Abstract
Purpose
The goal of this paper is to describe the green conversion of agricultural waste products, such as molasses and corn steep liquor, into large amounts of D(-) lactic acid using a facilitated multipulse fed-batch strategy and affordable pH neutralizer. This is a very low-cost process because there is no need for hydrolysis of the waste products. The fed-batch strategy increases lactic acid productivity by avoiding inhibition caused by a high initial substrate concentration, and the selected controlling agent prevents cell stress that could be caused by high osmotic pressure of the culture media.
Methods
The effects of different carbon and nitrogen sources on lactic acid production were investigated, and the best concentrations of the medium components were determined. To optimize the culture conditions of the Lactobacillus delbrueckii strain, the effects of pH control, temperature, neutralizing agent, agitation, and inoculum size in batch cultures were investigated. Fed-batch strategies were also studied to improve production and productivity.
Result
A high titer of D(-) lactic acid (162g/liter) was achieved after 48 hours of fermentation. Productivity at this point was 3.37 g/L·h. The optimum conditions were a temperature of 39°C, pH 5.5 controlled by the addition of Ca(OH)2, agitation at 150 rpm, and inoculum size of 25% (v/v).
Conclusion
The production of high optical purity D(-) lactic acid through L. delbrueckii fermentation with molasses and corn steep liquor is a promising economical alternative process that can be performed on the industrial scale.
1. Introduction
Lactic acid has gained considerable attention due to its role as a monomer for the production of biodegradable polylactic acid (PLA). It is also widely used in the food, cosmetic, pharmaceutical, leather, textile, and chemical industries [1–4]. Several methods have been studied in an attempt to improve lactic acid fermentation with the goal of achieving higher yields [5].
Lactic acid has two optical isomers: L(+) and D(-). Optically pure L(+) or D(-) lactic acid can be achieved through microbial fermentation using an appropriate microorganism that is capable of producing one of these isomers. In contrast, chemical synthesis always leads to the production of racemic DL-lactic acid [6, 7]. The optical purity of lactic acid is an important physical property that has attracted the attention of different industries that blend L(+) or D(-) polymers to polymerize high-crystalline PLA, thereby improving the mechanical properties of this polymer [8, 9].
There are numerous investigations on the development of biotechnological processes for the production of L(+) lactic acid and industrial-scale production has been established [10–14]. However, fewer studies have investigated D(-) lactic acid production by fermentation processes. On the industrial scale, lactic acid is generally produced using batch fermentation, but this method has the disadvantage of decreasing production and productivity due to inhibition by the high substrate concentration [15, 16]. Thus, different fed-batch fermentation strategies have been explored to enhance productivity and avoid the inhibitory effects of the substrate on lactic acid production [17–19].
Several bacterial species belonging to the genus Lactobacillus, such as plantarum, delbrueckii, sakei, casei, and lactis, have been widely used for the production of lactic acid [19–23]. The production cost and demand of lactic acid to make it an environmentally feasible process are important aspects driving the search for improvements in this technology [24]. Studies report the production of lactic acid using raw materials as substrate, such as rice starch, barley, wheat, corn, cottonseed meal, potato peel waste, fruit, and vegetable waste [17, 25–29]. However, these sources usually require pretreatment in order to release fermentable sugars, which is economically unfavorable [30]. The use of waste products from industrial processes, such as molasses, which contains fermentable sugars, and corn steep liquor, is a very economical alternative, and these waste products can be readily used as nutritional sources in fermentation media, thereby decreasing the overall production cost [22, 30, 31].
Molasses is a byproduct of the sugar industry that contains 40 to 60% sucrose, which can be converted into lactic acid through fermentation [6, 30]. Molasses is used mainly as a carbon source and requires supplementation with nitrogen and minerals in order to be used in fermentation media [32]. Corn steep liquor is a waste product generated by the corn industry that contains amino acids, vitamins, and minerals, which makes it an excellent nitrogen source that can used by microorganisms [33].
Corn is currently the most widely produced cereal in the world, with an estimated 989.30 million tons produced in the 2015/2016 season. Brazil is among the three largest producers of corn, along with China and the USA [34]. Brazil is also the largest sugarcane producer and the world leader in sugar production. In this process, large amounts of molasses are generated as a byproduct produced at the proportion of 40 to 60 kilograms per ton of processed sugarcane [35, 36]. In an attempt to establish cost-effective technologies, this paper describes the efficient conversion of agroindustrial waste products (molasses and corn steep liquor) into lactic acid by L. delbrueckii using fed-batch fermentation.
2. Material and Methods
2.1. Microorganisms and Seed Cultivation Conditions
L. delbrueckii used in the present study was isolated from commercial yogurt and stored using a previously reported technique [37]. The seed medium was cultured in GYP medium (glucose (2%), yeast extract (1%), peptone (1%), sodium acetate (1%), and 0.5% of salt solution that comprises MgSO4.7 H2O (4%), MnSO4. (0.2%), FeSO4. 7H2O (0.2%), and NaCl (0.2%); all of these reagents are of Analytical Purity-Synth) for 15 h at 35°C under stationary conditions until OD600 reached 5.0 and then inoculated with 10% (v/v) in Erlenmeyer flasks or the fermentor (except in the test on the influence of inoculum size on lactic acid production).
2.2. Optimization of Fermentation Medium
2.2.1. Influence of Carbon and Nitrogen Sources on Lactic Acid Fermentation
The effect of different carbon sources on lactic acid production by L. delbrueckii was studied using molasses, commercial sucrose, and whey as the substrate at a concentration of 140 g/L of sugar content based in the GYP medium. After selecting the carbon source, different nitrogen sources were evaluated, such as corn steep liquor, Proflo, yeast autolysate, peanut flour, soybean flour, ammonium sulfate, urea, and ammonium nitrate. The concentration of the nitrogen sources was based on the GYP medium. Fermentation was carried in 125 mL flasks containing 50 mL of the medium at pH 7.0 and 10% inoculum (v/v) under stationary conditions at 35°C with incubation for 48 hours. To avoid the decrease in pH due to the production of lactic acid, 5% CaCO3 was added to each flask before sterilization. The experiments were run in duplicate, and the results were expressed as mean values.
2.2.2. Response Surface Methodology (RSM)
The concentrations of the carbon source, nitrogen source, and mineral salts were studied using RSM, which is a set of experimental strategies applied to determine the best concentration of medium components that exert an influence on D(-) lactic acid production. For such, 17 experiments were run, including three replicates at the center point. The independent variables selected for the study were the concentration of molasses, corn steep liquor, and mineral salts (the same used in the GYP medium).
The dependent variable (D(-) lactic acid production) was fitted using a second-order equation, and response surface graphs were then created. Lactic acid fermentation was performed under the same conditions listed in the previous topic. The experimental design was determined using the Statistica 7 software program (StatSoft, Tulsa, USA), which was also used for the analysis of the results.
2.2.3. Study of D(-) Lactic Acid Fermentation Conditions
Fermentations were performed in a 750 mL bioreactor (Infors HT Multifors 2) with an initial working volume of 300 mL of optimized medium containing 150 g/L of molasses (except for the agitation and inoculum size experiments, which began with 160 g/L), 65 mL/L of corn steep liquor, and 5 mL/L of mineral salts. The system was equipped with pH and temperature sensors as well as feed and base pumps. The effects of the pH-controlling agent (Ca(OH)2, CaCO3, NaOH, NH4OH, and KOH), temperature (31°, 33°, 35°, 37°, 39°, and 41°), pH (5.0, 5.5, 6.0, 6.5, and 7.0), agitation (50, 75, 100, 125, and 150 rpm), and inoculum size (5, 10, 15, 20 25, and 30%) on lactic acid production were investigated.
The results are described below. The fermentation products were sampled periodically and analyzed to determine the concentrations of the substrate and lactic acid.
2.2.4. Neutralizing Agents
Lactic acid produced through fermentation has to be continuously neutralized, and the pH-controlling agent exerts an influence on lactic acid production. The effect of acid neutralization on fermentation performance was investigated using different pH-controlling agents: Ca(OH)2 9N, NaOH 10N, NH4OH 6N, CaCO3 5%, and KOH 10N. The reactors were mechanically stirred at 100 rpm and maintained at 35°C. The pH was maintained at 6.0 by the automatic addition of each neutralizing agent. The medium (270 mL) was inoculated with 30 mL of standardized culture.
2.2.5. Effects of Fermentation Parameters on Production of D(-) Lactic Acid
A set of experiments was conducted to evaluate the effects of temperature, pH, agitation, and inoculum size on lactic acid fermentation. The cultivation parameters used in these experiments are given in Table 1. Fermentations were carried out for 70 hours, and Ca(OH)2 9N was used as the pH-controlling agent.
Table 1.
Culture conditions on the study of the parameters' effect on lactic acid production.
| Parameter | T (°C) | pH | Agitation (rpm) | Inoculum (%) | Change rate |
|---|---|---|---|---|---|
| Temperature range | 31-41 | 6.0 | 100 | 10 | 2°C |
| pH range | 39 | 5.0-7.0 | 100 | 10 | 0.5 units |
| Agitation range | 39 | 5.5 | 50-150 | 10 | 25 rpm |
| Inoculum size range | 39 | 5.5 | 150 | 5-30 | 5% |
2.2.6. Fed-Batch Fermentations
Fed-batch fermentation is used to prevent inhibition due to a high initial substrate concentration. For such, nutrients are supplied either intermittently or continuously to the bioreactor throughout the cultivation process [38, 39].
In the present study, different fed-batch fermentations of L. delbrueckii were implemented using pulse, multipulse, and continuous feeding rate strategies. The fermentations were carried out in a 750 mL bioreactor (Infors HT Multifors 2) with an initial medium containing 65 mL/L of corn steep liquor, 5 mL/L of mineral salt solutions (the same as the GYP medium), and different concentrations of molasses, as described below. The processes were run under the previously optimized conditions at 39°C, 150 rpm, and pH 5.5 controlled by the automatic addition of Ca(OH)2 9N. The initial working volume was 300 mL with 25% inoculum (v/v). The feed solution was composed of 585 g/L of sucrose from molasses and 65 mL/L of corn steep liquor and was pumped into the bioreactor by a computer-controlled peristaltic pump as required. For the comparison of the processes, batch fermentation was performed under the same culture conditions containing 160 g/L of initial sucrose from molasses, with the addition of only the pH-controlling agent during the process.
In the pulse fed-batch process, the initial molasses concentration was set to 100 g/L and after 15 hours; when no residual sucrose was present, a single pulse was applied into the bioreactor with the addition of 60 g/L of sucrose in the feed solution. Another single-pulse fermentation process was carried out in which the initial concentration of molasses was 150 g/L, and 55 g/L of sucrose were added when the residual sugar was near 25 g/L. The multipulse fed-batch process began with 100 g/L of molasses, and the feed solution was supplied twice, when the residual sugar was below 10 g/L; 45 g/L and 30 g/L of sucrose from the feed solution were added at 15 and 24 hours, respectively. In constant feed rate fed-batch fermentation, when all the initial sucrose was consumed (at 15 hours), the feed solution was continuously pumped into the bioreactor at a rate of 7.58 mL/h until completing 24 hours of fermentation. Fermentations were conducted for 70 hours. Samples were collected periodically to determine the concentrations of lactic acid and sugar as well as cell growth.
2.2.7. Analysis of Sugars, Lactic Acid and Cell Growth
The samples collected were centrifuged at 7000xg for 15 minutes, microfiltered through a 0.22 μm membrane, and the supernatants were used to determine the concentration of residual sugar and D(-) lactic acid. These concentrations and the optical purity of the lactic acid were determined using high-performance liquid chromatography (HPLC), as reported elsewhere [37].
The cell concentration was determined using a calibration curve (y = 1.1297x − 0.045) correlating the optical density at 600 nm (measured in the spectrophotometer) to the dry cell weight, which was determined by centrifugation at 7000xg for 15 minutes, washing twice (first with HCl 0.3 N and then with distilled water), followed by drying at 100°C for 24 hours. The sugar from whey is lactose that induced the lower concentration of lactic acid on the present study; on the other hand, it was reported that the highest yield of D(-) lactic acid from lactose substrate derived from whey.
3. Results and Discussion
3.1. Effects of Carbon and Nitrogen Sources on D(-) Lactic Acid Production
L. delbrueckii was able to produce D(-) lactic acid using all the carbon sources studied; however, the highest production (112.84 ± 3.07 g/L) was achieved in the presence of molasses, with 12.12 ± 1.22 g/L of residual sucrose. Commercial sugar led to a similar lactic acid concentration (111.37 ± 1.15 g/L) but higher residual sucrose value (24.98 ± 1.22), as displayed in Table 2. The sugar from whey is lactose, which in this study induces the lower D(-) lactic acid production (100.08 ± 2.06). On the other hand, efficient conversion of this sugar into lactic acid by another strain of L. delbrueckii has been reported [40–42]. Another author reported that among various carbon sources including glucose, sucrose, molasses, sugarcane juice, and bagasse hydrolysate, greater lactic acid production was observed using sucrose as the carbon source [43].
Table 2.
Effects of carbon and nitrogen sources on D(-) lactic acid production, productivity, and yield by L. delbrueckii.
| Carbon sources | Carbohydrates | D(-) lactic acid (g/L) | Productivity (g/L·h) | Yield Yp/s (g/g) | Residual sugar (g/L) |
|---|---|---|---|---|---|
| Sugar molasses | 46.9 (g/100 mL)a | 112.84 ± 3.07 | 2.35 ± 0.06 | 0.88 ± 0.01 | 12.12 ± 1.22 |
| Commercial sucrose | 100 (g/100 g)a | 111.37 ± 1.15 | 2.32 ± 0.02 | 0.96 ± 0.02 | 24.98 ± 1.22 |
| Whey | 76.59 (g/100 g)b | 100.08 ± 2.06 | 2.08 ± 0.04 | 0.89 ± 0.02 | 28.32 ± 0.51 |
| Nitrogen sources | Percentage of N | ||||
| Yeast autolysate | 7.2% | 102.79 ± 1.92 | 2.14 ± 0.04 | 0.87 ± 0.00 | 22.08 ± 1.88 |
| Corn steep liquor | 4.69% | 101.06 ± 3.36 | 2.10 ± 0.07 | 0.86 ± 0.05 | 23.41 ± 1.85 |
| Proflo | 11.15% | 75.74 ± 1.20 | 1.57 ± 0.03 | 0.75 ± 0.04 | 39.11 ± 4.04 |
| Peanut flour | 7.63% | 66.03 ± 0.47 | 1.37 ± 0.01 | 0.68 ± 0.03 | 44.11 ± 1.84 |
| Soybean flour | 6.40% | 62.65 ± 1.38 | 1.30 ± 0.03 | 0.71 ± 0.07 | 52.43 ± 3.47 |
| Ammonium nitrate | 34% | 52.60 ± 3.40 | 1.09 ± 0.07 | 0.57 ± 0.13 | 48.92 ± 2.36 |
| Urea | 46.62% | 49.16 ± 1.30 | 1.02 ± 0.03 | 0.66 ± 0.04 | 66.56 ± 1.07 |
| Ammonium sulfate | 21% | 37.09 ± 0.10 | 0.77 ± 0.00 | 0.56 ± 0.03 | 74.46 ± 1.23 |
Culture conditions: GYP medium with 140 g/L of carbon source and 15.25% of nitrogen contained on each nitrogen sources, 5% of CaCO3, 50 mL of work volume, at 35°C, and stationary fermentation with an initial pH of 7.0 for 48 hours. Types of carbohydrate: asucrose and blactose.
Molasses may provide greater cell growth due to the presence of nitrogen in its composition as well as its buffering action, as it contains calcium [30]. Molasses is a byproduct of the sugar industry. It is a syrupy material left after the removal of sugar from the mother syrup and is available in large amounts in Brazil, which is the largest sugarcane grower and sugar producer on the global scale, accounting for more than half of the sugar sold worldwide. Molasses offers a considerable advantage over current readily fermentable sources of sucrose, as it does not require pretreatment or hydrolysis [36, 44, 45]. Previous studies have reported the conversion of molasses to lactic acid by L. delbrueckii [44, 45]. However, some researchers submitted the molasses to hydrolysis with H2SO4 [30–46]. In the present study, L. delbrueckii was able to metabolize molasses without hydrolysis, which makes the process more economical.
Among the nitrogen sources studied, yeast autolysate and corn steep liquor led to the highest lactic acid production and productivity, performing similarly. However, corn steep liquor is a byproduct of the corn industry and is therefore a very inexpensive material. The use of agroindustrial waste products in fermentation processes is an environmentally friendly approach that enables the conversion of waste into added-value products [47]. Lactic acid production was not favored by ammonium nitrate, ammonium sulfate, or urea, as these substances are inorganic nitrogen sources, and considering that the base medium does not contain any type of vitamin, which is important to the maintenance of microbial cells. Assavasirijinda et al. [9] report similar results in an investigation of D(-) lactic acid production by an engineered Bacillus sp. strain, in which inorganic nitrogen sources did not lead to satisfactory levels of production, whereas the highest lactate production was achieved using peanut meal as the nitrogen source. In the present study, higher lactic acid concentrations were found when using waste products (corn steep liquor and Proflo), which have vitamins in their compositions, such as thiamine, riboflavin, niacin, pantothenic acid, choline, pyridoxine, biotin, inositol, carotene, tocopherols, ascorbic acid, and folic acid. Thus, molasses and corn steep liquor can be considered alternative substrates for lactic acid fermentation, as these products satisfy the nutrient requirements of L. delbrueckii.
3.2. Optimization of Fermentation Medium Using Response Surface Methodology
The mathematical relationship for D(-) lactic acid production was developed by considering three independent variables (concentrations of molasses, corn steep liquor, and mineral salt solution) and one dependent variable (D(-) lactic acid concentration in final medium fermentation). The response values of productivity, yield, and residual sucrose for each run are also shown (Table 3).
Table 3.
RSM design matrix with real/coded values and experimental results.
| Run | Dependent variables | D(-) lactic acid | Productivity | Y p/s | Residual sucrose | ||
|---|---|---|---|---|---|---|---|
| X 1 | X 2 | X 3 | g/L | g/L·h | g/g | g/L | |
| 1 | 100.00 (-1) | 35.00 (-1) | 2.20 (-1) | 82.30 | 1.71 | 0.86 | 21.66 |
| 2 | 200.0/0 (1) | 35.00 (-1) | 2.20 (-1) | 82.61 | 1.72 | 0.73 | 107.22 |
| 3 | 100.00 (-1) | 95.00 (1) | 2.20 (-1) | 79.53 | 1.66 | 0.95 | 32.40 |
| 4 | 200.00 (1) | 95.00 (1) | 2.20 (-1) | 82.99 | 1.73 | 0.98 | 130.36 |
| 5 | 100.00 (-1) | 35.00 (-1) | 7.80 (1) | 88.00 | 1.83 | 0.98 | 25.44 |
| 6 | 200.00 (1) | 35.00 (-1) | 7.80 (1) | 85.35 | 1.78 | 0.91 | 122.48 |
| 7 | 100.00 (-1) | 95.00 (1) | 7.80 (1) | 88.85 | 1.85 | 0.94 | 21.26 |
| 8 | 200.00 (1) | 95.00 (1) | 7.80 (1) | 80.93 | 1.69 | 0.93 | 128.96 |
| 9 | 150.00 (0) | 65.00 (0) | 0.13 (-1.68) | 102.03 | 2.13 | 0.97 | 60.27 |
| 10 | 150.00 (0) | 65.00 (0) | 9.87 (+1.68) | 110.21 | 2.30 | 0.99 | 53.55 |
| 11 | 150.00 (0) | 14.60 (-1.68) | 5.00 (0) | 82.39 | 1.72 | 0.97 | 80.36 |
| 12 | 150.00 (0) | 115.40 (+1.68) | 5.00 (0) | 102.77 | 2.14 | 0.98 | 60.77 |
| 13 | 66.00 (-1.68) | 65.00 (0) | 5.00 (0) | 68.88 | 1.44 | 0.97 | 10.61 |
| 14 | 234.00 (+1.68) | 65.00 (0) | 5.00 (0) | 75.02 | 1.56 | 0.49 | 111.38 |
| 15 | 150.00 (0) | 65.00 (0) | 5.00 (0) | 124.56 | 2.60 | 0.96 | 36.30 |
| 16 | 150.00 (0) | 65.00 (0) | 5.00 (0) | 118.75 | 2.47 | 0.94 | 39.28 |
| 17 | 150.00 (0) | 65.00 (0) | 5.00 (0) | 122.56 | 2.55 | 0.96 | 37.79 |
X 1 = sugar molasses (g/L of sucrose), X2 = corn steep liquor (mL/L), X3 = salt solution (mL/L), and Yp/s = yield. Culture conditions: initial pH at 7.0, 5% of CaCO3 used as pH neutralizer, 50 mL of work volume, 10% inoculum (v/v), and 35°C. Fermentation was carried under stationary condition for 48 hours. Coded values were present in parenthesis.
At the end of the fermentation process, the highest titer of lactic acid (124.56 g/L), highest productivity (2.60 g/L·h), and residual sugar of 36.30 g/L occurred in run 15. Central point values were used in this assay. Table 4 displays the results of the analysis of variance for the response surface quadratic model.
Table 4.
ANOVA for the regression model with estimated regression coefficients for D(-) lactic acid production.
| D(-) lactic acid production | |||||
|---|---|---|---|---|---|
| Terms | Sum of squares | Degree of freedom | Media of squares | F calc | p |
| Model | 4505.71 | 9 | 500.63 | 13.08 | 0.001 |
| Error | 267.86 | 7 | 38.26 | - | - |
| Lack of fit | 250.44 | 5 | 50.08 | 5.74 | 0.154 |
| Pure error | 17.42 | 2 | 8.71 | - | - |
| Total | 4773.57 | 16 | - | - | - |
| R2 = 0.94 | |||||
| Terms | Coefficient | p value | |||
| Intercept | 122.24 | 0.0000∗ | |||
| X 1 | 0.25 | 0.8817 | |||
| X 1 X 1 | -18.67 | 0.0000∗ | |||
| X 2 | 2.07 | 0.2554 | |||
| X 2 X 2 | -11.37 | 0.0004∗ | |||
| X 3 | 2.15 | 0.2385 | |||
| X 3 X 3 | -6.59 | 0.0090∗ | |||
| X 1 X 2 | -0.26 | 0.9069 | |||
| X 1 X 3 | -1.79 | 0.4394 | |||
| X 2 X 3 | -0.14 | 0.9481 | |||
X 1 = sugar molasses (g/L of sucrose), X2 = corn steep liquor (mL/L), X3 = salt solution (mL/L). ∗Statistically significant at 95% of probability level.
The regression equation had an R2 value of 0.94, which demonstrates the satisfactory fit of the quadratic model to the experimental data and indicates that the model explains 94% of the variability in the response. The model was significant, as demonstrated by Fisher's F calc test (13.08) > Ft (3.39), and had a very low probability value (Pmodel = 0.001). Moreover, the lack-of-fit value was nonsignificant at the 5% level (p > 0.05), indicating the satisfactory predictability of the model. A probability (p) value < 0.05 is indicative of a statistically significant coefficient.
Interactions X1X1, X2X2, and X3X3 were significant with 95% probability, demonstrating a negative effect. The high sucrose content of molasses can induce catabolic repression. Moreover, high mineral salt concentrations affect the membrane permeability of bacterial cells as well as the osmotic pressure of the medium, which may lead to cell lysis or hinder the passage of essential nutrients through the microbial cell membrane. Corn steep liquor contains minerals in its composition (calcium (0.14%), copper (1.5 mg/100 g), manganese (2.0 mg/100 g), iron (10 mg/100 g), magnesium (0.6%), potassium (2.8%), sodium (0.1%), and phosphorus (1.8%)) as well as impurities [48], which, when combined with the mineral salt solution used in this experiment, results in a high salt concentration and may have caused the effects abovementioned in the cells of L. delbrueckii. The results achieved were submitted to multiple linear regression analysis to generate the following regression equation (1):
| (1) |
The evaluation of interactions among factors that comprise a process is very important. RSM detects these interactions through three-dimensional response surfaces, which demonstrate the effects of two factors on the response at a given time and assist in determining the degree of parametric interactions on the desired responses [49]. The effects of the independent variables and their interactions on lactic acid production are illustrated in the response surface graphs (Figures 1(a)– 1(c)) constructed from the equation generated according to the coefficient of the linear regression model.
Figure 1.
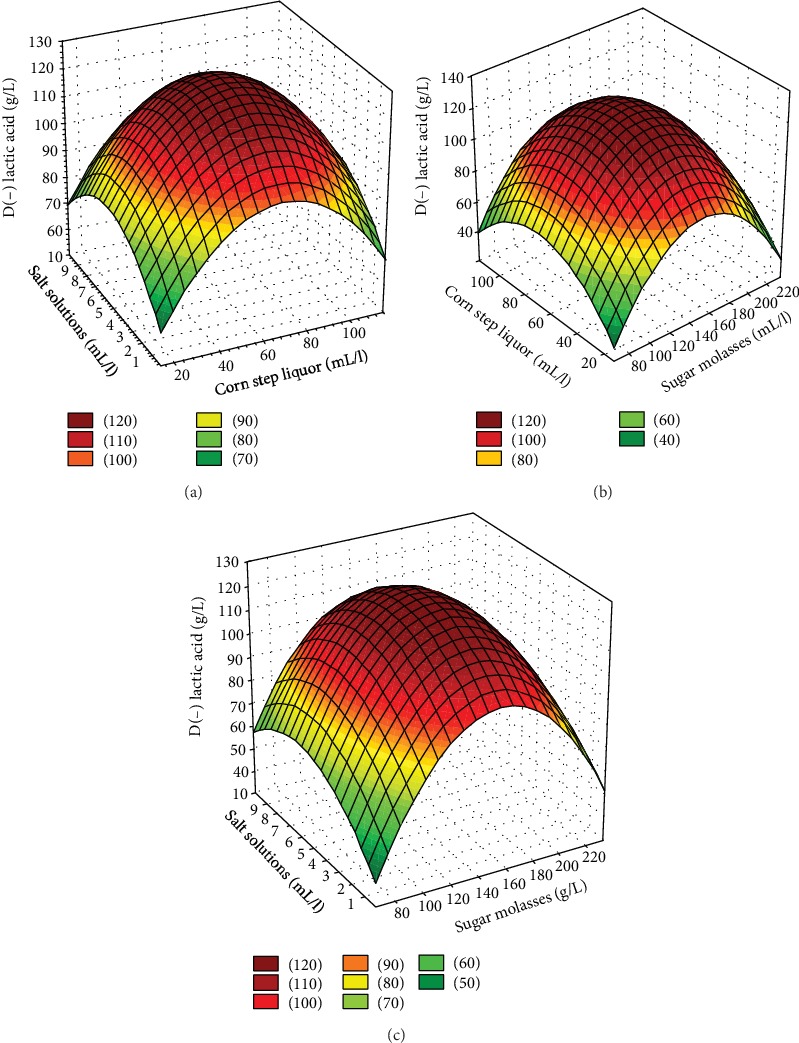
The interaction of media elements on lactic acid production using RSM. (a) Corn steep liquor and salt solution, (b) corn steep liquor and sugar molasses, and (c) salts solution and sugar molasses.
The maximum concentration of lactic acid (120 g/L) was achieved when the initial concentration of sucrose from molasses was approximately 150 g/L, the concentration of corn steep liquor was 65 mL/L, and the concentration of the mineral salt solution was around 5 mL/L. These concentrations correspond to the central point values used in the experimental design. Lactic acid production was reduced when less than 60 g/L of corn steep liquor was provided, as product formation is partially associated with cell growth in this process, in which the nitrogen source plays an important role. This result is in agreement with data described by Lima et al. [50], who applied RSM and achieved maximum D(-) lactic acid production (41.42 g/L) by Lactobacillus SMI8 using yeast autolysate and corn steep liquor and found that greater and lower concentrations of the nitrogen source led to a reduction in lactic acid production.
Lactic acid production increased with an increase in the concentration of molasses up to 160 g/L. The reduction in lactic acid production at higher concentrations was likely due to substrate inhibition, which is often reported in batch fermentation processes. Dumbrepatil et al. [30] describe similar results using a mutant Lactobacillus delbrueckii to produce lactic acid from molasses, reporting 166 g/L of lactic acid from 190 g/L of molasses and a substantial decrease in lactic acid production when fermentation was carried out with a molasses concentration of 240 g/L.
The mineral salts studied could serve as enzymatic activators, as reported by Lino et al. [21], who found that lactic acid dehydrogenase activity was promoted when adding sodium acetate to the medium for the production of lactic acid by species of Lactobacillus.
3.3. Effect of Neutralizing Agents on D(-) Lactic Acid Production
Figure 2 displays the effects of different neutralizing agents on the batch fermentations.
Figure 2.
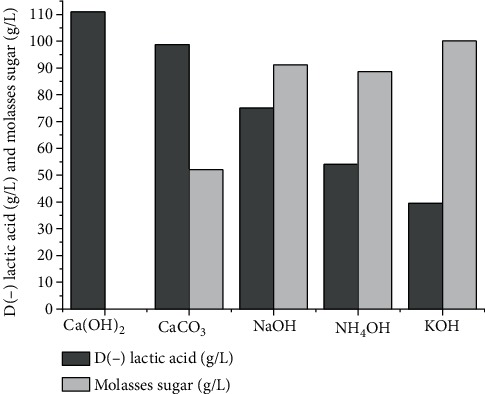
Effect of neutralizing agents on D(-) lactic acid production and residual sugar content by fermentation process with molasses sugar (150 g/L), corn steep liquor (65 mL/L), and salt solution (5 mL/L). Culture conditions: 35°C, 100 rpm, 10% inoculum (v/v), pH 6.0, and 300 mL of initial work volume.
The sucrose from the molasses was completely consumed when the pH was controlled by Ca(OH)2, yielding the highest lactic acid concentration (111.02 g/L). In the presence of CaCO3, 98.82 g/L of lactic acid was achieved, but the residual sucrose was substantial (52.00 g/L). When NaOH, NH4OH, and KOH were used, the sucrose was not completely converted into lactic acid by L. delbrueckii, resulting in low yields. These results are in agreement with data reported by Liu et al. [51], who found that Ca(OH)2 significantly facilitated D(-) lactic acid production compared to KOH or NH4OH using Escherichia coli. According to Nakano et al. [52], the pH of the medium is more efficiently neutralized by a divalent cation (Ca2+) compared to a monovalent cation (Na+, NH3+).
Some authors consider NaOH-based fermentation to be an environmentally friendly process that avoids generating precipitated waste [9, 53]. However, this agent could increase the osmotic pressure of the medium, causing stress to the microbial cells. Osmotic stress influences cell growth and organic acid production during fermentation, demonstrating an inhibition effect mainly in the late stage of the process [54, 55]. Ca(OH)2 and CaCO3 are generally applied on the industrial lactic acid fermentation scale, because these neutralizing agents make the downstream process easier and less expensive when compared to other pH-controlling agents. They are also available at a more affordable price than other neutralizing agents [56].
3.4. Effect of Temperature and pH on Lactic Acid Fermentation
Some important factors, such as temperature, pH, agitation, and inoculum size, have to be considered for the efficient production of lactic acid. Temperature and pH are closely related to bacterial growth and consequently affect the production of lactic acid [57]. pH affects the activity of enzymes and the transport of nutrients to microbial cells and also exerts an influence on RNA and protein syntheses [58]. In the present study, fermentations were carried out at 31°C to 41°C, with the optimum temperature for lactic acid production determined to be 39°C, yielding 115.13 g/L. In contrast, higher (41°C) and lower (31 and 33°C) temperatures did not assist in the satisfactory performance of L. delbrueckii with regard to lactic acid production (Figure 3(a)).
Figure 3.
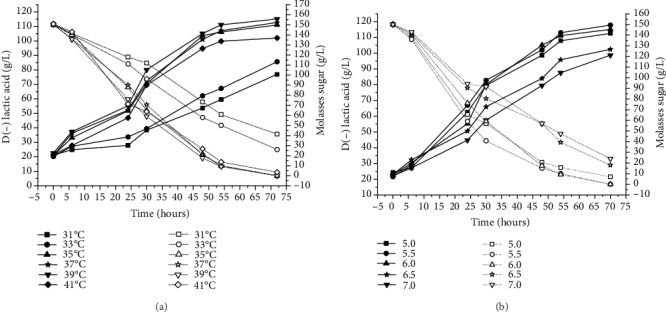
The effects of temperature (a) and pH (b) on lactic acid fermentation. Open symbol: molasses sugar; closed symbol: lactic acid.
According to results shown in Figure 3(b), the greatest lactic acid production occurred when the pH was maintained at 5.5, reaching a concentration of 117.88 g/L. Higher pH (6.5 and 7.0) had a negative effect on D(-) lactic acid production (Figure 3(b)).
The genus Lactobacillus comprises several species for which the optimum temperature for growth is between 35 and 40°C and pH is between 5.5 and 6.0 [59]. Several studies using Lactobacillus sp. have demonstrated that the best pH and temperature for lactic acid fermentation are within these ranges. Wee et al. [60] found that the optimum temperature and pH for a batch culture of Lactobacillus sp. RKY2 was 6.0 and 36°C, respectively, achieving 153.9 g/L of lactic acid using a medium containing glucose and yeast extract. Tang et al. [10] found that the highest lactic acid yield (0.46 g/g) by Lactobacillus sp. was obtained at 37°C and pH 6. Studying lactic acid produced from whey lactose and corn steep liquor by Lactobacillus sp. LMI8, Lima et al. [61] found the best temperature and pH to be 39.6°C and 5.9, respectively.
3.5. Effects of Agitation and Inoculum Size on Production of D(-) Lactic Acid
Lactic acid production was investigated in some batch fermentation experiments when varying the agitation speed and size of the inoculum, as shown in Figures 4(a) and 4(b).
Figure 4.
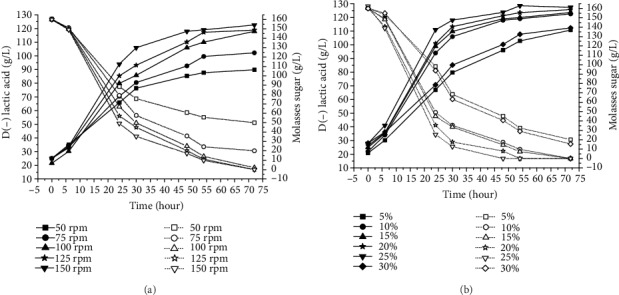
The effects of agitation (a) and inoculum size (b) on lactic acid fermentation. Open symbol: molasses sugar; closed symbol: lactic acid.
To determine the effect of inoculum size on lactic acid production, the optimized medium was inoculated with different percentages of culture seed (Figure 4(b)). The smallest (5%) and biggest (30%) inoculum size did not lead to satisfactory lactic acid production. A small inoculum size is reported to result in the low conversion to lactic acid due to inadequate enzymatic efficiency [37, 62]. Panesar et al. [58] report similar findings, with an increase in lactic acid formation and lactose utilization by Lactobacillus casei with the increase in inoculum size.
In the present study, the best results were found when the inoculum size was 25% and agitation was 150 rpm, achieving 122.55 g/L and 127.35 g/L of D(-) lactic acid, respectively. As the medium was composed of waste products with particles that tend to precipitate, more vigorous agitation promoted greater homogenization, thereby increasing contact between the cells and components of the medium. A lower agitation speed could result in an insufficient homogenization and the substrate would not be completely utilized, as demonstrated when 50 rpm and 75 rpm were used. Indeed, no residual sugar was observed at the end of the process when the highest agitation levels were used. Agitation is also important for mass and heat transfer [63].
3.6. Study of Fed-Batch Fermentation Strategies
The multipulse fed-batch strategy was performed with a low initial molasses concentration, and applying two pulses when the sucrose content was almost or completely exhausted. With this method, the highest concentration of D(-) lactic acid (161.93 g/L) was achieved after 48 hours of fermentation. However, production of 136.15 g/L of lactic acid was found at 30 hours, with the best productivity (4.54 g/L·h). When combined with the control of pH by Ca(OH)2, this method avoids excessive osmotic pressure that medium components could cause in the bacterial cells. Therefore, the phenomenon of substrate inhibition was avoided by providing small amounts of sucrose from molasses in the log phase of L. delbrueckii growth. The fed-batch strategy promotes a 33.73% increase in lactic acid production compared to a batch culture. Many studies report improvements in fermentation performance employing fed-batch strategies. Using a mutant Bacillus strain grown in a glucose and peanut meal medium, Assavasirijinda et al. ([9] performed multipulse fed-batch fermentation and found productivity of approximately 3.02 g/L·h at 16 h, reaching a final D(-) lactic acid concentration of 142.05 g/L. Using the twice fed-batch feeding strategy for Sporolactobacillus laevolacticus DSM442, Li et al. [64] achieved 144.4 g/L of D(-) lactic at 35 hours of fermentation as well as productivity of 4.13 g/L·h. Table 5 displays the best result of each fed-batch strategy in the present study, which was achieved at different fermentation times.
Table 5.
Fed-batch fermentation strategies on D(-) lactic acid production.
| Batch culture | Pulse fed-batch I | Pulse fed-batch II | Multipulse fed-batch | Constant feed rate fed-batch | |
|---|---|---|---|---|---|
| Fermentation time (h) | 33 | 48 | 54 | 48 | 30 |
| D(-) lactic acid (g/L) | 121.08 | 138.84 | 139.42 | 161.93 | 118.79 |
| Productivity (g/L·h) | 3.36 | 2.89 | 2.58 | 3.37 | 3.96 |
| Yield Yp/s (g/g) | 0.61 | 0.78 | 0.55 | 0.81 | 0.61 |
| Residual sucrose (g/L) | 0 | 0 | 7.97 | 0 | 31.63 |
| Cell growth (g/L) | 22.55 | 26.57 | 25.80 | 29.42 | 27.07 |
| Final broth volume (ml) | 400 | 520 | 480 | 580 | 550 |
Pulse fed-batch I started with 95 g/L of initial molasses sugar; pulse fed-batch II started with 150 g/L of initial molasses sugar.
It is evident that a high substrate concentration at the beginning of the process is not advantageous [22, 65]. In the present study, the single-pulse strategy that was initiated with a higher initial substrate concentration (pulse fed-batch II) took more time to reach the same concentration of lactic acid compared to the pulse fed-batch with a low initial substrate concentration (fed-batch I). Likewise, Ye et al. [65] found an improvement in productivity and production (3.2 g/L h and 140.4 g/L, respectively) using a single-pulse fed-batch strategy compared to a batch culture (2.6 g/L h and 118.2 g/l, respectively) involving Bacillus coagulans C106. Figure 5 displays the fed-batch fermentation kinetics, demonstrating D(-) lactic acid production, sucrose consumption, and cell growth rate.
Figure 5.
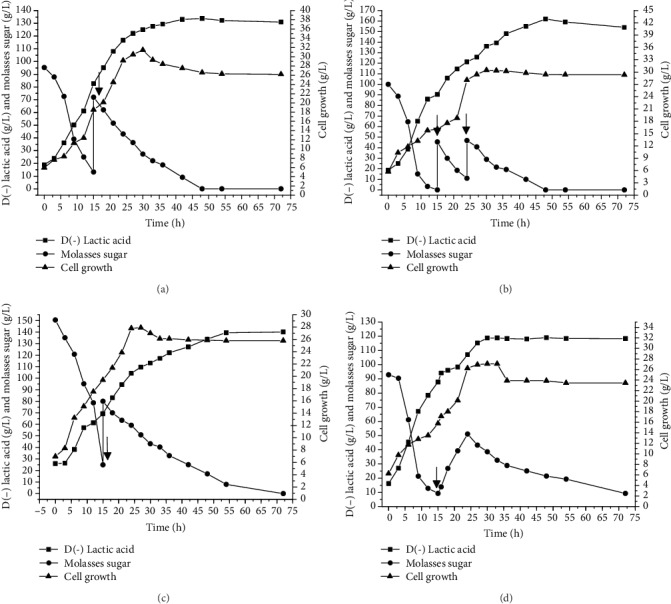
Fed-batch fermentations of D(-) lactic acid by L. delbrueckii. (a, c) Pulse. (b) Multipulse. (d) Continuous feed rate. Culture conditions: 39°C, 150 rpm, 25% inoculum (v/v), 300 mL of initial work volume, and pH 5.5 controlled by Ca(OH)2. The arrows represent when the pulse occurred (a–c) or the beginning of the fed-batch phase (d).
The constant feed rate fed-batch was not satisfactory, yielding the lowest D(-) lactic acid production among all fed-batch strategies. When the feed supply was stopped, the microbial cells were entering the stationary phase, declining thereafter. This result is in agreement with data described by Ding and Tan [19], who also did not achieve good results when employing this strategy on Lactobacillus casei fermentation.
The final culture medium (from multipulse fed-batch fermentation) was analyzed and high optical purity of the D(-) lactic acid was found (97.66% of D(-) and 2.34% of L(+) lactic acid).
4. Conclusion
The present findings demonstrate an efficient and low-cost process for the production of high optical purity D(-) lactic acid by L. delbrueckii, which was able to grow and convert sucrose from waste products (molasses and corn steep liquor) into D(-) lactic acid. This is a very economical process, since no pretreatment of the waste products is required. High levels of lactic acid and high productivity were achieved. Moreover, the pH neutralizer employed [Ca(OH)2] has the advantage of being available at a very affordable cost and contributes to maintaining low osmotic pressure in the culture medium, which is very important in avoiding stress in microbial cells. The multipulse fed-batch strategy conducted with the optimum conditions studied is an easy, effective method to improve D(-) lactic acid production.
Acknowledgments
This work was supported by Braskem/Fapesp (2010/52416-8) and CNPq (301567/2012-3).
Data Availability
All data in the manuscript are available.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
The authors also give full permission for the publication.
References
- 1.Wee Y. J., Kim J. N., Ryu H. W. Biotechnological production of lactic acid and its recent applications. Food Technology and Biotechnology. 2006;44:163–172. [Google Scholar]
- 2.Gao C., Ma C., Xu P. Biotechnological routes based on lactic acid production from biomass. Biotechnology Advances. 2011;29(6):930–939. doi: 10.1016/j.biotechadv.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Pang X., Zhuang X., Tang Z., Chen X. Polylactic acid (PLA): research, development and industrialization. Biotechnology Journal. 2010;5(11):1125–1136. doi: 10.1002/biot.201000135. [DOI] [PubMed] [Google Scholar]
- 4.Zhao B., Wang L., Ma C., Yang C., Xu P., Ma Y. Repeated open fermentative production of optically pure L-lactic acid using a thermophilic Bacillus sp. strain. Bioresource Technology. 2010;101(16):6494–6498. doi: 10.1016/j.biortech.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 5.Ghaffar T., Irshad M., Anwar Z., et al. Recent trends in lactic acid biotechnology: a brief review on production to purification. Journal of Radiation Research and Applied Science. 2014;7(2):222–229. doi: 10.1016/j.jrras.2014.03.002. [DOI] [Google Scholar]
- 6.Hofvendahl K., Hahn-Hägerdal B. Factors affecting the fermentative lactic acid production from renewable resources. Enzyme and Microbial Technology. 2000;26(2-4):87–107. doi: 10.1016/s0141-0229(99)00155-6. [DOI] [PubMed] [Google Scholar]
- 7.Kadam S. R., Patil S. S., Bastawde K. B., Khire J. M., Gokhale D. V. Strain improvement of Lactobacillus delbrueckii NCIM 2365 for lactic acid production. Process Biochemistry. 2006;41(1):120–126. doi: 10.1016/j.procbio.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Lunt J. Large-scale production, properties and commercial applications of polylactic acid polymers. Polymer Degradation and Stability. 1998;59(1-3):145–152. doi: 10.1016/S0141-3910(97)00148-1. [DOI] [Google Scholar]
- 9.Assavasirijinda N., Ge D. Y., Yu B., Xue Y. F., Ma Y. H. Efficient fermentative production of polymer-grade D-lactate by an engineered alkaliphilic Bacillus sp strain under non-sterile conditions. Microbial Cell Factories. 2016;15(1):1–10. doi: 10.1186/s12934-015-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang J., Wang X., Hu Y., Zhang Y., Li Y. Lactic acid fermentation from food waste with indigenous microbiota: effects of pH, temperature and high OLR. Waste Management. 2016;52:278–285. doi: 10.1016/j.wasman.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 11.Moon S. K., Wee Y. J., Choi G. W. A novel lactic acid bacterium for the production of high purity L-lactic acid, Lactobacillus paracasei subsp. paracasei CHB2121. Journal of Bioscience and Bioengineering. 2012;114(2):155–159. doi: 10.1016/j.jbiosc.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Calabia B. P., Tokiwa Y., Aiba S. Fermentative production of L-(+)-lactic acid by an alkaliphilic marine microorganism. Biotechnology Letters. 2011;33(7):1429–1433. doi: 10.1007/s10529-011-0573-0. [DOI] [PubMed] [Google Scholar]
- 13.Okano K., Zhang Q., Shinkawa S., et al. Efficient production of optically pure D-lactic acid from raw corn starch by using a genetically modified L-lactate dehydrogenase gene-deficient and alpha-amylase-secreting Lactobacillus plantarum strain. Applied and Environmental Microbiology. 2009;75(2):462–467. doi: 10.1128/AEM.01514-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michelson T., Kask K., Jogi E., Talpsep E., Suitso I., Nurk A. L(+)-lactic acid producer Bacillus coagulans SIM-7 DSM 14043 and its comparison with Lactobacillus delbrueckii ssp. lactis DSM 20073. Enzyme and Microbial Technology. 2006;39(4):861–867. doi: 10.1016/j.enzmictec.2006.01.015. [DOI] [Google Scholar]
- 15.Zhou S., Causey T. B., Hasona A., Shanmugam K. T., Ingram L. O. Production of optically pure D-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Applied and Environmental Microbiology. 2003;69(1):399–407. doi: 10.1128/aem.69.1.399-407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hujanen M., Linkos S., Linko Y.-Y., Leisola M. Optimisation of media and cultivation conditions for L(+)(S)-lactic acid production by Lactobacillus casei NRRL B-441. Applied Microbiology and Biotechnology. 2001;56(1-2):126–130. doi: 10.1007/s002530000501. [DOI] [PubMed] [Google Scholar]
- 17.Bai Z., Gao Z., Sun J., Wu B., He B. D-lactic acid production by Sporolactobacillus inulinus YBS1-5 with simultaneous utilization of cottonseed meal and corncob residue. Bioresource Technology. 2016;207:346–352. doi: 10.1016/j.biortech.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Wang L., Zhao B., Li F., et al. Highly efficient production of D-lactate by Sporolactobacillus sp. CASD with simultaneous enzymatic hydrolysis of peanut meal. Applied Microbiology and Biotechnology. 2011;89(4):1009–1017. doi: 10.1007/s00253-010-2904-9. [DOI] [PubMed] [Google Scholar]
- 19.Ding S., Tan T. L-lactic acid production by Lactobacillus casei fermentation using different fed-batch feeding strategies. Process Biochemistry. 2006;41(6):1451–1454. doi: 10.1016/j.procbio.2006.01.014. [DOI] [Google Scholar]
- 20.Yang P.-B., Tian Y., Wang Q., Cong W. Effect of different types of calcium carbonate on the lactic acid fermentation performance of Lactobacillus lactis. Biochemical Engineering Journal. 2015;98:38–46. doi: 10.1016/j.bej.2015.02.023. [DOI] [Google Scholar]
- 21.Lino T., Uchimura T., Komagata K. The effect of sodium acetate on the growth yield, the production of l- and d-lactic acid, and the activity of some enzymes of the glycolytic pathway of Lactobacillus sakei NRIC 1071T and Lactobacillus plantarum NRIC 1067T. The Journal of General and Applied Microbiology. 2002;48(2):91–102. doi: 10.2323/jgam.48.91. [DOI] [PubMed] [Google Scholar]
- 22.Kotzamanidis C., Roukas T., Skaracis G. Optimization of lactic acid production from beet molasses by Lactobacillus delbrueckii NCIMB 8130. World Journal of Microbiology and Biotechnology. 2002;18(5):441–448. doi: 10.1023/A:1015523126741. [DOI] [Google Scholar]
- 23.Bai D. M., Wei Q., Yan Z. H., Zhao X. M., Li X. G., Xu S. M. Fed-batch fermentation of Lactobacillus lactis for hyper-production of L-lactic acid. Biotechnology Letters. 2003;25(21):1833–1835. doi: 10.1023/a:1026276925649. [DOI] [PubMed] [Google Scholar]
- 24.Singhvi M., Joshi D., Adsul M., Varma A., Gokhale D. D-(-)-lactic acid production from cellobiose and cellulose by Lactobacillus lactis mutant RM2-24. Green Chemistry. 2010;12(6):1106–1109. doi: 10.1039/b925975a. [DOI] [Google Scholar]
- 25.Lee C. W. Production of D-lactic acid by bacterial fermentation of rice. Fibers and Polymers. 2007;8(6):571–578. doi: 10.1007/BF02875992. [DOI] [Google Scholar]
- 26.Oh H., Wee Y. J., Yun J. S., Ho Han S., Jung S., Ryu H. W. Lactic acid production from agricultural resources as cheap raw materials. Bioresource Technology. 2005;96(13):1492–1498. doi: 10.1016/j.biortech.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y. Y., Ma H. L., Zheng M. Y., Wang K. J. Lactic acid production from acidogenic fermentation of fruit and vegetable wastes. Bioresource Technology. 2015;191:53–58. doi: 10.1016/j.biortech.2015.04.100. [DOI] [PubMed] [Google Scholar]
- 28.Liang S. B., Mcdonald A. G., Coats E. R. Lactic acid production with undefined mixed culture fermentation of potato peel waste. Waste Management. 2014;34(11):2022–2027. doi: 10.1016/j.wasman.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Busairi A. M. Effect of nitrogen source and initial sugar concentration on lactic acid fermentation of pineapple waste using L.delbrueckii. Teknik. 2010;31:31–34. [Google Scholar]
- 30.Dumbrepatil A., Adsul M., Chaudhari S., Khire J., Gokhale D. Utilization of molasses sugar for lactic acid production by Lactobacillus delbrueckii subsp. delbrueckii mutant Uc-3 in batch fermentation. Applied and Environmental Microbiology. 2008;74(1):333–335. doi: 10.1128/AEM.01595-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Batista A. C. L., Silva M. C. F., Batista J. B., Nascimento A. E., Campos-Takaki G. M. Eco-friendly chitosan production by Syncephalastrum racemosum and application to the removal of acid orange 7 (AO7) from wastewaters. Molecules. 2013;18(7):7646–7660. doi: 10.3390/molecules18077646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amorim R. V. S., Pedrosa R. P., Kazutaka F., Martínez C. R., Ledingham W. M., Campos-Takaki G. M. Alternative carbon sources from sugar cane process for submerged cultivation of Cunninghamella bertholletiae to produce chitosan. Food Technology and Biotechnology. 2006;44:519–523. [Google Scholar]
- 33.Berger L. R. R., Cardoso A., Stamford T. C. M., Cavalcante H. M. M., Macedo R. O., Campos-Takaki G. M. Agroindustrial waste as alternative medium in the production of chitin and chitosan by Rhizopus arrhizus—a factorial design. Asian Chitin Journal. 2011;7:83–90. [Google Scholar]
- 34.Workshop Jornalismo agropecuário. 2016. July 2016, http://www.imea.com.br/upload/pdf/arquivos/Paper_jornalistas_Milho_AO.pdf.
- 35.Agência Embrapa de Informação Tecnológica. 2016. July 2016, http://www.agencia.cnptia.embrapa.br/gestor/cana-de-acucar/arvore/CONTAG01_108_22122006154841.html.
- 36.Ministério da Agricultura, Cana-de-açúcar. 2015. December 2015, http://www.agricultura.gov.br/vegetal/culturas/cana-de-acucar.
- 37.Beitel S. M., Sass D. C., Coelho L. F., Contiero J. High D(−) lactic acid levels production by Sporolactobacillus nakayamae and an efficient purification. Annales de Microbiologie. 2016;66(4):1367–1376. doi: 10.1007/s13213-016-1224-4. [DOI] [Google Scholar]
- 38.Yamanè T., Shimizu S. Fed-batch techniques in microbial processes. Advances in Biochemical Engineering/Biotechnology. 1984;30:147–194. doi: 10.1007/BFb0006382. [DOI] [Google Scholar]
- 39.Lee J., Lee S. Y., Park S., Middelberg A. P. J. Control of fed-batch fermentations. Biotechnology Advances. 1999;17(1):29–48. doi: 10.1016/s0734-9750(98)00015-9. [DOI] [PubMed] [Google Scholar]
- 40.Sahoo T. K., Jayaraman G. Co-culture of Lactobacillus delbrueckii and engineered Lactococcus lactis enhances stoichiometric yield of D-lactic acid from whey permeate. Applied Microbiology and Biotechnology. 2019;103(14):5653–5662. doi: 10.1007/s00253-019-09819-7. [DOI] [PubMed] [Google Scholar]
- 41.el-Sabaeny A. H. Influence of medium composition on lactic acid production from dried whey by Lactobacillus delbrueckii. Microbiología. 1996;12(3):411–416. [PubMed] [Google Scholar]
- 42.Rojas A. M., Montaño L. P., Bastidas M. J. Producción De Ácido Láctico A Partir Del Lactosuero Utilizando Lactobacillus delbrueckii subsp. bulgaricus Y Streptococcus THERMOPHILUS. Revista Colombiana de Química. 2015;44(3):5–10. doi: 10.15446/rev.colomb.quim.v44n3.55604. [DOI] [Google Scholar]
- 43.Rangaswamy V., Ramakrishna S. V. Lactic acid production by Lactobacillus delbrueckii in a dual reactor system using packed bed biofilm reactor. Letters in Applied Microbiology. 2008;46(6):661–666. doi: 10.1111/j.1472-765X.2008.02362.x. [DOI] [PubMed] [Google Scholar]
- 44.Farooq U., Anjum F. M., Zahoor T., et al. Optimization of lactic acid production from cheap raw material: sugarcane molasses. Pakistan Journal of Botany. 2012;44:333–338. [Google Scholar]
- 45.Calabia B. P., Tokiwa Y. Production of D-lactic acid from sugarcane molasses, sugarcane juice and sugar beet juice by Lactobacillus delbrueckii. Biotechnology Letters. 2007;29(9):1329–1332. doi: 10.1007/s10529-007-9408-4. [DOI] [PubMed] [Google Scholar]
- 46.Srivastava A. K., Tripathi A. D., Jha A., Poonia A., Sharma N. Production, optimization and characterization of lactic acid by Lactobacillus delbrueckii NCIM 2025 from utilizing agro-industrial byproduct (cane molasses) Journal of Food Science and Technology. 2015;52:3571–3578. doi: 10.1007/s13197-014-1423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ezejiofor T. I. N., Uchechi E., Chika O. Waste to Wealth- Value recovery from Agro-food Processing wastes using biotechnology: a review. British Biotechnology Journal. 2014;4(4):418–481. doi: 10.9734/BBJ/2014/7017. [DOI] [Google Scholar]
- 48.Corn Products Brasil. Solução concentrada de Milhocina. 1998, 3f. São Paulo: Technical Bulletin; 1998. [Google Scholar]
- 49.Zhu M., Wang C. J., Wang X., Chen S. H., Zhu H., Zhu H. M. Extraction of polysaccharides from Morinda officinalis by response surface methodology and effect of the polysaccharides on bone-related genes. Carbohydrate Polymers. 2011;85(1):23–28. doi: 10.1016/j.carbpol.2011.01.016. [DOI] [Google Scholar]
- 50.Lima C. J. B., Coelho L. F., Blanco K. C., Contiero J. Response surface optimization of D(-)-lactic acid production by Lactobacillus SMI8 using corn steep liquor and yeast autolysate as an alternative nitrogen source. African Journal of Biotechnology. 2009;8:5842–5846. [Google Scholar]
- 51.Liu Y., Gao W., Zhao X., et al. Pilot scale demonstration of D-lactic acid fermentation facilitated by Ca(OH)2 using a metabolically engineered Escherichia coli. Bioresource Technology. 2014;169:559–565. doi: 10.1016/j.biortech.2014.06.056. [DOI] [PubMed] [Google Scholar]
- 52.Nakano S., Ugwu C. U., Tokiwa Y. Efficient production of D-(-)-lactic acid from broken rice by Lactobacillus delbrueckii using Ca(OH)2 as a neutralizing agent. Bioresource Technology. 2012;104:791–794. doi: 10.1016/j.biortech.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 53.Meng Y., Xue Y. F., Yu B., Gao C. H., Ma Y. Efficient production of l-lactic acid with high optical purity by alkaliphilic Bacillus sp. WL-S20. Bioresource Technology. 2012;116:334–339. doi: 10.1016/j.biortech.2012.03.103. [DOI] [PubMed] [Google Scholar]
- 54.Liu L., Xu Q. L., Li Y., et al. Enhancement of pyruvate production by osmotic-tolerant mutant of Torulopsis glabrata. Biotechnology and Bioengineering. 2007;97(4):825–832. doi: 10.1002/bit.21290. [DOI] [PubMed] [Google Scholar]
- 55.Tian X., Wang Y., Chu J., Zhuang Y., Zhang S. L-Lactic acid production benefits from reduction of environmental osmotic stress through neutralizing agent combination. Bioprocess and Biosystems Engineering. 2014;37(9):1917–1923. doi: 10.1007/s00449-014-1166-9. [DOI] [PubMed] [Google Scholar]
- 56.Kelkar S. T., Mahanwar P. A. Production of lactic acid from tamarind kernel by Lactobacillus casei. International Journal of Technology Enhancements and Emerging Engineering Research. 2015;3:25–31. [Google Scholar]
- 57.Ani I., Suzana W. Effect of temperature and pH on lactic acid fermentation from liquid pineapple waste using immobilized Lactobacillus delbrueckii ATCC 9649. Bangkok, Thailand: Regional Symposium on Chemical Engineering; 2004. [Google Scholar]
- 58.Panesar P. S., Kennedy J. F., Knill C. J., Kosseva M. Production of L(+) lactic acid using Lactobacillus casei from whey. Brazilian Archives of Biology and Technology. 2010;53(1):219–226. doi: 10.1590/S1516-89132010000100027. [DOI] [Google Scholar]
- 59.Carvalho TO. Quais probióticos podem ser adicionados a alimentos industrializados? 2016. July 2016, http://www.nutritotal.com.br/perguntas/?acao=bu&categoria=27&id=456.
- 60.Wee Y. J., Kim J. N., Yun J. S., Ryu H. W. Optimum conditions for the biological production of lactic acid by a newly isolated lactic acid bacterium Lactobacillus sp. RKY2. Biotechnology and Bioprocess Engineering. 2005;10(1):23–28. doi: 10.1007/BF02931178. [DOI] [Google Scholar]
- 61.Lima C. J. B., Coelho L. F., Contiero J. The use of response surface methodology in optimization of lactic acid production: focus on medium supplementation, temperature and pH control. Food Technology and Biotechnology. 2010;48:175–181. [Google Scholar]
- 62.Shi G., Wang G., Chen X., Li C. Optically pure L-lactic acid production directly from leftover bits and pieces of potato starch using an amylolytic pellet-form complex Rhizopus oryzae ASC081. Journal of Applied Science and Engineering. 2013;16:205–210. doi: 10.6180/jase.2013.16.2.12. [DOI] [Google Scholar]
- 63.Senthuran A., Senthuran V., Hatti-Kaul R., Mattiasson B. Lactic acid production by immobilized Lactobacillus casei in recycle batch reactor: a step towards optimization. Journal of Biotechnology. 1999;73(1):61–70. doi: 10.1016/s0168-1656(99)00133-9. [DOI] [PubMed] [Google Scholar]
- 64.Li Y., Wang L. M., Ju J. S., Yu B., Ma Y. H. Efficient production of polymer-grade D-lactate by Sporolactobacillus laevolacticus DSM442 with agricultural waste cottonseed as the sole nitrogen source. Bioresource Technology. 2013;142:186–191. doi: 10.1016/j.biortech.2013.04.124. [DOI] [PubMed] [Google Scholar]
- 65.Ye L. D., Zhou X. D., Hudari M. S. B., Li Z., Wu J. C. Highly efficient production of L-lactic acid from xylose by newly isolated Bacillus coagulans C106. Bioresource Technology. 2013;132:38–44. doi: 10.1016/j.biortech.2013.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data in the manuscript are available.


