Abstract
Objective:
The hemodynamic benefits of catheter-directed thrombolysis for acute pulmonary embolism have not been clearly defined beyond the periprocedural period. The objective of this study is to report midterm outcomes of catheter-directed thrombolysis for treatment of acute pulmonary embolism.
Methods:
Records of all patients undergoing catheter-directed thrombolysis for high- or intermediate-risk pulmonary embolism were retrospectively reviewed. Endpoints were clinical success, procedure-related complications, mortality, and longitudinal echocardiographic parameter improvement.
Results:
A total of 69 patients underwent catheter-directed thrombolysis (mean age 59 ± 15 y, 56% male). Eleven had high-risk and 58 intermediate-risk pulmonary embolism. Baseline characteristics did not differ by pulmonary embolism subtype. Fifty-two percent of patients underwent ultrasound-assisted thrombolysis, 39% standard catheter-directed thrombolysis, and 9% other interventional therapy; 89.9% had bilateral treatment. Average treatment time was 17.7 ± 11.3 h with average t-Pa dose of 28.5 ± 19.6 mg. The rate of clinical success was 88%. There were two major (3%) and six minor (9%) periprocedural bleeding complications with no strokes. All echocardiographic parameters demonstrated significant improvement at one-year follow-up. Pulmonary embolism-related in-hospital mortality was 3.3%, and estimated survival was 81.2% at one year.
Conclusions:
Catheter-directed thrombolysis is safe and effective for treatment of acute pulmonary embolism, with sustained hemodynamic improvement at one year. Further prospective large-scale studies are needed to determine comparative effectiveness of interventions for acute pulmonary embolism.
Keywords: Pulmonary embolism, deep venous thrombosis, thrombolysis, endovascular therapy
Introduction
Acute pulmonary embolism is a morbid condition responsible for approximately 200,000 hospitalizations per year. Most patients with a low-risk presentation and treated with systemic anticoagulation have a benign disease course and do well; however, more severe pulmonary emboli can affect cardiovascular function and cause hemodynamic instability and cardiovascular collapse with potential progression to death. These patients often receive treatment with systemic thrombolytic therapy which has been shown in some studies to improve the amount of cardiovascular strain, potentially decreasing mortality and risk of further cardiovascular instability in some patient populations.1-3 This treatment modality, although effective, carries a 9% risk of major and nearly 30% risk of minor bleeding complications, with the potential for intracranial hemorrhage.3
New methods of invasively treating acute PE have been developed as a result of the progression of endovascular techniques, but complications and mid- to long-term outcomes of these therapies have not been well characterized. The objective of this study was to assess the midterm performance of catheter-directed interventions (CDIs) for the treatment of acute intermediate- and high-risk PE.
Methods
This single-institution retrospective study was exempted from informed consent and approved by the quality improvement committee at our institution.
Patients
All patients from 2009 to 2014 carrying the diagnosis of acute PE requiring CDI were identified through an electronic medical record search and included in the study. All patients underwent computed tomographic angiography for diagnosis or confirmation of acute PE. Patients were classified by both the American Heart Association guidelines4 (massive/submassive) and European Society for Cardiology guidelines5 (high/intermediate risk). No low-risk patients undergoing CDI for acute PE were identified.
Procedures
Catheter-directed thrombolysis (CDT) procedures requiring continuous infusion of thrombolytic were accomplished using the Cragg-McNamara (Boston Scientific, Marlborough, MA) or Unifuse (Angiodynamics, Latham, NY) catheters for standard CDT and the EKOSonic system (EKOS Corp, Bothell, WA) for ultrasound-accelerated thrombolysis (USAT).
Other primary catheter-directed procedures included aspiration thrombectomy with the Angio-Vac device (Angiodynamics, Latham, NY) or Pronto catheter (Vascular Solutions Inc, Minneapolis, MN), rheolytic therapy using the Angiojet device (Boston Scientific, Marlborough, MA), and angiographic catheter-directed administration of thrombolytic without initiation of an infusion. The use of rheolytic systems for the treatment of acute PE was discontinued in our institution after the issuance of an FDA black-box warning in 2009.
All catheter-directed procedures were performed in either a hybrid operating room with a fixed imaging system or a standard angiography suite. Ultrasound guidance was used to establish central venous access at the common femoral vein or internal jugular vein depending on procedure and physician preference. Each intervention was then performed after identifying areas of thrombus burden within the pulmonary vasculature on angiography. The thrombectomy and rheolysis procedures were performed according to the device specifics, whereas for CDT and USAT, multi-sidehole catheters were advanced across the heaviest clot burden (uni- or bilateral) and infusion and/or ultrasound acceleration initiated. Alteplase was the sole medication used during procedures requiring thrombolytic therapy.
Patients undergoing continuous infusion of thrombolytic for CDT or USAT were observed in a cardiovascular ICU for the duration for the procedure. Patients were maintained on an alteplase infusion at 0.5–1 mg/h along with an unfractionated heparin infusion for the duration of the infusion. Prior to 2014, a standard 500 unit/h heparin infusion was used, whereas after 2014, the heparin infusion was titrated for an activated partial thromboplastin time of 60–80. Patients were transitioned to long-term anticoagulation therapy (warfarin, enoxaparin, or a Factor Xa inhibitor) after termination of thrombolysis.
Our institutional protocol for CDT infusion procedures was continually updated through the study period; as a result, criteria for infusion termination varied during this time. Criteria from 2009 to 2011 were based primarily on angiographic findings demonstrating significant (>50%) resolution of clot burden. Subsequent to 2011, these were revised to rely primarily on clinical markers of improvement instead; these included hemodynamics and respiratory status, echocardiographic right heart function, and dose of lytic administered.
Outcome measures
Major outcomes analyzed were clinical success, perioperative death, postoperative hemodynamic stabilization, perioperative stroke, and overall survival. Clinical success was defined as improvement in existing hypotension or decrease in vasopressor requirement in patients with massive or high-risk PE, or freedom from hemodynamic decompensation in patients with intermediate-risk PE, without a major adverse event or in-hospital death. Hemodynamic decompensation was in turn defined as new-onset sustained hypotension, new requirement for inotropes or vasopressors, or continued or worsening hypotension despite treatment. Major bleeding events were defined as bleeding requiring either transfusion of packed red blood cells or an intervention or operation; minor bleeding events were defined as all other clinically significant bleeding events without need for transfusion or intervention. Major adverse events were defined as treatment-related events requiring surgical treatment or transfusion, any stroke, need for dialysis, or perioperative death.
Echocardiogram parameters measured were qualitative assessment of right ventricular (RV) systolic dysfunction, measured values of right/left ventricular size ratio (RV/LV ratio), tricuspid regurgitant jet velocity, and the estimated systolic pulmonary artery pressure, as determined by the interpreting cardiologist. Measurement of ventricular size and estimation of the RV/LV ratio for each subject was performed and verified by two independent abstractors according to protocol reported in previous studies.6 Available echocardiograms at baseline, immediately after completion of the procedure, and at the latest follow-up within one year were reviewed for each individual study subject.
Statistical analysis
Standard statistical tests were used to analyze discrete data. Paired t-tests were used for analysis of repeated measures echocardiogram data. The Kaplan–Meier method was used to analyze time-to-event survival data. All statistical testing were performed using Stata SE 13.1 (College Station, TX).
Results
A total of 69 patients met inclusion criteria and were included in the study. The average age was 59 ± 15, and the majority were female (n = 39, 57%). Ten patients (14%) presented with high-risk PE; the remainder was classified as intermediate risk. Almost all patients had bilateral PE (n = 65, 94%). Thirty-six patients (52%) had concurrent deep vein thrombolysis (DVT) (Table 1).
Table 1.
Baseline characteristics.
| Factor | All PE N = 69 |
High-risk PE N = 10 |
Intermediate-risk PE N = 59 |
|---|---|---|---|
| Age, mean ± SD | 59.20 ± 15.38 | 68.2 ± 8.95 | 57.68 ± 15.77 |
| Female, sex | 39 (57%) | 8 (80%) | 31 (53%) |
| PE type | |||
| High-risk | 10 (14%) | – | – |
| Intermediate-risk | 59 (86%) | – | – |
| PE Laterality | |||
| Unilateral | 3 (4%) | 0 (0%) | 3 (5%) |
| Bilateral | 66 (96%) | 10 (100%) | 56 (95%) |
| Hypercoagulable state | 4 (6%) | 0 (0%) | 4 (7%) |
| Recent surgery | 16 (23%) | 4 (40%) | 12 (20%) |
| Cancer | 10 (14%) | 3 (30%) | 7 (12%) |
| Hormonal therapy | 5 (7%) | 0 (0%) | 5 (8%) |
| Recent travel | 12 (17%) | 1 (10%) | 11 (19%) |
| Recent trauma | 1 (1%) | 0 (0%) | 1 (2%) |
| Concurrent DVT | 36 (52%) | 5 (50%) | 31 (53%) |
| History of DVT | 11 (16%) | 0 (0%) | 11 (19%) |
| History of PE | 10 (14%) | 1 (10%) | 9 (15%) |
| Coronary artery disease | 8 (12%) | 1 (10%) | 7 (12%) |
| Heart failure history | 3 (4%) | 1 (10%) | 2 (3%) |
| Pulmonary hypertension history | 2 (3%) | 0 (0%) | 2 (3%) |
| Pulmonary disease history | 7 (10%) | 1 (10%) | 6 (10%) |
PE: pulmonary embolism; DVT: deep vein thrombolysis.
Thirty-six patients (52%) underwent USAT, 27 patients (39%) had standard CDT, and six patients (9%) had aspiration thrombectomy, rheolysis, or catheter-directed on-table single-dose thrombolytic therapy performed. Patients undergoing continuous thrombolytic infusion (CDT or USAT) had an average of 1.7 ± 0.7 trips to the angio suite or operating room, and a total lysis time of 17.7 ± 11.3 h for an average total alteplase dose of 28.5 ± 19.6 mg.
The mean follow-up time for the cohort was 394.5 ± 370.6 days. The estimated overall survival was 96.8% at 30 days, 90.3% at 90 days, and 81.2% at one year (Figure 1). Two patients died perioperatively: one patient with intermediate-risk PE successfully completed USAT with a good initial result but suffered a sudden cardiac arrest and death just prior to anticipated discharge. The other patient presented with high-risk PE and hemodynamic instability, arresting and expiring on the operating room table after initial placement of catheters and delivery of alteplase loading dose but prior to initiation of continuous lytic infusion.
Figure 1.
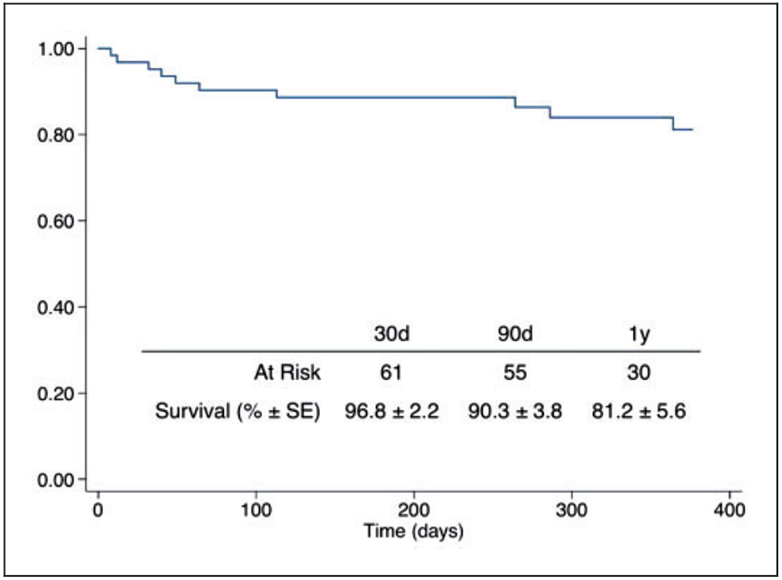
Estimated survival of the total cohort undergoing CDI. Y-axis: survival (%).
Six patients (n = 63, 91.3%), all undergoing CDT or USAT, met criteria for hemodynamic decompensation after initiation of catheter-directed therapy. Of these six, three had high-risk PE with preexisting hemodynamic instability (high-risk group), and three had new-onset postoperative hypotension (intermediate-risk group). Two of these patients died as mentioned previously, one before and one after initiation of continuous lytic infusion; three patients (4%) progressed to surgical embolectomy; and one patient recovered without intervention and was discharged to a long-term acute care facility. The median length of ICU stay was two days (IQR 2–3).
There were two major bleeding events (3%) requiring intervention or transfusion and six (9%) minor bleeding events. There were no recorded strokes. In total, eight patients (11%) met criteria for a major non-mortality adverse event: three surgical embolectomy, two major bleeding events, two patients with respiratory failure requiring tracheostomy, and one patient with a tricuspid valve rupture after suction thrombectomy with the Angio-Vac device (Angiodynamics, Marlborough, MA) requiring valve replacement.
The overall rate of clinical success, defined as freedom from hemodynamic decompensation, perioperative death, or major adverse event, was 84% (n = 58). Clinical success rates were 60% (n = 6) in the high-risk group and 88% (n = 52) in the intermediate-risk group. Two patients (3%) had documented recurrent PE events during the follow-up period; no subject had a recurrent PE during the same admission.
Echocardiogram parameters were measured at baseline, postoperatively, and at the latest follow-up visit within one year. Average baseline parameters were all abnormal; subsequent studies improvement in the postoperative period with normalization of the average values at the follow-up study. The change for each patient with a recorded postoperative or follow-up echocardiogram was analyzed, demonstrating statistically significant improvement from baseline for both postoperative and follow-up studies (Table 2).
Table 2.
Echocardiogram parameters.
| Baseline Mean ± SD |
Post-op, n = 23 |
Follow-up, n = 29 |
Difference: baseline vs. post-op |
Difference: baseline vs. follow-up |
|
|---|---|---|---|---|---|
| RV/LV ratio | 1.1 ± 0.2 | 0.9 ± 0.1 | 0.8 ± 0.2 | 0.4 ± 0.2 P < 0.001 |
0.3 ± 0.2 P < 0.001 |
| Tricuspid regurgitation Jet velocity (m/s) |
3.1 ± 0.6 | 2.8 ± 0.7 | 2.5 ± 0.8 | 0.5 ± 0.4 P < 0.001 |
0.8 ± 0.9 P = 0.003 |
| Systolic PA pressure (mmHg) | 50.1 ± 16.6 | 42.6 ± 17.9 | 37.5 ± 14.9 | 13.5 ± 7.8 P < 0.001 |
18 ± 19 P = 0.002 |
| RV dilation, n (%) | 58 (88%) | 15 (65%) | 8 (28%) | P = 0.03 | P < 0.001 |
| RV systolic dysfunction, n (%) | 46 (70%) | 6 (26%) | 1 (3%) | P < 0.001 | P < 0.001 |
RV/LV: right/left ventricular size ratio.
Discussion
Many society guidelines recommend the usage of systemic thrombolytics for the treatment of acute PE in the high-risk groups, with a weaker recommendation for intermediate-risk patients.4,5 Most studies have focused on the efficacy of this approach for either improving mortality or hemodynamic decompensation rates1,3,7 while acknowledging the increased bleeding risks presented by such therapies. As a result, CDIs for the treatment of acute PE have been utilized with increasing frequency, but limited data exists to verify both the short- and midterm benefits of these procedures.8 Nearly all of the data for comparative effectiveness of thrombolysis comes from systemic thrombolysis trials. Although meta-analysis of this data showed potential mortality benefits for the usage of systemic thrombolytics,3,7 extension of this benefit to CDI must be extrapolated due to lack of existing data. Comparative effectiveness studies have likewise not been undertaken for the comparison of CDIs against systemic thrombolysis or against each another.9
Our cohort represents the evolution of several treatment paradigms in our institution over the course of five years. Throughout this time, the workhorse and eventually sole type of catheter-directed therapy for treatment of acute PE has been continuous infusion therapy, both CDT and USAT. This has been in part due to our anecdotal experiences with the technical difficulty and equipment requirements of suction thrombectomy, and the disappointing results and subsequent FDA black-box warning for rheolytic systems as a result of complications reported in the literature.8 In addition, several studies have questioned the clinical applicability of radiographic measures of thrombus burden as a prognostic indicator.10-12 Our paradigm for management of acute PE treatment has similarly shifted from the traditional emphasis on measures such as Miller score to managing treatment based on physiologic measurements of cardiac function obtained from echocardiogram readings, as well as patient clinical condition.6,12,13
In addition, we show that the hemodynamic benefits of CDIs are sustained through the one-year period, with survival and postoperative adverse event rates that correspond with those in the existing literature. This series also demonstrates the outcomes of practical applications of CDIs to the treatment of acute PE. Although the sample size remains relatively small, the lower rate of hemorrhagic outcomes, including low rates of major bleeding and no strokes, demonstrate a sufficient level of safety to continue utilizing these interventions in a practical setting within the algorithmic framework of a multidisciplinary PE team.
In this study, we also evaluate a clinical success outcome based on prevention or resolution of hemodynamic compensation in the absence of perioperative death or major adverse event. Our overall rates of clinical success were satisfactory and have improved as changes to the institutional protocol have been implemented as part of a quality improvement initiative based on this data. More moderate success rates in the high-risk group were not attributable to major bleeding or intracranial hemorrhage but due to an immediate on-table arrest and death prior to initiation of lytic infusion, two instances of respiratory failure despite hemodynamic recovery, and progression of hemodynamic instability requiring surgical thrombectomy. Identifying factors correlating to the eventual necessity for surgical thrombectomy is difficult; the incidence of surgical intervention was spread out over the course of the study period, which may reflect a potential inevitability of a part of the population to progress despite any lytic therapy. Due to the low number of high-risk patients receiving CDI (n = 10), further analysis or interpretation of these findings is difficult. Success rates of 88% in the intermediate-risk group showed a low rate of major bleeding complications and no strokes or intracranial hemorrhage.
A subsidiary measure related to clinical success, hemodynamic decompensation, has been utilized as a primary outcome in some systemic thrombolytic studies1,10 but has not yet gained widespread usage in evaluation of CDIs and is applicable only for those with intermediate-risk PE. Quantitative measures of right heart dysfunction may be easier to measure or define, but improvements in these values have not been definitively correlated to improvement in any clinical patient outcomes. Our definition of clinical success may carry more clinically relevant information as a true patient-centered outcome especially when evaluating intervention for patients with intermediate-risk PE. A similar measure of hemodynamic stabilization has been utilized by Kuo et al.10 as a major clinical endpoint in a recently published analysis of prospectively collected registry data. These composite outcomes may provide a better overall view of the performance of CDIs rather than mortality or echocardiographic outcomes alone.
Data are lacking for the comparative effectiveness of CDIs for the treatment of acute PE, despite a plethora of small series. A meta-analysis by Kuo of CDI for acute high-risk (massive) PE consisting of 35 non-randomized studies ranging from the early 1990 to 2008.8 These studies included any catheter-directed therapy and utilized a primary composite clinical success endpoint of hemodynamic stabilization, resolution of hypoxia, and in-hospital survival, but did not include major adverse events. The pooled study demonstrated a clinical success rate of 86.5% and a major and minor adverse event rate of 2.4% and 7.9%, respectively, although any inference drawn from the pooled results must be limited due to the uncontrolled nature of the included studies and moderate heterogeneity. Definitive prospective comparative studies with anticoagulation therapy or systemic thrombolysis in this high-risk population have not yet been carried out, but comparison with historical rates of mortality in anticoagulation-only cohorts14,15 (25–50%) and with historical adverse event rates in those undergoing systemic thrombolysis3,7 (9.1% and 22.7%) suggest that there may be clinical benefit with lower rates of adverse events for treatment with CDI.
For intermediate-risk PE, published results are even less clear. Various endpoints have been used to attempt to quantify the overall benefit derived from CDI as the mortality benefit, if any, is expected to be small and is primarily extrapolated from meta-analyses of randomized systemic thrombolysis trials.7 Common outcome measures have included radiographic measures of thrombus burden such as the modified Miller score and echocardiographic measures of right ventricular function.6,11 Several studies have demonstrated the ability of CDIs to reduce echocardiographic signs of cardiac burden including tricuspid regurgitant jet velocity, RV/LV ratio, and estimated systolic pulmonary artery pressure,6,8,11,13,16 although the significance of these changes for the immediate and long-term clinical status of the patient remains unclear.17
Residual functional deficits and quality of life related to long-term hemodynamic abnormalities have been shown in patients with acute PE undergoing treatment with anticoagulation, with nearly half of patients estimated to have some kind of functional impairment six months to three years after the initial event.18 The effect of CDIs on these outcomes, however, has not been well studied. In our institution, long-term follow-up and measurement of functional outcomes have only recently become the standard of practice for patients post-discharge from treatment for acute PE. Midterm echocardiographic outcomes in our cohort generally demonstrated an improvement on average to a normal range. However, several patients continued to have abnormal hemodynamic parameters on follow-up, but functional and quality of life was unable to be extracted from their records. Further study of functional deficits and their relationship to residual hemodynamic abnormalities is needed.
Limitations
There are several limitations to this study. The retrospective nature of the analysis does not allow for a uniform treatment protocol; indeed, the continuously improving treatment specifics, although useful from a practical standpoint, limits interpretation of the results. For this same reason, not all patients received postoperative or follow-up echocardiograms and so a comparison of the entire cohort for these two periods was not possible. In addition, functional and ∥quality-of-life outcomes were not collected routinely and were not included in the analysis.
Conclusion
This study demonstrates safe and effective practical utilization of CDIs for the treatment of acute PE in a single institution over a five-year period. Patients who undergo CDI for acute PE have a rapid normalization of hemodynamic parameters as expected, but also are demonstrated in this study to have continued improvement at midterm follow-up. Further study of these interventions is needed, including prospective examination of the comparative effectiveness of these interventions for the improvement of an appropriate patient-centered outcome.
Acknowledgments
Funding
Nathan Liang received grant funding from NIH T32HL098036-06.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370: 1402–1411. [DOI] [PubMed] [Google Scholar]
- 2.Stein PD and Matta F. Thrombolytic therapy in unstable patients with acute pulmonary embolism: saves lives but underused. Am J Med 2012; 125: 465–470. [DOI] [PubMed] [Google Scholar]
- 3.Wan S, Quinlan DJ, Agnelli G, et al. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation 2004; 110: 744–749. [DOI] [PubMed] [Google Scholar]
- 4.Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123: 1788–1830. [DOI] [PubMed] [Google Scholar]
- 5.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35: 3033–3069. (3069a–3069k). [DOI] [PubMed] [Google Scholar]
- 6.Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129: 479–486. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014; 311: 2414–2421. [DOI] [PubMed] [Google Scholar]
- 8.Kuo WT. Endovascular therapy for acute pulmonary embolism. J Vase Interv Radiol; 23: 167–179. [DOI] [PubMed] [Google Scholar]
- 9.Lin PH, Annambhotla S, Bechara CF, et al. Comparison of percutaneous ultrasound-accelerated thrombolysis versus catheter-directed thrombolysis in patients with acute massive pulmonary embolism. Vascular 2009; 17(Supplement 3): S137–S147. [DOI] [PubMed] [Google Scholar]
- 10.Kuo WT, Banerjee A, Kim PS, et al. Pulmonary embolism response to fragmentation, embolectomy, and catheter thrombolysis (PERFECT). CHEST J 2015; 23: 575–575. [DOI] [PubMed] [Google Scholar]
- 11.Engelhardt TC, Taylor AJ, Simprini LA, et al. Catheter-directed ultrasound-accelerated thrombolysis for the treatment of acute pulmonary embolism. Thromb Res; 128: 149–154. [DOI] [PubMed] [Google Scholar]
- 12.Sardi A, Gluskin J, Guttentag A, et al. Saddle pulmonary embolism: is it as bad as it looks? A community hospital experience. Crit Care Med 2011; 39: 2413–2418. [DOI] [PubMed] [Google Scholar]
- 13.Piazza G, Hohlfelder B, Jaff MR, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism. JACC Cardiovasc Interv 2015; 8: 1382–1392. [DOI] [PubMed] [Google Scholar]
- 14.Ng ACC, Chung T, Yong ASC, et al. Long-term cardiovascular and noncardiovascular mortality of 1023 patients with confirmed acute pulmonary embolism. Circ Cardiovasc Qual Outcomes 2011; 4: 122–128. [DOI] [PubMed] [Google Scholar]
- 15.Goldhaber SZ, Visani L and De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999; 353: 1386–1389. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy RJ, Kenney HH and Dunfee BL. Thrombus resolution and hemodynamic recovery using ultrasound-accelerated thrombolysis in acute pulmonary embolism. J Vasc Interv Radiol 2013; 24: 841–848. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J 2008; 29: 1569–1577. [DOI] [PubMed] [Google Scholar]
- 18.Klok FA, van der Hulle T, den Exter PL, et al. The post-PE syndrome: a new concept for chronic complications of pulmonary embolism. Blood Rev 2014; 28: 221–226. [DOI] [PubMed] [Google Scholar]


