Abstract
The present study identified the threshold concentration of xylo-oligosaccharides (XOS) that resulted in minimal quality changes (rheology, color, water activity, pH, and total soluble solids) in strawberry puree. Optimization of XOS concentration to 5% (w/w) did not significantly alter the quality attributes of the strawberry puree. In addition, this study also monitored the rheological properties, composition (total soluble solids, total phenolic content, flavonoids, and tannin content), physicochemical attributes (color, water activity, pH) and sensorial properties of XOS-enhanced (5%, w/w) strawberry puree after thermal processing (HTST: 75 °C, 15s and UHT: 121 °C, 2s) and storage after 1, 15, and 36 days at 4 °C and 55 °C. At 5% (w/w) concentration, the addition of XOS increased consumer preference without significantly compromising quality attributes. Thermally treated strawberry puree (HTST and UHT) were less preferred by consumers than fresh puree. However, all strawberry samples incorporated with XOS (5%, w/w) received statistically higher scores than the samples without the XOS addition. Thus, the proposed supplementation of strawberry puree with XOS could be a viable solution to increase consumers’ dietary fiber intake with little need for behavioral changes.
Keywords: Food science, Food technology, Xylo-oligosaccharides, Strawberry puree, Thermal treatment, Sensory evaluation, Nutritional value, Shelf-life analysis
Food science; Food technology; Xylo-oligosaccharides; Strawberry puree; Thermal treatment; Sensory evaluation; Nutritional value; Shelf-life analysis.
1. Introduction
Xylo-oligosaccharides (XOS), a group of prebiotics that constitutes dietary fiber, are sugar oligomers linked by β 1–4 bonds with 2–6 degree of polymerization (mDP). These oligomers are widely present in honey, fruits, vegetables, milk and bamboo shoots [1]. Categorized as emerging prebiotics, XOS has already shown several advantages over some well-established and applied prebiotics (such as inulin) regarding their stability to both acidity and heat in food processing, allowing their application in low-pH food products [2, 3]. In addition, xylo-oligosaccharides exhibit other characteristics, such as sweetness [4] and prebiotic effects [5, 6]. The prebiotic effect of XOS may contribute to gastrointestinal health and reduce the risk of colorectal cancer (CRC) [7].
CRC has been listed as the third leading cancer among males and females in the United States [8]. The incidence of colorectal cancer is projected to increase by 60% to more than 2.2 million cases with a 50% death rate by 2030 [9]. A diet consisting of fruits, vegetables, and high dietary fiber may ameliorate gut microbiota composition, which may reduce the risk of cancer development [6,10]. However, 90% of American adults fail to meet the daily recommended level (DRI) of fiber and only 12.2% of the populations reached the recommended level of fruits and vegetable intake [11, 12]. Therefore, supplementing foods with dietary fiber to increase daily consumption could offer protective health effects that Americans are not currently experiencing. Xylo-oligosaccharides were first introduced in foods in the Japanese market as a functional food ingredient. With growing consumer awareness of the benefits of functional foods, food manufacturers recognized the contribution of functional ingredients to the enhancement of a food product's value. Consequently, the functional food market has exhibited impressive growth all over the world [13, 14, 15]. Since sensory attributes of products are usually the major factor that determines consumers' food choice in general, the addition of bioactive ingredients to introduce health benefits cannot be made at the expense of a foods' sensory properties.
Fruit purees are some of the ideal vehicles to carry XOS into varieties of food models. Being an ingredient of many foods, fruit purees are used in confections, beverages, ice creams, fruit snacks, baked goods, and baby food [16, 17, 18]. Also, fruit smoothies have been particularly popular in the past few years according to US industry report 31211C [19]. It was reported that blend fruit juice and smoothies were expected to generate 23.0% of the industry revenue in the year of 2019 [19]. Strawberry, being a preferable fruit in smoothies, is widely accepted in North America as fruit drink flavors [20]. Furthermore, strawberries are inherently one of the richest sources of phenolic compounds and active nutrients, which exhibits biological potentials in human health [21]. Therefore, it is necessary to study the quality attributes of dietary fiber fortified strawberry products.
Most of the food will undergo thermal processing in order to ensure food safety and prolong shelf-life. However, as mentioned before, the quality attributes as well as the sensorial properties of fruit purees should not be sacrificed by adding new ingredients such as XOS. Therefore, the quality of fruit puree in different thermal processing conditions and the sensorial profile of each thermally treated samples should be simulated and collected to verify no significant quality changes are introduced after the incorporation of XOS.
The objective of this study was to identify the threshold concentration of XOS that caused a minimum disturbance to the quality and stability of strawberry puree. The effects of thermal treatment and storage on the quality attributes of XOS enriched strawberry puree were also studied to assess the potential use of XOS in actual industrial and commercialization settings. Lastly, sensory tests were conducted to validate the consumer acceptance of XOS-enhanced strawberry puree.
2. Materials and methods
2.1. Chemicals and reagents
Catechin, Folin-Ciocalteu reagent (FCR), 2,2-Azinobis(2-methyl-propionamidine) dihydrochloride (AAPH), Gallic acid and fluorescein sodium salt were purchased from Sigma-Aldrich (St. Louis, MO, USA). Trolox (6-hydroxy-2,5,7,8-tetramethylchroman2-carboxylic acid) was obtained from Acros Organics (Morris Plains, New Jersey, USA). Unless mentioned, all other chemicals and reagents used in this study were of analytical grade.
2.2. Preparation of strawberry puree
Frozen strawberries (Fragaria × ananassa) were sourced from a local supplier (Stop & Shop, Hadley, MA, USA). Xylo-oligosaccharides (95P), which were manufactured by Shandong Longlive Bio-technology Co., (Qingdao, China), were sourced from the AIDP corporation (City of Industry, CA, USA). The frozen strawberries were thawed before blending in a Coolife HS-767 blender (Shenzhen, China) at low speed for 5 min. The resulting puree was then transferred into different beakers and mixed with xylo-oligosaccharides at different concentrations (0, 2.5, 5, 7.5 and 10% w/w) for an additional minute using a Cuisinart smart stick blender (East Windsor, U.S.) at low blend speed to complete sample preparation. Puree samples were subjected to texture and color analysis right after sample preparation to avoid any texture and color change.
2.3. Thermal treatment of strawberry puree
All production trials were processed with 48 h. For thermal treatment, larger volumes of puree were required to be processed. Therefore, a new puree preparation procedure was used in a scaled-up pilot plant process. Strawberry purees were prepared by a mechanical blender (Chemineer, Dayton, OH, USA), incorporated with 5% XOS (w/w) and stored at 4 °C before thermal treatments. The prepared puree was then processed through the Ultra high temperature/High temperature short time (UHT/HTST) Direct & Indirect Processing System (MicroThermics, Raleigh, NC, USA) for thermal treatments. The detailed parameters are shown in Table 1, which was based on the Guidance for Industry: Juice HACCP Hazards and Controls Guidance First Edition; Final Guidance [22]. Samples were processed and filled using the Clean-Fill Hood & Sterile Product Outlets (MicroThermics, City, NC, USA) in sterilized 9 oz glass jars. UHT samples were hot filled and positioned bottom side up for samples to cool down to room temperature. HTST samples were cooled in the MicroThermics system before filling. Control samples (fresh puree) were cold filled and stored in 4 °C refrigerator.
Table 1.
Parameters of the thermal processes applied to strawberry puree samples.
| Treatments | Processing time (s) | Processing temperature (°C) | Filling temperature (°C) |
|---|---|---|---|
| Control | - | - | 4 |
| HTST | 15 | 75 | 25 |
| UHT | 2 | 131 | 80 |
Processing temperature and processing time were slightly raised and extended to confidently achieve a 5-log reduction for E.coli O157:H7, Salmonella, Listeria monocytogenes, and Cryptosporidium parvum in acidified food, i.e., fruit puree (Mazzotta, 2001).
2.4. Imperfect lubricated squeezing flow (ILSF) viscometry of strawberry puree
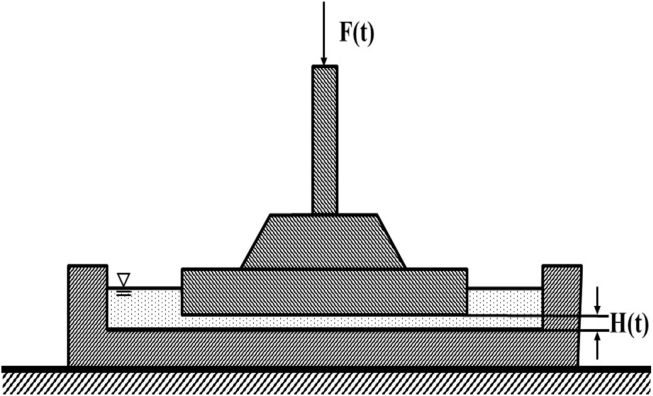
Imperfect lubricated squeezing flow viscometry (ILSF) was selected to characterize the rheological properties of these samples [23]. ILSF tests were conducted using a TA.TX Plus Texture Analyzer (Texture Technologies Corp., Hamilton, MA, USA) equipped with a 50 kg load cell. All measurements were performed at room temperature, i.e., 22 ± 1 °C with a sample volume of 75ml for each measurement. Samples were gently transferred into a shallow Teflon container (140 mm in diameter) and were compressed using a Teflon plate (100mm in diameter). The difference in diameter of the upper and lower plate allows minimizing the effect of the annular flow on the results [23]. All samples were compressed to a final height of 0.8 mm at a speed of 0.5 mm s−1 (Figure 1). After reaching its final position, the crosshead was held at the final height for 130 s and the residual force was recorded during that time. All samples were measured twice in triplicate.
Figure 1.
Schematic view of the imperfect squeezing flow array.
The apparent stress at two heights within the squeezing flow regime, namely 1.5 and 2 mm, were determined from the recorded force vs. height relationships as follows:
| (1) |
where σApp @ height is the apparent stress at the indicated height, F is the force recorded at each height in Newtons and R is the radius of the upper plate in meters.
The consistency of different strawberry samples was compared based on the estimated apparent compressive stress (σApp @ height) at each selected height (Eq. (1)) [24]. The sample's residual apparent stress provides information about the specimen's structural solidity and can be calculated as,
| (2) |
where σApp @ t is the residual apparent stress after the samples have been allowed to relax at a given height for a given time, F is the force recorded at that given time in seconds and R is the radius of the upper plate in meters.
The apparent stress at 1.5mm was used to represent the consistency of strawberry puree samples, due to its high sensitivity and low susceptibility to being affected by the presence of solid particles such as individual strawberry seeds or seed clusters. In addition, apparent stress at 60s and 120s were used to reflect the solidity of the strawberry puree specimens.
2.5. Color of strawberry puree samples
A HunterLab ColorFlex EZ (HunterLab, Reston, VA, USA) was used to investigate the changes in color of the untreated and heat-treated samples after preparation and during storage. The instrument was calibrated against a white and black tile prior to measurements, and all measurements were conducted at ambient temperature.
A low reflectance glass sample cup was used to hold the samples directly above the colorimeter sensor. Samples (30mL) were measured in a dark background using CIE-Lab color space, in which L∗ (lightness), a∗ (redness) and b∗ (yellowness) values were collected for each sample. Furthermore, the total color differences (ΔE) of samples during storage were compared against the control sample (fresh puree) using Eq. (3) [25].
| (3) |
2.6. Quality attributes of strawberry puree
Samples were pulled from storage at the selected time intervals and tested after the sample's temperature had reached room temperature. The water activity of strawberry puree was analyzed by an AquaLab 4TE Dew Point water activity meter (METER Group Inc, Pullman, WA, USA). A Metrohm 827 pH lab meter (Metrohm, Herisau, Switzerland), equipped with Unitrode with Pt1000 probe, was calibrated with standard buffer solutions (pH = 4, pH = 7, Fisher Scientific, City, State. the USA) prior to pH analysis. Total Soluble Solids (TSS), expressed in °Brix (total soluble solids g/100g) were analyzed using a Milwaukee refractometer (MA871, Rocky Mount NC, USA), calibrated with distilled water prior to measurements.
2.7. Total phenolic content, flavonoid, and tannin content analysis
2.7.1. Extract preparation
Puree samples of the whole strawberries (pulp and seeds) were pulled over time from each storage condition (4 °C, 55 °C). Fresh puree samples were extracted with acetone (80% v/v acetone, 5% v/v acetic acid in deionized water) for 24h on a Labquake tube shaker (ThermoFisher Scientific, U.S.A.) at 4 °C [26]. The supernatant and fruit pulp were separated in an Eppendorf centrifuge 5810 R (Eppendorf, Hauppauge, NY, USA.) at 1500g for 10min. The supernatant was then concentrated using a Heidolph Heizbad Hei-VAP rotary evaporator (Schwabach, Germany). Concentrated samples were further diluted with 50% methanol and stored at -20 °C for further phenolic, flavonoids, and tannin content analysis [27].
2.7.2. Determination of total phenolics, flavonoid, and tannin in strawberry puree extracts
Total phenolic, flavonoid, and tannin content were measured based on the Folin-Ciocalteu, aluminum chloride, and vanillin colorimetric method, respectively [28, 29]. The standard curves for total phenolic content, flavonoids, and tannins were developed based on gallic acid (0, 6.25, 12.5, 25, 50 and 100 mg/mL, for total phenolics) and catechin (0, 6.25, 12.5, 25, 50 and 100 mg/mL, for flavonoids and tannins) standard solutions, respectively.
Total phenolic, flavonoid, and tannin contents were calculated by introducing the absorbance in to the linear regression equation of the standard curve. The results were expressed as mg of gallic acid equivalent (GAE) g−1, mg of catechin equivalents (CE) g−1, and mg of catechin equivalents (CE) g−1 of fresh weight of puree, respectively.
2.8. Antioxidant activity in strawberry puree extract
The antioxidant activity of the samples was determined using the Oxygen Radical Absorbance Capacity (ORAC) assay [30]. Trolox standard solutions (0, 6.25, 12.5, 25, 50, and 100 μmol/mL) were prepared in diluted phosphate buffer to calculate the antioxidant activity [30]. Net areas under the quenching curve of the standard solutions were calculated by Eq. (4), where f0 is the initial fluorescence intensity and ft is the fluorescence intensity at the given time t. A standard curve, which describes the relationship between net area and Trolox equivalent concentration, was later generated.
| (4) |
The final antioxidant activity was calculated by solving the equation of standard curve for each samples’ net area value and expressed as μmol of Trolox equivalent (TE) g−1 of fresh weight of puree.
2.9. Sensory evaluation of strawberry puree
A sensory test with untrained panelists (n = 105) was conducted to examine the acceptance of XOS in differently processed strawberry puree using a 9-point Hedonic liking test [31]. Approval from the University of Massachusetts Institutional Review Board (IRB) for the Protection of Human Subjects was obtained prior to fielding these experiments. Concent was obtained from all panelists prior to the sensory evaluation. Untreated and thermally treated (fresh, HTST and UHT) samples with and without 5% w/w XOS were used in this sensory evaluation. The test was set up as a block design, using the Sensory Information Management System 2000 (SIMS 2000) software Version 6.0 (Sensory Computer Systems LLC, Berkeley Heights, NJ, USA). Each of the panelists randomly evaluated 3 of the 6 samples to reduce sensory fatigue. Subjects assessed the samples one after another at isolation stations to maintain a consistent test environment and reduce bias from the presence of other participants. During the 9-point (1 = extremely dislike, 5 = neutral, and 9 = extremely like) hedonic test, the recruited participants were asked to evaluate the overall acceptance, appearance, color, aroma, flavor, sweetness, and texture for each individual puree sample. All non-heat-treated samples were made 30 min prior to the study and stored in ice baths. All heat-treated samples were prepared 12 h before the evaluation and stored in 9-oz sterilized jars at 4 °C. Samples were then transferred from the fridge to ice baths 30 min prior to the study. Temperature variations throughout the study did not exceed 1.9–2.2 °C. Each subject evaluated one control sample (no heat or XOS addition) and two other samples in 3-oz plastic serving cups with a serving size of 1oz. Water and saltine crackers (unsalted) were provided between each sample.
2.10. Statistical analysis
The physicochemical properties of strawberry puree with different XOS concentrations were measured twice independently and in triplicate both times. For the scale-up pilot plant processing, two separate batches of thermally treated strawberries were produced, and each individual treatment had three sets of parallel samples for measurement. Values were reported as mean ± standard deviation (SD). All statistical analyses were performed using Statistical Analysis System software 9.4 (SAS Institute, Cary, NC, USA).
The hedonic sensory study was conducted in one session with 105 participants. Sensorial data from the entire group (105 participants) was evaluated using Duncan analysis to identify a difference in liking scores amongst the processing conditions and the control.
3. Results and discussion
3.1. Effect of XOS dosage on the quality attributes of strawberry puree
The goal of this study was to identify the threshold concentration of XOS that could be added to strawberry puree without altering its overall quality. Rheological, color, composition (TTS) and physicochemical (water activity, pH) attributes of strawberry puree samples with different XOS concentrations (0, 2.5, 5, 7.5 and 10%, w/w) were measured. Table 2 shows the rheological properties of fresh strawberry puree samples. An increase of xylo-oligosaccharides content resulted in a decrease (p < 0.05) in the consistency of strawberry puree samples expressed as apparent stress at 1.5, or 2mm height (Table 2). A Duncan test revealed that the consistency of the samples with an addition of 2.5% (334.0 ± 13.3Pa) and 5% (334.4 ± 23.7Pa) w/w xylo-oligosaccharides was statistically similar to the control (341.8 ± 5.3Pa) at 1.5mm. During the relaxation period, the residual stress was not influenced by XOS content, regardless of its concentration (Table 2).
Table 2.
Rheological parameters, color analysis and quality attributes of strawberry puree with different XOS ratios.
| XOS ratio (w/w) | Apparent stress @ 2mm (Pa) | Apparent stress @ 1.5mm (Pa) | Apparent stress @ 60s (Pa) | Apparent stress @ 120s (Pa) | L∗ | a∗ | b∗ | TCD (ΔE) | Water Activity | pH | Total Soluble Solids (TSS) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.0% | 252.72 ± 3.93A | 341.84 ± 5.33A | 71.35 ± 12.50A | 61.30 ± 16.88A | 30.24 ± 0.32A | 40.54 ± 0.82A | 24.3 ± 0.78A | 0.00 | 0.9902 ± 0.0013A | 3.35 ± 0.02A | 7.68 ± 0.37E |
| 2.5% | 247.00 ± 10.91AB | 333.98 ± 13.33AB | 53.07 ± 36.35A | 63.51 ± 11.67A | 29.51 ± 0.26B | 40.13 ± 0.82B | 24.16 ± 0.62A | 1.08 ± 0.16 | 0.9885 ± 0.0025A | 3.36 ± 0.03A | 9.97 ± 0.35D |
| 5.0% | 244.06 ± 11.08AB | 334.35 ± 23.71AB | 61.18 ± 7.63A | 56.24 ± 7.85A | 28.84 ± 0.28C | 39.88 ± 0.86C | 24.19 ± 0.72A | 1.66 ± 0.20 | 0.9882 ± 0.0016A | 3.36 ± 0.03A | 12.18 ± 0.58C |
| 7.5% | 233.56 ± 5.98BC | 321.18 ± 8.64B | 61.25 ± 5.96A | 55.97 ± 7.01A | 28.09 ± 0.24D | 39.40 ± 1.03D | 24.10 ± 0.94A | 2.55 ± 0.36 | 0.9841 ± 0.0028B | 3.36 ± 0.02A | 14.53 ± 0.28B |
| 10.0% | 225.43 ± 14.06C | 318.40 ± 23.70B | 67.43 ± 4.11A | 63.69 ± 4.79A | 27.45 ± 0.35E | 39.10 ± 0.86E | 23.98 ± 0.65A | 3.45 ± 0.29 | 0.9838 ± 0.0014B | 3.36 ± 0.02A | 16.80 ± 0.33A |
Note: In each column, values followed by different letters are statistically different accoridng to Duncan test (p < 0.05).All TCD values are calculated based on Eq. (3).
Strawberry puree contains a considerable number of polysaccharides and disrupted plant cells in a clear serum. For puree samples of the same weight, the incorporation of oligosaccharides will alter the composition of the fruit puree, i.e., large polysaccharides are replaced by smaller oligosaccharides. This change mimics, to a certain extent, fruit ripening, where large molecules like polysaccharides are broken into small oligosaccharides and monosaccharides by enzymes [32]. The composition change in strawberry puree might cause the softening of the sample.
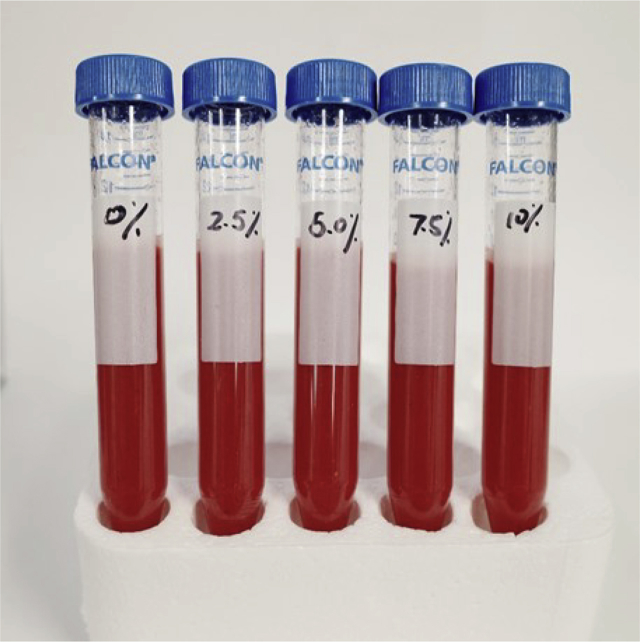
As summarized in Table 2, both L∗ and a∗ values decreased with higher xylo-oligosaccharides additions. However, the incorporation of XOS did not statistically affect the b∗ value. Thus, the addition of xylo-oligosaccharides turned the samples darker and less red compared to the fresh sample. Similar results were reported by Grigelmo-Miguel and Martın-Belloso (1999), where L∗ and a∗ values of strawberry jam negatively correlated with nominal soluble solids (peach dietary fiber) [33]. Total color difference (TCD) parameters, expressed as ΔE, were used to determine the color change [25,34, 35]. 2.5% w/w XOS incorporation showed a small color difference whereas samples with 5% and 7.5% w/w XOS additions showed a distinct difference. Samples with 10% w/w XOS content had a very distinct color difference compared to control (Table 2). It should be noted, however, that the color difference was not evident based on visual observations, see Figure 2. The color of strawberry fruit is affected by various factors such as pH, water activity, as well as composition. The addition of small molecular size components to a food product may alter the transmittance of the sample, which exhibits a darkening effect in sample color analysis (Table 2).
Figure 2.
Strawberry puree samples with different XOS concentration.
Table 2 summarizes the water activity, pH and total soluble solids of different strawberry puree samples. The observed pH and TSS values of untreated strawberry puree were similar to the results reported in different studies [36, 37, 38, 39]. Not surprisingly, total soluble solids (°Brix) yield a linear relationship (r2 = 0.999, data not shown) with the incorporation of xylo-oligosaccharides. Xylo-oligosaccharides are water soluble dietary fibers; therefore, the addition of XOS will increase the total soluble matter within the puree system, hence increase the TSS value. The pH of the product is not only an essential organoleptic characteristic but also affects the color expression of anthocyanin pigments in strawberry products [40]. In this study, the addition of XOS did not affect the overall pH of any of the strawberry purees (Table 2). This might be due to the buffering effect of the inherent organic acids in strawberry.
The water activity measurements indicated that the addition of xylo-oligosaccharides had a negative correlation with the samples' water activity (Table 2). Similar results were also reported by Ayyappan et al. (2016) where XOS was incorporated in cookies [41]. However, the water binding capacity was not very strong, an increase of 10% w/w XOS concentration only resulted in 0.65% water activity drop as compared to fresh puree (data not shown). The pH of all tested samples was about 3.4. The addition of XOS, whose solution pH is 4.05 ± 0.09 [42], did not alter the pH of strawberry puree. Grigelmo-Miguel et al. (1999) reported a similar pH response (3.08–3.29) when applying peach dietary fiber (pH = 3.91) in strawberry jams [33]. Since most oligo-saccharides are soluble in aqueous environments, an increased concentration of oligosaccharides will increase the total soluble solids content. Higher TSS content leads to a decrease in water activity in products such as strawberry puree (Table 2). The aw reduction can contribute to the final product's stability. In addition, fiber and water can interact with each other by different means. In this case, the extensive availability of hydroxyl groups contributed by the addition of XOS molecules, might play a unique role in binding and entrapping water molecules through hydrogen bonds.
Adding new functional ingredients to a food product has always been a challenge for the industry, because the addition of one or several substances may greatly impact the original properties of the food. Based on the results, strawberry puree samples with higher doses of XOS (7.5 and 10% w/w) exhibited significant quality changes on color, rheological properties, and water activity. Meanwhile, there were small or no differences in product characteristics when XOS dosages of 5% or below were applied. Therefore, a 5% XOS concentration was chosen for further testing during thermal treatments, storage and sensorial analysis.
3.2. Effects of thermal treatment and storage on of strawberry puree's attributes
3.2.1. TPC, TFC, tannin content and antioxidant activity in strawberry purees
The total phenolic content (TPC) during a 36-day shelf-life study for all samples is reported in Table 3. The TPC value of unprocessed strawberry puree (2.36 ± 0.048 mg GAE g−1 fresh weight) was comparable to the values obtained by other authors that reported TPC values within the 2.30–3.40 mg GAE g−1 fresh weight range [43, 44]. Total flavonoid and tannin contents from all storage samples are shown in Table 3.
Table 3.
Total Phenolic, flavonoid, tannin and antioxidant activity measurements in strawberry samples during storage.
| Processing conditions | Storage (day) | Total phenolic (mg GAE g−1 fresh puree) |
Total flavonoids (mg CE g-1 fresh puree) |
Total tannin (mg CE g-1 fresh puree) |
Antioxidant activity (μmol TE g-1 fresh puree) |
||||
|---|---|---|---|---|---|---|---|---|---|
| 4 °C storage | 55 °C storage | 4 °C storage | 55 °C storage | 4 °C storage | 55 °C storage | 4 °C storage | 55 °C storage | ||
| Control | 1 | 2.36 ± 0.048BCD | - | 0.73 ± 0.13A | - | 2.34 ± 0.043BCD | - | 24.31 ± 0.59F | - |
| HTST | 1 | 2.37 ± 0.05BCD | 2.05 ± 0.06A | 0.47 ± 0.01DEFG | 0.43 ± 0.03A | 2.40 ± 0.024B | 1.78 ± 0.127B | 32.90 ± 1.06E | 34.09 ± 1.54B |
| 15 | 2.29 ± 0.04BCDE | 1.40 ± 0.07CD | 0.49 ± 0.04CDEFG | 0.26 ± 0.03C | 2.33 ± 0.083BCD | 0.40 ± 0.064D | 35.90 ± 1.88DE | 23.54 ± 1.49EF | |
| 36 | 2.23 ± 0.09CDEF | 1.15 ± 0.04E | 0.52 ± 0.01CDEF | 0.21 ± 0.01D | 2.15 ± 0.127DEF | not detectedF | 33.28 ± 1.81E | 18.35 ± 1.49G | |
| HTST (XOS) | 1 | 2.73 ± 0.18A | 2.14 ± 0.18A | 0.55 ± 0.06BCDE | 0.41 ± 0.04A | 2.65 ± 0.033A | 1.89 ± 0.072A | 27.06 ± 4.61FG | 33.52 ± 5.17BC |
| 15 | 2.41 ± 0.14B | 1.65 ± 0.09B | 0.48 ± 0.01DEFG | 0.32 ± 0.03B | 2.59 ± 0.271A | 0.41 ± 0.054D | 35.72 ± 5.34DE | 26.95 ± 5.11DE | |
| 36 | 2.38 ± 0.01BC | 2.08 ± 0.12BC | 0.60 ± 0.05BC | 0.27 ± 0.04C | 2.38 ± 0.098BC | not detectedF | 33.17 ± 2.61E | 21.08 ± 4.28FG | |
| UHT | 1 | 2.08 ± 0.20FGH | 1.92 ± 0.12A | 0.49 ± 0.04CDEFG | 0.43 ± 0.01A | 2.20 ± 0.173BCDEF | 1.80 ± 0.042AB | 37.66 ± 0.85BCD | 39.74 ± 1.18A |
| 15 | 2.14 ± 0.13EFGH | 1.28 ± 0.03DE | 0.51 ± 0.04CDEFG | 0.26 ± 0.03C | 2.26 ± 0.110BCDE | 0.51 ± 0.072D | 42.33 ± 1.26A | 27.84 ± 0.87DE | |
| 36 | 2.11 ± 0.19FGH | 1.09 ± 0.08E | 0.54 ± 0.02BCDE | 0.21 ± 0.01D | 2.12 ± 0.120EFG | 0.23 ± 0.024E | 41.41 ± 0.85AB | 20.17 ± 0.82FG | |
| UHT (XOS) | 1 | 2.14 ± 0.09EFGH | 1.94 ± 0.04A | 0.44 ± 0.002EFG | 0.41 ± 0.01A | 2.01 ± 0.120FG | 1.62 ± 0.064C | 36.99 ± 0.96CDE | 39.13 ± 0.78A |
| 15 | 2.17 ± 0.04EFG | 1.45 ± 0.06BCD | 0.41 ± 0.02G | 0.26 ± 0.004C | 1.91 ± 0.024GH | 0.25 ± 0.024E | 40.78 ± 0.79ABC | 29.26 ± 0.10CD | |
| 36 | 2.20 ± 0.02DEFG | 1.12 ± 0.04E | 0.43 ± 0.01FG | 0.19 ± 0.003D | 1.80 ± 0.087H | not detectedF | 38.37 ± 2.45ABCD | 19.3 ± 0.72FG | |
Note: For each column, values followed by different letters indecates different groups according to the ANOVA and Duncan test (p < 0.05).
The TPC values in UHT-treated samples were significantly lower than in HTST-treated samples across storage conditions regardless of XOS addition. Also, the Duncan test revealed that TPC levels in HTST-treated samples were more similar to the control sample regardless of XOS incorporation (Table 3). Similar TPC reductions were reported in the literature for heat-treated vegetable and fruit samples. For example, Terefe et al. (2013) observed that the TPC level decreased by 14–24% in thermally treated strawberry puree compared to fresh puree [45]. Yao., & Ren (2011) also found a significant loss of about 48% in TPC when boiling Shengjie celery for 10 min [46]. Storage time and temperature jointly affect the TPC level of all samples. TPC in most samples decreased as storage time increased. Low-temperature storage (4 °C) helped retain the level of phenolic compounds in the strawberry puree as compared to high-temperature storage (55 °C).
Total flavonoids content (TFC) of HTST- and UHT-treated strawberry puree were significantly lower than flavonoids content in the fresh puree, regardless of XOS incorporation (Table 3). Tannin content followed the same trend as flavonoids content. Flavonoid content did not significantly decrease in samples stored at 4 °C over time, regardless of thermal treatment or XOS addition. However, flavonoid degradation was observed during storage at 55 °C. As expected, TFC levels in samples stored at 55 °C dropped significantly as storage time increased. Tannin content decreased over the storage period was examined regardless of storage temperature and XOS incorporation. Tannins were below the detection level in most samples stored at 55 °C for 36 days.
Collected data showed that thermal treatment increased the measurable antioxidant activity in strawberry puree specimens. Higher antioxidant activity was detected both in UHT and HTST samples as compared to the control (Table 3). Cao et al. (2011) also reported that thermal treatment (70 °C for 2min) increased the detection of catechin by 42% (p < 0.05) [47]. Keenan et al. (2010) observed increased 2,2-diphenyl-1-picrylhydrazyl (DPPH) levels, a marker of higher antioxidant activity, in fruit smoothies by 15% (p < 0.05) after thermal treatment (P70 ≥ 10 min). Antioxidant activity was significantly affected by storage conditions [48]. A significant decrease in ORAC level was detected in samples stored at 55 °C regardless of XOS incorporation. However, no change or a slight increase was observed in samples stored at 4 °C (Table 3). van der Sluis et al. (2001) also observed that the antioxidant activity of four apple cultivars was not affected by long-term storage at 4 °C [49].
Thermal treatments are widely used in the food industry to produce shelf-stable products as well as generating aroma and color. However, for fruit products, thermal treatment can lead to significant losses in nutritional value. As shown in this study, both thermal treatment and high-temperature storage decreased the nutritional quality of strawberry puree samples (Table 3). However, the antioxidant activity of strawberry samples was increased after thermal treatment. This phenomenon might be due to the increased extractability of antioxidant compounds by thermal treatments. It has been suggested that thermal processing such as cooking or pasteurization might help break down large polymers in the cell walls [47]. Antioxidant compounds within the plant cell might be released and consequently the treatment increased the antioxidant activity of the processed product. On the other hand, the antioxidant activity of the strawberry puree samples kept at 55 °C decreased as the storage time elapsed, indicating the degradation of water-soluble antioxidants during storage at this relatively high temperature. It should be noted that although vitamin C concentration plays an important parameter in strawberry products, the overall antioxidant capacity of the samples provided a better depiction of the potential health benefits associated with this product and the potential loss of these benefits during storage and processing. In addition, the ascorbic acid concentration contributes to the overall ORAC value [50], making the ORAC value a better parameter to monitor product quality degradation.
The addition of XOS did not provide a protective effect on the product's phenolic content, color, or texture during storage. It should be noted, however, that all samples with the XOS addition showed higher TPC values during storage (Table 3). This phenomenon was due to the limitations of the FC method, since XOS also has hydroxyl groups and shows similar chemical structures as phenolic compounds. The similarity in molecular structure may result from the increased TPC value in XOS incorporated samples. Alternative quantification strategies such as high-performance liquid chromatography (HPLC) should thus be considered.
3.2.2. Rheological properties
HTST and UHT treatment of all samples resulted in a significant decrease in consistency, i.e., apparent stress at 1.5mm, as compared to the control (340.4 ± 11.5Pa) (Table 4). In addition, all HTST HTST-treated samples exhibited higher apparent stress at 1.5mm than UHT treated samples. The apparent stress at 60s and 120s of the HTST and UHT-treated samples were statistically similar.
Table 4.
Rheological parameters of strawberry samples during storage.
| Processing conditions | Storage (day) | Apparent stress @ 2 mm (Pa) |
Apparent stress @ 1.5 mm (Pa) |
Apparent stress @ 60 s (Pa) |
Apparent stress @ 120 s (Pa) |
||||
|---|---|---|---|---|---|---|---|---|---|
| 4 °C storage | 55 °C storage | 4 °C storage | 55 °C storage | 4 °C storage | 55 °C storage | 4 °C storage | 55 °C storage | ||
| Control | 1 | 249.85 ± 9.53A | - | 340.36 ± 11.53A | - | 87.06 ± 8.89A | - | 85.64 ± 7.10A | - |
| HTST | 1 | 211.40 ± 6.75C | 195.71 ± 5.70A | 310.57 ± 8.29C | 273.88 ± 3.79A | 60.53 ± 0.56BC | 60.77 ± 1.91BCD | 52.89 ± 2.40C | 57.58 ± 3.08AB |
| 15 | 230.63 ± 2.61B | 136.16 ± 0.50D | 324.31 ± 3.14B | 182.85 ± 0.97D | 72.91 ± 19.38B | 74.74 ± 14.79A | 55.61 ± 2.09BC | 76.08 ± 16.16A | |
| 36 | 206.27 ± 2.96D | 104.67 ± 4.03FG | 294.03 ± 2.69D | 151.83 ± 5.21F | 65.33 ± 8.01BC | 56.90 ± 2.24CD | 64.64 ± 9.56BC | 58.00 ± 1.51AB | |
| HTST (XOS) | 1 | 223.60 ± 3.66BC | 193.58 ± 6.86A | 311.96 ± 6.97C | 274.42 ± 8.19A | 63.58 ± 1.17BC | 62.84 ± 5.11BCD | 60.63 ± 1.53BC | 61.10 ± 4.14AB |
| 15 | 224.71 ± 2.21BC | 139.28 ± 6.13CD | 315.69 ± 2.91C | 186.05 ± 7.48D | 55.81 ± 4.03C | 58.00 ± 3.04CD | 60.67 ± 12.99BC | 76.08 ± 16.16AB | |
| 36 | 198.30 ± 1.14E | 101.01 ± 2.05G | 286.16 ± 2.07E | 147.80 ± 3.78F | 56.38 ± 2.04C | 56.65 ± 2.88D | 55.86 ± 2.69BC | 58.00 ± 1.51AB | |
| UHT | 1 | 193.38 ± 4.86EF | 163.54 ± 9.67B | 265.15 ± 6.07G | 227.39 ± 10.06C | 63.34 ± 15.21BC | 58.51 ± 3.39CD | 55.17 ± 4.12BC | 54.14 ± 2.95B |
| 15 | 182.04 ± 10.86G | 143.12 ± 11.66CD | 249.31 ± 15.44H | 185.95 ± 14.90D | 64.54 ± 7.41BC | 64.52 ± 3.46BC | 65.30 ± 8.49B | 64.52 ± 5.18AB | |
| 36 | 177.16 ± 2.65H | 118.37 ± 9.52E | 249.89 ± 2.93H | 167.69 ± 10.54E | 58.78 ± 2.62BC | 56.65 ± 3.98CD | 56.27 ± 4.43BC | 56.68 ± 5.76AB | |
| UHT (XOS) | 1 | 188.02 ± 7.76FG | 170.17 ± 4.65B | 265.12 ± 10.56G | 240.81 ± 5.64B | 63.75 ± 6.94BC | 66.56 ± 9.41B | 61.54 ± 6.82BC | 66.36 ± 7.13AB |
| 15 | 198.76 ± 5.50E | 145.26 ± 2.77C | 276.16 ± 6.68F | 191.50 ± 4.70D | 61.52 ± 1.41BC | 67.04 ± 1.15B | 58.91 ± 2.45BC | 63.51 ± 0.94AB | |
| 36 | 180.01 ± 4.06H | 110.97 ± 5.03F | 256.75 ± 5.60H | 160.51 ± 5.42E | 56.44 ± 3.45C | 57.63 ± 1.35CD | 59.41 ± 6.44BC | 56.27 ± 1.58AB | |
Note: Values followed by different letters in each column indecates different groups according to the ANOVA and Duncan test (p < 0.05).
A loss in consistency, expressed as a decrease in apparent stress at 1.5mm, was detected at both storage temperatures over the length of the storage period, regardless of XOS incorporation. In addition, the reduction in consistency was accelerated during storage at 55 °C than 4 °C. The incorporation of XOS did not result in statistically different rheological properties of strawberry puree during storage at high temperature (55 °C). As mentioned before, the destruction of plant cell wall due to thermal processing may contribute to polymer breakdown in the samples, resulting in a significant decrease in consistency among heat treated samples (Table 4).
3.2.3. The total color difference of strawberry puree
Changes in color of the untreated- and thermally treated strawberry purees during storage are shown in Table 5. Overall, UHT treatments contributed to a significant decrease in L∗, a∗ and b∗ values of the specimens, indicating that UHT treatments will produce darker, less red, and less yellow puree compared to HTST-treated fresh puree. Also, prolonged storage at 55 °C had a significant impact on sample discoloration. Meanwhile, the color profile of all HTST-treated specimens stored at 4 °C was closer to the control (fresh puree) according to TCD results (Table 5). This result was also confirmed during the sensory evaluation (Table 7), where HTST samples received similar ratings in color preference as the control. The addition of XOS resulted in lower L∗ value, regardless of the type of thermal treatment applied (Table 5), indicating darker samples. This result was similar to previous findings (Table 2), where sample lightness decreased with greater XOS concentration.
Table 5.
Color of strawberry samples during storage.
| Processing conditions | Storage (day) | L∗ |
a∗ |
b∗ |
TCD (ΔE) |
||||
|---|---|---|---|---|---|---|---|---|---|
| 4 °C storage | 55 °C storage | 4 °C storage | 55 °C storage | 4 °C storage | 55 °C storage | 4 °C storage | 55 °C storage | ||
| Control | 1 | 29.41 ± 0.26FG | - | 37.96 ± 0.24AB | - | 22.53 ± 0.18C | - | 0 | - |
| HTST | 1 | 34.31 ± 0.24A | 33.27 ± 0.20A | 38.36 ± 0.54A | 30.59 ± 1.24A | 23.59 ± 0.48A | 20.51 ± 0.08A | 5.13 ± 0.18 | 8.61 ± 1.54 |
| 15 | 32.03 ± 0.20C | 24.38 ± 0.41E | 37.53 ± 0.35AB | 16.20 ± 0.82C | 22.97 ± 0.31BC | 19.46 ± 0.51B | 2.77 ± 0.20 | 22.54 ± 0.78 | |
| 36 | 32.39 ± 0.37C | 22.32 ± 0.13H | 34.71 ± 0.34DE | 14.32 ± 0.072DEF | 20.45 ± 0.35E | 18.55 ± 0.036C | 4.93 ± 0.18 | 24.99 ± 0.09 | |
| HTST (XOS) | 1 | 33.20 ± 0.48B | 32.81 ± 0.37A | 37.45 ± 1.83B | 31.11 ± 1.58A | 23.41 ± 0.83AB | 21.11 ± 0.16A | 4.35 ± 0.72 | 7.85 ± 1.25 |
| 15 | 30.88 ± 0.22D | 23.04 ± 0.52G | 37.88 ± 0.20AB | 15.62 ± 0.31CDE | 23.41 ± 0.14AB | 18.42 ± 0.17C | 1.78 ± 0.78 | 23.58 ± 0.19 | |
| 36 | 31.14 ± 0.17D | 21.11 ± 0.48I | 35.64 ± 0.15C | 13.93 ± 0.017F | 21.49 ± 0.049D | 18.56 ± 0.19C | 3.11 ± 0.11 | 25.72 ± 0.12 | |
| UHT | 1 | 30.68 ± 1.00D | 30.18 ± 0.62B | 33.06 ± 1.22G | 29.09 ± 0.27B | 18.59 ± 0.86GH | 18.22 ± 0.79C | 6.53 ± 1.31 | 9.93 ± 0.16 |
| 15 | 29.69 ± 0.25F | 25.49 ± 0.47D | 33.52 ± 0.34FG | 15.77 ± 0.47CD | 19.00 ± 0.17FG | 14.92 ± 1.11F | 5.67 ± 0.32 | 23.80 ± 0.34 | |
| 36 | 30.23 ± 0.37E | 23.46 ± 0.57FG | 31.45 ± 0.29H | 14.21 ± 0.35DEF | 17.59 ± 0.33I | 16.18 ± 0.40E | 8.22 ± 0.38 | 25.29 ± 0.55 | |
| UHT (XOS) | 1 | 29.09 ± 0.37GH | 28.66 ± 0.25C | 34.86 ± 0.79CD | 30.97 ± 0.48A | 20.08 ± 0.61E | 19.03 ± 0.23BC | 3.98 ± 0.99 | 7.86 ± 0.40 |
| 15 | 27.85 ± 0.05I | 24.06 ± 0.06EF | 33.94 ± 0.23EF | 17.08 ± 1.63C | 19.28 ± 0.17F | 17.29 ± 0.13D | 5.39 ± 0.25 | 22.18 ± 1.50 | |
| 36 | 28.68 ± 0.16H | 21.99 ± 0.12H | 32.05 ± 0.36H | 14.12 ± 0.031EF | 18.35 ± 0.33H | 17.12 ± 0.19D | 7.28 ± 0.48 | 25.54 ± 0.09 | |
Note: For each L∗ a∗ b∗ column, values followed by different letters indecates different groups according to the ANOVA and Duncan test (p < 0.05) All TCD values are calculated based on Eq. (3).
Differences in total perceivable color can be statistically classified as very distinct (ΔE > 3), distinct (1.5 < ΔE < 3) and small (0.5 < ΔE < 1.5) (Drlange, 1994).
Table 7.
Duncan results of 9-Point Hedonic test scores.
| Processing conditions | Overall | Appearance | Color | Aroma | Flavor | Sweetness | Texture |
|---|---|---|---|---|---|---|---|
| NO Heat | 5.86 ± 1.93AB | 7.30 ± 1.47A | 7.74 ± 1.04A | 6.51 ± 1.94AB | 5.53 ± 2.03AB | 5.06 ± 2.16AB | 6.33 ± 1.98AB |
| NO Heat (XOS) | 6.42 ± 1.52A | 7.38 ± 1.41A | 7.88 ± 1.09A | 6.91 ± 1.71A | 6.05 ± 1.76A | 5.81 ± 2.16A | 6.95 ± 1.40A |
| HTST | 5.28 ± 2.09B | 7.20 ± 1.55A | 7.44 ± 1.38A | 6.51 ± 2.18AB | 4.63 ± 2.34B | 4.56 ± 2.35B | 6.42 ± 2.20AB |
| HTST (XOS) | 5.71 ± 2.12AB | 7.47 ± 1.64A | 7.47 ± 1.59A | 7.30 ± 1.58A | 5.45 ± 2.28AB | 5.51 ± 2.24A | 6.67 ± 2.09AB |
| UHT | 4.05 ± 1.84C | 6.00 ± 1.99B | 6.11 ± 2.21B | 4.98 ± 2.26C | 3.52 ± 1.59C | 3.68 ± 2.01C | 5.77 ± 2.12B |
| UHT (XOS) | 5.60 ± 2.13AB | 6.43 ± 2.12B | 6.64 ± 2.14B | 6.00 ± 2.18B | 5.43 ± 2.38AB | 5.29 ± 1.91AB | 6.50 ± 1.81AB |
Note: In each column, values followed by different letters are statistically different accoridng to Duncan test (p < 0.05).
Strawberries are rich in phenolic pigments that are sensitive to thermal processing and oxidation. Therefore, the discoloring of the specimens could be from various factors, for example, oxidation of phenolic compounds by native enzymes, degradation of anthocyanins by enzymes or heat, or Maillard reactions during or after thermal processing and pH [47, 51]. In this study, ddiscoloration may be caused predominantly by the thermal degradation of anthocyanins and Maillard reactions during thermal processing and storage since most enzymatic activities should have been inhibited during thermal processing, and pH showed no significant change regardless of different treatments and storage conditions (Table 6).
Table 6.
Physicochemical characteristic evolution of strawberry samples during storage.
| Processing conditions | Storage (day) | pH |
Total soluble solids (TSS) |
Water activity |
|||
|---|---|---|---|---|---|---|---|
| 4 °C storage | 55 °C storage | 4 °C storage | 55 °C storage | 4 °C storage | 55 °C storage | ||
| Control | 1 | 3.44 ± 0.02B | - | 7.55 ± 0.08G | - | 0.9969 ± 0.0010AB | - |
| HTST | 1 | 3.50 ± 0.01A | 3.54 ± 0.03A | 7.48 ± 0.08H | 7.50 ± 0.30B | 0.9972 ± 0.0013A | 0.9923 ± 0.0018B |
| 15 | 3.43 ± 0.03B | 3.54 ± 0.01A | 7.55 ± 0.05G | 7.67 ± 0.25B | 0.9956 ± 0.0004CD | 0.9920 ± 0.0008B | |
| 36 | 3.53 ± 0.02A | 3.54 ± 0.02AB | 7.60 ± 0.14F | 7.47 ± 0.32B | 0.9942 ± 0.0005EF | 0.9934 ± 0.0013B | |
| HTST (XOS) | 1 | 3.51 ± 0.03A | 3.51 ± 0.02AB | 12.03 ± 0.30D | 12.37 ± 0.15A | 0.9938 ± 0.0012F | 0.9933 ± 0.0007B |
| 15 | 3.44 ± 0.03B | 3.53 ± 0.01AB | 12.17 ± 0.20C | 12.57 ± 0.21A | 0.9938 ± 0.0012F | 0.9917 ± 0.0017B | |
| 36 | 3.52 ± 0.02A | 3.50 ± 0.04B | 12.40 ± 0.09B | 12.40 ± 0.00A | 0.9918 ± 0.0007G | 0.9923 ± 0.0014B | |
| UHT | 1 | 3.50 ± 0.01A | 3.50 ± 0.04AB | 7.48 ± 0.19H | 7.77 ± 0.06B | 0.9973 ± 0.0005A | 0.9950 ± 0.0013AB |
| 15 | 3.43 ± 0.03B | 3.53 ± 0.01AB | 7.60 ± 0.00F | 7.60 ± 0.20B | 0.9962 ± 0.0008BC | 0.9971 ± 0.0021A | |
| 36 | 3.52 ± 0.01A | 3.51 ± 0.01AB | 7.67 ± 0.05E | 7.80 ± 0.17B | 0.9943 ± 0.0007EF | 0.9936 ± 0.0022B | |
| UHT (XOS) | 1 | 3.51 ± 0.01A | 3.52 ± 0.04AB | 12.38 ± 0.12B | 12.53 ± 0.25A | 0.9942 ± 0.0005EF | 0.9939 ± 0.0013B |
| 15 | 3.44 ± 0.02B | 3.52 ± 0.01AB | 12.40 ± 0.11B | 12.30 ± 0.17A | 0.9940 ± 0.0006DE | 0.9923 ± 0.0023B | |
| 36 | 3.52 ± 0.02A | 3.52 ± 0.01AB | 12.45 ± 0.18A | 12.60 ± 0.10A | 0.9921 ± 0.0008G | 0.9929 ± 0.0018B | |
Note: For each column, values followed by different letters indicates different groups according to the ANOVA and Duncan test (p < 0.05).
Based on the Duncan test, consumers could not identify differences in color between strawberry puree samples with or without XOS addition that have undergone the same heat treatment, which suggests the potential acceptability of XOS as a functional ingredient in strawberry puree. This phenomenon was also confirmed in previous findings, where the addition of XOS may not be distinguishable in terms of color expression at 5% w/w (Figure 2). Despite the fact that the TCD value was largely affected by XOS addition, the results from both visual observations and consumer evaluation indicated that customers could not distinguish the sample with XOS addition at 5% w/w.
3.2.4. pH, total soluble solids and water activity of strawberry puree
pH, total soluble solids, and water activity of all strawberry puree samples are shown in Table 6. The pH remained 3.4–3.6, regardless of the treatment applied or the storage conditions. As mentioned before, the addition of XOS significantly increased the TSS content and lowered water activity. Conversely, both thermal treatments (without XOS incorporation) decreased the TSS content (HTST: 7.48 ± 0.08; UHT: 7.48 ± 0.19) and increased water activity (HTST: 0.9972 ± 0.0013; UHT: 0.9973 ± 0.0005) as compared to fresh puree (TSS: 7.55 ± 0.08; aw: 0.9969 ± 0.001) (Table 6). TSS content reduction with thermal treatment was also reported by Cheng et al. (2014) where TSS decreased after heat pasteurization (90 °C, 60s) in strawberry samples [36]. This phenomenon may be due to the thermal and mechanical destruction of plant cell vacuoles during the sample processing. The destruction of vacuoles could potentially increase the water activity and decrease the TSS level by releasing more fluid into the sample (Table 6).
3.3. Effects of XOS incorporation and thermal treatment on sensorial properties of strawberry puree
The sensory study was fielded at the University of Massachusetts Amherst using untrained panelists recruited from campus (Male-42, Female-63, ages from 18-65). The present study revealed that 76% of the panelists failed to meet the DRI level of fruit and vegetables (USDA, 2015) (data not shown). Surprisingly, among the participants who failed to reach the DRI level, nearly 70% of the participants claimed to be fruit-drinks consumers (data not shown). The results of the 9-point hedonic sensory evaluation are shown in Table 7. The UHT-treated sample, without XOS incorporation, received the lowest rating in every sensory attribute, whereas fresh puree with XOS incorporation yielded the highest scores in most attributes except for appearance and aroma. The panel's preference ranked in the following order: fresh puree > HTST treated puree > UHT treated puree. They also preferred samples with XOS incorporation consistently among all treatments (Table 7). Thermal treatments, especially UHT-treatment, resulted in significant alterations in samples' appearance, color, aroma, flavor, and overall scores. Sweetness and texture were not significantly affected by thermal treatments according to the results of the sensory evaluation.
The sweetness of the untreated- and thermally-treated strawberry puree samples was significantly affected by the incorporation of XOS, due to the inherently sweet profile of XOS [4]. In addition, there was a close correlation between sweetness scores and overall scores of the samples (Table 7), suggesting that the overall liking of the product is largely affected by the sweetness of the sample. The unique sweet profile of XOS can constitute an asset since it would be possible to develop a food product with both health benefits as well as high consumer acceptance.
4. Conclusion
The present study investigated the possibility of using a fruit-based food vehicle, i.e. strawberry puree, to increase the consumption of beneficial compounds such as xylo-oligosaccharides (XOS). The results showed that the addition of XOS at 5% w/w could increase the nutritional value of strawberry puree as well as consumer acceptability of this product, without significantly compromising its quality attributes. Overall, several nutritional values (TPC, TFC, tannin content, and antioxidant activity) and the consistency of strawberry puree were decreased with different thermal processing and high temperature storage. Thermal treatment also affected the color expression of the samples. However, the Duncan test revealed that HTST-treated samples with XOS received the same score in overall liking, appearance, color, flavor, and texture as the fresh strawberry puree sample.
The result of this research could provide the industry with guidance on using xylo-oligosaccharides as a functional ingredient in strawberry products in terms of dosage and thermal and storage stability and consumer acceptance.
Declarations
Author contribution statement
Haochen Dai: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Caroline E. Leung: Performed the experiments; Wrote the paper.
Maria G. Corradini, Hang Xiao: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Amanda J. Kinchla: Analyzed and interpreted the data; Conceived and designed the experiments.
Funding statement
This work was supported by the Massachusetts Agricultural Experiment Station and the Food Science department of the University of Massachusetts Amherst (MAS00440).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The author would like to thank Dr. Corradini's, Dr. McClements', and Dr. Xiao's groups for analytical equipment support.
References
- 1.Kumar V., Satyanarayana T. Applicability of thermo-alkali-stable and cellulase-free xylanase from a novel thermo-halo-alkaliphilic bacillus halodurans in producing xylooligosaccharides. Biotechnol. Lett. 2011;33(11):2279. doi: 10.1007/s10529-011-0698-1. [DOI] [PubMed] [Google Scholar]
- 2.Courtin C.M., Swennen K., Verjans P., Delcour J.A. Heat and pH stability of prebiotic arabinoxylooligosaccharides, xylooligosaccharides and fructooligosaccharides. Food Chem. 2009;112(4):831–837. [Google Scholar]
- 3.Vázquez M.J., Alonso J.L., Domínguez H., Parajó J.C. Xylooligosaccharides: manufacture and applications. Trends Food Sci. Technol. 2000;11(11):387–393. [Google Scholar]
- 4.Kim M., Yoo S., Jung S., Park M., Hong J. Relative sweetness, sweetness quality, and temporal profile of xylooligosaccharides and Luo han guo (siraitia grosvenorii) extract. Food Sci. Biotechnol. 2015;24(3):965–973. [Google Scholar]
- 5.Hsu C., Liao J., Chung Y., Hsieh C., Chan Y. Xylooligosaccharides and fructooligosaccharides affect the intestinal microbiota and precancerous colonic lesion development in rats. J. Nutr. 2004;134(6):1523–1528. doi: 10.1093/jn/134.6.1523. [DOI] [PubMed] [Google Scholar]
- 6.Santos A., San Mauro M., Díaz D.M. Prebiotics and their long-term influence on the microbial populations of the mouse bowel. Food Microbiol. 2006;23(5):498–503. doi: 10.1016/j.fm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Lin D., Peters B., Sinha R., Goedert J.J., Hayes R., Ahn J. Association of dietary fiber intake and gut microbiota in healthy adults. J. Clin. Oncol. 2017;35(15_suppl) 1569-1569. [Google Scholar]
- 8.Stewart W. Bernard, Wild P. Christopher. 2014. World Health Organization/International Agency for Research on Cancer. World Cancer Report 2014. [Google Scholar]
- 9.Arnold M., Sierra M.S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 10.Platz E.A., Willett W.C., Colditz G.A., Rimm E.B., Spiegelman D., Giovannucci E. Proportion of colon cancer risk that might be preventable in a cohort of middle-aged US men. Cancer Causes Control. 2000;11(7):579–588. doi: 10.1023/a:1008999232442. [DOI] [PubMed] [Google Scholar]
- 11.Clemens R., Kranz S., Mobley A.R., Nicklas T.A., Raimondi M.P., Rodriguez J.C., Warshaw H. Filling America's fiber intake gap: summary of a roundtable to probe realistic solutions with a focus on grain-based foods. J. Nutr. 2012;142(7):1401S. doi: 10.3945/jn.112.160176. [DOI] [PubMed] [Google Scholar]
- 12.Lee-Kwan S.H., Moore L.V., Blanck H.M., Harris D.M., Galuska D. Disparities in state-specific adult fruit and vegetable consumption — United States, 2015. MMWR. Morbidity and Mortality Weekly Report. 2017;66(45):1241–1247. doi: 10.15585/mmwr.mm6645a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granato D., Branco G.F., Nazzaro F., Cruz A.G., Faria J.A.F. Functional foods and nondairy probiotic food development: trends, concepts, and products. Compr. Rev. Food Sci. Food Saf. 2010;9(3):292–302. doi: 10.1111/j.1541-4337.2010.00110.x. [DOI] [PubMed] [Google Scholar]
- 14.Menrad Klaus. Market and marketing of functional food in europe. J. Food Eng. 2003;56(2-3):181–188. [Google Scholar]
- 15.Son C.G. Progress of functional food market in korea and strategy of Korean medicine. J. Korean Med. 2014;35(1):68–74. [Google Scholar]
- 16.Bajwa U.A., Huma Nuzhat, Ehsan Babar, Jabbar Kashif, Khurrama A. Effect of different concentration of strawberry pulp on the properties of ice cream. Int. J. Agric. Biol. 2003;15:635–637. [Google Scholar]
- 17.Coyle, S., Morse, B., Flanyak, J., & Fox, C. (2003). U.S. Patent No. 6,528,102. Washington, DC: U.S. Patent and Trademark Office.
- 18.Langer, D. W., & Langer, N. (1988). U.S. Patent No. 4,737,367. Washington, DC: U.S. Patent and Trademark Office.
- 19.Amir A. 2019, June. U.S. Industry (NAICS) Report 31211C: Juice Production in the US. Retrieved from IBISWorld database. [Google Scholar]
- 20.Jones R. 2019, May. Infographic: the Most Popular Smoothie in North America [Re-post] Retrieved from infor.com. [Google Scholar]
- 21.Giampieri F., Forbes-Hernandez T.Y., Gasparrini M., Alvarez-Suarez J.M., Afrin S., Bompadre S., Battino M. Strawberry as a health promoter: an evidence based review. Foog Funct. 2015;6(5):1386–1398. doi: 10.1039/c5fo00147a. [DOI] [PubMed] [Google Scholar]
- 22.Food and Drug Administration/Center for Food Safety and Applied Nutrition . 2004. Guidance for Industry: Juice HACCP Hazards and Controls Guidance. first ed.: 5001 Campus Drive College Park, MD 20740: Michael E. Kashtock. [Google Scholar]
- 23.Campanella O.H., Peleg M. Squeezing flow viscometry for nonelastic semiliquid foods--theory and applications. Crit. Rev. Food Sci. Nutr. 2002;42(3):241–264. doi: 10.1080/10408690290825547. [DOI] [PubMed] [Google Scholar]
- 24.Suwonsichon T., Peleg M. Rheological characterisation of almost intact and stirred yogurt by imperfect squeezing flow viscometry. J. Sci. Food Agric. 1999;79(6):911–921. [Google Scholar]
- 25.Sulaiman A., Farid M., Silva F.V. Strawberry puree processed by thermal, high pressure, or power ultrasound: process energy requirements and quality modeling during storage. Food Sci. Technol. Int.= Ciencia Y Tecnologia De Los Alimentos Internacional. 2017;23(4):293–309. doi: 10.1177/1082013216685485. [DOI] [PubMed] [Google Scholar]
- 26.Han Y., Huang M., Li L., Cai X., Gao Z., Li F., Xiao H. Non-extractable polyphenols from cranberries: potential anti-inflammation and anti-colon-cancer agents. Food Funct. 2019;10(12):7714–7723. doi: 10.1039/c9fo01536a. [DOI] [PubMed] [Google Scholar]
- 27.Dewanto V., Wu X., Adom K.K., Liu R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002;50(10):3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- 28.Butler L.G., Price M.L., Brotherton J.E. Vanillin assay for proanthocyanidins (condensed tannins): modification of the solvent for estimation of the degree of polymerization. J. Agric. Food Chem. 1982;30(6):1087–1089. [Google Scholar]
- 29.Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559. [Google Scholar]
- 30.Cao G., Alessio H.M., Cutler R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993;14(3):303–311. doi: 10.1016/0891-5849(93)90027-r. [DOI] [PubMed] [Google Scholar]
- 31.Deliza R., MacFie H.J.H. 1996. The Generation of Sensory Expectation by External Cues and its Effect on Sensory Perception and Hedonic Ratings: A Review. [Google Scholar]
- 32.Knee M. Polysaccharide changes in cell walls of ripening apples. Phytochemistry. 1973;12(7):1543–1549. [Google Scholar]
- 33.Grigelmo-Miguel N., Martın-Belloso O. Influence of fruit dietary fibre addition on physical and sensorial properties of strawberry jams. J. Food Eng. 1999;41(1):13–21. [Google Scholar]
- 34.Adekunte A.O., Tiwari B.K., Cullen P.J., Scannell A.G.M., O’donnell C.P. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010;122(3):500–507. [Google Scholar]
- 35.Drlange . 1994. Color Review. Drlange Application Report No. 8.0 e. [Google Scholar]
- 36.Cheng X.F., Zhang M., Adhikari B. Changes in quality attributes of strawberry purees processed by power ultrasound or thermal treatments. Food Sci. Technol. Res. 2014;20(5):1033–1041. [Google Scholar]
- 37.Kafkas E., Koşar M., Paydaş S., Kafkas S., Başer K.H.C. Quality characteristics of strawberry genotypes at different maturation stages. Food Chem. 2007;100(3):1229–1236. [Google Scholar]
- 38.Osorio O., Martinez-Navarrete N., Moraga G., Carbonell J.V. Effect of thermal treatment on enzymatic activity and rheological and sensory properties of strawberry purees. Food Sci. Technol. Int. 2008;14(5_suppl):103–108. [Google Scholar]
- 39.Sturm K., Koron D., Stampar F. The composition of fruit of different strawberry varieties depending on maturity stage. Food Chem. 2003;83(3):417–422. [Google Scholar]
- 40.Holcroft D.M., Kader A.A. Controlled atmosphere-induced changes in pH and organic acid metabolism may affect color of stored strawberry fruit. Postharvest Biol. Technol. 1999;17(1):19–32. [Google Scholar]
- 41.Ayyappan P., Abirami A., Anbuvahini N.A., Tamil Kumaran P.S., Naresh M., Malathi D., Antony U. Physicochemical properties of cookies enriched with xylooligosaccharides. Food Sci. Technol. Int. 2016;22(5):420–428. doi: 10.1177/1082013215617567. [DOI] [PubMed] [Google Scholar]
- 42.NutraSource I. Ltd.'s Xylooligosaccharides; 2013. GRAS Notification for Shangdong Longlive Biotechnology. [Google Scholar]
- 43.Aaby K., Skrede G., Wrolstad R.E. Phenolic composition and antioxidant activities in flesh and achenes of strawberries (Fragaria ananassa) J. Agric. Food Chem. 2005;53(10):4032–4040. doi: 10.1021/jf048001o. [DOI] [PubMed] [Google Scholar]
- 44.Lin J.Y., Tang C.Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101(1):140–147. [Google Scholar]
- 45.Terefe N.S., Kleintschek T., Gamage T., Fanning K.J., Netzel G., Versteeg C., Netzel M. Comparative effects of thermal and high pressure processing on phenolic phytochemicals in different strawberry cultivars. Innovat. Food Sci. Emerg. Technol. 2013;19:57–65. [Google Scholar]
- 46.Yao Y., Ren G. Effect of thermal treatment on phenolic composition and antioxidant activities of two celery cultivars. LWT - Food Sci. Technol. 2011;44(1):181–185. [Google Scholar]
- 47.Cao X., Zhang Y., Zhang F., Wang Y., Yi J., Liao X. Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. J. Sci. Food Agric. 2011;91(5):877–885. doi: 10.1002/jsfa.4260. [DOI] [PubMed] [Google Scholar]
- 48.Keenan D.F., Brunton N.P., Gormley T.R., Butler F., Tiwari B.K., Patras A. Effect of thermal and high hydrostatic pressure processing on antioxidant activity and colour of fruit smoothies. Innovat. Food Sci. Emerg. Technol. 2010;11(4):551–556. [Google Scholar]
- 49.van der Sluis A.A., Dekker M., de Jager A., Jongen W.M. Activity and concentration of polyphenolic antioxidants in apple: effect of cultivar, harvest year, and storage conditions. J. Agric. Food Chem. 2001;49(8):3606–3613. doi: 10.1021/jf001493u. [DOI] [PubMed] [Google Scholar]
- 50.Atala E., Vásquez L., Speisky H., Lissi E., López-Alarcón C. Ascorbic acid contribution to ORAC values in berry extracts: an evaluation by the ORAC-pyrogallol red methodology. Food Chem. 2019;113(1):331–335. [Google Scholar]
- 51.Wesche-Ebeling P., Montgomery M.W. Strawberry polyphenoloxidase: its role in anthocyanin degradation. J. Food Sci. 1990;55(3):731–734. [Google Scholar]