Abstract
Patient: Female, 52-year-old
Final Diagnosis: Metastatic lung adenocarcinoma
Symptoms: Fatigue • vision loss
Medication: —
Clinical Procedure: —
Specialty: Endocrinology and metabolic
Objective:
Rare co-existance of disease or pathology
Background:
Sellar masses are most commonly pituitary adenomas, however, about 1% of surgical resected pituitary lesions are found to be metastatic disease. It is hard to distinguish pituitary adenomas from metastatic disease. The most common primary sources for pituitary metastases are breast and lung cancer.
Case Report:
In this paper, we report the case of a woman who presented with right-sided vision loss who was found to have a sellar mass and panhypopituitarism. Subsequent imaging showed a large mass in her left upper lung with additional lesions in the spleen and adrenal glands. Neurosurgery performed an urgent transsphenoidal resection, with pathology confirming lung adenocarcinoma.
Conclusions:
This is an unusual presentation of metastatic lung cancer, with the patient’s primary symptoms being related to her pituitary metastasis and panhypopituitarism. Pituitary metastases are most commonly asymptomatic, although they can present with visual disturbances, diabetes insipidus, or panhypopituitarism. Pituitary metastases should be on the differential for sellar masses, particularly with specific radiographic findings, visual disturbances, and/or the presence of diabetes insipidus.
MeSH Keywords: Hypopituitarism, Lung Neoplasms, Pituitary Diseases
Background
Sellar masses are a common occurrence, with pituitary adenomas found to be present in 10.6% of autopsies [1]. Guidelines recommend that all patients found to have a pituitary incidentaloma undergo clinical and laboratory evaluation for hormone hypersecretion [2]. The large majority of sellar masses are pituitary adenomas, which account for 91% of cases in a series of sellar masses that required surgery [3]. Pituitary metastases are rare and account for 1% of surgically resected pituitary lesions [4]. The most common primary sources for pituitary metastases are breast cancer, lung cancer, thyroid cancer, and renal cell carcinoma, as described in Table 1 [5–10].
Table 1.
Most common primary sources for pituitary metastases [5].
| 1. Breast cancer |
|
| 2. Lung cancer |
|
| 3. Thyroid cancer |
| 4. Renal cell carcinoma |
| • Much lower prevalence than the other common causes of metastases to the pituitary, suggesting a preference for spread to the pituitary gland [10] |
| 5. Other cancers |
| • Several other cancers have been found to be sources for pituitary metastases, but their incidence was too low to suggest any preference or homing to the pituitary and is most likely due to random seeding |
Lung cancer is the leading cause of death from cancer worldwide and is often diagnosed in the advanced stages, with the most frequent sites of metastases being the nervous system, bone, liver, respiratory system, and adrenal gland [11]. Its most common presentation includes dyspnea, cough, and chest or rib pain [12]. Here we will describe a case of metastatic lung cancer that presented with vision loss and panhypopituitarism secondary to a pituitary metastasis.
Case Report
A 52-year-old female presented to the Emergency Department complaining of progressive right-sided vision loss over the previous 2 weeks. She first noticed a small visual defect in the right eye that rapidly progressed to complete right eye vision loss. She also described 3 months of fatigue, daily headaches, dizziness, intermittent nausea, and amenorrhea. Past medical history included alcoholism for which she was admitted to intensive outpatient treatment 4 times but continued to consume 3 to 4 drinks daily. She also smoked 1 pack per day. She lived with her boyfriend locally and did not work. Family history was notable for a gastrointestinal cancer of unknown type.
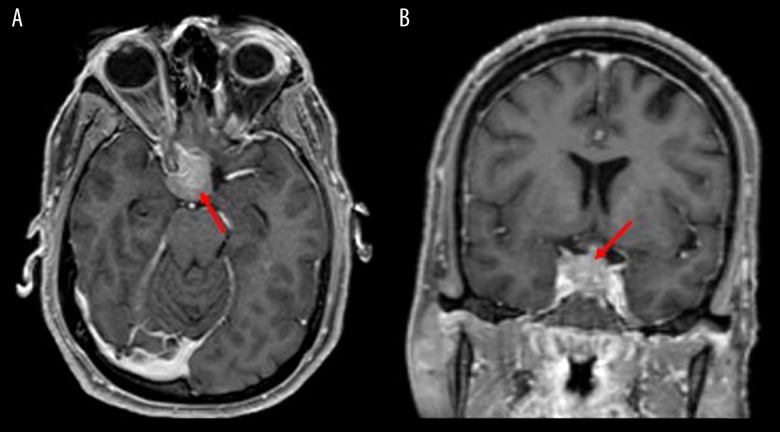
Upon presentation she was hemodynamically stable. On physical examination, she was awake and alert, with a full neurologic examination notable for a right afferent pupillary defect, full extraocular movements, loss of visual field in the right temporal aspect as well as right nasal aspect, and diminished peripheral vision in the left temporal field. A magnetic resonance imaging (MRI) of the head with and without contrast was performed, which demonstrated a sellar and suprasellar mass up to 2.2 cm that invaded the right cavernous sinus (Figure 1). Computed tomography (CT) of the chest, abdomen, and pelvis were subsequently obtained, which showed a 5.3×4.0 cm left upper lung mass with additional lesions in the spleen, bilateral adrenal glands, and enlarged retroperitoneal lymph nodes (Figure 2).
Figure 1.
(A) Axial and (B) coronal magnetic resonance imaging showing “expansile sellar and suprasellar mass which measures 22×20×19 mm” (arrows).
Figure 2.
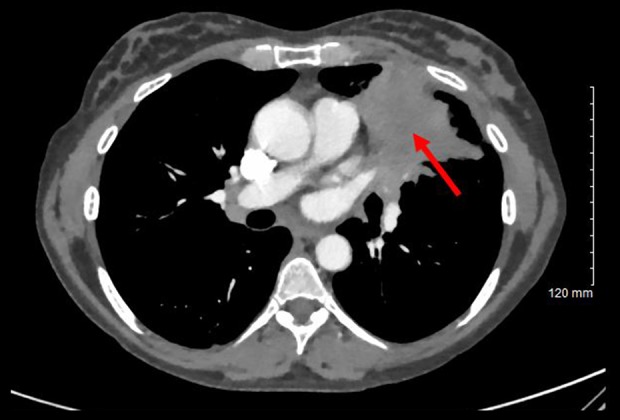
Computed tomography chest with contrast that highlights the 5.3×4.0 cm left upper lobe mass (arrow) that “abuts and may invade the left mediastinum”.
Hormonal evaluation was obtained at 23: 56 the day of presentation at the Emergency Department, and revealed that adrenocorticotropic hormone (ACTH) was <5 pg/mL; cortisol was 1 ug/dL; insulin-like growth factor 1 (IGF-1) was 124 ng/mL; follicle-stimulating hormone (FSH) was 2.5 mIU/mL; luteinizing hormone (LH) was 0.3 mIU/mL; prolactin was 69.2 ng/mL; thyroid-stimulating hormone (TSH) was 2.42 mIU/mL; total triiodothyronine (T3) was 87 ng/dL; free (thyroxine) T4 was 0.4 ng/dL. A repeat cortisol the following morning was also 1 ug/dL.
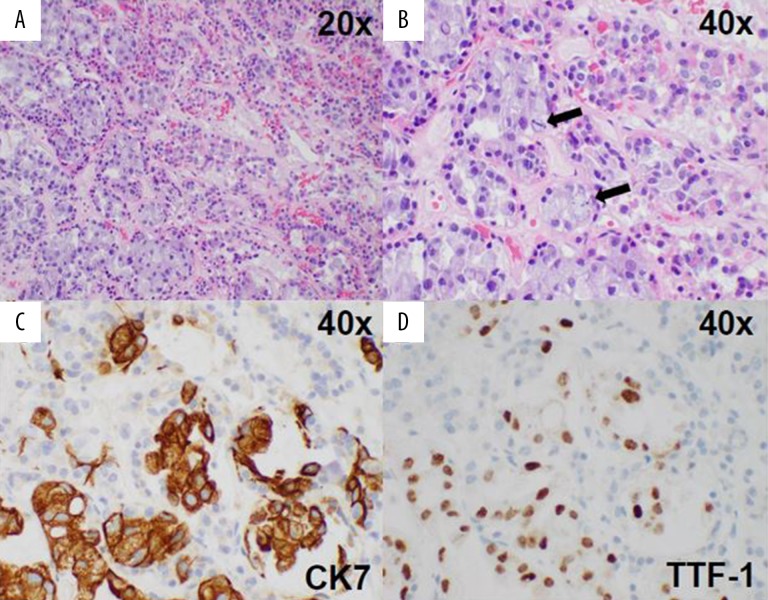
The patient was evaluated by neurosurgery while in the Emergency Department and an urgent endoscopic endonasal transsphenoidal resection of the tumor was performed to decompress her right optic nerve. Pathology showed lung adeno-carcinoma (Figure 3). The patient was started on dexamethasone and transitioned to prednisone, which was weaned down to 5 mg daily as her maintenance dose. She was also started on levothyroxine 75 mcg daily. She developed post-operative diabetes insipidus, which was managed with 2 mcg desmopressin and resolved prior to her discharge.
Figure 3.
(A, B) Histology shows gland-forming carcinoma cells (lower left) invading normal anterior pituitary tissue (upper right). Mitoses are apparent at higher power (arrows in B). Tumor cells are positive for CK7 (C) and TTF-1 (D), consistent with adenocarcinoma of lung origin.
Unfortunately, repeat MRI of her brain 3 weeks after discharge showed a new left frontal metastasis with a stable pituitary lesion. She had not recovered any vision, and she complained of left sided vision loss as well. She received post-operative radiation therapy. Her tumor showed a PDL1 score of 1 to 10% with a solid tumor gene panel positive for pathogenic KRAS variant and concomitant STK11 variant predicted to result in loss of function. Her oncologist planned to initiate chemoimmunotherapy with carboplatin, pemetrexed, and pembrolizumab.
Discussion
Pituitary metastases are rarely the presenting symptom of metastatic cancer, however, when they are, the most common presenting symptom is visual disturbances, found in 49% of cases when pituitary metastases are the first sign of disease [5]. The second most common presenting symptom is new onset diabetes insipidus [5], seen in 29% to 71% of cases of symptomatic pituitary metastases [7]. The high incidence of diabetes insipidus in pituitary metastases is most likely due to the preference of metastases to seed the posterior pituitary gland over the anterior pituitary gland – the posterior pituitary is involved in 85% of pituitary metastases [13], while the anterior pituitary is involved in 24% to 40% of all pituitary metastases [14], a finding that is thought to be due to the blood supply to the gland. The posterior pituitary receives blood directly from systemic circulation via the hypophyseal arteries, while the anterior pituitary only receives venous blood via the hypophyseal portal system. Metastases in the posterior pituitary can disrupt the hypothalamic axons that transport neurosecretory granules containing anti-diuretic hormone (ADH) to the posterior pituitary, leading to central diabetes insipidus [5]. Considering that only 1% of primary pituitary adenomas cause diabetes insipidus, its presence should be an indication of possible pituitary metastasis [5]. While this patient did present with visual disturbances, she did not have evidence of diabetes insipidus until postoperatively.
The third most common presenting symptoms of pituitary metastases is panhypopituitarism [5]. This is the most difficult presentation to workup given the nonspecific signs and symptoms, including fatigue, low muscle strength, cold intolerance, changes in menses, and slowing of mentation [15].
The diagnosis of metastatic disease in the pituitary cannot be made without a surgical biopsy of the mass and histologic examination. However, there are several features that indicate an increased probability of a pituitary mass being due to metastatic disease. These clinical features include headaches, abnormal eye motility, visual field defects, and diabetes insipidus [9]. Signs found on imaging that suggest a metastatic source for the pituitary mass include thickening of the pituitary stalk, invasion of the cavernous sinus, loss of the posterior lobe bright spot, and sclerosis of the surrounding sella turcica [7]. Blood flow status is another important feature, with hyper-perfusion of the tumor favoring a metastatic tumor rather than a pituitary adenoma [16]. If any of these suggestive signs or symptoms are present, then a workup to find the primary cancer should be considered.
There are no standardized protocols for the treatment of pituitary metastases. Surgery is done to relieve the symptoms of pituitary metastases and to make a tissue diagnosis. Surgery has no effect on survival time in these patients but does improve quality of life [15]. Chemotherapy or radiation can be considered based on the primary cancer and can be started immediately upon diagnosis of pituitary metastases [17]. A recent systemic review of pituitary metastases found systemic chemotherapy results in significantly improved survival rates, but these chemotherapy regimens differ based on primary tumor and patient characteristics. It also found that radiotherapy provides an improvement in survival times, although there is no evidence to suggest stereotactic radiotherapy is any better than conventional multifractionated radiotherapy [18]. All therapy modalities (surgery, chemotherapy, and/or radiation) can potentially lead to panhypopituitarism, so monitoring the patient’s hormone levels is important, as is treatment with hormone replacement therapy when indicated. Regardless of treatment, the mean survival time after diagnosis of pituitary metastasis is between 11.8 and 13.6 months [15].
Conclusions
Sellar masses are commonly found and the majority represent pituitary adenomas [3]. A much smaller subset represented metastatic disease, which can look very similar to pituitary adenomas on imaging. There are imaging findings such as the loss of the posterior lobe bright spot, thickening of the pituitary stalk, sellar diaphragm invasion, and tumor hyper-per-fusion that can be found in pituitary metastases [19]. In addition, visual disturbances and diabetes insipidus are more common in pituitary metastases compared to pituitary adenomas [5]. These clinical findings should be considered in cases with an unclear diagnosis.
Footnotes
Conflict of interest
Matthew P. Gilbert has worked as a consultant for Novo Nordisk and Sanofi, USA. Kelsey H. Sheahan, Gunnar C. Huffman, and John DeWitt declare that there are no conflicts of interest regarding the publication of this article.
References:
- 1.Molitch ME. Nonfunctioning pituitary tumors and pituitary incidentalomas. Endocrinol Metab Clin North Am. 2008;37:151–71. doi: 10.1016/j.ecl.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Freda PU, Beckers AM, Katznelson L, et al. Pituitary incidentaloma: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:894–904. doi: 10.1210/jc.2010-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freda PU, Post KD. Differential diagnosis of sellar masses. Endocrinol Metab Clin North Am. 1999;28:81–117. doi: 10.1016/s0889-8529(05)70058-x. [DOI] [PubMed] [Google Scholar]
- 4.Zoli M, Mazzatenta D, Faustini-Fustini M, et al. Pituitary metastases: Role of surgery. World Neurosurg. 2013;79:327–30. doi: 10.1016/j.wneu.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Javanbakht A, D’Apuzzo M, Badie B, et al. Pituitary metastasis: A rare condition. Endocr Connect. 2018 doi: 10.1530/EC-18-0338. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cieszynski L, Obolonczyk L, Sworczak K, et al. Diabetes insipidus as a main symptom of cancer. Arch Med Sci. 2014;10(2):401–5. doi: 10.5114/aoms.2014.42590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fassett DR, Couldwell WT. Metastases to the pituitary gland. Neurosurg Focus. 2004;16:E8. [PubMed] [Google Scholar]
- 8.He W, Chen F, Dalm B, et al. Metastatic involvement of the pituitary gland: A systematic review with pooled individual patient data analysis. Pituitary. 2015;18:159–68. doi: 10.1007/s11102-014-0552-2. [DOI] [PubMed] [Google Scholar]
- 9.Al-Aridi R, El Sibai K, Fu P, et al. Clinical and biochemical characteristic features of metastatic cancer to the sella turcica: An analytical review. Pituitary. 2014;17:575–87. doi: 10.1007/s11102-013-0542-9. [DOI] [PubMed] [Google Scholar]
- 10.Gopan T, Toms SA, Prayson RA, et al. Symptomatic pituitary metastases from renal cell carcinoma. Pituitary. 2007;10:251–59. doi: 10.1007/s11102-007-0047-5. [DOI] [PubMed] [Google Scholar]
- 11.Riihimaki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86:78–84. doi: 10.1016/j.lungcan.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton W, Peters TJ, Round A, et al. What are the clinical features of lung cancer before the diagnosis is made? A population based case-control study. Thorax. 2005;60:1059–65. doi: 10.1136/thx.2005.045880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCormick PC, Post KD, Kandji AD, et al. Metastatic carcinoma to the pituitary gland. Br J Neurosurg. 1989;3:71–9. doi: 10.3109/02688698909001028. [DOI] [PubMed] [Google Scholar]
- 14.Komninos J, Vlassopoulou V, Protopapa D, et al. Tumors metastatic to the pituitary gland: Case report and literature review. J Clin Endocrinol Metab. 2004;89:574–80. doi: 10.1210/jc.2003-030395. [DOI] [PubMed] [Google Scholar]
- 15.Novak V, Hrabalek L, Hampl M, et al. [Metastatic pituitary disorders] Klin Onkol. 2017;30:273–81. doi: 10.14735/amko2017273. [Article in Czech] [DOI] [PubMed] [Google Scholar]
- 16.Tanaka T, Hiramatsu K, Nosaka T, et al. Pituitary metastasis of hepatocellular carcinoma presenting with panhypopituitarism: A case report. BMC Cancer. 2015;15:863. doi: 10.1186/s12885-015-1831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ono R, Ito R, Nakagawa K, et al. Hypothalamic hypopituitarism secondary to suprasellar metastases from small cell lung cancer: A case report and review of the literature. J Med Case Rep. 2018;12:342. doi: 10.1186/s13256-018-1871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng S, Fomekong F, Delabar V, et al. Current status and treatment modalities in metastases to the pituitary: A systematic review. J Neurooncol. 2020;146:219–27. doi: 10.1007/s11060-020-03396-w. [DOI] [PubMed] [Google Scholar]
- 19.Vasilev V, Rostomyan L, Daly AF, et al. Management of endocrine disease: pituitary ‘incidentaloma’: Neuroradiological assessment and differential diagnosis. Eur J Endocrinol. 2016;175:R171–84. doi: 10.1530/EJE-15-1272. [DOI] [PubMed] [Google Scholar]