Abstract
Purpose
Premature ovarian insufficiency (POI), which is characterized by early menopause before the age of 40 years, affects approximately 1–5% of women. Cytoplasmic polyadenylation element binding protein 1 (CPEB1) is a post-transcriptional regulatory protein that is highly expressed in germ cells and promotes oocytes maturation, and several studies have found microdeletions of chromosome 15q25.2, which contains the CPEB1 gene, in POI patients. However, the deleted region also includes other plausible genes, and thus the contribution of CPEB1 to POI is uncertain. The present study aimed to determine the relationship between CPEB1 deletion and POI in a Chinese cohort.
Material and methods
Quantitative real-time polymerase chain reaction (qPCR) with primers for exon 4 and exon 11 of CPEB1 was performed to detect the CPEB1 deletion in 323 patients with POI and in 300 healthy controls. Subsequent qPCR with primers for each exon of CPEB1 was performed to precisely localize the deletion locus.
Results
One patient with primary amenorrhea was found to carry a heterozygous deletion of exons 8–12 of the CPEB1 gene.
Conclusion
Our study is the first to search for CPEB1 deletions in POI patients using a simple qPCR method, and we show that CPEB1 deletion is not a common cause for POI in a Chinese cohort.
Keywords: CPEB1, POI, Microdeletion, Meiosis, Polyadenylation
Introduction
Premature ovarian insufficiency (POI) is characterized by cessation of menstruation before 40 years of age combined with a serum level of follicle stimulating hormone (FSH) above 25 IU/L [1]. Approximately 1–5% of women under 40 years old are affected by POI and are at high risk of osteoporosis, cardiovascular disease, and other long-term health complications due to estrogen deficiency, thus adversely affecting women’s health both physiologically and psychologically [2].
The etiology of POI is heterogeneous, including chromosome abnormalities, gene mutations, autoimmune diseases, and iatrogenic factors; however, most cases are idiopathic. Genetic disorders account for 20–25% of POI cases, and chromosomal abnormalities explain 10–15% [3]. Mutations have been identified in the genes participating in meiosis, folliculogenesis, and steroid hormone synthesis, such as STAG3, SYCE1, MSH5, GDF9, NOBOX, and FSHR. However, only a minority of the mutations have been verified through functional assays [3, 4].
The genes involved in chromosome synapsis and homologous recombination during meiosis I are essential for oogenesis and for the maintenance of ovarian function. Deficiencies in the proteins encoded by these genes lead to oocyte apoptosis and infertility. Cytoplasmic polyadenylation element binding protein 1 (CPEB1) has been found to regulate the translation of essential genes – such as SYCP1 and SYCP3 – that are responsible for synaptonemal complex formation and homologous chromosome recombination [5]. Cpeb1-knockout mice show atrophic ovaries devoid of oocytes, mimicking the phenotype of human POI [6]. Previous studies found that CPEB1 heterozygous deletions occur in 0.33–2.9% of POI patients of Caucasian descent using array comparative genomic hybridization assay (CGH array) or quantitative multiplex PCR of short fluorescent fragments techniques (Table 1). Here, we used quantitative real-time polymerase chain reaction (qPCR) to locate the precise deletion region of CPEB1 in 323 Chinese patients with sporadic POI.
Table 1.
Previous studies of CPEB1 deletions in POI patients
| Case No. | Deletion | Type of amenorrhea | Familial history | Other features | Prevalence | Citation |
|---|---|---|---|---|---|---|
| Case 1 | 15q25.2 | Secondary | – | – | 0.33% | Tsuiko et al. |
| Case 2 | 15q25.2 | Primary | – | Behavioral disorders, progressive intellectual deficiency | 1.15% | Hyon et al. |
| Case 3 | 15q25.2 | Secondary | – | – | ||
| Case 4 | 15q25.2 | Primary | Yes | – | ||
| Case 5 | 15q25.2 | Primary | – | – | 1.12% | McGuire et al. |
| Case 6 | 15q25.2 | Primary | Yes | – | 2.9% | Bestetti et al. |
| Case 7 | 15q25.2 | Primary | Yes | – |
Materials and methods
Participant population
The inclusion criteria for POI were cessation of menstruation before 40 years of age and at least twice serum follicle stimulating hormone (FSH) concentrations exceeding 25 IU/L. In the present study, 123 patients with primary amenorrhea and 200 patients with secondary amenorrhea were recruited from Shandong Provincial Hospital Affiliated to Shandong University. Women with a history of pelvic surgery, chemotherapy, radiation therapy, or chromosome abnormalities were excluded. Three hundred women above 40 years of age with normal menorrhea and delivery history were recruited as controls. Written informed consents were obtained from all participants. The study was approved by the Institutional Review Board of Center for Reproductive Medicine, Shandong University.
Quantitative real-time polymerase chain reaction
Genomic DNA was extracted from the peripheral blood of all participants. The CPEB1 gene includes 12 exons, and qPCR with primers recognizing sequences in exon 4 and exon 11 was performed to screen for the gene deletion according to a previous CGH array with probes targeting exon 4 of CPEB1.
The 114 bp fragment targeting the ACTB gene was used as the normalization control (Table 2). DNA samples from women with no microdeletions according to the CGH array were used as the negative control in each assay. To determine the gene dosage of every amplified region, dosage quotients were obtained by dividing the ratio of the sample (2-∆∆CT of the CPEB1 fragment compared to ACTB) by the corresponding ratio of the negative control DNA. This equation provided a theoretical dosage quotient value of 1.0 for two copies and a value of 0.5 for deletions. Three independent replicates were performed for each case, and independent samples t-tests were used to test for differences. We identified a CPEB1 deletion in one patient, and qPCR with primers for each exon of CPEB1 was performed to localize the deletion region.
Table 2.
Primers for qPCR of CPEB1 and ACTB
| Forward primer | Reverse primer | |
|---|---|---|
| Exon 1 | 5′-AGCGGCTCGTAGGAGCTTCAT-3’ | 5′-TTACCAGCGGGAACGCCAT-3’ |
| Exon 2 | 5′-GATAAAAGATTGCTGGGACAACC-3’ | 5′-CAGATGCCTACCACGTTCAAGT-3’ |
| Exon 3 | 5′-GCTTTTCCCAACCTCTGCG-3’ | 5′-TACCCCAGCCAACTCATTCTC-3’ |
| Exon 4 | 5′-AGTTTCCAGCACCCTCAGTTAG-3’ | 5′-CACAATAATCTCCACTCCTCCC-3’ |
| Exon 5 | 5′-CTTCGCATTTCTCCACCTCTG-3’ | 5′-TGGTTGGGGAGGGAGTGACT- 3’ |
| Exon 6 | 5′-AAGCCACCTGTACCTGGAGTG-3’ | 5′-GCCCCACCCTTCAACTCTTA-3’ |
| Exon 7 | 5′-CTTCTGCCATTCTTTTCTGTCTC-3’ | 5′-GTGCCCACCATGTTACCAAC-3’ |
| Exon 8 | 5′-CTGTCCGATCCTTGCTTCA-3’ | 5′-CCTGCCTATCCACCTACCAC-3’ |
| Exon 9 | 5′-AGGGCGTTAGCTTAGCTTCAG-3’ | 5′-GGCATACACCACTCCACCAA-3’ |
| Exon 10 | 5′-CCAACGGAGTTACCTGAAAGC-3’ | 5′-CGTACAAAGACCAAGCCCAC-3’ |
| Exon 11 | 5′-TGAATCCCAGAGGCATCCAG-3’ | 5′-ACAGAAGAAAGGACCAGGCT-3’ |
| Exon 12 | 5′-TGTAACAAGGATGGTGGGTTTG-3’ | 5′-GTTTGGAGAAGGGTGGGAGAC-3’ |
| ACTB | 5′-ATTCCTATGTGGGCGACGA-3’ | 5′-TGTGGTGCCAGATTTTCTCC-3’ |
Results
In our qPCR of CPEB1 exon 4 and exon 11 in 323 patients and 300 controls, we found that the DNA dosage of exon 11 was reduced by half in one patient. Further qPCR for each exon of CPEB1 identified a heterozygous deletion of exons 8–12(Fig. 1).
Fig. 1.
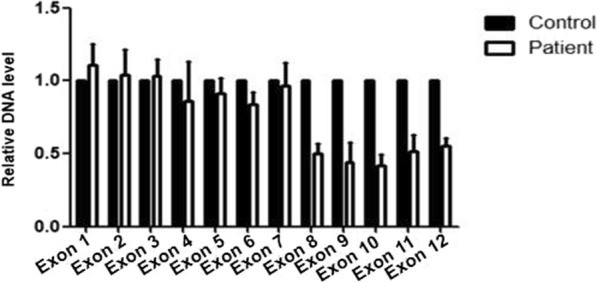
Heterozygous deletion of CPEB1 exons 8–12 was identified in one POI patient
The patient carrying the CPEB1 deletion was diagnosed with POI at 18 years of age presenting with primary amenorrhea and elevated FSH level. Ultrasound examination showed small ovaries and no visible follicles.
Discussion
Our qPCR experiment in 323 cases with sporadic POI provides the first precise identification of a CPEB1 microdeletion in a Chinese population, supporting the existence of CPEB1 haploinsufficiency consisted in POI pathogenesis, albeit with a low frequency of 0.31%.
CPEB1 is an important post-transcriptional regulatory protein that binds to the CPE region of target mRNAs and promotes the polyadenylation and translation of the mRNAs [7, 8]. CPEB1 is preferentially expressed in germ cells and regulates the translation of crucial genes involved in meiosis and oogenesis, such as the synapsis complex protein genes SYCP1 and SYCP3. Oocytes that are deficient in CPEB1 arrest at the pachytene stage due to abnormal synaptonemal complex formation, resulting in ovarian atresia and infertility [6]. Moreover, in Cpeb1-knockdown oocytes, the expression of growth differentiation factor 9 (GDF9), which is required for folliculogenesis, is significantly reduced due to a shortened mRNA poly(A) tail, indicating a pivotal role for CPEB1 in oogenesis after birth [9]. Our results combined with previous studies support the hypothesis that CPEB1 deficiency in humans might accelerate follicle atresia due to disrupted meiosis and oogenesis, thus resulting in ovarian failure at an earlier age, as is the case in POI.
In previous studies using CGH array or SNP array analysis, a total of seven POI cases were found to carry a heterozygous deletion of chromosome 15q25.2, where CPEB1 is partially localized (Table 1). Hyon et al. [10] reported three patients carrying the 15q25.2 deletion in 259 (1.15%) POI cases, and two of them presented with primary amenorrhea and the other presented with secondary amenorrhea. McGuire et al. [11] screened 89 POI patients and identified the 15q25.2 deletion in one patient (1.12%) suffering from primary amenorrhea. Tsuiko et al. [12] observed one case with the 15q25.2 deletion in the cohort samples from a bio bank (1/301, 0.33%). Finally, the study by Bestetti et al. [13] used a high-resolution CGH-array method and found 2 patients among 67 POI cases (2.9%) harboring the deletion. However, the deleted region of 15q25.2 contains more than 10 genes, and whether the CPEB1 deletion was completely or partially responsible for POI remained uncertain. Moreover, the probes used previously to target the CPEB1 gene were localized to exons 1–5, which could not identify any deficiencies in other exons. In addition, the breakpoint of the deletion was ambiguous. Therefore, the causative role of CPEB1 deletion in the pathogenesis of POI required more evidence. In the present study, two pairs of qPCR primers recognizing sequences in CPEB1 exon 4 and exon 11 were designed, which expanded the probe-targeting region. Similarly to previous studies, the frequency of CPEB1 microdeletion (1/323, 0.31%) in our cohort was low, indicating that CPEB1 microdeletion is not a common cause for POI in a Chinese population. Furthermore, we identified the specific deletion region of CPEB1 as being exons 8–12, which indicates the vital role of this region for the function of CPEB1.
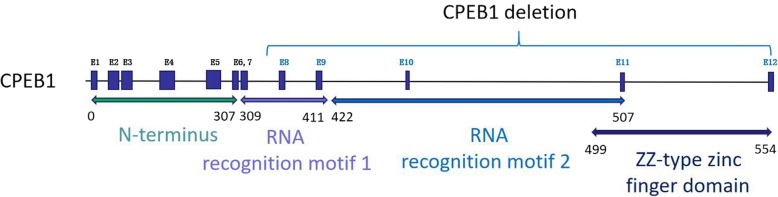
The CPEB1 protein is composed of three regions – an amino-terminal portion with no obvious functional motifs, two RNA recognition motifs (RRMs), and a zinc finger motif [14]. Previous studies have demonstrated that the RRMs and the zinc finger motifs are essential for the RNA binding capacity of CPEB1 [15], and Merkel et al. [16] found that the C-Terminal region of CPEB1 has the potential to recruit other proteins during the assembly of the ribonucleoprotein complex. Because the RRMs, zinc finger motifs, and C-terminal region are all encoded by exons 8–12, it can be assumed that the CPEB1 protein translated from the allele without these exons is non-functional (Fig. 2), and thus haploinsufficiency of CPEB1 due to the heterozygous microdeletion is likely to be the causative factor for the POI phenotype in a small subset of patients.
Fig. 2.
The gene structure of CPEB1 and the deletion region identified in the present study
The present study has some limitations that must be noted. The primary qPCR only tested exon 4 and exon 11 of CPEB1, which could have missed microdeletions outside this region. Moreover, the functional study was not performed given the fact that the proteins regulated by CPEB1 are expressed in oocytes during meiosis or folliculogenesis, which cannot be cultured in vitro. Considering the limitation, animal models with CPEB1 deletion in exons 8–12 might be generated to further illustrate the pathogenic mechanism of such deficiency on mammalian oogenesis and ovarian function.
Conclusion
Taken together, our results further illustrate that deficiency in CPEB1, which is required for synaptonemal complex formation and oogenesis, contributes to only a small minority of POI cases.
Acknowledgments
The authors thank all of the participants involved in this study.
Authors’ contributions
T.G. and Y. Q collected participants’ samples and clinical data. W.J., R. L and T. G performed the qPCR and statistical analysis under the guidance of S.Z. and Y.Q. T.G. and W.J. wrote the manuscript under the supervision of Y.Q. All authors contributed to this work, discussed the results, and critically reviewed and revised the manuscript. The author(s) read and approved the final manuscript.
Funding
This study was supported by grants from the National Key Research & Developmental Program of China (2017YFC1001100), the National Natural Science Foundation of China (81571406, 81522018, 81771541, and 31601198), the Science Foundation for Distinguished Young Scholars of Shandong (ZR201702150261), and the Key Research and Development Plan of Shandong Province (2018GSF118219).
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ting Guo, Email: gtlyp2008@126.com.
Yingying Qin, Email: qinyingying1006@163.com.
References
- 1.Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, et al. ESHRE guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31:926–937. doi: 10.1093/humrep/dew027. [DOI] [PubMed] [Google Scholar]
- 2.De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. The Lancet. 2010;376:911–921. doi: 10.1016/S0140-6736(10)60355-8. [DOI] [PubMed] [Google Scholar]
- 3.Jiao X, Ke H, Qin Y, Chen ZJ. Molecular genetics of premature ovarian insufficiency. Trends Endocrinol Metab. 2018;29:795–807. doi: 10.1016/j.tem.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Laven JS. Primary ovarian insufficiency. Semin Reprod Med. 2016;34:230–234. doi: 10.1055/s-0036-1585402. [DOI] [PubMed] [Google Scholar]
- 5.Gebauer F, Hentze MW. Fertility Facts: Male and Female Germ Cell Development Requires Translational Control by CPEB. Mol Cell. 2001;8:247–249. doi: 10.1016/S1097-2765(01)00326-4. [DOI] [PubMed] [Google Scholar]
- 6.Tay J, Richter JD. Germ cell differentiation and Synaptonemal complex formation are disrupted in CPEB knockout mice. Dev Cell. 2001;1:201–213. doi: 10.1016/S1534-5807(01)00025-9. [DOI] [PubMed] [Google Scholar]
- 7.Reyes JM, Ross PJ. Cytoplasmic polyadenylation in mammalian oocyte maturation. Wiley Interdiscip Rev RNA. 2016;7:71–89. doi: 10.1002/wrna.1316. [DOI] [PubMed] [Google Scholar]
- 8.Mendez R, Richter JD. Translational control by CPEB: a means to the end. Nat Rev Mol Cell Biol. 2001;2:521–529. doi: 10.1038/35080081. [DOI] [PubMed] [Google Scholar]
- 9.Racki WJ, Richter JD. CPEB controls oocyte growth and follicle development in the mouse. Development. 2006;133:4527–4537. doi: 10.1242/dev.02651. [DOI] [PubMed] [Google Scholar]
- 10.Hyon C, Mansour-Hendili L, Chantot-Bastaraud S, Donadille B, Kerlan V, Dodé C, et al. Deletion of CPEB1 gene: a rare but recurrent cause of premature ovarian insufficiency. J Clin Endocrinol Metab. 2016;101:2099–2104. doi: 10.1210/jc.2016-1291. [DOI] [PubMed] [Google Scholar]
- 11.McGuire MM, Bowden W, Engel NJ, Ahn HW, Kovanci E, Rajkovic A. Genomic analysis using high-resolution single-nucleotide polymorphism arrays reveals novel microdeletions associated with premature ovarian failure. Fertil Steril. 2011;95:1595–1600. doi: 10.1016/j.fertnstert.2010.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tšuiko O, Nõukas M, Žilina O, Hensen K, Tapanainen JS, Mägi R, et al. Copy number variation analysis detects novel candidate genes involved in follicular growth and oocyte maturation in a cohort of premature ovarian failure cases. Hum Reprod. 2016;31:1913–1925. doi: 10.1093/humrep/dew142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bestetti I, Castronovo C, Sironi A, Caslini C, Sala C, Rossetti R, et al. High-resolution array-CGH analysis on 46,XXpatients affected by early onset primary ovarian insufficiency discloses new genes involved in ovarian function. Hum Reprod. 2019;34:574–583. doi: 10.1093/humrep/dey389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richter JD. CPEB: a life in translation. Trends Biochem Sci. 2007;32:279–285. doi: 10.1016/j.tibs.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Hake LE, Richter JD. CPEB is a specificity factor that mediates cytoplasmic polyadenylation during Xenopus oocyte maturation. Cell. 1994;79:617–627. doi: 10.1016/0092-8674(94)90547-9. [DOI] [PubMed] [Google Scholar]
- 16.Merkel DJ, Wells SB, Hilburn BC, Elazzouzi F, Pérez-Alvarado GC, Lee BM. The C-terminal region of cytoplasmic polyadenylation element binding protein is a ZZ domain with potential for protein-protein interactions. J Mol Biol. 2013;425:2015–2026. doi: 10.1016/j.jmb.2013.03.009. [DOI] [PubMed] [Google Scholar]