Abstract
Background
Normothermic machine perfusion (NMP) can provide access to evaluate and resuscitate high-risk donor livers before transplantation. The purpose of this study was to determine the efficacy of NMP in preservation and assessment of extended-criteria donor (ECD) livers in China.
Case Reports
From September 2018 to March 2019, 4 liver grafts from 3 transplant center defined as ECD were subjected to NMP, and then were transplanted successfully. During perfusion, perfusion parameters such as vascular flow, glucose level, lactate clearance, and bile production/composition were recorded to assess graft viability. All recipients were followed up 6 months after transplantation.
Conclusions
NMP provides a potential tool for preservation and assessment of ECD livers in China.
MeSH Keywords: Donor Selection, Liver Transplantation, Organ Preservation
Background
Liver transplantation (LT) is the standard therapy for end-stage liver diseases [1]. However, there is an urgent need to increase the number of livers for transplantation, due to the high waiting-list mortality [2]. To enlarge the donor organ pool, extended-criteria donor (ECD) grafts, such as livers from donation after circulatory death, as well as elderly and steatotic grafts, are more frequently used [3]. The use of ECD livers is associated with higher risks of post-transplant complications, such as early allograft dysfunction (EAD), primary nonfunction (PNF), and cholangiopathies [4,5]. To effectively make use of ECD livers, a better preservation method is required, along with the ability to assess graft viability and repair graft injuries.
Recently, ex vivo normothermic machine perfusion (NMP) as a preservation method has attracted great attention [6]. The novel preservation technique can provide nutrition and oxygen to the livers at a physiological temperature. NMP can maintain metabolic activity and remove the harmful substances caused by ischemic injuries of grafts [7]. A number of animal experiments and clinical trials have shown that NMP is superior to cold storage in protection of liver grafts [8–13].
The national deceased organ donation system has been established in China since January 2015. There are 3 categories of organ donation, including donation after brain death (DBD), donation after circulatory death (DCD), and Organ Donation after Brain Death followed by Circulatory Death (DBCD) [14]. Due to having less experience in maintenance techniques of potential donors, the incidence of EAD is 55.91%, which is higher in China than the incidence (23%) in the United States [15,16]. In this circumstance, NMP might provide a better method than cold storage to preserve donor livers in China. Herein, we reported the first series of ECD liver transplantations using NMP preservation.
Case Reports
Donor livers
This study retrospectively analyzed the first 4 ECD livers with NMP preservation in the First Affiliated Hospital of Sun Yat-sen University. The transplants were performed between September 2018 and March 2019. The definition of warm ischemia time (WIT) was from cardiac arrest to in situ cold perfusion. The 4 liver grafts defined as ECD were respectively satisfied with DCD, DCD with peak serum natrium >165 mmol/L, DCD with poor in situ perfusion, and DBD with serum total bilirubin >3 mg/dL [17].
Four liver grafts were procured in 3 organ procurement organizations in Guangdong Province. Ice-cold University of Wisconsin (UW) solution was flushed in situ into the superior mesenteric vein and abdominal aorta. At this time, the color of the liver and the effluent from the inferior vena cava were observed, and the texture of the liver was felt to judge whether the liver was adequately and uniformly perfused.
Once it arrived at our center, each liver was assessed by an experienced transplant surgeon. The first liver graft was from a DCD donor, with a WIT of 22 min. The recipient suffered from acute liver failure (ALF). For patient safety, NMP was used to preserve the donor liver. The second liver graft was also from a DCD donor, with a WIT of 10 min, accompanied with a very high sodium level (173.3 mmol/L). The third liver felt hard to the touch and the graft appearance was not normal because of poor in situ cold perfusion. Moreover, it was also a DCD liver. Therefore, it was considered for NMP preservation. The fourth liver had a high serum total bilirubin level (141.5 umol/L) before procurement. To ensure the recipient’s safety, we used NMP to assess the viability of the graft.
Normothermic machine perfusion
The NMP device (Liver Assist, Groningen, The Netherlands) consists of a rotary pump, oxygenator, heat exchanger, connecting pipeline and other components. Two rotary pumps provide a pulsatile flow to the hepatic artery (HA) and a continuous flow to the portal vein (PV). The system is pressure and temperature controlled. Pressure was set at 50 mmHg in the hepatic artery and 11 mmHg in the portal vein. The perfusate was warmed to 37°C before perfusion [18]. The perfusate consisted of 6 units of leucocyte-depleted washed red blood cells, 0.6 L succinylated gelatin, 100 mL sodium bicarbonate, 37500 units heparin, 2 g Imipenem and Cilastatin Sodium (Pfizer, USA), 20 ml calcium chloride, 3 ml magnesium sulphate and 125 ml amino acid. The flow, pressure and temperature are displayed on the device in real time, and are recorded every 20 min. Blood gas of perfusate was measured every 10 min for the first 40 min, and then every 20 min. The oxygen flow was controlled through an oxygenator supplied with a mixture of O2 and air, with the aimed perfusate PaO2 of 200 mmHg. According to the results of blood gas analysis of perfusate, oxygen supply concentration/flow and perfusion fluid composition can be adjusted over time. Bile was collected from the biliary draining tube and the volume was measured every hour. Bile was collected hourly and was also subjected to blood gas analysis. Machine perfusion was maintained until the recipient’s liver was removed. Liver graft viability was assessed by the following criteria: the perfusate lactate level was below 2.5 mmol/L within 4 h of perfusion, the liver had to produce bile, stable artery flow was more than 150 ml/min and portal venous flow was more than 500 ml/min, and perfusate pH was greater than 7.3 [19]. The liver graft was flushed with 2 L of cold saline. Vascular and bile duct cannulas were removed, and liver biopsies were taken at the end of perfusion. The graft was immediately implanted and reperfused in the standard manner.
Transplant recipients
The recipients were patients listed for liver transplantation at the First Affiliated Hospital of Sun Yat-sen University, China. All patients received an explanation about the principles of NMP while obtaining informed consent for liver transplantation. When an extended-criteria liver graft was available, the consultant surgeon re-explained the procedures in detail and obtained additional patient consent to accept the graft. The NMP proceeded only when the patient fully understood and accepted the possible risks and benefits. Informed consent was obtained from every patient. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University.
After surgery, the recipients were transferred to the Intensive Care Unit (ICU) for post-transplant care, including respiratory and circulatory support therapy, and liver function monitoring. Liver and coagulation function indicators were measured every day for the first 7 days after the operation. The duration of ICU and hospital stays were recorded. Postoperative complications such as biliary complications and early allograft dysfunction (EAD) were also documented. EAD was defined as satisfying any of the following characteristics: serum total bilirubin (Tbil) >10 mg/dL on day 7 after the operation (excluding biliary stricture), serum international standardized ratio (INR) >1.6 on day 7 after the operation, the peak level of serum glutamic oxaloacetic transaminase aspartate aminotransferase (AST) within the first 7 days after the operation was > 2000 IU/L, and the peak level of serum alanine aminotransferase (ALT) within the first 7 days after the operation was >2000 IU/L [20]. After discharge from the hospital, the patients were followed up in the outpatient clinic monthly for the first year.
Histology
Biopsies were obtained from donor livers at 3 time points: before NMP, after NMP, and after reperfusion. Paraffin-embedded slides of liver biopsies were prepared for hematoxylin and eosin (HE) staining. Liver biopsies were assessed for sinusoidal congestion, hepatocyte necrosis and ballooning degeneration, based on the Suzuki criteria [21]. All samples were examined by an experienced liver pathologist.
Donor characteristics
In total, 4 ECD livers were procured, successfully preserved using NMP and transplanted. None of the donors had history of hepatitis B infection. The median donor age was 21 (19–26) years. Three grafts were from DCD donors and 1 was from a DBD donor. The detailed donor characteristics are summarized in Table 1. Donor 1 was admitted with cerebral ischemia, having normal liver function. Donor 2 suffered from brain injury due to intracranial hemorrhage. Donor 3 was a 26-year-old male with a diagnosis of intracranial hemorrhage and a BMI of 17.86 kg/m2. Donor 4 was a 19-year-old female admitted with head trauma.
Table 1.
The characteristics of donors and recipients.
| Characteristic | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Donor | ||||
| Age, y | 22 | 20 | 26 | 19 |
| Sex | Female | Male | Male | Female |
| Blood type | B | O | AB | O |
| BMI | 19.53 | 21.22 | 17.86 | 20.81 |
| Cause of brain injury | Cerebral ischemia | Intracranial hemorrhage | Intracranial hemorrhage | Head trauma |
| HBV | (−) | (−) | (−) | (−) |
| Na (mmol/L) | 155 | 173.3 | 134 | 148 |
| ALT (U/L) | 11 | 36.9 | 16.8 | 79 |
| AST (U/L) | 33 | 94.4 | 27.1 | 239 |
| TBiL (umol/L) | 8.8 | 8.9 | 27.6 | 141.5 |
| DBD/DCD | DCD | DCD | DCD | DBD |
| WIT (min) | 22 | 10 | 6 | 0 |
| CIT (min) | 638 | 536 | 423 | 334 |
| NMP time (min) | 260 | 260 | 380 | 360 |
| Recipient | ||||
| Age, y | 31 | 63 | 58 | 56 |
| Sex | M | M | F | M |
| HBV | (+) | (+) | (+) | (+) |
| MELD | 39 | 9 | 40 | 14 |
| Reason for transplantation | ALF | Cirrhosis | Cirrhosis | Cirrhosis |
| Operation duration (min) | 365 | 350 | 375 | 405 |
| Anhepatic phase (min) | 31 | 25 | 38 | 37 |
| Total blood loss (mL) | 3000 | 200 | 500 | 3000 |
| Blood transfusion (mL) | 1200 | 0 | 0 | 1600 |
| Post-transplantation complication | Biliary stricture* | EAD | NA | NA |
| ICU stay (h) | 85 | 36 | 60 | 23 |
| Hospital stay (day) | 29 | 26 | 17 | 26 |
Biliary anastomosis stricture.
BMI – body mass index; HBV – hepatitis B virus; DBD – donor after brain death; DCD – donor after cardiac death; WIT – warm ischemia time; ALT – alanine transaminase; AST – aspartate aminotransferase; TBIL – total bilirubin; CIT – cold ischemia time; NMP – nomorthermic machine perfusion; MELD – model for end-stage liver disease; ALF – acute liver failure; EAD – early allograft dysfunction; NA – not available.
Perfusion characteristics
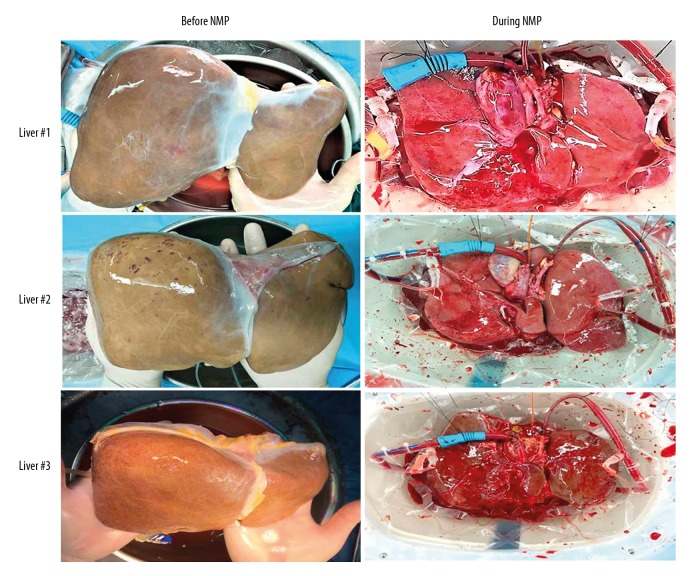
The median cold ischemia time (CIT) was 479.5 (334–638) min in this series. Prior to NMP, all liver grafts were flushed with 3 L of warm saline. There were no technical complications during NMP. The livers before and during perfusion are shown in Figure 1 (pictures of liver 4 were not available). All livers were ruddy and softer after perfusion.
Figure 1.
Graft appearance before and during normothermic machine perfusion.
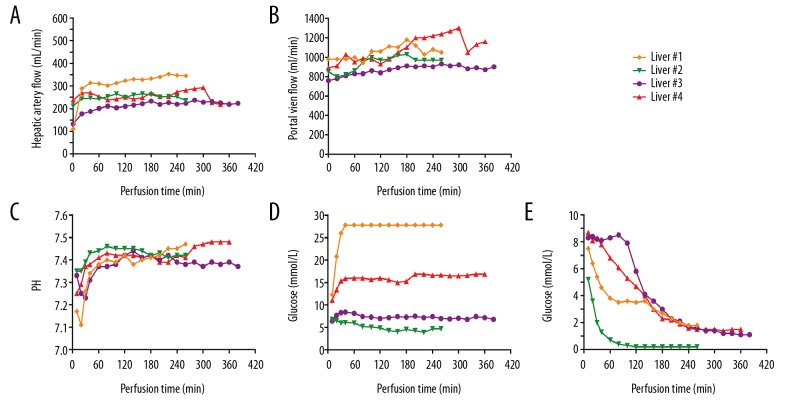
Median NMP time was 310 (260–380) min (Table 1). The initial hemoglobin concentration was 54 (50–58) g/L and the final hemoglobin concentration was 51 (47–54) g/L. The HA flows were variable between livers, but along with perfusion, they gradually tended to be stable (Figure 2A). The median of HA flow was 247.5 (201–310) mL/min at 1 h after perfusion. The HA flow in liver 1 was higher than in livers 2, 3, and 4. PV blood flow showed a slight upward trend, with a median arterial flow of 1010 (900–1160) mL/min at the end of perfusion (Figure 2B).
Figure 2.
Perfusion parameters and markers of viability assessment during normothermic machine perfusion. (A, B) Change of vascular flow during perfusion. (C) pH in perfusate. At the beginning of perfusion, pH was low and then rose to normal levels gradually. (D) Glucose level in perfusate. During perfusion, liver 1 and liver 4 presented a high level of glucose, while liver 2 and liver 3 demonstrated a relatively normal level. During perfusion, in all grafts, the glucose in perfusate remained stable. (E) Lactate clearance during perfusion. All livers showed active condition. At the end of perfusion, perfusate lactate levels dropped below 2.5 mmol/L.
After 1 h of perfusion, the pH value reached a relatively stable level, with a median value of 7.39 (range 7.37–7.44) (Figure 2C). The glucose levels of perfusate of livers 2 and 3 were steady during the whole perfusion process, but increased rapidly at the beginning of perfusion in livers 1 and 4, and finally stabilized at 27.8 and 16.9 mmol/L, respectively (Figure 2D). At the beginning of perfusion, lactate concentrations in perfusate were as high as 7.9 (5.2–8.7) mmol/L, but finally decreased to normal level, reflecting active metabolism of the livers (Figure 2E).
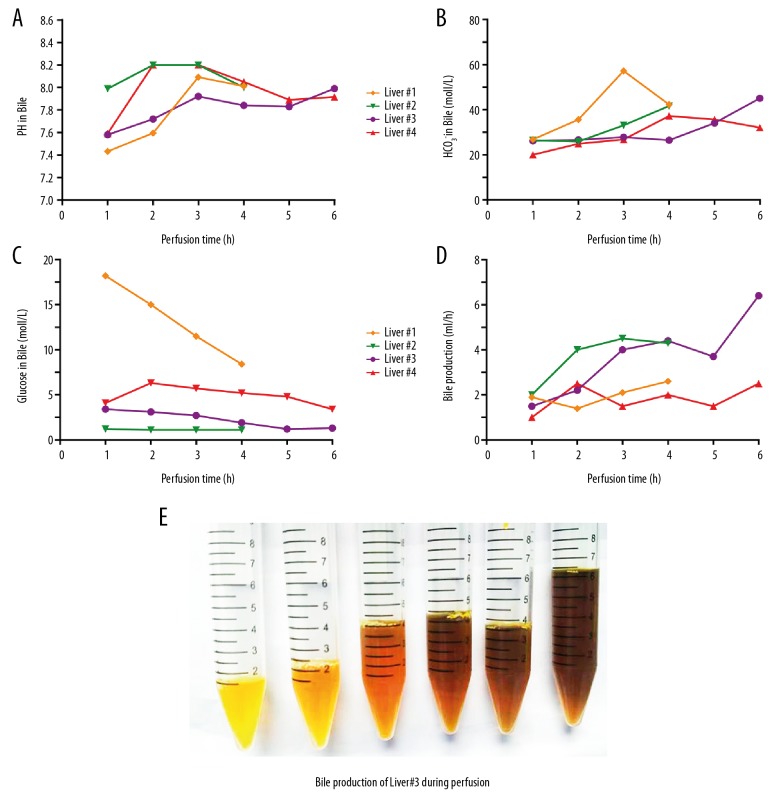
Bile production was observed throughout the entire perfusion period (Figure 3E). The median bile production after the first hour was 1.7 (1.0–2.0) mL (Figure 3D). There was a gradual change in color to a darker shade (Figure 3E). During perfusion, the pH values of bile produced by the 4 livers were quite different in the beginning, but at the end of perfusion, they were all around 8 (7.995, 7.915–8.011) (Figure 3A). The concentration of bicarbonate increased, but it fluctuated in the bile produced by liver 1. The glucose concentration in bile continued to decrease (Figure 3C). This was particularly evident in the bile of liver 1 (Figure 3A), with a level of 18.2 mmol/L at the beginning to 8.4 mmol/L at the end.
Figure 3.
Bile production and results of bile gas analysis during normothermic machine perfusion. (A–C) show pH values, HCO3−, and glucose levels in the bile every hour. (D) Bile production during perfusion. (E) Centrifuge tubes containing bile from liver 3. The bile production was increasing and the color of bile gradually turned darker.
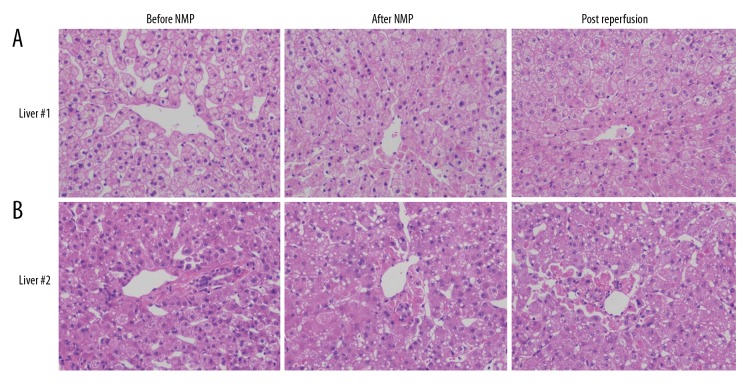
As liver biopsies were taken from liver 1 and 2, histological examination of HE staining liver biopsies (×100) showed that there was no significant difference between biopsies taken before and after NMP and after reperfusion (Figure 4). Liver 1 showed no signs of sinusoidal damage and hepatocyte necrosis. Mild ballooning degeneration was present after machine perfusion and after reperfusion. In contrast, liver 2 presented no obvious change in sinusoidal damage, hepatocyte necrosis, and ballooning degeneration after machine perfusion and after reperfusion.
Figure 4.
Liver biopsies with hematoxylin and eosin staining before and after normothermic machine perfusion and after reperfusion. (A, B) Shows the livers were well-preserved after perfusion, and the microscopic architecture was little changed.
Post-transplant outcomes and liver graft function
Recipient characteristics, transplant indications, and MELD scores are shown in Table 1. All recipients had a history of hepatitis B infection. One of them underwent liver transplantation for acute liver failure and the other 3 for cirrhosis. The median operation duration was 370 (350–405) min, and the median anhepatic phase was 34 (25–38) min. The median ICU stay was 48 (23–85) h, and the median length of hospital stay was 26 (17–29) days. In addition, recipient 1 in the study experienced postreperfusion syndrome (PRS), which was diagnosed by a decrease of 33% in mean arterial pressure (MAP) after anhepatic phase for 5 min with the use of norepinephrine (0.2 ug/kg/min) within the first 5 min after reperfusion.
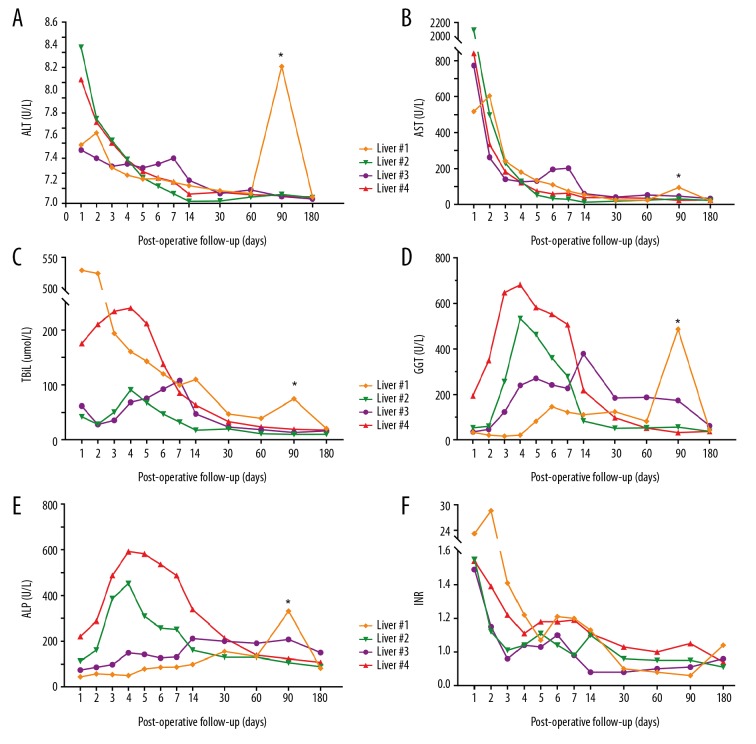
The results of postoperative liver function tests are shown in Figure 5. The serum ALT and AST levels decreased during the first week after transplantation, and values were nearly normal at 30 days after transplantation. The Tbil in recipients 2, 3, 4 initially increased to a temporary peak within 1 week after surgery, and then rapidly decreased to normal levels. The Tbil level of recipient 1 decreased continuously during the first 60 days after the operation. Serum glutamyltransferase (GGT) and alkaline phosphatase (ALP) levels tended to increase at first and then decreased after the operation. At 60 days after surgery, the serum GGT levels of recipients 1, 2, and 4 almost fell to normal levels, but the ALP concentrations were still higher than the normal range in all recipients. The INR was a little higher than the upper limit, but it decreased rapidly within 3 days. Due to their liver functions after transplant, no patients were diagnosed as PFN. The peak value of AST was 2106 U/L during the first 7 days after transplant for recipient 2, meeting the diagnostic criteria of EAD. At day 90 after transplantation, the liver enzymes and Tbil of recipient 1 increased significantly, which was confirmed by imaging studies as anastomostic biliary stricture. The stricture was cured by biliary stent placement. To date, no other biliary complications have occurred.
Figure 5.
Presentation of post-transplant liver function from 4 recipients. (A, B) show the post-transplant transaminase release after machine perfusion. In all recipients, ALT and AST recovered to normal within the first month. (C–E) Demonstrate the change of Tbil and ALP, GGT as markers of bile duct damage after transplantation. (F) Recovery of INR as marker of liver coagulation function. * Recipient #1 had the complication of anastomotic stenosis post-transplant follow-up 90 days, resulting in abnormal elevation of transaminase, Tbil, ALP, and GGT. ALT – alanine transaminase; AST – aspartate transaminase; Tbil – total bilirubin; GGT – gamma-glutamyl transferase; ALP – alkaline phosphatase; INR – international normalized ratio.
Discussion
In this study, we successfully transplanted 4 ECD livers for recipients with end-stage liver disease after ex situ preservation and assessment using NMP. The results from short-term follow-up suggest the efficacy and safety of NMP in ECD liver transplantation in China.
Although great advancements have been made in organ transplantation in recent decades, the organ donor shortage is a severe problem all over the world [22], and high-risk donor livers are increasingly used in clinical settings. It is difficult for surgeons to decide whether to use an ECD liver for transplantation or to discard it due to an expected high risk of PNF, based on surgeon experience [23]. Fortunately, advances have been made in machine perfusion (MP) technology, including hypothermic machine perfusion (HMP), hypothermic oxygenated perfusion (HOPE), subnormothermic machine perfusion (SNMP), and NMP [11,24–26]. HMP of kidneys can reduce the incidence of delayed graft function (DGF) in renal transplantation [27]. HMP is also feasible and effective to preserve high-risk liver grafts [28]. However, it is controversial whether HMP can improve graft survival [29]. In contrast to HMP, HOPE can ensure a supply of dissolved oxygen to liver grafts, and it minimizes preservation injury and replenishes adenosine triphosphate (ATP) stores to improve organ viability [30]. For SNMP, the rate of metabolism of liver graft can increase to close to 70% of the normal rate. Among these methods, only NMP can provide normothermic oxygenated blood supply to the organs, creating an environment close to physiological conditions, which enables the method to repair the injuries and also assess graft viability [6]. However, the NMP technology is more complicated and expensive. Therefore, the choice of machine perfusion technique needs to be based on the quality of organs, cost-effectiveness analysis, and technology availability.
Skaro et al. reported that livers from DCD had 2.1 times greater risk of graft failure, and 3.2 times greater risk of re-transplantation, compared with DBD recipients. In addition, DCD recipients have a 31.6% higher incidence of biliary complications and a 35.8% higher incidence of ischemic cholangiopathy [31]. In this study, the liver grafts from DCD were subjected to 22-min WIT and were transplanted successfully. As reported, grafts from DCD donors with 20–30 min WIT were defined as intermediate-risk ECD grafts [32]. Since the first recipient suffered from acute liver failure, the liver underwent NMP for safety. The recovery was uneventful, but an anastomotic stricture of the extrahepatic bile duct occurred at 3 months after transplantation. The second donor liver was subjected to 10-min WIT and hypernatremia. It has been shown that donor serum sodium level is correlated with liver transaminase level after transplant [33]. Figueras et al. reported that serum sodium levels >155 mmol/L in donors was associated with higher incidences of liver re-transplantation and graft loss within 1 month after transplantation [34]. As a consequence, we considered NMP for this organ. Despite the presence of EAD (due to the peak AST level of 2106 U/L), the recipient is without complications to date. The third donor liver was subjected to insufficient cold perfusion during procurement with 6-min WIT. Insufficient cold perfusion can result in microthrombus, which increases the risk of graft failure. Before machine perfusion, the donor liver felt hard to the touch and the color was abnormal; we therefore used NMP preservation for this organ. The fourth donor had high serum bilirubin levels. High bilirubin in blood indicates that metabolic function of the donor liver is insufficient, and a donor with high serum bilirubin has elevated risk of graft failure after transplantation [35]. Thus, we attempted to use NMP to resuscitate it. To date, there have been no complications in the recipients of livers from donors 3 and 4.
NMP appears to have excellent potential as a technique to predict the viability and suitability of ECD organs. In the majority of cases, donor characteristics and gross surface appearance are used to decide to either use or discard donor livers [36]. As a consequence, patients might suffer from PNF if these assessments are not accurate enough [37]. NMP can now address this issue by ex vivo assessment of graft viability before transplantation. During perfusion, parameters like perfusate PH value, lactate level, bile production/components, hepatic artery and portal vein flow, and liver enzyme level in perfusate can be monitored [11,12]. According to the criteria reported by Mergental et al., the perfusion parameters of our 4 donor livers suggest their viability [38]. Importantly, lactate is used as a significant marker to assess the function of liver grafts. During perfusion, our 4 liver grafts presented an interesting phenomenon of lactate clearance. The first liver graft showed a stable level of lactate during the middle of perfusion. The second showed a rapid decrease to below 2.5 mmol/L of lactate clearance. Compared with the situation of lactate in the first liver graft, the lactate level was slightly elevated during the initial perfusion, and lactate clearance rapidly decreased after 1.5 h. The fourth liver showed a gradual decrease of lactate. Finally, all lactate levels were below 2.5 mmol/L at the end of perfusion, meeting the criteria of Mergental et al. [38]. Although the lactate clearance was stable in the first liver graft, the peak post-transplant AST within 7 days was highest among these liver grafts. On the other hand, the presentation of lactate clearance in liver 1 during perfusion indicated that the liver graft parenchymal injury might be more serious because the liver could not clear lactate rapidly in the middle of perfusion. However, the peak post-transplant AST level within 7 days was the lowest, indicating the resuscitation function of NMP. Therefore, lactate clearance as a marker to evaluate liver viability might be incomplete. The rate of lactate clearance measured per unit of liver weight might better indicate viability [39].
During perfusion, perfusate glucose level increased early and stayed at a stable high level. Adding insulin did not affect glucose levels, which is inconsistent with a previous report [39]. Another group showed that bile pH values >7.5 are a better indicator of healthy cholangiocytes [13]. In all the 4 donor livers, the bile PH values were >7.5 at the end of perfusion. The glucose levels in bile of liver 1 indicated severe warm ischemia injury before perfusion, and the decreasing trend explained the recovery of cholangiocytes. Glucose levels in bile of the other livers were very low during perfusion. These results suggest that cholangiocytes secreted bicarbonate, resulting in an alkalotic pH, and resorbed the glucose in bile [35]. In addition, bile production is used as a marker of good liver function. The 4 donor livers showed differences in bile production. Nasralla et al. showed that although 18 livers produced minimal bile during perfusion, all presented with good post-transplant liver function, and there was no association between bile production and post-transplant liver function [13].
In liver transplantation, PRS is a common complication of transient hemodynamic changes occurring after liver graft reperfusion. According to the definition by Hilmi et al. [40], 1 case had PRS in this study. Studies have showed that, compared to static cold storage (SCS), NMP can reduce the incidence of PRS. In a study by Angelico et al. [41], 6 patients receiving grafts with NMP had lower incidence of PRS (0% versus 16.7%) compared to 12 matched controls preserved by SCS. Nasralla et al. also showed a significant reduction in PRS rate (12.4% versus 33%, p<0.001) in the NMP group versus the SCS group [13]. However, Watson et al. found that high oxygen tension in the perfusate during NMP can also lead to PRS [42]. In their study, in the first 6 cases, 5 cases experienced PRS when the mean PaO2 was between 621 and 671 mmHg. In contrast, PRS did not occur in the subsequent 6 cases when the level of PaO2 was reduced to 153–187 mmHg. Therefore, in our study, the target PaO2 level of 200 mmHg might be still too high for the liver graft. However, the optimal PaO2 level is unknown.
Our study has some limitations. The number of cases was small, which prevented us from forming strong conclusions. The UK DCD risk scores of cases 1, 2, and 3 were 7, 5, and 4, respectively. The ECD livers were not so severe. This is our preliminary attempt to use NMP to preserve donor livers in China. For the patient safety, we did not try NMP in more severe ECD livers in the beginning. Another limitation is the short follow-up of the patients after transplantation. These limitations need to be addressed in future studies.
Conclusions
This study is the first attempt to use NMP in liver transplantation in China. NMP is feasible as a preservation technique for assessment of ECD livers. Because there are many more DCD and DBCD donors in China, this novel preservation method might provide a valuable strategy to expand the organ pool and improve transplant outcomes.
Abbreviations
- EAD
early allograft dysfunction
- PNF
primary nonfunction
- NMP
normothermic machine perfusion
- WIT
warm ischemic time
- LT
liver transplantation
- DCD
donation after cardiac death
- IRI
ischemia reperfusion injury
- BMI
body mass index
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- UW
University of Wisconsin
- HA
hepatic artery
- PV
portal vein
- Tbil
total bilirubin
- ICU
Intensive Care Unit
- INR
international standardized ratio
- DBD
donation after brain death
- CIT
cold ischemic time
- MELD
model for end-stage liver disease score
- GGT
glutamyltransferase
- ALP
alkaline phosphatase
- PRS
postreperfusion syndrome
- SCS
static cold storage
- MAP
mean arterial pressure
Footnotes
Conflicts of interest
None.
Source of support: This study was supported by the following grants: the National Natural Science Foundation of China (81970564, 81471583 and 81570587), the Guangdong Provincial Key Laboratory Construction Projection on Organ Donation and Transplant Immunology (2013A061401007 and 2017B030314018), the Guangdong Provincial Natural Science Funds for Major Basic Science Culture Project (2015A030308010), the Guangdong Provincial Natural Science Funds for Distinguished Young Scholars (2015A030306025), the Special Support Program for Training High-Level Talents in Guangdong Province (2015TQ01R168), and the Science and Technology Program of Guangzhou (201704020150)
References
- 1.Starzl TE, Fung JJ. Themes of liver transplantation. Hepatology. 2010;51:1869–84. doi: 10.1002/hep.23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.OPTN/SRTR 2012 Annual Data Report: Deceased Organ Donation. http://srtr.transplant.hrsa.gov. [DOI] [PubMed]
- 3.Merion RM, Goodrich NP, Feng S. How can we define expanded criteria for liver donors? J Hepatol. 2006;45:484–88. doi: 10.1016/j.jhep.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Detry O, Deroover A, Meurisse N, et al. Donor age as a risk factor in donation after circulatory death liver transplantation in a controlled withdrawal protocol programme. Br J Surg. 2014;101:784–92. doi: 10.1002/bjs.9488. [DOI] [PubMed] [Google Scholar]
- 5.Jay CL, Lyuksemburg V, Ladner DP, et al. Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: A meta-analysis. Ann Surg. 2011;253:259–64. doi: 10.1097/SLA.0b013e318204e658. [DOI] [PubMed] [Google Scholar]
- 6.Ravikumar R, Leuvenink H, Friend PJ. Normothermic liver preservation: A new paradigm? Transpl Int. 2015;28:690–99. doi: 10.1111/tri.12576. [DOI] [PubMed] [Google Scholar]
- 7.Marecki H, Bozorgzadeh A, Porte RJ, et al. Liver ex situ machine perfusion preservation: A review of the methodology and results of large animal studies and clinical trials. Liver Transpl. 2017;23(5):679–95. doi: 10.1002/lt.24751. [DOI] [PubMed] [Google Scholar]
- 8.Boehnert MU, Yeung JC, Bazerbachi F, et al. Normothermic acellular ex vivo liver perfusion reduces liver and bile duct injury of pig livers retrieved after cardiac death. Am J Transplant. 2013;13:1441–49. doi: 10.1111/ajt.12224. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q, Nassar A, Farias K, et al. Sanguineous normothermic machine perfusion improves hemodynamics and biliary epithelial regeneration in donation after cardiac death porcine livers. Liver Transpl. 2014;20:987–99. doi: 10.1002/lt.23906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nassar A, Liu Q, Farias K, et al. Ex vivo normothermic machine perfusion is safe, simple, and reliable: Results from a large animal model. Surg Innov. 2015;22:61–69. doi: 10.1177/1553350614528383. [DOI] [PubMed] [Google Scholar]
- 11.Ravikumar R, Jassem W, Mergental H, et al. Liver transplantation after ex vivo normothermic machine preservation: A phase 1 (first-in-man) clinical trial. Am J Transplant. 2016;16:1779–87. doi: 10.1111/ajt.13708. [DOI] [PubMed] [Google Scholar]
- 12.Bral M, Gala-Lopez B, Bigam D, et al. Preliminary single centre Canadian experience of human normothermic ex vivo liver perfusion: Results of a clinical trial. Am J Transplant. 2017;17(4):1071–80. doi: 10.1111/ajt.14049. [DOI] [PubMed] [Google Scholar]
- 13.Nasralla D, Coussios CC, Mergental H, et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557(7703):50–56. doi: 10.1038/s41586-018-0047-9. [DOI] [PubMed] [Google Scholar]
- 14.Huang JF. Pragmatic solution for organ donation in response to challenges faced by the Chinese society: Summary for the National Donation after Circulatory Death Pilot Program. Chin Med J (Engl) 2013;126(3):569–73. [PubMed] [Google Scholar]
- 15.Yang L, Xin EY, Liao B, et al. Development and validation of a nomogram for predicting incidence of earlyallograft dysfunction following liver transplantation. Transplant Proc. 2017;49(6):1357–63. doi: 10.1016/j.transproceed.2017.03.083. [DOI] [PubMed] [Google Scholar]
- 16.Deschênes M, Belle SH, Krom RA, et al. Early allograft dysfunction after liver transplantation: a definition and predictors of outcome. National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Transplantation. 1998;66(3):302–10. doi: 10.1097/00007890-199808150-00005. [DOI] [PubMed] [Google Scholar]
- 17.Lozanovski VJ, Khajeh E, Fonouni H, et al. The impact of major extended donor criteria on graft failure and patient mortality after liver transplantation. Langenbecks Arch Surg. 2018;403:719–31. doi: 10.1007/s00423-018-1704-z. [DOI] [PubMed] [Google Scholar]
- 18.op den Dries S, Karimian N, Sutton ME, et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am Transplant. 2013;13:1327–35. doi: 10.1111/ajt.12187. [DOI] [PubMed] [Google Scholar]
- 19.Laing RW, Mergental H, Yap C, et al. Viability testing and transplantation of marginal livers (VITTAL) using normothermic machine perfusion: Study protocol for an open-label, non- randomised, prospective, single-arm trial. BMJ Open. 2017;7(11):e017733. doi: 10.1136/bmjopen-2017-017733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olthoff KM, Kulik L, Samstein B, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943–49. doi: 10.1002/lt.22091. [DOI] [PubMed] [Google Scholar]
- 21.Ge M, Yao W, Yuan D, et al. Brg1-mediated Nrf2/HO-1 pathway activation alleviates hepatic ischemia-reperfusion injury. Cell Death Dis. 2017;8(6):e2841. doi: 10.1038/cddis.2017.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barshes N, Horwitz I, Franzini L, et al. Waitlist mortality decreases with increased use of extended criteria donor liver grafts at adult liver transplant centers. Am J Transplant. 2007;7:1265–70. doi: 10.1111/j.1600-6143.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- 23.Xia W, Ke Q, Wang Y, et al. Donation after cardiac death liver transplantation: Graft quality evaluation based on pretransplant liver biopsy. Liver Transpl. 2015;21(6):838–46. doi: 10.1002/lt.24123. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz-Hernández M, Gómez-Dos Santos V, Díaz-Pérez D, et al. Experience with hypothermic machine perfusion in expanded criteria donors: Functional outcomes. Transplant Proc. 2019;51(2):303–6. doi: 10.1016/j.transproceed.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Dutkowski P, Polak WG, Muiesan P, et al. First comparison of hypothermic oxygenated perfusion versus static cold storage of human donation after cardiac death liver transplants: An international-matched case analysis. Ann Surg. 2015;262:764–70. doi: 10.1097/SLA.0000000000001473. [DOI] [PubMed] [Google Scholar]
- 26.Bruinsma BG, Yeh H, Ozer S, et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am J Transplant. 2014;14(6):1400–9. doi: 10.1111/ajt.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moers C, Pirenne J, Paul A, Ploeg RJ Machine Preservation Trial Study Group. Machine perfusion or cold storage in deceased donor kidney transplantation. N Engl J Med. 2012;366:770–71. doi: 10.1056/NEJMc1111038. [DOI] [PubMed] [Google Scholar]
- 28.Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am J Transplant. 2015;15:161–69. doi: 10.1111/ajt.12958. [DOI] [PubMed] [Google Scholar]
- 29.Deng R, Gu G, Wang D, et al. Machine perfusion versus cold storage of kidneys derived from donation after cardiac death: A meta-analysis. PLoS One. 2013;8(3):e56368. doi: 10.1371/journal.pone.0056368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutkowski P, Schlegel A, de Oliveira M, et al. HOPE for human liver grafts obtained from donors after cardiac death. J Hepatol. 2014;60:765–72. doi: 10.1016/j.jhep.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 31.Skaro AI, Jay CL, Baker TB, et al. The impact of ischemic cholangiopathy in liver transplantation using donors after cardiac death: The untold story. Surgery. 2009;146(4):543–52. doi: 10.1016/j.surg.2009.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Czigany Z, Lurje I, Tolba RH, et al. Machine perfusion for liver transplantation in the era of marginal organs – New kids on the block. Liver Int. 2019;39(2):228–49. doi: 10.1111/liv.13946. [DOI] [PubMed] [Google Scholar]
- 33.Tector AJ, Mangus RS, Chestovich P, et al. Use of extended criteria livers decreases wait time for liver transplantation without adversely impacting posttransplant survival. Ann Surg. 2006;244(3):439–50. doi: 10.1097/01.sla.0000234896.18207.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Figueras J, Busquets J, Grande L, et al. The deleterious effect of donor high plasma sodium and extended preservation in liver transplantation. A multivariate analysis. Transplantation. 1996;61(3):410–13. doi: 10.1097/00007890-199602150-00016. [DOI] [PubMed] [Google Scholar]
- 35.Saidi RF. Utilization of expanded criteria donors in liver transplantation. Int J Organ Transplant Med. 2013;4(2):46–59. [PMC free article] [PubMed] [Google Scholar]
- 36.Jay CL, Skaro AI, Ladner DP, et al. Comparative effectiveness of donation after cardiac death versus donation after brain death liver transplantation: Recognizing who can benefit. Liver Transpl. 2012;18(6):630–40. doi: 10.1002/lt.23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imber CJ, St Peter SD, Lopez De Cenarruzabeitia I, et al. Advantages of normothermic perfusion over cold storage in liver preservation. Transplantation. 2002;73(5):701–9. doi: 10.1097/00007890-200203150-00008. [DOI] [PubMed] [Google Scholar]
- 38.Mergental H, Perera MTPR, Laing RW, et al. Transplantation of declined liver allografts following normothermic ex-situ evaluation. Am J Transpl. 2016;16(11):3235–45. doi: 10.1111/ajt.13875. [DOI] [PubMed] [Google Scholar]
- 39.Watson CJE, Kosmoliaptsis V, Pley C, et al. Observations on the ex situ perfusion of livers for transplantation. Am J Transplant. 2018;18:2005–20. doi: 10.1111/ajt.14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilmi I, Horton CN, Planinsic RM, et al. The impact of postreperfusion syndrome on short-term patient and liver allograft outcome in patients undergoing orthotopic liver transplantation. Liver Transpl. 2008;14:504–8. doi: 10.1002/lt.21381. [DOI] [PubMed] [Google Scholar]
- 41.Angelico R, Perera MT, Ravikumar R, et al. Normothermic machine perfusion of deceased donor liver grafts is associated with improved postreperfusion hemodynamics. Transplant Direct. 2016;2:e97. doi: 10.1097/TXD.0000000000000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson CJE, Kosmoliaptsis V, Randle LV, et al. Normothermic perfusion in the assessment and preservation of declined livers before transplantation: hyperoxia and vasoplegia-important lessons from the first 12 cases. Transplantation. 2017;101:1084–98. doi: 10.1097/TP.0000000000001661. [DOI] [PMC free article] [PubMed] [Google Scholar]