Abstract
Background:
Electronic cigarettes (ECIGs) are a class of tobacco products that produce different effects (e.g., nicotine delivery), depending on the device, liquid, and behavioral factors. However, the influence of the two primary ECIG liquid solvents, propylene glycol (PG) and vegetable glycerin (VG), on ECIG acute effects is unknown.
Methods:
Thirty ECIG-experienced, ≥ 12-h nicotine- abstinent participants completed four conditions consisting of two ECIG-use bouts (10 puffs, 30 s interpuff-interval) differing only by liquid PG:VG ratio (2PG:98VG, 20PG:80VG, 55PG:45VG, 100PG). Device power (7.3 W) and liquid nicotine concentration (18 mg/ml) remained constant. Nicotine delivery, subjective effects, heart rate (HR), and puff topography were assessed.
Results:
In the 100PG condition, participants took shorter and smaller puffs but obtained significantly more nicotine relative to the two VG-based conditions. Total nicotine exposure (i.e., area under the curve) was also significantly higher during use of the two PG-based liquids. However, participants reported that the 100 PG liquid was significantly less “pleasant” and “satisfying” relative to the other liquids (all ps < .05). Increases in HR and decreases in abstinence symptoms (e.g., “craving”) did not differ across conditions.
Conclusions:
PG:VG ratio influenced nicotine delivery, some subjective effects, and puff topography. Lower overall product satisfaction associated with the 100PG liquid suggests factors other than nicotine delivery (e.g., aerosol visibility) may play a role in maintaining ECIG use. Regulating ECIG acute effects such as nicotine delivery and subjective effects may require simultaneous attention to liquid PG:VG ratio as well as device, liquid, and behavioral factors known to influence these outcomes.
Keywords: Electronic cigarettes, Propylene glycol, Vegetable glycerin, Nicotine delivery, Puff topography
1. Introduction
The use of electronic cigarettes (ECIGs) has increased exponentially in the U.S. (Jamal et al., 2017; Schoenborn and Gindi, 2015) and globally (Adkison et al., 2013) in recent years. ECIGs share several common features such as an electrical element that heats an often nicotine-containing liquid to produce an inhalable aerosol. However, ECIGs and their associated liquids can vary substantially on factors such as electrical power output (i.e., wattage) and the concentration of nicotine, flavorants, and solvents such as propylene glycol (PG) and vegetable glycerin (VG). A comprehensive understanding of how these various ECIG device/liquid characteristics, in conjunction with user puffing behaviors, influence ECIG users’ acute effects (e.g., nicotine delivery, subjective effects) may inform the regulation of these products.
Broadly, ECIG acute effects can be altered by three factors: device features, liquid components, and user puffing behaviors. For example, ECIGs operating at higher wattages deliver nicotine to the user more effectively than lower wattage devices (Wagener et al., 2017). In addition, increasing ECIG liquid nicotine concentration can increase nicotine delivery (Dawkins et al., 2016; Hiler et al., 2017) and alter subjective effect profiles, including greater suppression of nicotine-abstinence symptoms (Dawkins et al., 2013; Hiler et al., 2017) and higher product satisfaction (Hiler et al., 2017). Lastly, longer and larger puffs typically observed in experienced ECIG users result in greater nicotine delivery and suppression of abstinence symptoms relative to less intensive puffs typical of inexperienced users (Farsalinos et al., 2015; Hiler et al., 2017). However, the influence of other ECIG components such as the solvents PG and VG which are found, alone or in combination, in most ECIG liquids on the market (Breland et al., 2017) remains uncertain.
PG and VG act as a vehicle to carry nicotine and flavorants to the user’s mouth, throat, and/or lungs. ECIG users can purchase liquids containing various combinations of PG and VG (Breland et al., 2017), and anecdotal reports suggest that using different PG:VG ratios can alter aspects of ECIG use (Li et al., 2016). For instance, ECIG users report that liquids containing more PG provide a better “throat hit” and deliver more flavor while liquids containing more VG produce more exhaled aerosol (referred to colloquially as “clouds” or “vapor;” Li et al., 2016). Additionally, limited pre-clinical evidence suggests that when relevant device, liquid, and puff topography factors are held constant, ECIG liquids with higher proportions of PG produce aerosols containing more nicotine relative to liquids containing predominantly VG (Baassiri et al., 2017; Kosmider et al., 2014b). Taken together, anecdotal reports from ECIG users and the available preclinical evidence suggest that ECIG liquid PG:VG ratio may also influence important acute effects in ECIG users such as nicotine delivery and/or suppression of nicotine-abstinence symptoms.
The present study is the first to explore the extent to which ECIG liquid PG:VG ratio influences the acute effects of ECIG use including nicotine delivery, subjective effects, and puff topography. Given the possibility that PG:VG ratio could influence nicotine delivery (based on the available pre-clinical evidence), heart rate (HR) was also included as an outcome measure, as nicotine delivery from ECIGs (Hiler et al., 2017) and other tobacco products (Benowitz et al., 1988) is commonly associated with increases in HR. Similar to prior clinical laboratory examinations of ECIGs (e.g., Hiler et al., 2017) several device, liquid, and puff topography factors were held constant while PG:VG ratio was manipulated systematically, in order to elucidate the individual influence of these liquid solvents on ECIG acute effects.
2. Method
2.1. Participants
This study was approved by Virginia Commonwealth University’s (VCU’s) institutional review board (IRB). Potential participants were recruited by advertisements (posted online, throughout campus, and at local vape shops) and word-of-mouth (some participants were informed of the study by other individuals and not via advertisement exposure). Eligible participants were healthy (determined via self-reported medical history), over 110 pounds, aged 18–55, used < 5 tobacco cigarettes daily, used ≥ 1 ml of ECIG liquid daily, used ≥ 6 mg/ml nicotine concentration, and had used their ECIG ≥ 3 months. Given reports (e.g., Wagener et al., 2017) that some ECIG users use liquids containing < 6 mg/ml but consume far more than 1 ml of liquid daily (particularly users of more advanced, higher-powered devices), participants were also eligible if they used ≥ 10 ml of liquid daily of any active liquid nicotine concentration (i.e., excluding non-nicotine containing liquids). Exclusion criteria included: history of chronic disease or psychiatric condition, positive pregnancy test at screening, regular use of a prescription medication, marijuana use > 10 and alcohol use > 25 days in the past 30 (as in Cobb et al., 2010; Hiler et al., 2017), or use of illicit drugs (e.g., cocaine, methamphetamine) in the past 30 days (all according to self-report). Additionally, participants were deemed ineligible at screening if their: resting HR exceeded 110 beats per minute (bpm), systolic blood pressure (BP) exceeded 140 mm Hg or diastolic BP exceeded 100 mm Hg.
Forty-one individuals provided informed consent for the present study. Of these 41 individuals, eleven did not complete the study and were not included in the final analyses: four were determined ineligible at screening (two had been using ECIGs < 3 months, one used < 1 ml of ECIG liquid per day, and one weighed < 110 pounds), and the remaining seven were discontinued before study completion (three failed to attend study sessions, three lacked venous access, and one exhibited an elevated HR). Thus, thirty experienced ECIG users (29 men; 21 White/Caucasian) completed the study (see Supplementary Table 1). An a priori power analysis revealed that 27 participants were required to detect moderate effect sizes and obtain power of at least 0.80, assuming a moderate correlation among repeated measures (i.e., r ≥ 0.50), and an alpha error probability of < .05 (Barcikowski and Robey, 1985). Thus, using these criteria, 30 participants were sufficient to detect within-group differences for all outcome measures in the present study.
2.2. Materials
For all experimental sessions, participants used an “eGo” (3.3 V) battery with a 1.5 ohm (Ω), dual-coil, 510 “cartomizer” (7.3 W; SmokTech; Shenzhen, China). “Cartomizers” were filled with 1 ml of ECIG liquid (“Virginia Pure” tobacco flavor), containing 18 mg/ml of nicotine (AVAIL Vapor, Richmond, VA). Liquid PG:VG ratio differed by session. The PG:VG ratios as labeled by the vendor were: 100:0, 70:30, 30:70, and 0:100. Subsequent independent analysis (see Peace et al., 2016), revealed that the ratios were: 100:0, 55:45, 20:80, and 2:98. Liquid nicotine concentrations were independently verified as ± 1 mg/ml of the labeled concentrations. All “cartomizers” were verified with an Ohmmeter as ± 0.1 Ω of the purported resistance.
2.3. Procedure
Participants completed four sessions lasting ~ 3.5 h and separated by ≥ 48 h at VCU’s Clinical Behavioral Pharmacology Laboratory (CBPL). Session order was determined by Latin square and participants were blinded to the PG:VG ratio during each session. Participants were instructed to abstain from nicotine/tobacco and/or ECIG use for ≥ 12 h prior to each session. Abstinence from combustible products was verified using participants’ expired air carbon monoxide (CO; ≤ 10 ppm; as in Breland et al., 2002) and abstinence from noncombustible products (e.g., ECIGs) was verified retrospectively by confirming participants’ baseline plasma nicotine concentration was ≤ 5 ng/ml (as in Hiler et al., 2017; Spindle et al., 2017). Additionally, because of prior non-compliance with abstinence requirements by ECIG users (see Hiler et al., 2017), all participants underwent a one-hour observation period prior to each study session during which no nicotine/tobacco product use was permitted. Three study completers were considered to have not abstained prior to at least one session but these participants were ultimately included in all final analyses because the overall study findings were unaffected upon their exclusion and the higher N improved statistical power.
Following the one-hour observation period, an intravenous catheter was inserted into a forearm vein of the participant and monitoring of HR commenced. Thirty minutes after catheter insertion, a baseline blood sample was taken, and participants completed a “directed” ECIG use bout consisting of 10 puffs with 30 s inter-puff-interval (IPI). Participants completed a second ECIG use bout (60 min after the first) to determine the reliability of the results observed after the first bout. Importantly, these “directed” puffing procedures (i.e., two 10-puff bouts with 30 s IPI, separated by 60 min) have been used in examinations of various tobacco products (e.g., little cigars/cigarillos: Blank et al., 2011; ECIGs: Hiler et al., 2017; and tobacco cigarettes: Vansickel et al., 2010), allowing for direct comparisons of acute effects (e.g., nicotine delivery) across products. Additional blood samples were taken at 5, 15, 30, 45, and 55 min after the onset of bout 1 and 5, 15, 30, and 45 min after the onset of bout 2. Subjective questionnaires were administered immediately following each blood sampling. Participants were compensated after each session (US $75 after first and second sessions, $150 after the third, and $200 after the fourth).
2.4. Outcome measures
2.4.1. Physiological measures
Blood samples were analyzed for nicotine concentrations by VCU’s Bioanalytical Shared Resource Laboratories using LC–MS/MS analysis; limit of quantitation (LOQ): 2 ng/ml (see Breland et al., 2006). HR was monitored using Criticare Systems model 507 (Waukesha, Wisconsin) and expired air CO was measured using a BreathCO monitor (Vitalo-graph, Lenexa, KS).
2.4.2. Puff topography
A mouthpiece-based topography device developed and manufactured at the American University of Beirut (used in prior ECIG studies: Hiler et al., 2017; Spindle et al., 2017) integrated flow rate data to generate values for puff number, duration, volume, IPI, and mean flow rate (see Shihadeh et al., 2004). Mouthpieces manufactured for the device were calibrated prior to each session using an automatic digital flow calibrator.
2.4.3. Subjective questionnaires
Four of the five subjective questionnaires were administered using a computerized visual analog scale (VAS), containing a word/phrase in the middle of a horizontal line with “not at all” on the left and “extremely” on the right. Participants recorded responses by clicking at any point on the line, with scores expressed as a percentage of total line length (i.e., 0–100). Nicotine abstinence symptoms were assessed using the Hughes-Hatsukami withdrawal scale (11 items; Hughes and Hatsukami, 1986) and the Tiffany Drobes-QSU Brief consisting of 10 items forming two factors: (1) intention to use one’s product and (2) anticipation from relief from abstinence symptoms (Tiffany and Drobes, 1991). Additional VAS questionnaires included the Direct Effects of Nicotine (10 items; Evans et al., 2006) and Direct Effects of ECIG-use scales (10 items; adapted from Pickworth et al., 1994; Foulds et al., 1992). The fifth questionnaire (the general labeled magnitude scale, gLMS; adapted from Green et al., 1993) assessed the flavor sensation, harshness/irritancy, and throat hit provided by the ECIG using a category-ratio scale with seven semantic labels ranging from (0) “no sensation” to (100) “strongest imaginable sensation of any kind.” This questionnaire was administered (via pen and paper) after the two ECIG bouts in each session.
2.5. Data preparation and analysis
Plasma nicotine values below the assay’s LOQ were replaced with the LOQ (2 ng/ml; as in Vansickel et al., 2010), providing a more conservative approach than assuming that each value below the LOQ was zero. Total nicotine exposure was assessed by calculating the area under the curve (AUC) for both 10-puff directed bouts (bout 1 AUC: timepoints 1–5; bout 2 AUC: timepoints 6–10) in each condition using the linear trapezoidal method (as in see Vaughan and Dennis, 1978). HR data were averaged to produce single values for the five minutes prior to each ECIG-use bout and blood sample. The topography recording equipment’s software used two data cleaning procedures automatically in real-time to correct for measurement error: merging puffs that were separated by 300 ms or less into a single puff and deleting puffs ≤ 300 ms. Remaining data for each topography variable were averaged for all participants to produce single values for each 10-puff bout.
Repeated measures analysis of variance (ANOVA) was used to examine all outcomes measures. Four (condition) by ten (time) repeated measures ANOVAs were used to examine plasma nicotine and HR; AUC analysis contained two levels of time (one for each ECIG-use bout). Questionnaire items (or factors for the QSU Brief) were examined individually using separate ANOVAs. Analyses conducted on the Hughes-Hatsukami, Tiffany Drobes-QSU, and Direct Effects of Nicotine scales had 10 levels of time, the Direct Effects of ECIG use scale had nine levels of time (scores could not be obtained before ECIG use), and the gLMS had two levels of time, as it was only administered after each ECIG-use bout. Puff topography variables that were not held constant (i.e., puff duration, puff volume, and flow rate) were analyzed using condition and time (two levels) as the within-subject factors. Only 29 participants were included in the puff topography analyses, as a malfunction of the topography recording device resulted in incomplete data for one participant.
Violations of sphericity were adjusted using Huynh-Feldt corrections. In order to maintain statistical power and limit type 1 error for plasma nicotine, HR, and subjective effects, planned contrasts (paired samples t-tests) were conducted across conditions at the two timepoints immediately after each ECIG-use bout (e.g., timepoints 2 and 7 for plasma nicotine). At these two post-bout timepoints, the mean value for each outcome measure in the 100 PG condition was compared to the corresponding mean values in the 2PG:98VG, 20PG:80VG, and 55 PG:45VG conditions. Because these comparisons were non-orthogonal, a Bonferroni correction was applied (Keppel, 1991). Because three comparisons were made at each timepoint, the threshold for statistical significance for these planned comparisons was: p < .017. For all other post-hoc tests, Tukey’s Honestly Significant Difference (HSD) was used to explore significant main effects and interactions; these calculations were performed manually using parameters from the respective repeated measures ANOVAs and a studentized range distribution (q) table: HSD = (Tukey, 1949). All other statistical analyses were performed using IBM SPSS (Version 24.0).
3. Results
Results from all outcome measures are described below. Supplementary Table 2 displays results from the statistical analyses (main effects and interactions) for plasma nicotine, AUC, and all subjective effect measures.
3.1. Plasma nicotine
Significant main effects of time and condition, but no time by condition interaction, were observed for plasma nicotine. Fig. 1a depicts the mean plasma nicotine results for each condition and timepoint. Collapsed across condition, mean (SD) plasma nicotine concentration increased significantly from 2.60 ng/ml (1.85) at baseline to 10.40 ng/ml (8.11) immediately after bout 1 and to 11.11 ng/ml (6.80) immediately after bout 2. Planned contrasts did not reveal significant differences across conditions after bout 1. However, after bout 2, the mean (SD) plasma nicotine concentration of 13.40 ng/ml (8.99) in the 100 PG condition was significantly higher relative to the mean plasma nicotine concentration of 9.59 ng/ml (7.95) in the 20 PG:80VG condition and 8.58 ng/ml (5.41) in the 2 PG:98VG condition [ts (29) > 2.56, p < .017].
Fig. 1.
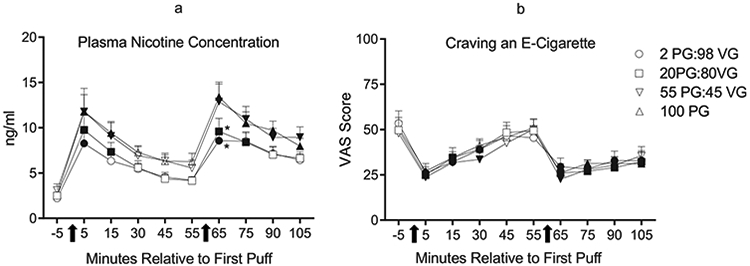
a: Mean plasma nicotine concentration (+SEM) from 30 ECIG-experienced participants during four independent sessions that differed only by PG:VG ratio. Arrows beneath the x-axis indicate the onset of each 10-puff ECIG use bout. Units on the x-axis represent the timepoints at which blood was sampled from participants relative to their first puff in bout 1. Filled symbols indicate a significant difference from baseline (−5 timepoint; Tukey’s HSD). Asterisks (*) indicate significant differences from the 100 PG:0VG condition at that timepoint (planned contrasts with Bonferroni correction: ps < .017). b: Mean ratings (+SEM) for the visual analog scale (VAS) item, “Craving an e-cigarette” from the Hughes-Hatsukami withdrawal scale. In all other respects the figure is identical to Fig. 1a.
Main effects of condition and time, but no time by condition interaction, were observed for AUC. For bout 1 (i.e., timepoints 1–5), the mean (SD) AUC for the 100 PG condition of 276.75 ng min/ml (221.49) was significantly greater relative to the mean AUC for the 2 PG:98VG condition of 178.32 ng min/ml (183.76; Tukey’s HSD, p < .05). For bout 2 (i.e., timepoints 6–10), the mean (SD) AUC for the 100PG condition of 373.24 ng min/ml (274.09) was significantly greater than the mean AUC of 257.75 ng min/ml (217.26) in the 2 PG:98VG condition and 251.93 ng min/ml (224.49) in the 20PG:80VG condition (Tukey’s HSD, p < .05; Supplementary Fig. 1). Collapsed across condition, mean AUC increased significantly from bout 1 to bout 2.
3.2. Heart rate
A significant main effect of time but no significant main effect of or interaction with condition was observed for HR. Collapsed across condition, mean (SD) HR increased significantly from 63.71 bpm (5.37) at baseline to 70.98 bpm (5.87) immediately after bout 1 and 70.92 bpm (6.86) immediately after bout 2 (Tukey’s HSD, p < .05). Planned contrasts did not reveal any significant differences across conditions after either ECIG bout.
3.3. Subjective measures: abstinence symptom suppression
3.3.1. Hughes-Hatsukami Withdrawal Scale
A significant main effect of time (but no significant main effect of condition or time by condition interaction) was observed for “Anxious,” “Craving,” “Difficulty concentrating,” “Drowsy,” “Hunger,” “Impatient,” “Irritable,” “Restlessness,” and “Urge.” Fig. 1b shows the results for “Craving” (the item with the largest F value for the main effect of time). Scores for “Anxious,” “Craving,” “Difficulty Concentrating,” “Drowsy,” and “Urge” were reduced significantly following both ECIG bouts relative to baseline (Tukey’s HSD, ps < .05). For example, collapsed across condition, mean (SD) VAS score for “Urge to use an ECIG” decreased significantly from 53.83 (32.71) at baseline to 28.49 (25.76) after bout 1 and 29.29 (26.97) after bout 2. Planned contrasts did not detect significant differences across conditions for any questionnaire item.
3.3.2. Tiffany Drobes QSU-brief
A significant main effect of time was observed for both QSU factors, and a significant main effect of condition was observed for factor 2 (a significant time by condition interaction was not observed for either factor). Despite the observed main effect of condition, planned contrasts did not reveal any significant differences across conditions at the post-ECIG use timepoints for factor 2. Collapsed across condition, factor 1 scores were reduced significantly after each directed bout relative to baseline. For example, the mean (SD) score for factor 1 was reduced significantly from 21.34 (8.28) at baseline to 13.96 (7.71) after bout 1 and 12.62 (7.73) after bout 2 (Tukey’s HSD, ps < .05).
3.4. Subjective measures: other ECIG sensory effects
3.4.1. Direct effects of nicotine
Significant main effects of time (but no main effects of or interactions with condition) were observed for “Dizzy” and “Lightheaded.” However, post-hoc testing for these items did not detect differences from baseline for any timepoint. Planned contrasts did not reveal differences across conditions for any questionnaire item.
3.4.2. Direct effects of ECIG use
A significant time by condition interaction was observed for the item “Awake;” participants reported feeling significantly less “Awake” in the 100PG condition relative to the 2PG:98VG condition after bout 1 [t (29) < −2.65, p < .017]. Significant main effects of time were observed for “Awake,” “Calm,” “Dizzy,” “Pleasant,” “Reduce Hunger,” “Right Now,” and “Satisfying” but no comparisons to baseline were possible for this questionnaire (see Method). Main effects of condition were observed for “Awake,” “Calm,” “Concentrate,” “Pleasant,” “Satisfying,” and “Taste Good.” Planned contrasts conducted at the two post-ECIG use timepoints revealed significantly lower scores for “Awake,” “Calm,” “Pleasant,” and “Satisfying” in 100PG condition relative to one or more of the other conditions (see Fig. 2a-d). For example, participants reported that the ECIG containing only PG was less “Pleasant” and less “Satisfying” than the 2 PG:98VG and 20PG:80VG liquids [ts (29) < −2.61, ps < .017].
Fig. 2.
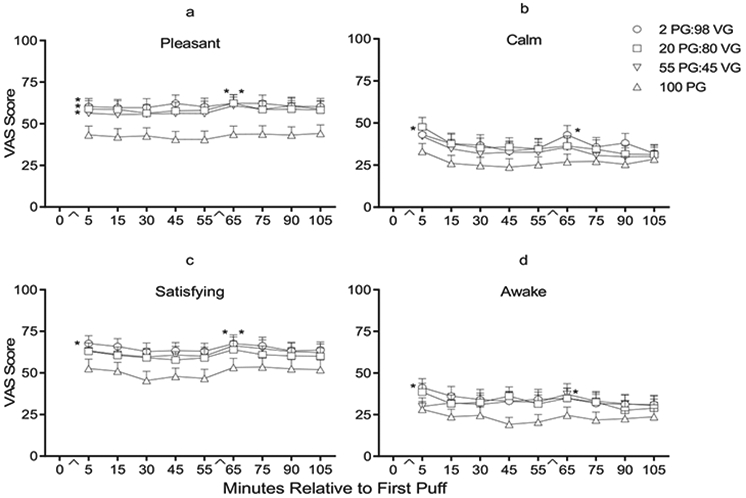
a–d: Mean ratings (+SEM) for four visual analog scale (VAS) items from the Direct Effects of ECIG use scale: “Was the ECIG pleasant?” (top left), “Did the ECIG make you feel more calm?” (top right), “Was the ECIG Satisfying?” (bottom left), and “Did the ECIG make you feel more awake?” (bottom right). In all other respects the figure is identical to Fig. 1a.
3.4.3. General labeled magnitude scale
Significant main effects of condition were observed for “Harshness/Irritancy” and “Throat Hit.” For “Harshness,” planned contrasts revealed that immediately after bout 2, the mean score observed in the 100 PG condition was significantly higher relative to those observed in the 2 PG:98VG and 20 PG:80VG conditions [ts (29) > 3.07, ps < .017]. For “Throat Hit,” mean scores in the 100 PG condition after bouts 1 and bout 2 were significantly greater relative to scores in the 2 PG:98VG and 20 PG:80VG conditions at the corresponding timepoints [ts (29) > 3.32, ps < .017].
3.5. Puff topography
Mean (SD) puff duration, puff volume, and flow rate values are displayed in Table 1. For puff duration, a significant condition by time interaction [F (3, 84) = 3.45, p < .05] and significant main effects of time [F (1, 28) = 28.33, p < .001] and condition [F (3, 84) = 12.34, p < .001] were observed. During bout 1, participants took significantly longer puffs in the 2 PG:98VG condition relative to the 55 PG:45VG and 100 PG conditions; during bout 2, participants took significantly longer puffs in the 2 PG:98VG condition relative to all other conditions (Tukey’s HSD, p < .05). In addition, within all conditions except the 100 PG condition, participants took longer puffs, on average, in the second bout relative to the first (Tukey’s HSD, p < .05).
Table 1.
Mean (SD) puff topography results by condition and bout.
| ECIG Liquid PG:VG Ratio | ||||||||
|---|---|---|---|---|---|---|---|---|
| Bout 1 |
Bout 2 |
|||||||
| 2:98 | 20:80 | 55:45 | 100:0 | 2:98 | 20:80 | 55:45 | 100:0 | |
| Puff Duration (sec) | 5.26* | 4.99* | 4.47 | 4.32 | 5.90*+ | 5.32*+ | 4.91*+ | 4.48 |
| (1.95) | (1.99) | (1.52) | (1.35) | (2.26) | (2.23) | (1.58) | (1.44) | |
| Puff Volume (ml) | 115.45* | 108.85* | 96.81 | 100.25 | 133.92*+ | 121.69*+ | 110.89*+ | 103.09 |
| (58.28) | (51.84) | (51.61) | (47.12) | (67.22) | (68.63) | (55.14) | (50.86) | |
| Flow Rate (ml/s) | 21.88 | 21.84 | 21.52 | 22.97 | 22.66 | 22.57 | 22.43 | 22.86 |
| (8.12) | (6.56) | (7.85) | (6.89) | (7.21) | (8.97) | (8.59) | (7.41) | |
Mean (SD) puff parameters for ECIG-experienced (N = 29) individuals for two 10-puff directed ECIG-use bouts (30 s IPI). A malfunction of the topography recording device resulted in incomplete data for one participant out of the 30 completers who were included in all other analyses. Asterisks
indicate significant differences from the 100 PG condition within that bout and plus signs
indicate differences from bout 1 within that condition (Tukeys HSD; ps < .05).
For puff volume, significant main effects of time [F (1, 28) = 33.78, p < .001] and condition [F (3, 84) = 3.97, p < .05] were observed but no significant time by condition interaction. Participants took significantly larger puffs in the 2 PG:98VG condition relative to the 55 PG:45VG and 100 PG conditions. During bout 2, participants took significantly larger puffs in the 2PG:98VG condition relative to all other conditions (Tukey’s HSD, p < .05). Additionally, within all conditions except the 100 PG condition, participants exhibited longer mean puff volume in the second bout relative to the first (Tukey’s HSD, p < .05). No significant main effects or interactions were observed for flow rate.
4. Discussion
The primary purpose of the present study was to elucidate the influence of ECIG liquid PG:VG ratio on nicotine delivery, HR, subjective effects, and puff topography in experienced ECIG users. PG-based liquids (i.e., 100 PG and 55 PG:VG) delivered more nicotine, despite participants taking significantly shorter and smaller puffs in these conditions, relative to VG-based liquids (i.e., 2 PG:98VG and 20 PG:80VG). Overall the pure PG liquid was perceived to be significantly less favorable relative to all other liquids. Increases in HR and suppression of abstinence symptoms were observed after ECIG use (as in Dawkins et al., 2016; Hiler et al., 2017), but these outcomes were not influenced by liquid PG:VG ratio. Collectively, results from this study suggest that ECIG liquid PG:VG ratio may be relevant to regulating ECIG user nicotine intake and other effects.
Consistent with pre-clinical examinations of ECIG nicotine emissions (e.g., Baassiri et al., 2017), the present study demonstrated that holding the relevant device and liquid factors constant, PG-based liquids delivered more nicotine to users relative to VG-based liquids. PG was also a more efficient vehicle for nicotine delivery, as participants took shorter and smaller puffs when using PG-based liquids but still obtained higher plasma nicotine concentrations in these conditions. These observed differences in nicotine delivery could be the result of PG having a lower threshold for evaporation, and thus greater volatility, than VG (Talih et al., 2017) and/or because aerosols generated from PG-based liquids contain significantly smaller particles (Baassiri et al., 2017) that likely are deposited to a greater extent in a user’s lungs and absorbed more readily into their bloodstream (Heyder, 2004; Zhang et al., 2013). Given that PG:VG ratio can also influence emissions of other toxicants (e.g., carbonyl compounds: Kosmider et al., 2014a; free radicals: Bitzer et al., 2017), future research should explore whether ECIG users’ toxicant exposure is affected by liquid PG and VG concentrations.
Abstinence symptoms were suppressed following ECIG use, although the magnitude of suppression did not differ across PG:VG ratio. However, when using the 100PG liquid, participants reported less overall product satisfaction as evidenced by significantly lower ratings on “Satisfaction” and “Pleasantness” and significantly higher ratings on “Harshness/Irritancy.” Thus, interestingly, participants favored the VG-based liquids over the 100PG liquid, despite these two liquids delivering less nicotine. The lower preference for the 100PG liquid may be because pure PG liquids produce little to no visible exhaled aerosol (Baassiri et al., 2017; Li et al., 2016). ECIG users cite the ability to produce large “clouds” of aerosol from their device as an important positive aspect of ECIG use (Kim et al., 2017). Furthermore, the presence of non-nicotine behavioral stimuli, such as the sight of exhaled aerosol/smoke, can increase product satisfaction for other tobacco products (e.g., cigarettes; Rose et al., 2003; Buchhalter et al., 2005). Additional research whereby participants are blinded as to whether their ECIG is producing an aerosol may be necessary to understand the importance of aerosol visibility on ECIG user subjective effects.
Interestingly, PG:VG ratio significantly influenced puff topography such that participants took significantly longer and larger puffs when using the two VG-based liquids. These observed alterations in puff topography could reflect an attempt by participants to titrate their dose of nicotine as seen in previous examinations of cigarette smokers (Ashton et al., 1979). For example, participants may have increased their puffing intensity when using the 2PG:98VG liquid in order to extract more nicotine from the ECIG. Conversely, increased perceptions of “Harshness/Irritancy” and “Throat Hit” may have made the 100PG liquid more difficult to inhale, resulting in shorter and smaller puffs in this condition. Future studies should examine whether these observed differences in puff topography across PG:VG ratio persist over longer periods of time and whether puff topography is also influenced by an additional device (e.g., Ohms) or liquid factors (e.g., liquid pH).
There were several limitations to this study. First, participants were not permitted to use their preferred ECIG and liquid. Further research is necessary to determine whether PG:VG ratio influences nicotine delivery from ECIGs operating at higher power settings than those used in the present study, as vaporization rates of PG and VG can become more comparable at higher temperatures that may be associated with newer ECIG models (Talih et al., 2017). Second, the controlled puffing parameters (i.e., 10-puffs, 30 s IPI) used may have altered study outcomes. ECIG users may increase their mean puff number, duration, and volume during ad libitum ECIG use (Spindle et al., 2017), suggesting PG:VG ratio may have further influenced ECIG effects under more naturalistic puffing conditions. However, directed puffing conditions maintained internal validity and made the results more interpretable. Third, puff duration and puff volume were not controlled, likely reducing the influence of PG:VG ratio on nicotine delivery, as participants took shorter and smaller puffs in the PG-based conditions. Future studies may consider holding puff duration and puff volume constant, as in previous examinations of cigarette smokers (e.g., Zacny and Stitzer, 1988). Conversely, the observed differences in puff topography across PG:VG ratio highlights the importance of pre-clinical studies using real-world puff topography data when generating aerosols in order to assess toxicant yields more precisely. Fourth, the present study relied primarily on participant self-report during recruitment. Future studies may benefit from objectively verifying aspects of participants’ preferred devices and liquids (e.g., testing liquid nicotine concentration or device wattage) and their status as ECIG users (e.g., examining urine cotinine at baseline). In a similar vein, objective testing for the use of other licit and illicit substances may also strengthen future examinations. Lastly, consistent with prior clinical laboratory examinations of ECIG users (e.g., Dawkins et al., 2016; Hajek et al., 2017) and several nationally representative surveys (e.g., Jamal et al., 2017; Syamlal, 2016) the majority of participants (29 of 30) in this study were men. The inclusion of additional women and other types of ECIG users (e.g., dual users of cigarettes and ECIGs) may have improved the generalizability of these findings.
Results from the present study have important implications for regulating ECIGs. Specifically, PG:VG ratio should now be added to the growing list of devices (e.g., battery voltage/heater resistance; Wagener et al., 2017), liquid (liquid nicotine concentration; Hiler et al., 2017) and puff topography factors (e.g., puff duration; Hiler et al., 2017) that have been shown to influence ECIG user nicotine delivery and thus should be considered by future regulations intending to control nicotine delivery from these products. Assigning a standard PG:VG ratio for all ECIG liquids may reduce the influence of this factor on ECIG effects, although more work is necessary to determine which PG:VG ratio would be optimal. Regulatory bodies may also want to consider approaches that can accurately predict ECIG nicotine emissions based on multiple device, liquid, and puff topography factors, such as the proposed nicotine flux model (Shihadeh and Eissenberg, 2015).
In conclusion, results from the present study revealed that the ECIG liquid containing PG as the only solvent delivered more nicotine, reduced mean puff duration and volume, and was less satisfying overall to participants than the two VG-based liquids. These results could suggest that a user’s overall satisfaction with an ECIG may be influenced by factors aside from nicotine delivery such as the sight of aerosol. Additionally, after ECIG use, similar increases in HR and suppression of abstinence symptoms were observed, regardless of the PG:VG ratio of the liquid being used. Regulatory efforts intending to control the acute effects of ECIG use such as nicotine delivery and subjective effects should consider liquid PG:VG ratio along with other device, liquid, and puff topography factors known to influence these outcomes.
Supplementary Material
Acknowledgments
Role of funding sources
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036105 and F31DA040319 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration. NIH, NIDA, and FDA had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit for publication.
Acknowledgements
This manuscript is based on research performed for a doctoral dissertation that was conducted, completed, and defended at Virginia Commonwealth University (VCU). The dissertation is published in its entirety on Scholar Compass and ProQuest. Portions of this work were presented at the 24th annual meeting of the Society for Research on Nicotine and Tobacco and the 79th annual meeting of the College on Problems of Drug Dependence. We would like to thank Barbara Kilgalen and Janet Austin for their assistance in data collection and management and Dr. Justin Poklis of VCU’s Department of Pharmacology and Toxicology for analyzing the PG and VG concentrations of the ECIG liquids used in this study.
Footnotes
Conflict of interests
Dr. Eissenberg and Dr. Shihadeh are paid consultants in litigation against the tobacco industry and are named on a patent application for a device that measures the puffing behavior of electronic cigarette users. All other authors have no conflicts to report.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.drugalcdep.2018.03.042.
References
- Adkison SE, O’Connor RJ, Bansal-Travers M, Hyland A, Borland R, Yong HH, Cummings KM, McNeill A, Thrasher JF, Hammond D, Fong GT, 2013. Electronic nicotine delivery systems: international tobacco control four-country survey. Am. J. Prev. Med 44 (3), 207–215. 10.1016/j.amepre.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton H, Stepney R, Thompson JW, 1979. Self-titration by cigarette smokers. Br. Med. J 2, 357–360. 10.1136/bmj.2.6186.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baassiri M, Talih S, Salman R, Karaoghlanian N, Saleh R, El Hage R, Saliba N, Shihadeh A, 2017. Clouds and throat hit: effects of liquid composition on nicotine emissions and physical characteristics of electronic cigarette aerosols. Aerosol. Sci. Technol 51, 1231–1239. 10.1080/02786826.2017.1341040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcikowski RS, Robey RR, 1985. Sample size selection in single group repeated measures analysis. In: Paper Presented at the Annual Meeting of the American Educational Research Association Chicago, IL: . Retrieved from: https://files.eric.ed.gov/fulltext/ED257860.pdf. [Google Scholar]
- Benowitz NL, Porchet H, Sheiner L, Jacob P, 1988. Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin. Pharmacol. Ther 44, 23–28. 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- Bitzer ZT, Goel R, Reilly SM, Foulds J, Muscat J, Elias RJ, Richie JP, 2017. Solvent and temperature effects on free radical formation in electronic cigarette aerosols. Chem. Res. Toxicol 31, 4–12. 10.1021/acs.chemrestox.7b00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank MD, Nasim A, Hart A Jr., Eissenberg T, 2011. Acute effects of cigarillo smoking. Nicotine Tob. Res 13, 874–879. 10.1093/ntr/ntr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland AB, Buchhalter AR, Evans SE, Eissenberg T, 2002. Evaluating acute effects of potential reduced-exposure products for smokers: clinical laboratory methodology. Nicotine Tob. Res 4, 131–140. 10.1080/1462220021000032780. [DOI] [PubMed] [Google Scholar]
- Breland AB, Kleykamp BA, Eissenberg T, 2006. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine Tob. Res 8, 727–738. 10.1080/14622200600789585. [DOI] [PubMed] [Google Scholar]
- Breland A, Soule E, Lopez A, Ramôa C, El-Hellani A, Eissenberg T, 2017. Electronic cigarettes: what are they and what do they do? Ann. N.Y. Acad. Sci 1394, 5–30. 10.1111/nyas.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchhalter AR, Acosta MC, Evans SE, Breland AB, Eissenberg T, 2005. Tobacco abstinence symptom suppression: the role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction 100, 550–559. 10.1111/j.1360-0443.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- Cobb CO, Shihadeh A, Weaver MF, Eissenberg T, 2010. Waterpipe tobacco smoking and cigarette smoking: a direct comparison of toxicant exposure and subjective effects. Nicotine Tob. Res 13, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins L, Turner J, Crowe E, 2013. Nicotine derived from the electronic cigarette improves time-based prospective memory in abstinent smokers. Psychopharmacology (Berl.) 227, 377–384. 10.1007/s00213-013-2983-2. [DOI] [PubMed] [Google Scholar]
- Dawkins LE, Kimber CF, Doig M, Feyerabend C, Corcoran O, 2016. Self-titration by experienced e-cigarette users: blood nicotine delivery and subjective effects. Psychopharmacology (Berl.) 233, 2933–2941. 10.1007/s00213-016-4338-2. [DOI] [PubMed] [Google Scholar]
- Evans SE, Blank M, Sams C, Weaver MF, Eissenberg T, 2006. Transdermal nicotine-induced tobacco abstinence symptoms suppression: nicotine dose and smokers’ gender. Exp. Clin. Psychopharmacol 14, 121–135. 10.1037/1064-1297.14.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Spyrou A, Stefopoulos C, Tsimopoulou K, Kourkoveli P, Tsiapras D, Kyrzopoulos S, Poulas K, Voudris V, 2015. Nicotine absorption from electronic cigarette use: comparison between experienced consumers (vapers) and naive users (smokers). Sci. Rep 5, 11269 10.1038/srep11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Feyerabend C, Vesey C, Jarvis M, Russell MA, 1992. Effect of transdermal nicotine patches on cigarette smoking: a double blind crossover study. Psychopharmacology (Berl.) 106, 421–427. 10.1007/BF02245429. [DOI] [PubMed] [Google Scholar]
- Green BG, Shaffer GS, Gilmore MM, 1993. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem. Senses 18, 683–702. 10.1093/chemse/18.6.683. [DOI] [Google Scholar]
- Hajek P, Przulj D, Phillips A, Anderson R, McRobbie H, 2017. Nicotine delivery to users from cigarettes and from different types of e-cigarettes. Psychopharmacology (Berl.) 234, 773–779. 10.1007/s00213-016-4512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyder J, 2004. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc. Am. Thorac. Soc 1, 315–320. 10.1513/pats.200409-046TA. [DOI] [PubMed] [Google Scholar]
- Hiler M, Breland A, Spindle TR, Maloney S, Lipato T, Karaoghlanian N, Shihadeh A, Lopez A, Ramôa C, Eissenberg T, 2017. Electronic cigarette user plasma nicotine concentration, puff topography, heart rate, and subjective effects: influence of liquid nicotine concentration and user experience. Exp. Clin. Psychopharmacol 25, 380–392. 10.1037/pha0000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D, 1986. Signs and symptoms of tobacco withdrawal. Arch. Gen. Psychiatry 43, 289–294. 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Jamal A, Gentzke A, Hu SS, Cullen KA, Apelberg BJ, Homa DM, King BA, 2017. Tobacco use among middle and high school students—United States, 2011–2016. Morb. Mortal. Wkly. Rep 65 10.15585/mmwr.mm6623a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G, 1991. Design and Analysis: A Researcher’s Handbook. Prentice Hall, New Jersey. [Google Scholar]
- Kim H, Davis AH, Dohack JL, Clark PI, 2017. E-cigarettes use behavior and experience of adults: qualitative research findings to inform e-cigarette use measure development. Nicotine Tob. Res 19, 190–196. 10.1093/ntr/ntw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, Goniewicz ML, 2014a. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob. Res 16, 1319–1326. 10.1093/ntr/ntu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider L, Sobczak A, Knysak J, Goniewicz ML, 2014b. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob. Res 16, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhan Y, Wang L, Leischow SJ, Zeng DD, 2016. Analysis of symptoms and their potential associations with e-liquids’ components: a social media study. BMC Public Health 16, 674 10.1186/s12889-016-3326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peace MR, Baird TR, Smith N, Wolf CE, Poklis JL, Poklis A, 2016. Concentration of nicotine and glycols in 27 electronic cigarette formulations. J. Anal. Toxicol 40, 403–407. 10.1093/jat/bkw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickworth WB, Bunker EB, Henningfield JE, 1994. Transdermal nicotine: reduction of smoking with minimal abuse liability. Psychopharmacology (Berl.) 115, 9–14. 10.1007/BF02244745. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Bates JE, Salley A, 2003. Pharmacologic and sensorimotor components of satiation in cigarette smoking. Pharmacol. Biochem. Behav 76, 243–250. 10.1016/j.pbb.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Schoenborn CA, Gindi RM, 2015. Electronic Cigarette Use Among Adults: United States, 2014. pp. 1–8. NCHS data brief 217. https://www.cdc.gov/nchs/data/databriefs/db217.pdf. [PubMed] [Google Scholar]
- Shihadeh A, Eissenberg T, 2015. Electronic cigarette effectiveness and abuse liability: predicting and regulating nicotine flux. Nicotine Tob. Res 17, 158–162. 10.1093/ntr/ntu175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihadeh A, Azar S, Antonius C, Haddad H, 2004. Towards a topographical model of narghile water-pipe café smoking: a pilot study in a high socioeconomic status neighborhood of Beirut, Lebanon. Pharmacol. Biochem. Behav 79, 75–82. 10.1016/j.pbb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Spindle T, Hiler M, Breland A, Karaoghlanian N, Shihadeh AL, Eissenberg T, 2017. The influence of a mouthpiece-based topography measurement device on electronic cigarette user’s plasma nicotine concentration, heart rate, and subjective effects under directed and ad libitum use conditions. Nicotine Tob. Res 19, 469–476. 10.1093/ntr/ntw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syamlal G, 2016. Electronic cigarette use among working adults—United States, 2014. Morb. Mortal. Wkly. Rep 65, 557–561. 10.15585/mmwr.mm6522a1. [DOI] [PubMed] [Google Scholar]
- Talih S, Balhas Z, Salman R, El-Hage R, Karaoghlanian N, El-Hellani A, Baassiri M, Jaroudi E, Eissenberg T, Saliba N, Shihadeh A, 2017. Transport phenomena governing nicotine emissions from electronic cigarettes: model formulation and experimental investigation. Aerosol. Sci. Technol 51, 1–11. 10.1080/02786826.2016.1257853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ, 1991. The development and initial validation of a questionnaire on smoking urges. Br. J. Addict 86, 1467–1476. 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Tukey JW, 1949. Comparing individual means in the analysis of variance. Biometrics 5, 99–114. 10.2307/3001913. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE, 2010. A clinical laboratory model for evaluating the acute effects of electronic cigarettes: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol. Biomark. Prev 19, 1945–1953. 10.1158/1055-9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan DP, Dennis M, 1978. Mathematical basis of point-area deconvolution method for determining in vivo input functions. J. Pharm. Sci 67, 663–665. 10.1002/jps.2600670524. [DOI] [PubMed] [Google Scholar]
- Wagener TL, Floyd EL, Stepanov I, Driskill LM, Frank SG, Meier E, Leavens EL, Tackett AP, Molina N, Queimado L, 2017. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob. Control 26, e23–e28. 10.1136/tobaccocontrol-2016-053041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Stitzer ML, 1988. Cigarette brand-switching: effects on smoke exposure and smoking behavior. J. Pharmacol. Exp. Ther 246, 619–627. [PubMed] [Google Scholar]
- Zhang Y, Sumner W, Chen DR, 2013. In vitro particle size distributions in electronic and conventional cigarette aerosols suggest comparable deposition patterns. Nicotine Tob. Res 15, 501–508. 10.1093/ntr/nts165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


