD/SOS is most commonly observed in the context of stem cell transplantation, exposure to toxins, pyrrolizidine alkaloids, onco‐therapeutic agents, and purine analogues. Thrombosis of the major hepatic veins in Budd‐Chiari can lead to massive congestion and ischemic necrosis. This is now an uncommon scenario, as it was associated with early formulations of contraceptive steroids.( 65 , 81 , 82 , 83 ) Nodular regenerative hyperplasia, typically identified with a reticulin stain, has been seen in cases of exposure to azathioprine (as a result of active metabolite 6‐thioguanine), oxaliplatin, and chlorambucil, in addition to various non–drug‐related disease states.( 33 , 65 , 81 )
Immune Phenotypes
Drug‐Induced AIH
In multiple case cohorts, 2%‐9% of cases of AIH were considered to be induced by drugs, and drug‐induced AIH is responsible for 9% of all cases of DILI.( 33 , 84 , 85 ) Differentiation between drug‐induced AIH and idiopathic AIH can be difficult for both the gastroenterologist and the pathologist, as patients often present with serologic and histologic markers of idiopathic AIH. As discussed previously, identification of DILI risk alleles can aide in the diagnosis of drug‐related AIH, such as HLA DRB1*15:01, which is positive in 57%‐67% of DILI cases from amoxicillin/clavulanate, in comparison with 15%‐20% cases in the general population. Other medications associated with drug‐induced AIH include minocycline, nitrofurantoin, hydralazine, infliximab, and methyldopa.( 33 , 81 )
Immunotherapy‐Related Liver Injury
Immune checkpoint inhibitors (ICIs) increase T‐cell responses and restore antitumor immune responses, which have been suppressed by cancer with the goal of inducing tumor rejection. The various targets for ICIs include cytotoxic T‐lymphocyte antigen 4 (CTLA‐4, the target for ipilimumab), programmed cell death 1 (PD‐1, the target for pembrolizumab and nivolumab), and programmed cell death ligand 1 (the target for avelumab, durvalumab, and atezoliumab).( 33 ) However, the therapeutic reversal of immune tolerance following administration of these agents comes at the expense of immune‐related adverse events (irAEs), including hepatotoxicity.( 86 ) A recent meta‐analysis showed a higher rate of all‐grade and high‐grade hepatotoxicity with CTLA‐4 inhibitors in comparison to PD‐1 inhibitors.( 87 ) In other clinical trials with ipilimumab treatment, 11% of patients had early discontinuation of treatment due to hepatotoxicity, and this rose to 30% early discontinuation in combination therapy with ipilimumab and nivolumab.( 88 , 89 ) The mechanisms of the specific irAEs, which include rash, diarrhea, colitis, hepatitis and endocrinopathies, are not fully understood. It is postulated that hepatitis occurs from immune T‐cell activation, leading to secretion of CD4 T‐helper cell cytokines and cytolytic CD8 T‐cell tissue infiltration.
Hepatotoxicity from ICIs varies from an asymptomatic elevation of aminotransferases to acute hepatitis and fulminant liver failure. The pattern of liver injury can be nonspecific as well, ranging from hepatocellular to cholestatic. ICI‐related hepatitis is typically a seronegative hepatitis in comparison to cases of idiopathic AIH.( 33 ) The 2018 American Society of Clinical Oncology Clinical Practice Guideline details the grading system for ICI‐related hepatotoxicity and its management.( 90 ) Table 3 details the grades of toxicity from ICIs and proposed management.
Table 3.
ICI Hepatotoxicity Grading and Management
| Parameter | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| ALT, AST (ULN) | 1‐3 | 4‐5 | 6‐20 | >20 |
| Bilirubin (ULN) | 1‐1.5 | 1.6‐3 | 4‐10 | >10 |
| Liver disease/decompensation | X | X | ||
| ICI treatment | Continue | Hold | Stop | Stop |
| Lab monitoring | Twice weekly | Twice weekly | Daily | Daily |
| DILI treatment | Prednisone | IV Solumedrol | IV Solumedrol |
Abbreviation: IV, intravenous.
Treatment
The most important step in management of suspected DILI is the discontinuation of the implicated agent and avoiding re‐exposure. In most cases (up to 90% or more), spontaneous recovery occurs as a result, without need for further treatment measures. Such improvement with discontinuation of a suspected offending agent is termed “dechallenge” and serves as good evidence of a causal association with injury.( 4 , 33 , 91 )
Short‐term administration of a bile acid resin can be used in DILI by enhancing drug clearance by interrupting enterohepatic circulation of leflunomide and terbinafine.( 33 , 92 ) Carnitine is an antidote to valproate hepatotoxicity. It regulates mitochondrial acetyl‐CoA levels, leading to enhanced fatty acid uptake and beta oxidation in the mitochondria.( 33 , 93 ) The role of N‐acetylcysteine (NAC) in APAP‐related liver injury has been well established. NAC should be considered in patients with ALF from IDILI. The U.S. ALF study group randomized 45 patients with ALF from non‐APAP DILI to receive NAC versus placebo infusion. The NAC group had 2‐times higher transplant‐free survival rates.( 94 ) The use of corticosteroids should be limited to DILI in the setting of drug‐induced AIH, immune therapy–related severe hepatitis, or in the presence of features of hypersensitivity. Corticosteroid treatment in one retrospective analysis was associated with lower survival in patients with more severe liver injury.( 95 ) When corticosteroids are used, close monitoring is essential, as a failure of response may either suggest no benefit or an alternative diagnosis, and they should be stopped. With improvement of liver injury, withdrawal of steroids should be performed gradually with close follow‐up to detect resurgence of liver injury or uncover an AIH that warrants ongoing therapy.
Ursodiol can be used as pretreatment prophylaxis for patients undergoing myeloablative hematopoietic stem cell transplantation with good tolerability. Defibrotide is used as both a treatment for severe SOS and pretreatment prophylaxis in patients at high risk of developing SOS.( 96 ) Liver transplantation remains the primary rescue treatment for ALF from DILI. Timely identification of ALF should prompt a referral to a liver transplant center. Table 4 details the targeted therapies for DILI.
Table 4.
Specific Treatments for DILI
| Agent | Treatment |
|---|---|
| APAP | NAC |
| Valproic acid | Carnitine |
| Leflunomide | Bile salt resin |
| Amanita (mushroom) | Silymarin |
| ICIs | Steroids |
| Sinusoidal obstruction syndrome | Ursodiol, defibrotide |
HDS‐Induced Liver injury
More than half of U.S. households use some form of dietary supplement, most commonly multivitamins and minerals.( 97 , 98 , 99 , 100 ) The incidence of hepatotoxicity from HDSs is steadily increasing in the United States, up to 20% in 2013‐2014.( 15 , 16 ) The current regulatory framework established by the Dietary Supplement Health and Education Act of 1994 is suboptimal. The requirements for HDS products before being marketed are significantly less stringent than for pharmaceutical products; specifically, there is no requirement for assessments of product efficacy and safety. The FDA and other regulatory bodies take action only after a supplement has been shown to have potential toxicity.( 101 )
Among HDSs, bodybuilding products are the most common cause of liver injury.( 16 ) The typical clinical scenario is that of prolonged jaundice in young men, with nonfatal outcomes from use of anabolic androgenic steroids.( 102 , 103 ) Non‐bodybuilding HDS‐related liver injury is commonly seen in women with a hepatocellular pattern of injury. This group of patients with HDS‐related liver injury tend to have worse outcomes, with transplantation rates up to 11%.( 16 ) Hillman found that in comparison to prescription medications, HDSs tend to have a higher transplantation rate and lower rate of ALF‐specific transplant‐free survival.( 104 ) Non‐bodybuilding HDSs can be either single‐ingredient or multi‐ingredient products. One of the most common single‐ingredient HDSs is green tea extract (GTE). Results from a randomized Minnesota study showed that the health of postmenopausal women without chronic liver disease were 7 times more likely to have ALT elevations with GTE use. GTE dechallenge leads to a downtrend in ALT levels, and ALT elevation recurred following GTE rechallenge,( 105 ) indicating a causal relationship between GTE and hepatotoxicity.
Multi‐ingredient products also pose a risk for HDS‐related liver injury.( 16 , 106 ) Given their unclear chemical descriptors, identification of the culprit agent is difficult. In addition, there can be contamination of HDSs with microbials, heavy metals, and mycotoxins. Large batch‐to‐batch variation in HDS product content is common.( 107 , 108 , 109 , 110 ) The U.S. DILIN using toxicologic analysis confirmed the suspicion of supplement mislabeling in multi‐ingredient products, with rates up to 50%‐80%.( 111 ) The potential for more severe liver injury with these products remains largely unknown. However, the risk for toxicity may result from the complex interactions among individual ingredients, supplement overuse/misuse, or combination use with prescription medications.
Summary
DILI remains the leading cause for ALF in the U.S. adult population. It is a diagnosis of exclusion and requires a meticulous process of evaluation and a high index of clinical suspicion to attribute a potential agent as the cause of the liver injury. The accurate identification of clinical and biochemical patterns of liver injury at the onset enables prognostication for individual cases. HDSs are also a common cause for DILI, although the exact ingredient responsible for injury is difficult to confirm. The DILINs have played a vital role in expanding our understanding of liver injury from both drugs and HDSs. With continued advancements and expansion of drug injury registries, our hope is that the understanding of DILI epidemiology, mechanism of injury, and establishment of causality will continue to improve.
Financial support: National Institutes of Health and National Institute of Diabetes and Digestive and Kidney Diseases (U01DK083027).
Potential conflict of interest: Nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002;137:947‐954. [DOI] [PubMed] [Google Scholar]
- 2. Kaplowitiz N. Drug‐induced liver disorders: implications for drug development and regulation. Drug Saf 2001;24:483‐490. [DOI] [PubMed] [Google Scholar]
- 3. Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug‐induced liver injury. Clin Pharmacol Ther 2011;89:806‐815. [DOI] [PubMed] [Google Scholar]
- 4. Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci 2016;17:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bunchorntavakul C, Reddy KR. Acetaminophen‐related hepatotoxicity. Clin Liver Dis 2013;17:587‐607. [DOI] [PubMed] [Google Scholar]
- 6. Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, et al. Incidence of drug‐induced hepatic injuries: a French population‐based study. Hepatology 2002;36:451‐455. [DOI] [PubMed] [Google Scholar]
- 7. De valle MB, Av klinteberg V, Alem N, Olsson R, Björnsson E. Drug‐induced liver injury in a Swedish University hospital out‐patient hepatology clinic. Aliment Pharmacol Ther 2006;24:1187‐1195. [DOI] [PubMed] [Google Scholar]
- 8. de Abajo FJ, Montero D, Madurga M, Rodriguez LAG. Acute and clinically relevant drug‐induced liver injury: a population based case‐control study. Br J Clin Pharmacol 2004;58:71‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bjornsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug‐induced liver injury in the general population of Iceland. Gastroenterology 2013;144:1419‐1425.e3. [DOI] [PubMed] [Google Scholar]
- 10. Wai CT, Tan BH, Chan CL, Sutedja DS, Lee YM, Khor C, et al. Drug induced liver injury at an Asian center: a prospective study. Liver Int 2007;27:465‐474. [DOI] [PubMed] [Google Scholar]
- 11. Shen T, Liu Y, Shang J, Xie Q, Li J, Yan M, et al. Incidence and etiology of drug‐induced liver injury in mainland China. Gastroenterology 2019;156:2230‐2241. [DOI] [PubMed] [Google Scholar]
- 12. Vega M, Verma M, Beswick D, Bey S, Hossack J, Merriman N, et al. The incidence of drug‐and herbal and dietary supplement‐induced liver injury: preliminary findings from gastroenterologist‐based surveillance in the population of the state of Delaware. Drug Saf 2017;40:783‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, Serrano J, et al.; DILIN Study Group . Drug‐induced liver injury network (DILIN) prospective study: rational, design and conduct. Drug Saf 2009;32:55‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, et al.; United States Drug Induced Liver Injury Network . Features and outcomes of 899 patients with drug‐induced liver injury: the DILIN prospective study. Gastroenterology 2015;147:1340‐1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Navarro VJ, Khan I, Björnsson E, Seeff LB, Serrano J, Hoofnagle JH. Liver injury from herbal and dietary supplements. Hepatology 2017;65:363‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Navarro VJ, Barnhart H, Bonkovsky HL, Davern T, Fontana RJ, Grant L, et al. Liver injury from herbals and dietary supplements in the U.S. drug induced liver injury network. Hepatology 2014;60:1399‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Danan G, Benichou C. Causality assessment of adverse reactions to drugs. I: A novel method based on the conclusions of international consensus meeting. J Clin Epidemiol 1993;46:1323‐1330. [DOI] [PubMed] [Google Scholar]
- 18. Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs. II: An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol 1993;46:1331‐1336. [DOI] [PubMed] [Google Scholar]
- 19. Saithanyamurthi H, Faust AJ. Drug‐induced liver disease: clinical course. Clin Liver Dis 2017;21:21‐34. [DOI] [PubMed] [Google Scholar]
- 20. Bessone F, Hernandez N, Lucena M, Andrade R. The Latin American DILI registry experience: a successful ongoing collaborative strategic initiative. Int J Mol Sci 2016;17:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bjornsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug‐induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005;40:1095‐1101. [DOI] [PubMed] [Google Scholar]
- 22. Zimmerman HJ. Drug‐induced liver disease In: Zimmerman HJ, ed. Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver, 2nd Edition Philadelphia, PA: Lippincott Williams & Wilkins;1999:427‐456. [Google Scholar]
- 23. Zimmerman HJ. The spectrum of hepatotoxicity. Perspect Biol Med 1968;12:135‐161. [DOI] [PubMed] [Google Scholar]
- 24. Black M, Mitchell JR, Zimmerman HJ, Ishak KG, Epler GR. Isoniazid‐associated hepatitis in 114 patients. Gastroenterology 1975;69:289‐302. [PubMed] [Google Scholar]
- 25. Robles‐Diaz M, Lucena MI, Kaplowitz N, Stephens C, Medina‐Cáliz I, González‐Jimenez A, et al. Use of Hy’s law and a new composite algorithm to predict acute liver failure in patients with drug‐induced liver injury. Gastroenterology 2014;147:109‐118. [DOI] [PubMed] [Google Scholar]
- 26. Bjornsson ES, Björnsson HK. Mortality associated with drug‐induced liver injury (DILI). Transl Gastroenterol Hepatol 2017;2:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teschke R. Idiosyncratic DILI: analysis of 46,266 cases assessed for causality by RUCAM and published from 2014 to early 2019. Front Pharmacol 2019;23:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andrade RJ, Lucena MI, Kaplowitz N, García‐Muņoz B, Borraz Y, Pachkoria K, et al. Outcome of acute idiosyncratic drug‐induced liver injury: long‐term follow up in a hepatotoxicity registry. Hepatology 2006;44:1581‐1588. [DOI] [PubMed] [Google Scholar]
- 29. Fontana RJ, Hayashi PH, Gu J, Reddy KR, Barnhart H, Watkins PB, et al. Idiosyncratic drug‐induced liver injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterology 2014;147:96‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Habib S, Shaikh OS. Liver transplantation for drug‐induced acute liver failure. Eur Rev med Pharmacol Sci 2017;21(Suppl. 1):37‐45. [PubMed] [Google Scholar]
- 31. Uetrecht J. Idiosyncratic drug reactions: current understanding. Annu Rev Pharmacol Toxicol 2007;47:513‐539. [DOI] [PubMed] [Google Scholar]
- 32. Lammert C, Einarsson S, Saha C, Niklasson A, Bjornsson E, Chalasani N. Relationship between daily dose of oral medications and idiosyncratic drug‐induced liver injury: search for signals. Hepatology 2008;47:2003‐2009. [DOI] [PubMed] [Google Scholar]
- 33. European Association for the Study of the Liver . EASL clinical practice guidelines: drug‐induced liver injury. J Hepatol 2019;70:1222‐1261. [DOI] [PubMed] [Google Scholar]
- 34. David S, Hamilton JP. Drug‐induced liver injury. US Gastroenterol Hepatol Rev 2010;1:73‐80. [PMC free article] [PubMed] [Google Scholar]
- 35. Uetrecht J. Idiosyncratic drug reactions: past, present, and future. Chem Res Toxicol 2008;21:84‐92. [DOI] [PubMed] [Google Scholar]
- 36. Njoku DB, Greenberg RS, Bourdi M, Borkowf CB, Dake EM, Martin JL, et al. Autoantibodies associated with volatile anesthetic hepatitis found in the sear of a large cohort of pediatric anesthesiologists. Anesth Analg 2002;94:243‐249. [DOI] [PubMed] [Google Scholar]
- 37. Kullak‐Ublick GA, Andrade RJ, Merz M, End P, Benesic A, Gerbes AL, et al. Drug‐induced liver injury: recent advances in diagnosis and risk assessment. Gut 2017;66:1154‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev 2000;174:21‐34. [DOI] [PubMed] [Google Scholar]
- 39. Cantor HM, Dumont AE. Hepatic suppression of sensitization to antigen absorbed into the portal system. Nature 1967;215:744‐745. [DOI] [PubMed] [Google Scholar]
- 40. Dara L, Liu Z‐X, Kaplowitz N. Mechanisms of adaptation and progression in idiosyncratic drug induced liver injury, clinical implications. Liver Int 2016;36:158‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vuppalanchi R, Chalasani N. Risk factors for drug‐induced liver disease In: Kaplowitz N, DeLeve OLD, eds. Drug‐Induced Liver Disease, 3rd Edition Waltham, MA: Academic Press; 2013:265‐274. [Google Scholar]
- 42. Guy J, Peters MG. Liver disease in women: the influence of gender on epidemiology, natural history, and patient outcomes. Gastroenterol Hepatol 2013;9:633‐639. [PMC free article] [PubMed] [Google Scholar]
- 43. deLemos A, Foureau D, Jacobs C, Ahrens W, Russo M, Bonkovsky H. Drug‐induced liver injury with autoimmune features. Semin Liver Dis 2014;34:194‐204. [DOI] [PubMed] [Google Scholar]
- 44. Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group . Drug‐induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010;52:2065‐2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, et al. Causes, clinical features, and outcomes from a prospective study of drug‐induced liver injury in the United States. Gastroenterology 2008;135:1924‐1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Andrade RJ, Lucena M, Fernandez M, Pelaez G, Pachkoria K, Garciaruiz E, et al.; Spanish Group for the Study of Drug‐Induced Liver Disease . Drug‐induced liver injury: an analysis of 461 incidences submitted to the Spanish Registry over a 10‐year period. Gastroenterology 2005;129:512‐521. [DOI] [PubMed] [Google Scholar]
- 47. Chalasani N, Reddy RKK, Fontana RJ, Barnhart H, Gu J, Hayashi PH, et al. Idiosyncratic drug induced liver injury in African‐Americans is associated with greater morbidity and mortality compared to Caucasians. Am J Gastroenterol 2017;112:1382‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lu NA, Rai SK, Terkeltaub R, Kim SC, Menendez ME, Choi HK. Racial disparities in the risk of Stevens‐Johnson Syndrome and toxic epidermal necrolysis as urate‐lowering drug adverse events in the United States. Semin Arthritis Rheu 2016;46:253‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kunelis CT, Peters JL, Edmondson HA. Fatty liver of pregnancy and its relationship to tetracycline therapy. Am J Med 1965;38:359‐377. [DOI] [PubMed] [Google Scholar]
- 50. Zimmerman HJ. Effects of alcohol on other hepatotoxins. Alcohol Clin Exp Res 1986;10:3‐15. [DOI] [PubMed] [Google Scholar]
- 51. Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, et al. Safety and efficacy of long‐term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post‐hoc analysis. Lancet 2010;376:1916‐1922. [DOI] [PubMed] [Google Scholar]
- 52. Han KH, Rha SW, Kang HJ, Bae JW, Choi BJ, Choi SY, et al. Evaluation of short‐term safety and efficacy of HMG‐CoA reductase inhibitors in hypercholesterolemic patients with elevated serum alanine transaminase concentrations: PITCH study (PITavastatin versus atorvastatin to evaluate the effect on patients with hypercholesterolemia and mild to moderate hepatic damage). J Clin Lipidol 2012;6:340‐351. [DOI] [PubMed] [Google Scholar]
- 53. Gomez‐Dominguez E, Gisbert JP, Moreno‐Monteagudo JA, García‐Buey L, Moreno‐Otero R. A pilot study of atorvastatin treatment in dyslipidemia, non‐alcoholic fatty liver patients. Aliment Pharmacol Ther 2006;23:1643‐1647. [DOI] [PubMed] [Google Scholar]
- 54. Antonopoulos S, Mikros S, Mylonopoulou M, Kokkoris S, Giannoulis G. Rosuvastatin as a novel treatment of non‐alcoholic fatty liver disease in hyperlipidemic patients. Atherosclerosis 2006;184:233‐234. [DOI] [PubMed] [Google Scholar]
- 55. Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe'er I, Floratos A, et al. HLA‐B 5701 genotype is a major determinant of drug‐induced liver injury due to flucloxacillin. Nat Genet 2009;41:816‐819. [DOI] [PubMed] [Google Scholar]
- 56. Lucena MI, Molokhia M, Shen Y, Urban TJ, Aithal GP, Andrade RJ, et al. Susceptibility to amoxicillin‐clavulanate induced liver injury is influenced by multiple HLA class I and II alleles. YGAST 2011;141:338‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Urban TJ, Nicoletti P, Chalasani N, Serrano J, Stolz A, Daly AK, et al. Minocycline hepatotoxicity: clinical characterization and identification of HLA‐B*35:02 as a risk factor. J Hepatol 2017;67:137‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lucena MI, Andrade RJ, Kaplowitz N, García‐Cortes M, Fernández MC, Romero‐Gomez M, et al. Phenotypic characterization of idiosyncratic drug‐induced liver injury: the influence of age and sex. Hepatology 2009;49:2001‐2009. [DOI] [PubMed] [Google Scholar]
- 59. Ghabril M, Chalasani N, Björnsson E. Drug‐induced liver injury: a clinical update. Curr Opin Gastroenterol 2010;26:222‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen M, Borlak J, Tong W. High lipophilicity and high daily dose of oral medications are associated with significant risk for drug‐induced liver injury. Hepatology 2013;58:388‐396. [DOI] [PubMed] [Google Scholar]
- 61. Maria VA, Victorino RM. Development and validation of a clinical scale for the diagnosis of drug‐induced hepatitis. Hepatology 1997;26:664‐669. [DOI] [PubMed] [Google Scholar]
- 62. Takikawa H, Takamori Y, Kumagi T, Onji M, Watanabe M, Shibuya A, et al. Assessment of 287 Japanese cases of drug induced liver injury by the diagnostic scale of the International Consensus Meeting. Hepatol Res 2003;27:192‐195. [DOI] [PubMed] [Google Scholar]
- 63. Rockey DC, Seeff LB, Rochon J, Freston J, Chalasani N, Bonacini M, et al. Causality assessment in drug‐induced liver injury using a structured expert opinion process: comparison to the Roussel‐Uclaf causality assessment method. Hepatology 2010;51:2117‐2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kleiner DE, Chalasani NP, Lee WM, Fontana RJ, Bonkovsky HL, Watkins PB, et al.;Drug‐Induced Liver Injury Network . Hepatic histological findings in suspected drug‐induced liver injury: systematic evaluation and clinical associations. Hepatology 2014;59:661‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kleiner DE. Histopathological challenges in suspected drug‐induced liver injury. Liver Int 2018;38:198‐209. [DOI] [PubMed] [Google Scholar]
- 66. Schmeltzer PA, Kosinski AS, Kleiner DE, Hoofnagle JH, Stolz A, Fontana RJ, et al. Liver injury from non‐steroidal anti‐inflammatory drugs in the United States. Liver Int 2016;36:603‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bond P, Llewellyn W, Van Mol P. Anabolic androgenic steroid‐induced hepatotoxicity. Med Hypotheses 2016;93:150‐153. [DOI] [PubMed] [Google Scholar]
- 68. Björnsson E, Kalaitzakis E, Olsson R. The impact of eosinophilia and hepatic necrosis on prognosis in patients with drug‐induced liver injury. Aliment Pharmacol Ther 2007;25:1411‐1421. [DOI] [PubMed] [Google Scholar]
- 69. Hautekeete ML. Hepatotoxicity of antibiotics. Acta Gastroenterol Belg 1995;58:290‐296. [PubMed] [Google Scholar]
- 70. Chounta A, Zouridakis S, Ellinas C, Tsiodras S, Zoumpouli C, Kopanakis S, et al. Cholestatic liver injury after glimepride therapy. J Hepatol 2005;42:944‐946. [DOI] [PubMed] [Google Scholar]
- 71. Woeber KA. Methimazole‐induced hepatotoxicity. Endocr Pract 2002;8:222‐224. [DOI] [PubMed] [Google Scholar]
- 72. Skoog SM, Smyrk TC, Talwalkar JA. Cephalexin‐induced cholestatic hepatitis. J Clin Gastroenterol 2004;38:8333. [DOI] [PubMed] [Google Scholar]
- 73. Björnsson ES, Gu J, Kleiner DE, Chalasani N, Hayashi PH, Hoofnagle JH. Azathioprine and 6‐mercaptopurine induced liver injury: clinical features and outcomes. J Clin Gastroenterol 2017;51:63‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bonkovsky HL, Kleiner DE, Gu J, Odin JA, Russo MW, Navarro VM, et al. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology 2017;65:1267‐1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Begriche K, Massart J, Robin M‐A, Borgne‐Sanchez A, Fromenty B. Drug‐induced toxicity on mitochondria and lipid metabolism: mechanistic diversity and deleterious consequences for the liver. J Hepatol 2011;54:773‐794. [DOI] [PubMed] [Google Scholar]
- 76. Trost LC, Lemasters JJ. The mitochondrial permeability transition: a new pathophysiological mechanism for Reye’s syndrome and toxic liver injury. J Pharmacol Exp Ther 1996;278:1000‐1005. [PubMed] [Google Scholar]
- 77. Silva MF, Ruiter JP, IJlst L, Jakobs C, Duran M, de Almeida IT, et al. Differential effect of valproate and its Delta2‐ and Delta4‐unsaturated metabolites, on the beta‐ oxidation rate of long‐chain and medium‐chain fatty acids. Chem Biol Interact 2001;137:203‐212. [DOI] [PubMed] [Google Scholar]
- 78. Card JW, Lalonde BR, Rafeiro E, Tam AS, Racz WJ, Brien JF, et al. Amiodarone‐induced disruption of hamster lung and liver mitochondrial function: lack of association with thiobarbituric acid‐reactive substance production. Toxicol Lett 1998;98:41‐50. [DOI] [PubMed] [Google Scholar]
- 79. Fromenty B, Fisch C, Berson A, Letteron P, Larrey D, Pessayre D. Dual effect of amiodarone on mitochondrial respiration. Initial protonophoric uncoupling effect followed by inhibition of the respiratory chain at the levels of complex I and complex II. J Pharmacol Exp Ther 1990;255:1377‐1384. [PubMed] [Google Scholar]
- 80. Lewis W, Levine ES, Griniuviene B, Tankersley KO, Colacino JM, Sommadossi JP, et al. Fialuridine and its metabolites inhibit DNA polymerase gamma at sites of multiple adjacent analog incorporation, decrease mtDNA abundance, and cause mitochondrial structural defects in cultured hepatoblasts. Proc Natl Acad Sci U S A 1996;93:3592‐3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kleiner DE. Drug‐induced liver injury: the hepatic pathologist’s approach. Gastroenterol Clin North Am 2017;46:273‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lewis JH, Tice HL, Zimmerman HJ. Chiari syndrome associated with oral contraceptive steroids. Review of treatment of 47 cases. Dig Dis Sci 1983;28:673‐683. [DOI] [PubMed] [Google Scholar]
- 83. Ulrickson M, Aldridge J, Kim HT, Hochberg EP, Hammerman P, Dube C, et al. Busulfan and cyclophosphamide (Bu/Cy) as a preparative regimen for autologous stem cell transplantation in patients with non‐Hodgkin : a single‐ institution experience. Biol Blood Marrow Transplant 2009;15:1447‐1454. [DOI] [PubMed] [Google Scholar]
- 84. Björnsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, et al. Drug‐induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology 2010;51:2040‐2048. [DOI] [PubMed] [Google Scholar]
- 85. Licata A, Maida M, Cabibi D, Butera G, Macaluso FS, Alessi N, et al. Clinical features and outcomes of patients with drug‐induced autoimmune hepatitis: a retrospective cohort study. Dig Liver Dis 2014;46:1116‐1120. [DOI] [PubMed] [Google Scholar]
- 86. Lleo A, Rimassa L, Colombo M. Hepatotoxicity of immune check point inhibitors: approach and management. Dig Liver Dis 2019;51:1074‐1078. [DOI] [PubMed] [Google Scholar]
- 87. Wang W, Lie P, Guo M, He J. Risk of hepatotoxicity in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta‐analysis of published data. Int J Cancer 2017;1:1018‐1028. [DOI] [PubMed] [Google Scholar]
- 88. Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety profiles of anti‐CTLA‐4 and anti‐PD‐1 antibodies alone and in combination. Nat Rev Clin Oncol 2016;13:473‐486. [DOI] [PubMed] [Google Scholar]
- 89. Larkin J, Chiarion‐Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. New Engl J Med 2015;373:23‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;10:1714‐1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yang H, Guo D, Xu Y, Zhu M, Yao C, Chen C, et al. Comparison of different liver test thresholds for drug‐induced liver injury: updated RUCAM versus other methods. Front Pharmacol 2019;19:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mallat A, Zafrani ES, Metreau JM, Dhumeaux D. Terbinafine‐induced prolonged cholestasis with reduction of interlobular bile ducts. Dig Dis Sci 1997;42:1486‐1488. [DOI] [PubMed] [Google Scholar]
- 93. Lheureux PER, Hantson P. Carnitine in the treatment of valproic acid‐induced toxicity. Clin Toxicol 2009;47:101‐111. [DOI] [PubMed] [Google Scholar]
- 94. Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, et al. Intravenous N‐acetylcysteine improves transplant‐free survival in early stage nonacetaminophen acute liver failure. Gastroenterology 2009;137:856‐864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Karkhanis J, Verna EC, Chang MS, Stravitz RT, Schilsky M, Lee WM, et al. Steroid use in acute liver failure. Hepatology 2014;59:612‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mohty M, Malard F, Abecasis M, Aerts E, Alaskar AS, Aljurf M, et al. Prophylactic, preemptive, and curative treatment for sinusoidal obstruction syndrome/veno‐occlusive disease in adult patients: a position statement from an international expert group. Bone Marrow Transplant 2020;55:485‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, et al. Dietary supplement use in the United States, 2003‐2006. J Nutr 2011;141:261‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Picciano MF, Dwyer JT, Radimer KL, Wilson DH, Fisher KD, Thomas PR, et al. Dietary supplement use among infants, children, and adolescents in the United States, 1999‐2002. Arch Pediatr Adolesc Med 2007;161:978‐985. [DOI] [PubMed] [Google Scholar]
- 99. Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999‐2012. JAMA 2016;316:1464‐1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999‐2000. Am J Epidemiol 2004;160:339‐349. [DOI] [PubMed] [Google Scholar]
- 101. Food and Drug Administration . Dietary supplements. Updated August 16, 2019. http://www.fda.gov/Food/DietarySupplements/default.htm. Accessed January 2, 2020. [Google Scholar]
- 102. Krishnan PV, Feng ZZ, Gordon SC. Prolonged intrahepatic cholestasis and renal failure secondary to anabolic androgenic steroid‐enriched dietary supplements. J Clin Gastroenterol 2009;43:672‐675. [DOI] [PubMed] [Google Scholar]
- 103. Kafrouni MI, Anders RA, Verma S. Hepatotoxicity associated with dietary supplements containing anabolic steroids. Clin Gastroenterol Hepatol 2007;5:809‐812. [DOI] [PubMed] [Google Scholar]
- 104. Hillman L, Gottfried M, Whitsett M, Rakela J, Schilsky M, Lee WM, et al. Clinical features and outcomes of complementary and alternative medicine induced acute liver failure and injury. Am J Gastroenterol 2016;111:958‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yu Z, Samavat H, Dostal AM, Wang R, Torkelson CJ, Yang CS, et al. Effect of green tea supplements on liver enzyme elevation: results from a randomized intervention study in the United States. Cancer Prev Res 2017;10:571‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Medina‐Caliz I, Garcia‐Cortes M, Gonzalez‐Jimenez A, Cabello MR, Robles‐Diaz M, Sanabria‐Cabrera J, et al. Herbal and dietary supplement‐induced liver injuries in the Spanish DILI registry. Clin Gastroenterol Hepatol 2018;16:1495‐1502. [DOI] [PubMed] [Google Scholar]
- 107. Liu J, Pei M, Zheng C, Li Y, Wang Y, Lu A, et al. A systems‐pharmacology analysis of herbal medicines used in health improvement treatment: predicting potential new drugs and targets. Evid Based Complement Alternat Med 2013;2013:938764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Stickel F, Droz S, Patsenker E, Bögli‐Stuber K, Aebi B, Leib SL. Severe hepatotoxicity following ingestion of Herbalife contaminated with Bacillus subtilis. J Hepatol 2009;50:111‐117. [DOI] [PubMed] [Google Scholar]
- 109. Miller GM, Stripp R. A study of western pharmaceuticals contained within samples of Chinese herbal/patent medicines collected from New York City's Chinatown. Leg Med (Tokyo) 2007;9:258‐264. [DOI] [PubMed] [Google Scholar]
- 110. Gurley BJ, Gardner SF, Hubbard MA. Content versus label claims in ephedra‐containing dietary supplements. Am J Health Syst Pharm 2000;57:963‐969. [DOI] [PubMed] [Google Scholar]
- 111. Navarro V. The frequency of herbal and dietary supplement mislabeling: experience of the drug induced liver injury network. AASLD LiverLearning 2017;24:201449. [Google Scholar]


