Abstract
Diapause is a state of arrested growth, which allows insects to adapt to diverse environments. Serine protease inhibitors (serpins) play an important role in various physiological processes, including blood coagulation, fibrinolysis, development, complement activation and extracellular matrix remodeling. We hypothesized that serpin may affect energy metabolism and thereby control diapause of migratory locust (Locusta migratoria) embryos by regulating protease cascades. A total of seven nonredundant serpin genes (named serpin1–serpin7) of L. migratoria were obtained through transcriptomic analysis. We further performed label‐free proteomic sequencing and analysis of diapause and nondiapause eggs of L. migratoria, revealing significant differences in serpin7 expression. A significant reduction in diapause rate under the short photoperiod was observed in insects treated with serpin7 double‐stranded RNA. Furthermore, knockdown of the serpin7 gene resulted in significant upregulation of the activity of polyphenol oxidase. We therefore propose that the observed serpin7 gene plays a crucial role in diapause, suggesting that control of energy metabolism may have potential as a future strategy for the reproductive control of insect pests.
Keywords: diapause, locust, Locusta migratoria, polyphenol oxidase, RNAi, serpin
Lm_Serpin7, a member of the Serpin protease inhibitor family, is highly expressed in diapause eggs and plays an important role in Locusta migratoria egg diapause. Knockdown of Lm_Serpin7 by injection with ds_serpin7 decreases egg diapause rate, suggesting that control of Lm_Serpin7 may have potential as a future strategy for the reproductive control of insect pests.
Abbreviations
- A
absorbance
- ddH2O
double‐distilled water
- dsRNA
double‐stranded RNA
- LB
lysogeny broth
- NJ
neighbor‐joining
- ORF
open reading frame
- pI
isoelectric point
- PO
phenol oxidase
- PPO
polyphenol oxidase
- proPO
prophenoloxidase
- RH
relative humidity
- RNAi
RNA interference
- RT‐qPCR
real‐time quantitative PCR
Diapause is an important behavior of insects that allows the insects to adapt to diversified environments. Migratory locust, Locusta migratoria L., belongs to the facultative diapause type of embryonic diapause insect [1, 2]. Temperature and photoperiod are the key factors affecting diapause of L. migratoria. The maternal L. migratoria can sense the environmental information and transmit this information in the form of signals to the offspring to cut off the developmental signal, which induces diapause of the eggs in the locusts [3].
Serine protease inhibitors (serpins) belong to the widely distributed protein family protease inhibitors [4, 5]. More than 1500 serpins have been extensively studied in animals, plants, bacteria and viruses [6]. Generally, the serpin could be 350–500 amino acids found in typical serpin, and irreversible inhibition occurs whenever serpin binds to its substrates [4, 7]. Studies showed that the serpins play an important role in blood coagulation, fibrinolysis, complement activation, inflammatory reactions, immunity, physiology, digestion, development and extracellular matrix remodeling [8, 9, 10, 11, 12]. Seven serpins were purified from hemolymph of tobacco moth, Ephestia elutella, that regulated the activation of phenol oxidase (PO) and played a role in immune defense [13, 14, 15]. In addition, serpins also play a crucial role in tissue synthesis and embryonic development of animals [16]. Spn27A regulates the formation of dorsal ventral axis of drosophila embryos in the early developmental stage by inhibiting the Toll signaling pathway [17, 18]. In addition, Spn88Ea is necessary for wing development in fruit flies [19]. Balance of SRP‐2 (serpin), a cross‐class inhibitor, is also important for postembryonic development of nematodes, Caenorhabditis elegans [20]. However, no extensive and comprehensive studies have been made on the effects of serpin in relation with insect diapause. Keeping in view the importance of serpins in other plants and animals, this novel study was designed to carry out the transcriptome analysis of diapause and nondiapause eggs of migratory locust, L. migratoria, especially for the serpin genes [21, 22]. We further performed label‐free proteomic sequencing on diapause and nondiapause eggs of migratory locust to understand the expression of serpin‐related genes [21, 22]. We hypothesized that serpin may affect energy metabolism and could control diapause of migratory locust embryos by regulating protease cascade reaction. To explore the role of serpin in diapause regulation, we performed RNA interference (RNAi)‐mediated silencing of serpin gene. We further planned to study the content of polyphenol oxidase (PPO) by applying RNAi of specific serpin gene. This study would provide a theoretical basis for further study on the diapause mechanism of the migratory locust through serpins.
Materials and methods
Insect materials
The L. migratoria L. colony used in this study was originally collected from the field at Cangzhou, Hebei, China (39°37′N, 98°30′E, 40 m above sea level) and was maintained by the State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chines Academy of Agricultural Sciences for successive years. Locust eggs were hatched in an artificial climate box (PRX‐250B‐30; Haishu Saifu Experimental Instrument Factory, Ningbo, China) at a temperature of around 30 °C with relative humidity of 60%. The photoperiodic regimen used for nondiapause locusts in the experiment was 16 h light : 8 h darkness. Similarly, to induce diapause, we reared locusts under a short photoperiod at 10 h light : 14 h darkness, 27 °C and 60% relative humidity [23, 24]. Freshly grown wheat seedlings were fed to the locusts in the laboratory.
Identification of serpin genes in the migratory locust
The transcriptome sequencing and analysis were performed on diapause and nondiapause eggs of migratory locusts in the State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chines Academy of Agricultural Sciences. A total of seven serpin genes (serpin1 to serpin7) were obtained. Molecular formula, molecular weight and isoelectric points of serpin proteins were analyzed by expasy software (Swiss Institute of Bioinformatics, Geneva, Switzerland). Meanwhile, we used wolf psort software (http://wolfpsort.org/) to predict the subcellular localization of serpins (Data S1).
Amino acid sequence alignment and construction of phylogenetic tree
dnaman software (version 7.212; Lynnon Corp., Quebec, QC, Canada) was used to translate the serpin sequence of migratory locust. We obtained the amino acid sequences of serpins’ open reading frame (ORF). Sequence alignment was performed between serpin amino acid sequences of the migratory locust (Data S2) and other serpins sequences of silkworm, Bombyx mori, and fruit fly, Drosophila melanogaster, published by Universal Protein Knowledgebase (UniProt). The neighbor‐joining (NJ) method was used to construct phylogenetic trees by mega 6.0 software (Molecular Evolutionary Genetics Analysis Version 6.0), and 1000 bootstrap tests were performed [25].
Advanced structure analysis of serpin proteins
Using the Self‐Optimized Prediction method With Alignment (SOPMA) online server (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_sopma.html), we analyzed the secondary structure of serpin proteins and predicted the tertiary structure of serpin proteins by expasy software (Swiss Institute of Bioinformatics).
Clone of serpin7 gene
Third‐instar nymph of locusts was dissected, and the digestive tract was clinically removed. The remaining tissues were used for the extraction of total RNA. TRIcom Reagent (Tianmo, Huailai, China) was used to extract RNA. Total RNA was isolated according to the manufacturer’s protocol. The quality was checked on a spectrophotometer with A 260/A 280 between 1.9 and 2.0, whereas the reliability of RNA was confirmed on 1% agarose gel, which gave three clear bands. cDNA was synthesized according to the PrimeScript™ II 1st strand cDNA Synthesis Kit (TaKaRa, Dalian, China). By analyzing transcriptome of the migratory locust, we obtained the sequence of serpin7 gene, and primers were then designed by dnaman software (version 7.212; Lynnon Corp.). Using cDNA of migratory locust as a template, we amplified the serpin7 gene by primers serpin7‐1F/serpin7‐1R. The PCR‐amplified fragment was 894 bp (Fig. 1). The obtained PCR product was purified by TIANgel Midi Purification Kit (Tiangen, Beijing, China) and was connected to the 1‐μL pMD19‐T vector (TaKaRa, Dalian, China), 6 μL solution (TaKaRa, Japan) and 3 μL DNA to incubate at room temperate for 6 h. Later, the recombinant was transformed into Escherichia coli Trans1‐T1 strain, and 500 μL LB (lysogeny broth) liquid medium was added. Notably, no restriction enzymes were used. The obtained product was allowed to shake at 200 r.p.m. at 37 °C for 2 h. A total of 100 μL bacterial solution was applied to LB solid medium, including 1% of ampicillin. The medium was incubated at 37 °C for 12 h. The recombinant colonies were transferred into liquid LB culture medium containing 1% ampicillin and were shaken for 3–6 h at 37 °C. Finally, the medium for PCR template was prepared. Primers for this particular study were synthesized by Sangon Biotech Co. Ltd. (Beijing, China) (Table 1).
Fig. 1.
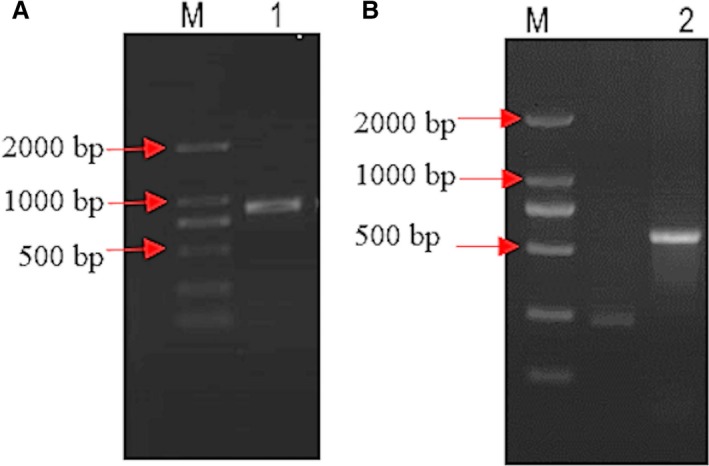
(A, B) Electrophoresis pattern of serpin7 by PCR and serpin7 dsRNA. 1, PCR fragment of serpin7 gene; 2, Serpin7 dsRNA; M, marker.
Table 1.
List of specific primers used and synthesized for this study.
| Primers | Primer sequences (5'‐3') | Intention |
|---|---|---|
| Serpin7‐1F | TTTCTCTCCAGCCAGCAT | Clone of serpin7 gene |
| Serpin7‐1R | GCTTCAGTGCCTTCCTCAT | |
| Serpin7‐2F | TAATACGACTCACTATAGG CTGCTCAGGAGATTGTAGAGG | Synthesis of serpin7 dsRNA |
| Serpin7‐2R | TAATACGACTCACTATAGG GACCATCATTCTTCTTTGGC | |
| Serpin7‐3F | GAAGGGAACTGATGACACGG | Serpin7 primer for RT‐qPCR |
| Serpin7‐3R | TCTTGCTCTCAACCCATTCA | |
| Easter‐F | CGCATCGGATACATCGGGT | Easter primer for RT‐qPCR |
| Easter‐R | TTCTTGAAGGCGGGCTTG | |
| Toll‐F | GGCTGTAATGAATGGGGAA | Toll primer for RT‐qPCR |
| Toll‐R | GTAAACTGGAACTGGTGCG | |
| Pelle‐F | CCAACACGGAGAATAGATAGT | Pelle primer for RT‐qPCR |
| Pelle‐R | TGGTAAAATTCCAAGGTAGA | |
| MyD88‐F | GGCTTCCTCCTCAGCATCT | MyD88 primer for RT‐qPCR |
| MyD88‐R | GACCTCCAACCAAATCACG | |
| Cactus‐F | CAGCGGTGCTTGCCTCTAC | Cactus primer for RT‐qPCR |
| Cactus‐R | TTTTCCTCCAACCTGTCCT | |
| Actin‐F | GTTACAAACTGGGACGACAT | Reference gene of RT‐qPCR |
| Actin‐R | AGAAAGCACAGCCTGAATAG |
Synthesis and injection of double‐stranded RNA of serpin7 gene
Recombinant plasmid including serpin7 gene fragment was extracted by using EZgene™ Plasmid Miniprep Kit (Biomiga, San Diego, CA, USA). Using the recombinant plasmid as template, the serpin7 gene was amplified by primers serpin7‐2F/serpin7‐2R (Table 1). The amplified PCR products were then purified with TIANgel Midi Purification Kit (Tiangen), followed by quantification through NanoPhotometer™ (Implen GmbH, Munchen, Germany). Serpin7 double‐stranded RNA (dsRNA) was synthesized using the T7 RiboMAX™ Express RNAi System Kit (Promega, Madison, WI, USA). The expected size of serpin7’s dsRNA was 602 bp (Fig. 1). dsRNA concentration of serpin7 was detected by a NanoPhotometer™ (Implen, GmbH, München, Germany), and the final concentration was adjusted to 1 μg·μL−1 for further analysis.
Twenty‐five female L. migratoria were selected from each photoperiod within 24 h after adults were injected with 10 μL serpin7 dsRNA (μg·μL−1) between the third and fourth abdominal segments. Double‐distilled water (ddH2O) as control was injected in a similar manner to the selected females. Dissecting the whole bodies of dsRNA‐injected and control group’s adult locusts after 36 h, we obtained hind leg, ovary and fat body. The efficiency of RNAi‐mediated knockdown was determined with real‐time quantitative PCR (RT‐qPCR).
RT‐qPCR
To check the efficiency of RNAi‐mediated knockdown in different tissues of L. migratoria, we dissected out the hind leg, ovary and fat body from each treatment. RNA was extracted from each sample using TRIcom Reagent (Tianmo) followed by estimating the RNA concentration through a NanoPhotometer™ (Implen, GmbH). For reverse transcription, 5 μL of total RNA was reverse transcribed with PrimeScript™ II 1st strand cDNA Synthesis Kit (TaKaRa, Dalian, China). To evaluate RNAi efficiency, we used primers serpin7‐3F/serpin7‐3R to amplify endogenous serpin7 gene on the ABI 7500 Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA). RT‐qPCR was performed with the Bester® SYBR Green qPCR MasterMix (DBI® Bioscience, Berlin, Germany). β‐actin was used as a reference gene in the study. A total of three technical replicates were set up. The relative mRNA level was calculated by method [26], where
ELISA
PPO activity was detected using Insect PPO ELISA Kit (Collodi Biotechnology Co., Ltd., Quanzhou, China) according to the manufacturer’s protocol. The standard curve was generated by plotting the average absorbance (A 450 nm) obtained for each of the six standard concentrations on the vertical (x) axis versus the corresponding concentration on the horizontal (y) axis. First, we calculated the mean A 450 value for each standard and sample. Later, all of the A 450 values were subtracted by the mean value of the blank well before interpretation of results. We constructed the standard curve using graph paper or statistical software. The A 450 value of the sample was substituted into the equation, and the concentration of the sample was then calculated.
Diapause rate
Remaining locusts were reared at 28 °C, until eggs laying. The numbers of hatched nymph of locusts (D 1) and the unhatched eggs (D 2) were counted, and the diapause rate (%) was calculated accordingly:
Statistical analysis
Independent samples t‐test was used for measuring mRNA levels, enzyme activities and diapause rate. Statistically significant differences were considered on an error probability of P < 0.05. Data are presented as means ± SE. Asterisks on the bars in the figures represent significant differences among the treatments and control. Statistical analyses were performed using spss software version 16.0 (SPSS Inc., Chicago, IL, USA), whereas graphpad prism software version 6.01 (GraphPad Software Inc., San Diego, CA, USA) was used for constructing the graphs.
Results
Identification of the serpin genes
Seven nonredundant serpin protein sequences were identified by transcriptome sequencing of diapause and nondiapause eggs in the migratory locust and were respectively named from serpin1 to serpin7 (Table 2). The encoding gene of serpin proteins ranged from 987 bp (serpin1) to 1500 bp (serpin5). The isoelectric point (pI) was between 5.13 (serpin3) and 8.36 (serpin4). Similarly, prediction of subcellular localization was performed by wolf psort software (http://wolfpsort.org/). Higher value means accurate prediction. After analysis, serpin1 was mainly distributed in the endoplasmic reticulum, nucleus and cytoplasm followed by a small amount of distribution in mitochondria and peroxisome. Among them, four proteins were predicted to be endoplasmic reticulum localization, including serpin2, serpin3, serpin5 and serpin6. Moreover, two of the serpin proteins were predicted to be localized in cytoplasm, including serpin4 and serpin7 (Table 3).
Table 2.
Characteristics and features of serpins in L. migratoria.
| Protein name | ORF | Amino acids | Molecular formula | Molecular weight | Theoretical pI |
|---|---|---|---|---|---|
| Serpin1 | 987 | 328 | C1655H2623N445O492S9 | 36.92 | 5.9 |
| Serpin2 | 1164 | 387 | C1945H3078N490O559S18 | 42.85 | 6.73 |
| Serpin3 | 1155 | 384 | C1966H3056N494O569S13 | 43.13 | 5.13 |
| Serpin4 | 1176 | 391 | C1997H3117N519O565S14 | 43.89 | 8.36 |
| Serpin5 | 1500 | 499 | C2543H3960N664O739S8 | 55.92 | 5.91 |
| Serpin6 | 1446 | 481 | C2402H3794N640O728S11 | 53.64 | 5.86 |
| Serpin7 | 1161 | 386 | C1933H3106N494O567S20 | 42.98 | 6.08 |
Table 3.
Subcellular localization and prediction of serpins using wolf psort software.
| Protein name | Plasma membrane | Endoplasmic reticulum | Nucleus | Cytoplasm | Mitochondria | Peroxisome | Lysosome | Secreted |
|---|---|---|---|---|---|---|---|---|
| Serpin1 | – | 8 | 9 | 6.5 | 3 | 2.5 | – | 2 |
| Serpin2 | 1 | 16 | 3 | – | – | 4 | – | 2 |
| Serpin3 | 2 | 10 | 4 | 5 | 1 | – | – | 6 |
| Serpin4 | 4 | 5 | – | 14 | 2 | 5 | – | 2 |
| Serpin5 | 7 | 11 | – | – | – | 1 | 2 | 11 |
| Serpin6 | 7 | 12 | – | – | – | 1 | 3 | 9 |
| Serpin7 | – | – | – | 22.5 | 8 | – | – | – |
Structure and phylogenetic tree of serpin protein
Amino acid sequence alignment of seven serpin proteins was performed using dnaman software (version 7.212; Lynnon Corp.). High similarity among seven serpin proteins was found (Fig. 2). The phylogenetic tree was constructed by comparing the amino acid sequences of seven serpin proteins of migratory locust (Data S2) using the NJ method (Fig. 3). Two of the serpin proteins matched with fruit fly, D. melanogaster, and two with silkworm, B. mori. Through phylogenetic analysis, it was found that serpin5 in the migratory locust is closely related to serpin27A of D. melanogaster, whereas the relationship between serpin1, serpin7, serpin3 and serpin4 was relatively close; however, among them, the relationship between serpin1 and serpin3 was much closer. Serpin1 was mainly distributed in the endoplasmic reticulum, nucleus and cytoplasm followed by a small amount of distribution in mitochondria and peroxisome predicted by wolf psort software (http://wolfpsort.org/).
Fig. 2.
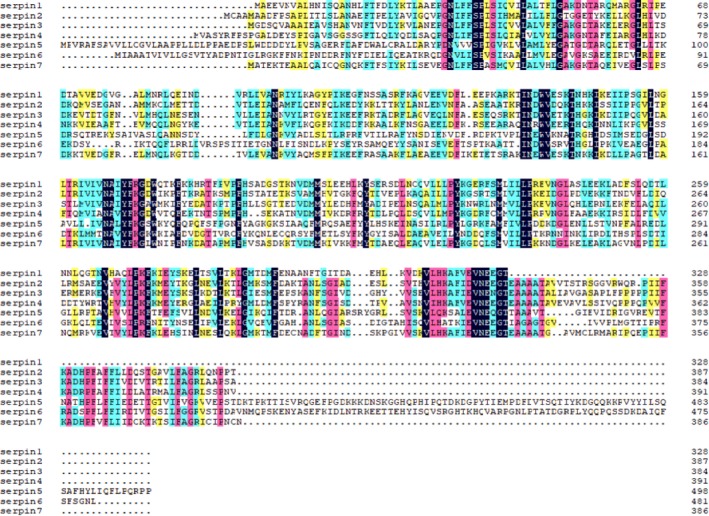
Homology of serpin proteins in L. migratoria constructed by using dnaman software.
Fig. 3.
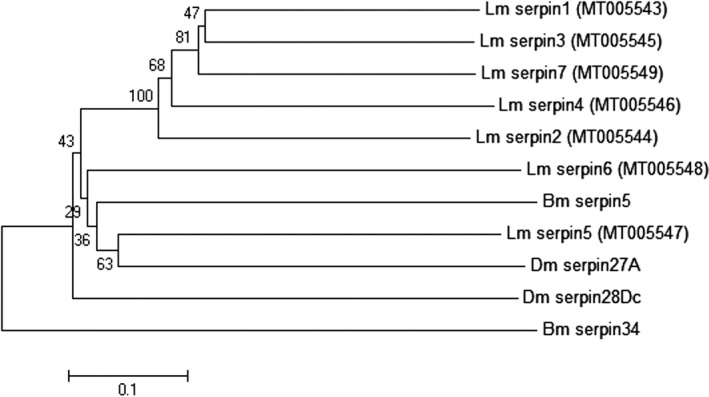
Phylogenetic tree of insect serpin proteins constructed by mega 6.0 and the NJ method. A species acronym is added before the name of each serpin protein, whereas Dm, Bm and Lm, respectively, represent D. melanogaster, B. mori and L. migratoria. GenBank accession numbers were provided for our nucleotide sequences, where serpin1, serpin2, serpin3, serpin4, serpin5, serpin6 and serpin7 are respectively numbered MT005543, MT005544, MT005545, MT005546, MT005547, MT005548 and MT005549.
Analysis of the advanced structure of serpin proteins in the migratory locust
The secondary and tertiary structures of the seven serpin proteins (serpin1–serpin7) were analyzed by the SOPMA online server and expasy software (Table 4; Fig. 4). There was a slight difference in the ratio of four secondary structures (α‐helix, extended strand, β‐turn and random coil) among the serpin proteins, in which the amino acids proportion of α‐helix was 34.79–54.27%, whereas β‐turn was 4.58–7.16%. Similarly, the amino acids proportion of extended chains was 10.37–16.80%. Moreover, the ratio of random coils ranged from 28.96% to 46.67%. Furthermore, secondary structures of α‐helix and random coils were dominant in serpin proteins. The tertiary structure of serpin was similar with three β‐turn and eight to nine α‐helices (Fig. 4).
Table 4.
Secondary structure of the serpin proteins in L. migratoria.
| Number/Percentage | ||||
|---|---|---|---|---|
| Random coil | α‐Helix | Extended strand | β‐Turn | |
| Serpin1 | 101/30.79 | 168/51.22 | 39/11.89 | 20/6.10 |
| Serpin2 | 131/33.85 | 175/45.22 | 60/15.50 | 21/5.43 |
| Serpin3 | 128/33.33 | 164/42.71 | 69/17.97 | 23/5.99 |
| Serpin4 | 138/35.29 | 182/46.55 | 56/14.32 | 15/3.84 |
| Serpin5 | 217/43.49 | 177/35.47 | 75/15.03 | 30/6.01 |
| Serpin6 | 215/44.70 | 180/37.42 | 67/13.93 | 19/3.95 |
| Serpin7 | 138/35.75 | 170/44.04 | 59/15.28 | 19/4.92 |
Fig. 4.
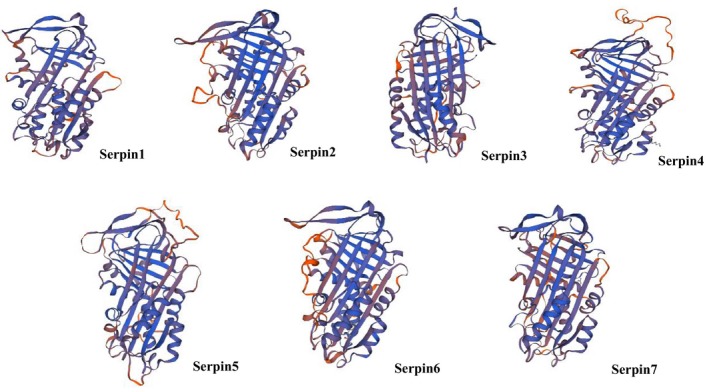
Tertiary structures of the serpin proteins obtained through expasy. Serpin1: α‐helix, 54.27%; extended strand, 10.37%; β‐turn, 6.40%; random coils, 28.96%. Serpin2: α‐helix, 45.99%; extended strand, 16.80%; β‐turn, 5.43%; random coils, 31.78%. Serpin3: α‐helix, 45.57%; extended strand, 15.36%; β‐turn, 6.25%; random coils, 32.81%. Serpin4: α‐helix, 44.50%; extended strand, 14.07%; β‐turn, 7.16%; random coils, 34.27%. Serpin5: α‐helix, 35.47%; extended strand, 16.23%; β‐turn, 6.01%; random coils, 42.28%. Serpin6: α‐helix, 34.79%; extended strand, 13.96%; β‐turn, 4.58%; random coils, 46.67%. Serpin7: α‐helix, 46.11%; extended strand, 16.32%; β‐turn, 5.96%; random coils, 31.61%.
RNAi efficiency
To verify the function of serpin7 on regulating locust diapause, we synthesized and subsequently injected dsRNA of serpin7 into female adults of L. migratoria to RNAi the serpin7 under long and short photoperiods. RNAi efficiency of serpin7 was confirmed through RT–qPCR. The results showed that the mRNA level of serpin7 gene in hind leg and fat body was significantly different as compared with control (P < 0.05) under both long (relative humidity [RH] 60%; 30 °C; 16 h light:8 h darkness) and short (RH 60%; 27 °C; 10 h light:14 h darkness) photoperiods (Fig. 5). The results further confirmed that the serpin7 gene was successfully interfered under two photoperiods. Under the long photoperiod, mRNA level of serpin7 gene in the hind leg decreased by 95.98% compared with that of the control, whereas mRNA level of serpin7 gene in fat body decreased by 99.08%. Furthermore, under the short photoperiod, mRNA level of serpin7 gene in hind leg decreased by 99.14% compared with that of the control, whereas mRNA level of serpin7 gene in fat body decreased by 93.85%.
Fig. 5.
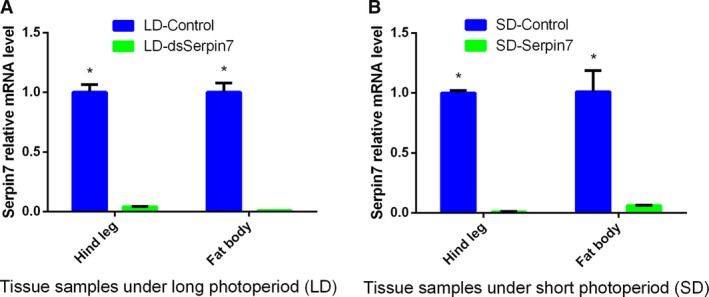
Relative mRNA levels of serpin7 after RNAi under two photoperiodic regimens: (A) long photoperiod and (B) short photoperiod. Three replicates are used for each treatment. Asterisks (*) indicate a standard error probability of P < 0.05 by Student’s t‐test.
Effects of serpin7 RNAi on the Toll pathway
dsserpin7 and ddH2O were injected into the female locusts within 24 h after adulthood under both long and short photoperiods. Locusts were dissected after 36 h to collect the fat body. The mRNA relative level of Easter, Toll, Pelle, MyD88 and Cactus genes in the Toll pathway was checked (Fig. 6). Under the long photoperiod, the mRNA level of Easter in fat body was significantly up‐regulated (P < 0.05), whereas the mRNA levels of Pelle, MyD88 and Cactus in fat body were significantly down‐regulated (P < 0.05). Similarly, the mRNA level of Toll gene in the treatment group was down‐regulated as compared with the control group, but with no significant difference. Under the short photoperiod, the variation trend of gene levels was similar to that of the long photoperiod. The mRNA relative levels of Toll, Pelle, MyD88 and Cactus genes were significantly lower than those of the control group (P < 0.05), whereas the mRNA level of Easter gene was increased but did not reach a level of significant difference.
Fig. 6.
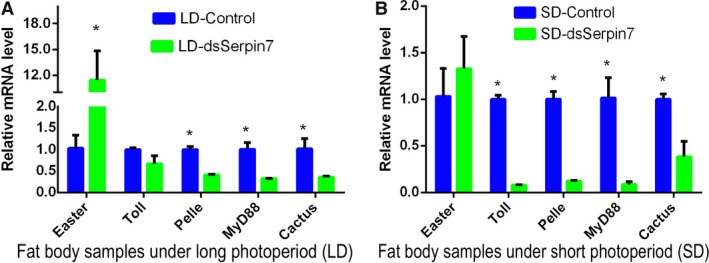
Data acquired from fat body samples and relative mRNA level of genes in the Toll pathway after serpin7 RNAi under two photoperiodic regimens: (A) long photoperiod and (B) short photoperiod. Three replicates are used for each treatment. Asterisks (*) indicate a standard error (SE) probability of P < 0.05 by Student’s t‐test.
Effect of RNAi‐mediated silencing of serpin7 gene on activity of PPO
Under long and short photoperiods, the female adults were injected with dsRNA of serpin7 and ddH2O, respectively. After 36 h, the female locusts were dissected to obtain the hind legs, fat bodies and ovaries. The PPO content was detected (Fig. 7). Results showed that the content of PPO in the hind leg, fat body and ovary significantly increased (P < 0.05) after serpin7 RNAi under both long and short photoperiods.
Fig. 7.
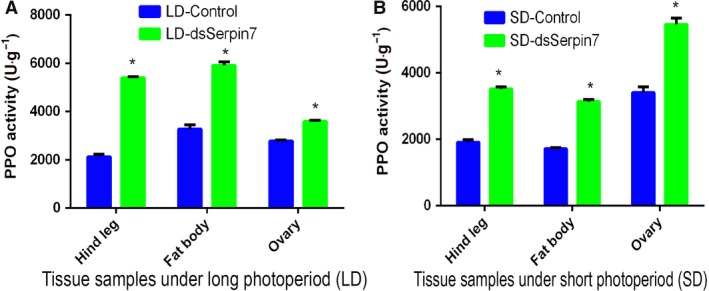
PPO activity in the migratory locust after serpin7 RNAi under two photoperiodic regimens: (A) long photoperiod and (B) short photoperiod. Three replicates are used for each treatment. Asterisks (*) indicate a standard error probability of P < 0.05 by Student’s t‐test.
Diapause rate
Under the long photoperiod, RNAi‐mediated silencing by injecting serpin7 dsRNA had no effect on diapause rate. However, under the short photoperiod, diapause rate (%) of the individuals injected with serpin7 dsRNA was significantly reduced (P < 0.05). Diapause rate in the dsserpin‐injected group was 84.19%, which was 13.25% lower than that of the control group. The result suggests that serpin7 may play a modulatory role in egg diapause of the migratory locust (Fig. 8).
Fig. 8.
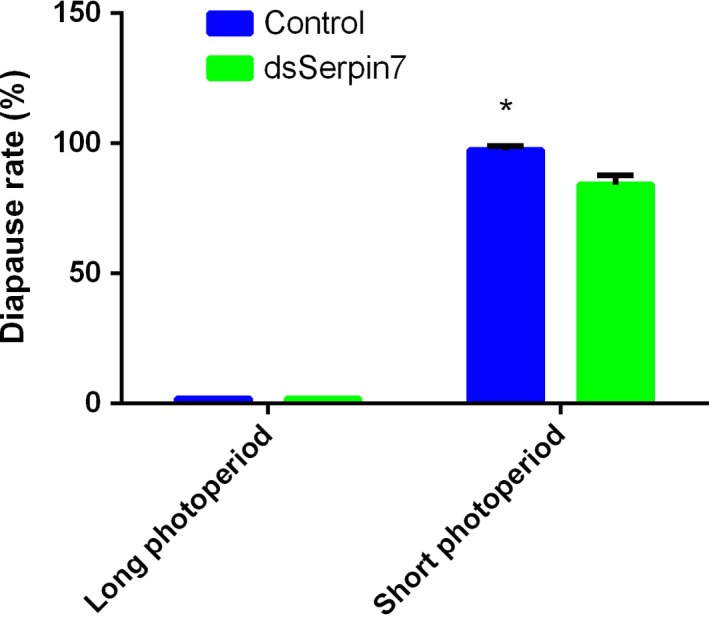
Diapause rate of migratory locusts by injecting dsserpin7 along with control under both long and short photoperiods. Three replicates are used for each treatment. Asterisk (*) indicates a standard error probability of P < 0.05 by Student’s t‐test.
Discussion
Serpins are a superfamily of proteases found in serine protease inhibitors, usually consisting of 350–400 amino acids [4]. At the C terminus of serpin, there is a reactive central ring exposed outside the main body [27]. Sequence of serpin is highly conserved, but its function is highly differentiated; that is why serpin has an irreversible inhibitory effect with a suicidal nature [7], just like trypsin inhibitors, which affect the growth, development and survival of insects [28]. Serpins play an important role in innate immunity of insects [19]. Currently, a negatively correlated serpin was found to be associated with the Toll signaling pathway of fruit flies [29, 30], and other serpins were mostly involved in melanization caused by PO cascade [31, 32]. In addition, a specific group of serpins is involved in tissue synthesis and embryonic development [17, 18, 20].
Serpins regulate some innate immune responses of insects by inhibiting endogenous protease [33]. In insects, serpins have been identified in several species, but extensive studies of insect serpins are mainly focused on fruit fly, D. melanogaster, and tobacco horn worm, Manduca sexta. So far, six M. sexta serpins have been characterized and shown to be inhibitory [13, 34, 35, 36, 37]. To investigate the effect of serpin gene on diapause of migratory locust, we subsequently cloned serpin7 of the migratory locust in this particular study. The ORF of serpin7 gene was 1161 bp, encoding 386 amino acid residues, and had the molecular formula of C1933H3106N494O567S20. The subcellular localization of serpin7 was predicted to be cytoplasmic and mitochondrial. Meanwhile, the molecular mass of the protein was 42.98 kDa, which was similar to that of serpin’s superfamily protein [38]. The theoretical pI of serpin7 was 6.08, and there was no signal peptide. It belongs to the serpin superfamily and was a typical inhibitory serpin. Multiple sequence alignment showed that seven serpin amino acid sequences of the migratory locust showed high similarity (Fig. 2). Phylogenetic analysis was performed on serpin amino acid sequences by the NJ method, including seven serpin proteins in the migratory locust, two serpin proteins in fruit flies and two serpin proteins in silkworms. We observed a close relationship between serpin1 and serpin7 of migratory locusts. Moreover, serpin5 of migratory locusts was closely related to that of serpin27A of Drosophila [39, 40]. In addition, serpin6 of the migratory locust was closely related to serpin28Dc of Drosophila [41]. Furthermore, serpin1, serpin7, serpin3 and serpin4 were closely related with each other (Fig. 3).
Down‐regulation of the expression of specific genes through RNAi has been widely used in entomological research for functional genomics in a variety of insects, and its potential for RNAi‐based pest control has been increasingly emphasized mainly because of its high specificity [42, 43]. We used the RNAi technology to mediate silencing of serpin7 gene of migratory locust for elaborating the effect of serpin on diapause of locust [23, 24, 44]. The RNAi efficiency of serpin7 was checked by RT–qPCR, and the results revealed successful knocking down of serpin7 gene. Our results showed the 100% hatching of all eggs under the long photoperiod, for both treated and untreated (control) groups with zero diapause. In contrast, under the short photoperiod, diapause rate of the individuals injected with serpin7 dsRNA was significantly reduced as compared with the control group. Results suggested that maternal serpin7 promotes the egg diapause process of L. migratoria. Serpins are an important regulator in PO cascade reaction [32, 39, 45]. To further understand the regulatory mechanism, we checked the effect of serpin7 RNAi on PPO activity in L. migratoria. Results showed that PPO activity increased significantly in the hind leg, fat body and ovary after serpin7 RNAi. PO plays an important role in melanization [31, 32] and synthesis of antimicrobial peptides, where the proPO mainly exists as an inactive precursor [46, 47]. proPO regulates various downstream factors, such as protease, protease inhibitors in Drosophila and Mandu casexta [48, 49]. We speculated that serpin7 negatively regulates PPO and affects diapause of migratory locust eggs by PO cascade reaction. In addition, we examined the effect of serpin7 gene RNAi on Toll pathway gene of the migratory locust. The results showed that after serpin7 gene RNAi under long and short photoperiods, the mRNA level of Easter increased in fat body, whereas mRNA levels of Toll, Pelle, MyD88 and Cactus genes decreased compared with the control group in fat body.
Conclusions
RNAi of serpin7 affected PPO activities in fat, hind leg and ovary of L. migratoria that ultimately revealed the possible role of serpin7 in locust diapause. Serpin7 may also be involved in the cascade reaction of the Toll signaling pathway, which needs to be further verified.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
JC, DC and HU conceptualized the study. KH performed formal analysis. JC, DC and KH were involved in the investigation. KH and DC contributed to the methodology of the study. XT and ZZ were involved in project administration. DC and HU wrote the original draft. KH and HU contributed to the writing, reviewing and editing of the manuscript.
Supporting information
Data S1. Selected serpins nucleic acid sequences.
Data S2. Selected serpins amino acid sequences.
Acknowledgements
We are thankful to Mark Richard McNeill (Ag Research, Lincoln Research Centre, Christchurch, New Zealand) for invaluable suggestions on manuscript organization and linguistic revision. Funding was provided by the China Agriculture Research System (CARS‐34‐07B) and National Natural Science Foundation of China of P.R. China (Grant No. 31672485). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Jun Chen, Dongnan Cui and Hidayat Ullah contributed equally to this article
Contributor Information
Xiongbing Tu, Email: xbtu@ippcaas.cn.
Zehua Zhang, Email: zhangzehua@caas.cn.
Data accessibility
The sequences of Serpin1, Serpin2, Serpin3, Serpin4, Serpin5, Serpin6 and Serpin7 were deposited in GenBank with the accession numbers MT005543, MT005544, MT005545, MT005546, MT005547, MT005548 and MT005549, respectively.
References
- 1. Uvarov BP (1966) Grasshoppers and Locusts, Vol. 1 Cambridge University Press, Cambridge, London. [Google Scholar]
- 2. Uvarov BP (1977) Grasshoppers and Locusts, Vol. 2 Centre for Overseas Pest Research, Cambridge University Press, Cambridge, London. [Google Scholar]
- 3. Jarwar AR, Hao K, Bitume EV, Ullah H, Cui D, Nong X, Wang G, Tu X and Zhang Z (2019) Comparative transcriptomic analysis reveals molecular profiles of central nervous system in maternal diapause induction of Locusta migratoria . G3 (Bethesda) 9, 3287–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Irving JA, Pike RN, Lesk AM and Whisstock JC (2000) Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res 10, 1845–1864. [DOI] [PubMed] [Google Scholar]
- 5. Rawlings ND, Tolle DP and Barrett AJ (2004) MEROPS: the peptidase database. Nucleic Acids Res 32, D160–D164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Law RH, Zhang Q, McGowan S, Buckle AM, Silverman GA, Wong W, Rosado CJ, Langendorf CG, Pike RN, Bird PI et al (2006) An overview of the serpin superfamily. Genome Biol 7, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gettins PG (2002) Serpin structure, mechanism, and function. Chem Rev 102, 4751–4804. [DOI] [PubMed] [Google Scholar]
- 8. Abraham EG, Pinto SB, Ghosh A, Vanlandingham DL, Budd A, Higgs S, Kafatos FC, Jacobs‐Lorena M and Michel K (2005) An immune‐responsive serpin, SRPN6, mediates mosquito defense against malaria parasites. Proc Natl Acad Sci USA 102, 16327–16332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gulley MM, Zhang X and Michel K (2013) The roles of serpins in mosquito immunology and physiology. J Insect Physiol 59, 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silverman GA, Whisstock JC, Bottomley SP, Huntington JA, Kaiserman D, Luke CJ, Pak SC, Reichhart JM and Bird PI (2010) Serpins flex their muscle I. Putting the clamps on proteolysis in diverse biological systems. J Biol Chem 285, 24299–24305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pauchet Y, Wilkinson P, Vogel H, Nelson DR, Reynolds SE, Heckel DG and Ffrench‐Constant RH (2010) Pyrosequencing the Manduca sexta larval midgut transcriptome: messages for digestion, detoxification and defence. Insect Mol Biol 19, 61–75. [DOI] [PubMed] [Google Scholar]
- 12. Richardson J, Viswanathan K and Lucas A (2006) Serpins, the vasculature, and viral therapeutics. Front Biosci 11, 1042–1056. [DOI] [PubMed] [Google Scholar]
- 13. Tong Y, Jiang H and Kanost MR (2005) Identification of plasma proteases inhibited by Manduca sexta serpin‐4 and serpin‐5 and their association with components of the prophenol oxidase activation pathway. J Biol Chem 280, 14932–14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanost MR (2007) Serpins in a lepidopteran insect, Manduca sexta In Molecular and Cellular Aspects of the Serpinopathies and Disorders in Serpin Activity (Silverman GA. and Lomas DA, eds), pp. 229–241. World Scientific Publishing Co., Hackensack, NJ. [Google Scholar]
- 15. Jiang H (2008) The biochemical basis of antimicrobial responses in Manduca sexta . Insect Sci 15, 53–66. [Google Scholar]
- 16. Jenkins G (2008) The role of proteases in transforming growth factor‐β activation. Int J Biochem Cell Biol 40, 1068–1078. [DOI] [PubMed] [Google Scholar]
- 17. Hashimoto C, Kim DR, Weiss LA, Miller JW and Morisato D (2003) Spatial regulation of developmental signaling by a serpin. Dev Cell 5, 945–950. [DOI] [PubMed] [Google Scholar]
- 18. Ligoxygakis P, Roth S and Reichhart JM (2003) A serpin regulates dorsal‐ventral axis formation in the Drosophila embryo. Curr Biol 13, 2097–2102. [DOI] [PubMed] [Google Scholar]
- 19. Reichhart JM, Gubb D and Leclerc V (2011) The Drosophila serpins: multiple functions in immunity and morphogenesis In Methods in Enzymology (Whisstock JC. and Bird PI, eds), Vol. 499, pp. 205–225. Academic Press, Cambridge, MA. [DOI] [PubMed] [Google Scholar]
- 20. Pak SC, Kumar V, Tsu C, Luke CJ, Askew YS, Askew DJ, Mills DR, Brömme D and Silverman GA (2004) SRP‐2 is a cross‐class inhibitor that participates in postembryonic development of the nematode Caenorhabditis elegans initial characterization of the clade l serpins. J Biol Chem 279, 15448–15459. [DOI] [PubMed] [Google Scholar]
- 21. Tu X, Wang J, Hao K, Whitman DW, Fan Y, Cao G and Zhang Z (2015) Transcriptomic and proteomic analysis of pre‐diapause and non‐diapause eggs of migratory locust, Locusta migratoria L. (orthoptera: acridoidea). Sci Rep 5, 11402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hao K, Wang J, Tu X, Whitman DW and Zang Z (2017) Transcriptomic and proteomic analysis of, Locusta migratoria, eggs at different embryonic stages: comparison for diapause and non‐diapause regimes. J Integr Agric 16, 1777–1788. [Google Scholar]
- 23. Hao K, Jarwar AR, Ullah H, Tu X, Nong X and Zhang Z (2019) Transcriptome sequencing reveals potential mechanisms of the maternal effect on egg diapause induction of Locusta migratoria . Int J Mol Sci 20, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hao K, Tu X, Ullah H, McNeill MR and Zhang Z (2019) Novel Lom‐dh genes play potential role in promoting egg diapause of Locusta migratoria L. Front Physiol 10, 767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tamura K, Stecher G, Peterson D, Filipski A and Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30, 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rau JC, Beaulieu LM, Huntington JA and Church FC (2007) Serpins in thrombosis, hemostasis and fibrinolysis. J Thromb Haemost 5, 102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Livak KJ and Schmittgen TD (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- 28. Zhao H, Ullah H, McNeill MR, Du G, Hao K, Tu X and Zhang Z (2019) Inhibitory effects of plant trypsin inhibitors Msti‐94 and Msti‐16 on Therioaphis trifolii (Monell) (Homoptera: Aphididae) in Alfalfa. Insects 10, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levashina EA, Langley E, Green C, Gubb D, Ashburner M, Hoffmann JA and Reichhart JM (1999) Constitutive activation of toll‐mediated antifungal defense in serpin‐deficient Drosophila . Science 285, 1917–1919. [DOI] [PubMed] [Google Scholar]
- 30. Reichhart JM (2005) Tip of another iceberg: Drosophila serpins. Trends Cell Biol 15, 659–665. [DOI] [PubMed] [Google Scholar]
- 31. Jiang R, Kim EH, Gong JH, Kwon HM, Kim CH, Ryu KH, Park JW, Kurokawa K, Zhang J, Gubb D et al (2009) Three pairs of protease‐serpin complexes cooperatively regulate the insect innate immune responses. J Biol Chem 284, 35652–35658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang H (2009) Regulation and function of the melanization reaction in Drosophila . Fly 3, 105–111. [DOI] [PubMed] [Google Scholar]
- 33. Söderhäll I, Wu C, Novotny M, Lee BL and Söderhäll K (2009) A novel protein acts as a negative regulator of prophenoloxidase activation and melanization in the freshwater crayfish Pacifastacus leniusculus . J Biol Chem 284, 6301–6310. [DOI] [PubMed] [Google Scholar]
- 34. Jiang H and Kanost MR (1997) Characterization and functional analysis of 12 naturally occurring reactive site variants of serpin‐1 from Manduca sexta . J Biol Chem 272, 1082–1087. [DOI] [PubMed] [Google Scholar]
- 35. Gan H, Wang Y, Jiang H, Mita K and Kanost MR (2001) A bacteria‐induced, intracellular serpin in granular hemocytes of Manduca sexta . Insect Biochem Mol Biol 31, 887–898. [DOI] [PubMed] [Google Scholar]
- 36. Wang Y and Jiang H (2004) Purification and characterization of Manduca sexta serpin‐6: a serine proteinase inhibitor that selectively inhibits prophenoloxidase‐activating proteinase‐3. Insect Biochem Mol Biol 34, 387–395. [DOI] [PubMed] [Google Scholar]
- 37. Zhu Y, Wang Y, Gorman MJ, Jiang H and Kanost MR (2003) Manduca sexta serpin‐3 regulates prophenoloxidase activation in response to infection by inhibiting prophenoloxidase‐activating proteinases. J Biol Chem 278, 46556–46564. [DOI] [PubMed] [Google Scholar]
- 38. Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, Irving JA, Lomas DA, Luke CJ, Moyer RW et al (2001) The serpins are an expanding superfamily of structurally similar but functionally diverse proteins evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem 276, 33293–33296. [DOI] [PubMed] [Google Scholar]
- 39. De Gregorio E, Han SJ, Lee WJ, Baek MJ, Osaki T, Kawabata SI, Lee BL, Iwanaga S, Lemaitre B and Brey PT (2002) An immune‐responsive serpin regulates the melanization cascade in Drosophila . Dev Cell 3, 581–592. [DOI] [PubMed] [Google Scholar]
- 40. Moussian B and Roth S (2005) Dorsoventral axis formation in the Drosophila embryo‐shaping and transducing a morphogen gradient. Curr Biol 15, R887–R899. [DOI] [PubMed] [Google Scholar]
- 41. Shakeel M, Xu X, De Mandal S and Jin F (2019) Role of serine protease inhibitors in insect‐host‐pathogen interactions. Arch Insect Biochem Physiol 102, e21556. [DOI] [PubMed] [Google Scholar]
- 42. Price DR and Gatehouse JA (2008) RNAi‐mediated crop protection against insects. Trends Biotechnol 26, 393–400. [DOI] [PubMed] [Google Scholar]
- 43. Yu N, Christiaens O, Liu J, Niu J, Cappelle K, Caccia S, Huvenne H and Smagghe G (2013) Delivery of dsRNA for RNAi in insects: an overview and future directions. Insect Sci 20, 4–14. [DOI] [PubMed] [Google Scholar]
- 44. Han P, Fan J, Liu Y, Cuthbertson AG, Yan S, Qiu BL and Ren S (2014) RNAi‐mediated knockdown of serine protease inhibitor genes increases the mortality of Plutella xylostella challenged by destruxin A. PLoS ONE 9, e97863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tang H, Kambris Z, Lemaitre B and Hashimoto C (2008) A serpin that regulates immune melanization in the respiratory system of Drosophila . Dev Cell 15, 617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kan H, Kim CH, Kwon HM, Park JW, Roh KB, Lee H, Park BJ, Zhang R, Zhang J, Söderhäll K et al (2008) Molecular control of phenoloxidase‐induced melanin synthesis in an insect. J Biol Chem 283, 25316–25323. [DOI] [PubMed] [Google Scholar]
- 47. Buchon N, Poidevin M, Kwon HM, Guillou A, Sottas V, Lee BL and Lemaitre B (2009) A single modular serine protease integrates signals from pattern‐recognition receptors upstream of the Drosophila Toll pathway. Proc Natl Acad Sci USA 106, 12442–12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lemaitre B and Hoffmann J (2007) The host defense of Drosophila melanogaster . Annu Rev Immunol 25, 697–743. [DOI] [PubMed] [Google Scholar]
- 49. Ling E, Rao XJ, Ao JQ and Yu XQ (2009) Purification and characterization of a small cationic protein from the tobacco hornworm Manduca sexta . Insect Biochem Mol Biol 39, 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Selected serpins nucleic acid sequences.
Data S2. Selected serpins amino acid sequences.
Data Availability Statement
The sequences of Serpin1, Serpin2, Serpin3, Serpin4, Serpin5, Serpin6 and Serpin7 were deposited in GenBank with the accession numbers MT005543, MT005544, MT005545, MT005546, MT005547, MT005548 and MT005549, respectively.


