Abstract
Cardiolipin oxidation and degradation by different factors under severe cell stress serve as a trigger for genetically encoded cell death programs. In this context, the interplay between cardiolipin and another mitochondrial factor—cytochrome c—is a key process in the early stages of apoptosis, and it is a matter of intense research. Cytochrome c interacts with lipid membranes by electrostatic interactions, hydrogen bonds, and hydrophobic effects. Experimental conditions (including pH, lipid composition, and post-translational modifications) determine which specific amino acid residues are involved in the interaction and influence the heme iron coordination state. In fact, up to four binding sites (A, C, N, and L), driven by different interactions, have been reported. Nevertheless, key aspects of the mechanism for cardiolipin oxidation by the hemeprotein are well established. First, cytochrome c acts as a pseudoperoxidase, a process orchestrated by tyrosine residues which are crucial for peroxygenase activity and sensitivity towards oxidation caused by protein self-degradation. Second, flexibility of two weakest folding units of the hemeprotein correlates with its peroxidase activity and the stability of the iron coordination sphere. Third, the diversity of the mode of interaction parallels a broad diversity in the specific reaction pathway. Thus, current knowledge has already enabled the design of novel drugs designed to successfully inhibit cardiolipin oxidation.
1. Introduction
Mitochondria—the so-called powerhouses of the cell—are responsible for a broad assortment of metabolic processes. Their key role in cells is reflected by the cornucopia of proteins involved in its function. In total, more than 1150 genes related to organelle function are recorded in the human MitoCarta. Furthermore, 1 in every 5000 people are effected by a mitochondrial disorder [1].
Mitochondria play a significant role in cell homeostasis by helping to modulate cell signaling pathways. On one hand, the activity of the electron transport chain (ETC) is related to the release of reactive oxygen species (ROS) [2] which are strong modifiers of cell constituents such as proteins, nucleic acids, and lipids. Dysregulation of ROS can lead to oxidative stress which in turn can initiate cell death programs [3, 4], in which lipid peroxidation and their products play a key role [5].
Cardiolipin (CL) oxidation by cytochrome c (Cc) at the onset of apoptosis is a decisive step [6]. During homeostasis, the soluble cationic hemeprotein is located in the mitochondrial intermembrane space, shuttling electrons between complexes III (CIII) and IV (CIV) in the ETC. Indeed, Cc is a key Janus catalyst of CL signaling rather than a passive messenger. Its ability to oxidize superoxide anions (O2-•) to molecular O2 along with its peroxidase activity in solution reduce the damage caused by oxidative stress [7–12]. However, rearrangement of the mitochondrial membrane triggered by t-Bid upon severe stress makes CL available to bind Cc [13] on the outer leaflet of the IMM. Thus, acyl chains of CL are oxidized due to the oxygenase activity of the hemeprotein [14]. In fact, oxygenase activity of Cc rises substantially in Cc-CL complexes [15]. Subsequent CL oxidation favors the release of Cc into the cytosol where it triggers apoptosis [16–18]. Furthermore, an array of products from Cc-mediated CL oxidation—e.g., hydroxy-, oxo-, and peroxipolyunsaturated fatty acids—act as cell fate decision signals [19].
Although major features of cell death signaling pathways converging on CL metabolism have been thoroughly characterized, understanding the intimate mechanism of CL oxidation by Cc remains challenging. Both CL-containing membranes and Cc display complex behaviors that depend on different factors, including experimental conditions and post-translational modifications (PTM) of the protein.
This review article aims to provide the readers with an overview of the interaction between Cc and CL and how it affects the peroxidase and oxygenase activities of the hemeprotein. Particular emphasis will be made on the conformational plasticity of Cc, which enables its Janus functionality. In addition, we will discuss free oxidation of CL, regulation of Cc activity, and its relationship with a diverse range of human diseases and recent strategies to combat them.
2. Cardiolipin: Properties and Role in the Mitochondrial Membranes
Cardiolipins (1,3-bis(sn-3′-phosphatidyl)-sn-glycerol) are a group of anionic phospholipids found in the plasma membrane of various bacteria and the inner mitochondrial membrane of eukaryotic cells [20]. These lipids contain two 1,2-diacyl-sn-glycero-3-phos-phoryl moieties bridged by a glycerol molecule. The two phosphatidyl groups are stereochemically nonequivalent, being respectively in pro-R and pro-S positions with respect to carbon 2 in the bridge [21]. The presence of 4 acylation sites—a fifth one at the central carbon of the glycerol bridge is also possible—would be consistent with a diverse range of CL species according to the distinct acyl chains available in a given organism. In humans, for instance, we would expect 144 CL derivatives. This contrasts with the rather lower diversity of CL compounds found in each organism [22].
Despite the presence of two phosphate groups in CL, it is thought that the single anionic species predominates. In this species, one proton is shared through a bicyclic resonance structure involving the central hydroxyl group [23]. In membranes, the glycerol hydroxyl forms intra- and interlipid hydrogen bonds with oxygen atoms from phosphate, not with carbonyl groups [24]. Early measurements of ionization constants yielded a first pKA value of 2.8 and a second one in the range between 7.5 and 9.5. A recent fourier transform infrared spectroscopy (FT-IR) analysis on liposomes also suggests two ionization steps with pKA values 4.7 and 7.9 [25, 26]. Density functional theory level computations indicate a wide gap between the two pKA values [27]. Other results indicate the opposite: both behave as strong dibasic acids with pKA values within the pH range 2-3 in solution [28] and membrane preparations [29, 30]. According to this data, membrane-embedded CL carries two negative charges at physiological pH values.
The behavior of CL-containing membranes is complex and strongly dependent on the composition [31] and experimental/simulation conditions [32]. For instance, the selected CL protonation state in deterministic simulations can influence results. Thermodynamic analyses with lipid mono- and bilayers indicate a decrease in the area compressibility modulus [33]. According to molecular dynamics (MD) simulations, their thickness—measured as interphosphate distances—decreases with CL content, as the electron density does [24, 34]. Furthermore, small-angle X-ray scattering (SAXS) and neutron scattering (SANS) have confirmed that CL-containing bilayers have a lower thickness. This may reflect the smaller head-group volume per phosphate. However, these membranes show larger distances between electron density maxima and a thicker hydrocarbon moiety [24]. Comparison of different MD trajectories of bilayers with PDB files suggests conformational selection takes place when CLs bind to membrane proteins [34]. The negative charge of CL and its four acyl groups strongly affect the phase preference of the lipid, which varies from lamellar (Lα) to inverted hexagonal (HII) depending on pH [30, 32].
CL is essential for the functionality of mitochondrial membranes and processes taking place therein—e.g., protein import and electron transport [6]. It represents between 5% and 20% of the total lipid content of the inner mitochondrial membrane (IMM) and is more abundant in the internal leaflet [35, 36] (Figure 1(a)). CL acts on membrane components of the ETC, aiding the assembly of the so-called respiratory supercomplexes [37, 38]. Supercomplexes modulate the performance of mitochondrial electron transport and oxidative phosphorylation [39]. Reportedly, CL is able to trap protons [40, 41], and it has been hypothesized to be important in the mechanism of CIII and IV acting as a proton exchanger [42–44]. The absence and/or modification of CL cause the development of several pathologies such as Barth's syndrome [37, 45–48]. Indeed, alteration of the IMM due to a decrease in the content of CL disrupts the ETC, increasing the generation of ROS [49] (Figure 1(b)). Remarkably, CL can be oxidized directly by ROS such as hydroxyl radicals and singlet oxygen, acting the products as proapoptotic signals [50].
Figure 1.

Role of cardiolipin in cell homeostasis and apoptosis. (a) Under homeostatic conditions, cardiolipin (CL) facilitates the assembly of respiratory supercomplexes (brown arrows) and maintains a population of Cc bound to the inner mitochondrial membrane (IMM). The efficiency of electron transfer is high (thick red arrow). (b) Under apoptotic stimuli, procaspase-8 is recruited to CL-enriched microdomains in the outer mitochondrial membrane (OMM). The activation of caspase-8 involves cleavage of the BID proapoptotic factor into two domains, namely, the N-terminal (n-Bid) and C-terminal fragments (t-Bid). Dissociation of these two fragments is required for the interaction of t-Bid with CL. Then, t-Bid promotes the formation of mitochondrial pores by assembling BAX–BAK oligomers. At the same time, ROS production increases and Cc acts as a ROS scavenger and pseudoperoxidase. Cc peroxidase activity results in oxidation of CL acyl chains, to which the hemeprotein is anchored, freeing Cc from the IMM, facilitating its subsequent release into the cytosol upon OMM permeabilization. The efficiency of electron transfer is low (dashed red arrow). In addition, CL can be degraded in part, losing one of its acyl chains, giving rise to monolysocardiolipin (MLCL).
CL is a mitochondrial stress-signaling factor in mitophagy and both the intrinsic and extrinsic apoptotic pathways [6, 51]. Under stress conditions (e.g., treatment with rotenone, staurosporine or cyclosporine A, and autophagic or apoptotic stimuli), CL molecules flip from the IMM to the outer mitochondrial membrane (OMM) [52–54] (Figure 1(b)). When eliciting the extrinsic apoptotic pathway in lymphoblastoid cells (type II cells) derived from Barth's syndrome patients and tafazzin knock-down HeLa cells, CL microdomains on the OMM recruit procaspase-8 to promote its activation [55, 56]. When caspase-8 becomes active, it cleaves the proapoptotic factor Bid, a BH3-only member of the Bcl-2 family [56]. The active C-terminal fragment of the Bid (t-Bid) targets CL or its degradation product monolyso-CL (MLCL) in mitochondria [57–60] and promotes OMM permeabilization [61]. During this process, the peroxidase activity of Cc results in the oxidation of CL (to which it is anchored) facilitating the release of Cc from the IMM and subsequent massive release into the cytosol at the onset of apoptosis [18, 62]. Extramitochondrial Cc molecules interact with a variety of targets in the cytosol and nucleus, leading to a point of no return in the programmed cell death regulation [63–77].
3. Cytochrome c Binds Cardiolipin: A Tale of Grooves, Cavities, and Melting
Cc belongs to the class I single-heme cytochrome c family, displaying the four typical α-helices conserved in the whole domain family [78]. In addition, Cc displays three Ω-loops, two of them providing axial ligands for the heme iron. His18 at the proximal side of the heme provides the imidazole ligand conserved among the class I family. At physiological pH values, Met80 thioether acts as a distal ligand. The heme porphyrin ring is covalently bound to the protein backbone by thioether bonds between the vinyl groups of the porphyrin and conserved cysteine residues in the CXXCH motif. For human Cc, conserved cysteine residues are Cys14 and Cys17. According to hydrogen exchange (HX) experiments, the apparently simple structure hides five folding units (called foldons) with different stabilities [79]. The most stable one (I) comprises the N- and C-terminal α-helices. Foldon II comprises ΩI (from Thr19 to Phe36) and α-helix 3, which comes before ΩIII. Foldon III (a.k.a. neck) comprises two short amino acid stretches with an extended conformation flanking the ΩII-loop. Notably, the latter faces heme propionates and is the least stable foldon (V), followed by the ΩIII region (IV) containing Met80.
The low stability of the loop containing Met80, comprising the sixth iron ligand, has a crucial role in Cc physiology. Recent ultrafast X-ray spectroscopy analyses have highlighted the weakness of the Met80-Sδ-Fe+2 bond and the lack of stability (4 kJ mol−1) provided by the protein matrix, most likely via hydrogen bonding [80].
At physiological pH, Cc has a net charge of +8 from its unevenly distributed ionizable groups [81, 82]. This favors interactions with negatively charged molecules, such as the polar head of phospholipids, including CL. This interaction was first analyzed by Kimelberg and Lee, using lecithin-CL vesicles [83]. Their analysis together with early HX measurements by solid-state nuclear magnetic resonance (ssNMR) indicated that Cc preferentially binds with CL [84]. They also suggested that during this interaction, CL destabilizes or unfolds the hemeprotein. Further relaxation time measurements by 31P NMR showed that Cc impacts CL dynamics [85]. Surface plasmon resonance and electrochemical experiments on planar lipid bilayers allowed Salamon and Tollin to propose a two-step mechanism [86–88]. According to their proposal, Cc first binds to membranes through electrostatic interactions and, subsequently, through hydrophobic interactions to promote changes in both the structure of Cc and the membrane. Then, electron paramagnetic resonance (EPR) and magnetic circular dichroism (MCD) analyses showed that Cc undergoes structural changes which affect Fe coordination and result in the appearance of a radical at high liposome concentrations [89]. Hence, mixing Cc with lipids may yield several species, found in recent fluorescence anisotropy analyses [90].
Apparently at odds with this proposed model, paramagnetic-quenching EPR experiments on horse heart Cc, spin-labelled at different lysine positions, indicated that the hemeprotein can weakly interact with 1,2-dioleoyl-sn-glycero-3-phosphoglycerol (DOPG) bilayers [91]. This study highlighted three lysine residues at ΩIII (K72, K86, and K87, a.k.a A-site; Figure 2(a)) adjacent to the DOPG membrane. Further fluorescence studies using vesicles containing fluorescent lipid probes identified a secondary CL-binding site at low pH values [93]. Contrary to the A-site, CL association at this novel region (a.k.a. C-site; Figure 2(a)) is unaffected by ionic strength or the presence of ATP. Data suggested CL binds to this site via hydrogen bonds at N52, and a single acyl chain of the phospholipid inserts into a nearby hydrophobic pocket while the others remain in the bilayer [94]. This proposed interaction mechanism is known as the extended lipid anchorage model and is supported by studies on the ability of natural and engineered phospholipids to quench the fluorescence of Zn-substituted Cc [95, 96]. Consistently, a N52I mutation heavily impacts the kinetics of the interaction between Cc and CL in CL-containing liposomes [97].
Figure 2.

Cardiolipin-binding sites in cytochrome c. (a) Ribbon representation of oxidized human Cc (PDB 2N9J) [92]. CL-binding sites are highlighted in orange (A-site or distal productive), green (L-site or proximal productive), purple (C-site), and cyan (N-site or proximal unproductive). The heme axial ligands H18 and M80 are highlighted as well. (b) Side chain representation of the positively charged Cc residues involved in the formation of the Cc-CL complex. Residues marked with an asterisk are reported to constitute the L- and N-sites. (c) Side chain representation of hydrophobic Cc residues, which ensure the tight interaction between Cc and CL acyl chains.
While, a combination of lysine modification, tryptic digestion, and MALDI-TOF analysis unveiled that horse heart Cc promotes the fusion of lipid vesicles via an interaction at a second positive patch. This region (L-site) comprises K22, K25, K27, H26, and H33, besides the previously reported A- and C-sites (Figure 2(a)) [98].
An additional UV-Vis analysis showed slight differences in the binding kinetics within a set of yeast Cc mutants [99]. Based on their own data and the solution structure of the protein (PDB 1AKK; [100]), the authors proposed a cleft defined by the ΩIII residues K72, K73, K86, and R91, matching A-site. Building on from this data, the ability of site-directed mutants of horse heart Cc to bind CL-containing liposomes was tested [101]. Notably, substituting K73 and K79 with asparagine alters the affinity of Cc towards these liposomes, whereas the same mutation at position 72 does not.
A common feature of these binding sites is the presence of several positively charged and hydrophobic residues (Figures 2(b) and 2(c)). In line with Tollin and Salamon's early postulates [88], the interaction between the positively charged residues of Cc and negatively charged phosphate groups of CL initiates the formation of the Cc-CL complex driven by electrostatic interactions [101–104]. After the initiation of the complex, hydrophobic Cc residues play a key role in the interaction with CL acyl chains, thereby establishing a tight binding between Cc and CL [15, 105].
A strong piece of evidence supporting the extended anchorage hypothesis is the presence of a channel formed by residues 52 to 74 in the tuna Cc XRD structure [81], where the highlighted cavities are only visible in the 4 Å model. An attempt to manually dock CL inside the structure of horse heart Cc yielded two acyl chains within the backbone [97]. Any assessment of clashes of these two acyl chains with internal residues was missing. Nevertheless, none of the two cavities in this structure appear in the updated structure at 1.5 Å resolution (PDB 5CYT; unpublished). Hence, the extended lipid anchorage model demands that Cc must undergo a substantial conformation change when interacting with lipid vesicles. A low-resolution analysis using monoclonal antibodies suggested that lipid-bound Cc displayed an alkaline-like conformation [106]. The alkaline form of oxidized Cc presents a conformation different to that of the native species, which relates to an exchange between the Met thioether ligand and a Lys amine. However, to our knowledge, the only Cc alkaline structure available (PDB 1LMS; [107]) lacks a channel in which the acyl chain may be lodged, although the heme moiety is more accessible to solvent than the native structure. Another possibility is the formation of Cc oligomers by domain swapping [108, 109]. Although these structures are highly variable depending on how the domain swapping is triggered by experimental conditions, a recent analysis has shown they are capable of encompassing an acyl chain [110]. Furthermore, Tyr67 in these structures would face C11 of docked linoleic (18 : 2Δ9,12) acid, a finding in agreement with peroxidation mechanisms. However, evidence of Cc oligomerization in the presence of membranes remains unavailable.
Many of the studies above report the loss of the Met80 coordination and at least partial denaturation—or transition to a molten globule state—of Cc when binding to CL-containing vesicles or liposomes [15, 84, 93, 95–97, 109–111]. Aside from CL, some other lipids can elicit similar such structural changes in Cc [105]. This makes the heme moiety more accessible to small substrates such as carbon monoxide or nitric oxide [105, 111]. Moreover, time-resolved fluorescence resonance energy transfer (trFRET) experiments show labelled residues—not previously reported to bind CL—moving further away from the heme group in the presence of CL-containing liposomes [103, 112]. Furthermore, changes in the CD spectra and Trp59 fluorescence indicate unfolding of at least the lowest energy foldons. Consistent with the loss of Met80 coordination, substantial negative shifts in the midpoint potential of Cc are observable in the presence of lipid vesicles [113, 114]. Disruption of the Fe-Met80 bond also correlates with a substantial increase in Cc peroxidase activity when Cc interacts with CL-containing vesicles [15, 105, 113]. An illustration of conformational changes, adapted from Muenzner et al. [112], is available in Figure 3(a).
Figure 3.
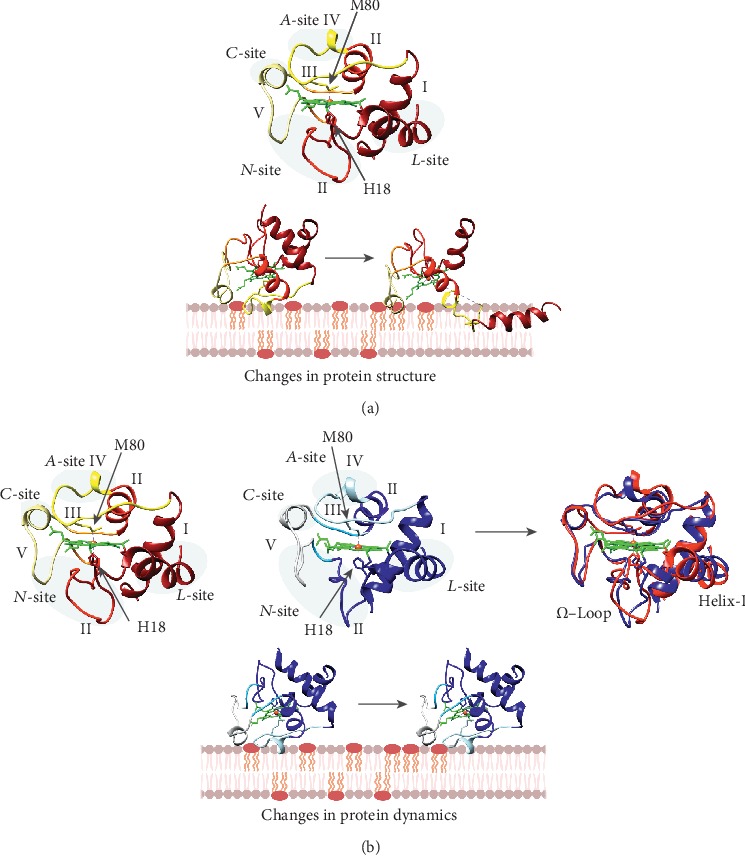
Proposed model for the interaction of cytochrome c with cardiolipin at pH values above 7. (a) Upper: structure of free Cc showing the foldon units (in red scale, PDB 1AKK [100]). Lower: the Cc-CL interaction promotes unfolding of the metalloprotein and dissociation of the axial ligand M80, thus increasing accessibility to the heme crevice. (b) Upper: structural comparison of free (in red scale, PDB 1AKK [100]) and CL-bound Cc (in blue scale, PDB 2N3B [115]). Lower: interaction of Cc with CL yields a slight difference in dynamics at the level of the Ω-loops and helix-I. The different foldon units of Cc are colored as a gradient from the most stable (dark colors) to the weakest region (light colors). The heme group is in green, and the iron atom in orange.
The current understanding of the effects of lipid binding on Cc structure (as described above) is controversial. For instance, depending on the nature of the CL-rich membrane preparations and experimental setup, the shift in redox potential can be positive [86] or negative [113, 114]. Recently, Wand and collaborators demonstrated that the mitochondrial crypts are concave surfaces, opposed to the convex lipoic vesicles often used in the analyses. They then analyzed the solution structure of oxidized horse heart Cc encapsulated in reverse lipid micelles by NMR [115]. They utilized pseudocontact shifts as experimental restraints, which are highly sensitive to distances and orientation with respect to the main axes of the iron coordination sphere. An overlay of the structures of free [100] and encapsulated horse Cc [115] (PDB 1AKK [100] and 2N3B [115], respectively) are shown in Figure 3(b). Notably, the structure of Cc remains unaltered, and the chemical-shift perturbation map [115] resembles that observed for interactions between Cc and its protein targets [116] and other class I single-heme cytochrome c family members [117]. In fact, this patch includes a novel region (N-site), not previously reported, comprising F36, G37, T58, W59, and K60, besides the known residues of the A- and L-sites (Figure 2(a)). Surprisingly, ssNMR spectra tracking the interaction between Cc and small unilamellar (convex) vesicles showed no feature indicating formation of either a molten globule or unfolding [118]. Consistently, solution HSQC (NMR) spectra of horse heart Cc in the presence and absence of similar vesicles overlapped quite well [119]. Although some signals broaden, while others were displaced—as expected for this interaction—no features of unfolding are apparent. Therefore, the overall curvature or the membrane may be irrelevant for the stability of bound Cc. Previous data on Cc unfolding in the presence of lipid vesicles might be reviewed in regard to membrane composition, lipid stability, and experimental setup. For instance, the dynamics of Cc can change depending on buffer conditions [119]. Similarly, the ionization state of CL also affects Cc binding [120].
Understanding the entire landscape of the data requires the rationale underpinning the studies to be taken into account. An equilibrium between unbounded native Cc species and membrane-bound populations—by either weak electrostatic interactions, hydrogen bonding, or hydrophobic interactions—can be observed. The relative weights of such populations vary with experimental conditions. Furthermore, our ability to detect different species relies on the sensitivity of each biophysical approach. As recently pointed out, many of the analyses above suffer from low-resolution data or the introduction of probes that could partially alter results [118]. Therefore, full understanding requires complete knowledge of experimental conditions.
4. Interplay between Lipid and Cytochrome c Dynamics: The Compact/Extended Model
Cc can undergo several structural transitions triggered by changes in experimental conditions such as pH [89, 90, 121–123]. For example, low-spin (S = 0) FeII species may turn into high-spin (S = 2) species in the presence of liposomes, as shown by EPR and MCD spectroscopies [89]. Notably, ionic strength and lipid-to-protein (L/P) ratio strongly influence the populations of the distinct species [15, 110, 124, 125]. These ratios relate to lipid surface coverage by Cc molecules [125, 126]. At low L/P ratios the Cc-coated micelles undergo coalescence—forming giant unilamellar vesicles—and precipitate. Whereas at moderate L/P ratios, Cc promotes interactions between small unilamellar vesicles [125, 127].
The dynamic of the Cc heme group is highly sensitive to spin state, axial ligand strength, and conformational changes. Therefore, Raman spectroscopy studies have been key in unveiling the complexity of Cc conformation equilibria under different conditions [123, 125–128]. Hildebrandt and collaborators detected native (B1; His-Met coordination) and altered (B2) states in the presence of DOPG vesicles [125]. The B2 state comprises different species: a low-spin (B2[6cLS]), His-His-coordinated species, and two high-spin species (a pentacoordinated (B2[5cHS]) and a hexa-coordinated species (B2[6cHS])) in which a water molecule acts as the sixth ligand. The populations of the different states change according to the L/P ratio. The B2 species predominates at high L/P ratios, whereas native B1 and His-His-coordinated B2[6cLS] coexist at lower ratios. These states are also detectable by MCD [114]. The B2 bis-His-coordinated species is detectable when Cc is absorbed onto self-assembled monolayers, with and without CL [129]. At increasing concentrations of DOPC/tetraoleoylcardiolipin (TOCL) micelles, the population of bis-His species increases, as confirmed by His-by-Asn mutations and spectroscopic analyses [130].
In addition to the L/P ratio, the content of CL and its composition influence Cc affinity and dynamics in the bound state. Fluorescence data has indeed revealed that increasing amounts of CL favors Cc binding to membranes [120]. The theoretical analysis therein suggests that the protonation state of CL may have a strong influence on populations of distinct membrane-bound Cc species. However, the authors acknowledge that the formalism does not include the effects that Cc exerts on CL distribution or membrane state (see below). Indeed, kinetic investigations have shown that the exchange rate between a native-like, compact (C) and the “extended” (E) conformations correlates with the amount of CL in the vesicles [131]. A more recent spectroscopic analysis on titration experiments by Pandiscia and Schweitzer-Stenner resulted in similar conclusions [90]. Additionally, the study highlighted that the L/P ratio also affects the relative weight of electrostatic interactions, hydrogen bonds, and hydrophobic interactions—according to ionic strength series. In summary, besides governing Cc conformational states, the L/P ratio modulates the nature of bilayer-protein interactions. Notably, all the studies highlighted above hint at a rather peripheral binding model, with little or no embedding of Cc into the membrane [90, 123, 125, 126, 128, 129, 131].
On the other hand, Cc does exert a strong influence on lipid head-group dynamics, as revealed by early 31P ssNMR studies which demonstrated an increase in acyl chains dynamics and a restraint in the polar head groups of phospholipids [132, 133]. Interestingly, Cc has little impact on the 31P ssNMR “powder” spectra of dioleoyl-phosphatidylcholine (DOPC), dioleoyl-phosphatidylethanolamine (DOPE), or DOPC/DOPE vesicles not containing CL [134]. Indeed, NMR data strongly supported CL undergoing phase separation—to form CL rafts—within mixed DOPC/CL preparations upon the addition of Cc [132, 135]. Further, freeze-fracture electron microscopy images highlighted the ability of Cc to promote the transition of phospholipid bilayers containing CL into non-bilayer structures, including inverted tubular (HII) states [133, 134]. A full isotropic signal in the 31P ssNMR spectra of phospholipid preparations in the presence of Cc evinced the formation of vesicular or micellar structures when the vesicles contained CL [132, 136]. Similarly, a downfield broad signal indicated that CL mediates the formation of the HII phase upon the addition of Cc. In this sense, molecular dynamics simulations in which Cc is in contact with a DOPC/CL membrane highlight the ability of this protein to recruit CL into rafts [118]. In addition, Cc can induce local changes in membrane curvature when the ratio of CL increases up to 20%. Furthermore, Cc induces pore formation in DOPC/CL giant unilamellar vesicles (GUV), as shown by confocal microscopy [137]. These pores are wide enough to allow Cc and dextran molecules to cross the membrane.
Nevertheless, the ability of Cc to induce membrane changes seems to be secondary regarding the activation of peroxidase activity. Addition of Cc to large DOPC/CL unilamellar vesicles at a ca. 6 CL/Cc ratio promotes peroxidase activity without substantially affecting 31P ssNMR spectra or the 13C frequencies of the lipid glycerol signals [138]. The major population—those accounting for less than 10% of the protein are not detectable—of Cc in these experiments displays the same structure as the native protein in solution. Chemical-shift perturbation analysis revealed that residues affected include the ΩIII-loop and some nearby residues (including A-site residues). However, changes in the dynamics of the ΩIII-loop as it couples with bilayer motions are observable. This, rather than an overall unfolding, is sufficient to trigger the peroxidase activity under these conditions. In accordance with this finding, the perturbation pattern shifts when the temperature is changed or when the vesicle phospholipids are unsaturated.
The formation of Cc-CL complex requires approximately 6 molecules of CL per Cc molecule [15, 112, 119, 138, 139]. The values of the apparent dissociation equilibrium constant for the Cc-CL-reduced complex are in the low micromolar range (1.4 μM at pH 8.1 and 2.2 μM at pH 6.5) [140], whereas in the oxidized form, they are in the high micromolar range in a two-step reaction (20 μM and 42 μM) [102]. Nevertheless, binding constants depend on CL composition. The affinity of Cc towards tetra-stearyl-cardiolipin-containing vesicles is several fold higher than that for TOCL ones [102], whereas tetra-myristoyl-cardiolipin barely interacts Cc [15]. Notably, the measured affinities correlate well with the peroxidase activity of Cc in the presence of the respective vesicles, rather than the degree of unsaturation in the acyl chains [102].
5. Cardiolipin Oxidation by Cytochrome c
CL is particularly sensitive to auto-oxidation processes—those directly initiated by inducers, such as ROS. The proximity of its four unsaturated acyl chains allow “arm-to-arm” propagation, enhancing its reactivity [141]. Auto-oxidation takes place in several steps. A free radical (e.g., a ROS molecule) contains an unpaired electron, and this semioccupied orbital is a sink for a second electron. Polyunsaturated fatty acids (PUFA), such as linoleic or linolenic composing CL, are particularly sensitive to ROS-induced oxidation due to conjugative effects. Radicals such as superoxide, peroxyl (ROO•), or hydroxyl (HO•) sequester a hydrogen atom from the α-methylene carbon with respect to the first (di-) vinyl group. The resulting radical reacts immediately with O2 to generate a peroxyl radical. The variation of electron vacancy in the lipid radical underlies the diversity of reaction products. No matter how the lipid radical originates, it tends to propagate via a reaction with molecular oxygen, water, or other lipids. Within the process of CL signaling, there are several reactions which stand out including the addition of oxygen (to form peroxides), transfer of hydrogen atoms, addition of peroxyl radicals, and intramolecular peroxide substitution [142].
Oxidative phosphorylation and certain mitochondrial enzymes are sources of O2-• radicals [143]. Superoxide is highly soluble in lipids but can be reduced within membranes by tocopherol and quinone and eliminated by superoxide dismutase (SOD), which transforms two superoxide molecules into a molecular oxygen and hydrogen peroxide [144]. In fact, enzymes like SOD, catalase, and peroxidases take part in active cell defense against oxidative stress [144]. H2O2 is a strong oxidant—the reduction potential for the H2O2/H2O pair is +1.35 V, at pH 7—but kinetically inefficient. However, the presence of iron chelates under certain pathological conditions can enhance H2O2 efficiency through Haber-Weiss-like reactions (see Equations (1)–(3)) [145]:
| (1) |
| (2) |
| (3) |
The hydroxyl radical product is highly reactive, sequestering hydrogen atoms from available substrates. The resulting carbon-centered radicals may react with molecular oxygen to generate (hydro-) peroxides (Equations (4)–(6)):
| (4) |
| (5) |
| (6) |
Nevertheless, homeostatic cells exert a tight control over metal chelation to avoid Fenton's reactions. Indeed, a set of antioxidant agents prevent a surge in the levels of ROS. Hence, except for pathological conditions, H2O2 requires the activity of peroxidases to function efficiently as an oxidant. Within the IMM, Cc displays both peroxidase and oxygenase activities, the latter promoting CL oxidation, while sparing other phospholipids [18, 146]. This event is crucial for the release of mitochondrial proapoptotic factors into the cytoplasm [18].
5.1. Peroxidase and Oxygenase Activities of Cytochrome c (Dr. Jeckyll and Mr. Hyde)
Heme peroxidases constitute a vital and ubiquitous group of heme enzymes catalyzing the two-electron oxidation of substrates using H2O2 as the ultimate electron acceptor [147, 148]. In canonical peroxidases, H2O2 is added to the pentacoordinated, high-spin FeIII. The resulting state—Compound I—is two oxidation equivalents above the resting state and is reduced back to the resting configuration in two steps through Compound II. Both states, Compounds I and II, are high valence oxoferryl (FeIV) derivatives, but the first comprises an additional π-cation radical (see Equations (7)–(9)) (Figure 4) [147].
| (7) |
| (8) |
| (9) |
Figure 4.

Peroxidase and oxygenase activities of cytochrome c. Reaction model merging the proposal from Kagan and collaborators [149] and the adapted catalytic model of cyclooxygenases as reviewed by Marnett [150]. Blue arrows correspond to the canonical peroxidase cycle [146]. Heterolytic cleavage of a peroxide substrate—preferentially for Cc, a lipid hydroperoxide—yields the corresponding hydroxyl derivative (or water when the substrate is H2O2) and Compound I, which is reduced back to the resting ferric state in two sequential single-electron transfers from A substrate. Red and green arrows indicate the reactions purportedly leading to oxygenase activity according to the literature. Spin trap experiments have detected Y48 radicals [151]. Dimers of tyrosines 67 and 74 and oxidation products of Y48 are detectable even in the absence of H2O2 [152]. The tyrosyl radical sequesters a hydrogen atom from an unsaturated fatty acid. Finally, O2 reacts with the alkyl radical to form an alkyl peroxide radical as an initial product undergoing further reactions.
In true peroxidases, a histidine residue acts as an acid-base catalyst at the distal side of the heme ring, while a highly conserved arginine residue stabilizes the alkoholate leaving group to favor the heterolytic cleavage of the peroxide O–O bond [153]. The orientation of these residues and the hydrogen-bond network at the heme distal side are critical for efficient formation of Compound I [154]. As recently pointed out by Vlasova [148], the composition of a true peroxidase active site prevents its damage by highly oxidizing intermediate compounds.
Cc and other hemeproteins can act as pseudoperoxidases; that is, under only certain stimuli they show peroxidase activity [148, 155]. Contrary to true peroxidases, the surroundings of heme moiety are unprotected against oxidation, so the peroxidase activity ends up damaging the protein. In the early 1990s, Radi and collaborators reported the ability of Cc to oxidize small compounds in solution [145] and to carry out lipid peroxidation [146] in the presence of H2O2. Nevertheless, the reactivity of Cc towards H2O2 was low, as it requires the absence of the sixth ligand. Thus, the Km value for H2O2 was very high—ca. 65 mM. In this sense, oxidative reactions showed a time lag after the addition of H2O2, indicative of an activation step. Moreover, peroxidase activity in solution decayed at pH values higher than 8 [145]. Poor reactivity may also result from the absence of histidine at the distal site (vide infra), as it is an acid-base residue “pumping” the heterolytic cleavage of H2O2 during Compound I formation. Based on chemiluminescence analyses of the reaction, Chance and coworkers proposed a homolytic reaction mechanism (Equations (10)–(12)) [156], supported by EPR spin trap studies of small organic hydroperoxides [157].
| (10) |
| (11) |
| (12) |
However, EPR spin trap experiments highlighted the generation of Cc tyrosine radicals upon treatment with H2O2 [18, 151, 155, 158]. Furthermore, spin trap experiments detecting radical products resulting from oxidation of different substrates by H2O2 strongly suggested the reaction being mediated by an oxoferryl [O=FeIV] intermediate [155]. Analysis of the orientation of this radical within the native state identified a tyrosine residue at the ΩII-loop—namely, Y48 in horse heart Cc and either Y46 or Y48 in human Cc [159]. The peroxidase activity of Cc in the presence of CL increases by three orders of magnitude when the driving oxidant is a lipid peroxide instead of H2O2 [149]. Spin trap analysis of reaction products by Kagan and collaborators indicates a diversity of catalytic mechanisms depending on the binding site of the substrate, namely, a homolytic peroxide cleavage minority mechanism and a major, heterolytic mechanism. Notably, the small hydroperoxide substrates involved in this pathway dock near R38 and H33. However, how the docked structure undergoes conformational changes to fulfil all geometrical constraints needed for Compound I formation remains unclear (Figure 4).
Binding of hydrogen peroxide to the heme iron is a key step in the reaction mechanism underlying peroxidase activity. The reactive species need to displace the thioether axial ligand. This takes place when a strong interaction between Cc and a membrane induce a substantial conformational change in the hemeprotein [15, 84, 93, 95–97, 109, 111]. Nevertheless, Kagan and coworkers also detected peroxidase activity in Cc at low CL/Cc ratios—at which the most interactions are weak electrostatic [15]. Furthermore, they found that the energy required to activate peroxidase activity is lower than that required for partial unfolding of the protein. In fact, as pointed out before, the bond joining iron to the thioether ligand is quite weak [160]. Thus, “breathing” fluctuations of ΩII- and ΩIII-loops may facilitate the replacement of the thioether ligand by small reactants—such as cyanide, carbon monoxide, water, or hydrogen peroxide—without demanding major structural changes. In fact, Cc peroxidase activity rises in the presence of H2O2 as the concentration of denaturant increases, as previously observed in a similar analysis with bacterial cytochrome c550 [161]. Statistical analysis of activity and unfolding slopes indicate that increasing the motions of the weakest Ω-loops correlates well with peroxidase activity in the “compact” Cc species [162].
The peroxidase activity of Cc can exert a protective role in mitochondria under certain conditions [11]. Indeed, reduction of lipoid hydroperoxide compounds to hydroxyl ones provides a way of relieving oxidative stress in the mitochondrial membrane while generating signaling molecules [149]. Moreover, O2-• reduces nitric oxide (•NO) generated in mitochondria under stress to form peroxynitrite (HOONO), a highly reactive species. Cc-CL complexes have been proposed to aid peroxynitrite detoxification to yield either nitrate or nitrite through an oxoferryl state [163].
Conversely, the peroxidase activity of Cc has been implicated in certain pathologies. For instance, oxidation of sulfite to its radical SO3-• is a key mechanism in sulfite toxicity [164]. Moreover, Cc mediates the formation of tyrosine radicals responsible for α-synuclein dimerization [66, 165], which leads to the development of the Lewy body diseases.
Canonical peroxidase activity involves two sequential one-electron oxidation steps and no transfer of oxygen from the oxoferryl complexes to the substrate [146]. Nevertheless, Compound I in certain heme enzymes—such as cytochrome P450—can transfer oxygen to certain substrates yielding hydroxy-derivatives. Interestingly, hydroxy-derivatives of CL cannot undergo peroxidation and inhibit the release of Cc [166]. Altogether, considering the findings above concerning tyrosyl radicals in Cc [151], Kagan and collaborators proposed that Cc acts as an oxygenase to produce CL peroxidation [167]. This activity would also be responsible for phosphatidylserine peroxidation affecting the plasma membrane during apoptosis [65]. This hypothesis suggests that hydrogen is transferred to Compound I from a nearby tyrosine residue to yield oxoferryl Compound II and the aforementioned tyrosyl radical (Figure 4). This mechanism is similar to that proposed for cyclooxygenases, in which a hydrogen atom is sequestered from an acyl chain, generating a carbon-centered radical capable of reacting with molecular oxygen [150].
Unlike true peroxidases, the environment of the heme moiety is unprotected from highly oxidizing species arising during the catalytic cycle [148]. When reacting with H2O2, degradation of the heme porphyrin often becomes apparent by a diminution of the Soret band intensity [146, 156]. A thorough mass spectrometry analysis of Cc residue adducts derived from H2O2 has been carried out by Flemmig and collaborators [152]. Several oxidation reactions can occur to produce a methyl-sulfoxide derivative from the methionine thioether, a sulfonic acid derivative from cysteines and 2-oxohistidine from histidine. While tyrosine residues can covalently cross-link or undergo oxidation to dihydroxyphenylalanine (DOPA) and subsequently to quinones, lysine residues can undergo carbonylation [152, 168, 169]. These changes occur when H2O2 is added to Cc samples [80, 152, 170–173].
Remarkably, different regions in the protein display different sensitivities to oxidation depending on the environment. For instance, specific M80 oxidation takes place in the presence of DOPC/DOPE micelles [174]. The ΩIII-loop is the first to be affected, whereas foldon I (helices I and IV) is the least affected by oxidation. Notably, the peroxidase activity of Cc increases in a time-dependent manner upon the addition of H2O2. Such increments in peroxidase activity may result from successive oxidation of M80 and lysine residues, as proposed by Yin and Konermann [80, 170]. Indeed, M80 oxidation promotes conformation exchange in Cc which impacts on heme ligation. With time, oxidative damage extends to the porphyrin ring, releasing iron capable of performing Fenton's reactions [173]. Finally, it is worth noting that CL peroxides can induce at least some of these oxidative PTM [152].
5.2. The Alkaline Transition of Cytochrome c and Peroxidase Activity
As mentioned above, previous data obtained using monoclonal antibodies highlighted that an alkaline-like conformation could interact with CL and exit mitochondria during apoptosis [106]. These antibodies also recognize the M80A mutant in the cell nucleus [175]. Notably, this mutant displays enhanced peroxidase activity. In addition, the peroxidase activity of Cc is somewhat pH dependent [145, 176]. Indeed, for horse heart Cc, peroxidase activity increases at acidic pH values [177] and slows beyond pH 8 [145]. Furthermore, the ability of Cc to oxidize O2-• falls at pH values above 7 [7]. The affinity of Cc towards membranes and the interaction patch involved also depend on pH [15, 98, 120, 127]. These effects illustrate how pH-dependent conformation changes modulate the different activities performed by Cc.
A number of mutations and PTM have been reported to simultaneously affect the peroxidase activity of Cc while bringing the so-called alkaline transition to lower even physiological pH values [178–182]. Loss of M80 coordination is evident from NMR spectra and UV-Vis spectra in all these studies. Cc peroxidase activity requires the heme iron to be pentacoordinated; the relationship with the pKA of the alkaline transition could be attributable to the lysine amine being weaker than methionine thioether in the ligand. However, at a neutral pH, lysine is a stronger ligand than methionine [183]. In fact, horse heart and human Cc show lower peroxidase activities at alkaline pH values [145, 182]. Moreover, mutation M100K in P. versutus cytochrome c550 makes the protein more stable at neutral pH while decreasing its peroxidase activity 20-fold [183].
Nevertheless, the shift in the alkaline transition towards lower pH values indicates destabilization or higher dynamics in the ΩII- and ΩIII-loops in the Met-coordinated species. Given the weakness of the thioether ligand bond towards iron, increasing fluctuations of these loops will increase the population of high-spin species and/or alternative low-spin (e.g., bis-His) species below the pKA value of the transition. This is observable in phosphomimic mutants, as well as in nitrated species of Cc [178–180, 184–186]. Furthermore, enhanced dynamics facilitate the access of small substrates to the heme iron [162].
5.3. Control of Cardiolipin Oxidation by Post-translational Modification of Cytochrome c
Protein PTM regulate tightly controlled cellular processes and increase the functional diversity of proteins, often acting as a cell response switch. Several post-translational modifications modulate Cc structure and functionality, such as sulfoxidation [187], carbonylation [152], homocysteinylation [188], nitration [179, 180], and phosphorylation [189] (Figure 5). Phosphorylation of tyrosine residues is associated with many human pathologies including cancer, ischemia, asthma, and sepsis. As highlighted earlier, tyrosine radicals are key for the oxygenase activity of Cc [157]. Thus, the amount of hydroxyl products from TOCL oxidation is lower when Y48E phosphomimic species instead of WT Cc acts as a catalyst [190]. Additionally, tyrosine phosphorylation impairs the formation of radicals, preventing dimerization [191]. This fact may be critical in pathological processes such as Parkinson's disease [66]. Therefore, the PTM that affect these residues are key in regulating Cc activity.
Figure 5.

Functional implications of PTM and mutations of cytochrome c. Left: chemical modifications of cytochrome c residues. Right: ribbon representation of oxidized human Cc (PDB 2N9J) [92]. Residues are colored by type of PTM: pink for nitration, purple for sulfoxidation, orange for phosphorylation, brown for carbonylation, cyan for N-homocysteinylation, and blue for point mutations. Y48 (asterisk) can be either phosphorylated, nitrated, or mutated for histidine, while Y97 (asterisk) can be phosphorylated or nitrated.
Given the difficulty in preserving the phosphorylation state of Cc outside of cell extracts, a common strategy to investigate consequences of phosphorylation is to mimic the modification by site-directed mutagenesis. All phosphomimetic Cc species, except a mutant at position 97, display altered affinity towards cardiolipin [178, 186, 190, 192–195]. The peroxidase activity of both free Cc and Cc-CL complexes increases in the phosphomimetic T28D and Y48pCMF. However, for the Y48E species, the increase only occurs with the free protein (Table 1) [178, 190, 192, 194]. In addition, at a high CL/lipid ratio, the peroxidase activity of the T28E mutant decreases [195]. A possible explanation may be that the greater population of CL versus other lipids in the liposome composition promote unfolding of the hemeprotein, acting as an off switch (Table 1) [131]. Hence, the negative charge at these positions could induce structural changes in the heme crevice which allow greater accessibility for hydrogen peroxide (Figure 5).
Table 1.
Effect of PTM and point mutations on cytochrome c peroxidase activity.
| Cc PTMs/mutation | Effect on peroxidase activity | References | |
|---|---|---|---|
| Sulfoxidationa | |||
| M80 | ↑ | [168, 187, 196] | |
| Nitrationb | |||
| Y46 | ↑ | [184] | |
| Y48 | ↑ | [184] | |
| Y67 | ↑ | [185, 197] | |
| Y74 | ↑ | [185, 197] | |
| Y97 | ↑ | [185, 197] | |
| Carbonylationc | |||
| K53 | ↑ | [152] | |
| K55 | ↑ | [152] | |
| K72 | ↑ | [152] | |
| K73 | ↑ | [152] | |
| Phosphorylation | |||
| T28 | T28D | ↑ | [192] |
| T28 | T28E | ↓ | [195] |
| S47 | S47D | ≈ | [192] |
| Y48 | Y48E | ↑/↓ | [176, 190] |
| Y48pCMF | ↑ | [194] | |
| Y97 | Y97E | ≈ | [176] |
| Y97pCMF | ≈ | [193] | |
| N-Homocysteinylationd | |||
| K8/K13 | ↑ | [198] | |
| K86/87 | ↑ | [198] | |
| K99 | ↑ | [198] | |
| K100 | ↑ | [198] | |
| Point mutation | |||
| G41S | ↑ | [181, 199] | |
| Y48H | ↑ | [181, 200] | |
aDetermined under oxidative stress. bDetermined after peroxynitrite treatment. cDetermined after chloramine-T treatment. dDetermined after homocysteine thiolactone treatment.
Peroxynitrite generated during nitrooxidative stress is a powerful amino acid modifier, affecting tyrosine residues among others [201]. Common products of the reaction between tyrosine and HOONO are 3,5-dinitrotyrosine, 3-nitrotyrosine, tyrosine radicals, and dityrosine. Nevertheless, treatment of Cc in vitro with peroxynitrite yields its 3-nitrotyrosine adducts, with the nitro group attaching to one of the Cε of the aromatic ring [202]. Nitration affects the redox potential of Cc as well as its electron-exchange kinetics, depending on the residue involved [203]. The nitration of Y46, Y48, Y74, and Y97 residues also increases the peroxidase activity of Cc and lowers the pKA value of the alkaline transition besides other functional properties [179, 180, 183, 185, 197, 204, 205] (Table 1). Nitration of Cc has been associated with several diseases, including chronic nephropathy [206].
All modifications/mutations of S47 and Y67 alter the peroxidase activity of Cc [79, 179, 185, 192, 195, 197]. Y67 is located close to Met80 and is part of the hydrophobic pocket which houses the acyl chains of CL. This residue is also key for the stability of the ΩIII − loop (Table 1).
Homocysteinylation is a PTM that involves the covalent bonding of a homocysteine thiolactone—an intermediate metabolite of methionine metabolism—with a lysine residue [188]. Upregulation of this PTM is implicated in several human pathologies including cancer and cardiopathies [198, 207]. The degree of homocysteinylation, as well as the rate at which this modification occurs in the presence of homocysteine thiolactone, is related to the number of lysine residues [188]. Human Cc displays a lysine content of 17.1% in its amino acid composition, which makes it sensitive to homocysteinylation. Homocysteinylation of surface lysines on Cc causes aggregation of the protein. However, N-homocysteinylated lysines adjacent to the heme cavity produce conformational changes, disrupt the coordination of the M80 axial ligand, and alter the redox state of Cc, reducing the iron of the heme group [196, 208]. These conformational changes increase Cc peroxidase activity (Table 1) [209].
As discussed before, Cc is modulated by several oxidative modifications due to its activity, eventually leading to changes in iron coordination. One oxidative modification is the carbonylation of lysine residues, which affects residues 72 and 73, both of which are involved in the alkaline transition of Cc [170] (Figure 5). Reportedly, successive carbonylation events at K53, K55, K72, and K73 lead to the formation of the pentacoordinated Cc species, resulting in increased Cc peroxidase activity (Table 1) [170]. Similarly, the sulfoxidation of M80 facilitates the formation of a Compound I-type intermediate that initiates the activity of Cc peroxidase (Table 1) [171, 210].
5.4. Peroxidase Activity of Cytochrome c and Diseases
Since the peroxidase activity of Cc relates to the activation of apoptosis, it is a clear target for the development of more efficient therapies against certain diseases or pathologies. There are several examples in the literature that shed light on this topic.
The point mutations Y48H and G41S in Cc cause the appearance of a special type of thrombocytopenia (thrombocytopenia 4) which is an asymptomatic disorder [199, 200]. Thrombocytopenia 4 is notable for normal platelet production. However, in thrombocytopenia platelets are not transported to the bone marrow and remain in the matrix, causing the effective platelet content to be lower. Both mutations cause an increase in peroxidase activity, which is related to an increase in the population of Cc in the pentacoordinated heme state [181, 211, 212] (Table 1).
Most neurodegenerative diseases are associated with cellular stress and apoptotic processes. Due to the double role played by Cc in the electron transport chain and apoptosis, it represents an amenable target for the development of therapies against neurodegenerative pathologies. For example, post-translational phosphorylation of Cc at Y97 is an excellent neuroprotective strategy following brain injury as it increases the efficiency of electron transport during hypoxic conditions [193, 213]. Moreover, Cc has been implicated in the development of Parkinson's disease—as it colocalizes with α-synuclein in the Lewy bodies [214]. In fact, the peroxidase activity of Cc plays an important role in the aggregation of α-synuclein by tyrosine dimerization [66, 174].
Minocycline is an antibiotic functional against both Gram-positive and Gram-negative bacteria. Additionally, it displays neuroprotective properties [215]. The antibiotic minocycline impairs the interaction between Cc and CL, inhibiting the activation of peroxidase activity and the consequent release of Cc into the cytosol to trigger apoptosis [216–218].
Nitric oxide is a well-known inhibitor of Cc peroxidase activity and thus may downregulate apoptosis [219, 220]. Flavonoids are excellent antioxidants that prevent cellular aging by ROS scavenger activity. In addition, they can inhibit Cc peroxidase activity, preventing proapoptotic events [221].
The protection of healthy cells during radiotherapy is a hot topic. In fact, novel synthetic compounds—e.g., imidazole-substituted fatty acids—are currently being trialed in order to preserve healthy cells by inhibiting the peroxidase activity of Cc during the irradiation process [222, 223]. These de novo compounds, mainly imidazole conjugates, block access to the heme crevice preventing the activation of peroxidase activity.
Finally, the activation of proapoptotic events, such as the release of Cc after the peroxidation of CL, may be a good target for the development of more efficient and specific therapies against cancer [224, 225].
6. Concluding Remarks
In this review, we have outlined major advances and hypotheses regarding the oxidation of CL by Cc. CL oxidation is a crucial event at the onset of a diverse range of pathologies, and thus, controlling it has become a key objective of current research. Targeting Cc—a key player in CL oxidation—has emerged as an important task.
Since its discovery last century, Cc has displayed great functional complexity despite its apparently simple structure. Indeed, its highly dynamic architecture, which enables conformation changes critical in regulating metabolism, signaling, and cell fate, still amazes the scientific community. Cc interacts with membranes in different ways depending on their composition and curvature, being able of modifying the latter. When the hemeprotein interacts with lipids, it may undergo subtle changes in the dynamics of its most flexible foldons or may even unfold. A plethora of factors, including PTM, control these phenomena.
The chemical activity of this protein ranges from the simplest reactions to diverse and complex reaction mechanisms to drive the oxidation of substrates including CL. In the recent years, we have witnessed concerted effort to unveil the intimate chemistry of this process. Solving this conundrum will require us to discriminate minority conformations in functional assays and elucidate how this activity is tuned under distinct conditions. Nevertheless, knowledge has already amassed on the subject enabling us to examine the inhibition of CL oxidation, which will aid the development of translational approaches.
Acknowledgments
This work was supported by the Ministry of Science and Innovation/FEDER–National Research Agency (PGC2018-096049-B-I00), European Social Fund, Andalusian Government (BIO-198, US-1257019, and US-1254317), and TA Instruments. G.P.M. was awarded a PhD fellowship from the Spanish Ministry of Education, Culture and Sport (FPU17/04604). Molecular graphics and analyses were performed with the UCSF Chimera software, developed by the Resource for Biocomputing, Visualization and Informatics at the University of California, San Francisco, with support from NIH (P41-GM103311).
Contributor Information
Antonio Díaz-Quintana, Email: qzaida@us.es.
Irene Díaz-Moreno, Email: idiazmoreno@us.es.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this review article.
References
- 1.Lee Y. M. Mitochondrial diseases. Journal of Epilepsy Research. 2012;2(1):1–4. doi: 10.14581/jer.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turrens J. F. Mitochondrial formation of reactive oxygen species. The Journal of Physiology. 2003;552(2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyurina Y. Y., St Croix C. M., Watkins S. C., et al. Redox (phospho)lipidomics of signaling in inflammation and programmed cell death. Journal of Leukocyte Biology. 2019;106(1):57–81. doi: 10.1002/JLB.3MIR0119-004RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kajarabille N., Latunde-Dada G. O. Programmed cell-death by ferroptosis: antioxidants as mitigators. International Journal of Molecular Sciences. 2019;20(19):p. 4968. doi: 10.3390/ijms20194968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kagan V. E., Tyurina Y. Y., Sun W. Y., et al. Redox phospholipidomics of enzymatically generated oxygenated phospholipids as specific signals of programmed cell death. Free Radical Biology and Medicine. 2020;147:231–241. doi: 10.1016/j.freeradbiomed.2019.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudek J. Role of cardiolipin in mitochondrial signaling pathways. Frontiers in Cell and Developmental Biology. 2017;5:p. 90. doi: 10.3389/fcell.2017.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler J., Jayson G. G., Swallow A. J. The reaction between the superoxide anion radical and cytochrome c. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1975;408(3):215–222. doi: 10.1016/0005-2728(75)90124-3. [DOI] [PubMed] [Google Scholar]
- 8.Atlante A., Calissano P., Bobba A., Azzariti A., Marra E., Passarella S. Cytochrome c is released from mitochondria in a reactive oxygen species (ROS)-dependent fashion and can operate as a ROS scavenger and as a respiratory substrate in cerebellar neurons undergoing excitotoxic death. Journal of Biological Chemistry. 2000;275(47):37159–37166. doi: 10.1074/jbc.M002361200. [DOI] [PubMed] [Google Scholar]
- 9.Pereverzev M. O., Vygodina T. V., Konstantinov A. A., Skulachev V. P. Cytochrome c, an ideal antioxidant. Biochemical Society Transactions. 2003;31(6):1312–1315. doi: 10.1042/bst0311312. [DOI] [PubMed] [Google Scholar]
- 10.Pasdois P., Parker J. E., Griffiths E. J., Halestrap A. P. The role of oxidized cytochrome c in regulating mitochondrial reactive oxygen species production and its perturbation in ischaemia. Biochemical Journal. 2011;436(2):493–505. doi: 10.1042/BJ20101957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sedlak E., Fabian M., Robinson N. C., Musatov A. Ferricytochrome c protects mitochondrial cytochrome c oxidase against hydrogen peroxide-induced oxidative damage. Free Radical Biology and Medicine. 2010;49(10):1574–1581. doi: 10.1016/j.freeradbiomed.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korshunov S. S., Krasnikov B. F., Pereverzev M. O., Skulachev V. P. The antioxidant functions of cytochromec. FEBS Letters. 1999;462(1-2):192–198. doi: 10.1016/S0014-5793(99)01525-2. [DOI] [PubMed] [Google Scholar]
- 13.Tyurin V. A., Tyurina Y. Y., Osipov A. N., et al. Interactions of cardiolipin and lyso-cardiolipins with cytochrome c and tBid: conflict or assistance in apoptosis. Cell Death & Differentiation. 2007;14(4):872–875. doi: 10.1038/sj.cdd.4402068. [DOI] [PubMed] [Google Scholar]
- 14.Huttemann M., Pecina P., Rainbolt M., et al. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: from respiration to apoptosis. Mitochondrion. 2011;11(3):369–381. doi: 10.1016/j.mito.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belikova N. A., Vladimirov Y. A., Osipov A. N., et al. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry. 2006;45(15):4998–5009. doi: 10.1021/bi0525573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shidoji Y., Hayashi K., Komura S., Ohishi N., Yagi K. Loss of molecular interaction between cytochrome c and cardiolipin due to lipid peroxidation. Biochemical and Biophysical Research Communications. 1999;264(2):343–347. doi: 10.1006/bbrc.1999.1410. [DOI] [PubMed] [Google Scholar]
- 17.Petrosillo G., Ruggiero F. M., Paradies G. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. The FASEB Journal. 2003;17(15):2202–2208. doi: 10.1096/fj.03-0012com. [DOI] [PubMed] [Google Scholar]
- 18.Kagan V. E., Tyurin V. A., Jiang J., et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nature Chemical Biology. 2005;1(4):223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 19.Tyurina Y. Y., Poloyac S. M., Tyurin V. A., et al. A mitochondrial pathway for biosynthesis of lipid mediators. Nature Chemistry. 2014;6(6):542–552. doi: 10.1038/nchem.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlame M. Thematic review series: glycerolipids. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. Journal of Lipid Research. 2008;49(8):1607–1620. doi: 10.1194/jlr.R700018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powell G. L., Jacobus J. nonequivalence of the phosphorus atoms in cardiolipin. Biochemistry. 1974;13(19):4024–4026. doi: 10.1021/bi00716a032. [DOI] [PubMed] [Google Scholar]
- 22.Schlame M., Ren M., Xu Y., Greenberg M. L., Haller I. Molecular symmetry in mitochondrial cardiolipins. Chemistry and Physics of Lipids. 2005;138(1-2):38–49. doi: 10.1016/j.chemphyslip.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Kates M., Syz J. Y., Gosser D., Haines T. H. pH-dissociation characteristics of cardiolipin and its 2′-deoxy analogue. Lipids. 1993;28(10):877–882. doi: 10.1007/BF02537494. [DOI] [PubMed] [Google Scholar]
- 24.Pan J., Cheng X., Sharp M., Ho C. S., Khadka N., Katsaras J. Structural and mechanical properties of cardiolipin lipid bilayers determined using neutron spin echo, small angle neutron and X-ray scattering, and molecular dynamics simulations. Soft Matter. 2015;11(1):130–138. doi: 10.1039/C4SM02227K. [DOI] [PubMed] [Google Scholar]
- 25.Hübner W., Mantsch H. H., Kates M. Intramolecular hydrogen bonding in cardiolipin. Biochimica et Biophysica Acta (BBA) - Biomembrane. 1991;1066(2):166–174. doi: 10.1016/0005-2736(91)90183-9. [DOI] [PubMed] [Google Scholar]
- 26.Hielscher R., Wenz T., Hunte C., Hellwig P. Monitoring the redox and protonation dependent contributions of cardiolipin in electrochemically induced FTIR difference spectra of the cytochrome bc1 complex from yeast. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2009;1787(6):617–625. doi: 10.1016/j.bbabio.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Dahlberg M., Marini A., Mennucci B., Maliniak A. Quantum chemical modeling of the cardiolipin headgroup. The Journal of Physical Chemistry A. 2010;114(12):4375–4387. doi: 10.1021/jp9110019. [DOI] [PubMed] [Google Scholar]
- 28.Olofsson G., Sparr E. Ionization constants pKa of cardiolipin. PLoS ONE. 2013;8(9, article e73040) doi: 10.1371/journal.pone.0073040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seddon J. M., Kaye R. D., Marsh D. Induction of the lamellar-inverted hexagonal phase transition in cardiolipin by protons and monovalent cations. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1983;734(2):347–352. doi: 10.1016/0005-2736(83)90134-7. [DOI] [Google Scholar]
- 30.Kooijman E. E., Swim L. A., Graber Z. T., Tyurina Y. Y., Bayır H., Kagan V. E. Magic angle spinning 31P NMR spectroscopy reveals two essentially identical ionization states for the cardiolipin phosphates in phospholipid liposomes. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2017;1859(1):61–68. doi: 10.1016/j.bbamem.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Róg T., Martinez-Seara H., Munck N., Oresic M., Karttunen M., Vattulainen I. Role of cardiolipins in the inner mitochondrial membrane: insight gained through atom-scale simulations. The Journal of Physical Chemistry B. 2009;113(11):3413–3422. doi: 10.1021/jp8077369. [DOI] [PubMed] [Google Scholar]
- 32.Dahlberg M. Polymorphic phase behavior of cardiolipin derivatives studied by coarse-grained molecular dynamics. The Journal of Physical Chemistry B. 2007;111(25):7194–7200. doi: 10.1021/jp071954f. [DOI] [PubMed] [Google Scholar]
- 33.Nichols-Smith S., Teh S. Y., Kuhl T. L. Thermodynamic and mechanical properties of model mitochondrial membranes. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2004;1663(1-2):82–88. doi: 10.1016/j.bbamem.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Dahlberg M., Maliniak A. Molecular dynamics simulations of cardiolipin bilayers. The Journal of Physical Chemistry B. 2008;112(37):11655–11663. doi: 10.1021/jp803414g. [DOI] [PubMed] [Google Scholar]
- 35.Ardail D., Privat J. P., Egret-Charlier M., Levrat C., Lerme F., Louisot P. Mitochondrial contact sites. Lipid composition and dynamics. Journal of Biological Chemistry. 1990;265(31):18797–18802. [PubMed] [Google Scholar]
- 36.Krebs J. J., Hauser H., Carafoli E. Asymmetric distribution of phospholipids in the inner membrane of beef heart mitochondria. Journal of Biological Chemistry. 1979;254:5309–5316. [PubMed] [Google Scholar]
- 37.Raja V., Greenberg M. L. The functions of cardiolipin in cellular metabolism–potential modifiers of the Barth syndrome phenotype. Chemistry and Physics of Lipids. 2014;179:49–56. doi: 10.1016/j.chemphyslip.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang M., Mileykovskaya E., Dowhan W. Gluing the respiratory chain Together. Journal of Biological Chemistry. 2002;277(46):43553–43556. doi: 10.1074/jbc.C200551200. [DOI] [PubMed] [Google Scholar]
- 39.Pfeiffer K., Gohil V., Stuart R. A., et al. Cardiolipin stabilizes respiratory chain supercomplexes. Journal of Biological Chemistry. 2003;278(52):52873–52880. doi: 10.1074/jbc.M308366200. [DOI] [PubMed] [Google Scholar]
- 40.Haines T. H. Anionic lipid headgroups as a proton-conducting pathway along the surface of membranes: a hypothesis. Proceedings of the National Academy of Sciences. 1983;80(1):160–164. doi: 10.1073/pnas.80.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haines T. H., Dencher N. A. Cardiolipin: a proton trap for oxidative phosphorylation. FEBS Letters. 2002;528(1-3):35–39. doi: 10.1016/S0014-5793(02)03292-1. [DOI] [PubMed] [Google Scholar]
- 42.Lange C., Nett J. H., Trumpower B. L., Hunte C. Specific roles of protein-phospholipid interactions in the yeast cytochrome bc1 complex structure. The EMBO Journal. 2001;20(23):6591–6600. doi: 10.1093/emboj/20.23.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Namslauer A., Brzezinski P. Structural elements involved in electron-coupled proton transfer in cytochrome c oxidase. FEBS Letters. 2004;567(1):103–110. doi: 10.1016/j.febslet.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 44.Arnarez C., Marrink S. J., Periole X. Identification of cardiolipin binding sites on cytochrome c oxidase at the entrance of proton channels. Scientific Reports. 2013;3(1) doi: 10.1038/srep01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlame M., Ren M. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Letters. 2006;580(23):5450–5455. doi: 10.1016/j.febslet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 46.Saric A., Andreau K., Armand A. S., Møller I. M., Petit P. X. Barth Syndrome: from mitochondrial dysfunctions associated with aberrant production of reactive oxygen species to pluripotent stem cell studies. Frontiers in Genetics. 2016;6:p. 359. doi: 10.3389/fgene.2015.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalvez F., D'Aurelio M., Boutant M., et al. Barth syndrome: cellular compensation of mitochondrial dysfunction and apoptosis inhibition due to changes in cardiolipin remodeling linked to tafazzin (TAZ) gene mutation. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2013;1832(8):1194–1206. doi: 10.1016/j.bbadis.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Wang G., McCain M. L., Yang L., et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nature Medicine. 2014;20(6):616–623. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paradies G., Paradies V., De Benedictis V., Ruggiero F. M., Petrosillo G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2014;1837(4):408–417. doi: 10.1016/j.bbabio.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Yin H., Zhu M. s. Free Radical Research. 2012;46(8):959–974. doi: 10.3109/10715762.2012.676642. [DOI] [PubMed] [Google Scholar]
- 51.Maguire J. J., Tyurina Y. Y., Mohammadyani D., et al. Known unknowns of cardiolipin signaling: the best is yet to come. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2017;1862(1):8–24. doi: 10.1016/j.bbalip.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chu C. T., Ji J., Dagda R. K., et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nature Cell Biology. 2013;15(10):1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Arriba G., Calvino M., Benito S., Parra T. Cyclosporine A-induced apoptosis in renal tubular cells is related to oxidative damage and mitochondrial fission. Toxicology Letters. 2013;218(1):30–38. doi: 10.1016/j.toxlet.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Garcia Fernandez M., Troiano L., Moretti L., et al. Early changes in intramitochondrial cardiolipin distribution during apoptosis. Cell Growth & Differentiation. 2002;13(9):449–455. [PubMed] [Google Scholar]
- 55.Gonzalvez F., Schug Z. T., Houtkooper R. H., et al. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. Journal of Cell Biology. 2008;183(4):681–696. doi: 10.1083/jcb.200803129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jalmar O., François-Moutal L., García-Sáez A. J., et al. Caspase-8 binding to cardiolipin in giant unilamellar vesicles provides a functional docking platform for Bid. PLoS ONE. 2013;8(2, article e55250) doi: 10.1371/journal.pone.0055250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lutter M., Fang M., Luo X., Nishijima M., Xie X. S., Wang X. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nature Cell Biology. 2000;2(10):754–756. doi: 10.1038/35036395. [DOI] [PubMed] [Google Scholar]
- 58.Esposti M. D., Cristea I. M., Gaskell S. J., Nakao Y., Dive C. Proapoptotic Bid binds to monolysocardiolipin, a new molecular connection between mitochondrial membranes and cell death. Cell Death & Differentiation. 2003;10(12):1300–1309. doi: 10.1038/sj.cdd.4401306. [DOI] [PubMed] [Google Scholar]
- 59.Gonzalvez F., Pariselli F., Jalmar O., et al. Mechanistic issues of the interaction of the hairpin-forming domain of tBid with mitochondrial cardiolipin. PLoS ONE. 2010;5(2, article e9342) doi: 10.1371/journal.pone.0009342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petit P. X., Dupaigne P., Pariselli F., et al. Interaction of the alpha-helical H6 peptide from the pro-apoptotic protein tBid with cardiolipin. FEBS Journal. 2009;276(21):6338–6354. doi: 10.1111/j.1742-4658.2009.07345.x. [DOI] [PubMed] [Google Scholar]
- 61.Raemy E., Martinou J. C. Involvement of cardiolipin in tBID-induced activation of BAX during apoptosis. Chemistry and Physics of Lipids. 2014;179:70–74. doi: 10.1016/j.chemphyslip.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Ott M., Robertson J. D., Gogvadze V., Zhivotovsky B., Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proceedings of the National Academy of Sciences. 2002;99(3):1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li P., Nijhawan D., Budihardjo I., et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91(4):479–489. doi: 10.1016/S0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 64.Zou H., Li Y., Liu X., Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. Journal of Biological Chemistry. 1999;274(17):11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 65.Bayir H., Fadeel B., Palladino M. J., et al. Apoptotic interactions of cytochrome c: redox flirting with anionic phospholipids within and outside of mitochondria. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2006;1757(5-6):648–659. doi: 10.1016/j.bbabio.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 66.Kumar A., Ganini D., Mason R. P. Role of cytochrome c in α-synuclein radical formation: implications of α-synuclein in neuronal death in Maneb- and paraquat-induced model of Parkinson's disease. Molecular Neurodegeneration. 2016;11(1):70–82. doi: 10.1186/s13024-016-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elena-Real C. A., Díaz-Quintana A., González-Arzola K., et al. Cytochrome c speeds up caspase cascade activation by blocking 14-3-3 ε-dependent Apaf-1 inhibition. Cell Death & Disease. 2018;9(3):365–377. doi: 10.1038/s41419-018-0408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martínez-Fábregas J., Díaz-Moreno I., González-Arzola K., et al. New Arabidopsis thaliana cytochrome c partners: a look into the elusive role of cytochrome c in programmed cell death in plants. Molecular & Cellular Proteomics. 2013;12(12):3666–3676. doi: 10.1074/mcp.M113.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martínez-Fábregas J., Díaz-Moreno I., González-Arzola K., Díaz-Quintana A., De la Rosa M. A. A common signalosome for programmed cell death in humans and plants. Cell Death & Disease. 2014;5(7):p. e1314. doi: 10.1038/cddis.2014.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martínez-Fábregas J., Díaz-Moreno I., González-Arzola K., et al. Structural and functional analysis of novel human cytochrome c targets in apoptosis. Molecular & Cellular Proteomics. 2014;13(6):1439–1456. doi: 10.1074/mcp.M113.034322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.González-Arzola K., Díaz-Moreno I., Cano-González A., et al. Structural basis for inhibition of the histone chaperone activity of SET/TAF-Iβ by cytochrome c. Proceedings of the National Academy of Sciences. 2015;112(32):9908–9913. doi: 10.1073/pnas.1508040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.González-Arzola K., Díaz-Quintana A., Rivero-Rodríguez F., Velázquez-Campoy A., de la Rosa M. A., Díaz-Moreno I. Histone chaperone activity of Arabidopsis thaliana NRP1 is blocked by cytochrome c. Nucleic Acids Research. 2017;45(4):2150–2165. doi: 10.1093/nar/gkw1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Díaz-Moreno I., Velázquez-Cruz A., Curran-French S., Díaz-Quintana A., De la Rosa M. A. Nuclear cytochrome c - a mitochondrial visitor regulating damaged chromatin dynamics. FEBS Letters. 2018;592(2):172–178. doi: 10.1002/1873-3468.12959. [DOI] [PubMed] [Google Scholar]
- 74.Nur-E-Kamal A., Gross S. R., Pan Z., Balklava Z., Ma J., Liu L. F. Nuclear translocation of cytochrome c during apoptosis. Journal of Biological Chemistry. 2004;279(24):24911–24914. doi: 10.1074/jbc.C400051200. [DOI] [PubMed] [Google Scholar]
- 75.Zalk R., Israelson A., Garty E. S., Azoulay-Zohar H., Shoshan-Barmatz V. Oligomeric states of the voltage-dependent anion channel and cytochrome c release from mitochondria. Biochemical Journal. 2005;386(1):73–83. doi: 10.1042/BJ20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nolin F., Michel J., Wortham L., et al. Stage-specific changes in the water, Na+, Cl- and K+ contents of organelles during apoptosis, demonstrated by a targeted cryo correlative analytical approach. PLoS ONE. 2016;11(2, article e0148727) doi: 10.1371/journal.pone.0148727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.González-Arzola K., Velázquez-Cruz A., Guerra-Castellano A., et al. New moonlighting functions of mitochondrial cytochrome c in the cytoplasm and nucleus. FEBS Letters. 2019;593(22):3101–3119. doi: 10.1002/1873-3468.13655. [DOI] [PubMed] [Google Scholar]
- 78.Bertini I., Cavallaro G., Rosato A. Cytochrome c: occurrence and functions. Chemical Reviews. 2006;106(1):90–115. doi: 10.1021/cr050241v. [DOI] [PubMed] [Google Scholar]
- 79.Maity H., Maity M., Walter Englander S. How cytochrome c folds, and why: submolecular foldon units and their stepwise sequential stabilization. Journal of Molecular Biology. 2004;343(1):223–233. doi: 10.1016/j.jmb.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 80.Yin V., Shaw G. S., Konermann L. Cytochromecas a peroxidase: activation of the precatalytic native state by H2O2-induced covalent modifications. Journal of the American Chemical Society. 2017;139(44):15701–15709. doi: 10.1021/jacs.7b07106. [DOI] [PubMed] [Google Scholar]
- 81.Dickerson R. E., Takano T., Eisenberg D., et al. Ferricytochrome c. I. General features of the horse and bonito proteins at 2.8 A resolution. Journal of Biological Chemistry. 1971;246(5):1511–1535. [PubMed] [Google Scholar]
- 82.Koppenol W. H., Margoliash E. The asymmetric distribution of charges on the surface of horse cytochrome c. Functional implications. Journal of Biological Chemistry. 1982;257(8):4426–4437. [PubMed] [Google Scholar]
- 83.Kimelberg H. K., Lee C. P. Binding and electron transfer to cytochrome c in artificial phospholipid membranes. Biochemical and Biophysical Research Communications. 1969;34(6):784–790. doi: 10.1016/0006-291X(69)90248-4. [DOI] [PubMed] [Google Scholar]
- 84.Spooner P. J. R., Watts A. Reversible unfolding of cytochrome c upon interaction with cardiolipin bilayers. I. Evidence from deuterium NMR measurements. Biochemistry. 1991;30(16):3871–3879. doi: 10.1021/bi00230a010. [DOI] [PubMed] [Google Scholar]
- 85.Spooner P. J., Duralski A. A., Rankin S. E., Pinheiro T. J., Watts A. Dynamics in a protein-lipid complex: nuclear magnetic resonance measurements on the headgroup of cardiolipin when bound to cytochrome c. Biophysical Journal. 1993;65(1):106–112. doi: 10.1016/S0006-3495(93)81048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Salamon Z., Tollin G. Interaction of horse heart cytochrome c with lipid bilayer membranes: effects on redox potentials. Journal of Bioenergetics and Biomembranes. 1997;29(3):211–221. doi: 10.1023/A:1022401825287. [DOI] [PubMed] [Google Scholar]
- 87.Salamon Z., Tollin G. Surface plasmon resonance studies of complex formation between cytochrome c and bovine cytochrome c oxidase incorporated into a supported planar lipid bilayer. II. Binding of cytochrome c to oxidase-containing cardiolipin/phosphatidylcholine membranes. Biophysical Journal. 1996;71(2):858–867. doi: 10.1016/S0006-3495(96)79287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salamon Z., Tollin G. Surface plasmon resonance studies of complex formation between cytochrome c and bovine cytochrome c oxidase incorporated into a supported planar lipid bilayer. I. Binding of cytochrome c to cardiolipin/phosphatidylcholine membranes in the absence of oxidase. Biophysical Journal. 1996;71(2):848–857. doi: 10.1016/S0006-3495(96)79286-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nantes I. L., Zucchi M. R., Nascimento O. R., Faljoni-Alario A. Effect of heme iron valence state on the conformation of cytochrome c and its association with membrane interfaces. A CD and EPR investigation. Journal of Biological Chemistry. 2001;276(1):153–158. doi: 10.1074/jbc.M006338200. [DOI] [PubMed] [Google Scholar]
- 90.Pandiscia L. A., Schweitzer-Stenner R. Coexistence of native-like and non-native partially unfolded ferricytochrome c on the surface of cardiolipin-containing liposomes. The Journal of Physical Chemistry B. 2015;119(4):1334–1349. doi: 10.1021/jp5104752. [DOI] [PubMed] [Google Scholar]
- 91.Kostrzewa A., Páli T., Froncisz W., Marsh D. Membrane location of spin-labeled cytochrome c determined by paramagnetic relaxation agents. Biochemistry. 2000;39(20):6066–6074. doi: 10.1021/bi992559l. [DOI] [PubMed] [Google Scholar]
- 92.Imai M., Saio T., Kumeta H., Uchida T., Inagaki F., Ishimori K. Investigation of the redox-dependent modulation of structure and dynamics in human cytochrome c. Biochemical and Biophysical Research Communications. 2016;469(4):978–984. doi: 10.1016/j.bbrc.2015.12.079. [DOI] [PubMed] [Google Scholar]
- 93.Rytomaa M., Kinnunen P. K. Evidence for two distinct acidic phospholipid-binding sites in cytochrome c. Journal of Biological Chemistry. 1994;269(3):1770–1774. [PubMed] [Google Scholar]
- 94.Rytomaa M., Kinnunen P. K. J. Reversibility of the binding of cytochrome c to liposomes. Implications for lipid-protein interactions. Journal of Biological Chemistry. 1995;270(7):3197–3202. doi: 10.1074/jbc.270.7.3197. [DOI] [PubMed] [Google Scholar]
- 95.Tuominen E. K. J., Zhu K., Wallace C. J. A., et al. ATP induces a conformational change in lipid-bound cytochrome c. Journal of Biological Chemistry. 2001;276(22):19356–19362. doi: 10.1074/jbc.M100853200. [DOI] [PubMed] [Google Scholar]
- 96.Tuominen E. K. J., Wallace C. J. A., Kinnunen P. K. J. Phospholipid-CytochromecInteraction. Journal of Biological Chemistry. 2002;277(11):8822–8826. doi: 10.1074/jbc.M200056200. [DOI] [PubMed] [Google Scholar]
- 97.Sinibaldi F., Howes B. D., Piro M. C., et al. Extended cardiolipin anchorage to cytochrome c: a model for protein-mitochondrial membrane binding. JBIC Journal of Biological Inorganic Chemistry. 2010;15(5):689–700. doi: 10.1007/s00775-010-0636-z. [DOI] [PubMed] [Google Scholar]
- 98.Kawai C., Prado F. M., Nunes G. L. C., di Mascio P., Carmona-Ribeiro A. M., Nantes I. L. pH-dependent interaction of cytochrome c with mitochondrial mimetic membranes: the role of an array of positively charged amino acids. Journal of Biological Chemistry. 2005;280(41):34709–34717. doi: 10.1074/jbc.M412532200. [DOI] [PubMed] [Google Scholar]
- 99.Kalanxhi E., Wallace C. J. A. Cytochrome c impaled: investigation of the extended lipid anchorage of a soluble protein to mitochondrial membrane models. Biochem J. 2007;407(2):179–187. doi: 10.1042/BJ20070459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Banci L., Bertini I., Gray H. B., et al. Solution structure of oxidized horse heart cytochrome c. Biochemistry. 1997;36(32):9867–9877. doi: 10.1021/bi970724w. [DOI] [PubMed] [Google Scholar]
- 101.Sinibaldi F., Howes B. D., Droghetti E., et al. Role of lysines in cytochrome c-cardiolipin interaction. Biochemistry. 2013;52(26):4578–4588. doi: 10.1021/bi400324c. [DOI] [PubMed] [Google Scholar]
- 102.Abe M., Niibayashi R., Koubori S., Moriyama I., Miyoshi H. Molecular mechanisms for the induction of peroxidase activity of the cytochrome c-cardiolipin complex. Biochemistry. 2011;50(39):8383–8391. doi: 10.1021/bi2010202. [DOI] [PubMed] [Google Scholar]
- 103.Hanske J., Toffey J. R., Morenz A. M., Bonilla A. J., Schiavoni K. H., Pletneva E. V. Conformational properties of cardiolipin-bound cytochrome c. Proceedings of the National Academy of Sciences. 2012;109(1):125–130. doi: 10.1073/pnas.1112312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hong J., Huang K., Wang W., et al. Direct electrochemistry of artificial peroxidase based on self-assembled cytochrome c-sds-nano-micelle. Analytical Letters. 2012;45(15):2221–2235. doi: 10.1080/00032719.2012.682234. [DOI] [Google Scholar]
- 105.Kapralov A. A., Kurnikov I. V., Vlasova I. I., et al. The hierarchy of structural transitions induced in cytochrome c by anionic phospholipids determines its peroxidase activation and selective peroxidation during apoptosis in cells. Biochemistry. 2007;46(49):14232–14244. doi: 10.1021/bi701237b. [DOI] [PubMed] [Google Scholar]
- 106.Jemmerson R., Liu J., Hausauer D., Lam K. P., Mondino A., Nelson R. D. A conformational change in cytochrome c of apoptotic and necrotic cells is detected by monoclonal antibody binding and mimicked by association of the native antigen with synthetic phospholipid vesicles. Biochemistry. 1999;38(12):3599–3609. doi: 10.1021/bi9809268. [DOI] [PubMed] [Google Scholar]
- 107.Assfalg M., Bertini I., Dolfi A., et al. Structural model for an alkaline form of ferricytochrome c. Journal of the American Chemical Society. 2003;125(10):2913–2922. doi: 10.1021/ja027180s. [DOI] [PubMed] [Google Scholar]
- 108.Hirota S., Hattori Y., Nagao S., et al. Cytochrome c polymerization by successive domain swapping at the C-terminal helix. Proceedings of the National Academy of Sciences. 2010;107(29):12854–12859. doi: 10.1073/pnas.1001839107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hirota S., Yamashiro N., Wang Z., Nagao S. Effect of methionine80 heme coordination on domain swapping of cytochrome c. JBIC Journal of Biological Inorganic Chemistry. 2017;22(5):705–712. doi: 10.1007/s00775-017-1446-3. [DOI] [PubMed] [Google Scholar]
- 110.McClelland L. J., Steele H. B. B., Whitby F. G., et al. Cytochrome c can form a well-defined binding pocket for hydrocarbons. Journal of the American Chemical Society. 2016;138(51):16770–16778. doi: 10.1021/jacs.6b10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kapetanaki S. M., Silkstone G., Husu I., Liebl U., Wilson M. T., Vos M. H. Interaction of carbon monoxide with the apoptosis-inducing cytochrome c-cardiolipin complex. Biochemistry. 2009;48(7):1613–1619. doi: 10.1021/bi801817v. [DOI] [PubMed] [Google Scholar]
- 112.Muenzner J., Toffey J. R., Hong Y., Pletneva E. V. Becoming a peroxidase: cardiolipin-induced unfolding of cytochrome c. The Journal of Physical Chemistry B. 2013;117(42):12878–12886. doi: 10.1021/jp402104r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Basova L. V., Kurnikov I. V., Wang L., et al. Cardiolipin switch in mitochondria: shutting off the reduction of cytochrome c and turning on the peroxidase activity. Biochemistry. 2007;46(11):3423–3434. doi: 10.1021/bi061854k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bradley J. M., Silkstone G., Wilson M. T., Cheesman M. R., Butt J. N. Probing a complex of cytochrome c and cardiolipin by magnetic circular dichroism spectroscopy: implications for the initial events in apoptosis. Journal of the American Chemical Society. 2011;133(49):19676–19679. doi: 10.1021/ja209144h. [DOI] [PubMed] [Google Scholar]
- 115.O'Brien E. S., Nucci N. V., Fuglestad B., Tommos C., Wand A. J. Defining the apoptotic trigger the interaction of cytochrome c and cardiolipin. Journal of Biological Chemistry. 2015;290(52):30879–30887. doi: 10.1074/jbc.M115.689406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Díaz-Moreno I., García-Heredia J. M., Díaz-Quintana A., De la Rosa M. A. Cytochrome c signalosome in mitochondria. European Biophysics Journal. 2011;40(12):1301–1315. doi: 10.1007/s00249-011-0774-4. [DOI] [PubMed] [Google Scholar]
- 117.Díaz-Moreno I., Díaz-Quintana A., Ubbink M., De la Rosa M. A. An NMR-based docking model for the physiological transient complex between cytochrome f and cytochrome c6. FEBS Letters. 2005;579(13):2891–2896. doi: 10.1016/j.febslet.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 118.Mandal A., Hoop C. L., DeLucia M., et al. Structural Changes and Proapoptotic Peroxidase Activity of Cardiolipin-Bound Mitochondrial Cytochrome c. Biophysical Journal. 2015;109(9):1873–1884. doi: 10.1016/j.bpj.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mohammadyani D., Yanamala N., Samhan-Arias A. K., et al. Structural characterization of cardiolipin-driven activation of cytochrome c into a peroxidase and membrane perturbation. Biochimica et Biophysica Acta (BBA) - Biomembrane. 2018;1860(5):1057–1068. doi: 10.1016/j.bbamem.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 120.Gorbenko G. P., Molotkovsky J. G., Kinnunen P. K. J. Cytochrome c interaction with cardiolipin/phosphatidylcholine model membranes: effect of cardiolipin protonation. Biophysical Journal. 2006;90(11):4093–4103. doi: 10.1529/biophysj.105.080150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Robinson J. B., Jr., Strottmann J. M., Stellwagen E. A globular high spin form of ferricytochrome c. Journal of Biological Chemistry. 1983;258(11):6772–6776. [PubMed] [Google Scholar]
- 122.Tonge P., Moore G. R., Wharton C. W. Fourier-transform infra-red studies of the alkaline isomerization of mitochondrial cytochrome c and the ionization of carboxylic acids. Biochemical Journal. 1989;258(2):599–605. doi: 10.1042/bj2580599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schweitzer-Stenner R. Internal electric field in cytochrome c explored by visible electronic circular dichroism spectroscopy. The Journal of Physical Chemistry B. 2008;112(33):10358–10366. doi: 10.1021/jp802495q. [DOI] [PubMed] [Google Scholar]
- 124.Pandiscia L. A., Schweitzer-Stenner R. Salt as a catalyst in the mitochondria: returning cytochrome c to its native state after it misfolds on the surface of cardiolipin containing membranes. Chemical Communications. 2014;50(28):3674–3676. doi: 10.1039/C3CC48709A. [DOI] [PubMed] [Google Scholar]
- 125.Oellerich S., Lecomte S., Paternostre M., Heimburg T., Hildebrandt P. Peripheral and integral binding of Cytochromecto phospholipids vesicles. The Journal of Physical Chemistry B. 2004;108(12):3871–3878. doi: 10.1021/jp036799t. [DOI] [Google Scholar]
- 126.Oellerich S., Wackerbarth H., Hildebrandt P. Spectroscopic characterization of nonnative conformational states of Cytochromec. The Journal of Physical Chemistry B. 2002;106(25):6566–6580. doi: 10.1021/jp013841g. [DOI] [Google Scholar]
- 127.Milorey B., Malyshka D., Schweitzer-Stenner R. pH dependence of ferricytochrome c conformational transitions during binding to cardiolipin membranes: evidence for histidine as the distal ligand at neutral pH. The Journal of Physical Chemistry. 2017;8(9):1993–1998. doi: 10.1021/acs.jpclett.7b00597. [DOI] [PubMed] [Google Scholar]
- 128.Schweitzer-Stenner R. Relating the multi-functionality of cytochrome c to membrane binding and structural conversion. Biophysical Reviews. 2018;10(4):1151–1185. doi: 10.1007/s12551-018-0409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ranieri A., Millo D., di Rocco G., et al. Immobilized cytochrome c bound to cardiolipin exhibits peculiar oxidation state-dependent axial heme ligation and catalytically reduces dioxygen. JBIC Journal of Biological Inorganic Chemistry. 2015;20(3):531–540. doi: 10.1007/s00775-015-1238-6. [DOI] [PubMed] [Google Scholar]
- 130.Capdevila D. A., Oviedo Rouco S., Tomasina F., et al. Active site structure and peroxidase activity of oxidatively modified cytochrome c species in complexes with cardiolipin. Biochemistry. 2015;54(51):7491–7504. doi: 10.1021/acs.biochem.5b00922. [DOI] [PubMed] [Google Scholar]
- 131.Hong Y., Muenzner J., Grimm S. K., Pletneva E. V. Origin of the conformational heterogeneity of cardiolipin-bound cytochrome c. ournal of the American Chemical Society. 2012;134(45):18713–18723. doi: 10.1021/ja307426k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Spooner P. J. R., Watts A. Cytochrome c interactions with cardiolipin in bilayers: a multinuclear magic-angle spinning NMR study. Biochemistry. 1992;31(41):10129–10138. doi: 10.1021/bi00156a037. [DOI] [PubMed] [Google Scholar]
- 133.Rietveld A., Sijens P., Verkleij A. J., de Kruijff B. Interaction of cytochrome c and its precursor apocytochrome c with various phospholipids. EMBO Journal. 1983;2(6):907–913. doi: 10.1002/j.1460-2075.1983.tb01520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.de Kruijff B., Cullis P. R. Cytochrome c specifically induces non-bilayer structures in cardiolipin- containing model membranes. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1980;602(3):477–490. doi: 10.1016/0005-2736(80)90327-2. [DOI] [PubMed] [Google Scholar]
- 135.Brown L. R., Wüthrich K. NMR and ESR studies of the interactions of cytochrome c with mixed cardiolipin-phosphatidylcholine vesicles. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1977;468(3):389–410. doi: 10.1016/0005-2736(77)90290-5. [DOI] [PubMed] [Google Scholar]
- 136.Pinheiro T. J. T., Watts A. Lipid specificity in the interaction of cytochrome c with anionic phospholipid bilayers revealed by solid-state 31P NMR. Biochemistry. 1994;33(9):2451–2458. doi: 10.1021/bi00175a013. [DOI] [PubMed] [Google Scholar]
- 137.Bergstrom C. L., Beales P. A., Lv Y., Vanderlick T. K., Groves J. T. Cytochrome c causes pore formation in cardiolipin-containing membranes. Proceedings of the National Academy of Sciences. 2013;110(16):6269–6274. doi: 10.1073/pnas.1303819110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li M., Mandal A., Tyurin V. A., et al. Surface-binding to cardiolipin nanodomains triggers cytochrome c pro-apoptotic peroxidase activity via localized dynamics. Structure. 2019;27(5):806–815.e4. doi: 10.1016/j.str.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Borisenko G. G., Kapralov A. A., Tyurin V. A., Maeda A., Stoyanovsky D. A., Kagan V. E. Molecular design of new inhibitors of peroxidase activity of cytochrome c/cardiolipin complexes: fluorescent oxadiazole-derivatized cardiolipin. Biochemistry. 2008;47(51):13699–13710. doi: 10.1021/bi801507s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ascenzi P., Marino M., Polticelli F., Santucci R., Coletta M. Cardiolipin modulates allosterically the nitrite reductase activity of horse heart cytochrome c. BIC Journal of Biological Inorganic Chemistry. 2014;19(7):1195–1201. doi: 10.1007/s00775-014-1175-9. [DOI] [PubMed] [Google Scholar]
- 141.Yin H., Zhu M. Free radical oxidation of cardiolipin: chemical mechanisms, detection and implication in apoptosis, mitochondrial dysfunction and human diseases. Free Radical Research. 2012;46(8):959–974. doi: 10.3109/10715762.2012.676642. [DOI] [PubMed] [Google Scholar]
- 142.Yin H., Xu L., Porter N. A. Free radical lipid peroxidation: mechanisms and analysis. Chemical Reviews. 2011;111(10):5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 143.Indo H. P., Yen H.-C., Nakanishi I., et al. A mitochondrial superoxide theory for oxidative stress diseases and aging. Journal of Clinical Biochemistry and Nutrition. 2015;56(1):1–7. doi: 10.3164/jcbn.14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Michiels C., Raes M., Toussaint O., Remacle J. Importance of SE-glutathione peroxidase, catalase, and CU/ZN-SOD for cell survival against oxidative stress. Free Radical Biology and Medicine. 1994;17(3):235–248. doi: 10.1016/0891-5849(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 145.Radi R., Thomson L., Rubbo H., Prodanov E. Cytochrome c-catalyzed oxidation of organic molecules by hydrogen peroxide. Archives of Biochemistry and Biophysics. 1991;288(1):112–117. doi: 10.1016/0003-9861(91)90171-E. [DOI] [PubMed] [Google Scholar]
- 146.Radi R., Turrens J. F., Freeman B. A. Cytochrome c-catalyzed membrane lipid peroxidation by hydrogen peroxide. Archives of Biochemistry and Biophysics. 1991;288(1):118–125. doi: 10.1016/0003-9861(91)90172-F. [DOI] [PubMed] [Google Scholar]
- 147.Dawson J. Probing structure-function relations in heme-containing oxygenases and peroxidases. Science. 1988;240(4851):433–439. doi: 10.1126/science.3358128. [DOI] [PubMed] [Google Scholar]
- 148.Vlasova I. Peroxidase activity of human hemoproteins: keeping the fire under control. Molecules. 2018;23(10):p. 2561. doi: 10.3390/molecules23102561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Belikova N. A., Tyurina Y. Y., Borisenko G., et al. Heterolytic reduction of fatty acid hydroperoxides by cytochrome c/cardiolipin complexes: antioxidant function in mitochondria. Journal of the American Chemical Society. 2009;131(32):11288–11289. doi: 10.1021/ja904343c. [DOI] [PubMed] [Google Scholar]
- 150.Marnett L. J. Cyclooxygenase mechanisms. Current Opinion in Chemical Biology. 2000;4(5):545–552. doi: 10.1016/S1367-5931(00)00130-7. [DOI] [PubMed] [Google Scholar]
- 151.Qian S. Y., Chen Y. R., Deterding L. J., et al. Identification of protein-derived tyrosyl radical in the reaction of cytochrome c and hydrogen peroxide: characterization by ESR spin-trapping, HPLC and MS. Biochemical Journal. 2002;363(2):281–288. doi: 10.1042/bj3630281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Barayeu U., Lange M., Méndez L., et al. Cytochrome c autocatalyzed carbonylation in the presence of hydrogen peroxide and cardiolipins. Journal of Biological Chemistry. 2019;294(6):1816–1830. doi: 10.1074/jbc.RA118.004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Poulos T. L., Kraut J. The stereochemistry of peroxidase catalysis. Journal of Biological Chemistry. 1980;255(17):8199–8205. [PubMed] [Google Scholar]
- 154.Banci L., Carloni P., Díaz A., Savellini G. G. Molecular dynamics calculations on peroxidases: the effect of calcium ions on protein structure. JBIC Journal of Biological Inorganic Chemistry. 1996;1(3):264–272. doi: 10.1007/s007750050052. [DOI] [Google Scholar]
- 155.Lawrence A., Jones C. M., Wardman P., Burkitt M. J. Evidence for the role of a peroxidase compound I-type intermediate in the oxidation of glutathione, NADH, ascorbate, and dichlorofluorescin by cytochrome c/H2O2. Implications for oxidative stress during apoptosis. Journal of Biological Chemistry. 2003;278(32):29410–29419. doi: 10.1074/jbc.M300054200. [DOI] [PubMed] [Google Scholar]
- 156.Cadenas E., Varsavsky A. I., Alberto B., Britton C. Low level chemiluminescence of the cytochrome c-catalyzed decomposition of hydrogen peroxide. FEBS Letters. 1980;113(2):141–144. doi: 10.1016/0014-5793(80)80577-1. [DOI] [PubMed] [Google Scholar]
- 157.Barr D. P., Mason R. P. Mechanism of radical production from the reaction of cytochrome c with organic hydroperoxides. An ESR spin trapping investigation. Journal of Biological Chemistry. 1995;270(21):12709–12716. doi: 10.1074/jbc.270.21.12709. [DOI] [PubMed] [Google Scholar]
- 158.Barr D. P., Gunther M. R., Deterding L. J., Tomer K. B., Mason R. P. ESR spin-trapping of a protein-derived tyrosyl radical from the reaction of cytochrome c with hydrogen peroxide. Journal of Biological Chemistry. 1996;271(26):15498–15503. doi: 10.1074/jbc.271.26.15498. [DOI] [PubMed] [Google Scholar]
- 159.Rajagopal B. S., Edzuma A. N., Hough M. A., et al. The hydrogen-peroxide-induced radical behaviour in human cytochrome c-phospholipid complexes: implications for the enhanced pro-apoptotic activity of the G41S mutant. Biochemical Journal. 2013;456(3):441–452. doi: 10.1042/BJ20130758. [DOI] [PubMed] [Google Scholar]
- 160.Mara M. W., Hadt R. G., Reinhard M. E., et al. Metalloprotein entatic control of ligand-metal bonds quantified by ultrafast X-ray spectroscopy. Science. 2017;356(6344):1276–1280. doi: 10.1126/science.aam6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Diederix R. E. M., Ubbink M., Canters G. W. Peroxidase activity as a tool for studying the folding of c-type cytochromes. Biochemistry. 2002;41(43):13067–13077. doi: 10.1021/bi0260841. [DOI] [PubMed] [Google Scholar]
- 162.Tomaskova N., Varhac R., Lysakova V., Musatov A., Sedlak E. Peroxidase activity of cytochrome c in its compact state depends on dynamics of the heme region. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2018;1866(11):1073–1083. doi: 10.1016/j.bbapap.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 163.Ascenzi P., Coletta M., Wilson M. T., et al. Cardiolipin-cytochrome c complex: switching cytochrome c from an electron-transfer shuttle to a myoglobin- and a peroxidase-like heme-protein. IUBMB Life. 2015;67(2):98–109. doi: 10.1002/iub.1350. [DOI] [PubMed] [Google Scholar]
- 164.Velayutham M., Hemann C. F., Cardounel A. J., Zweier J. L. Sulfite oxidase activity of cytochrome c: role of hydrogen peroxide. Biochemistry and Biophysics Reports. 2016;5:96–104. doi: 10.1016/j.bbrep.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bayır H., Kapralov A. A., Jiang J., et al. Peroxidase mechanism of lipid-dependent cross-linking of synuclein with cytochrome c: protection against apoptosis versus delayed oxidative stress in Parkinson disease. Journal of Biological Chemistry. 2009;284(23):15951–15969. doi: 10.1074/jbc.M900418200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nomura K., Imai H., Koumura T., Kobayashi T., Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochemical Journal. 2000;351(1):183–193. doi: 10.1042/bj3510183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kagan V. E., Borisenko G. G., Tyurina Y. Y., et al. Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Radical Biology and Medicine. 2004;37(12):1963–1985. doi: 10.1016/j.freeradbiomed.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 168.Kim N. H., Jeong M. S., Choi S. Y., Kang J. H. Oxidative modification of cytochrome c by hydrogen peroxide. Molecules and Cells. 2006;22(2):220–227. [PubMed] [Google Scholar]
- 169.Nugraheni A. D., Ren C., Matsumoto Y., Nagao S., Yamanaka M., Hirota S. Oxidative modification of methionine80 in cytochrome c by reaction with peroxides. Journal of Inorganic Biochemistry. 2018;182:200–207. doi: 10.1016/j.jinorgbio.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 170.Yin V., Mian S. H., Konermann L. Lysine carbonylation is a previously unrecognized contributor to peroxidase activation of cytochromecby chloramine-T. Chemical Science. 2019;10(8):2349–2359. doi: 10.1039/C8SC03624A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Parakra R. D., Kleffmann T., Jameson G. N. L., Ledgerwood E. C. The proportion of Met80-sulfoxide dictates peroxidase activity of human cytochrome c. Dalton Transactions. 2018;47(27):9128–9135. doi: 10.1039/C8DT02185F. [DOI] [PubMed] [Google Scholar]
- 172.Zhong F., Pletneva E. V. Ligation and reactivity of methionine-oxidized Cytochromec. Inorganic Chemistry. 2018;57(10):5754–5766. doi: 10.1021/acs.inorgchem.8b00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Harel S., Salan M. A., Kanner J. Iron release from metmyoglobin, methaemoglobin and cytochrome c by a system generating hydrogen peroxide. Free Radical Research Communications. 1998;5(1):11–19. doi: 10.3109/10715768809068554. [DOI] [PubMed] [Google Scholar]
- 174.Capdevila D. A., Marmisollé W. A., Tomasina F., et al. Specific methionine oxidation of cytochrome c in complexes with zwitterionic lipids by hydrogen peroxide: potential implications for apoptosis. Chemical Science. 2015;6(1):705–713. doi: 10.1039/C4SC02181A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Godoy L. C., Muñoz-Pinedo C., Castro L., et al. Disruption of the M80-Fe ligation stimulates the translocation of cytochrome c to the cytoplasm and nucleus in nonapoptotic cells. Proceedings of the National Academy of Sciences. 2009;106(8):2653–2658. doi: 10.1073/pnas.0809279106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.McClelland L. J., Mou T.-C., Jeakins-Cooley M. E., Sprang S. R., Bowler B. E. Structure of a mitochondrial cytochrome c conformer competent for peroxidase activity. Proceedings of the National Academy of Sciences. 2014;111(18):6648–6653. doi: 10.1073/pnas.1323828111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Balakrishnan G., Hu Y., Oyerinde O. F., Su J., Groves J. T., Spiro T. G. A conformational switch to β-sheet structure in cytochrome c leads to heme exposure. Implications for cardiolipin peroxidation and apoptosis. Journal of the American Chemical Society. 2007;129(3):504–505. doi: 10.1021/ja0678727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.García-Heredia J. M., Díaz-Quintana A., Salzano M., et al. Tyrosine phosphorylation turns alkaline transition into a biologically relevant process and makes human cytochrome c behave as an anti-apoptotic switch. JBIC Journal of Biological Inorganic Chemistry. 2011;16(8):1155–1168. doi: 10.1007/s00775-011-0804-9. [DOI] [PubMed] [Google Scholar]
- 179.Abriata L. A., Cassina A., Tórtora V., et al. Nitration of solvent-exposed tyrosine 74 on CytochromecTriggers heme iron-methionine 80 bond disruption. Journal of Biological Chemistry. 2008;284(1):17–26. doi: 10.1074/jbc.M807203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cassina A. M., Hodara R., Souza J. M., et al. Cytochrome c nitration by peroxynitrite. Journal of Biological Chemistry. 2000;275(28):21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 181.Deacon O. M., Svistunenko D. A., Moore G. R., Wilson M. T., Worrall J. A. R. Naturally occurring disease-related mutations in the 40-57 Ω-loop of human cytochrome c control triggering of the alkaline isomerization. Biochemistry. 2018;57(29):4276–4288. doi: 10.1021/acs.biochem.8b00520. [DOI] [PubMed] [Google Scholar]
- 182.Nold S. M., Lei H., Mou T. C., Bowler B. E. Effect of a K72A mutation on the structure, stability, dynamics, and peroxidase activity of human cytochrome c. Biochemistry. 2017;56(26):3358–3368. doi: 10.1021/acs.biochem.7b00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Worrall J. A. R., van Roon A. M. M., Ubbink M., Canters G. W. The effect of replacing the axial methionine ligand with a lysine residue in cytochrome c-550 from Paracoccus versutus assessed by X-ray crystallography and unfolding. FEBS Journal. 2005;272(10):2441–2455. doi: 10.1111/j.1742-4658.2005.04664.x. [DOI] [PubMed] [Google Scholar]
- 184.Díaz-Moreno I., García-Heredia J. M., Díaz-Quintana A., Teixeira M., De la Rosa M. A. Nitration of tyrosines 46 and 48 induces the specific degradation of cytochrome c upon change of the heme iron state to high-spin. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2011;1807(12):1616–1623. doi: 10.1016/j.bbabio.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 185.García-Heredia J. M., Díaz-Moreno I., Nieto P. M., et al. Nitration of tyrosine 74 prevents human cytochrome c to play a key role in apoptosis signaling by blocking caspase-9 activation. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2010;1797(6-7):981–993. doi: 10.1016/j.bbabio.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 186.Guerra-Castellano A., Díaz-Quintana A., Moreno-Beltrán B., et al. Mimicking tyrosine phosphorylation in human cytochromecby the evolved tRNA synthetase technique. Chemistry. 2015;21(42):15004–15012. doi: 10.1002/chem.201502019. [DOI] [PubMed] [Google Scholar]
- 187.Ivanetich K. M., Bradshaw J. J., Kaminsky L. S. Methionine sulfoxide cytochrome c. Biochemistry. 1976;15(5):1144–1153. doi: 10.1021/bi00650a029. [DOI] [PubMed] [Google Scholar]
- 188.Jakubowski H. Protein homocysteinylation: possible mechanism underlying pathological consequences of elevated homocysteine levels. FASEB Journal. 1999;13(15):2277–2283. doi: 10.1096/fasebj.13.15.2277. [DOI] [PubMed] [Google Scholar]
- 189.Kalpage H. A., Wan J., Morse P. T., et al. Cytochrome c phosphorylation: control of mitochondrial electron transport chain flux and apoptosis. The International Journal of Biochemistry & Cell Biology. 2020;121:p. 105704. doi: 10.1016/j.biocel.2020.105704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pecina P., Borisenko G. G., Belikova N. A., et al. Phosphomimetic substitution of cytochrome c tyrosine 48 decreases respiration and binding to cardiolipin and abolishes ability to trigger downstream caspase activation. Biochemistry. 2010;49(31):6705–6714. doi: 10.1021/bi100486s. [DOI] [PubMed] [Google Scholar]
- 191.Gmeiner B. M. K., Seelos C. C. C. Tyrosine phosphorylation blocks tyrosine free radical formation and, hence, the hormonogenic iodination reaction. Free Radical Biology and Medicine. 1996;21(3):349–351. doi: 10.1016/0891-5849(96)00016-0. [DOI] [PubMed] [Google Scholar]
- 192.Guerra-Castellano A., Díaz-Moreno I., Velázquez-Campoy A., De la Rosa M. A., Díaz-Quintana A. Structural and functional characterization of phosphomimetic mutants of cytochrome c at threonine 28 and serine 47. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2016;1857(4):387–395. doi: 10.1016/j.bbabio.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 193.Guerra-Castellano A., Díaz-Quintana A., Pérez-Mejías G., et al. Oxidative stress is tightly regulated by cytochromecphosphorylation and respirasome factors in mitochondria. Proceedings of the National Academy of Sciences. 2018;115(31):7955–7960. doi: 10.1073/pnas.1806833115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Moreno-Beltrán B., Guerra-Castellano A., Díaz-Quintana A., et al. Structural basis of mitochondrial dysfunction in response to cytochromecphosphorylation at tyrosine 48. Proceedings of the National Academy of Sciences. 2017;114(15):E3041–E3050. doi: 10.1073/pnas.1618008114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mahapatra G., Varughese A., Ji Q., et al. Phosphorylation of cytochrome c threonine 28 regulates electron transport chain activity in kidney: implications for AMP kinase. Journal of Biological Chemistry. 2017;292(1):64–79. doi: 10.1074/jbc.M116.744664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shervedani R. K., Foroushani M. S. Direct electrochemistry of cytochrome c immobilized on gold electrode surface via Zr(IV) ion glue and its activity for ascorbic acid. Bioelectrochemistry. 2014;98:53–63. doi: 10.1016/j.bioelechem.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 197.Batthyány C., Souza J. M., Durán R., Cassina A., Cerveñansky C., Radi R. Time course and site(s) of cytochrome c tyrosine nitration by peroxynitrite†. Biochemistry. 2005;44(22):8038–8046. doi: 10.1021/bi0474620. [DOI] [PubMed] [Google Scholar]
- 198.Hasan T., Arora R., Bansal A. K., Bhattacharya R., Sharma G. S., Singh L. R. Disturbed homocysteine metabolism is associated with cancer. Experimental & Molecular Medicine. 2019;51(2):1–13. doi: 10.1038/s12276-019-0216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Morison I. M., Cramer Bordé E. M., Cheesman E. J., et al. A mutation of human cytochrome c enhances the intrinsic apoptotic pathway but causes only thrombocytopenia. Nature Genetics. 2008;40(4):387–389. doi: 10.1038/ng.103. [DOI] [PubMed] [Google Scholar]
- 200.de Rocco D., Cerqua C., Goffrini P., et al. Mutations of cytochrome c identified in patients with thrombocytopenia THC4 affect both apoptosis and cellular bioenergetics. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2014;1842(2):269–274. doi: 10.1016/j.bbadis.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 201.Alvarez B., Radi R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids. 2003;25(3-4):295–311. doi: 10.1007/s00726-003-0018-8. [DOI] [PubMed] [Google Scholar]
- 202.Díaz-Moreno I., Nieto P. M., delConte R., et al. A non-damaging method to analyze the configuration and dynamics of nitrotyrosines in proteins. Chemistry. 2012;18(13):3872–3878. doi: 10.1002/chem.201103413. [DOI] [PubMed] [Google Scholar]
- 203.Rodríguez-Roldán V., García-Heredia J. M., Navarro J. A., De la Rosa M. A., Hervás M. Effect of nitration on the physicochemical and kinetic features of wild-type and monotyrosine mutants of human respiratory Cytochromec†. Biochemistry. 2008;47(47):12371–12379. doi: 10.1021/bi801329s. [DOI] [PubMed] [Google Scholar]
- 204.García-Heredia J. M., Díaz-Moreno I., Díaz-Quintana A., et al. Specific nitration of tyrosines 46 and 48 makes cytochrome c assemble a non-functional apoptosome. FEBS Letters. 2012;586(2):154–158. doi: 10.1016/j.febslet.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 205.Ly H. K., Utesch T., Díaz-Moreno I., García-Heredia J. M., De La Rosa M. A., Hildebrandt P. Perturbation of the redox site structure of cytochrome c variants upon tyrosine nitration. The Journal of Physical Chemistry B. 2012;116(19):5694–5702. doi: 10.1021/jp302301m. [DOI] [PubMed] [Google Scholar]
- 206.MacMillan-Crow L. A., Cruthirds D. L., Ahki K. M., Sanders P. W., Thompson J. A. Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free Radical Biology and Medicine. 2001;31(12):1603–1608. doi: 10.1016/S0891-5849(01)00750-X. [DOI] [PubMed] [Google Scholar]
- 207.Beltowski J. Protein homocysteinylation: a new mechanism of atherogenesis? Postępy Higieny i Medycyny Doświadczalnej. 2005;59:392–404. [PubMed] [Google Scholar]
- 208.Perła-Kaján J., Marczak L., Kaján Ł., Skowronek P., Twardowski T., Jakubowski H. Modification by homocysteine thiolactone affects redox status of cytochrome c. Biochemistry. 2007;46(21):6225–6231. doi: 10.1021/bi602463m. [DOI] [PubMed] [Google Scholar]
- 209.Sharma G. S., Singh L. R. Conformational status of cytochrome c upon N-homocysteinylation: implications to cytochrome c release. Archives of Biochemistry and Biophysics. 2017;614:23–27. doi: 10.1016/j.abb.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 210.Wang Z., Ando Y., Nugraheni A. D., Ren C., Nagao S., Hirota S. Self-oxidation of cytochrome c at methionine80 with molecular oxygen induced by cleavage of the Met-heme iron bond. Molecular BioSystems. 2014;10(12):3130–3137. doi: 10.1039/C4MB00285G. [DOI] [PubMed] [Google Scholar]
- 211.Karsisiotis A. I., Deacon O. M., Wilson M. T., et al. Increased dynamics in the 40-57 Ω-loop of the G41S variant of human cytochrome c promote its pro-apoptotic conformation. Scientific Reports. 2016;6(1):30447–30459. doi: 10.1038/srep30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Deacon O. M., Karsisiotis A. I., Moreno-Chicano T., et al. Heightened dynamics of the oxidized Y48H variant of human CytochromecIncreases its peroxidatic activity. Biochemistry. 2017;56(46):6111–6124. doi: 10.1021/acs.biochem.7b00890. [DOI] [PubMed] [Google Scholar]
- 213.Sanderson T. H., Mahapatra G., Pecina P., et al. Cytochrome c is tyrosine 97 phosphorylated by neuroprotective insulin treatment. PLoS ONE. 2013;8(11, article e78627) doi: 10.1371/journal.pone.0078627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hashimoto M., Takeda A., Hsu L. J., Takenouchi T., Masliah E. Role of Cytochromecas a stimulator of α-synuclein aggregation in lewy body disease. Journal of Biological Chemistry. 1999;274(41):28849–28852. doi: 10.1074/jbc.274.41.28849. [DOI] [PubMed] [Google Scholar]
- 215.Garrido-Mesa N., Zarzuelo A., Gálvez J. Minocycline: far beyond an antibiotic. British Journal of Pharmacology. 2013;169(2):337–352. doi: 10.1111/bph.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Scarabelli T. M., Stephanou A., Pasini E., et al. Minocycline inhibits caspase activation and reactivation, increases the ratio of XIAP to smac/DIABLO, and reduces the mitochondrial leakage of cytochrome c and smac/DIABLO. Journal of the American College of Cardiology. 2004;43(5):865–874. doi: 10.1016/j.jacc.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 217.Patriarca A., Polticelli F., Piro M. C., et al. Conversion of cytochrome c into a peroxidase: inhibitory mechanisms and implication for neurodegenerative diseases. Archives of Biochemistry and Biophysics. 2012;522(1):62–69. doi: 10.1016/j.abb.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 218.Firsov A. M., Kotova E. A., Antonenko Y. N. Minocycline prevents peroxidative permeabilization of cardiolipin-containing bilayer lipid membranes mediated by cytochrome c. Biochemical and Biophysical Research Communications. 2018;507(1-4):510–513. doi: 10.1016/j.bbrc.2018.11.078. [DOI] [PubMed] [Google Scholar]
- 219.Vlasova I. I., Tyurin V. A., Kapralov A. A., et al. Nitric oxide inhibits peroxidase activity of cytochrome c-cardiolipin complex and blocks cardiolipin oxidation. Journal of Biological Chemistry. 2006;281(21):14554–14562. doi: 10.1074/jbc.M509507200. [DOI] [PubMed] [Google Scholar]
- 220.Silkstone G., Kapetanaki S. M., Husu I., Vos M. H., Wilson M. T. Nitric oxide binding to the cardiolipin complex of ferric cytochrome c. Biochemistry. 2012;51(34):6760–6766. doi: 10.1021/bi300582u. [DOI] [PubMed] [Google Scholar]
- 221.Lagoa R., Samhan-Arias A. K., Gutiérrez-Merino C. Correlation between the potency of flavonoids for cytochrome c reduction and inhibition of cardiolipin-induced peroxidase activity. BioFactors. 2017;43(3):451–468. doi: 10.1002/biof.1357. [DOI] [PubMed] [Google Scholar]
- 222.Atkinson J., Kapralov A. A., Yanamala N., et al. A mitochondria-targeted inhibitor of cytochrome c peroxidase mitigates radiation-induced death. Nature Communications. 2011;2(1) doi: 10.1038/ncomms1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bakan A., Kapralov A. A., Bayir H., Hu F., Kagan V. E., Bahar I. Inhibition of peroxidase activity of Cytochromec: de novo compound discovery and validation. Molecular Pharmacology. 2005;88(3):421–427. doi: 10.1124/mol.115.097816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kagan V. E., Bayır H. A., Belikova N. A., et al. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radical Biology and Medicine. 2009;46(11):1439–1453. doi: 10.1016/j.freeradbiomed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Vladimirov Y. A., Proskurnina E. V., Alekseev A. V. Molecular mechanisms of apoptosis. Structure of cytochrome c-cardiolipin complex. Biochemistry. 2013;78(10):1086–1097. doi: 10.1134/s0006297913100027. [DOI] [PubMed] [Google Scholar]


