Abstract
Polymeric nanoparticles prepared using high pressure homogenizer (HPH) present some unique challenges during manufacturing which can be better understood by application of quality by design (QbD) approaches. The present review highlights the ways to identify the critical material attributes which includes the anticancer drugs, polymers, surfactants, solvent system and dispersion system. A comprehensive understanding of the critical processing parameters like pressure and number of cycles during the working of HPH used in putting forward the critical quality attributes such as size, shape, surface charge or droplet stabilization. Such QbD approach will involve development of an effective control strategy for would ensure safe encapsulation of anticancer drugs for successful product development. Proper addressing of the issues related to scaling-up would lead to successful commercialization of the nano-sized formulations loaded with anticancer drugs.
Keywords: QbD, Polymeric nanoparticles, Anticancer drugs, High pressure homogenizer, Nanotechnology, Nanomaterials, Pharmaceutical science, Cancer research, Oncology
QbD; Polymeric nanoparticles; Anticancer drugs; High pressure homogenizer; Nanotechnology; Nanomaterials; Pharmaceutical science; Cancer research; Oncology
1. Introduction
Nanotechnology based research is the ability to operate on properties of materials at molecular level where the particle sizes are around 100 nm and this multidisciplinary technology is driven by innovation to enhance the quality of human life [1]. The role of nanotechnology in medicine has been able to allow beneficial interaction of the biomolecules for treating many diseases [2]. It's just not only the nanometre size which plays the determinant role in medicine but also the way the communication and interaction impact the therapeutic outcomes in terms of improved therapeutics [3].
Nanotechnology in pharmaceuticals has profoundly contributed in formulation development of differentiated products with enhanced therapeutic efficacies. The application of nanotechnology to develop formulations of anticancer drugs has led to an arena of cancer nanotherapeutics which has shown a tremendous exponential growth in the last two decades [4,5]. Unlike the other active molecules, the anticancer drugs have issues of being cytotoxic and hence necessitate the need to be safely encapsulated and have ability to reach their target sites [6]. The use of nanoparticles allows possibilities of all of these. In addition, nanoparticles offer distinctive features of loading poorly soluble anticancer drugs, protecting the active moiety from harsh in vivo environment, sustaining their release, modifying their biodistribution, targeting specific cells/tissues and enhancing the blood circulation time. Even though there are overwhelming number of research papers on development of nanoparticles for anticancer drugs not many have reached the market. This is due to poor scalability of the methods used. The application of quality by design (QbD) approach has led to development of standardized procedures which are formulation-driven for getting the optimized product [7]. The benefit of scaling-up such nanoparticles would improve the therapeutic outcome of not only the traditional drugs but also the next generation agents. Proper addressing of the issues related to scaling-up would lead to successful commercialization of the nano-sized formulations loaded with anticancer drugs [8,9,10].
In this context the present review highlights the series of obstacles faced during scaling up of nanoparticles and the feasibility to overcome them. Other advantages associated with use of QbD principles that may be listed include a better clarity and understanding of the processes/unit operations at every step [11]. It is a scientifically driven risk-based approach which assures an easier regulatory process as both the approval time and number of audits needed are reduced. The US FDA's initiative to bring a risk-based approach utilizing both the sound science and knowledge space to convey the concept of enhanced understanding of process and product in pharmaceuticals. The best part is the achievement of desired target profiles due to minimum changes due to real time release testing complimented with monitoring by the appropriate control strategy, overall consistent commercial manufacturing.
Due to the ability of polymeric nanoparticles to encapsulate, provide stealthing properties, dual loading and targeting by attaching ligands they are preferred over other novel drug delivery systems [12]. In addition, variety and development of new biodegradable polymers makes it more interesting to play with retention time and cellular uptake of the drug. Even after having so many advantages, very few products have been able to reach the market because of toxicity caused in clinical trials, not choosing an appropriate model in preclinical studies and failure in reproducing results on industrial scale. For developing a quality product of commercial acceptance, it is important to choose a right experimental model. Choosing a right experimental model will also reduce wastage of time during scale-up, will provide cost effective manufacturing, development of less time-consuming methods for and time-bound product launch [13].
The term homogenization is derived from the greek word homogenous (homos + genos); homos meaning same or even and genos meaning kind. Thus, Homogenization is a process for the formation of evenly distributed particles. This is usually done using an instrument called as homogenizer. In pharmaceutical industry, these homogenizers are used widely used for particle size reduction and formation of evenly distributed emulsion, dispersions or suspensions. The high energy generated in the process is responsible for the size reduction of particles and work by breakdown of larger particles into smaller particles. Different type of instruments providing such high energy include rotor-stator homogenizer, high power ultrasonicator, high shear homogenizer, high pressure homogenizer etc [14]. The HPH instrument is widely used in biotechnology, pharmaceutics, and food industry [15].
The success of any commercial sized batches lies in the ability to fulfil all quality parameters especially the quality control tests shown during lab scale. This is facilitated by systematically planned Critical Quality Attributes (CQAs) with appropriate design space [16].
CQAs by definition are the characteristic properties shown by the physical, chemical, biological, or microbiological which needs to be controlled within a range to ensure expected quality in the end product. However, the CQAs are just more than the analytical tests as they are the critical limits which when kept under appropriate limits will bring the needful results in product. In addition, the CQAs are arguably the most difficult step in the implementation of QbD. In this context, the present review highlights the ways to identify the critical processing steps during working of HPH to get the desired product quality which can be scaled up.
2. QbD approach
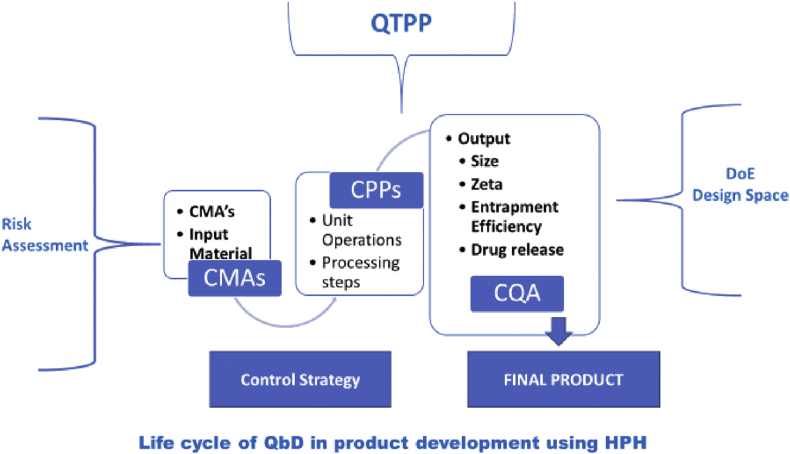
In a QbD approach setting up the Quality Target Product Profile – (QTPP) would be the first step in product development cycle to get the desired quality characteristics. This phase involves assigning well defined attributes to a given drug product. In QTPP, CQAs becomes a subset that defines few critical parameters that involves in development of product. The critical parameters that likely to be changed are based on the variation in raw materials and processes is depicted in Figure 1. This has to be observed and evaluated through the phase of product development to ensure that drug product remains within safe and effective levels. The factors that affect the drug product in terms of CQAs [17].
Figure 1.
Life cycle of QbD in product development using HPH.
For any quality product, the characteristics properties of the raw materials that are going to be introduced in the process technology plays a vital role. For example, the input materials need to be identified as Critical Material Attributes (CMAs). In-process material properties is considered as CQAs of one step process and this becomes CMAs for a downstream manufacturing process. The identification of Critical Process Parameters (CPPs) becomes a part of production process parameters and finally the risk assessment of the drug substance attributes to the development of the product.
3. Critical material attributes: identification and optimization
Critical Material Attributes (CMA), its identification and optimization in selection of anticancer drugs along with selection of excipients and emulsifying system for development of product is discussed in this section. For any product development, if we consider the critical elements of drug substance attributes as well as drug product critical quality attributes, then the solid-state form and particle size will be the main critical material attributes that effects the effectiveness of the final product. Identification and Optimization of these critical material attributes is important. In-depth knowledge about the material, its chemistry, mechanism of action, complexity involved at the molecular level need to be addressed during the optimization stage. The technology used for manufacturing of the dosage form along with the right selection of materials makes a huge impact on the product quality.
3.1. Anticancer drugs
Drugs that act on cancerous cells can be classified into four types: Cytotoxic drugs, vaccines endocrine (hormonal) therapy and small molecules/antibodies that are used in targeted therapies. During the selection of the drug candidate, formulator should consider the following [18].
-
i.
The drug candidate should possess potential dose range and the mechanism of action at the target site.
-
ii.
The compound's polymorphic form should be explained.
-
iii.
The scale-up possibility of the developed formulations needs to be looked into.
-
iv.
It should be a commercially sustainable product for the benefit of the human life.
-
v.
With novel approaches built -in the product, the developed product should show more effective therapies with safety and efficacy.
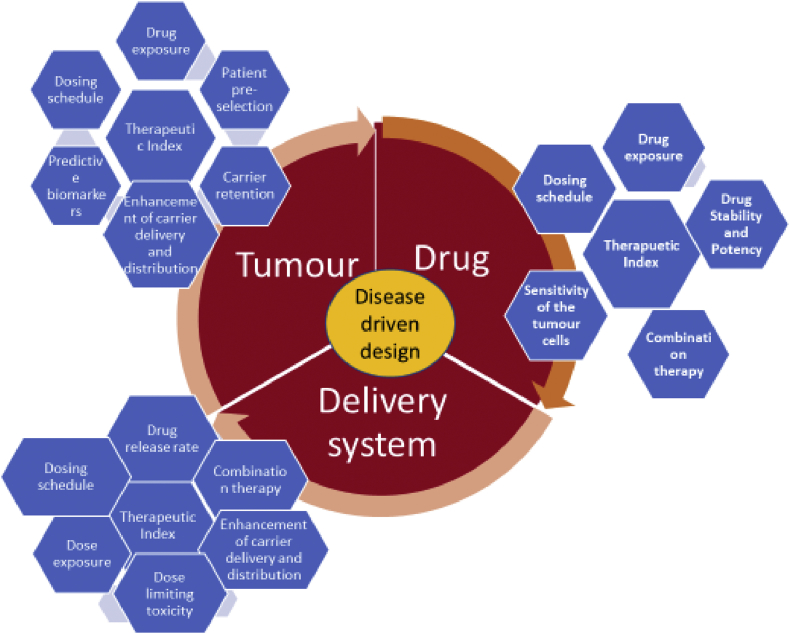
Figure 2 describes the parameters to be selected for developing an anticancer nano product for selecting a drug and delivery systems. The drug development process is based on two approaches, i.e. Structure and Target based approach. In case of the structure-based approach, the concept for drug design and its selection depends on how the drug structure interaction and its respective target receptors indicates the therapeutic effect. This can be interpreted by NMR and X-ray crystallography. Target based approach are identified as per their ability to bind to the respective target receptor indicated by the particular disease. Computer aided drug design (CADD) is used to study the probable interaction between the drug molecule and target receptors through this software.
Figure 2.
Parameters to be selected for developing an anticancer Nano product for selecting a Drug and delivery systems.
One more approach known as ligand-based drug designing using the QSAR i.e quantitative structure activity relationship (QSAR) mechanism is also explored by many researchers. In this approach, the elucidation of pharmacophore on bio-molecules tends to target receptor. This bind and modifies the biological progression to induce the disease state. Interaction of drug molecules with the genes need to be identified during targeting anticancer moiety. This study is known as pharmacogenomics. This will enhance the selection of right drug candidate and its target receptors. The information can be applied further for knowing the potency of that molecule for the target disease. Targets may be in terms of receptors, transporters, enzymes, as play a role in pathological response and role in signal transduction. In-silico models can be designed to study the target and validate the disease susceptibility. Knowledge of drug metabolizing enzymes, its polymorphic forms need to be cleared as to understand the drug stability with their genes. Few examples for metabolic enzymes are cytochrome P450 (CYP), aldehyde oxidase, monoamine oxidase (MAO), etc. Proteins that are adhered to and enable the movement of biomolecules are the membrane bound transport system that makes the drug candidate reach the target site [19]. The material attribute of an active should possess the ability to withstand the pressure involved in the high-pressure homogenization technology. During the manufacturing process, the degradation of ingredients may be observed due to the strong cavitation and shear forces that are generated and the intense energy the materials are subjected during the process. Certain molecules like insulin and enzymes are able to with stand the process pressure but may be sensitive to temperature or heat. Lipid layer may be lost due to the interference by adsorption of the lipids on the system walls. With reference to the advance complexity of the instrument the working and tearing of the system (erosion) of the homogenizer may lead to the contamination of the preparation mixtures.
The various factors that influence the particle size in formulation of polymeric nanoparticles and the success of the homogenization process are:
-
•
Uniformity in the initial product.
-
•
Design of the Homogenizer.
-
•
Ionic strength of the medium
-
•
Number of cycles in the process and with its pressure
-
•
Process temperature
-
•
Excipient content and composition especially polymers and lipid.
Controlling all these above factors will ensure the stability, potency and product integrity. The Figure 2, gives the parameters to be selected for developing an anticancer nano product for selecting a drug and delivery systems.
3.2. Excipients
In excipients, polymers play the most significant part in formulation development of polymeric nanoparticles. Researchers have now been able to synthesize polymers as per the need of delivery by polymerization, complexation or by modifying functional group. This concept has enhanced the target drug delivery and hence this has hallmarked the enhanced therapeutic efficacy. The selection of the polymers is primarily based on the solubility of the drug in the polymers since the higher the solvent capacity is the higher the drug loading potential.
Polymers as drug carriers in cancer chemotherapy:
Polymers have now become one of the essential parts in formulation development of carriers for cancer chemotherapy. Right from polymers characteristics of them being responsive to their ability to form conjugates with drugs. Nevertheless, their ability to provide sustained and controlled release of the entrapped drug for long periods enhances the therapeutic regimen. Moreover, the ability to entrap diverse molecules form both hydrophilic and hydrophobic drugs to proteins and peptides with tunable drug release.
Polymeric formulation can improve the delivery of anticancer drugs by following ways [20]:
-
1.
Increase the solubility of the hydrophobic drugs
-
2.
Reduce the excretion by lowering the renal filtration rate and hence the circulation period is prolonged.
-
3.
Premature degradation of gene or any kind of proteins, hence drug protection.
-
4.
Avoiding the drug into macrophage uptake and the reticuloendothelial system (RES) by masking, coating or shielding the drug delivery carrier (example use of PEG to shield nanoparticles).
-
5.
Coating or surface protection of the drug by shielding or masking the surface.
-
6.
Reduction of drug efflux by multidrug-resistant (MDR) cells.
-
7.
Passive targeting of drug delivery and explore the tumors structure. This permits enhanced permeability and retention (EPR) of nanoparticles of specific sizes which would be collected more in the tumor tissue compared to normal tissues.
-
8.
Active targeting of drug delivery to overexpress the tumour-associated antigens.
Polymeric nanoparticles are soft biomaterials that can be used in drug delivery because of their simplistic synthesis process and easy structural alterations to form a desired property to target deliver or surface modification to improve drug loading efficacy etc. Here bio-distribution, therapeutic efficacy can be enhanced since they are biocompatible and often biodegradable systems in colloidal systems with nanoscale dimensions [21,22]. Polymeric nanoparticles explored in anticancer product development are from various sources like natural origin such as chitosan, collagen, gelatin, dextran and water soluble polymers such as human serum albumin(HAS), lectins, poly(aminoacids), poly (ethylene glycols) etc. Synthetic polymers such as biodegradable like poly (lactic acid) (PLA), poly (glycolic acid) (PGA), poly(ε-caprolactone) (PCL), co-polymers poly (lactic-co-glycolic acid), N-(2-hydroxypropyl)-methacrylamide copolymer (HPMA) and poly (styrene-maleic anhydride) copolymer, polyamide-amine (PAMAM) dendrimers are also extensively used in cancer treatments. During the formulation development, the drugs can either be encapsulated or dispersed in the polymeric matrix system or drug can be attached to the polymer molecule for modified delivery the anticancer drugs. The drug release mechanism may follow diffusion through polymer matrix, surface or bulk erosion, swelling or stimuli response delivery. With the objective to provide protection from rapid clearance and enzymatic digestion, control release delivery of the actives using polymer mediated delivery systems are designed. These have shown huge potentials even in targeting the actives at site of action. For example: Polymeric nanoparticles, polymer micellar systems, polymer –drug conjugates and nanoscale hydrogels to name few. Ren et al. developed cellulose nanoparticles with microcrystalline cellulose by acid hydrolysis reaction using HPH [23]. In this study, they observed the quality of nanoparticles with the effect on various types of acid in the reaction, the acid – microcrystalline (MCC) ratio, reaction time and the cycles used in the HPH process with the effect of various pressure. The impact of all these parameters were studied on the morphology and thermal stability of nanoparticles. They concluded that the concentrated acid in the reaction produced rod shaped particles of size 10nm diameter and length of 150–200nm. With increase in number of homogenization cycles, there was a reduction in nanocrystal size that was formed. Gupta S et al. investigated various surfactant system for solid lipid nanoparticles (SLN) system using HPH and observed that poloxamer 188 (Pluronic F68) gave minimum particle size and polydispersity index with maximum entrapment efficiency [24].
3.3. Surfactants/solubilizers
The major component for stable nanoemulsions is the selection of surfactant or solubiliser based on their hydrophilic lipophilic balance (HLB) value. It acts as driving force as it has a range that is required to develop a formulation with oil or water as continuous phase or one can also go with choice of mixed surfactants that gives an ideal blend for stabilizing the formulated system. Higher the HLB value more hydrophilic the system. Chong et al., investigated the effect of mixed surfactants ranging with HLB value 10–15, and they observed reduction in droplet size from 96.47-130.90nm to 88.95–112.20nm while using 5 wt% and 10 wt% of surfactants respectively [25]. The stability studies were also performed on these formulated emulsions and they observed that HLB values with 13 and 15 showed unstable emulsion over 35days of storage as droplet size changed significantly. The difference in the head group sizes of surfactants influences the synergistic effects of mixed surfactants [26]. Studies have shown that the differences in larger group size on the head portion of the surfactant influence to larger synergistic effects, since small molecule surfactants can enclose better at the interface of large surfactant between oil and water phase [26]. For example, use of span and tweens. Dispersity and solubility in dispersion medium is enhanced by using mixed surfactants and this can strengthen the interfacial film in between the two phases. Chong WT et al., investigated the effect of mixed surfactants over the droplet size and showed whenever surfactant concentration is more than 7.5 wt% and glycerol concentration as a solvent is more than 20 wt% in aqueous phase, a significant reduction in droplet size of nanoemulsion is achieved.
3.4. Solvent system
HPH avoids the selection of organic solvents, hence the regulatory aspects of residual solvents and safety concern with respect to the product profile is screened off. Glycerol when used as co-solvent in the formulation system it can increase the viscosity of aqueous phase. Thus, an addition of co-solvent can reduce the droplet size due to its solubility in dispersion medium and hence this dispersion medium reduces its viscosity and gives a smaller droplet sized system [27]. In case if the co-solvent has both the hydrophilic and hydrophobic nature, they can diffuse into the monolayer of surfactant that will lead to change in optimum curvature, flexibility of surfactant and interfacial tension. Vivek et al. investigated the application of glyceryl monostearate with various concentration and its effect on homogenization pressure of solid lipid nanoparticles prepared [28]. They found that the pressure ranges from 5000psi -10000psi gives a reduced nanosize particle range and could be observed due to the cavitational forces developed in the homogenization stage resulting in reduction of lipid droplets to the nanosize range.
3.5. Selection of an emulsification/dispersion system
In HPH process, and a good emulsification system the achievable droplet sizes depend on the flow pattern of the disruption unit that influences the droplet to break. This disruption units depends on the flow guidance into radial diffusers, counterjet dispergators and axial nozzle aggregates [29]. The energy density equals the pressure drop in the disruption unit. The increasing pressure difference or increasing energy density will give decreased droplet sized diameter unless unless coalescence occur. Juttulapa et al. investigated the various types of pectin type that can be used as an emulsifying agent for HPH process [30]. The study indicated that the droplet size of emulsion was dependent on methoxy content of pectin. Lower droplet size with good stability was observed with high methoxy content, this was due to good emulsifying property.
4. Critical processing parameters in working of High-Pressure Homogenizer (HPH)
4.1. Working principle of HPH
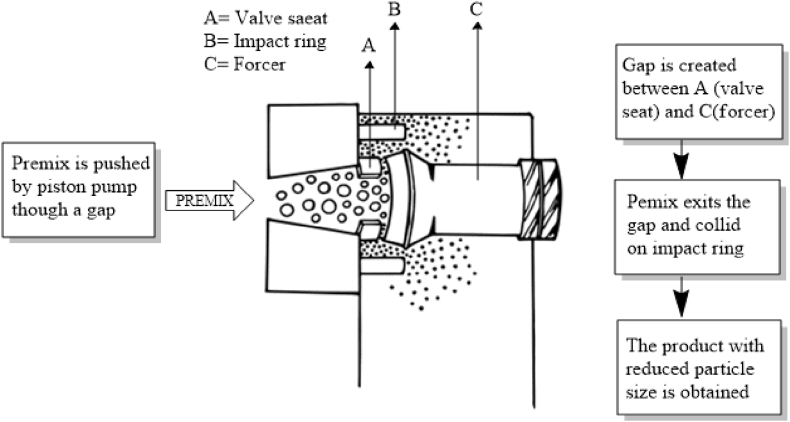
For the preparation of nanoparticles using HPH, a premix used could be coarse emulsion, dispersion or suspension. They are prepared by dispersing one phase of liquid (dispersed phase) in other phase (dispersion medium) either with the help of mechanical shear or by providing physical energy. In case of suspension and dispersions with particles of larger size, the premix could be pre-processed with high energy systems like ultra-sonification, rotor stator, high shear homogenizer or membrane emulsifying system before subjecting to high pressure homogenizer. Pre-processing of the premix is necessary as large particles may clog the gap. The working principle of an HPH is depicted in Figure 3.
Figure 3.
Schematic illustration of a homogenization process (adapted from Ref. [31]).
As described, in HPH this premix is forced from inlet chamber to outlet chamber through a narrow gap under high pressure. The homogenized product which collected can be recirculated again into the feeder making the process of homogenization continuous for obtaining further size reduction. The final product which is obtained is then is lyophilized. The cavitation forces developed during this process are responsible for breaking the polymeric particles into nanoparticles.
The delivery of anticancer drugs based on nanoparticles has been extensively investigated. However, a very important process for research and development in any pharmaceutical industry is the scaling of nanoparticles formulation techniques in order to produce large batches for preclinical and clinical trials. For preparing batches on large scales it is important to optimize certain process parameters and material parameter which has direct effect on final product. Such parameters are called as critical parameters.
4.2. Homogenization pressure
Homogenization pressure is pressure that forces the liquid to pass through a narrow gap and ranges between (10–500 MPa). The droplets of the liquid experience shear, cavitation and turbulence which are responsible for breakdown into smaller droplets. Droplets are broken only when a sufficient amount of shear to overcome the Laplace's pressure is experienced.
Increase in pressure creates a higher pressure drop that would easily overcome the Laplace's pressure and lead to increased size reduction.
In case of paclitaxel HSA nanoparticles, reduction in size from 337.7 ± 14.8 upto 248.2 ± 6.6 nm at 15,000 psi and 254.7 ± 15.5 nm at 20,000 psi was achieved. No noticeable difference in size was attained at 15000 psi and 20000 psi [32].
Yadav and Sawant were able to achieve size of approximately 98 nm with 10,000 psi [33]. Kushwah et al. reported particle size of approximately 147.2 nm for gemcitabine- Bovine Serum Albumin conjugate at 20,000 psi [34].
Thus, increase in the pressure leads to decrease in mean diameter size up to certain extent which depends on the method of formulation of emulsion, combination of polymers as well as the quantity of drug used. Variation in size will be seen with varying concentration of drug, solvent used, type of polymers and number of times the premix is recirculated.
4.3. Number of cycles
One cycle is said to be completed after collected premix is again recirculated through a narrow gap under specified conditions of pressure. as the liquid experiences all the shear, cavitation and turbulence all over again the size of the droplets is also reduced further. Thus, number of cycles/recirculation's shows variation in direct relation with mean diameter size at constant pressure [35]. The time required to complete one cycle is dependent on the viscosity of the liquid.
In case of paclitaxel Huan Serum Albumin nanoparticles, after evaluating the effect of 6, 9, 12 and 15 cycles, 12 cycles were applied for further process. 12 cycles were selected as, there was no much difference in mean diameter size with further increasing the number of cycles. At the same time effect of solvent used and the quantity of drug added cannot be ignored which influenced the particle size [32]. Thus, increasing the number of cycles will reduce the size of droplets to some extent depending on the different material attributes of the emulsion under constant pressure. Increasing the number of cycles reduces the size of the droplets further.
Number of cycles can also affect the encapsulation efficiency. In case of PLGA paclitaxel nanoparticles, as the number of cycles were increased the reduced encapsulation efficiency was reported [36].
5. Critical quality attributes
The Critical Quality Attributes (CQA) would be beneficial in optimization of mean droplet diameter, polydispersity index, shape, surface charge and the droplet stabilization are some of the attributes that need to be understood.
5.1. Mean particle size/mean droplet size
In general, there are two basic methods of defining particle size. The first method is to inspect the particles and make actual measurements of their dimensions. Microscopic techniques, for example, measure many dimensional parameters from particle images. The second method utilizes the relationship between particle behaviour and its size. This often implies an assumption of equivalent spherical size developed using a size-dependent property of the particle and relating it to a linear dimension [37].
Mean particle Size of polymeric nanoparticles is expected to be in between 10-100nm. nanoparticles of size less than 20 nm have a deep penetration into the perivascular region of the tumor cell under hydraulic pressure [38].
Other than the size, the geometry and shape also have a significant role in transport of nanoparticles across intestinal cells [39,40].
The Mean particle size can be measured either by using dynamic light dispersion (DLS) or by using the Nanoparticle Tracking System (NTA). DLS is advantageous for evaluating a 1nm-3000 nm monodisperse sample solution in about 2–5 min with reproducible results. While NTA is suitable for evaluation of monodisperse as well as polydisperse solutions showing accurate size distribution. But, NTA is more time consuming with detecting size range up to 30nm–1000nm and requires skill full sample handling [41]. Presence of contaminant i.e. thread or dust particles shows drastic variation in results obtained by DLS which is not the case with size detection by NTA. Even though sample preparation by DLS is not as tricky as NTA, a monodisperse sample (single particle suspension) should be prepared so that particles are not agglomerating.
Particle scattering diffusometry (PSD) is one of the ways of detecting particle size, diffusion pattern and of nanoparticles. Using this method particles of size less than 30 nm can also be measured [42]. The method makes use of images captured by camera depending on the Brownian motion of suspended particles. PSD provides data regarding size, heterogeneity, surface modifications done on nanoparticles with the number of layer present on it. PSD is less time consuming and there is no requirement of skilled labour for sample handling [43,44].
Particles of size lass than 100nm are able to pass through leaky vasculature of tumor because of enhanced permeability and retention (EPR) effect and it becomes easier to passively target the tumour cells [45].
5.2. Polydispersity index (PDI)
The PDI is defined as the standard deviation (σ) of the particle diameter distribution divided by the mean particle diameter (d) as shown in Eq. (1).
| (1) |
The smaller is the PDI more is the monodispersity of the samples. The PDI is an estimation of the uniformity of the samples [46].
5.3. Shape
Bulk properties of the particles like size and shape affect the performance of the system [47,48,49]. These surface properties can be varied with the choice of method adopted for nanoparticles preparation and their critical parameters involved in the method. It is reported that nanoparticles are prepared in various shapes like rod, disc, hollow etc., impacts the in-vitro and in-vivo release of the drug from its system. Major factors like cell uptake of nanoparticles, circulation time, tissue penetration and distribution, target binding to cells or tissues, intracellular trafficking behaviour and ability to overcome biological barriers depends on the shape of nanoparticles [50]. Along with the shape, shape related parameters like aspect ratio or edge geometry affects the biological interactions with respect to transport characteristics, cell particle interactions that in turn influence the release kinetics [51].
But, for polymeric nanoparticles, spherical shape is the ideal requirement to meet the quality criteria of finished product. Compared with nanospheres, oblong-shaped nanoparticles are able to form a greater number of multivalent interactions [52]. Shape of the nanoparticles can be controlled by lithography based methods, membrane stretching methods [53].
Nanoparticle size suggestively influences the interactions with biological targets and the ability to cross various cellular barriers [54]. Among the different shapes, the spherical particles are the ones showing maximum therapeutic applications [55].
5.4. Surface charge
Cell membrane possesses a slight negative charge and cell uptake is driven by electrostatic attractions [56]. This electrostatic attraction between membranes and nanoparticles that are positively charged favours adhesion to the surface of the cell leading to absorption [57]. The surface load induces the reconstruction of lipid bilayers for larger nanoparticles (4–20 nm) [58]. The surface charge can be tailored by appropriate selection of polymer and surfactant combination so as to suit the delivery system.
The interactions of the nanoparticles with cancer cell membranes is highly dependent on the surface charge. The cationic nanoparticles can enter the cell membranes. Whereas, the anionic ones are not able to penetrate the lipid membrane. However, the anionic nanoparticles give them different functions such as ability to survive the harsh conditions of pH [59,60].
5.5. Droplet stabilization
For the formation of stable emulsions and preventing the coalescence, flocculation and Ostwald ripening of the particles various stabilizing agents are used [61,62]. In case of polymeric nanoparticles non-ionic polymers like PVA and Pluronics are the commonly used stabilizing agents. They act by decreasing the interfacial tension and thereby stabilizing the emulsion. HLB value becomes a criterion for selection of stabilizing agent.
5.6. Drug loading and entrapment efficiency
Anti-cancer drugs should be loaded efficiently so that both the drug loading and entrapment efficiency is successful in reaching the optimized values [63].
It is generally understood that the entrapment efficiency (EE%) of drugs in nanoparticle is a measure of the total drug added minus the free or the non-entrapped drug over the total drug added [30,64,65,66].
In nano-formulations, two critical parameters are drug loading content and drug loading efficiency. Drug loading content defines the mass ratio of drugs in given nanoformulations whereas drug loading efficiency defines the exact amount of drug in the feed during the process of nanoformulation and are given in Eqs. (2) and (3).
| Drug loading (% w/w) = (Mass of the drug in nanoformulation)/(initial mass of the nanoformulation)∗100 | (2) |
| Drug loading efficiency (% w/w) = (Mass of the drug in nanoformulation)/(Mass of drug in feed)∗100 | (3) |
With respect to drug loading it depends on the physicochemical properties and the structure of the carrier material, and efficiency is depended on the mechanism of loading, molecular weight of the drug/s in the feed as well as other process variables. Hence one has to optimise a process with high loading efficiency in order to get high drug loading content. This can be achieved by crystallization and covalent/co-ordinate bonds rather than physical and electrostatic adsorption wherein the drug loading capacity is low [67,68].
6. Development of control strategy
In pharmaceuticals, a control strategy may be defined as the efficient materials and process controls set to ensure consistent quality of the finished product right from the scale up to commercial production. Hence, every process or a procedure will have a set parameter which would be fixed along with appropriate use of materials. The appropriate set of input material controls and process controls is confirmed by getting the desired critical quality attributes (CQAs). The control strategy can be developed via several iterations as the level of process understanding increases during the product life cycle.
Designing a control strategy involves ensuring various material attributes and critical quality attributes are within the set limits as discussed in above sections.
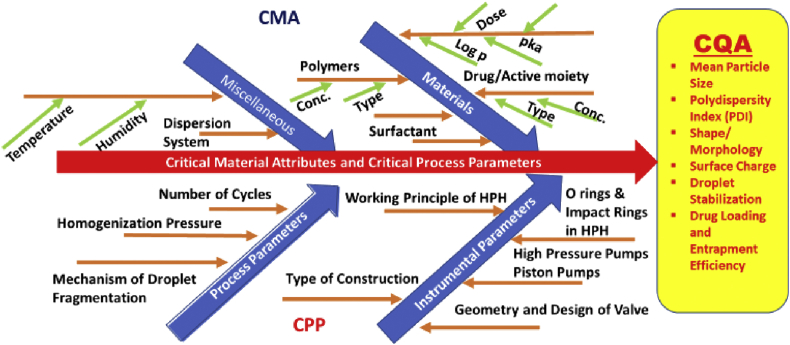
Correct implementation of the control strategy would be after appropriate setting up of the design space and completion of all risk assessment parameters. The risk assessment can be effectively done by using the Ishikawa Fishbone diagram. The Ishikawa diagram is drawn to understand cause-and-effect of a process that would be useful in tracing down the reasons for failures or variations. Figure 4 illustrates the Ishikawa diagram for mapping of CMA and CPP to CQA in formulation of polymeric nanoparticles using HPH. These controls are suited for both during and at the end of production using the HPH. The possibility of having a well-defined control strategy can be best evaluated using an online monitoring system which can continuously check the product quality parameters. An effective control strategy would reduce the need for end-product testing and very well implement the desired real-time testing methods.
Figure 4.
Mapping of CMA and CPP to CQA in High-Pressure Homogenizer (HPH) for formulation of Polymeric Nanoparticles using Ishikawa Diagram.
7. Some examples of anticancer drugs loaded in nanoparticles using HPH
7.1. Doxorubicin
Doxorubicin is very old anticancer drug which acts by inhibiting topoisomerase 2 necessary for growth of cancerous cells. It is used in treating many cancers either alone or in combination with other chemotherapeutic agents.
In case of doxorubicin-HSA (cationic and mannose modified) nanoparticles, a homogenized (at 10,000 rpm for 2 min using a WiseTis HG15D homogenizer) primary emulsion was fed to high pressure homogenizer (Avestin B15) at 20000 psi and 9 cycles. The solvents used as organic phase were chloroform and ethanol. After filtering this nanoparticle through 200nm pore size they were able to achieve particle size of 80.3 ± 4.2, 198.1 ± 6.7, 88.5 ± 3.8, and 90.5 ± 3.1 nm for HSA NP, c-HSA NP, m-HSA NP and c/m-HSA NP preparation, respectively [69].
Doxorubicin -PLGA and PLGA-cyanin 5.5 nanoparticles were prepared by double emulsion technique, first emulsion containing dichloromethane was processed under ultra turrax and then after addition of aqueous solution of PVA in phosphate buffer saline secondary emulsion as processed through HPH at 1000 bar. The final homogenized product was they were able to achieve approx. 120, 114 and 143 for dox-PLGA, dox-PLGAcyanin 5.5 and PLGA-dil respectively [70].
Dox-PLGA nanoparticles were produced by double emulsion and solvent evaporation method. Primary emulsion (dox in 0.001n HCl and PLGA in DCM) was processed through ultra turrax (23,000–24,000 rpm, 5 min) and double emulsion obtained after addition PVA in PBS solution was again homogenized in Ultra Turrax T-25 (23,000–24,000 rpm, 20 min) then passed through HPH 10000 psi and 3 cycles. They were able to achieve particles with mean hydrodynamic diameter of approximately 94 nm [71].
Thus, from the work performed in above three cases it can be understood that with the change of polymer type change in particles was observed. And the pre-processing of premix with ultraturrax has further led to size reduction.
7.2. Paclitaxel
Paclitaxel, a plant alkaloid and sold under the brand name taxol has shown the effectiveness in various types of cancer. Researchers have worked a lot on the formulation development of polymeric nanoparticles. In case of paclitaxel HSA nanoparticles, reduction in size from ~337.7 upto ~248.2 nm at 15,000 psi and 254.7 ± 15.5 nm at 20,000 psi was achieved. No noticeable difference in size was attained at 15000 psi and 20000 psi. Thus, the limiting effect of pressure on the size reduction of particles can be observed. At the same time effect of solvent used and the quantity of drug added cannot be ignored which influenced the particle size. Paclitaxel (PTX-NE) and PTX hyaluronan coated (PTX-HNE) nanoemulsion prepared by using suitable oil phase and aqueous phase was processed through HPH at 15000 psi seven times. They were able to achieve approximately 52nm and 72 nm for PTX-NE and PTX-HNE respectively [72].
7.3. Etoposide
Etoposide is an antineoplastic agent used as first line chemotherapy drug for lung cancer, especially for small cell lung cancer may be alone or with other antineoplastic agents. Yadav and Sawant have shown the effectiveness of homogenization pressure and cycles on reduction of particle size. Their study reports that homogenization pressure is more influential or effective than the number of cycles for reducing the particle size [73]. Here, they showed the size of polymeric nanoparticles of etoposide with PLGA-mPEG and Pluronic was 94.027 ± 3.4 nm and 148.07 ± 2.1 nm respectively using different process variables.
The Table 1 explains effect of variation in polymers, homogenization pressure on the particle size of nanoparticles. After obtaining emulsion with evenly distributed nanosized droplets it can be easily converted into powder form by lyophilization technique. Use of different HPH parameters can help getting the desired particle size. It is also seen that type of solvent system along with aqueous: non-aqueous ratio plays significant role in deciding the particle size of the formed nanoparticles.
Table 1.
Formulation of Polymeric Nanoparticles loaded with Anticancer.
| Drug loaded polymeric nanoparticles | Aqueous phase | Organic phase | Homogenization pressure and recirculation's | Instrument | Size obtained | Reference |
|---|---|---|---|---|---|---|
| TRAIL- PTX [(TNF related apoptosis-inducing ligand)Paclitaxel] | HSA+ TRAIL+ Deionized water and PBS 7.4 [Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL/PTX HSA-NP)] |
PTX+ Chloroform+ Ethanol [PTX - Paclitaxel ] |
20000 psi and 9 cycles | EmulsiFlex-B15 | 0.2%, 1.0%, and 2.0% TRAIL/PTX HSA-NPs was 160 ± 12.2nm, 172 ± 6.1nm, 180 ± 7.7nm, and 236 ± 5.8 nm, respectively |
[74] |
| Ceramide – chitosan PTX nanoparticles [PTX- Paclitaxel] | CS-CE in acetic acid solution [Ceramide-Chitosan (CS-CE)] | PTX + DCM [Paclitaxel + Dichloromethane] |
3 bar and 10 cycles | EmulsiFlex-B15 | 305.83± 16.27 nm |
[75] |
| Doxorubicin and paclitaxel co-bound lactosylated albumin (Lac-BSA) nanoparticles | BSA and Lac-BSA in de-ionized water. (Bovine serum albumin and lactosylated albumin) |
DOX and PTX in chloroform ethanol (9:1) | 20000 psi and 9 cycles | EmulsiFlex-B15 | DOX-PTX BSA = 109.2nm np DOX-PTX Lac-BSA = 130–148 nm |
[76] |
| Paclitaxel and curcumin co-bound nanoparticls (PTX- CCM) | BSA in deionized water (Bovine serum albumin) | PTX and curcumin in Chloroform: ethanol ratio of 9:1 | 20,000psi and 9 cycles | EmulsiFlex-B15 | 234.4nm | [77] |
| Etoposide –BSA (Bovine serum albumin) | BSA + distilled water | EPEG in TCM (Etoposide in Trichloromethane) | After homogenizing in (high-speed cutting of 12,000 rpm for 3 min) then processed through 1500 bar and 15 cycles |
DIAX900 homogenizer | Before lyophilization 190.2 ± 7.3nm After lyophilization 182.3 ± 8.9nm |
[78] |
| Gemcitabine-HSA-NP [Gemcitabine-loaded human serum albumin nanoparticle] | HSA + Pure water |
Gemcitabin + Chloroform Saturated with pure water |
20,000 psi 9 cycles | Nano DeBEE; BEE International | Approximately 150–175 nm | [79] |
| Concurrent delivery of tocotrienols and simvastatin for adenocarcinoma | Primary and secondary emulsifiers in deionized water + glycerol | α-Tocopherol + medium chain triglyceride (70/30 ratio by weight) and simvastatin | After using ultraturrax for 2min Passed through HPH for 25000 psi and 25 cycles |
EmulsiFlex®-C3, Avestin Inc. | Approx. 224nm | [80] |
| 10-hydroxycamptothecin loaded glycyrrhizic acid-conjugated BSA (10-HCPT) | GA-BSA in deionized water [Glycyrrhizic acid-conjugated bovine serum albumin (GL-BSA)] | 10-hydroxycamptothecin in chloroform | 800 bar and 7cycles | AH-100D ultra-high-pressure nano homogenizer | ~157.5nm | [81] |
| Docetaxel + gemcitabine self-assembled albumin nanoparticles | Anacardic acid-gemcitabine-albumin conjugate in water[AA- anacardic acid] | Docetaxel in hanol:chloroform(1:1) ratio | 20,000 psi and 10 cycles | High pressure homogenizer | ~163 ± 8 nm | [82] |
8. Concluding remarks
Use of QbD approach in product design and manufacturing will enhance the patient compliance as it meets the quality of the product as well as the ease of manufacturing will help the pharmaceutical industry to abide with the various regulatory guidance. This also help pharmaceutical industries to widen their innovations in novel technology. For example, QbD approach used in formulation development and scale-up for polymeric nanoparticles using HPH. Advances in HPH technology and its applications have no doubt widen the scope and hence applying QbD approach will definitely benefit the pharma companies. For example, the orifices and valves of the HPH can be geometrically modified in order to enhance the energy efficiency of the technology process to yield a good quality product. These innovations in the technology will help to enhance its process variables that can be well planned during the formulation development stage that would not only provide better clarity over the quality target product profile (QTPP) but also be exploited for various pharmaceutical applications. QbD approach can be used for both manufacturing and scale up of polymeric nanoparticles using high pressure homogenization (HPH) by identifying the critical processing steps and suitability of characterization that confirms the reproducibility of the product. Formulations with anticancer drugs needs special attention with respect to many areas, among which processing parameters during formulation development would play an important role. In conclusion, the application of QbD approach would lead to successful commercialization of nano-formulations with proper scale-up of nanoparticles ensuring proper control over all critical parameters.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Doran J., Ryan G. Does nanotechnology research generate an innovation premium over other types of research? Evidence from Ireland. Technol. Soc. 2019;59:101183. [Google Scholar]
- 2.Mukherjee A., Bhattacharyya S. Biotechnology Business-Concept to Delivery. Springer; Cham: 2020. Nanotechnology in medicine; pp. 57–64. 2020. [Google Scholar]
- 3.Soni G., Yadav K.S. Modeling, Methodologies and Tools for Molecular and Nano-Scale Communications. Springer; Cham: 2017. Communication of drug loaded nanogels with cancer cell receptors for targeted delivery; pp. 503–515. [Google Scholar]
- 4.Utreja P., Verma S., Rahman M., Kumar L. Use of nanoparticles in medicine. Curr. Biochem. Eng. 2020;6(1):7–24. [Google Scholar]
- 5.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33(9):941. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Canc. 2017;17(1):20. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soni G., Yadav K.S., Gupta M.K. QbD based approach for formulation development of spray dried microparticles of erlotinib hydrochloride for sustained release. J. Drug Deliv. Sci. Technol. 2020:101684. [Google Scholar]
- 8.Gad A., Kydd J., Piel B., Rai P. Targeting cancer using polymeric nanoparticle mediated combination chemotherapy. Int. J. Nanomed. Nanosurg. 2016;2(3) doi: 10.16966/2470-3206.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doppalapudi S., Jain A., Domb A.J., Khan W. Biodegradable polymers for targeted delivery of anti-cancer drugs. Expet Opin. Drug Deliv. 2016;13(6):891–909. doi: 10.1517/17425247.2016.1156671. [DOI] [PubMed] [Google Scholar]
- 10.Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14(2):282–295. doi: 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soni G., Yadav K.S., Gupta M.K. Design of experiments (DoE) approach to optimize the sustained release microparticles of gefitinib. Curr. Drug Deliv. 2019;16(4):364–374. doi: 10.2174/1567201816666181227114109. [DOI] [PubMed] [Google Scholar]
- 12.Muthu M.S., Wilson B. Challenges posed by the scale-up-up of nanomedicines. Nanomedicine (Lond.) 2012;7(3):307–309. doi: 10.2217/nnm.12.3. [DOI] [PubMed] [Google Scholar]
- 13.Schultz S., Wagner G., Urban K., Ulrich J. High-pressure homogenization as a process for emulsion formation. Chem. Eng. Technol. 2004;27(4):361–368. [Google Scholar]
- 14.Jafari S.M., Assadpoor E., He Y., Bhandari B. Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocolloids. 2008;22(7):1191–1202. [Google Scholar]
- 15.Stang M., Schuchmann H., Schubert H. Emulsification in high-pressure homogenizers. Eng. Life Sci. 2001;1(4):151–157. [Google Scholar]
- 16.Rathore A.S., Winkle H. Quality by design for biopharmaceuticals. Nat. Biotechnol. 2009;27(1):26. doi: 10.1038/nbt0109-26. [DOI] [PubMed] [Google Scholar]
- 17.Guideline I.C.H. Pharmaceutical development Q8. Curr. Step. 2005 Nov;4:11. [Google Scholar]
- 18.Hawthorn J., Redmond K. Oncology community by AstraZeneca; 2006. A Guide to Cancer Drug Development and Regulation. [Google Scholar]
- 19.Incecayir T., Sun J., Tsume Y., Xu H., Gose T., Nakanishi T., Tamai I., Hilfinger J., Lipka E., Amidon G.L. Carrier-mediated prodrug uptake to improve the oral bioavailability of polar drugs: an application to an oseltamivir analogue. J. Pharm. Sci. 2016;105(2):925–934. doi: 10.1016/j.xphs.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senapati S., Mahanta A.K., Kumar S., Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct.Target Ther. 2018;3(1):7. doi: 10.1038/s41392-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee E.S., Shin J.M., Son S., Ko H., Um W., Song S.H., Lee J.A., Park J.H. Recent advances in polymeric nanomedicines for cancer immunotherapy. Adv. Healthc. Mater. 2019;8(4):1801320. doi: 10.1002/adhm.201801320. [DOI] [PubMed] [Google Scholar]
- 22.Choi Y., Lim S., Yoon H.Y., Kim B.S., Kwon I.C., Kim K. Tumor-targeting glycol chitosan nanocarriers: overcoming the challenges posed by chemotherapeutics. Expet Opin. Drug Deliv. 2019;16(8):835–846. doi: 10.1080/17425247.2019.1648426. [DOI] [PubMed] [Google Scholar]
- 23.Ren S., Sun X., Lei T., Wu Q. The effect of chemical and high-pressure homogenization treatment conditions on the morphology of cellulose nanoparticles. J. Nanomater. 2014;(168):1–12. 2014. [Google Scholar]
- 24.Gupta S., Kesarla R., Chotai N., Misra A., Omri A. Systematic approach for the formulation and optimization of solid lipid nanoparticles of efavirenz by high pressure homogenization using design of experiments for brain targeting and enhanced bioavailability. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/5984014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong W.T., Tan C.P., Cheah Y.K., Lajis A.F., Dian N.L., Kanagaratnam S., Lai O.M. Optimization of process parameters in preparation of tocotrienol-rich red palm oil-based nanoemulsion stabilized by Tween80-Span 80 using response surface methodology. PloS One. 2014;13(8) doi: 10.1371/journal.pone.0202771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu Z., Liu M., Xu J., Wang Q., Fan Z. Stabilization of water-in-octane nano-emulsion. Part I: stabilized by mixed surfactant systems. Fuel. 2010;89(10):2838–2843. [Google Scholar]
- 27.Hecht L.L., Wagner C., Landfester K., Schuchmann H.P. Surfactant concentration regime in miniemulsion polymerization for the formation of MMA nanodroplets by high-pressure homogenization. Langmuir. 2011;27(6):2279–2285. doi: 10.1021/la104480s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vivek K., Reddy H., Murthy R.S. Investigations of the effect of the lipid matrix on drug entrapment, in vitro release, and physical stability of olanzapine-loaded solid lipid nanoparticles. AAPS PharmSciTech. 2007;8(4):16–24. doi: 10.1208/pt0804083. [DOI] [PubMed] [Google Scholar]
- 29.Gall V., Runde M., Schuchmann H. Extending applications of high-pressure homogenization by using simultaneous emulsification and mixing (SEM)—an overview. Processes (Basel). 2016;4(4):46. [Google Scholar]
- 30.Juttulapa M., Piriyaprasarth S., Takeuchi H., Sriamornsak P. Effect of high-pressure homogenization on stability of emulsions containing zein and pectin. Asian J. Pharm. Sci. 2017;12(1):21–27. doi: 10.1016/j.ajps.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yadav K.S., Kale K. High pressure homogenizer in pharmaceuticals: understanding its critical processing parameters and applications. J. Pharm. Innov. 2019 [Google Scholar]
- 32.Lomis N., Westfall S., Farahdel L., Malhotra M., Shum-tim D., Prakash S. Human serum albumin nanoparticles for use in cancer drug Delivery : process optimization and in vitro characterization. Nanomaterials. 2016;6(6):1–17. doi: 10.3390/nano6060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yadav K.S., Sawant K.K. Formulation optimization of etoposide loaded PLGA nanoparticles by double factorial design and their evaluation. Curr. Drug Deliv. 2010;7(1):51–64. doi: 10.2174/156720110790396517. [DOI] [PubMed] [Google Scholar]
- 34.Kushwah V., Agrawal A.K., Dora C.P., Mallinson D., Lamprou D.A., Gupta R.C., Jain S. Novel gemcitabine conjugated albumin nanoparticles: a potential strategy to enhance drug efficacy in pancreatic cancer treatment. Pharm. Res. 2017;34(11):2295–2311. doi: 10.1007/s11095-017-2238-8. [DOI] [PubMed] [Google Scholar]
- 35.Peng J., Dong W.J., Li L., Xu J.M., Jin D.J., Xia X.J., Liu Y.L. Effect of high-pressure homogenization preparation on mean globule size and large-diameter tail of oil-in-water injectable emulsions. J. Food Drug Anal. 2015;23(4):828–835. doi: 10.1016/j.jfda.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong Y., Feng S.S. Poly (D, L-lactide-co-glycolide)(PLGA) nanoparticles prepared by high pressure homogenization for paclitaxel chemotherapy. Int. J. Pharm. 2007;342(1-2):208–214. doi: 10.1016/j.ijpharm.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 37.Akbari B., Tavandashti M.P., Zandrahimi M. Particle size characterization of nanoparticles–a practical approach. Iran J. Mater. Sci. Eng. 2011;8(2):48–56. [Google Scholar]
- 38.Albanese A., Tang P.S., Chan W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 39.Kurd M., Sadegh Malvajerd S., Rezaee S., Hamidi M., Derakhshandeh K. Oral delivery of indinavir using mPEG-PCL nanoparticles: preparation, optimization, cellular uptake, transport and pharmacokinetic evaluation. Artif. Cells Nanomed. Biotechnol. 2019;47(1):2123–2133. doi: 10.1080/21691401.2019.1616553. [DOI] [PubMed] [Google Scholar]
- 40.Banerjee A., Qi J., Gogoi R., Wong J., Mitragotri S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Contr. Release. 2016;238:176–185. doi: 10.1016/j.jconrel.2016.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filipe V., Hawe A., Jiskoot W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010;27(5):796–810. doi: 10.1007/s11095-010-0073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srinivas N.S.K., Verma R., Kulyadi G.P., Kumar L. A quality by design approach on polymeric nanocarrier delivery of gefitinib: formulation, in vitro, and in vivo characterization. Int. J. Nanomed. 2017;12:15. doi: 10.2147/IJN.S122729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clayton K.N., Salameh J.W., Wereley S.T., Kinzer-Ursem T.L. Physical characterization of nanoparticle size and surface modification using particle scattering diffusometry. Biomicrofluidics. 2016;10(5):05410711–05410714. doi: 10.1063/1.4962992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.System and Methods of Analyzing Particles in a Fluid. 2017. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2017136664 [Google Scholar]
- 45.Fang J., Nakamura H., Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011;63(3):136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Hughes J.M., Budd P.M., Grieve A., Dutta P., Tiede K., Lewis J. Highly monodisperse, lanthanide-containing polystyrene nanoparticles as potential standard reference materials for environmental “nano” fate analysis. J. Appl. Polym. Sci. 2015;132(24):42061–42069. [Google Scholar]
- 47.Rezaei G., Daghighi S.M., Raoufi M., Esfandyari-Manesh M., Rahimifard M., Mobarakeh V.I., Kamalzare S., Ghahremani M.H., Atyabi F., Abdollahi M., Rezaee F. Synthetic and biological identities of polymeric nanoparticles influencing the cellular delivery: an immunological link. J. Colloid Interface Sci. 2019;556:476–491. doi: 10.1016/j.jcis.2019.08.060. [DOI] [PubMed] [Google Scholar]
- 48.Culver K.S., Shin Y.J., Rotz M.W., Meade T.J., Hersam M.C., Odom T.W. Shape-dependent relaxivity of nanoparticle-based T 1 magnetic resonance imaging contrast agents. J. Phys. Chem. C Nanomater. Interfaces. 2016;120(38):22103–22109. doi: 10.1021/acs.jpcc.6b08362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rampersaud S., Fang J., Wei Z., Fabijanic K., Silver S., Jaikaran T., Ruiz Y., Houssou M., Yin Z., Zheng S., Hashimoto A. The effect of cage shape on nanoparticle-based drug carriers: anticancer drug release and efficacy via receptor blockade using dextran-coated iron oxide nanocages. Nano Lett. 2016;16(12):7357–7363. doi: 10.1021/acs.nanolett.6b02577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hussain Z., Khan S., Imran M., Sohail M., Shah S.W.A., de Matas M., PEGylation A promising strategy to overcome challenges to cancer-targeted nanomedicines: a review of challenges to clinical transition and promising resolution. Drug Deliv. Transl. Res. 2019;9(3):721–734. doi: 10.1007/s13346-019-00631-4. [DOI] [PubMed] [Google Scholar]
- 51.Caldorera-Moore M., Guimard N., Shi L., Roy K. Designer nanoparticles: incorporating size, shape and triggered release into nanoscale drug carriers. Expet Opin. Drug Deliv. 2010;7(4):479–495. doi: 10.1517/17425240903579971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Decuzzi P., Ferrari M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials. 2006;27(30):5307–5314. doi: 10.1016/j.biomaterials.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 53.Williford J.M., Santos J.L., Shyam R., Mao H.Q. Shape control in engineering of polymeric nanoparticles for therapeutic delivery. Biomater Sci. 2015;3(7):894–907. doi: 10.1039/C5BM00006H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vyas S.P., Goswami R. Size-dependent cellular uptake and TLR4 attenuation by gold nanoparticles in lung adenocarcinoma cells. Nanomedicine. 2019;14(3):229–253. doi: 10.2217/nnm-2018-0266. [DOI] [PubMed] [Google Scholar]
- 55.Wu M., Guo H., Liu L., Liu Y., Xie L. Size-dependent cellular uptake and localization profiles of silver nanoparticles. Int. J. Nanomed. 2019;14:4247. doi: 10.2147/IJN.S201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y., Yang B., Zhang X. Oral delivery of imatinib through galactosylated polymeric nanoparticles to explore the contribution of a saccharide ligand to absorption. Int. J. Pharm. 2019;568:118508. doi: 10.1016/j.ijpharm.2019.118508. [DOI] [PubMed] [Google Scholar]
- 57.Wang J., Tian S., Petros R.A., Napier M.E., DeSimone J.M. The complex role of multivalency in nanoparticles targeting the transferrin receptor for cancer therapies. J. Am. Chem. Soc. 2010;132(32):11306–11313. doi: 10.1021/ja1043177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arvizo R.R., Miranda O.R., Thompson M.A., Pabelick C.M., Bhattacharya R., Robertson J.D., Rotello V.M., Prakash Y.S., Mukherjee P. Effect of nanoparticle surface charge at the plasma membrane and beyond. Nano Lett. 2010;10(7):2543–2548. doi: 10.1021/nl101140t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao J., Zheng D., Tao Y., Li Y., Wang L., Liu J., He J., Lei J. Self-assembled pH-responsive polymeric nanoparticles based on lignin-histidine conjugate with small particle size for efficient delivery of anti-tumor drugs. Biochem. Eng. J. 2020;156:107526. [Google Scholar]
- 60.Pang L., Pei Y., Uzunalli G., Hyun H., Lyle L.T., Yeo Y. Surface modification of polymeric nanoparticles with M2pep peptide for drug delivery to tumor-associated macrophages. Pharm. Res. 2019;36(4):65. doi: 10.1007/s11095-019-2596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y., Zheng Y., Zhang L., Wang Q., Zhang D. Stability of nanosuspensions in drug delivery. J. Contr. Release. 2013;172(3):1126–1141. doi: 10.1016/j.jconrel.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 62.de Oca-Ávalos J.M.M., Candal R.J., Herrera M.L. Nanoemulsions: stability and physical properties. Curr. Opin. Food Sci. 2017;16:1–6. [Google Scholar]
- 63.Lei C., Davoodi P., Zhan W., Chow P.K.H., Wang C.H. Development of Nanoparticles for Drug Delivery to Brain Tumor: the effect of surface materials on penetration into brain tissue. J. Pharm. Sci. 2019;108(5):1736–1745. doi: 10.1016/j.xphs.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 64.Vandervoort J., Yoncheva K., Ludwig A. Influence of the homogenisation procedure on the physicochemical properties of PLGA nanoparticles. Chem. Pharm. Bull. (Tokyo). 2004;52(11):1273–1279. doi: 10.1248/cpb.52.1273. [DOI] [PubMed] [Google Scholar]
- 65.Yadav K.S., Chuttani K., Mishra A.K., Sawant K.K. Effect of size on the biodistribution and blood clearance of etoposide-loaded PLGA nanoparticles. PDA J. Pharm. Sci. Technol. 2011;65(2):131–139. [PubMed] [Google Scholar]
- 66.Shen S., Wu Y., Liu Y., Wu D. High drug-loading nanomedicines: progress, current status, and prospects. Int. J. Nanomed. 2017;12:4085. doi: 10.2147/IJN.S132780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang R.Y., Xing R.R., Jiao T.F. Carrier-free, chemophotodynamic dual nanodrugs via self-assembly for synergistic antitumor therapy. ACS Appl. Mater. Interfaces. 2016;8(21):13262–13269. doi: 10.1021/acsami.6b02416. [DOI] [PubMed] [Google Scholar]
- 68.Soni G., Yadav K.S. High encapsulation efficiency of poloxamer-based injectable thermoresponsive hydrogels of etoposide. Pharmaceut. Dev. Technol. 2014;19(6):651–661. doi: 10.3109/10837450.2013.819014. [DOI] [PubMed] [Google Scholar]
- 69.Byeon H.J., Lee S., Min S.Y., Lee E.S., Shin B.S., Choi H.G., Youn Y.S. Doxorubicin -loaded nanoparticles consisted of cationic-and mannose-modified-albumins for dual-targeting in brain tumors. J. Contr. Release. 2016;225:301–313. doi: 10.1016/j.jconrel.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 70.Malinovskaya Y., Melnikov P., Baklaushev V., Gabashvili A., Osipova N., Mantrov S., Ermolenko Y., Maksimenko O., Gorshkova M., Balabanyan V., Kreuter J. Delivery of doxorubicin-loaded PLGA nanoparticles into U87 human glioblastoma cells. Int. J. Pharm. 2017;524(1-2):77–90. doi: 10.1016/j.ijpharm.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 71.Pereverzeva E., Treschalin I., Treschalin M., Arantseva D., Ermolenko Y., Kumskova N., Maksimenko O., Balabanyan V., Kreuter J., Gelperina S. Toxicological study of doxorubicin-loaded PLGA nanoparticles for the treatment of glioblastoma. Int. J. Pharm. 2019;554:161–178. doi: 10.1016/j.ijpharm.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 72.Kim J.E., Park Y.J. High paclitaxel-loaded and tumor cell-targeting hyaluronan-coated nanoemulsions. Colloids Surf. B Biointerfaces. 2017;150:362–372. doi: 10.1016/j.colsurfb.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 73.Yadav K.S., Chuttani K., Mishra A.K., Sawant K.K. Long circulating nanoparticles of etoposide using PLGA-MPEG and PLGA-pluronic block copolymers: characterization, drug-release, blood-clearance, and biodistribution studies. Drug Dev. Res. 2010;71(4):228–239. [Google Scholar]
- 74.Min S.Y., Byeon H.J., Lee C., Seo J., Lee E.S., Shin B.S., Choi H.G., Lee K.C., Youn Y.S. Facile one-pot formulation of TRAIL-embedded paclitaxel-bound albumin nanoparticles for the treatment of pancreatic cancer. Int. J. Pharm. 2015;494(1):506–515. doi: 10.1016/j.ijpharm.2015.08.055. [DOI] [PubMed] [Google Scholar]
- 75.Battogtokh G., Ko Y.T. Self-assembled chitosan-ceramide nanoparticle for enhanced oral delivery of paclitaxel. Pharm. Res. 2014;31(11):3019–3030. doi: 10.1007/s11095-014-1395-2. [DOI] [PubMed] [Google Scholar]
- 76.Lee C., Kim B., Lee S., Kim T.H., Kim J.O., Lee E.S., Oh K.T., Choi H.G., Yoo S.D., Youn Y.S. Doxorubicin and paclitaxel co-bound lactosylated albumin nanoparticles having targetability to hepatocellular carcinoma. Colloids Surf. B Biointerfaces. 2017;152:183–191. doi: 10.1016/j.colsurfb.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 77.Kim B., Lee C., Lee E.S., Shin B.S., Youn Y.S. Paclitaxel and curcumin co-bound albumin nanoparticles having antitumor potential to pancreatic cancer. Asian J. Pharm. 2016;11(6):708–714. [Google Scholar]
- 78.Wang Z., Li Z., Zhang D., Miao L., Huang G. Development of etoposide-loaded bovine serum albumin nanosuspensions for parenteral delivery. Drug Deliv. 2015;22(1):79–85. doi: 10.3109/10717544.2013.871600. [DOI] [PubMed] [Google Scholar]
- 79.Yu X., Di Y., Xie C., Song Y., He H., Li H., Pu X., Lu W., Fu D., Jin C. An in vitro and in vivo study of gemcitabine-loaded albumin nanoparticles in a pancreatic cancer cell line. Int. J. Nanomed. 2015;10:6825–6834. doi: 10.2147/IJN.S93835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alayoubi A.Y., Anderson J.F., Satyanarayanajois S.D., Sylvester P.W., Nazzal S. Concurrent delivery of tocotrienols and simvastatin by lipid nanoemulsions potentiates their antitumor activity against human mammary adenocarcenoma cells. Eur. J. Pharmaceut. Sci. 2013;48(3):385–392. doi: 10.1016/j.ejps.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 81.Zu Y., Meng L., Zhao X., Ge Y., Yu X., Zhang Y., Deng Y. Preparation of 10-hydroxycamptothecin-loaded glycyrrhizic acid-conjugated bovine serum albumin nanoparticles for hepatocellular carcinoma-targeted drug delivery. Int. J. Nanomed. 2013;8:1207. doi: 10.2147/IJN.S40493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kushwah V., Katiyar S.S., Dora C.P., Agrawal A.K., Lamprou D.A., Gupta R.C., Jain S. Co-delivery of docetaxel and gemcitabine by anacardic acid modified self-assembled albumin nanoparticles for effective breast cancer management. Acta Biomater. 2018;73:424–436. doi: 10.1016/j.actbio.2018.03.057. [DOI] [PubMed] [Google Scholar]