Abstract
Iron overload affects the cell cycle of various cell types, but the effect of iron overload on human pluripotent stem cells has not yet been reported. Here, we show that the proliferation capacities of human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) were significantly inhibited by ferric ammonium citrate (FAC) in a concentration‐dependent manner. In addition, deferoxamine protected hESCs/hiPSCs against FAC‐induced cell‐cycle arrest. However, iron overload did not affect pluripotency in hESCs/hiPSCs. Further, treatment of hiPSCs with FAC resulted in excess reactive oxygen species production and DNA damage. Collectively, our findings provide new insights into the role of iron homeostasis in the maintenance of self‐renewal in human pluripotent stem cells.
Keywords: cell cycle, DNA damage, human embryonic stem cells, human induced pluripotent stem cells, iron overload, ROS
We demonstrated in this study that iron overload could severely impair the proliferation of human embryonic stem cells/human induced pluripotent stem cells through excess reactive oxygen species production and DNA damage. These results imply a role of iron homeostasis in maintaining the self‐renewal of human embryonic stem cells/human induced pluripotent stem cells.
Abbreviations
- ALP
alkaline phosphatase
- DFO
deferoxamine
- EdU
5‐ethynyl‐20‐deoxyuridine
- FAC
ferric ammonium citrate
- hESC
human embryonic stem cell
- hiPSC
human induced pluripotent stem cell
- hPSC
human pluripotent stem cell
- PFA
paraformaldehyde
- PSC
pluripotent stem cell
- ROS
reactive oxygen species
- SEM
standard error of the mean
Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) have the ability to self‐renew and differentiate into almost all types of somatic cells in vitro [1, 2]. Therefore, ESCs and iPSCs could be used for a broad range of clinical applications in regenerative medicine [3, 4, 5, 6]. It has been reported that genetic and epigenetic networks synergistically maintain the self‐renewal status of human embryonic stem cells (hESCs)/human induced pluripotent stem cells (hiPSCs) [7, 8]. However, the details of the mechanism of self‐renewal maintenance in PSCs are still not yet fully understood.
Iron is one of the most essential microelements in the body. However, it has been reported that excess iron can initiate some signaling pathways that are essential for cell death because it has the ability of the redox activity and can produce reactive oxygen species (ROS) [9, 10]. In recent years, numerous studies have revealed that unbalanced iron homeostasis was closely connected with certain common human diseases, such as anemia of chronic disease and obesity [11, 12, 13]. In addition, many studies have reported that iron homeostasis can influence the functions of some kinds of stem cells. For example, it has been clarified that specific targeting of iron homeostasis in cancer stem cells can increase the cancer therapy [14]. Besides, our group discovered that iron deficiency caused inhibition of pluripotency in hESCs/hiPSCs [15]. However, the effect of iron overload in iPSCs or ESCs has not yet been reported.
Here, we studied the function of iron overload in human pluripotent stem cells (hPSCs). We investigated that iron overload inhibits the proliferation of hPSCs through excess ROS production and DNA damage. Our study provides novel insights into the maintenance of hPSC self‐renewal.
Materials and methods
Cell culture
Human ESCs (H1‐ESCs) and iPSCs (AC‐iPSCs) were cultured as described in our previous study [15]. In brief, cells were cultured in E8 pluripotent stem cell culture medium (STEMCELL Technology, Vancouver, British Columbia, Canada) at a temperature of 37 °C in 5% CO2. When the cell density reached 70%, we subcultured the cells with EDTA acid (CELLAPY, Beijing, China).
Quantitative real‐time PCR
A quantitative real‐time PCR assay was performed as described previously [16]. Total RNA was isolated using TRIzol reagent (Ambion, Life Technologies, Camarillo, CA, USA) according to the manufacturer’s protocol. The FastStart Universal SYBR Green Master (Rox) and 7500 Fast Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA) were used in real‐time PCR for relative quantification of RNA. Every sample was tested four times. The primers are listed in Table S1.
Alkaline phosphatase staining
A 5‐bromo‐4‐chloro‐3‐indolyl phosphate/Nitro Blue tetrazolium/alkaline phosphatase (ALP) color development kit (C3206; Beyotime, Nantong, Jiangsu, China) was used for ALP staining. In brief, 4% paraformaldehyde (PFA) was used to immobilize cells for 30 min at 37 °C. The cells were then washed three times in Dulbecco’s PBS. Then, cells were incubated in the dark in 5‐bromo‐4‐chloro‐3‐indolyl phosphate/Nitro Blue tetrazolium solution for 7 min. Distilled water was immediately added to end the reaction. Cells were photographed under a standard light microscope (Eclipse TS100; Nikon, Melville, NY, USA).
5‐Ethynyl‐20‐deoxyuridine assay
The effect of iron overload on cell proliferation was determined by 5‐ethynyl‐20‐deoxyuridine (EdU) assay. Cells were cultured in glass‐bottomed cell culture dishes. Then, 4% PFA was used to immobilize cells for 15 min and after 0.4% Triton X‐100 was used to permeabilize the cells for 20 min. Then, a cell proliferation assay was carried out using an EdU assay kit (Ribobio Co., Ltd., Guangzhou, China). The cells were photographed with a confocal fluorescence microscope (FV10C‐W3; Olympus, Tokyo, Japan). The cell nuclei of double‐labeled EdU and 4′,6‐diamidino‐2‐phenylindole (DAPI) were considered markers of positive cells.
Cellular iron content assay
An iron assay kit (K390‐100; BioVision, Milpitas, CA, USA) was used to detect cellular iron content. First, cells were lysed with 120 μL iron assay buffer (K3900‐100‐1; BioVision). Subsequently, the liquid was collected and centrifuged at 16 000 g for 10 min. Then, it was added along with 35 μL sample and 65 μL assay buffer to 96‐well plates, and 5 μL iron reducer (K3900‐100‐3; BioVision) was added to each well. The cells were incubated at 25 °C for 30 min. Next, 100 μL iron probe (K3900‐100‐2; BioVision) was added, and the solution was mixed and then incubated in the dark at 25 °C for 30 min. The absorbance was measured at 593 nm using a microplate reader.
Western blotting
Cells were lysed with radioimmunoprecipitation assay (RIPA) buffer (P0013; Beyotime). Subsequently, proteins were separated via 12.5% SDS/PAGE and transferred to a nitrocellulose blotting membrane (Life Science, Mexico Assembly). The membrane was blocked and then incubated with primary antibodies overnight at 4 °C. It was then incubated with secondary antibody for 1 h at room temperature. The membrane was then washed three times with PBST and examined using odyssey version 1.2 software (LI‐COR Biosciences, Lincoln, NE, USA). The antibodies are listed in Table S2.
Measurement of ROS production
Intracellular levels of ROS production were measured using a ROS Assay Kit (S0033; Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instructions. In brief, after washing cells with PBS, cells were incubated with 10 μm 2′,7′‐Dichlorodihydrofluorescein diacetate (DCFH‐DA) probes for 30 min at 37 °C. Then, the cells were fixed in 4% PFA for 30 min and stained with DAPI (20 μg·mL−1) for 10 min. The cells were then imaged with a fluorescence microscope (Olympus Optical).
Immunofluorescence assays
Cells were fixed with 4% PFA for 15 min at 37 °C. Then, cells were washed with PBS and penetrated with 0.3% Triton X‐100 for 15 min. Next, cells were blocked with goat serum. After, the cells were stained with ROS overnight. Then, the cells were stained with secondary antibody for 1 h. Cell nuclei were stained with DAPI. The cells were then observed under a confocal fluorescence microscope (FV10C‐W3; Olympus). The antibodies are listed in Table S2.
Statistics
Error bars represent the standard error of the mean (SEM). Data were analyzed using prism 7 (GraphPad Software Inc., San Diego, CA, USA). The significance of differences was analyzed using one‐way ANOVA and presented with the following levels of significance: *P < 0.05, **P < 0.01 and ***P < 0.005.
Results
Iron overload inhibits self‐renewal of human PSCs
To characterize the role of iron overload in human PSCs, we treated hESCs/hiPSCs with different concentrations (0, 10, 20 and 50 μm) of ferric ammonium citrate (FAC) and 50 μm iron chelator deferoxamine (DFO). Interestingly, we observed that FAC‐treated cells exhibited a dramatically slower rate of proliferation compared with the control group (Fig. 1A). We first measured cellular iron content after FAC treatment. The results showed that the iron content of the hPSCs was increased by FAC treatment in a concentration‐dependent manner (Fig. 1B). As shown by ALP staining, iron overload significantly inhibited the proliferation of hESCs/hiPSCs, but cells treated with FAC were maintained with undifferentiated colony morphology (Fig. 1C). The cell counting assay also showed that the proliferation of hESCs/hiPSCs was significantly inhibited after FAC treatment. However, DFO 50 μm protected hESCs/hiPSCs from cell‐cycle arrest induced by FAC 50 μm (Fig. 1D). Meanwhile, we used an EdU incorporation assay to assess cell proliferation capacity. As expected, there were more EdU‐positive cells in the control group than in the FAC group (Fig. 1E). Together, these results indicate that iron overload inhibited self‐renewal of hPSCs.
Fig. 1.
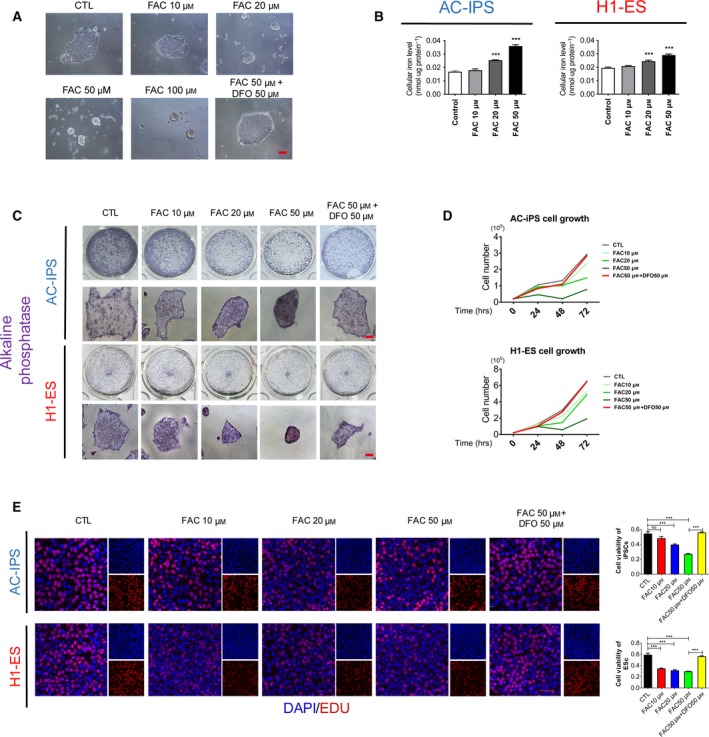
(A) Phase images of hiPSCs treated with 10, 20, 50 or 100 μm FAC or 50 μm FAC + 50 μm DFO. Scale bar: 100 μm. (B) The cellular iron content of AC‐iPSCs and H1‐ESCs treated with different concentrations of FAC for 16 h. Data are shown as mean ± SEM (n = 3). One‐way ANOVA was applied. ***P < 0.001 compared with the control. (C) Morphology and ALP staining of hiPSCs/hESCs treated with different concentrations of FAC or 50 μm DFO supplemented with 50 μm FAC for 16 h. Scale bars: 100 μm. (D) Cell counting assay of AC‐iPSCs and H1‐ESCs supplemented with 10, 20 or 50 μm FAC or 50 μm FAC + 50 μm DFO. (E) EdU assay of hiPSCs/hESCs treated with different concentrations of FAC or 50 μm DFO supplemented with 50 μm FAC for 16 h. Scale bar, 50 μm. Data are shown as mean ± SEM (n = 3). One‐way ANOVA was applied. ***P < 0.001.
Iron overload did not affect pluripotency in hPSCs
We next investigated whether iron overload affected the pluripotency of hPSCs. We performed quantitative RT‐PCR analysis to detect the mRNA expression of pluripotency factors in hiPSCs after exposure to different concentrations of FAC for 16 h. The results show that after treatment with FAC, hiPSCs exhibited similar expression of pluripotency factors to the control group (Fig. 2A). This finding was also supported by our western blot analysis (Fig. 2B,C). The earlier results suggest that iron overload inhibits the proliferation but has no influence on the pluripotency of hESCs/hiPSCs.
Fig. 2.
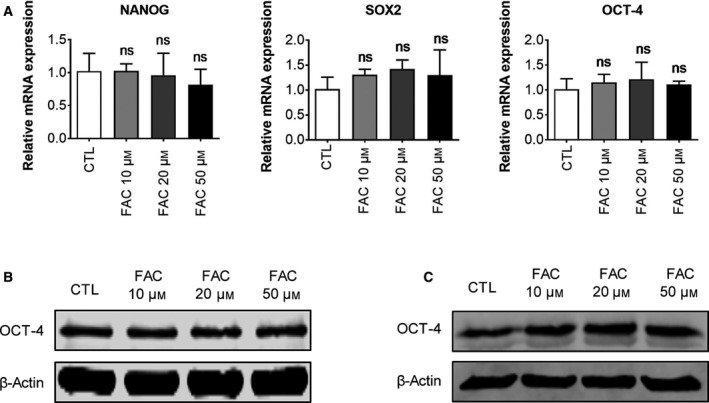
(A) Quantitative RT‐PCR analysis of NANOG, SOX2 and OCT‐4 expression in AC‐iPSCs treated with different concentrations of FAC for 16 h. Data are shown as mean ± SEM (n = 3). One‐way ANOVA was applied. (B) Western blot analysis of OCT‐4 expression in AC‐iPSCs treated with different concentrations of FAC for 16 h. (C) Western blot analysis of OCT‐4 expression in H1‐ESCs treated with different concentrations of FAC for 16 h.
Increased intracellular ROS levels in hPSCs after FAC treatment
Next, we focused on exploring the mechanism by which iron overload regulates self‐renewal in hPSCs. Intracellular ROS levels play crucial roles in the proliferation of various cell types. It has been reported that excess ROS generation is involved in iron dyshomeostasis‐induced cell injury [17, 18, 19]. To further evaluate the effects of iron overload on ROS production, we performed DCFH‐DA staining in hPSCs after exposure to different concentrations of FAC. As shown in Fig. 3, FAC 50 μm significantly induced ROS production in hiPSCs. Meanwhile, 50 μm DFO and 5 mm ROS scavenger N‐acetyl‐L‐cysteine (NAC) suppressed FAC‐induced ROS production. In addition, we found that DFO‐induced iron deficiency also increased ROS production in hiPSCs (Fig. S1). These data indicate that iron dyshomeostasis resulted in a significant increase in ROS in hPSCs.
Fig. 3.
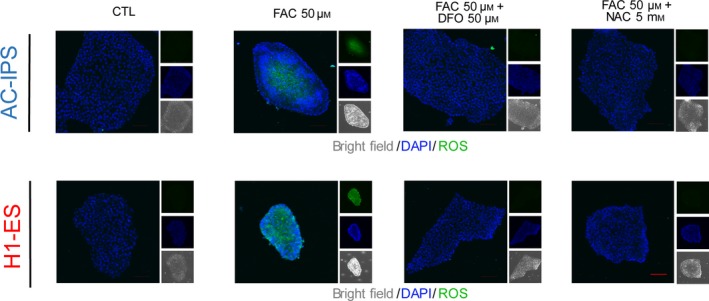
DCFH‐DA staining of AC‐iPSCs and H1‐ESCs treated with 50 μm FAC, 50 μm FAC + 50 μm DFO and 50 μm FAC + 5 mm NAC. Scale bar, 100 μm.
Iron overload leads to DNA damage in hESCs/hiPSCs
Previous studies have indicated that increased ROS production resulted in DNA damage [20]. We thus examined whether iron overload causes DNA damage in hiPSCs and hESCs. We stained cells with γ‐H2A.X (phosphorylation status of H2A.X), a marker of DNA damage. As shown in Fig. 4A, after exposure to FAC for 16 h, iPSCs/ESCs exhibited a significant increase in DNA damage. However, DFO and NAC treatment suppressed the increase of γ‐H2A.X‐positive cells upon FAC treatment. The protein expression of γ‐H2A.X also increased after treatment with different concentrations of FAC (Fig. 4B). These results indicate that iron overload results in a sharp increase in DNA damage in hPSCs.
Fig. 4.
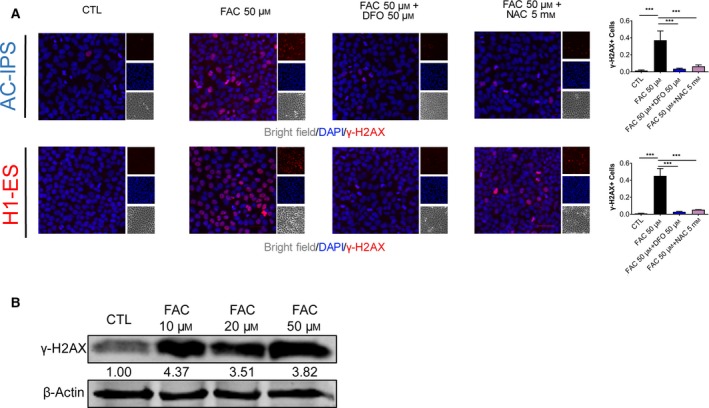
(A) Immunofluorescence analysis of DNA damage in AC‐iPSCs and H1‐ESCs treated with 50 μm FAC, 50 μm FAC + 50 μm DFO and 50 μm FAC + 5 mm NAC. Scale bars, 50 μm. Data are shown as mean ± SEM (n = 4). One‐way ANOVA was applied. ***P < 0.001. (B) Western blot analysis of γ‐H2A.X expression in H1‐ESCs treated with different concentrations of FAC for 16 h.
Discussion
This study reveals that iron overload inhibited the proliferation of hESCs/iPSCs without affecting pluripotency. The underlying mechanisms of this phenomenon were associated with DNA damage and excessive ROS production. These findings provide a better understanding of the maintenance of self‐renewal in hPSCs.
Iron overload is mainly caused by certain genetic diseases and high‐iron diets. Excessive iron accumulation in the body can cause liver damage, cardiovascular damage, neurodegenerative diseases and many aging‐related diseases [21]. Iron overload has a profound effect on immature hematopoietic cells and stromal cells, and thereby destroys the hematopoietic process [22]. Previous studies have indicated that iron homeostasis is crucial for various kinds of stem cell. For example, iron overload can impact the self‐renewal and function of hematopoietic stem cells and progenitor cells [23, 24], whereas iron deficiency impairs the pluripotency of hESCs/hiPSCs [15]. Besides, it has been reported that iron deficiency inhibited the expression of stemness markers and spherogenesis in cancer stem cells [25]. However, our findings indicate that iron overload can restrict self‐renewal in hPSCs via excessive ROS production and DNA damage, which provides a new perspective for studying the role of iron homeostasis in the maintenance of hPSC self‐renewal.
hESCs and iPSCs have the ability to self‐renew and differentiate into all cell types. Therefore, human PSCs have emerged as a promising cell resource for regenerative medicine and a particular experimental model [26]. Accumulated evidence from studies of hESCs suggests that the self‐renewal of ESCs was regulated via ROS to a certain degree [27, 28]. Meanwhile, a previous study indicated that stem cells were highly susceptible to DNA damage and cause DNA damage accumulation in the microenvironment of growing stem cells, which was partly responsible for catastrophic consequences for tissue and body homeostasis [29]. For example, the accumulation of DNA damage affected the genomic stability of hematopoietic stem cells and may increase the incidence of hematological malignancies, especially with age [30, 31]. The production of ROS is also one of the reasons for the accumulation of DNA damage. Although our study revealed that hPSC proliferation was inhibited because iron overload caused excessive ROS production and DNA damage, the molecular mechanism of cell proliferation induction requires further study.
In summary, our study shows that iron overload inhibited self‐renewal capacity of hESCs and hiPSCs, which provides a new insight into the potential mechanisms of the self‐renewal of PSCs.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
ZH, WM and YL conceived the study and designed the experiments. ZH, ZX, LC, DY, YY, YZ, YC, DB, X. Gao, X. Guo, WZ, MY, SL, GY, MJ, QH, XW, BH, CF and FY contributed to the data collection, performed the data analysis and interpreted the results. ZH wrote the manuscript. WM and YL contributed to the critical revision of this article. All authors read and approved the final manuscript.
Supporting information
Fig. S1 . DFO‐induced iron deficiency promotes ROS generation in hiPSCs. DCFH‐DA staining of AC‐IPSCs treated with 150 μm DFO for 24 h. Scale bar, 100 μm.
Table S1 . Primers used in quantitative RT‐PCR.
Table S2 . Antibodies used in this study.
Acknowledgements
This work was supported by the Young Creative Talents Training Program of Heilongjiang Provincial Universities (Grant No. UNPYSCT‐2015034), National Key R&D Program of China (Grant No. 2017YFC1307403) and the National Natural Science Fund of China (Grant No. 81573434/81730012).
Zhenbo Han, Zihang Xu and Lei Chen contributed equally to this article
Contributor Information
Wenya Ma, Email: 787973558@qq.com.
Yu Liu, Email: rainfall1982@163.com.
References
- 1. Thomson JA, Itskovitz‐Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS and Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147. [DOI] [PubMed] [Google Scholar]
- 2. Takahashi K and Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. [DOI] [PubMed] [Google Scholar]
- 3. Musunuru K, Domian IJ and Chien KR (2010) Stem cell models of cardiac development and disease. Annu Rev Cell Dev Biol 26, 667–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Masuda S, Miyagawa S, Fukushima S, Nakamura T, Khurram MA, Ishikawa T, Saito A and Sawa Y (2016) Expandable progenitors from induced pluripotent stem cells. Nat Rev Cardiol 13, 574. [DOI] [PubMed] [Google Scholar]
- 5. Lalit PA, Salick MR, Nelson DO, Squirrell JM, Shafer CM, Patel NG, Saeed I, Schmuck EG, Markandeya YS, Wong R et al (2016) Lineage reprogramming of fibroblasts into proliferative induced cardiac progenitor cells by defined factors. Cell Stem Cell 18, 354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Y, Cao N, Huang Y, Spencer CI, Fu J‐D, Yu C, Liu K, Nie B, Xu T, Li K et al (2016) Expandable cardiovascular progenitor cells reprogrammed from fibroblasts. Cell Stem Cell 18, 368–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li M, Liu GH and Izpisua Belmonte JC (2012) Navigating the epigenetic landscape of pluripotent stem cells. Nat Rev Mol Cell Biol 13, 524–535. [DOI] [PubMed] [Google Scholar]
- 8. Shiraki N, Shiraki Y, Tsuyama T, Obata F, Miura M, Nagae G, Aburatani H, Kume K, Endo F and Kume S (2014) Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell Metab 19, 780–794. [DOI] [PubMed] [Google Scholar]
- 9. Ray PD, Huang BW and Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24, 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bogdan AR, Miyazawa M, Hashimoto K and Tsuji Y (2016) Regulators of iron homeostasis: new players in metabolism, cell death, and disease. Trends Biochem Sci 41, 274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MacKenzie EL, Iwasaki K and Tsuji Y (2008) Intracellular iron transport and storage: from molecular mechanisms to health implications. Antioxid Redox Signal 10, 997–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hentze MW, Muckenthaler MU, Galy B and Camaschella C (2010) Two to tango: regulation of Mammalian iron metabolism. Cell 142, 24–38. [DOI] [PubMed] [Google Scholar]
- 13. Tussing‐Humphreys L, Pusatcioglu C, Nemeth E and Braunschweig C (2012) Rethinking iron regulation and assessment in iron deficiency, anemia of chronic disease, and obesity: introducing hepcidin. J Acad Nutr Diet 112, 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El Hout M, Dos Santos L, Hamai A and Mehrpour M (2018) A promising new approach to cancer therapy: targeting iron metabolism in cancer stem cells. Semin Cancer Biol 53, 125–138. [DOI] [PubMed] [Google Scholar]
- 15. Han Z, Yu Y, Xu J, Bao Z, Xu Z, Hu J, Yu M, Bamba D, Ma W, Ding F et al (2018) Iron homeostasis determines fate of human pluripotent stem cells via glycerophospholipids‐epigenetic circuit. Stem Cells 37, 489–503. [DOI] [PubMed] [Google Scholar]
- 16. Bao Z, Han Z, Zhang B, Yu Y, Xu Z, Ma W, Ding F, Zhang L, Yu M, Liu S et al (2019) Arsenic trioxide blocked proliferation and cardiomyocyte differentiation of human induced pluripotent stem cells: implication in cardiac developmental toxicity. Toxicol Lett 309, 51–58. [DOI] [PubMed] [Google Scholar]
- 17. Nakamura T, Naguro I and Ichijo H (2019) Iron homeostasis and iron‐regulated ROS in cell death, senescence and human diseases. Biochim Biophys Acta Gen Subj 1863, 1398–1409. [DOI] [PubMed] [Google Scholar]
- 18. Chen C, Wang S and Liu P (2019). Deferoxamine enhanced mitochondrial iron accumulation and promoted cell migration in triple‐negative MDA‐MB‐231 breast cancer cells via a ROS‐dependent mechanism. Int J Mol Sci 20, 4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fang S, Yu X, Ding H, Han J and Feng J (2018) Effects of intracellular iron overload on cell death and identification of potent cell death inhibitors. Biochem Biophys Res Commun 503, 297–303. [DOI] [PubMed] [Google Scholar]
- 20. Yuan Y, Yan G, Gong R, Zhang L, Liu T, Feng C, Du W, Wang Y, Yang F, Li Y et al (2017) Effects of blue light emitting diode irradiation on the proliferation, apoptosis and differentiation of bone marrow‐derived mesenchymal stem cells. Cell Physiol Biochem 43, 237–246. [DOI] [PubMed] [Google Scholar]
- 21. Levi S and Taveggia C (2014) Iron homeostasis in peripheral nervous system, still a black box? Antioxid Redox Signal 21, 634–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tanaka H, Espinoza JL, Fujiwara R, Rai S, Morita Y, Ashida T, Kanakura Y and Matsumura I (2019) Excessive reactive iron impairs hematopoiesis by affecting both immature hematopoietic cells and stromal cells. Cells 8, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jin X, He X, Cao X, Xu P, Xing Y, Sui S, Wang L, Meng J, Lu W, Cui R et al (2018) Iron overload impairs normal hematopoietic stem and progenitor cells through reactive oxygen species and shortens survival in myelodysplastic syndrome mice. Haematologica 103, 1627–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muto Y, Nishiyama M, Nita A, Moroishi T and Nakayama KI (2017) Essential role of FBXL5‐mediated cellular iron homeostasis in maintenance of hematopoietic stem cells. Nat Commun 8, 16114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katsura Y, Ohara T, Noma K, Ninomiya T, Kashima H, Kato T, Sato H, Komoto S, Narusaka T, Tomono Y et al (2019) A novel combination cancer therapy with iron chelator targeting cancer stem cells via suppressing stemness. Cancers 11, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kimbrel EA and Lanza R (2015) Current status of pluripotent stem cells: moving the first therapies to the clinic. Nat Rev Drug Discov 14, 681–692. [DOI] [PubMed] [Google Scholar]
- 27. Bigarella CL, Liang R and Ghaffari S (2014) Stem cells and the impact of ROS signaling. Development 141, 4206–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang CK, Yang S‐C, Hsu S‐C, Chang F‐P, Lin Y‐T, Chen S‐F, Cheng C‐L, Hsiao M, Lu FLand Lu J (2017) CHAC2 is essential for self‐renewal and glutathione maintenance in human embryonic stem cells. Free Radic Biol Med 113, 439–451. [DOI] [PubMed] [Google Scholar]
- 29. Behrens A, van Deursen JM, Rudolph KL and Schumacher B (2014) Impact of genomic damage and ageing on stem cell function. Nat Cell Biol 16, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rossi DJ, Jamieson CH and Weissman IL (2008) Stems cells and the pathways to aging and cancer. Cell 132, 681–696. [DOI] [PubMed] [Google Scholar]
- 31. Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M and Alt FW (2005) DNA repair, genome stability, and aging. Cell 120, 497–512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 . DFO‐induced iron deficiency promotes ROS generation in hiPSCs. DCFH‐DA staining of AC‐IPSCs treated with 150 μm DFO for 24 h. Scale bar, 100 μm.
Table S1 . Primers used in quantitative RT‐PCR.
Table S2 . Antibodies used in this study.


