Abstract
Background:
Biphasic tumors of the central nervous system are rarely described and mainly consisted out of the glial and mesenchymal component. The tumor originated out of both astrocytes and pinealocytes, best to our knowledge, has not been described. We present a case of a brain tumor consisted out of pilocytic astrocytoma (PA) and pineocytoma as components situated in the pineal region and posterior cranial fossa in young adult.
Case Description:
We present a 21-year-old patient with a history of intermittent headache, followed by nausea and vomiting, double vision, and dextropulsion. Magnetic resonance imaging revealed an extensive cystic-solid expansive formation in the posterior cranial fossa with a solid part in the area of the pineal gland. The patient underwent surgical resection. The pathohistological analysis showed two types of tumor cells; the major part of tumor showed features of PA, while minor part corresponded to pineocytoma.
Conclusion:
PA accounts for 5% of all gliomas and is most common in children and young adults. It usually occurs in the cerebellum, the optic pathway, third ventricular region, etc. Pineocytomas are rare, accounting up to 1% of all intracranial tumors. Since tumors origin is different, there must be complex molecular events or mutations that can lead to cell rearrangements and generation of two histologically different tissues in the same tumor mass. The course of treatment options is different for PA and pineocytoma; therefore, the case of brain mass consisted out of two different tissues can be helpful when deciding about the treatment of tumors in posterior cranial fossa and pineal region.
Keywords: Biphasic tumor, Brain tumor, Pilocytic astrocytoma, Posterior cranial fossa, Pyneocitoma

INTRODUCTION
The central nervous system (CNS) biphasic tumors occur rarely. According to the literature, only few cases of biphasic tumor have been described.[4,6,7] Pilocytic astrocytoma (PA) is a rare, slowly growing glioma, makes up approximately 5% of all gliomas, and is most common in children and young adults. PA can arise anywhere in the CNS, although it most frequently occurs in the cerebellum.[2,3,9] Pineocytomas are rare, relatively benign tumor, accounting for 0.4–1% of all intracranial tumors that arise from pineal parenchymal cells.[1,5,8] We present a unique case of brain tumor consisted out of PA and pineocytoma as tumor components situated in the pineal region and posterior cranial fossa in young adult.
CASE DESCRIPTION
History
A 21-year-old male patient presented with a 2 weeks history of intermittent headache followed by nausea and vomiting, double vision, discrete horizontal nystagmus bilaterally, and discrete dextropulsion. Initial computerized tomography (CT) and magnetic resonance imaging (MRI) revealed an extensive cystic-solid expansive formation in the posterior cranial fossa with a solid part in the area of the pineal gland. After the administration of intravenous contrast, the solid mass showed heterogeneous enhancement [Figure 1]. Neuroradiologically, differential diagnoses included hemangioblastoma.
Figure 1:
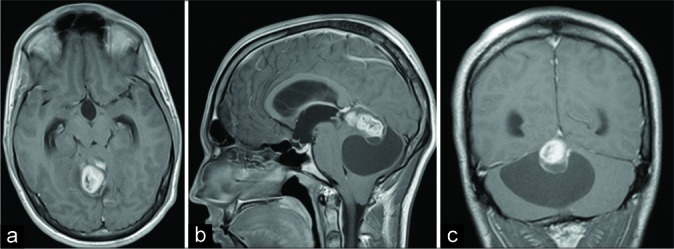
Initial brain magnetic resonance imaging showing right- sided extra-axial high parietal parasagittal mass in (a) transverse plain, T1-weighted image; (b) sagittal plain, T1-weighted image; and (c) coronal plain, T1-weighted image. Note the marginal heterogeneous enhancement of the tumor after the administration of intravenous contrast.
Operation
The patient underwent a paramedian occipital and suboccipital craniotomy and total tumor removal. The surgery went without any complications. A control CT scan performed on the 1st postoperative day, revealed a satisfactory finding without any signs of ischemia, hemorrhage, or residual tumor. The patient recovered uneventfully. Tissue samples acquired during the surgical procedure underwent pathohistological and cytological analysis. Cytological analysis showed no signs of malignant cells. The pathohistological analysis showed two types of tumor cells. A major part of the tumor, more than 75% of total tumor tissue, consisted out of compacted bipolar piloid astrocyte. Focal microcystic changes were observed. The vascular network was pronounced, and the mitosis was not found. Scattered eosinophilic granular bodies and Rosenthal fibers were observed under higher magnification. Proliferation index Ki67 was up to 3% diffusely and up to 10% focally [Figure 2]. According to the World Health Organization (WHO) classification, major tumor part corresponded to a PA WHO Grade I. Minor part of the tumor, which was approximately around 25% of total tumor tissue, showed moderate cellularity. It consisted of uniform cells resembling pinealocytes, with a round core and medium abundant cytoplasm. Tumor cells form clusters. Immunohistochemical analysis showed focally slightly positive glial fibrillary acidic protein (GFAP), positive synaptophysin, and negative chromogranin A staining. The proliferation index Ki67 was around 1% [Figure 3]. According to the WHO classification, minor tumor part corresponded to a pineocytoma WHO Grade I. There was no indication for further oncological treatment.
Figure 2:
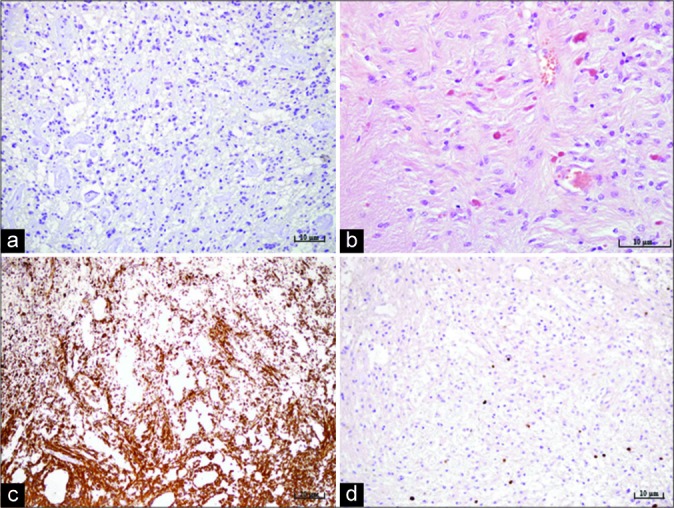
Microphotography of a pathohistological section showing histological and immunohistochemical features of a pilocytic astrocytoma stained with (a) IDH-1, original magnification of ×200, showing the absence of IDH mutation, (b) hematoxylin and eosin, original magnification of ×400, showing characteristic Rosenthal fibers and eosinophilic granular bodies, (c) strong positive reaction for glial fibrillary acidic protein staining, original magnification of ×100, (d) proliferation index Ki67, original magnification of ×200, showing up to 3% diffusely and up to 10% focally.
Figure 3:
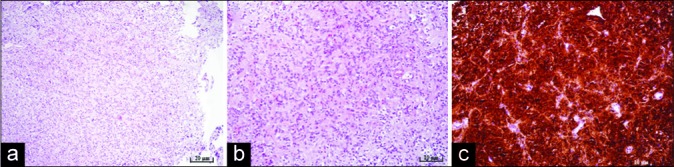
Microphotography of a pathohistological section showing histological and immunohistochemical features of a pineocytoma stained with (a) hematoxylin and eosin, original magnification of ×100, showing moderate cellularity, (b) hematoxylin and eosin, original magnification of ×200, showing pineocytomas rosette formation, (c) strong positive reaction for synaptophysin immunohistochemistry staining, original magnification of ×200.
Postoperative course
Five months afterward, an MRI and neurosurgical control was performed. The patient was without any neurological deficits. MRI revealed a satisfying postoperative result without the recurrence of intracranial masses [Figure 4].
Figure 4:
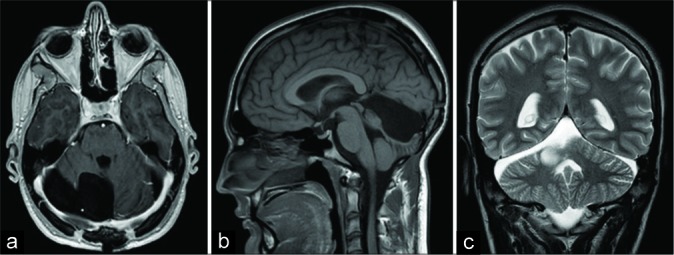
Brain magnetic resonance imaging scans, 5 months postoperatively, (a) transverse plain, T1-weighted image; (b) sagittal plain, T1-weighted image; (c) coronal plain, T2-weighted image. Note how there is no recurrence or additional intracranial mass in the images.
DISCUSSION
Biphasic tumors of the CNS are rarely described and mainly consisted out of the glial and mesenchymal component.[4,6,7] Tumor originated out of both astrocytes and pinealocytes, best to our knowledge, has not been described in the literature.
PA, clinically referred to by several terms, including cerebellar astrocytoma, optic glioma, and infundibuloma, is a rare glioma, classified as Grade I by the WHO. PA accounts for 15.4% of children and adolescents and 17.6% of childhood primary brain tumors. Nevertheless, PA may occur at any age, becoming increasingly uncommon with advancing years. PA most frequently occurs in the cerebellum, followed by the supratentorial compartment, the optic pathway, third ventricular/hypothalamic region, brainstem, and the spinal cord.[1,2,9] Pineocytomas are rare tumor that arises from pineal parenchymal cells and their appearance in the literature is primarily composed of case reports and small case series.[1,5] Neuroradiologically, PA may contain cysts or a tumor nodule in a cyst. On MRI, PAs are iso- or hypo-intense on T1-weighted sequences and hyperintense on T2-weighted images. Furthermore, they show strong and diffuse enhancing.[2,3,9] Unlike PA, pineocytoma imaging is nonspecific; they can present as well-circumscribed homogeneously enhancing solid masses, centered on the pineal gland. Just like PS, pineocytoma can be presented with a cystic or partially cystic appearance.[1,5] For PA, symptoms will be dependent on localization and include ataxia, cranial nerve defects, and signs of increased intracranial pressure.[3] Signs of increased intracranial pressure and Parinaud syndrome can point to mass in the pineal region.[5]
Gross examination of PA shows relatively soft-tissue tumor, white-grey in sections, as in major part of removed tumor. A cyst is usually present either within or around the tumor tissue, which can later result in a typical finding of cyst consisting of a tumor nodule. Patohistologically, PA is generally low to the moderate cellular tumor, characterized by a biphasic pattern with alternating densely and loosely packed areas. Micro cyst, granular bodies, and protoplasmic astrocyte-like cells (multipolar cells) with a round to oval nuclei can be in loose areas, while compact areas consist out of bipolar cells with the presence of Rosenthal fibers. Rosenthal fibers consist out of cells with long bipolar processes and elongated cytologically bland nuclei. Other characteristic includes vascular proliferation, sclerotic vessels, calcification, and chronic inflammation, while nuclear pleomorphism, mitosis, and necrosis are rare.[3,8,9]
As mentioned above, the major part of the removed tumor in our patient showed the pathohistological characteristic of PA. Patohistologically, pineocytomas are composed of small cells similar in appearance to normal pinealocytes, arranged in sheets, pineocytoma pseudo rosettes which are not characteristic and in normal pineal gland tissue. Immunohistochemically, they show a strong positive reaction to synaptophysin, neurofilament and neuron- specific enolase, while NeuN usually shows a negative reaction. Other neuronal markers, such as chromogranin A, are variable.[1,5,8] Minor part of removed tumor corresponded to the pathohistological characteristic of pineocytomas. Since the mentioned tumors origin is different, there must be complex molecular events or mutations that can lead to cell rearrangements and generation of two histologically different tissues in the same tumor mass. Although molecular pathways have been studied, so far, we have not found a specific pattern that can be used as a diagnostic biomarker.[3] In addition, regarding presented both histological sections and MRI, we cannot exclude the possibility that both of the mentioned tumors developed independently.
PA is primarily treated by surgery, and the survival rates for patients are excellent. Radiotherapy or chemotherapy can be used as therapy of choice. In the case of pineocytomas, gross total resection is the appropriate treatment when anatomically possible because it is affiliated with long term survival. Adjuvant radiotherapy is a therapy of choice.
CONCLUSION
Since the course of treatment options are slightly different for PA and pineocytoma, we believe that this unique case of brain mass consisted out of two pathohistologically different tumors can be helpful when deciding about the treatment of tumors in posterior cranial fossa and pineal region.
Footnotes
How to cite this article: Almahariq F, Raguz M, Romic D, Dlaka D, Oreskovic D, Sesar P, et al. A biphasic tumor in posterior cranial fossa and the pineal region in young adult. Surg Neurol Int 2020;11:64.
Contributor Information
Fadi Almahariq, Email: fadi.almahariq@gmail.com.
Marina Raguz, Email: marinaraguz@gmail.com.
Dominik Romic, Email: dominikromic00@gmail.com.
Domagoj Dlaka, Email: domagojdlaka@gmail.com.
Darko Oreskovic, Email: darkoreskov@gmail.com.
Patricija Sesar, Email: patricijasesar@yahoo.com.
Darko Chudy, Email: darko.chudy@gmail.com.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Clark AJ, Sughrue ME, Aranda D, Parsa AT. Contemporary management of pineocytoma. Neurosurg Clin N Am. 2011;22:403–7. doi: 10.1016/j.nec.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Collins VP, Jones DT, Giannini C. Pilocytic astrocytoma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129:775–88. doi: 10.1007/s00401-015-1410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cyrine S, Sonia Z, Mounir T, Badderedine S, Kalthoum T, Hedi K, et al. Pilocytic astrocytoma: A retrospective study of 32 cases. Clin Neurol Neurosurg. 2013;115:1220–5. doi: 10.1016/j.clineuro.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Dutta G, Gupta R, Garg M, Singh D, Singh H, Srivastava AK, et al. Giant parieto-occipital lobe pediatric gliosarcoma: Report of a rare entity and review of literature. Surg Neurol Int. 2018;9:111. doi: 10.4103/sni.sni_31_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fakhran S, Escott EJ. Pineocytoma mimicking a pineal cyst on imaging: True diagnostic dilemma or a case of incomplete imaging? AJNR Am J Neuroradiol. 2008;29:159–63. doi: 10.3174/ajnr.A0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feigin IH, Gross SW. Sarcoma arising in glioblastoma of the brain. Am J Pathol. 1955;31:633–53. [PMC free article] [PubMed] [Google Scholar]
- 7.Jang SJ, Kim J, Cho JM, Noh S, Park SH, Kim SH. A biphasic tumor consisting of pilocytic astrocytoma with an anaplastic solitary fibrous tumor component in the pineal region: A case report and literature review. Neuropathology. 2013;33:288–91. doi: 10.1111/j.1440-1789.2012.01347.x. [DOI] [PubMed] [Google Scholar]
- 8.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 world health organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–20. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 9.Wade A, Hayhurst C, Amato-Watkins A, Lammie A, Leach P. Cerebellar pilocytic astrocytoma in adults: A management paradigm for a rare tumour. Acta Neurochir (Wien) 2013;155:1431–5. doi: 10.1007/s00701-013-1790-1. [DOI] [PubMed] [Google Scholar]


