Highlights
-
•
An asymptomatic 2019-coronavirus disease patient with normal radiography throughout appeared in this study.
-
•
Lopinavir has a positive effect on 2019-coronavirus disease patients.
-
•
Eosinophil counts presented potentiality as a predictor on the progression of 2019-coronavirus disease.
Keywords: 2019-Coronavirus disease, Lopinavir, Asymptomatic infection, Eosinophil
Abstract
Objectives
To explore the epidemiological information, clinical characteristics, therapeutic outcomes and temporal progression of laboratory findings in 2019-coronavirus disease (COVID-19) patients exposed to lopinavir.
Methods
We collected data from ten COVID-19 patients admitted between January 22, 2020 and February 11, 2020 at Xixi hospital in Hangzhou, China.
Results
Of ten patients, secondary, tertiary and quartus patients emerged; the incubation period was 3–7 days. Mainly initial symptoms were cough and low fever (37.3–38.0 °C). An asymptomatic case presented normal radiography, the others had ground glass opacities. All cases (three transferred, seven discharged) were exposed to lopinavir on initial hospitalization. Three patients stopped lopinavir because of adverse effects, two of them deteriorated, one was hospitalized longer than others who with sustained lopinavir use. Levels of potassium, albumin, and lymphocytes were low, but increased persistently after treatment. Eosinophil values were low on initial hospitalization, then all returned to normal before discharge. Viral load of SARS-CoV-2, radiography and eosinophil improved continuously in 3–14, 6–8 and 7–9 days, respectively.
Conclusions
Increasing eosinophils may be an indicator of COVID-19 improvement. The COVID-19 patients may benefit from sustained lopinavir use. More research on a larger scale is needed to verify these points.
Introduction
At the end of 2019, coronavirus disease (COVID-19) was reported in Wuhan, China. COVID-19 was confirmed to be the pathogenic cause of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The disease is highly contagious, and some cases infected with SARS-CoV-2 were rapidly fatal (The 2019-nCoV Outbreak Joint Field Epidemiology Investigation Team, 2020The 2019-nCoV Outbreak Joint Field Epidemiology Investigation Team, 2020). More than 74,000 cases from 31 provinces in China have been confirmed so far (National Health Commission, 2020). Confirmed cases including those in America, France, Thailand, Japan and twenty other countries have also been reported. On January 27 of 2020, the World Health Organization (WHO) issued a global vigilance and worldwide surveillance of the outbreak from China, and provided the first edition of infection prevention and control (IPC) guidance, although this interim guidance needs updated information (WHO, 2020). And for COVID-19, clinical, epidemiological and laboratory data, especially on temporal progression of abnormal laboratory findings, remains limited. Herein, we report epidemiological information, clinical characteristics, therapeutic outcomes and the temporal progression of laboratory findings in COVID-19 patients exposed to lopinavir (LPV) for use in case management.
Methods
Patients and data collection
Between January 22 and February 11, 2020, 10 patients whose disease was confirmed by two consecutive positive results of SARS-CoV-2 RNA tests were screened for eligibility. These patients were admitted through the outpatient clinic in Xixi hospital (a designated hospital for COVID-19, Hangzhou), or were transferred by ambulance from other hospitals in Hangzhou, and isolated in a negative-pressure ward immediately. Protective equipment for aerosol generation was used by medical workers. This study was approved by the Institutional Review Board of Xixi Hospital.
Epidemiological information was obtained in detail from face-to-face interview of these patients. Medical records and laboratory test results were standardized as a uniform tabulation which was double checked independently by two researchers.
Radiologic assessment
The chest radiograph of each patient was evaluated without consulting clinical data. High-resolution computed tomography (HRCT) scanning of the chest was available and was evaluated in all patients. The presence of opacification was classified as air-space shadowing (ground-glass opacity, focal consolidation or patchy consolidation), reticular or diffuse (all lung involvement). Pleural effusions were also marked.
Detection of SARS-CoV-2
Four respiratory samples (two nasal swabs and two throat swabs) were collected from each patient. A total 300 μL sample from nasal swab tubes (150 μL per tube) was added into short-time cracking reaction fluid (MVR01, Liferiver, Shanghai, China); throat swabs were treated in the same way. Total RNA was extracted according to the manufacturer's instructions and 5 μL RNA was used for real-time RT-PCR, which targeted E gene (first line screen assay), RdRP gene (confirmatory assay) and N gene (additional confirmatory assay) by using Multiple Fluorescence Quantitative PCR reagent. The reaction conditions of real-time RT-PCR started with 45 °C for 10 min and 95 °C for 3 min, followed by 45 cycles of amplification at 95 °C for 15 s and 58 °C for 30 s. The test results of SARS-CoV-2 were reported as positive (Cycle threshold; Ct ≤ 35), weak positive (35 < Ct ≤ 43) and negative (Ct > 43).
Definitions
The definitions of this research were acute myocardial injury (AMI, troponin I > 0.2 μg/L) (Dous et al., 2017), acute kidney injury (AKI, serum creatinine >26.5 μmol/L in 48 h or urine volume <0.5 mL/kg/h for 6 h) (Kellum et al., 2012), respiratory failure (arterial partial oxygen pressure <60 mm Hg) (Hamp et al., 2017), digestive adverse effect (the clinical symptoms of diarrhea, nausea and vomiting), low potassium (serum potassium <3.5 mmol/L) and hypoalbuminemia (serum albumin <35 g/L) (Touma and Bisharat, 2019).
Statistical analysis
Continuous variables were represented with median (interquartile range, IQR). Linear regression model was used to estimate the variable change over time. All analyses were performed using the R software, version 3.3.1 (http://www.R-project.org).
Results
The median age of the 10 patients (6 females) was 42 (IQR, 34–50). Except as noted below, all patients had inconspicuous medical history. Patient 3 had hypertension and cardiovascular disease, and was taking metoprolol and nifedipine. Patient 7 had chronic liver disease, and was taking tenofovir. Nine patients had no smoking history. Patient 7 was a current smoker, 20 cigarettes per day for twenty years.
Contact history
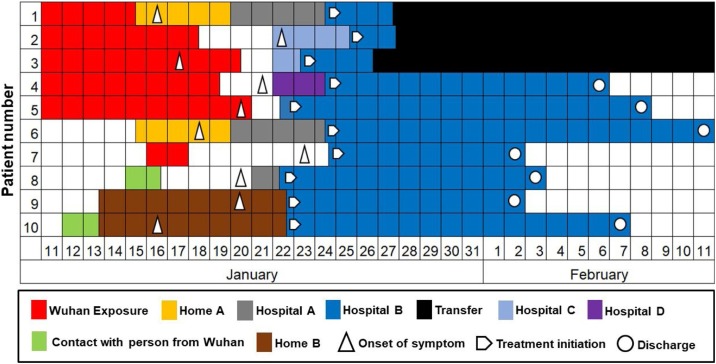
The contact history of 10 patients is shown in Figure 1 . Patient 1, a Hangzhou resident, was a clothing salesperson who went to Wuhan garment market on January 11, 2020, and got back to Hangzhou on January 15. One day later, fever as the earliest symptom occurred. She was admitted to hospital A with pneumonia. Patient 6 was the husband of patient 1 and had close contact with patient 1 for more than two days at home before the onset of symptoms. The couple (patients 1and 6) were transferred to hospital B (Xixi hospital) from hospital A on January 24 by ambulance after the laboratory test of SARS-CoV-2-RNA came back as positive. Patients 2, 3, 4 and 5 were all Wuhan residents. They came to Hangzhou for a family reunion. Patients 3 and 5 had already had the symptom of fever when they were in Wuhan. Patient 7 was a Hangzhou resident who went to Wuhan for a business investigation for two days. He had the symptom of chest congestion on 8th day after Wuhan exposure. Patient 8, a woman living in Hangzhou, had a meeting with her colleagues from Wuhan on January 15. She had the symptom of fever on January 20. Patients 9 and 10 were a couple. Patient 10 went to Beijing for a conference for two days and had close contact with her colleagues who came from Wuhan, then returned to Hangzhou on January 13. Patient 9 lived with patient 10 at home for more than six days before the onset of symptoms.
Figure 1.
Contact history and timeline of the onset of symptoms in COVID-19 patients.
As shown in Figure 1, all patients had a direct or indirect history of Wuhan exposure. The maximum incubation period, which was estimated as the number of days from likely exposure to the onset of symptoms, could be accurately assessed for patients 1, 6, 7, 8, 9 and 10 (five, three, seven, five, six and four days, respectively). The contact history of patients 2, 3, 4 and 5 was indistinct, and was not estimated precisely.
Clinical and other characteristics
Most patients presented cough and fever (mainly low fever, temperature ranges from 37.3 to 38.0) (Table 1 ). Four patients had the symptom of productive cough (white phlegm) and sore throat. Three patients reported headache and nausea. One patient only showed chest congestion (patient 7). None of the patients presented with rhinorrhea, rigor, diarrhea and dyspnea. Leukopenia (less than 3.5 × 109 leucocytes per liter) and lymphopenia (less than 1.1 × 109 lymphocytes per liter) were observed in two patients, respectively.
Table 1.
Characteristics of COVID-19 patients at presentation.
| Patient no. |
Summary | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Sex | F | F | M | F | F | M | M | F | M | F | − |
| Age (year) | 45 | 30 | 62 | 53 | 51 | 47 | 40 | 33 | 34 | 35 | 42 (34–50) |
| Current smoking | − | − | − | − | − | − | + | − | − | − | 1+ |
| Symptoms | |||||||||||
| Cough | + | + | + | + | + | + | − | + | + | − | 8+ |
| White phlegm | + | + | + | − | − | + | − | − | − | − | 4+ |
| Headache | + | + | − | + | − | − | − | − | − | − | 3+ |
| Nausea | + | + | − | − | − | − | − | + | − | − | 3+ |
| Sore throat | − | − | − | + | + | − | − | − | + | + | 4+ |
| Rhinorrhea | − | − | − | − | − | − | − | − | − | − | − |
| Rigor | − | − | − | − | − | − | − | − | − | − | − |
| Diarrhea | − | − | − | − | − | − | − | − | − | − | − |
| Chest congestion | − | − | − | − | − | − | + | − | − | − | 1+ |
| Fever | + | + | − | + | − | + | − | + | + | + | 7+ |
| Highest Temperature | |||||||||||
| <37.3 | − | − | + | − | + | − | + | − | − | − | 3+ |
| 37.3–38.0 | + | − | − | + | − | + | − | − | + | + | 5+ |
| 38.0–39.0 | − | + | − | − | − | − | − | + | − | − | 2+ |
| >39.0 | − | − | − | − | − | − | − | − | − | − | — |
| Anxiety | − | − | − | − | − | − | + | − | − | − | 1+ |
| Dyspnea | − | − | − | − | − | − | − | − | − | − | — |
| Comorbidity | |||||||||||
| Diabetes | − | − | − | − | − | − | − | − | − | − | — |
| Hypertension | − | − | + | − | − | − | − | − | − | − | 1+ |
| Cardiovascular disease | − | − | + | − | − | − | − | − | − | − | 1+ |
| Malignancy | − | − | − | − | − | − | − | − | − | − | — |
| Chronic liver disease | − | − | − | − | − | − | + | − | − | − | 1+ |
| Renal dysfunction | − | − | − | − | − | − | − | − | − | − | — |
| Physical signs | |||||||||||
| WBC decreaseda | − | − | − | + | − | − | − | − | + | − | 2+ |
| CRP increasedb | − | + | + | + | − | − | − | − | − | − | 3+ |
| Lymphocyte decreasedc | + | − | − | − | − | − | − | − | + | − | 2+ |
| Systolic pressure (mm Hg) | 116 | 132 | 178 | 136 | 150 | 126 | 120 | 102 | 132 | 100 | 129 (117–135) |
| Respiratory rate (times/min) | 19 | 20 | 18 | 17 | 17 | 19 | 19 | 18 | 18 | 18 | 18 (18–19) |
| Current medicine | |||||||||||
| Antibiotics | + | + | − | + | − | + | − | + | − | − | 5+ |
| Antipyretics | + | − | − | − | − | + | − | + | − | − | 3+ |
| Exposure | |||||||||||
| Wuhan exposure | + | + | + | + | + | − | + | − | − | − | 6+ |
| Contact with person from Wuhan | − | − | − | − | − | + | − | + | + | + | 4+ |
A plus sign represents the data or symptom was present, and a minus sign indicates absent. WBC: white blood cell count.
WBC decreased: below the normal range of WBC (3.5–9.5 × 109 cells/L)
CRP increased: above the normal range of C-reactive protein (0–10 mg/L)
Lymphocyte decreased: below the normal range of lymphocyte (1.1–3.2 × 109 cells/L)
Among these patients, five patients (patients 1, 2, 3, 6 and 8) developed serious illness (resting oxygen saturation less than 93% or arterial partial oxygen pressure <60 mm Hg or respiratory rate more than 30 breaths per minute), three (patients 1, 2 and 3) of them were transferred to the First Affiliated Hospital of Zhejiang University hospital for further treatment. Seven patients (patients 4, 5, 6, 7, 8, 9 and 10) were cured and discharged from hospital, on February 6, 8, 11, 2, 3, 2 and 7, respectively. Two severe cases (patients 1 and 2) had multiple symptoms (cough, white phlegm, headache, nausea and fever) before admission. In discharged patients, patient 7 had just one symptom (chest congestion), while developing anxiety after admitted to hospital.
Five patients had not received any treatment before the onset of symptoms. The results of cardiovascular, abdominal, and neurologic examinations were normal in all patients, in consideration of their preexisting conditions.
Radiologic assessment
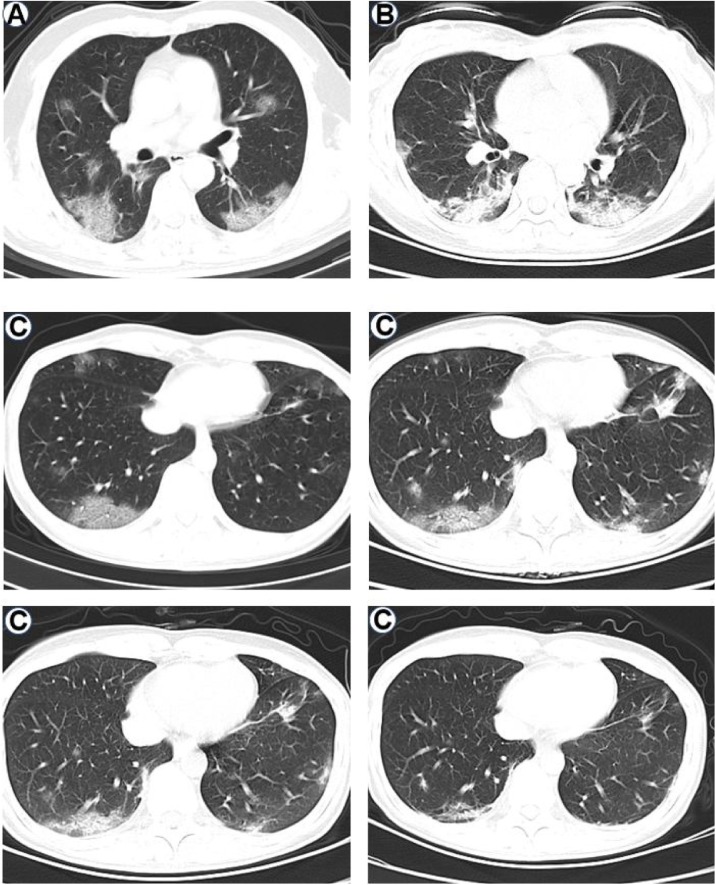
All patients had abnormal chest radiographs except patient 7. The main abnormalities in chest CT images were air-space shadowing: patchy ground-glass opacities (in patients 2, 3, 6 and 9) (Figure 2A) or patchy hyperdense areas (patients 1, 4, 5, 8 and 10) (Figure 2B) on the initial chest radiograph. The radiographic opacities were mostly in the lower lung zones (patients 1, 2, 3, 4, 5, 6, 8 and 9). Patient 6 presented with pleural effusions.
Figure 2.
Chest radiographs showing patchy ground-glass opacities (panel A: patient 3), patchy hyperdense areas (panel B: patient 1) and multifocal opacity changes over time (panel C: patient 8).
The radiographic lesions progressed throughout in size, extent and severity in three patients (patients 1, 2 and 3) after admission. Patchy consolidation was found in the lower lung zone in patient 6. Some improvement on lesions occurred in patients 4, 5, 8, 9 and 10 within the first eight days after presentation. The CT-images of patient 8 on the second, fourth, sixth and eighth day after admission are shown in Figure 2C; the progression of the lesions occurred on the 4th day and improvement appeared on the 6th day, the opacities shrank significantly by the 8th day after admission.
Laboratory findings
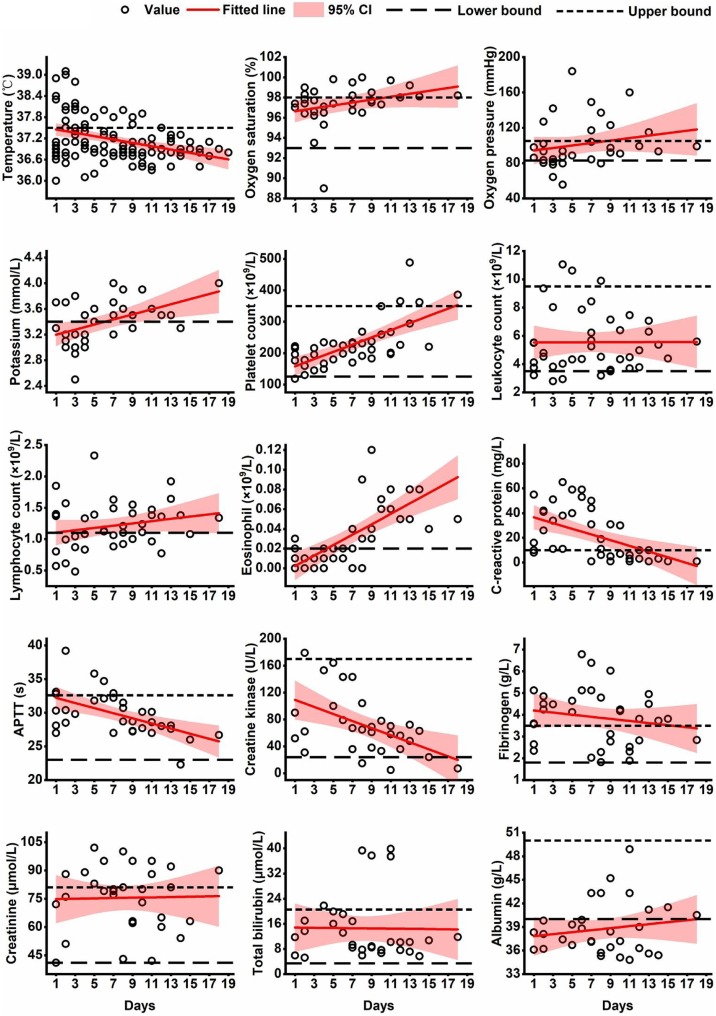
Figure 3 reveals the changes over time in fifteen laboratory indicators after the beginning of therapy. As can be seen from the figure, most values of temperature dropped below 37.5 degrees Celsius for four days of treatment. During the therapeutic period, over half of the lymphocyte counts (normal range: 1.1–3.5 × 109 per liter), eosinophil counts (normal range: 0.02–0.52 × 109 per liter), artery potassium concentrations (normal range: 3.4–5.5 mmol/L), and serum albumin levels (normal range: 40–50 g/L) were under the lower threshold of normal range, but all presented a rising trend, particularly evident in eosinophils.
Figure 3.
The temporal changes of laboratory indicators in COVID-19 patients after initiation of LPV-combined therapy.
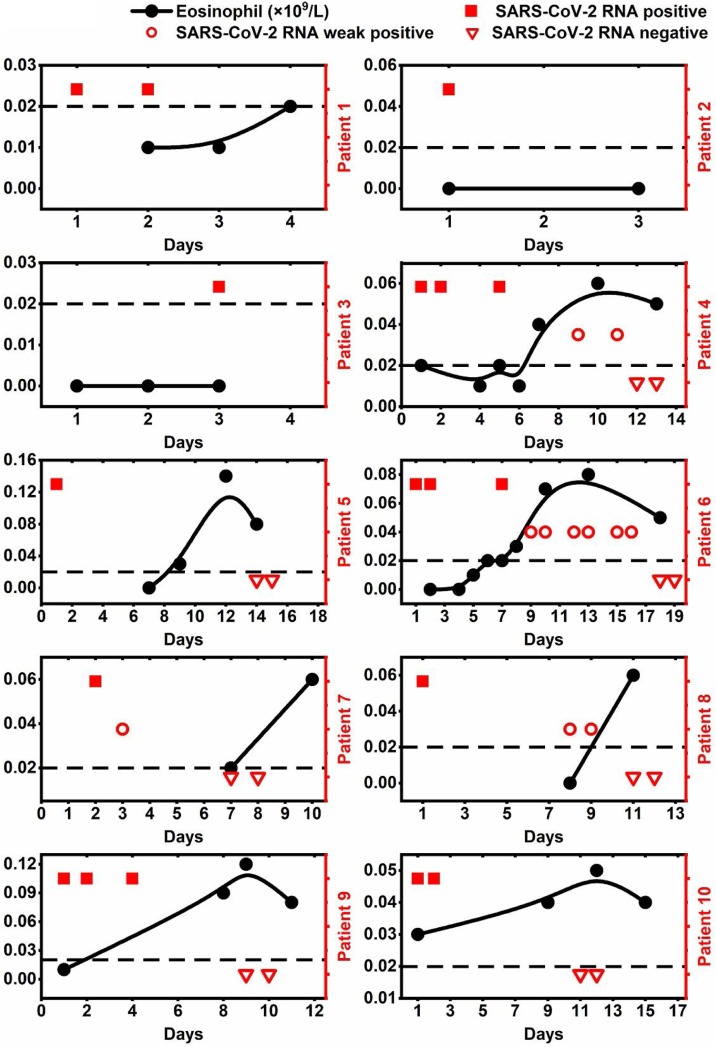
The eosinophil values of patients 1, 2 and 3 were all less than the lower bound of normal range before they were transferred to the First Affiliated Hospital of Zhejiang University (Figure 4 ). Other patients, except for patient 10, also had similar findings at the early stage of COVID-19. Eosinophil counts of patients 4, 5, 6, 7, 8 and 9 returned to normal in nine days after treatment (Figure 3), and were discharged within 7, 9, 12, 3, 2 and 4 days after the persistent improvement sign of eosinophil count appeared (Figure 4). Two mild patients (patients 7 and 9) had a shorter improvement period of eosinophil count than the other two severe cases (patients 6 and 8).
Figure 4.
The changes over time on eosinophil level and SARS-CoV-2 viral load in each COVID-19 patient.
The results of SARS-CoV-2-RNA were positive in patients 1, 2 and 3 before they were transferred. Patient 7 first showed signs of improvement in virology on 3th day after treatment, and others presented such signs between 7 and 14 days after admission. The negative results of SARS-CoV-2-RNA of patients 7 and 9 occurred within 7 and 9 days after admission, earlier than that of patients 4, 5, 6, 8 and 10 (12, 14, 18, 11 and 11 days, respectively). During the period of lower eosinophil, SARS-CoV-2-RNA presented positive, and after eosinophil increased to normal, SARS-CoV-2-RNA turned to negative within 5 days, except for patient 6 (11 days).
Most values of C-reactive protein (CRP, normal range: 0–10 mg/L) were above the upper bound of normal range, and over half of the fibrinogen levels (FIB, normal range: 1.8–3.5 g/L) were high during the observed period (Figure 3). Leukocyte count (normal range: 3.5–9.5 × 109 cells/L), platelet count (PLT, normal range: 125–350 × 109 cells/L), activated partial thromboplastin time (APTT, normal range: 23–32.6 s), total bilirubin level (normal range: 3.42–20.52 mmol/L), creatinine concentration (normal range: 41–81 μmol/L) and creatine kinase value (normal range: 24–170 U/L) were mainly normal in these cases (Figure 3). An increasing trend toward improved oxygen saturation, oxygen partial pressure (PO2) and platelet count was found after initiating therapy.
Treatments and outcomes
The median duration between the onset of symptom and initiating therapy was five days (IQR, 3–6). All patients were treated with combined therapy (lopinavir, LPV, 400 mg every twelve hours and interferon α2b atomization inhalation, 5 million U twice daily) or single drug LPV (in patient 7 only) (Table 2 ). Before the initiation of treatment, patients 1, 2, 4, 6 and 8 received antibiotics for a median of three days (IQR, 2–4) (Table 1), which did not ease the symptoms of fever and cough.
Table 2.
Treatments and outcomes of COVID-19 patients.
| Patient no. |
Summary | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Complications | |||||||||||
| Acute myocardial injury | − | − | − | − | − | − | − | − | − | − | — |
| Acute kidney injury | − | − | − | − | − | − | − | − | − | − | — |
| Respiratory failure | + | − | − | − | − | − | − | + | − | − | 2+ |
| Digestive adverse effect | + | + | − | − | − | + | − | + | − | + | 5+ |
| Low potassium | + | + | − | + | + | + | − | + | − | + | 7+ |
| Hypoalbuminemia | − | − | − | − | − | − | − | + | − | − | 1+ |
| Treatment | |||||||||||
| LPV | + | + | + | + | + | + | + | + | + | + | 10+ |
| RH-interferon α2b | + | + | + | + | + | + | − | + | + | + | 9+ |
| Antibiotics | − | − | + | + | − | − | − | − | − | − | 2+ |
| Glucocorticoid | + | + | − | − | − | − | − | + | − | − | 3+ |
| Immunoglobulin | + | + | − | − | + | + | − | + | − | − | 5+ |
| Oxygen support | |||||||||||
| Nasal cannula | + | + | + | + | + | + | − | + | + | + | 9+ |
| Non-invasive ventilation | − | − | − | − | − | − | − | − | − | − | − |
| Invasive mechanical ventilation | − | − | − | − | − | − | − | − | − | − | − |
| Prognosis | |||||||||||
| Hospitalization days | 4 | 3 | 4 | 14 | 18 | 19 | 10 | 13 | 12 | 17 | 13 (4–17) |
| Intensive transfers | + | + | + | − | − | − | − | − | − | − | 3+ |
| Chest radiographs | |||||||||||
| Bilateral lesions of lung | + | + | + | + | + | + | − | + | − | + | 8+ |
| Pneumonia progression >50% | + | − | − | − | − | − | − | − | − | − | 1+ |
LPV: lopinavir; RH-interferon α2b: Recombinant Human Interferon α2b Injection; Pneumonia progression >50%: Lung pattern of the patient progressed more than 50% in 48 h.
As indicated in Table 2, the median observed period was 13 days (IQR, 4–17). During this period, all of the patients had no AMI and AKI, and five patients reported digestive adverse effects (diarrhea or emesis). Seven patients had hypokalemia. All patients received oxygen inhalation through a nasal tube except patient 7.
Patients 1 and 2 had severe digestive adverse effects (diarrhea and emesis) and hypokalemia within four days of treatment (LPV + interferon α2b). The combined therapy was changed empirically to interferon α2b atomization inhalation (5 million U twice daily) and arbidol hydrochloride granules (AHG, 0.2 g, three times daily) and human immunoglobulin intravenous injection (IVIG, 20 g every day) and methylprednisolone (40 mg, every twelve hours). But, the clinical symptoms and radiography still presented deterioration. Patient 3 had complex underlying diseases combined with mycoplasma pneumonia. Because of deterioration, patients 1, 2 and 3 were transferred to the First Affiliated Hospital of Zhejiang University within four days (Table 2).
Among discharged cases, patient 7 had unremarkable radiographic patterns throughout after admission. The opacities on CT images of patients 4, 5, 9 and 10 improved persistently after eight, seven, six and seven days of LPV-combined regimen exposure, respectively. Another discharged case (patient 8) presented severe diarrhea, hypokalemia, and respiratory failure on the 4th day and hypoproteinemia on the 5th day of taking LPV and interferon α2b. In view of this, somac (one tablet daily), Human Albumin Solution (HAS, 10 g per day), AHG (0.2 g, three times daily), IVIG (20 g every day) and methylprednisolone (40 mg, every twelve hours) were added to the original treatment regimen, without stopping LPV. The adverse reaction, complications and radiographic opacities (Figure 2C) of patient 8 were significantly improved in two days. Patient 6 stopped the usage of LPV due to digestive adverse reaction after four days of treatment. The period of improvement of radiographic lesions was 14 days, longer than others (8 days). Because the results of SARS-CoV-2 RNA presented weakly positive, he remained hospitalized when the other six patients were discharged. The results of SARS-CoV-2-RNA of patient 6 presented negative on 18th day after admission, later than other discharged cases. Fitness for discharge was based on improvement of chest radiographic evidence, with viral clearance in respiratory samples and abatement of fever for at least 3 days.
Discussion
Our report of these 10 patients reveals that COVID-19 is a contagious and rapidly progressive disease. Among these cases, secondary (patients 1, 2, 3, 4, 5 and 7), tertiary (patients 8 and 10) and quartus (patients 6 and 9) patient emerged. Four patients had a history of interaction with source patients in a room, which confirmed that contact (however trivial) is one of the routes of transmission. There was no further transmission among our healthcare workers in our hospital after we implemented appropriate isolation precautions involving use of isolation gowns, protective suits, N95 masks, gloves, and hands disinfecting before and after contact with patients. Which protective approach has an effect on the decrease in transmission remains uncertain. These findings revealed that person-to-person transmission and interprovincial spread are possible, which is consistent with the results of the latest research (Huang et al., 2020, Chan et al., 2020); control of travel and strict protection are essential prerequisites.
The incubation period of COVID-19 was between 3 and 7 days in those patients who could be accurately assessed. Such incubation period is similar to the recent report which estimated ranges from 3 to 6 days (Chan et al., 2020), but longer than that of SARS (Wang et al., 2004). This further complicates the epidemiological investigation and singles out at-risk exposure to source patients even without any symptoms as the key factor in the diagnosis of COVID-19.
In this cluster of 10 patients, most of them presented with fever, cough and bilateral lesions of the lung. Two severe cases were combined with multiple early symptoms: cough, white phlegm, headache, nausea and fever. In seven discharged patients, we could not find a universal characteristic of clinical symptoms before admission. Patient 7 had no symptoms except for chest congestion and anxiety. It is also of note that the radiographic images of patient 7 were normal throughout until discharge. For the above points, it is suspected that chest congestion may be caused by anxiety. However, this finding is different from another asymptomatic infection that was reported in a child aged 10 years without any clinical symptoms, but had ground-glass lung opacities (Chan et al., 2020). From this point of view, asymptomatic patients like in our study will make the diagnosis of COVID-19 even more difficult, and diagnostic delays may further lead to the spread of the epidemic. However, whether asymptomatic infection actually exists needs more clinical evidence.
The radiological appearance of COVID-19 is mostly ground-glass opacity with patchy areas, predominantly affecting the lower lobe. Focal absorption occurs in size, extent, and severity, followed by features of the disease entering a fibrotic phase (Figure 2C). In most patients, radiological lesions changed evidently and rapidly in a few days after onset of symptoms. Hence, timely radiographic observation and evaluation are necessary in realizing the progression and recovery of COVID-19.
Most patients had hypokalemia and digestive adverse effects in four days after the beginning of treatment, which could be caused by LPV exposure ((Dybul et al., 2002). In view of this, three severe cases (patients 1, 2 and 6) were taken off LPV empirically; instead, their therapies were replaced with a glucocorticoid and interferon-backbone combination regimen. However, the conditions of patients 1 and 2 had no improvement, and were even aggravated after the withdrawal of LPV. The results of SARS-CoV-2-RNA of patient 6 remained positive for four days after that of the other six discharged cases who with continuous use of LPV turned to negative. Noticeably, one severe case (patient 8) received support care and symptomatic treatment after the adverse reactions occurred, rather than stopping LPV, with improvement appearing in two days. The experience therefore suggests that receiving LPV is effective in disease recovery; the efficacy of glucocorticoid and interferon is not evident on cases infected with SARS-CoV-2 (Wang et al., 2020).
In this study, the findings of high CRP level (mostly between 10 and 100 mg/L) and increased FIB concentration were consistent with those of a recent study (Chan et al., 2020), and the counts of white blood cells and PLT were normal in most cases, as was also shown in another research study of COVID-19 (the normal percentages were 45% and 95%, respectively) (Huang et al., 2020). However, a large proportion of our cases presented low levels of potassium, albumin, lymphocyte and eosinophil in the early stage of COVID-19, particularly that almost all eosinophil values were less than the lower threshold of normal range within the first week of hospitalization. This symptom could not be attributed to glucocorticoid exposure, since seven patients had not received such treatment at initial admission. However, the reduction of eosinophils may be related to a mechanism of stress response in the condition of acute lung injury caused by SARS-CoV-2, which results in inhibiting the release of eosinophils in the marrow through glucocorticoid secretion. Interestingly, there was continuous improvement on eosinophils in the later stage of COVID-19, which was in sync with the symptom of radiographic and virology improvements in all discharged cases, and the time of eosinophil improvement of mild cases was earlier than that of severe cases, which suggests that lower and increasing eosinophils may be the signs of COVID-19 progression and recovery. Combined with the above abnormal indicators, whether these can be regarded as diagnostic characteristics of COVID-19 needs more research to determine. Although such abnormalities existed in the early stage of COVID-19, there was an improving trend of these indicators after receiving LPV-combined regimen. This was further evidence that LPV has a positive effect on the patients infected with SARS-CoV-2.
Our study has limitations; the inherent shortcomings due to a retrospective observational single-center study, small sample size, and short term follow-up make it difficult to address the relationships between COVID-19, LPV and eosinophil, and to reach a firm conclusion. All above limitations require further study; nonetheless, our primary results provided moderate yet important insight for this topic.
Conclusions
Considering the information we describe, taking appropriate isolated precautions with source patients suspected to have COVID-19 is vitally important. Asymptomatic infection (as reported in patient 7) appears possible; strict infection control is crucial for avoiding a second pandemic. No effective therapy is known for COVID-19 at present. The use of LPV appears effective in this study, but digestive adverse effects and hypokalemia should be a concern by clinicians after beginning this drug. More clinical evidence is also needed imminently to assess the efficacy and safety of LPV in patients with SARS-CoV-2 infection. In addition, eosinophil counts presented as a potential predictor in the development process of COVID-19 in this study. Further investigation and research on a larger scale are worth conducting to verify this point.
Conflict of interest statement
The authors declare that they have no competing interests.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributors
LF and ZJ conceived, designed, and organized the study, interpreted the results, and drafted the manuscript. XAF helped supervise the study. The other authors contributed to collect the data on site.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Xixi Hospital. All data were anonymized to comply with the provisions of personal data protection legislation. Due to the retrospective nature of this study and due the fact that only historical medical data were collected, written informed consent was not required.
Availability of data and materials
The data set used for this manuscript will be available from the corresponding author upon reasonable request.
Acknowledgments
We thank all study participants and staff of all participating sites.
References
- Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020 doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dous G.V., Grigos A.C., Grodman R. Elevated troponin in patients with acute stroke–is it a true heart attack? Egypt Heart J. 2017;69(3):165–170. doi: 10.1016/j.ehj.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybul M., Fauci A.S., Bartlett J.G., Kaplan J.E., Pau A.K. Guidelines for Using Antiretroviral Agents among HIV-Infected Adults and Adolescents: The Panel on Clinical Practices for Treatment of HIV*. Ann Intern Med. 2002;51(RR-7):1–55. [PubMed] [Google Scholar]
- Hamp D.B., Leženić R., Duper H.M., Goranović T. Respiratory failure as a result of chronic impaired ventilation in actualization of insufficient respiration: a case report. Trends Anaesth Crit Care. 2017;16:32–33. [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum J.A., Lameire N., Aspelin P., Barsoum R.S., Burdmann E.A., Goldstein S.L. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138. [Google Scholar]
- National Health Commission . China CDC weekly; Beijing: 2020. Tracking the epidemic. Available from: http://weekly.chinacdc.cn/news/TrackingtheEpidemic.htm#NHCJan27 [accessed 27.01.20] [Google Scholar]
- The 2019-nCoV Outbreak Joint Field Epidemiology Investigation Team, Li Q. An outbreak of NCIP (2019-nCoV) infection in China — Wuhan, Hubei Province, 2019–2020. China CDC Wkly. 2020;2(5):79–80. [PMC free article] [PubMed] [Google Scholar]
- Touma E., Bisharat N. Trends in admission serum albumin and mortality in patients with hospital readmission. Int J Clin Pract. 2019;73(6):e13314. doi: 10.1111/ijcp.13314. [DOI] [PubMed] [Google Scholar]
- World Health Organization . The Organization; Geneva: 2020. Infection prevention and control during health care when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. Available from: https://www.who.int/publications-detail/infection-prevention-and-control-during-health-care-when-novel-coronavirus-(ncov)-infection-is-suspected-20200125 [accessed 27.01.20] [Google Scholar]
- Wang J.T., Wang-Huei S., Chi-Tai F., Yee-Chun C., Jiun-Ling W., Chong-Jen Y. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis. 2004;10(5):818–824. doi: 10.3201/eid1005.030640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set used for this manuscript will be available from the corresponding author upon reasonable request.